Abstract
Human papillomavirus (HPV) vaccines are among the most effective vaccines available, the first to prevent infection by a mucosatropic sexually transmitted infectious agent and to do so without specific induction of mucosal immunity. Currently available prophylactic HPV vaccines are based on virus-like particles that self-assemble spontaneously from the L1 major capsid protein. The first HPV vaccine was licensed in 2006. All vaccines target HPV-16 and HPV-18, types which cause the majority of HPV-attributable cancers. As of 2020, HPV vaccines had been introduced into national immunization programs in more than 100 countries. Vaccination polices have evolved; most programs target vaccination of young adolescent girls, with an increasing number also including boys. The efficacy and safety found in prelicensure trials have been confirmed by data from national immunization programs. The dramatic impact and effectiveness observed has stimulated interest in ambitious disease reduction goals.
Keywords: human papillomavirus, HPV, HPV vaccine
Human papillomavirus (HPV) vaccines are among the most effective prophylactic vaccines available and have established several important landmarks in human vaccinology. They are the first vaccines to prevent infection by a mucosatropic sexually transmitted infectious agent and do so without specific induction of mucosal immunity [1].They are also the first subunit vaccines to consistently induce long-term (more than a decade) stable serum antibody responses. HPV vaccines appear to induce sterilizing immunity from initial infection for a least a decade without additional booster vaccination [1]. The high efficacy found in prelicensure clinical trials has been confirmed by dramatic impact and effectiveness observed in national immunization programs over the past decade [2]. There are now ambitious goals for reduction of HPV-associated disease [3]. In contrast to prophylactic vaccines, vaccines to treat HPV infections or induced neoplasia have had limited clinical success to date, have not been commercialized, and will not be further discussed in this review.
HPV is a DNA virus that replicates in stratified squamous epithelia [4]. Mucosal types are sexually transmitted, mainly through skin to skin contact. HPV is the most common sexually transmitted infection [5]. Although most HPV infections become undetectable within 2 years and do not result in clinical disease, persistent infections with oncogenic or high-risk types can lead to precancerous lesions and cancer [6]. HPV-associated cancers include cancer of the cervix, vulva, vagina, penis, anus, and oropharynx. Cervical cancer is the most common HPV-associated cancer worldwide, with the majority of cases and deaths occurring in lower-income countries where screening for cervical precancer and treatment are limited. Worldwide, an estimated 630 000 cancers are attributable to HPV annually, including 570 000 cancers in women [6]. Oropharyngeal cancer attributable to HPV has been increasing in high-income countries, particularly among men [7]. In the United States, an estimated 35 000 HPV-attributable cancers occur each year [8]. Cervical cancer has been decreasing over the past several decades owing to cervical cancer screening, while oropharyngeal cancer has been increasing, likely because of changes in sexual behaviors. Oropharyngeal cancer is now the most common HPV-attributable cancer in the United States [8, 9].
More than 40 HPV types that infect mucosal epithelium have been identified. Twelve are defined as oncogenic (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and 8 others as probably or possibly oncogenic (types 26, 53, 66, 67, 68, 70, 73, and 82) [10]. There is a range of oncogenicity across these types, with HPV-16 having markedly highest risk for progression to cancer. HPV-16 and HPV-18 are responsible for approximately 70% of cervical cancers and an even greater percentage of other HPV-attributable cancers [6]. HPV-induced cancers are primarily driven by the continued expression of 2 viral oncogenes, E6 and E7, which interact with multiple cell targets, including p53 and pRb, respectively. However, additional cellular alternations are also required, since only a small minority of infections progress to cancer, even for HPV-16 [11]. Anogenital warts and recurrent respiratory papillomatosis, other conditions caused by HPV, are caused mainly by HPV-6 and HPV-11, types not considered oncogenic [12].
HISTORY OF HPV VACCINE DEVELOPMENT
After identification of HPV as the primary cause of cervical cancer in the 1980s, work ensued to develop a vaccine [13, 14]. In the 1980s and 1990s, studies in animal models demonstrated that animals could be protected against papillomavirus lesions using purified virions, that neutralizing antibody was necessary and sufficient for protection against viral challenge, and that protection was likely specific to HPV type [15]. Vaccine development focused on subunit approaches owing to the challenges in propagating papillomaviruses and because of the oncogenes contained in the viral genome [16].
The licensed HPV vaccines are based on virus-like particles (VLPs), which self-assemble spontaneously from 72 pentamers of the L1 major capsid protein (Table 1) [17–20]. Because they are produced from a single virion protein, they are noninfectious and nononcogenic. The VLPs are morphologically similar to authentic virus and induce high titers of virion neutralizing antibodies [21].
Table 1.
Characteristics of Licensed Human Papillomavirus Vaccines
| Brand Name and Valency | VLP Types | Manufacturer and Date of First Licensure | Adjuvant | Expression System | Administration and Doses Recommendeda |
|---|---|---|---|---|---|
| Gardasilb; quadrivalent | HPV-6 (20 µg); HPV-11 (40 µg); HPV-16 (40 µg); HPV-18 (20 µg) |
Merck & Co; 2006 | Amorphous aluminum hydroxyphosphate sulfate (225 µg) | Saccharomyces cerevisiae (Baker’s yeast) expressing L1 | Intramuscular; 2 or 3 doses, depending on age at initiation |
| Cervarixc; bivalent | HPV-16 (20 µg); HPV-18 (20 µg) | GlaxoSmithKline; 2007 | Aluminum hydroxide (500 µg); 3-O-desacyl-4’ monophosphoryl lipid A (AS04) (50 µg) | Trichoplusia ni insect cell line infected with L1 encoding recombinant baculovirus | Intramuscular; 2 or 3 doses, depending on age at initiation |
| Gardasil 9d; nonavalent | HPV-6 (30 µg); HPV-11 (40 µg); HPV-16 (60 µg); HPV-18 (40 µg); HPV-31 (20 µg); HPV-33 (20 µg); HPV-45 (20 µg); HPV-52 (20 µg); HPV-58 (20 µg) |
Merck & Co; 2014 | Amorphous aluminum hydroxyphosphate sulfate (500 µg) | S. cerevisiae (Baker’s yeast) expressing L1 | Intramuscular; 2 or 3 doses, depending on age at initiation |
| Cecoline; bivalent | HPV-16 (40 µg); HPV-18 (20 µg) |
Xiamen Innovax Biotech; 2020 | Aluminum hydroxide (208 µg) | Escherichia coli expressing L1 | Intramuscular; 2 or 3 doses, depending on age at initiation |
Abbreviations: HPV, human papillomavirus; L1, capsid protein of human papillomavirus; VLP, virus-like particle.
a Gardasil, Cervarix, and Gardasil 9 were originally licensed with 3-dose schedules: Cervarix: 0, 1, and 6 months; Gardasil and Gardasil 9: 0, 2, and 6 months.
b Package insert available online (https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil) [17].
cPackage insert available online (https://www.fda.gov/vaccines-blood-biologics/vaccines/cervarix) [18]. Cervarix was licensed in the United States in 2009.
dPackage insert available online (https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9) [19].
eLicensed in China [20]. Pending WHO prequalification.
