Abstract
Background and Aims
Byttneria is one of the few climbing genera in Malvaceae. Some Byttneria are known for their lobed stems. We explore the development of these stems, how they have evolved within the group and their relevance in the evolution of the climbing growth form in Malvaceae.
Methods
We combine developmental anatomical work with phylogenetic comparative methods. We use Byttneria divaricata and B. filipes as models in the anatomical work, a review of herbarium vouchers, and the most recent phylogeny of Byttneria and allies to elucidate how these stems evolved within the clade under maximum-likelihood and Bayesian approaches. We use Pagel94 tests to analyse the correlated evolution of lobed stems and prickles.
Key Results
Each lobe coincides with one of the five vascular bundles. By augmented activity of the fascicular cambium in the lobes coupled with reduced activity of the interfascicular cambium in the interlobes, secondary growth increases the lobulation already present during primary growth. Within Byttneria and allies, lobed young stems appeared at least three times, once in Ayenia and twice in the paraphyletic Byttneria. Lobed adult stems were conserved in Byttneria s.s., where lobed adult stems in combination with prickles were shown to have evolved as a climbing mechanism within the group; prickles were lost once within Byttneria s.s., in a shrubby subclade. Byttneria Clade 2 comprises climbers with twining cylindrical adult stems and no prickles, which constitutes a different climbing mechanism in the group.
Conclusions
We provide evidence of one of the few cambial variants known whose secondary body reflects the primary body vasculature and show that lobed adult stems and prickles in Byttneria could be used in the new delimitation of genera in the group. Lobed stems independently appeared in climbing Grewia, suggesting a convergence favouring the climbing growth form.
Keywords: Climbing plants, lianas, cambial variants, non-cylindrical stems, lobed stems, ontogeny, cambium differential activity, Malvaceae, Byttnerioideae, Byttneria
INTRODUCTION
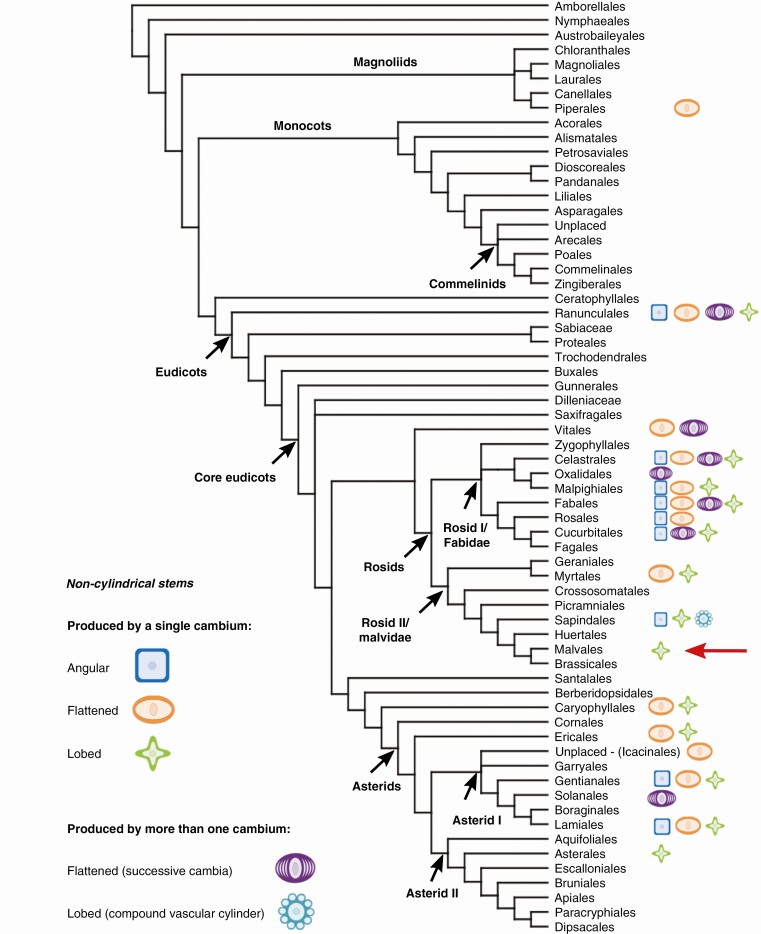
Most woody plants exhibit cylindrical stems throughout their lives. Such cylindrical shape is already established in the primary body and is typically maintained during secondary growth by the activity of a vascular cambium that produces tissues bidirectionally and at constant rates across its girth (Evert, 2006). However, alternatives to this pattern have appeared multiple times in the history of vascular plants and have been named cambial variants (Carlquist, 2001; Angyalossy et al., 2012, 2015). One group of such variations are the non-cylindrical stems, which can be encountered scattered across the entire phylogeny of woody plants; they are present in gymnosperms, such as lianescent Ephedra (Ephedraceae) (Cabanillas et al., 2014), as well as in numerous angiosperm lineages (summarized in Fig. 1, listed in Supplementary Data Table S1). Although much more common in climbing plants, where they are thought to contribute in anchoring the plant to other plants or supports while climbing (Angyalossy et al., 2015), non-cylindrical stems may be also found in shrubs and trees, such as in some trees of Aspidosperma (Apocynaceae), Haematoxylum (Fabaceae) (Schenck, 1893; Angyalossy et al., 2012) and several buttress trees of rainforests, where they are thought to provide more mechanical stability to these trees which commonly occur in flood-prone regions of unstable soils (Francis, 1924).
