Highlights
-
•
MVP level were up-regulated in temozolomide-resistant glioblastoma cells and glioblastoma stem cells.
-
•
MVP decreased the sensitization to temozolomide of glioblastoma cells and glioblastoma stem cells.
-
•
Knockdown of MVP reduced temozolomide-resistance, sphere formation ability and invasive capacity.
-
•
Negative correlation between MVP expression and prognosis of glioblastoma patients
Keywords: Cancer stem cells, Glioblastoma, Major vault protein, Tumorsphere, Temozolomide
Abstract
The resistance of highly aggressive glioblastoma multiforme (GBM) to chemotherapy is a major clinical problem resulting in a poor prognosis. GBM contains a rare population of self-renewing cancer stem cells (CSCs) that proliferate, spurring the growth of new tumors, and evade chemotherapy. In cancer, major vault protein (MVP) is thought to contribute to drug resistance. However, the role of MVP as CSCs marker remains unknown and whether MVP could sensitize GBM cells to Temozolomide (TMZ) also is unclear. We found that sensitivity to TMZ was suppressed by significantly increasing the MVP expression in GBM cells with TMZ resistance. Also, MVP was associated with the expression of other multidrug-resistant proteins in tumorsphere of TMZ-resistant GBM cell, and was highly co-expressed with CSC markers in tumorsphere culture. On the other hands, knockdown of MVP resulted in reduced sphere formation and invasive capacity. Moreover, high expression of MVP was associated with tumor malignancy and survival rate in glioblastoma patients. Our study describes that MVP is a potentially novel maker for glioblastoma stem cells and may be useful as a target for preventing TMZ resistance in GBM patients.
Introduction
Glioblastoma multiforme (GBM), classified as a grade IV tumor by the World Health Organization, is the most malignant and aggressive primary brain tumor. Despite conventional treatments such as surgical resection, radiation therapy, and adjuvant chemotherapy, the average survival is less than 2 years, and relapses are virtually inevitable. Temozolomide (TMZ) is one of the few medicines with a proven efficiency against GBM by inducing tumor cell death. However, TMZ treatment also results in drug resistance, contributing to unsatisfactory prognosis for glioma patients [1]. Therefore, therapeutic strategies targeting resistant GBM cells are important. Various factors contribute to the recurrence of brain tumors, such as issues with complete resection, resistance to chemotherapy, and the blood–brain barrier, and the existence of glioblastoma stem cells (GSCs), which are particularly chemical and radiation resistant [2].
GBM is characterized by heterogeneity, increased invasiveness, a high recurrence rate, and resistance to therapy; these properties have been attributed to the presence of GSCs in tumors [37]. GSCs display the stem cell properties of self-renewal and multi-lineage differentiation and are involved in tumor maintenance by conferring resistance to chemotherapy [3]. Indeed, expression of multiple CSC markers in GBM is negatively associated with overall survival in GBM patients. Therefore, targeting CSCs is considered a promising therapeutic strategy [4].
Effective chemotherapeutic treatment of brain tumors is primarily limited by the blood–brain barrier and the expression of ABC-binding cassette (ABC) transporter proteins, which act as drug efflux pumps [5]. Major vault protein (MVP), also known as lung resistance protein, is also frequently associated with drug resistance [6]. MVP is a primary component of the vault complex, a ribonucleoprotein particle with a hollow barrel-shaped structure that exports drugs from the nucleus for sequestration in cytosolic vesicles [7,8]. Several in vitro studies have shown that MVP is commonly overexpressed in drug-resistant human cancer cells selected after treatment with various chemotherapeutic agents [9], [10], [11]. Multiple proteins are reported to interact with MVP, including the estrogen receptor, Src, SHP2, COP1, and Shc3 [[12], [13], [14], [15],34]. Furthermore, MVP is involved in pathways related to tumor development and multidrug resistance (MDR), such as mTOR, PI3K/AKT, MAPK/ERK, and Notch signaling [7,10,15].
In this study, there was no difference in survival rate according to high or low MVP expression in the group without TMZ treatment. However, among the patients who received chemotherapy, those with high MVP expression had poor prognoses. MVP negatively affects the sensitization of GBM cells to TMZ. Here, we suggest that MVP, which is associated with MDR proteins, offers a specific approach to target GSCs. We aimed to identify new functions of MVP as a novel CSC marker in GBM. To examine the clinical significance of MVP and its role in regulating GSCs, we evaluated the contribution of MVP in sustaining the stemness, and increasing the invasiveness, of GSCs. In addition, we analyzed the expression of MVP in clinical glioma specimens and its association with patient prognosis. Collectively, our study provides insight into the defense strategies of GBM cells, which may provide therapeutic targets for GSCs.
Materials and methods
Cell culture and culture conditions
Human GBM cell lines U87 (HTB-14), U118 (HTB-15), U138 (HTB-16), and LN-229 (CRL-2611) were obtained from the American Type Culture Collection (Manassas, VA, USA), and U251 (300385) cells were obtained from the CLS Cell Lines Service (Eppelheim, Germany). All the cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. All the cells were maintained at 37°C in a 5% CO2 humidified incubator.
G418 selection and expression of stably transfected U251 cells
The cells were seeded onto 48-well plates following transfection with pcDNA3.1-MVP vector for 48 h. G418 selection was started at day 2 post-transfection. To determine the optimal concentration of G418, the cells were passaged for three to four generations. Different dosages of G418 arranging from 100 to 1000 µg/mL were used to incubate with the cells. The cells which integrated the transfected plasmid are supposed to survive the G418 selection while cells without transfected plasmid integration will be eliminated. The cells were examined daily and medium changed every 2 days. Then the proportion of cell death was observed under an inverted microscope (Olympus, Tokyo, Japan). After 1 month, MVP-positive clones were selected for further expansion in G418 selection medium.
Sphere formation assay
Cells were seeded at the density of 10,000 cells/mL in 75-T flask in DMEM/F12 (SH30023.01, HyClone) supplemented with 0.04% modified B27 (17504044, Invitrogen, Carlsbad, CA, USA), 1% l-glutamine (25030081, Invitrogen), 20 ng/mL basic fibroblast growth factor (100–18B, PeproTech, Rocky Hill, NJ, USA) and 20 ng/mL epidermal growth factor (GMP100–15, PeproTech). Cells were incubated at 37℃ in a humidified 5% CO2 atmosphere, and the fresh culture medium was added once a week until cells started to form floating aggregates. The spheres with a diameter > 50 μm were counted under microscopy, and were collected after 14 days. Spheroid formation was confirmed under an eclipse TS100 inverted microscope (Nikon, Tokyo, Japan). Also, IncuCyte Live-Cell Imaging System (Sartorius, Göttingen Germany) was used to monitor the tumorsphere formation, and images were taken each time for 6 h.
Temozolomide chemoresistance assay
Cells were seeded into 96-well plates at a density of 20,000 cells/well with complete growth medium. Temozolomide (TMZ, Sigma Aldrich, MO, USA) was added at different concentrations (62.5, 125, 250, 500, 1000, 2000 μM) and DMSO (solvent) was added to the control batch of cells. Cell viability was assessed by CCK-8 (Cell Counting Kit, Dongin-LS, Korea) assay. For CCK-8 assay, cells were given 24 h to attach and were treated TMZ for 24, 48, 72 h. After treatment, cells were incubated with 100 µl/well of the CCK reagent for 1 h at 37 °C and were measured the absorbance at 450 nm. All values were normalized to the vehicle control-treated wells.