Initial commercial development of HPV vaccines was undertaken by 2 companies, GlaxoSmithKline Biologicals (GSK) and Merck & Co [22]. GSK developed a bivalent vaccine (Cervarix), composed of HPV-16 and HPV-18 VLPs. Merck developed a quadrivalent vaccine (Gardasil), with HPV-16 and HPV-18 as well as HPV-6 and HPV-11 VLPs. Other differences between the 2 vaccines are the producer cells for the viral L1 proteins and the adjuvants. For Cervarix, the proteins are produced in L1-recombinant baculovirus-infected insect cells and for Gardasil in yeast (Saccharomyces cerevisiae). Cervarix has a proprietary adjuvant AS04, composed of aluminum hydroxide plus 3-deacylated mono-phosphoryl lipid A, a detoxified form of lipopolysaccharide and a Toll-like receptor 4 agonist, while Gardasil contains an aluminum salt adjuvant (aluminum hydroxyphosphate sulfate). Merck later developed a nonavalent vaccine, Gardasil 9, similar to Gardasil but containing L1 VLPs of 5 addition oncogenic types HPV 31, 33, 45, 52, and 58 and so has the potential to provide type-specific protection against approximately 90% of cervical cancers worldwide [6].
More recently, L1 VLP HPV vaccines are being developed by manufacturers in China, India, and other countries [23]. A bivalent vaccine (Cecolin), containing HPV-16 and HPV-18 VLPs, developed by Xiamen Innovax Biotech, was licensed in China in 2020 and is currently under review by the World Health Organization (WHO) [20]. The L1 proteins are produced in Escherichia coli, and the vaccine has an alum adjuvant.
MECHANISM OF ACTION
While HPV VLP vaccines induce both B-cell and T-cell responses, they are thought to function primarily, if not exclusively, by the induction of antibodies that bind the virions and thereby prevent initial infection [1]. The systemic antibodies, mostly immunoglobulin (Ig) G, induced by intramuscular injection can reach the sites of cervicovaginal infection by 2 mechanisms. One is transudation of IgG across the epithelial barrier into mucosal secretions via the neonatal Fc receptor, which is pronounced at the cervix [24]. A second mechanism is the direct exudation of serum and interstitial antibodies at the sites of trauma that permit virion binding to the basement membrane, an activity that appears to be essential for initiating the infectious process [25]. The latter mechanism is likely sufficient to prevent infection, since Gardasil and Gardasil 9 are highly effective at preventing anogenital warts, many of which occur on skin surfaces that are not bathed in mucus. Secretory IgA, which is not efficiently induced by intramuscular injection, is not thought to play a substantial role in protection.
Immune monitoring in vaccine trials has largely centered on measurement of vaccine-induced serum antibodies. VLP binding antibodies were primarily measured by enzyme-linked immunosorbent assay (ELISA) (in GSK trials) or Luminex assays based on competition with type-specific monoclonal antibodies (competitive Luminex immunoassay [cLIA]) (in Merck trials). Functionality of the induced antibodies has largely been assessed by in vitro neutralization assays based on HPV pseudovirions, L1/L2-based vectors that transfer maker gene-expressing plasmids, because there is no ready source of authentic virions and their infection does not induce easily scored phenotypic changes in cultured cells [26].
HPV VACCINE CLINICAL TRIALS
All licensed HPV vaccines completed a range of safety, immunogenicity and efficacy trials before licensure [27–30]. The first efficacy trials were randomized controlled trials (RCTs) among young women aged 15–26 years [31–36]. Although the target group for HPV vaccination programs is preadolescents and young adolescents, efficacy trials were not feasible in that age group, primarily because it would take too long to accrue a sufficient number of sexually transmitted infections or lesions. HPV vaccines were licensed in young adolescents based on bridging immunogenicity trials, as discussed below, and safety data [37–40]. Later, RCTs were conducted among women >26 years old [41–44] and among men [45, 46].
Appropriate end points for efficacy trials in women were discussed extensively, with input from WHO and national regulatory authorities [47]. Cervical cancer could not be used as an end point because participating women would receive active follow-up including treatment of any precancer lesions detected. The agreed-upon primary end points included a combination of cervical intraepithelial neoplasia (CIN) grade 2 or higher (CIN2+) and cervical adenocarcinoma in situ caused by incident infection of the vaccine-targeted types [47]. The relationship between cervical HPV infection, CIN and cervical cancer had been well established in natural history studies and this surrogate end point for cancer was widely accepted [14]. Additional end points were included in efficacy trials, including anogenital warts for Gardasil, because that vaccine also targets HPV-6 and HPV-11 (Table 2).
Table 2.
Prelicensure Human Papillomavirus (HPV) Vaccine Efficacy Trials: Results for Primary and Selected Other End Points, Per-Protocol Analyses for Prevention of HPV Vaccine-Type Disease Outcomes, End-of-Study Analyses (if Available)a
| Vaccine and Trial Population | Trialb | Location | Vaccine Type–Attributable End Point | Vaccine Group | Control Group | Vaccine Efficacy (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Total No. | No. With Outcome | Total No. | No. With Outcome | |||||
| Gardasil; quadrivalent (Merck) | ||||||||
| Women aged 15–26 y | FUTURE I and II [35, 36] NCT00092521 NCT00092534 | 16 and 13 Countries in Asia Pacific, Europe, North America, Latin America | CIN2+ c | 7864 | 2 | 7865 | 110 | 98.2% (93.3–99.8) |
| Anogenital warts | 7665 | 2 | 7669 | 190 | 99.0% (95.8–99.7) | |||
| Women aged 24–45 y | FUTURE III [42] NCT00090220 | Colombia, France, Germany, Philippines, Spain, Thailand, United States | Incidence persistent infection, CIN1+, or EGLsd | 1581 | 10 | 1584 | 86 | 88.7% (78.1–94.8) |
| CIN2+ | 1581 | 1 | 1584 | 6 | 83.3% (−37.6 to 99.6) | |||
| Men aged 16–26 y | Efficacy trial in men [45] NCT00090285 | 18 Countries in Asia Pacific, Europe, North America, Latin America | Anogenital warts | 1397 | 3 | 1408 | 28 | 89.4% (65.5–97.9) |
| Substudy in men who have sex with men [46] | Australia, Brazil, Canada, Croatia, Germany, Spain, United States | AIN1/2/3 | 194 | 5 | 208 | 24 | 77.5% (39.6–93.3) | |
| AIN2/3 | 194 | 3 | 208 | 13 | 74.9% (8.8–95.4) | |||
| Cervarix; bivalent (GSK) | ||||||||
| Women aged 15–25 y | PATRICIA [33] NCT00122681 | 14 Countries in Asia Pacific, Europe, North America, Latin America | CIN2+ e | 7338 | 5 | 7305 | 97 | 94.9% (87.7–98.4) |
| Women aged 18–25 y | CVT [48, 49] NCT00128661 | Costa Rica | Incident persistent infection | 2635 | 8 | 2677 | 89 | 90.9% (82.0–95.9) |
| CIN2+ | 2635 | 1 | 2677 | 10 | 89.9% (39.5–99.5) | |||
| Women aged ≥26 y | VIVIANE [44] NCT00294047 | 12 Countries in Asia Pacific, Europe, North America, Latin America | Incidence persistent infection or CIN1+ | 1852 | 7 | 1818 | 71 | 90.5% (78.6–96.5) |
| CIN2+ | 1852 | 1 | 1818 | 6 | 83.7% (−46.5 to 99.7) | |||
| Gardasil 9; nonavalent (Merck) | ||||||||
| Women aged 16–26 y | NCT00543543 [50] | 18 Countries in Asia Pacific, Europe, North America, Latin America | HPV types 31, 33, 45, 52, 58 CIN2+, VIN2+, VaIN2+ | 6016 | 1 | 6017f | 38f | 97.4% (85.0–99.9) |
| Cecolin; bivalent (Xiamen Innovax) | ||||||||
| Women aged 18–45 y | Efficacy trial in women [20] | China | CIN2+, VIN2+, VaIN2+ | 3306 | 0 | 3296 | 10 | 100% (55.6–100) |
Abbreviations: AIN, anal intraepithelial neoplasia; CI, confidence interval 95%; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2 or higher or adenocarcinoma in situ; EGLs, external genital lesions; GSK, GlaxoSmithKline; HPV, human papillomavirus; VaIN, vaginal intraepithelial neoplasia grade 2; VIN, vulvar intraepithelial neoplasia.