Fig. 1.
Non-cylindrical stems within woody angiosperm orders (monocots not considered). Three main categories are recognized: angular, flattened and lobed stems; a single or multiple cambia may be involved in their development. To the best of our knowledge, Malvales only exhibits lobed stems in some Malvaceae genera, i.e. Byttneria, Gossypioides, Gossypium and Grewia (red arrow). Topology follows Stevens (2001 onwards), and Supplementary Data Table S1 lists in detail all the taxa used for mapping.
Three main categories of non-cylindrical stems can be recognized (Fig. 1): (1) angular stems, which may be triangular to hexagonal; (2) flattened stems, unilaterally or bilaterally, ranging from slightly oval to markedly flattened; and (3) lobed stems, ranging from bilobed to multilobed (Schenck, 1893; Obaton, 1960; Carlquist, 2001; Acevedo-Rodríguez et al., 2015 onwards). Non-cylindrical stems as a whole commonly start cylindrical and remain as such for a certain period of time after the vascular cambium is established. However, later their cambium stops having equal activity throughout its girth with some sectors starting to produce more wood and/or bark than others, resulting in non-cylindrical stems. The most common case is that of non-cylindrical stems derived from the unequal activity of a single cambium. This is the case of species of Heteropterys subsect. Aptychia (Malpighiaceae) (Amorim, 2003; Pace, 2015) and lianescent Coccoloba (Polygonaceae) (Caballé, 1993). Rarely, stems can already begin non-cylindrical, such as in a number of species of Schnella (previously known as climbing Bauhinia; Löffler, 1914; Wagner, 1946; Basson and Bierhorst, 1967; Fisher and Blanco, 2014), Serjania (Johnson and Truscott, 1956; Cunha Neto et al., 2017) and Paullinia (Cunha Neto et al., 2017; Chery et al., 2020b) (Sapindaceae). These initially lobed stems are either maintained and present in adult stems or lost when secondary growth starts and progresses (Johnson and Truscott, 1956; Cunha Neto et al., 2017; Chery et al., 2020b), and in Paullinia this phenomenon has been shown to be important in the formation of complex stems in Sapindaceae (Chery et al., 2020b).
The second potential generator of non-cylindrical stems is the formation of multiple cambia in a single stem, either compound stems in Sapindaceae or successive cambia in Convolvulaceae, Fabaceae, Menispermaceae and Vitaceae (Radlkofer, 1875; Schenck, 1893; Caballé, 1993; Carlquist, 2007; Jacques and De Franceschi, 2007; Tamaio and Angyalossy, 2009; Rajput et al., 2012, 2017; Pace et al., 2018; Dias-Leme et al., 2020; Chery et al., 2020b). Only a few works have explicitly shown non-cylindrical stems to be taxonomically informative, such as that of Amorim (2003), where the author shows that they are diagnostic for a set of species of Heteropterys subsect. Aptychia. However, it is likely that a closer examination will prove many non-cylindrical stems to be phylogenetically conserved.