Isolation of a temozolomide-resistant cell lines
Parental U251 and LN229 cells were gradually re-exposed to an incremental TMZ pulse from initiation of 50 μg/ml, reaching a concentration of 500 μg/ml. In short, cells were plated in 6-well plates in their usual medium and allowed to attach overnight, and then the medium was replaced with a medium containing TMZ every 72 h per week. The live cells were seed at the new plate and grew into a medium containing a double concentration of TMZ. The cycle was repeated for 2 months. When there was no obvious cell loss observed, cells were collected and performed to the extreme limiting dilution analysis (ELDA).
Extreme limiting dilution assay
GBM cells were dissociated into single-cell suspension and then plated into 96-well plates with sequentially decreasing densities (1–100 cells per well). Cells were incubated at 37 °C for 7 to 14 days. At the time of quantification, each well was examined for formation of tumorsphere-like cell aggregates with microscope (Olympus, Tokyo, Japan). Stem cell frequency was calculated using the ELDA software [31].
Tumorspheres immunofluorescence staining
Spheres were fixed in 3.8% formalin for 20 min, then permeabilized with Triton X-100 for 5 min, and blocked with 5% normal goat serum albumin. Rabbit anti-human primary antibodies, including CD133 (sc-30220; 1:50; Santa Cruz, CA), Nanog (#4903; 1:50; Cell Signaling, Danvers, MA, USA), Oct4 (#2840; 1:50; Cell Signaling, Danvers, MA, USA) and Sox2 (#3579; 1:50; Cell Signaling, Danvers, MA, USA), Mouse anti-human primary antibody (MVP, sc-23916; 1:50; Santa Cruz, CA), were added and incubated overnight at 4 °C using a shaking table. Subsequent to washing the tumorspheres three times with phosphate-buffered saline(PBS), donkey anti-rabbit secondary antibodies conjugated with Cy3 (Cat.406402; 1:100; Biolegend, CA, USA) and goat anti-mouse secondary antibodies conjugated with dylight (Cat.405310; 1:100; Biolegend, CA, USA) were added, and the tumorspheres were incubated at room temperature for 1 h. After washing with PBS three times, spheres were counterstained with DAPI (Vector Labs, CA, USA) [16,17]. Images were obtained using Confocal-A1 fluorescence microscopy (Nikon, Japan).
Human glioma cancer patients tissue microarrays
High-density multiple organ human tissue microarray (TMA) derived from human brain cancer was purchased from US Biomax, Inc. (Cat#GL208). This High-density TMA contains brain primary tumor tissues and normal tissues microarray, triplicated cores per case (69 cases/208 cores) from glioma patients, whose clinical data, including age, sex, pathology diagnosis and grade, were informed by the additional file; Table 1. The tumor tissues were fixed with formalin, paraffin-embedded, and sectioned by a microtome to a 5 µm thickness
Invasion assay
Transwell assay was used to evaluate the invasion abilities of the GBM cells. Twenty-four-well transwell chambers with an 8 µm pore size polycarbonate membrane (Corning Inc., Corning, NY, USA) were used in these assays. For the invasion assay, cells (10,000 cells/well) were suspended and dissociated from its sphere forms in 200 μl of serum-free DME/F-12 1:1. Cells were seeded in the upper transwell chamber, which was coated with Matrigel matrix (Corning Inc., Corning, NY, USA) with the lower well filled with 700 μl of DMEM-F12 containing 10% FBS. The invasion assay was performed at 37 °C in a humidified atmosphere with 5% CO2 for 48 h.
After incubation, cells on the top surface of the interface membrane were removed using a cotton swab. Invaded cells on the lower surface of the membrane were fixed with 10% formaldehyde, stained with a hematoxylin and eosin kit (Sysmex Corporation, Kobe, Japan), and counted under an optical microscope (100×, Nikon, Tokyo, Japan) from four random fields using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experiments were performed with Microsoft Excel 2016 or GraphPad Prism 5 analytical tools (GraphPad software, Inc.), and results were presented as the means ± SD from least three independent samples. Student's unpaired two-tailed t-test for independent analysis was applied to evaluate the differences. Survival rate analyses were performed by drawing curves and calculating log-rank p test using the Kaplan–Meier method. A p-value less than 0.05 was considered statistically significant. * P < 0.05, ** P < 0.01, *** P < 0.001.
Results
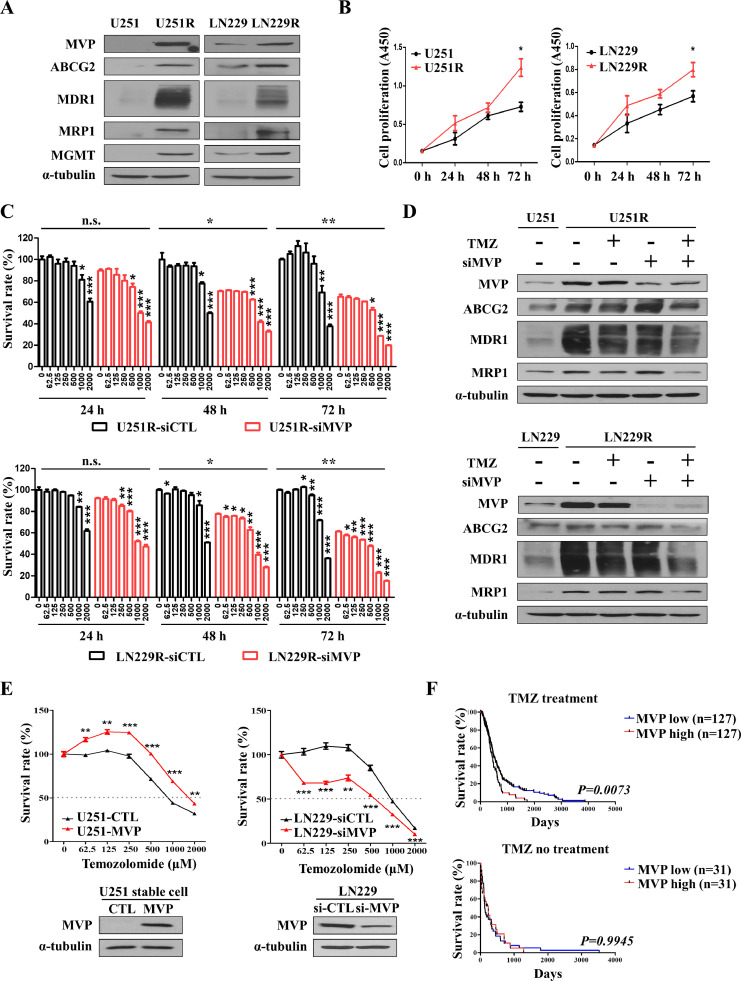
Upregulation of chemoresistance-related MVP promotes temozolomide resistance in glioblastoma cells
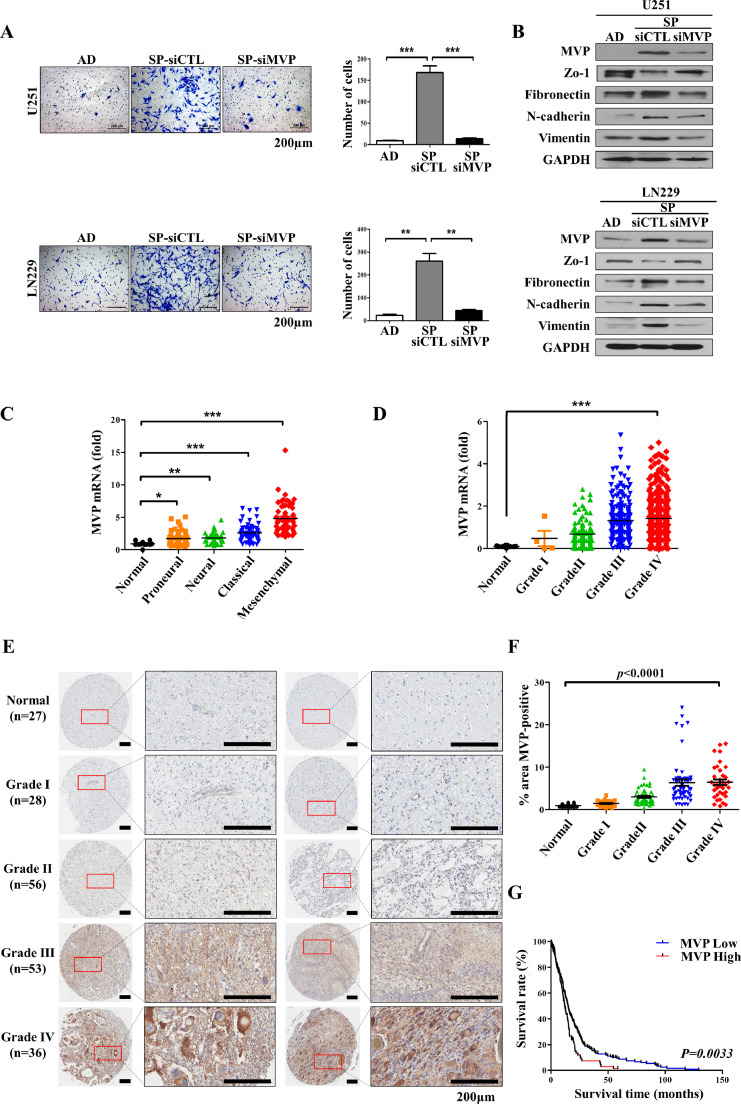