aPer-protocol population includes participants who received all 3 vaccine doses, were seronegative at day 1 and DNA negative to the respective HPV type at day 1 through month 7, with case counting starting 1 day or 1 month after the last dose.
bClinicaltrials.gov numbers are included, if available.
cCIN2+ outcomes include data from a phase II study in the referenced publication.
dDenominators from a June 2018 Advisory Committee on Immunization Practices presentation. EGLs include VIN, VaIN, vaginal, vulvar disease, and anogenital warts.
eData from Lehtinen et al [33, supplementary appendix, table 3].
fThe comparison group for Gardasil 9 was Gardasil.
Vaccine Efficacy in Young Adult Women
The first phase III efficacy trials were large, multisite, international RCTs including thousands of young women (aged 15–16 through 26 years) (Table 2). The trials for Gardasil and Cervarix were designed and conducted by the respective manufacturers, with women receiving 3 doses via intramuscular injection over a course of 6 months and then followed over approximately 4 years [31–36]. In addition, a trial of Cervarix, the Costa Rica Vaccine Trial, was undertaken by the US National Cancer Institute in cooperation with the Costa Rican government [48, 49]. Clinical end points were based on histologic determination of CIN/adenocarcinoma in situ and type-specific HPV DNA detection. After licensure of Cervarix and Gardasil by the US Food and Drug Administration (FDA) and the European Medicine Agency, efficacy trials were also conducted in some other countries, as required by local regulatory authorities [51–53].
While the designs were similar for the Cervarix and Gardasil efficacy trials, specific aspects differed, including enrollment criteria (eg, number of lifetime partners), follow-up for disease outcomes and HPV assays [30]. Because Gardasil and Cervarix already had been licensed and recommended, the Gardasil 9 prelicensure trial was designed to compare Gardasil 9 with Gardasil [50, 54]. The primary disease end point was high-grade cervical, vulvar, or vaginal intraepithelial lesions attributable to HPV type 31, 33, 45, 52, or 58 (types not included in Gardasil). In addition, noninferiority of the geometric mean antibody titers (GMTs) (value >0.67 for the lower bound of 95% confidence interval [CI] of the Gardasil 9/Gardasil GMT ratio) to the 4 types common to both vaccines was required. The efficacy trial design for the bivalent vaccine recently licensed in China (Cecolin) was similar to those for Gardasil and Cervarix.
Women in the efficacy trials were enrolled without regard to HPV infection status. The primary efficacy analyses included women who were negative for cervicovaginal HPV DNA and serum antibody (at least for types in the vaccine) throughout the period of vaccination and received 3 doses: the per-protocol (PP) or according-to-protocol (ATP) cohorts. Analyses including all randomized women who received ≥1 vaccine dose, the total vaccinated (TV) or intention-to-treat (ITT) cohorts, were also conducted. Results in the PP/ATP analyses reflect the anticipated efficacy in adolescent girls, vaccinated before onset of sexual activity.
Efficacy against vaccine type–attributable CIN2+ ranged from 90% to 98% in the Gardasil and Cervarix ATP or PP cohorts (Table 2) [31–36, 49]. For Gardasil, high efficacy was also reported for vaginal and vulvar precancers and anogenital warts [34, 36]. Efficacy in the TV/ITT cohorts was lower because these analyses included women infected at the time of vaccination. An important observation from the initial trials was that HPV vaccines are not therapeutic and do not prevent progression of infection present at the time of vaccination to disease [55, 56]. Consequently, in the 4-year trial span, many CIN2+ lesions arose from prevalent infection. Efficacy in TV/ITT cohorts depend on HPV prevalence in the trial population at the time of vaccination as well as other parameters. Observed efficacy in TV/ITT analyses increased with time since vaccination, as new disease occurred from incident infection mainly in the control group [33, 35]. Efficacy also increased over time in ATP/PP cohorts, with most “breakthrough” infection detected in the first year after vaccination, apparently because they were the result of emergence of infections acquired, but not detected, before vaccination.
While not primary end points in Cervarix clinical trials, efficacy against oral, anal and vulvar HPV infection have been reported from the Costa Rica Vaccine Trial [28]. Evaluations of oral and anal infection were based on one-time detection at 4 years of follow-up. Vaccine efficacy (among women who were cervical HPV-16/18 DNA-negative and seronegative at baseline) against HPV-16/18 anal infection was 83.6% (95% CI, 66.7%–92.8%) and against oral infection was 93.3% (95% CI, 62.5%–99.7%).