Within Malvaceae, the development of lobed stems was recently described in a single species of climbing Grewia from South Africa, raising interest in what is probably the first record of a cambial variant in the family and its possible correlation to climbing growth form (Gama, 2020; Gama and Oskolski, 2021). Lobed stems are not exclusive to Grewia though, being well known to occur also in Byttneria Loefl. (Cristóbal, 1976), which indicates that lobed stems are probably a cambial variant that evolved more than once in Malvaceae. Byttneria is a pantropical genus which contains ~130 species, composed mostly of climbers, which are either lianas or leaning shrubs, shrubs, subshrubs and more rarely small trees, and many of them have often non-cylindrical, lobed stems (Fig. 2) (Cristóbal, 1976, 1985; Barnett and Dorr, 1990). Byttneria is not monophyletic and is embedded within a larger clade (Whitlock and Hale, 2011; Sharber, 2018), which we refer to as ABRM, by the first initials of the genera within this clade (Ayenia, Byttneria, Rayleya and Megatritheca) (Byttneria s.l. according to Bayer and Kubitzki, 2003).
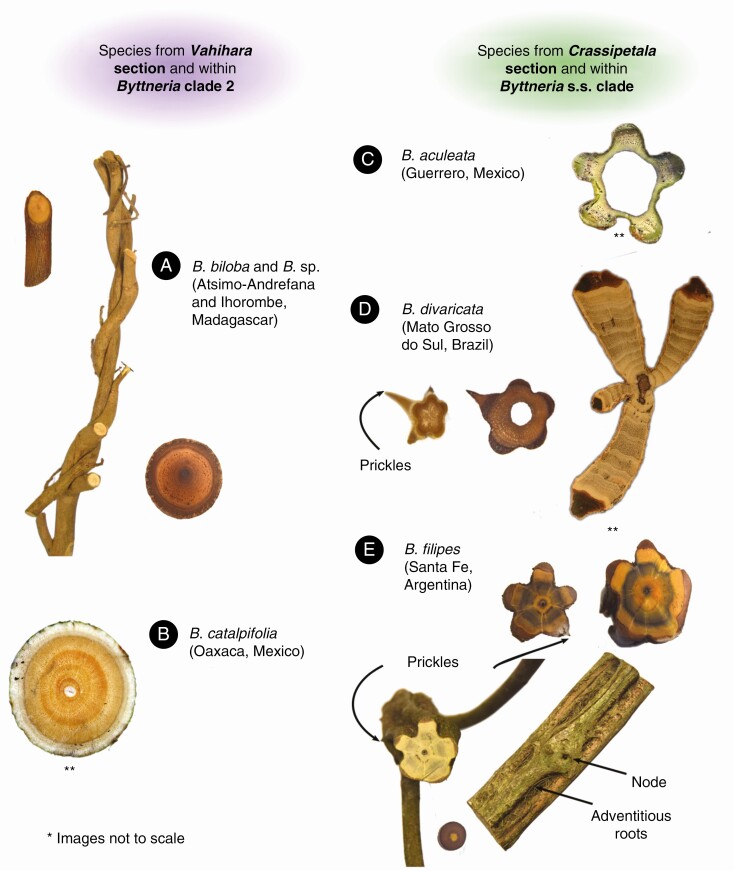
Fig. 2.
An overview of stem diversity within climbing Byttneria. (A, B) Regular stemmed species from Madagascar (Pace 1051 and Pace 1037) and Mexico (Acevedo-Rodríguez 16352), respectively. (A) Note their twining stems. (C, D, E) Lobed stemmed species from Mexico (Acevedo-Rodríguez 16123), Brazil (Acevedo-Rodríguez 16697) and Argentina (Pace 1159), respectively; these species have prickles and at nodes two lobes may fusion into one lobe. (C) Note species may be myrmecophytes, hence the hollow stem. (D) Young stems are lobed and prickles grow exclusively upon lobes. (E) This species may be regular in younger branches. Note that adventitious roots grow exclusively on the interlobe areas, while prickles have the opposite pattern. **B, C and D (only adult four-lobed) images by P. Acevedo-Rodríguez, added with permission of the author.