The high recurrence rate and low survival rate of GBM is because it is resistant to chemotherapy due to the presence of cancer stem cells [35]. TMZ is the most important chemotherapy drug for GBM. However, the emergence of drug resistance limits its clinical efficacy. To identify the molecules responsible for TMZ resistance, firstly, we treated U251 and LN229 cells with a low dose of TMZ in culture medium for 2 months and established TMZ-resistant cells, designated U251R and LN229R. Consistent with the increased resistance, protein levels of multidrug resistance related proteins (i.e., MVP, ABC transporters ABCG2, MDR1, and MRP1), and MGMT were elevated in U251R and LN229R cells (Fig. 1A). The morphology of U251R and LN229R cells differed from that of the parental control cells; larger cells with irregular morphology and long protrusions were observed (Supplementary Fig. 1A). TMZ-resistant U251R and LN229R cells further promoted the proliferation, compared with those parental control cells (U251 or LN229) for 24 h, 48 h, and 72 h (Fig. 1B). To determine whether MVP contributes to the development of drug resistance in GBM cells, the cells were transfected with si-MVP to downregulate its expression and the sensitivity of GBM cells to TMZ was then evaluated. To assess TMZ resistance in MVP knockdown cells of U251R and LN229R, we monitored their responses to TMZ treatment at three time points (24, 48, and 72 h). Eventually, MVP knockdown cells were more sensitive to TMZ than their control cells (U251R and LN229R) (Fig. 1C). We were performed additional experiments to rule out the effect of MVP on the survival of glioblastoma cells not exposed to TMZ. TMZ (500 μM) was treated for 72 h in LN229 cells and TMZ-resistant LN229 cells, respectively, and MVP was knocked down to compare with control. There was also a paper that MVP affects the survival of cancer cells [10], but it was possible to prove the TMZ resistance effect of MVP through this experiments (Supplementary Fig. 1C). Even when TMZ was treated on TMZ resistant cell, MVP, ABCG2, MDR1, and MRP1 all remained unchanged (lane 2, 3). However, knockdown of MVP, which increases resistance to TMZ, made resistant cells more sensitive to TMZ, and treatment with TMZ on it reduced drug resistant proteins (lane 3, 4) (Fig. 1D). We next sought to assess the drug resistance effect of MVP on GBM cells. Stable expression of MVP in U251 cells (i.e., U251-MVP) was achieved after approximately 30 days of cultivation and G418 selection (Supplementary Fig. 1B). TMZ, the standard therapeutic drug for GBM chemotherapy, further inhibited the viability of U251 and LN229 cells expressing a low level of MVP, compared with those expressing a high level, in a dose-dependent manner (Fig. 1E, Supplementary Fig. 1D). Also, GBM patients in The Cancer Genome Atlas (TCGA) database were categorized into low/high MVP expression groups depending on whether the patients received chemotherapy. We compared the survival rates of the MVP-low and -high subgroups using Kaplan–Meier analysis and found that the MVP-high group had a poor survival rate compared with the MVP-low group in the chemotherapy dataset. There was no difference in survival rate between the MVP-low and -high groups among the patients who did not undergo chemotherapy, suggesting that MVP is related to drug resistance in GBM (Fig. 1F). These data suggested that chemoresistance-related MVP down-regulation enhances the sensitivity of GBM cells to TMZ, and may improve the prognosis of GBM patients.
Fig. 1.
Upregulation of chemoresistance-related MVP promotes temozolomide resistance in glioblastoma cells. (A) Western blot analysis of MVP, ATP binding cassette (ABC) transporters (ABCG2, MDR1, and MRP), and MGMT in TMZ-resistant (U251R/LN229R) cells. α-Tubulin was used as a loading control. (B) U251/U251R (left) and LN229/LN229R (right) cells cultured in 96-well plates. CCK-8 assay was performed to determine the proliferation of TMZ-resistant cells. (C) Cell survival rate (CCK-8 assay) in U251R (top) and LN229R (bottom) cells transfected with control (siCTL) or MVP targeting siRNA (siMVP). Cells were treated with increasing concentrations of TMZ for 24, 48, 72 h. (D) Cells were processed for immunoblot analysis using the indicated antibodies. α-Tubulin was used as a loading control. (E) Control U251 cells (U251-CTL), U251 cells stably transfected with MVP (U251-MVP) (left), and siCTL- or siMVP-transfected LN229 cells (right) cultured in 96-well plates and treated with the indicated concentration of TMZ for 48 h. The viability of cells treated with TMZ was determined by CCK-8 assay (upper). Cells were processed for immunoblot analysis using the indicated antibodies. α-Tubulin was used as a loading control (bottom). (F) Kaplan–Meier plot of the survival of patients according to chemotherapy status and low versus high expression of MVP (blue and red, respectively). MVP mRNA expression in GBM patients was quantified using data from the web interface Betastasis. Data are presented as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control cells.