Vaccine Efficacy in Women Aged >26 Years
The first RCTs in women older than 25 or 26 years also enrolled participants from multiple countries (Table 2). The Gardasil trial, FUTURE III, included 3800 women [41, 42] and the Cervarix trial, VIVIANE, included about 5700 women [43, 44]. The trial designs were similar with women enrolled without regard to HPV infection status or number of lifetime partners, and the primary end points were vaccine type 6-month persistent infection or vaccine type–attributable CIN1+ (and external genital lesions for the Gardasil trial). For the primary end points, efficacy in the PP/ATP populations was statistically significant and similar in the Gardasil and Cervarix trials, 88.7% and 90.5%, respectively. For vaccine-type CIN2+, the point estimates of efficacy were high, but not statistically significant owing to small numbers. In the ITT population, Gardasil efficacy was 47.2% (95% CI, 33.5%–58.2%) against a combined end point of persistent infections, extragenital lesions, and/or CIN1+ attributable to HPV type 6, 11, 16, or 18. For Cervarix, efficacy was 86.5% (95% CI, 74.4%–93.6%) for a combined end point of persistent infection and/or CIN1+ attributable to HPV-16 or HPV-18.
Vaccine Efficacy in Men
The only prelicensure efficacy trial in men was a Gardasil trial that included 4065 men aged 16–26 years [45]. Participants were enrolled without regard to baseline HPV status, but with 5 or fewer lifetime sex partners. In the PP analysis, efficacy for prevention of vaccine type–attributable anogenital warts was 89.3% (Table 2). Similar to findings in women, no efficacy was observed among participants infected with the respective HPV type at baseline. About 400 men who have sex with men were included in a substudy; outcomes were anogenital warts, anal intraepithelial neoplasia (AIN) of any grade and AIN grade 2/3 (AIN2/3, considered anal precancer [46]. The PP efficacy was 88.1% for prevention of vaccine type–attributable condyloma and 74.9% for AIN2/3 (Table 2). In the ITT population, efficacy was lower, 54.2% (95% CI, 18.0%–75.3%), for prevention of AIN2/3 attributable to HPV type 6, 11, 16 or 18. Penile/perineal/perianal intraepithelial neoplasia grade 1/2/3 outcomes were evaluated, but there were few cases.
Although there was no prelicensure Gardasil 9 efficacy trial in men, immunogenicity studies found that >99% of men were seropositive for all 9 types 1 month after the third vaccine dose and GMTs were noninferior to those of women who received the vaccine in efficacy trials. In an immunogenicity trial comparing Gardasil and Gardasil 9 in men, seroconversion and GMTs for HPV types 6, 11, 16, and 18 were similar [57]. Gardasil 9 was licensed in men based on safety and immunobridging data [57, 58].
No studies have evaluated efficacy for prevention of oropharyngeal cancer, the most common HPV-attributable cancer in men in the United States [8]. The lack of a precursor lesion for oropharyngeal cancers has precluded study of that outcome. However, based on accumulated clinical trial data showing high vaccine efficacy against a wide range of HPV-associated outcomes, as well as effectiveness against oral infection in men and women in post hoc analyses of some trials and in observational studies, in 2020 Gardasil 9 received an indication from the US FDA for prevention of oropharyngeal cancer and some other head and neck cancers caused by HPV [59, 60]. An RTC of Gardasil 9 in men, with a primary end point of persistent vaccine-type oral infection, started in 2020 (ClinicalTrials.gov identifier NCT04199689).
Immunogenicity
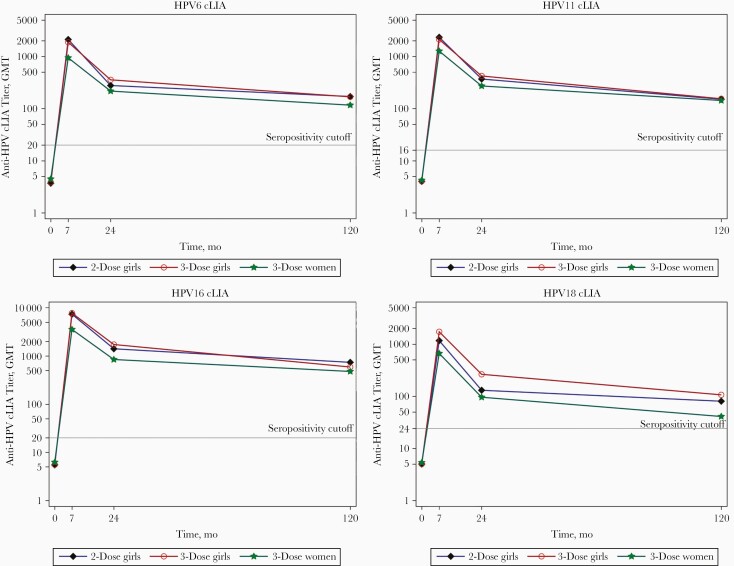
HPV vaccines produce robust antibody responses, with peak titers much higher than the generally low titers observed after natural infection [61, 62]. Seroconversion was close to 100% for all types after the 3-dose series in clinical trials, and GMTs were high and comparable across racial/ethnic groups and region of residence [63]. Titers increase after each dose, decline over time after the last dose, and plateau after about 2 years [61, 62, 64] (Figure 1). Antibodies appear stable over time, with studies showing persistence of antibody levels through 11 or 12 years after vaccination [65, 66]. Antibody titers produced by HPV vaccination are inversely related to age [41, 63, 67]. The higher titers among adolescent girls and boys (ie, 9 to 14 or 15 years), compared with those among women of the age enrolled in efficacy trials, allowed licensure of the vaccines in the younger age group through immunobridging [38, 40]. Immunogencity data have also contributed to other appprovals. As noted above, antibody response to the 4 types included in Gardasil were noninferior among Gardasil 9 vaccine recipients, an important finding in the prelicensure trial [54]. Immunobridging data were also important for licensure of Gardasil 9 in men aged 27–45 years [27, 68].
Figure 1.
Kinetics of competitive Luminex immunoassay (cLIA) antibody titers over time. Geometric mean titers (GMTs) for girls receiving 2 or 3 doses and women receiving 3 doses, up to 120 months after quadrivalent human papillomavirus (HPV) vaccination (modified from Donken et al [64]).
Because the initial trials used different antibody assays, it was difficult to compare immunogenicity results from Gardasil trials, which used predominantly the cLIA, and Cervarix trials, which used a VLP ELISA [26]. While the ELISA is sensitive, it does not distinguish neutralizing from nonneutralizing antibodies. The cLIA measures serum antibody competition for binding a type-specific neutralizing monoclonal antibody to the VLPs. Head-to-head immunogenicity trials were later conducted using the same assay, showing that Cervarix produced higher titers [69]. The higher titers after Cervarix are thought to be due to the AS04 adjuvant. The clinical implications are not clear, because both vaccines produce antibody substantially higher than natural infection and there is no evidence of waning protection, even when antibody wanes to undetectable levels, as has occurred for HPV-18 in a subset of Gardasil vaccinees [61]. No minimum level of protective antibody has been established, because “breakthrough” infections are uncommon and cannot be distinguished from emergence of prevalent infection or reactivation of latent infection, and they are not correlated with serum antibody levels. In addition, immune serum passive transfer studies in a mouse cervicovaginal challenge model suggest that very low antibodies levels are protective, levels likely below the level of detection of currently available assays [30].