At present it is unknown how lobed stems are ontogenetically formed in Byttneria and what is their distribution in the phylogeny of the group. In addition, it is unknown if the convergence of lobed stems in Grewia and Byttneria occurs also at the ontogenetic level and their relationship to the climbing growth form. To fill this gap, this work aimed to (1) describe in detail the ontogeny of non-cylindrical lobed stems of Byttneria, using B. divaricata and B. filipes as models, (2) delineate the distribution of lobed stems within the genus by an analysis of Byttneria and allies, (3) unravel if lobed stems evolved in a convergent or conserved fashion within Byttneria and allies, and (4) determine the probable functional role of lobed stems in the evolution of the climbing growth form in the group and its systematic relevance.
MATERIALS AND METHODS
Nomenclature adopted
Macro stem characters. (i) Growth form (non-climbing/climbing)
. We consider ‘climbers’ or ‘climbing plants’ in a broad way, as plants that germinate on the ground, do not self-support themselves and rise during their growth on an erect plant or inert object (Font Quer, 2001; Cabanillas and Hurrell, 2012; Sperotto et al., 2020). These includes species described as ‘scandent shrub’, ‘leaning shrub’, ‘climbing shrub’, ‘liana’ and ‘decumbent shrub’ (Cristóbal, 1960, 1976).
(ii) Climbing plant type (by climbing mechanisms, by twining stem/with aid of prickles)
. Within climbers, we included plants both with ‘specialized’ or ‘active’ climbing mechanisms, e.g. twining stems [plants described by Cristóbal (1976) as ‘lianas’] as well as ‘non-specialized’ or ‘passive’ climbing mechanisms, such as prickles [plants described by Cristóbal (1976) as ‘scandent shrub’, ‘leaning shrub’, ‘highly branched shrubs’ or ‘climbing shrub’] (Darwin, 1865; Schenck, 1893; Cabanillas and Hurrell, 2012; Sperotto et al., 2020).
(iii) Young stem shape (cylindrical/lobed)
. Stems under primary growth or, in herbarium specimens, portions near the stem apex, from either the principal axis or lateral branches, ideally before five up to ten nodes, with any sign of primary growth.
(iv) Adult stem shape (cylindrical/lobed)
. Stems with secondary growth or, in herbarium specimens, portions near the stem base, from either the principal axis or lateral branches or with any sign of secondary growth.
(v) Cylindrical (young or adult) stem
. Plants with a cylindrical stem outline and a smooth surface.
(vi) Lobed (young or adult) stem
. Plants with a non-cylindrical stem outline, with a variable number of lobes. These include adult stems previously described in the literature of the genus as ‘angular’, ‘canaliculate’ ‘square’ and ‘pentagonal’, with ‘wings’, ‘strands’, ‘concavities’ or ‘bulges’(Cristóbal, 1976).
(vii) Prickles (absence/presence)
. As reported by Cristóbal (1976). Does not include other kinds of spiny structures of different origins, such as modified organs (spine s.s.) or stinking hairs.
Plant material
Samples of entire stems from the apex (~1.5 mm diameter at interlobes, ~2.5 mm diameter at lobes) to the thickest portion (~15 mm diameter at interlobes, ~45 mm diameter at lobes) of two species with lobed stems from the Byttneria s.s. clade were collected from natural populations. Byttneria divaricata was collected along the margins of the Paraguay River, north of Corumbá, Mato Grosso do Sul, Brazil (voucher deposited at US and COR Herbaria, Acevedo-Rodríguez 16697). Byttneria filipes was collected along the margins of the Colastiné River, in South Colastiné, Santa Fe, Argentina (voucher deposited at CTES and MEXU Herbaria, Pace 1159). For anatomical procedures, stems were fixed in FAA 70 (5 % formaldehyde, 5 % acetic acid, 90 % ethanol 70) (modified from Johansen, 1940) and after 2 d were transferred to 70 % ethanol for conservation.