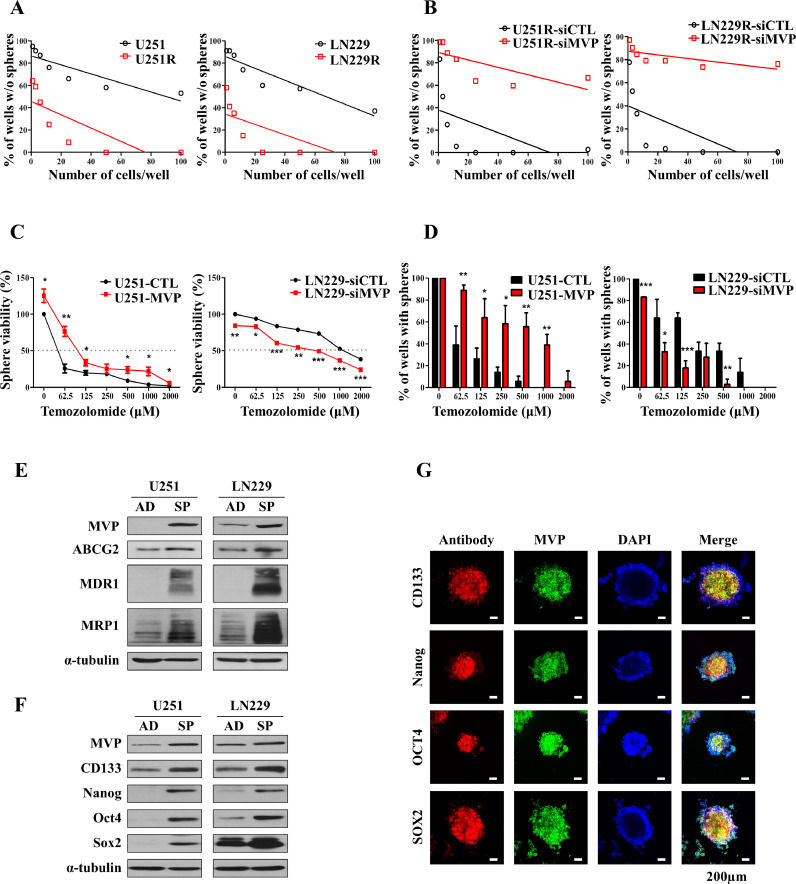
Temozolomide resistant cells promote glioblastoma stem cells formation by upregulating MVP associated with cancer stem cell markers
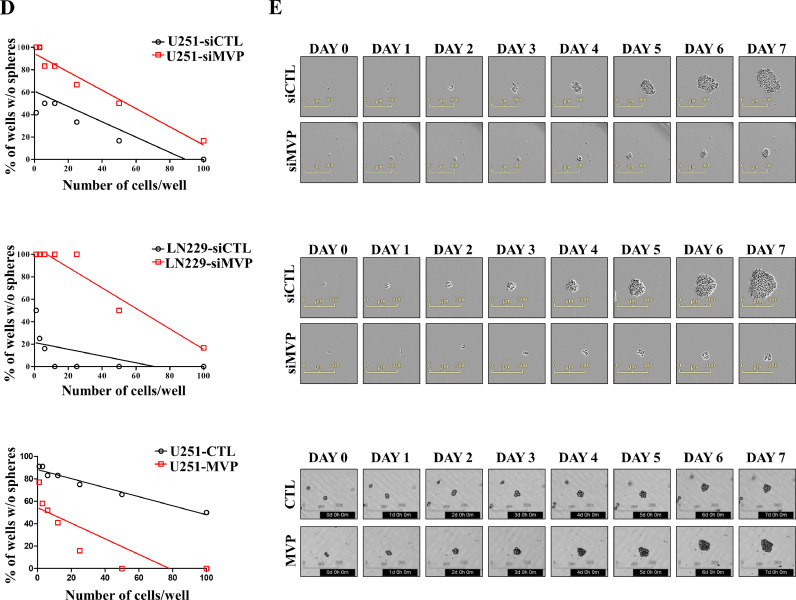
It is well known that GSCs is a major cause of tumor recurrence after chemotherapy with TMZ [35]. To further investigate the effect of TMZ on the self-renewal of GBM cells, we performed a sphere-formation assay in U251/U251R and LN229/LN229R cells. These results were performed using an extreme limiting dilution assay (ELDA), in which U251R and LN229R cells were evaluated for sphere formation 7 days after cell plating. Consistent with the ELDA results, a significant increase in tumorsphere formation was observed in TMZ-resistant cells compared with control cells (Fig. 2A). Moreover, U251R and LN229R cells transfected with siCTL or siMVP resulted in decreased tumorsphere formation in TMZ-resistant cells transfected with siMVP (Fig. 2B). Also, we sought to evaluate the sensitivity of MVP to TMZ on the viability of tumorspheres using the CCK-8 assay. Tolerance to TMZ treatment was greater in sphere-forming U251-MVP cells than U251-CTL cells. Our results suggest that sphere-forming U251-MVP cells have a survival advantage when exposed to cytotoxic TMZ (Fig. 2C, left). Stable overexpression of MVP in U251 cells also resulted in improved sphere formation compared with U251-CTL cells despite TMZ treatment (Fig. 2D, left). Conversely, U251 and LN229 derived spheres transfected with siMVP exhibited significantly reduced resistance to TMZ and sphere-forming capacity (Fig. 2C-right and D-right, and Supplementary Fig. 1E, 1F and 1 G). As a positive control, we confirmed that tumorsphere viability was increased in U251-MVP cells treated with the CSC inhibitor BBI-608 (Selleckchem, #S7977). The mode of action of BBI-608 is a mechanism that consequently suppresed cancer stemness by inhibiting STAT3, which plays an important role in cancer stem cells (Supplementary Fig. 1H). We experimented with spheroid culture and used this experimental method to mimic the environment of GSCs in vitro. AD refers to adherent growth of cells under conventional 10% serum medium, and SP refers to adherent cells induced to form non-adherent spheres cultured in CSC culture medium (Supplementary Fig. 1I). We confirmed the protein expression of MVP, as well as the MDR proteins and ABC transporters ABCG2, MDR1, and MRP1. In tumorsphere culture, expression of both MVP and the ABC transporters was increased (Fig. 2E). CSCs and ABC transporters are known to be associated with treatment resistance and outcomes in cancer patients [30]. We hypothesized that MVP is a novel CSC marker. The presence of a subpopulation of stem cell-like cells in GBM, known as GSCs, is a major factor in recurrence and drug resistance [18]. To explore the role of MVP in different GBM cell lines, we examined the expression of MVP as well as CSC markers. The GBM cell line-derived spheres showed different expression patterns of CSC markers compared with adherent cultured cells. Expression of MVP and CSC markers was determined by western blot analysis after 14 days of culture. Protein expression levels of MVP, CD133, Nanog, Oct4, and Sox2 were significantly higher in sphere cells than in adherent cultured cells (Fig. 2F and Supplementary Fig. 1J). Exogenous transfection of MVP in U251 cells did not affect the levels of CSC markers, confirming that the increase in CSC markers was due to the formation of spheroids and not MVP (Supplementary Fig. 1K). Immunofluorescence assays confirmed that MVP and CSC markers were correlated in spheres (Fig. 2G). Therefore, the MVP expression involved in chemoresistance is positively related to the cancer stem cell markers.
Fig. 2.
Temozolomide resistant cells induce MVP associated with cancer stem cell markers to promote the formation of glioblastoma stem cells. (A) Tumorsphere-forming ELDAs in U251/U251R (left) and LN229/LN229R (right) cells. Cells were plated at varying densities (1–100 cells/well) and cultured. The wells containing no spheroids were counted and plotted. (B) Tumorsphere-forming ELDAs in U251R (left) and LN229R (right) cells transfected with control (siCTL) or MVP targeting siRNA (siMVP). Cells were plated at varying densities (1–100 cells/well) and cultured. The wells containing no spheroids were counted and plotted. (C) Tumorsphere viability of MVP-overexpressing (left) or knockdown (right) cells treated with the indicated concentration of TMZ and cultured with sphere formation medium determined by CCK-8 assay. (D) Sphere formation assay performed in U251 cells stably transfected with pcDNA3.1 (control empty vector) or pcDNA3.1-MVP (left) and LN229 cells transfected with siCTL or siMVP (right). (E) Protein levels of MVP and the ATP binding cassette (ABC) transporters ABCG2, MDR1, and MRP1 in U251 and LN229 adherent cultured cells (AD) and tumorspheres (SP) assessed using western blot analysis. α-Tubulin was used as a loading control. (F) Protein levels of MVP and CD133, Nanog, Oct4, and Sox2 in adherent cultured cells (AD) and tumorspheres (SP) of U251 and LN229 GBM cell lines assessed by western blot analysis. α-Tubulin was used as a loading control. (G) Higher expression of MVP (green) and CSC markers (red) in U251 human glioma cells that formed tumorspheres. Nuclei were stained with DAPI (blue). Data are presented as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control cells.