Cross-Protection Against HPV Types Not Targeted by the Vaccines
Protection against HPV types not specifically targeted by HPV vaccines was explored in prelicensure clinical trials and in postlicensure evaluations. In the first efficacy trials, there was evidence for partial cross-protection against infection as well as disease due to some nonvaccine HPV types. The most consistent protection for Cervarix was for HPV-31 and HPV-33, types closely related to HPV-16, and HPV-45, a type closely related to HPV-18 [48, 70]. For Gardasil, protection was observed mainly for HPV-31 [71]. Although initial data suggested that the cross-protection might be short term, data from follow-up of Cervarix studies show cross-protection against at least HPV types 31, 33, 45, and to a lesser extent other nonvaccine types, through 11 years [72], and sustained seropositivity to HPV-31 and HPV-45 [73]. Data from clinical trials have been substantiated by postlicensure monitoring in Scotland and the Netherlands, countries that introduced Cervarix, showing declines in prevalence of these 3 types in addition to HPV-16 and HPV-18 [74, 75].
Investigations of HPV Type Replacement
Although type replacement has occurred after introduction of other vaccines [76], HPV is a stable virus and types appear to act independently, suggesting it unlikely that an ecological niche created by decreasing vaccine-targeted types would lead to type replacement [77]. Studies to date have not identified any consistent evidence of type replacement, although some increases in individual types have been reported in various studies in different age groups [78]. Detection of higher prevalence of individual types could be due to “unmasking” as a result of reduced competition for reagents in HPV assays. In national surveillance monitoring with stable HPV DNA testing methods there has been no evidence of high-risk type replacement [79, 80]. Continued monitoring for potential type replacement is prudent in case this emerges as a problem as vaccine coverage increases.
Number of Doses
The high efficacy of 3-dose schedules in prelicensure vaccine trials and a post hoc analysis of the trial in Costa Rica suggesting similar high efficacy in persons randomized to receive 3 doses who did not complete the series and those who did [81], led to trials of 2-dose schedules. Trials were designed to evaluate noninferior immunogenicity of 2 doses in 9–14-year-olds compared with 3 doses in women the same age as those in the original efficacy trials. Outcomes included seroconversion and GMTs using either an ELISA or cLIA. These designs were chosen because antibody titers are higher with younger age at vaccination and noninferiority, required by regulatory agencies, would be easier to demonstrate comparing these age groups.
Studies of Gardasil, Cervarix, Gardasil 9, and Cecolin all found noninferiority with 2 doses administered at a 6- or 12-month interval [82–85]. For example, the Gardasil 9 trial compared a 2-dose schedule (0 and 6 or 0 and 12 months) in girls and boys aged 9–14 years, with a 3-dose schedule (0, 2, and 6 months) in women aged 16–26 years [82]. Among about 1500 participants, ≥97.9% seroconverted to all 9 types and GMT noninferiority criteria were met (value >0.67 for the lower bound of the 95% CI of the GMT ratio for 3 doses in women to 2 doses in girls and boys). Not only were noninferiority criteria met, but GMTs were significantly higher among persons aged 9–14 years who received 2 doses than in women aged 16–26 years who received 3 doses. GMTs were generally higher for 2 doses administered at an interval of 12 months than for those administered at an interval of 6 months.
Questions remain about reduced dose schedules for older age groups and whether a single dose might be sufficient for high and long-lasting immunity; these are the focus of current research [86]. A single-dose schedule would have logistical, cost and acceptability advantages. Post hoc evaluation of randomized clinical trials, in which some participants did not complete their vaccination schedules, have allowed comparison of 1, 2, and 3 doses [66, 87]. To date, efficacy against prevalent infection is similar in all dose groups and antibody titers have been stable for up to 11 years after vaccination [66]. However, a single-dose produces lower antibody titers (about 5-fold lower) than 2 or 3 doses, precluding studies based on immunological noninferiority. Postlicensure observational studies have examined effectiveness by number of doses, some showing differences by number of doses and other not [88]. Differential risk of prevalent HPV at the time of vaccination in women who did not complete the recommended series likely biased the results in some studies. Several large clinical trials are ongoing to rigorously evaluate single-dose vaccination [89–91].
Long-Term Immunogenicity and Protection
When HPV vaccines were first licensed, data from the phase III efficacy trials were available through ≤4 years; however, available evidence suggested that the protection from vaccination would be long lasting. Those data included the relatively stable antibody titers over time and evidence of immune memory based on an anamnestic response to an additional vaccine dose 5 years after the initial series [30, 92]. Data, now through >10 years after vaccination, show no waning of protection and suggest that booster doses will not be needed. Evidence suggests that the long-lasting duration of protection after HPV vaccination is due to long-lived plasma cells, not memory B or T cells [1]. Because of the slow initial stage of the HPV life cycle, very low levels of neutralizing antibody seem to be needed to prevent HPV infection at the epithelial basement membrane.
The longest follow-up in the randomized clinical trials was 9.4 years for Cervarix, 5 years for Gardasil, and 9.5 years for a monovalent HPV-16 vaccine [62, 93, 94]. While control group participants were vaccinated after completion of the large efficacy trials, follow-up of some participants continued for disease outcomes and immunogenicity [62, 65, 95–97]. Women from 4 Nordic countries who received 3 doses of Gardasil in the FUTURE II trial were followed up through national registries, and effectiveness against HPV-16/18–attributable CIN2+ was estimated by comparing incidence with the expected incidence using historical registry data. After >10 years of follow-up, there were no cases of HPV-16/18–attributable CIN2+ among 2121 participants [97]. Vaccine effectiveness of 100% (95% CI, 94.7%–100%) was demonstrated for at least 12 years.
Adolescents who participated in immunogenicity studies have also been followed up for antibody persistence and, in some studies, for infection or disease after they became sexually active. Among girls vaccinated at age 10–14 years with 3 doses of Cervarix, there was high and sustained antibody responses through 10 years [73]. For Gardasil and Gardasil 9, seropositivity also remained high, with no vaccine-type high-grade cervical disease during 10 or 8 years of follow-up, respectively [98, 99]. While these are follow-up data after a 3-dose schedule, immunogenicity studies show similar antibody kinetics after 3 and 2 appropriately spaced doses, suggesting that a 2-dose schedule will provide the same duration of protection [64, 87] (Figure 1).
HPV VACCINE LICENSURE AND POLICY
Since the first HPV vaccine was licensed in 2006, there have been changes in regulatory indications and vaccination policy [100]. Cervarix and Gardasil were first licensed by the US FDA and many other regulatory authorities for girls and women from age 9 or 10 through age 25 or 26 years. Because the initial efficacy trials were conducted only in women, it was not until 2009, after data were available in men, that Gardasil was licensed for boys and men in the United States. When trials were completed in persons older than 26 years, some authorities outside the United States extended the licensed age indications through age ≥45 years. The FDA did not approve any HPV vaccine for use above age 26 years until 2018, when the Gardasil 9 manufacturer submitted a supplemental application to extend the age through 45 years [59].