Stem characters (growth form, climbing mechanism, young and adult stem shapes, presence of prickles) were reviewed in illustrations and descriptions treating Ayenia (Cristóbal, 1960), Byttneria (Cristóbal, 1976, 1985; Barnett and Dorr, 1990), Rayleya (Arbo, 1981; Cristóbal, 1981), Megatritheca (Cristóbal, 1965) and the outgroups (Bayer and Kubitzki, 2003; Cheek and Dorr, 2007; Rahman et al., 2012). In addition, all vouchers of Ayenia and Byttneria deposited in the National Herbarium of Mexico (MEXU) and the Tsimbazaza Herbarium (TAN) in Antananarivo, Madagascar, were studied in situ. In order to consider multiple specimens and other species not found in MEXU or TAN collections, such as various Ayenia spp., Byttneria spp., Rayleya bahiensis, Megatritheca grossedenticulata, plus the outgroups, Abroma augustum and Kleinhovia hospita, we reviewed the stem shapes of digitized specimens made available by different virtual herbaria (BR, COI, F, G, K, MO, MPU, NY, P, RB, S, US, W; acronyms follow Thiers, 2016) (see information on herbaria consulted in Supplementary Data Table S2; see authorities for ABRM species studied here, character states per species as well as particular sources in Table S3). Literature and herbaria reviews were carried out before studying the ontogeny and were subsequently verified, at different stages of this work.
Anatomical procedures and analyses
After making numerous hand sections and establishing the main switches in the developmental trajectory of Byttneria lobed stems, we were able to delimit three main ontogenetic stages: primary growth, initial secondary growth and advanced secondary growth. Several stem pieces were polished with sandpaper under water to illustrate the overall stem microanatomy following Barbosa et al. (2021). For microanatomical analyses, stem samples from different regions were gradually embedded in PEG 1500 (polyethylene glycol) following Rupp (1964). Histological sections (25–30 µm) were obtained using a sliding microtome (American Optical Corp. 860 or Leica SM2010 R), with permanent steel knives polished with sandpaper (Barbosa et al., 2018), and by applying a coat of polystyrene foam resin (packaging Styrofoam dissolved in either xylene or butyl acetate) upon the embedded stem block (Barbosa et al., 2010) to ensure that soft and stiff tissues would remain connected and unaltered. Once the sections were put in a Petri dish with water, PEG 1500 was dissolved and the histological sections were subsequently doubled stained in Safrablau [1 safranin : 9 astra blue in 50 % ethanol; Bukatsch (1972) as modified by Kraus and Arduin (1997)], and then dehydrated in an ethanol series (50 %, 70 %, 96 %, 100 %). On the last step, polystyrene coating was removed in either xylene or butyl acetate and permanent slides were mounted using a synthetic resin (Hycel 7987) or Canada balsam (Hycel 866). Prickle sections were made by hand, following the same stain to mounting method, but without the use of polystyrene foam resin.
All sections were analysed under a compound microscope (Leica DM2500 and Velab prime VE-B50). Photomicrographs were taken with different cameras and software coupled to compound microscopes. Photographs and figures were edited using GIMP 2.10.18 (Kimball et al., 1995–2020) and Inkscape 1.0 (Inkscape Developers, 2020) software. Measurements and counting were made using ImageJ 1.53a (Schneider et al., 2012) and following IAWA guidelines (IAWA Committee, 1989).
Phylogenetic ancestral character state reconstructions and correlation analysis
This work is focused on Byttneria; however, as mentioned in the Introduction this genus is not monophyletic and is embedded within a larger clade (Whitlock and Hale, 2011; Sharber, 2018), which has been named here ABRM. This clade is organized in four main lineages: the Byttneria s.s clade is sister to monophyletic Ayenia, and both are sister to Rayleya bahiensis and a clade of Byttneria species exclusively from section Vahihara; this last clade will herein be referred to as Byttneria clade 2. This entire clade is sister to the genus Megatritheca (Sharber, 2018). All of them comprise the ABRM clade and are collectively considered in our reconstructions. This phylogeny is well supported and well resolved, contains 46 taxa and was reconstructed from four chloroplast phylogenetic markers: the trnH-psbA intergenic spacer, trnL intron, trnL-trnF intergenic spacer, and ~700-bp coding region of matK (Sharber, 2018). In this work we use the paraphyletic nomenclature of Byttneria, as new taxonomic designations are underway by the second and third authors.