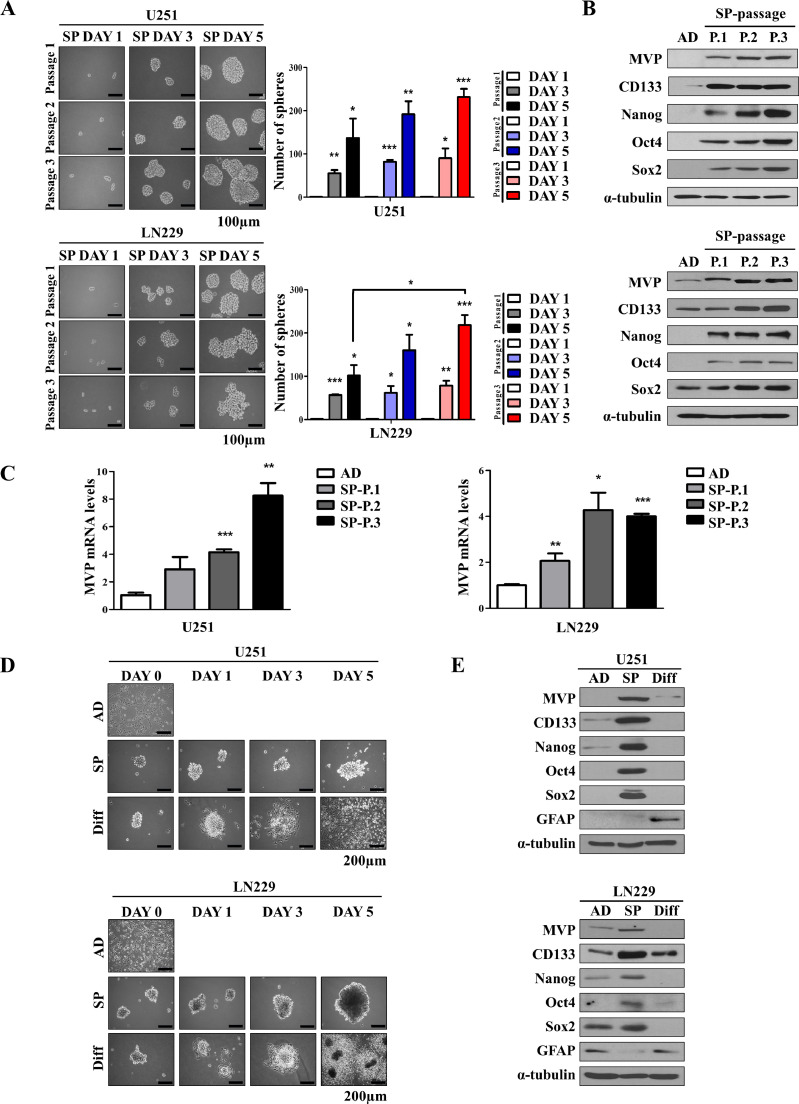
MVP is associated with stemness of glioblastoma stem cells
CSCs were initially described as a subpopulation of cancer cells with unlimited self-renewal capacity and ability to differentiate and repopulate the entire tumor [36]. Moreover, self-renewal, a characteristic feature of CSCs, was enhanced with serial passages, and the protein levels of MVP and CSC markers were increased (Fig. 3A and B, and Supplementary 2A). mRNA levels of MVP were also increased with serial passages (Fig. 3C). Upon differentiation, CSCs can regenerate into tumors that are phenotypically similar to the primary tumor. The differentiation conditions, originally developed for embryonic stem cells [17], involved incubating CSCs with 10% fetal bovine serum for 5 days. Primary GBM adherent cells cultured as tumorspheres in CSC culture medium expressed high levels of stem cell markers including CD133, Nanog, Oct4, and Sox2, and low levels of astrocytic differentiation markers, including GFAP. By contrast, the expression of stem cell markers overall appeared to decrease, and the expression of GFAP increased, following serum-induced differentiation (Fig. 3D and E, and Supplementary Fig. 2B). Taken together, these results suggest that MVP is a potential marker of GSCs.
Fig. 3.
MVP is associated with stemness of glioblastoma stem cells. (A) Sphere formation from cells seeded at a density of 10,000 cells/mL into 6-well plates. Sphere size was observed every 2 days until day 5. Representative images of spheres are shown on the left, and sphere-forming efficiency is shown in the right. (B) MVP and CSC marker expression was assessed by western blot analysis. α-Tubulin was used as a loading control. (C) qRT-PCR analysis of the expression levels of MVP mRNA in U251 and LN229 cells after serial passages. (D) Representative morphology of adherent (AD), tumorsphere (SP), and differentiated (Diff.) U251 (upper) and LN229 (bottom) cells. (E) Western blot analysis of MVP, CSC markers, and GFAP in U251 (upper) and LN229 (bottom) cells. α-Tubulin was used as a loading control. Data are presented as the mean ± standard error of the mean (SEM) of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control cells.
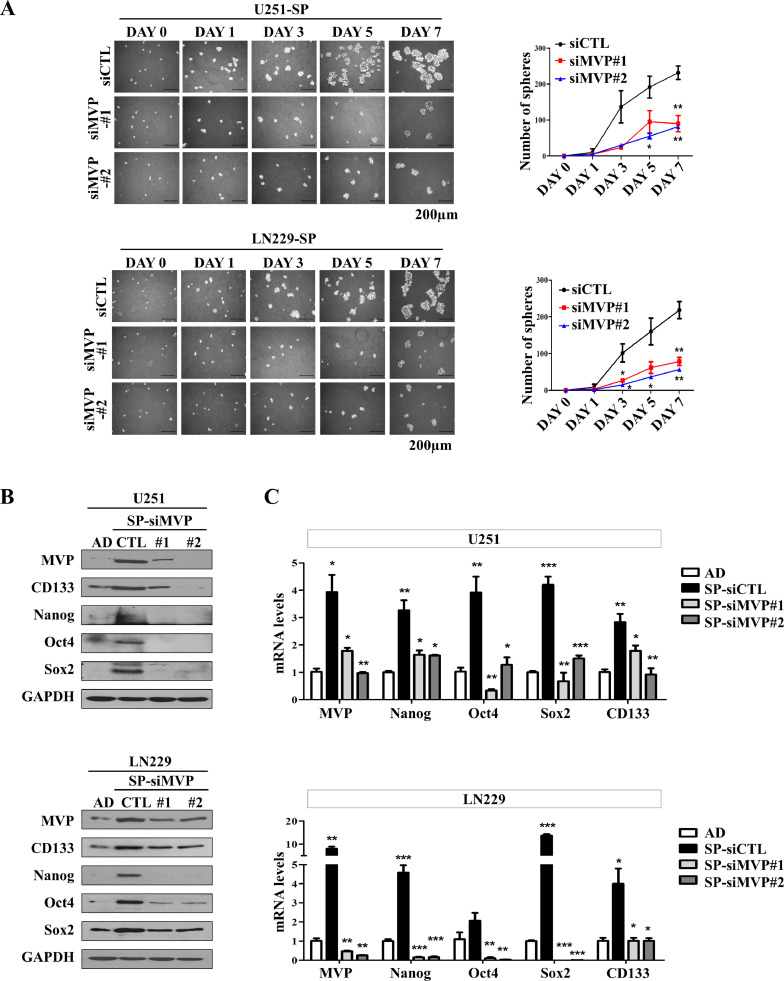
MVP is essential for the maintenance of self-renewal in glioblastoma stem cells
Given the role of MVP as a CSC marker in GBM, we assessed the effect of MVP knockdown on GSCs. To determine whether MVP is required for the maintenance of GSC self-renewal, siRNA transfection was performed to decrease MVP expression in U251, LN229, and U87 GSCs. A sphere formation assay was performed to confirm the self-renewal capacity of MVP-depleted GSCs at the single cell level. Compared with control cells, MVP-knockdown stem cells formed spheres significantly more slowly and formed fewer spheres > 50 μm within 7 days (Fig. 4A and Supplementary Fig. 2C). To confirm these results, protein and mRNA levels of CSC markers in GSCs transfected with control and MVP siRNAs (siCTL, siMVP#1, and siMVP#2) were compared with those of adherent cultured cells. Levels of CD133, Nanog, Oct4, and Sox2 were decreased due to reduced sphere formation in MVP-knockdown stem cells (Fig. 4B and C). These results were confirmed using an ELDA, in which U251 and LN229 cells transfected with siCTL or siMVP were evaluated for sphere formation 7 days after cell plating. Consistent with the ELDA results, a significant decrease in tumorsphere formation was observed in MVP-knockdown cells compared with control cells. Moreover, stable overexpression of MVP in U251 or control cells resulted in increased tumorsphere formation in U251 cells transfected with the pcDNA3.1-MVP expression vector, named U251-MVP (Fig. 4D and E, and Supplementary Fig. 2D and E). IncuCyte live-cell analysis was used to investigate the tumorsphere formation of MVP-knockdown or -overexpressing cells generated from single cells at 6-h intervals for 7 days. Two live imaging movies were taken of cells in a well originally containing 100 cells. Cells with MVP knockdown or stable MVP overexpression had high spheroid formation ability (Supplementary Video. S1 and S2). These results highlight that MVP is crucial to the maintenance of stemness in GSCs.