The changes in HPV vaccination recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) [101–106] and WHO [107–110] are shown in Table 3. In the United States, routine vaccination has been recommended since 2006 for girls at age 11 or 12 years; vaccination can be started at age 9 years. Vaccination is also recommended through age 26 years for girls and women not vaccinated previously (catch-up vaccination).
Table 3.
Recommendations for Human Papillomavirus Vaccination From the Advisory Committee on Immunization Practices and the World Health Organization
| Yeara | Recommendations | HPV Vaccinesb |
|---|---|---|
| ACIP | (Available in the United States) | |
| 2006 [101] | Females: routine vaccination with a 3-dose schedule at age 11 or 12 y; series can be started at age 9 y; vaccination recommended through age 26 y for those not vaccinated previously | Gardasil |
| 2009 [102] | Females: recommendations as in 2006 Males: may be vaccinated at age 9–26 y |
Gardasil, Cervarix |
| 2011 [103] | Females: Recommendations as in 2006 Males: Routine vaccination at age 11 or 12 y with Gardasil; series can be started at age 9 y; vaccination recommended through age 21 y in those not vaccinated previously and through age 26 y for men who have sex with men and persons with immunocompromising conditions, including HIV |
Gardasil, Cervarix |
| 2015 [104] | Gardasil 9 recommended as a vaccine that can be used for females and males | Gardasil, Cervarix, Gardasil 9 |
| 2016 [105] | 2-dose schedule, if starting series at age 9–14 y; 3-dose schedule for older age groups and for persons with immunocompromising conditions | Gardasil, Cervarix, Gardasil 9 |
| 2019 [106] | Vaccination recommended through age 26 y, for those not vaccinated previously; shared clinical decision making for persons aged 27–45 y | Gardasil 9 |
| WHO | (Available through Gavi) | |
| 2009 [107] | Routine vaccination of a single birth cohort of girls aged 9–13 y with a 3-dose schedule | Gardasil, Cervarix |
| 2014 [108] | 2-dose schedule, if starting series at age 9–14 y; 3-dose schedule for older girls/women or for immunocompromised persons | Gardasil, Cervarix |
| 2017 [109] | Vaccination of multiple cohorts of girls aged 9–14 y when vaccine first introduced; vaccination of other populations (females aged ≥15 y or males), only if feasible, affordable, cost-effective, and not diverting resources from primary target population or from cervical cancer screening programs | Gardasil, Cervarix |
| 2019 [110] | Owing to the global vaccine supply/demand imbalance, target girls aged 13 or 14 y, before they age out of the recommended primary target population, or schedule an extended interval of 3–5 y between the 2 doses, with dose 1 given to girls aged 9 or 10 y | Gardasil, Cervarix |
Abbreviations: ACIP, Advisory Committee on Immunization Practices; Gavi, The Vaccine Alliance (Gavi); HIV, human immunodeficiency virus; HPV, human papillomavirus; WHO, World Health Organization.
aYear of vote by ACIP or decision by WHO or WHO’s Strategic Advisory Group of Experts on Immunization.
bNo preference stated for any vaccine. Gardasil is a quadrivalent, Cervarix a bivalent, and Gardasil 9 a nonavalent HPV vaccine.
In 2011, routine HPV vaccination was recommended for boys and men aged 11–12 through 21 years for those not vaccinated previously [103]. The burden of disease among males in the United States and the cost-effectiveness and impact of vaccinating males when vaccination coverage levels are low in females, as in the United States in 2011, were considered, along with equity, in the decision to include males in the routine immunization program [100, 111–113]. Health economic data also informed the different upper age for catch-up vaccination in men compared with women. In late 2016, ACIP recommended a 2-dose series (with the second dose 6–12 months after the first), for most persons starting the series before their 15th birthday [105]. The most recent recommendation change was in 2019, after licensure of Gardasil 9 for persons through age 45 years. ACIP did not expand catch-up vaccination through age 45 years but recommended shared clinical decision making regarding potential vaccination [106]. At that time, recommendations for catch-up vaccination were harmonized through age 26 years for everyone, primarily for simplification of the schedule.
There was never a preferential recommendation among the 3 HPV vaccines licensed in the United States; however, almost all vaccine used through 2015 was Gardasil [100]. In 2014, Gardasil 9 was licensed for use in females and males, and in 2015 was recommended by ACIP among 3 vaccines for use in females and 2 for use in males [104]. By the end of 2016, only Gardasil 9 was marketed in the United States.
In all countries with funded national HPV vaccine immunization programs, vaccination was initially recommended for young adolescent girls. Over time, more countries have included males in their routine vaccination program. For example, in 2007 Australia recommended vaccination for 12–13-year-old girls, with catch-up vaccination through age 26 years for 2 years at the beginning of the program. Boys were included in the national program in 2013 [114]. There is a range of recommended age groups across Europe [115]. Some countries target adolescent girls in a narrow age range, with no catch-up. In the United Kingdom, vaccine has been recommended for girls aged 12–13 years since 2008; catch-up vaccination through age 17 years was recommended for the first 2–3 years of the program. In 2015, vaccination was recommended for gay and bisexual men up to age 45 years seen at sexual health clinics, before gender-neutral routine vaccination was recommended in 2018 [116].
WHO first issued a recommendation for HPV vaccination in 2009, targeting a single birth cohort of girls aged 9–13 years with a 3-dose schedule [107] (Table 3). In 2014, WHO recommended a 2-dose HPV vaccination schedule if the series is initiated before age 15 years [108]. The majority of low- and middle-income countries that introduced the HPV vaccine followed the WHO guidelines and focused on a single age cohort of young adolescent girls and also changed to a 2-dose schedule. To increase direct protection and herd immunity, WHO updated recommendations in 2017, stating that multiple cohorts of girls aged 9–14 years be vaccinated when vaccine is first introduced [109, 117]. Vaccination of other populations was only recommended under some conditions. In 2019, because of a global HPV vaccine shortage, WHO’s Strategic Advisory Group of Experts on Immunization recommended a temporary pause to multiple-age cohort vaccination and proposed alternative vaccination strategies to allow vaccine introductions to reach girls most at risk, including (1) targeting girls at age 13 or 14 years, before they age out of the recommended primary target population or (2) adopting an extended interval of 3–5 years between the 2 doses, with the first dose given to girls at age 9 or 10 years [110].
HPV VACCINATION PROGRAMS
Through January 2020, more than 104 (54%) countries had introduced HPV vaccination [118], including 37 countries or territories with a gender-neutral immunization schedule [119]. High-income countries were the first to introduce HPV vaccination, and there remain disparities by income with >85% of high-income countries having introduced vaccination, compared with only 30% of lower-income countries [120].