Using information gathered from different sources (as described in Plant material), we delimited the following characters and character states in both Mesquite and BayesTraits: (1) growth form (non-climbing/climbing) and climbing plant type (non-climbing/scandent or leaning shrub/liana), (2) young stem shape (cylindrical/lobed), (3) adult stem shape (cylindrical/lobed) and (4) prickles (absence/presence). We analysed stem shapes from Byttneria (with representatives from five of its six sections, 24 spp.), Ayenia (with representatives from all three sections, 17 spp.), Rayleya bahiensis (monospecific genus) and Megatritheca grossedenticulata (one of the two species in the genus). We performed analyses using the majority clade consensus chronogram for the ABRM clade and a sample of 20 001 trees obtained from the phylogenetic reconstruction of Sharber (2018). We performed an ancestral character state reconstruction using a maximum-likelihood (ML) method for discrete characters, under two models of evolution, Asymmetrical Markov k-state 2 parameter model (AsymmMk) and Markov k-state 1 parameter model (Mk1) (Lewis, 2001), as implemented in Mesquite 3.61 (Maddison and Maddison, 2019). We present the different probabilities in all cases. To account for phylogenetic uncertainty, we used RStudio (R Core Team, 2020; RStudio Team, 2020) and library ape 5.4-1 (sample function) (Paradis and Schliep, 2019) to sample 1000 trees from the 20 001 obtained by Sharber (2018). Then in BayesTraits v3.0.2 (Pagel and Meade, 2019) we performed Bayesian inference (BI) analyses for each character with the 1000-tree sample, we reconstructed all 45 nodes of the ABRM phylogeny, reconstructing specific nodes if present (AddNode command), under the Multistate model of evolution and the reversible-jump Markov chains Monte Carlo (rjMCMC) method, we performed each reconstruction three times, with 10 000 000 iterations and discarding 10 % as burn-in (1 000 000) for each run, and we specified the same hyper-prior for all reconstructions (revjumphp exp 0 10). We checked for convergence in Tracer v1.7.1. (Rambaut et al., 2018). We visualized probabilities on the majority clade consensus tree using RStudio 1.3.1093 (R Core Team, 2020; RStudio Team, 2020) and libraries ape 5.4-1 (Paradis and Schliep, 2019), phangorn 2.5.5 (Schliep, 2011),- and phytools 0.7-70 (Revell, 2012). Because we used a large set of phylogenies for the BI, nodes with low support were not present in all possible phylogenies, and therefore in our reconstructions of ancestral states we also present the possibility that a certain node was absent in a certain percentage of the trees, in which therefore no ancestral state would be reconstructed, since the node would be absent (no-node probabilities). To test character correlations, we performed a Pagel94 test for discrete character evolution (Pagel, 1994) as implemented in Mesquite (Maddison and Maddison, 2019). We tested the correlation between prickles (absent/present) and adult stem shape (cylindrical/lobed). Vessel width and vessel frequency means were compared between the lobular and interlobular areas using an unpaired two-tailed t-test.
Micro stem characters
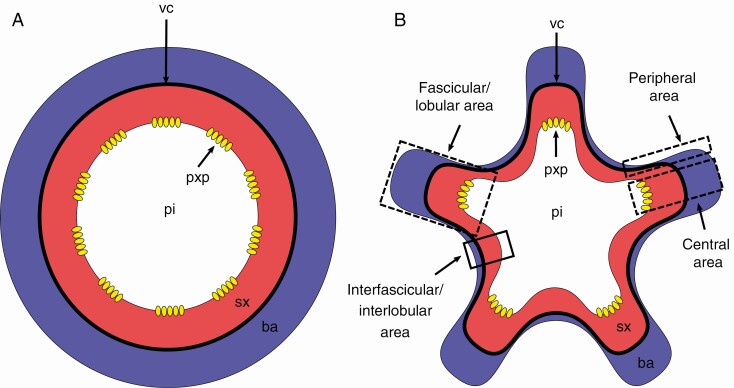
. Within adult lobed stems cambial production follows the same bidirectional pattern as in adult regular stems (Fig. 3A), namely secondary xylem centripetally and secondary phloem centrifugally, but with two main areas: a lobular area that coincides with the place where the vascular bundles are located, therefore a fascicular area, and an interlobular area, which coincides with the interfascicular area (Fig. 3B). Within a fascicular/lobular area, there is a central area and a peripheral area (Fig. 3B). We preferred the use of the word ‘area’ since there are no clear-cut boundaries between lobe and interlobe, but rather a smooth transition between them.