Fig. 4.
MVP is essential for the maintenance of self-renewal in glioblastoma stem cells. (A) Sphere formation from cells seeded at a density of 5000 cells/well into 6-well plates with tumorsphere culture medium. Sphere size was observed every 2 days until day 7. A sphere formation assay was performed in U251 (upper) and LN229 (bottom) cells transfected with control or MVP siRNAs (siCTL, siMVP#1, and siMVP#2). Representative images are shown on the left, and sphere-forming efficiency is shown on the right. (B) Western blot analysis using the indicated antibodies in adherent cells (AD) transfected with siCTL, siMVP#1, and siMVP#2. GAPDH was used as a loading control. (C) qRT-PCR analysis of the expression of MVP, Nanog, Oct4, Sox2, and CD133 mRNA in U251 (upper) and LN229 (bottom) cells transfected with siCTL, siMVP#1, or siMVP#2. (D) Tumorsphere-forming extreme limiting dilution assays (ELDAs) in U251 (upper) and LN229 (middle) cells transfected with siCTL or siMVP, and U251 cells stably transfected with pcDNA3.1 (control empty vector) or pcDNA3.1-MVP (bottom). Cells were plated at varying densities (1–100 cells/well) and cultured for 7 days. The wells containing no spheroids were counted and plotted. (E) Representative images of MVP-knockdown or MVP-overexpression tumorspheres using the IncuCyte Live-Cell Analysis System. Data are presented as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control cells.
MVP expression of glioblastoma is associated with invasive outgrowth and poor prognosis of glioma patients
To confirm whether MVP is also involved in the invasive ability, another property of CSCs, we performed an invasion assay and assessed changes in gene expression following MVP knockdown. The invasion ability was significantly increased in sphere cultured cells than adherent cells; however, there was no significant change in the invasion of MVP-knockdown cells compared with adherent cells (Fig. 5A). Expression of invasion-related genes was also affected in sphere cultured cells, whereas in MVP-knockdown cells, there was little change in expression compared with control cells (Fig. 5B), indicating that MVP is directly involved in the invasiveness of GSCs. Among the identified transcriptional subtypes, the mesenchymal subtype has been found associated with more aggressive, invasive, and multidrug-resistant features than other transcriptional subtypes [32]. Also, it is known that the higher the glioma grade, the higher the malignancy and invasiveness [33], and we have checked the expression of MVP by grade in the glioma patient tissues. When we assessed MVP expression in TCGA data, we found that patients with mesenchymal subtype and higher grades exhibited higher expression of MVP (Fig. 5C and D). To explore the expression of MVP, which shows potential as a GSC marker, in GBM patients, we assessed MVP expression in the tissues of 200 glioma patients by immunohistochemistry. We found that higher-grade tumors exhibited greater MVP expression (Fig. 5E and F). GBM patients from TCGA database were divided into MVP-low and -high subgroups. Kaplan–Meier analysis indicated overall survival in the MVP-high than MVP-low group (Fig. 5G). Supplementary Fig 3A shows H&E images of the PDX model from GSCs of GBM patients. As a result of orthotopic injection of GSC cells, which are primary GSCs, tumor mass was generated in the injected hemisphere, and infiltration into the opposite hemisphere was confirmed. In addition, we investigated the expression of MVP in PDX tumor tissue, and the expression of MVP was confirmed not only in the tumor mass but also in the subpopulation of the infiltrated portion. These data suggested that we can provide direct evidence that MVP is a novel marker of GSC (Supplementary Fig 3A). Altogether, these data suggest that MVP is involved in chemoresistance of GBM, and that MVP enhanced the resistance of GSCs to TMZ, and MVP acts as a novel CSC marker, as illustrated in Fig. 6.
Fig. 5.
MVP expression of glioblastoma is associated with invasive outgrowth and poor prognosis of glioma patients. (A) Invasion assay of adherent (AD) cells and tumorspheres (SP) transfected with control (siCTL) or MVP targeting siRNA (siMVP) using Transwell chambers coated with Matrigel. Cells were seeded at a density of 10,000 cells/well and incubated in serum-free DMEM-F12 for 48 h. Relative cell numbers are shown (right). (B) Protein expression of epithelial/mesenchymal markers in U251 (upper) and LN229 (bottom) cells determined by western blot analysis. GAPDH was used as a loading control. (C) Quantification of MVP mRNA expression according to GBM subtype in GBM patients in the TCGA database. (D) Quantification of MVP mRNA expression according to grade in glioma patients in the TCGA database. (E) Correlation of MVP immunohistochemical expression in glioma tissue arrays of normal brain tissues (n = 27) or glioma specimens (n = 173) with tumor grade. (F) Quantification of MVP expression according to grade in normal and glioma tissues. (G) Kaplan–Meier plot of survival of glioma patients according to low and high expression of MVP (blue and red, respectively) (*P = 0.0033). Data are presented as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control cells.
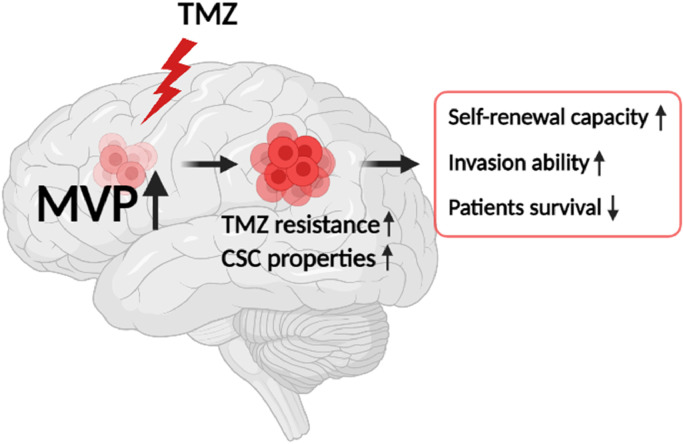
Fig. 6.