Determining global and country-specific coverage is challenging owing to different age groups targeted and changing dose recommendations. WHO has collected data and calculated coverage measures since 2010 for females and since 2019 for males [121]. In general, countries with school-based vaccination programs have achieved higher coverage (mean, 20% higher) than those with health facility-based programs.
In the United States, HPV vaccine is administered mainly in primary care, health facility settings. HPV vaccination coverage among females paralleled other vaccines recommended for adolescents in the first years of the program. However, HPV vaccination coverage then increased more slowly and remained lower than for other vaccines recommended in the same age group [122]. After the routine recommendation for males in 2011, coverage among boys aged 13–17 years increased at a faster rate than among girls. In 2019, among girls and boys, ≥1-dose coverage was 73% and 70%, respectively, and up-to-date coverage was 57% and 52%. Several factors contribute to the low HPV vaccination coverage, including parent- and health system–level factors. Because a provider recommendation is strongest predictor for accepting vaccination, efforts to increase coverage have focused on providing education, tools and communication messages for vaccine providers [123–125].
The high cost of HPV vaccines initially limited introduction in many countries and there were also other impediments, such as completing health priorities and other new vaccine introductions. The target adolescent age group, not part of most immunization programs, also presented challenges. In many low- and middle-income countries, vaccination was initially in subnational or national demonstration projects through vaccine donations or other support [126]. Gavi, the Vaccine Alliance (Gavi) began supporting HPV vaccine in 2012 [127]. At that time, 53 Gavi-eligible countries could apply for either small-scale demonstration projects or national introduction (if they had experience with delivering a multidose vaccine to adolescents) for a single year age cohort. In 2016, after WHO’s updated recommendations, Gavi supported national HPV vaccine introductions, including multiage cohort vaccination in the first year of the program [109].
Tiered pricing and other purchasing programs also made vaccine more affordable in some countries. For example, in 2011, through the Pan American Health Organization’s Revolving Fund, HPV vaccines were available for member countries at a substantially lower price than in high-income countries. Almost all countries in Central and South America introduced HPV vaccine by 2018 [128].
While progress has been made, with more countries being able to introduce HPV vaccine with Gavi support, there has been a global HPV vaccine shortage since 2018 [129]. Based on current manufacturing capacity, the global supply will not be able to meet demand until 2023. Some countries have had to delay vaccine introduction. The 2 primary manufacturers have committed to increasing capacity and new manufacturers are likely to enter the global HPV vaccine market [20, 23].
Many countries have had challenges with their vaccination programs owing to safety and other concerns. Initially, theories emerged about vaccination promoting risky sexual behavior; this has not been found in multiple studies [130–132]. There were also concerns about infertility related to vaccination, which have not been substantiated [133]. In some countries, specific vaccine safety concerns have resulted in decreases in coverage or completely unsuccessful introductions. For example, in Japan, case reports about chronic regional pain syndrome resulted in suspension of government HPV vaccination recommendations in 2013, less than a year after the vaccine was introduced [134]. As of 2020, recommendations had not been reinstated. In Denmark, where sustained high coverage was initially achieved in a health facility-based program, concerns about postural orthostatic tachycardia syndrome resulted in a precipitous drop in coverage after negative media reports starting in 2013. A strong national information campaign was able to reverse the decline in coverage [135]. In Colombia, mass psychogenic illness during school-based immunization programs resulted in coverage falling from 80% to 5% in 2016 [136]. Educational efforts directed to medical staff and the public were successful in reversing this trend. These and other events have underscored the importance of planning and implementing safety communication strategies.
VACCINE SAFETY
There are robust data on the safety of HPV vaccination from both prelicensure trials and postlicensure monitoring and evaluations [137–140]. The prelicensure trials raised no consistent concerns. All found the expected adverse effects, which include mainly fever and injection site reactions [139, 140]. Routine safety monitoring as well as special studies have been conducted in many national vaccination programs [138]. These data provide extensive reassuring information regarding Gardasil and Cervarix. Because the United States was the first country to introduce Gardasil 9, at present the only postlicensure safety data are from the United States [141, 142].
In the United States, the major routine monitoring systems, as well as special studies, were used to evaluate HPV vaccine safety [137]. In addition, postlicensure safety studies were conducted by manufacturers as FDA postmarketing commitments. Early monitoring data from the Vaccine Adverse Event Reporting System showed that syncopal episodes can occur after HPV vaccination, as with other adolescent vaccinations; recommendations were made for adolescents to be seated when vaccinated and observed afterward [143]. Through 2019, >120 million doses of HPV vaccines had been distributed in the United States, including 29 million doses of Gardasil 9. Additional reviews of Vaccine Adverse Event Reporting System data for all 3 licensed HPV vaccines have not identified new concerns [142, 144]. Rapid cycle analyses in the Vaccine Safety Datalink were conducted for Gardasil and more recently for Gardasil 9 [137, 141]. These active investigations can look at specific associations and no safety concerns have been confirmed. Other large population-based evaluations of general safety, death, and multiple autoimmune and neurologic conditions have been reassuring with no other confirmed safety signals [137].
In response to safety concerns in Europe, the European Medicine Agency reviewed data on postural orthostatic tachycardia syndrome and chronic regional pain syndrome, finding no relationship with HPV vaccination [145]. The WHO’s Global Advisory Committee on Vaccine Safety reviewed safety of HPV vaccines first in 2007 and multiple times since. In 2017, it commissioned a comprehensive assessment and systematic review focusing on serious events after Cervarix and Gardasil vaccination [146]. The review included 26 RCTs and 6 postlicensure cohort studies. Subsequently, further safety studies along with systematic reviews, including evaluation of many autoimmune diseases and specific conditions such as Guillain-Barré syndrome, primary ovarian insufficiency and chronic fatigue, have been published [147]. A 2018 review identified 109 studies, including 15 population-based studies in over 2.5 million vaccinated individuals across 6 countries [138]. Data continue to accumulate showing that HPV vaccines have an excellent safety profile.