Fig. 3.
Terminology adopted for description of lobed stems. (A) Cylindrical stem (regular) during secondary growth. (B) Non-cylindrical lobed stem during secondary growth, with fascicular/lobular (dashed rectangles) areas and interfascicular/interlobular areas (solid lined rectangles). The fascicular/lobular area comprises a central area and a peripheral area (dashed lines). Blue filling corresponds to the bark (ba), which encompasses all tissues outside of the vascular cambium (vc, bold black line), namely secondary phloem, cortex and periderm, red to secondary xylem (sx) and white to the pith (pi), while yellow ovals represent protoxylem poles (pxp), indicating the location of vascular bundles during primary growth.
RESULTS
Phylogenetic distribution of stem characters and their ancestral states within the ABRM clade
Here we present the trees with the Bayesian reconstructions only (Figs 4–6), but we discuss in the text the few cases where the reconstruction probabilities were different. Moreover, nodes in the phylogeny whose probabilities under both ML and BI were congruent are indicated with an arrowhead, while results indicated with a simple arrow had contrasting probabilities between the two methods and/or lower probabilities than those indicated with arrowheads; probabilities are described in the text and can be found in Supplementary Data Table S4. For each reconstruction we present the Bayesian posterior probabilities (PP), and proportional likelihoods from both ML reconstructions under AsymmMk and Mk1 models with different and equal rates between the transitions of states, respectively. Both posterior probabilities (BI) and proportional likelihoods (ML) are presented as percentages.
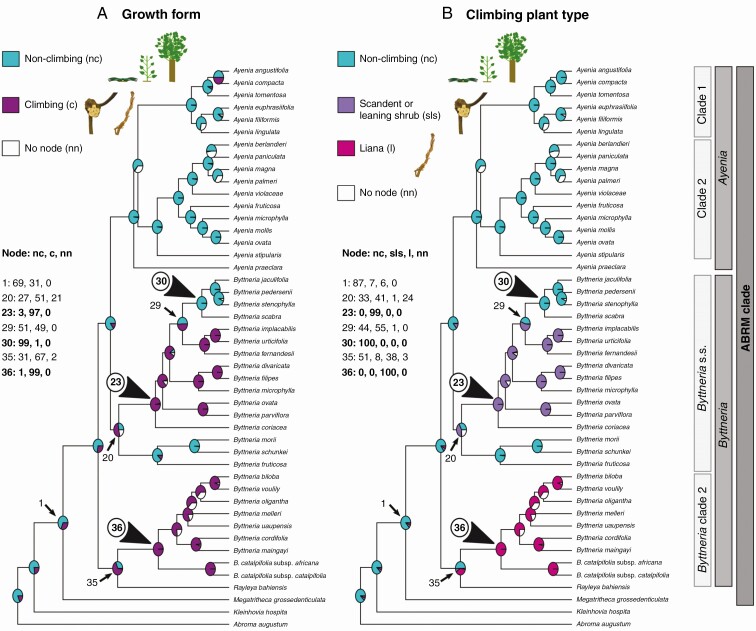
Fig. 4.
Ancestral character state reconstruction of (A) growth form and (B) climbing plant type within ABRM. Posterior probabilities of occurrence are expressed in pie charts on nodes. Turquoise blue filling represents non-climbing plants (tree, shrub, subshrub); magenta to purple colours represent represent climbing plants (scandent/leaning shrub, liana); and white represents the node no-occurrence probabilities from the sample of trees used in the Bayesian analyses. Climbers probably appeared twice in Byttneria only (nodes 23 and 36), but there is also considerable probability that climbers evolved once in node 35, which includes Rayleya. The climbing growth form was lost once in Byttneria s.s (node 30, top arrowhead).