Schematic illustration of the contribution of MVP in glioblastoma stem cells.
Discussion
GBM is the most malignant brain tumor in adults. Because tumor cells infiltrate peripheral brain tissue, complete surgical resection is virtually impossible. Even with surgical intervention, the prognosis remains poor. TMZ resistance is considered to be one of the major reasons responsible for GBM therapy failure. In addition, the heterogeneity present in GBM contributes to cancer drug resistance by mechanisms that are poorly understood [19]. New treatment strategies that can identify and accurately destroy dispersed tumor cells are needed. GSCs, a subset of tumor cells with stem cell characteristics such as stem cell marker expression and enhanced self-renewal, are important in tumorigenesis, progression, and recurrence [20,21]. Compared with conventional treatments, developing novel treatment strategies targeting CSCs may effectively eliminate malignancies, resistance to TMZ, and reduce the risk of recurrence. The present study showed that MVP is highly expressed in TMZ-resistant GBM cells and GSCs and contributes to their stemness. We also showed that MVP expression is associated with the GBM grade and a poorer prognosis, suggesting that MVP acts as a novel marker of GSCs.
With respect to malignant progression, increased levels of MVP and vault particles have been reported in several cancer types. However, it is still controversial that the increased MVP/vault expression is related to chemotherapy resistance [38], but it is certain that MVP is associated with resistance according to several papers [10,22]. MVP is a major component of the vault complex, which plays a pivotal role in chemoresistance by allowing intracellular drugs to enter the nucleus and by regulating MAPK/ERK and phosphoinositide 3-kinase/Akt signaling [17,23,24]. In addition, Xiao et al. intensively investigated the mechanism underlying the chemoresistance of MVP. According to their report, MVP functioned in vesicular transport of drug and could activate the mTOR pathway, and induce EMT leading to chemoresistance in breast cancer cell [7]. It was confirmed that the mTOR was phosphorylated due to the increased MVP in GSCs, and it was also activated in the exogenous overexpression cells (Supplementary Fig. 2D and E). However, it is not known exactly whether this pathway was observed in GSCs. MVP is also associated with MDR in cancer, although the exact mechanism remains unclear [25,26]. The protein expression of ABC transporters, including ABCG2, MDR1, and MRP1, was assessed after increasing MVP expression to determine a possible correlation with MDR proteins. While there was no effect of MVP upregulation on the expression of ABC transporter-related proteins, expression of both MVP and ABC transporter proteins was increased under tumorsphere culture conditions (Fig. 2E and Supplementary Fig. 1L). Furthermore, MVP may be useful as a novel CSC marker independent of the ABC transporter proteins currently used as CSC markers. It is remarkable that the mechanical contribution of MVP to malignant phenotypes, especially with respect to CSCs, has not yet been explored.
To date, the role of MVP in chemotherapy responses or in relation to the survival of GBM patients in clinical settings has not been investigated extensively. In the present study, we found that analysis of the original dataset of TCGA-derived gene expression omnibuses revealed that Kaplan–Meyer survival analysis significantly supports this perspective, and MVP expression in tumors was higher in patients undergoing chemotherapy than in those not undergoing chemotherapy. Also, TMZ-resistant GBM cells exhibited upregulation of MVP expression and promoted the viability and sphere formation ability (Figs. 1 and 2). These results support the notion that by targeting CSCs, MVP is an important predictor of a poor chemotherapy response and prognosis in GBM patients. Moreover, the expression of MVP was elevated in GSCs, compared with the parental control cells, and was essential for stemness (Figs. 3 and 4). Another significant observation was that MVP regulates the invasive capacity of GSCs, which could contribute in part to chemotherapy tolerance (Fig. 5). An increasing number of studies have indicated that the epithelial–mesenchymal transition (EMT) is associated with chemoresistance in different cancers, including non-small cell lung, pancreatic, breast, and ovarian cancers [27,28]. Cancer cells undergoing EMT are converted to a stem cell-like mesenchymal phenotype. This cell subpopulation is thought to contribute to chemoresistance [29,30]. Thus, MVP may contribute to the chemoresistance of GSCs via the EMT.
In conclusion, our data demonstrate for the first time that a potential role for MVP as a CSCs marker of increasing the TMZ resistance of GBM tumors. In GBM, we demonstrated that MVP expression is almost constitutively activated during resistance acquirement to TMZ and sphere formation, improving drug resistance and invasion potential. In addition, we found that knockdown of MVP reduces self-renewal and leads to a loss of stemness. Moreover, upregulation of MVP was associated with reduced survival of glioma patients (Fig. 6). Our results provide a new potential strategy to overcome TMZ resistance in GBM patients. Also, MVP may be considered as a prognostic marker for GSCs.
Declaration of Competing Interest
The authors have no conflicting both financial and non-financial interests related to this work.
Acknowledgments
Authors' contributions
K. H. Noh and S. K. Ye: conceptualization, manuscript writing-original draft
K. H. Noh: methodology, validation, visualization, software, data curation
K. H. Noh, S. H. Lee, A. J. Jeong and H. Lee: formal analysis, investigation
K.O. Kim, H.M. Shin, H.R. Kim: data curation
J. Lee, M.-J Park, J.B. Park: resources
S. K. Ye: supervision, project administration
Acknowledgements
This work was carried out with the support of the Seoul National University Hospital (SNUH) Research Fund (04-2020-0230), the R&D Program for Forest Science Technology (Project No. 2020195A00-2122-BA01) provided by the Korea Forest Service (Korea Forestry Promotion Institute), the National Research Foundation of Korea (NRF) funded by the Korean government (NRF-2018R1A5A2025964), and the "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01589402 and No. PJ016020012021)" Rural Development Administration, Republic of Korea.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101255.