IMPACT OF VACCINATION PROGRAMS
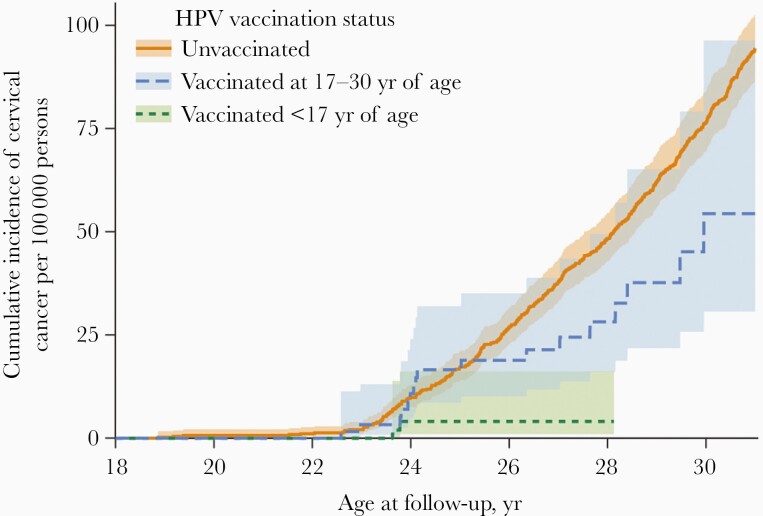
The high efficacy of HPV vaccines observed in clinical trials has been reflected in dramatic impact of vaccination programs. Prevention of HPV-associated cancer is the main goal of HPV vaccination programs; however, impact on this outcome was not expected to be observed for a decade or more. In many countries, monitoring more proximal HPV-associated outcomes has been conducted. A systematic review and meta-analysis published in 2019, including data from 65 studies in 14 high-income countries, showed large decreases in these outcomes within 5–8 years after vaccine introduction [2]. The prevalence of HPV-16/18 decreased significantly by 83% among girls aged 13–19 years, and by 66% among women aged 20–24 years. Anogenital warts decreased significantly by 67% among girls aged 15–19 years and by 54% among women aged 20–24 years. Decreases were also observed in boys and men. After 5–9 years of vaccination, among women screened for cervical cancer, CIN2+ decreased significantly by 51% among 15–19-year-olds and by 31% among 20–24-year-olds. Recently, HPV vaccine effectiveness for prevention of cervical cancer was demonstrated. In Sweden, a country where Gardasil had been introduced, the risk of cervical cancer was 88% lower among women who had initiated vaccination before age 17 years and 53% lower among those who initiated vaccination at age 17–30 years, compared with unvaccinated women (Figure 2) [148].
Figure 2.
Cumulative incidence of invasive cervical cancer according to human papillomavirus (HPV) vaccination status. This study used data from nationwide Swedish demographic and health registers, 2006–2017, to evaluate vaccine effectiveness in the national program (reprinted with permission from Lei et al [148]).
Data from postlicensure monitoring have provided additional information on several aspects of vaccination that could not be determined in the prelicensure clinical trials. For example, in Australia, a country that achieved high coverage early in their program, a large and dramatic decline in anogenital warts was observed among young women (93%) as well as young men who have sex with women (82%), although at that time the vaccination program only included girls and women. No declines were observed in men who have sex with men [149]. This was the first demonstration of strong herd effects. Postlicensure studies from several countries have examined possible cross-protection and type replacement that could not be adequately explored in clinical trials, as discussed earlier in this review [74, 78, 80].
In the United States, despite modest coverage in the first years of the vaccination program, a 56% decline in HPV vaccine-type prevalence among girls and women aged 14–19 years was observed within the first 4 years of the program [150]. By 10 years after introduction, there was a 86% decrease among 14–19-year-olds and a 71% decrease among 20–24-year-olds [151]. The greater than expected impact observed given the low 3-dose coverage suggested high efficacy with <3 doses and/or impact through herd effects. There also have been decreases in anogenital warts, consistent with the drop in vaccine-type prevalence [152]. In claims data from US health plans, from 2006 to 2014 there were declines in anogenital warts among women: a 61% decline among 14–19-year-olds and a 44% decline among 20–24-year-olds [152]. Cervical cancer precursor lesions are being monitored in the United States through several efforts and all show declines in lesions among young adult women [153–155]. In a 5-site project that includes active population-based surveillance of CIN2+, 50% and 36% declines have been reported in CIN2+ per estimated women screened among 18–20- and 21–24-year-olds, respectively, between 2008–2009 and 2014–2015 [155].
FUTURE GOALS AND RESEARCH
The availability of highly effective HPV vaccines, as well as tools for cervical cancer screening and treatment, has raised interest in setting disease reduction goals. In May 2018, the WHO director general called for action toward the elimination of cervical cancer as a public health problem, and a strategy was adopted by the World Health Assembly in 2020 [120, 156]. The target is ≤4 cases per 100 000 women-years ,with 2030 goals of 90% HPV vaccine coverage of girls in the target age group, 70% coverage of twice lifetime cervical cancer screening of women at age 35 and 45 years, and 90% delivery of treatment needed for cervical precancer and cancer. Modeling studies predict that most lower- and lower-middle-income countries could achieve this goal by the end of the century, and that it would prevent 74 million of the estimated 93.5 million new cervical cancer cases predicted to occur during that time [3]. Country-specific modeling, using current vaccination and cervical cancer screening coverage, suggest that <4 cases per 100 000 person-years could be achieved in Australia between 2021 and 2035 and in the United States between 2038 and 2046 [157, 158].
There are outstanding questions about HPV vaccines. Current research is addressing some of these, including reduced dose schedules, efficacy and effectiveness in various immunocompromised populations and the possible role of HPV vaccine to prevent recurrent disease. Questions also remain about how cervical cancer screening recommendations should be changed in areas with high vaccine coverage as vaccinated girls age into screening programs. As for all vaccines, there are challenges related to the coronavirus disease 2019 pandemic regarding new HPV vaccine introductions and maintaining coverage in existing programs [159].
HPV vaccines have demonstrated remarkable efficacy in clinical trials and impact in real-world settings across a spectrum of HPV-associated disease. Robust data show that HPV vaccines are safe, although concerns about safety have impeded vaccine introductions or coverage goals in some countries. HPV vaccination policy has evolved as new data have become available and ambitious disease reduction goals are being discussed. Although the current demand for HPV vaccines exceeds supply, increased production capacity from current manufacturers as well as new manufacturers in late stages of vaccine development should alleviate the current shortage and allow realization of further global disease prevention goals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
The complete references are available as online Supplemental Material.
Supplementary Material
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Cancer Institute. Use of trade names is for identification only and does not imply endorsement by the Public Health Services or the US Department of Health and Human Services.
Supplement sponsorship. This supplement is sponsored by the Bill and Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 2. Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group . Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020; 395:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30(suppl 5):F123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–27. [DOI] [PubMed] [Google Scholar]
- 32. Paavonen J, Jenkins D, Bosch FX, et al. ; HPV PATRICIA study group . Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161–70. [DOI] [PubMed] [Google Scholar]
- 43. Skinner SR, Szarewski A, Romanowski B, et al. ; VIVIANE Study Group . Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014; 384:2213–27. [DOI] [PubMed] [Google Scholar]
- 45. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011; 364:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet 2017; 390:2143–59. [DOI] [PubMed] [Google Scholar]
- 64. Donken R, Dobson SRM, Marty KD, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis 2020; 71:1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA 2016; 316:2411–21. [DOI] [PubMed] [Google Scholar]
- 96. Kjaer SK, Nygård M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis 2018; 66:339–45. [DOI] [PubMed] [Google Scholar]
- 100. Markowitz LE, Gee J, Chesson H, Stokley S. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr 2018; 18:S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf 2018; 41:329–46. [DOI] [PubMed] [Google Scholar]
- 148. Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 2020; 383:1340–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.