Appendix. Supplementary materials
References
- 1.Weller M., Butowski N., Tran D.D., Recht L.D., Lim M., Hirte H., Ashby L., Mechtler L., Goldlust S.A. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 2.Chauvel A.K., Andersson V.G., Baricault L., Martin E., Delmas C., Toulas C., Moyal E.C.J., Seva C. Alpha6-integrin regulates FGFR1 expression through the ZEB1/YAP1 transcription complex in glioblastoma stem cells resulting in enhanced proliferation and stemness. Cancers (Basel) 2019;11(3):406. doi: 10.3390/cancers11030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarmishyn A.A., Yang Y.P., Lu K.H., Chen Y.C., Chien Y., Chou S.J., Tsai P.H., Ma H.I.L., Chien C.S., Chen M.T. Musashi-1 promotes cancer stem cell properties of glioblastoma cells via upregulation of YTHDF1. Cancer Cell Int. 2020;20(1):597. doi: 10.1186/s12935-020-01696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q., Fu W.J., Tang X.P., Wang L., Niu Q., Wang S., Lin Y., Cao M.F., Hu R., Wen H.Y. ADP-ribosylation factor like GTPase 4C (ARL4C) augments stem-like traits of glioblastoma cells by upregulating ALDH1A3. J. Cancer. 2021;12(3):818–826. doi: 10.7150/jca.45052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res. Rev. 2001;35(2):161–204. doi: 10.1016/s0165-0173(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Steiner E., Holzmann K., Elbling L., Micksche M., Berger W. Cellular functions of vaults and their involvement in multidrug resistance. Curr. Drug Targets. 2006;7(8):923–934. doi: 10.2174/138945006778019345. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y.S., Zeng D., Liang Y.K., Wu Y., Li M.F., Qi Y.Z., Wei X.L., Huang W.H., Chen M., Zhang G.J. Major vault protein is a direct target of Notch1 signaling and contributes to chemoresistance in triple-negative breast cancer cells. Cancer Lett. 2019;440-441:156–167. doi: 10.1016/j.canlet.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Han M., Lv Q., Tang X.J., Hu Y.L., Xu D.H., Li F.Z., Liang W.Q., Gao J.Q. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex. J. Contr. Release. 2012;163(2):136–144. doi: 10.1016/j.jconrel.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Kickhoefer V.A., Rajavel K.S., Scheffer G.L., Dalton W.S., Scheper R.J., Rome L.H. Vaults are up-regulated in multidrug-resistant cancer cell lines. J. Biol. Chem. 1998;273(15):8971–8974. doi: 10.1074/jbc.273.15.8971. [DOI] [PubMed] [Google Scholar]
- 10.Lotsch D., Steiner E., Holzmann K., Kreinecher S.S., Pirker C., Hlavaty J., Petznek H., Hegedus B., Garay T., Mohr T. Major vault protein supports glioblastoma survival and migration by upregulating the EGFR/PI3K signalling axis. Oncotarget. 2013;4(11):1904–1918. doi: 10.18632/oncotarget.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z., Zhang W., Phillips J.B., Arora R., McClellan S., Li J., Kim J.H., Sobol R.W., Tan M. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;1:88–102. doi: 10.1038/s41388-018-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E., Lee S., Mian M.F., Yun S.U., Song M., Yi K.S., Ryu S.H., Suh P.G. Crosstalk between Src and major vault protein in epidermal growth factor-dependent cell signaling. FEBS J. 2006;273(4):793–804. doi: 10.1111/j.1742-4658.2006.05112.x. [DOI] [PubMed] [Google Scholar]
- 13.Kolli S., Zito C.I., Mossink M.H., Wiemer E.A.C., Bennett A.M. The major vault protein is a novel substrate for the tyrosine phosphatase SHP-2 and scaffold protein in epidermal growth factor signaling. J. Biol. Chem. 2004;279:29374–29385. doi: 10.1074/jbc.M313955200. [DOI] [PubMed] [Google Scholar]
- 14.Yi C., Li S., Chen X., Wiemer E.A.C., Wang J., Wei N. Major vault protein, in concert with constitutively photomorphogenic 1, negatively regulates c-Jun-mediated activator protein 1 transcription in mammalian cells. Cancer Res. 2005;65:5835–5840. doi: 10.1158/0008-5472.CAN-05-0423. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Zhang X., Yang B., Zhuang H., Guo H., Wei W. Demethylation-induced overexpression of Shc3 Drives c-Raf-independent activation of MEK/ERK in HCC. Cancer Res. 2018;78:2219–2232. doi: 10.1158/0008-5472.CAN-17-2432. [DOI] [PubMed] [Google Scholar]
- 16.Ma X.L., Sun Y.F., Wang B.L., Shen M.N., Zhou Y., Chen J.W., Hu B., Gong Z.J., Zhang X., Cao Y. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19(1):760. doi: 10.1186/s12885-019-5963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z.W., Chen L., Liu J.X., Huang J.W., Wu G., Zheng .Y.F., Yao K.T. A novel three-dimensional tumorsphere culture system for the efficient and low-cost enrichment of cancer stem cells with natural polymers. Exp. Ther. Med. 2018;15(1):85–92. doi: 10.3892/etm.2017.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begicevic R.R., Falasca M. ABC transporters in cancer stem cells: beyond chemoresistance. Int. J. Mol. Sci. 2017;18(11):2362. doi: 10.3390/ijms18112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Yang K., Xie Q., Wu Q., Mack S.C., Shi Y., Kim L.J.Y., Prager B.C., Flavahan W.A., Liu X. Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat. Neurosci. 2017;20:661–673. doi: 10.1038/nn.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batlle E., Clevers H. Cancer stem cells revisited. Nat. Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 21.Vescovi A.L., Galli R., Reynolds B.A. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 22.Losert A., Lötsch D., Lackner A., Koppensteiner H., Vörösmarty B.P., Steiner E., Holzmann K., Grunt T., Schmid K., Maria B. The major vault protein mediates resistance to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Lett. 2012;319:164–172. doi: 10.1016/j.canlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Tomiyasu H., Watanabe M., Koshino Y.G., Fujino Y., Ohno K., Sugano S., Tsujimoto H. Regulation of expression of ABCB1 and LRP genes by mitogen-activated protein kinase/extracellular signal-regulated kinase pathway and its role in generation of side population cells in canine lymphoma cell lines. Leuk Lymphoma. 2013;54(6):1309–1315. doi: 10.3109/10428194.2012.751529. [DOI] [PubMed] [Google Scholar]
- 24.Park K. The role of major vault protein (MVP) in drug resistance. J. Contr. Release. 2012;163:266. doi: 10.1016/j.jconrel.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Steiner E., Holzmann K., Elbling L., Micksche M., Berger W. Cellular functions of vaults and their involvement in multidrug resistance. Curr. Drug Targets. 2006;7(8):923–934. doi: 10.2174/138945006778019345. [DOI] [PubMed] [Google Scholar]
- 26.Moiseeva N.I., Susova O.Y., Mitrofanov A.A., Panteleev D.Y., Pavlova G.V., Pustogarov N.A., Stavrovskaya A.A., Rybalkina E.Y. Connection between proliferation rate and temozolomide sensitivity of primary glioblastoma cell culture and expression of YB-1 and LRP/MVP. Biochemistry (Mosc) 2016;6:628–635. doi: 10.1134/S0006297916060109. [DOI] [PubMed] [Google Scholar]
- 27.Du B., Shim J.S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21(7):965. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T., Choi H., El Rayes T., Ryu S., Troeger J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung E.H., Lee H.N., Han G.Y., Kim M.J., Kim C.W. Targeting ROR1 inhibits the self-renewal and invasive ability of glioblastoma stem cells. Cell Biochem. Funct. 2016;34(3):149–157. doi: 10.1002/cbf.3172. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y., Kim Y.K. Cancer stem cells as a potential target to overcome multidrug resistance. Front Oncol. 2020;10:764. doi: 10.3389/fonc.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Smyth G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y., Varn F.S., Park S.H., Yoon B.W., Park H.R., Lee C., Verhaak R.G.W., Paek S.H. Perspective of mesenchymal transformation in glioblastoma. Acta Neuropathol. Commun. 2021;9:50. doi: 10.1186/s40478-021-01151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di K., Linskey M.E., Bota D.A. TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth. Oncogene. 2013;32:5038–5047. doi: 10.1038/onc.2012.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbondanza C., Rossi V., Roscigno A., Gallo L., Belsito A., Piluso G., Medici N., Nigro V., Molinari A.M., Moncharmont B. Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell. J. Cell Biol. 1998;141(6):1301–1310. doi: 10.1083/jcb.141.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auffinger B., Spencer D., Pytel P., Ahmed A.U., Lesniak M.S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev. Neurother. 2015;15(7):741–752. doi: 10.1586/14737175.2015.1051968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safa A.R., Saadatzadeh M.R., Cohen-Gadol A.A., Pollok K.E., Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2(2):152–163. doi: 10.1016/j.gendis.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joo K.M., Jin J., Kim E., Kim K.H., Kim Y., Kang B.G., Kang Y.J., Lathia J.D., Cheong K.H., Song P.H. MET signaling regulates glioblastoma stem cells. Cancer Res. 2012;72(15):3828–3838. doi: 10.1158/0008-5472.CAN-11-3760. [DOI] [PubMed] [Google Scholar]
- 38.Mossink M.H., Zon A.V., Scheper R.J., Sonneveld P., Wiemer E.A. Vault: a ribonucleoprotein particle involved in drug resistance? Oncogene. 2003;22:7458–7467. doi: 10.1038/sj.onc.1206947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.