Fig. 6.
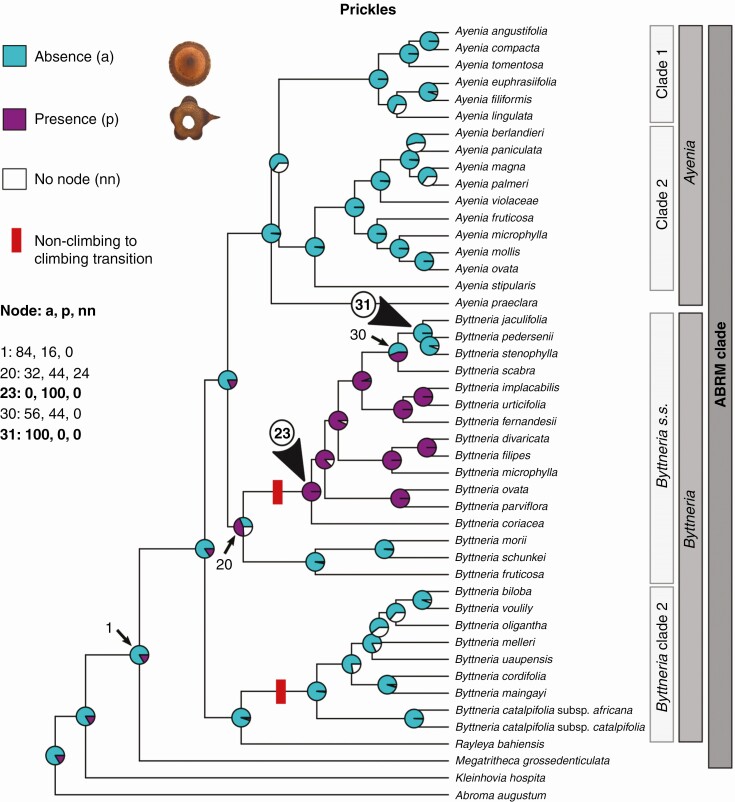
Ancestral character state reconstruction of prickles within ABRM. Probabilities of occurrence are expressed in pie charts on nodes. Turquoise blue filling represents plants without prickles; magenta represents armed plants with prickles; and white represents the node no-occurrence probabilities. Only within Byttneria s.s. did prickles appear (node 23, bottom arrowhead) and were then lost once (node 31, top arrowhead).
Growth form and climbing plant type ( Fig. 4A, B)
We present here probabilities from growth form (Fig. 4A); climbing plant type can be seen in Fig. 4B with the same main transitions. Our results indicate that the ancestral state of the ABRM clade was non-climbing [Fig. 4A, node 1, 69 PP with BI, 82 % (AsymmMk) and 90 % (Mk1) with ML]. Climbers probably evolved at least twice within two different clades of Byttneria, once in Byttneria s.s. [Fig. 4A, node 23, 97 PP with BI, 97 % (AsymmMk) and 96 % (Mk1) with ML], which are scandent or leaning shrubs (Fig. 4B), and once in Byttneria clade 2 [Fig. 4A, node 36, 99 PP with BI, 96 % (AsymmMk) and 95 % (Mk1) with ML], which are twining lianas (Fig. 4B). Within Byttneria s.s., the climbing growth form was lost once in the subclade composed of erect shrubs and subshrubs in B. jaculifolia (erect or decumbent), B. pedersenii, B. stenophylla, and B. scabra [Fig. 4A, node 30, 99 PP with BI, 93 % (AsymmMk) and 95 % (Mk1) with ML]. The remaining species from Byttneria s.s. are shrubs or erect small trees in B. morii, B. schunkei and B. fruticosa. Here there is also considerable probability under BI that climbers evolved one node down at node 35 (Fig. 4A, 67 PP climbing with BI), a clade that includes Byttneria clade 2 and Rayleya. However, ML suggests the opposite scenario, with a 70 % (AsymmMk) and 80 % (Mk1) chance that this node was instead non-climbing.
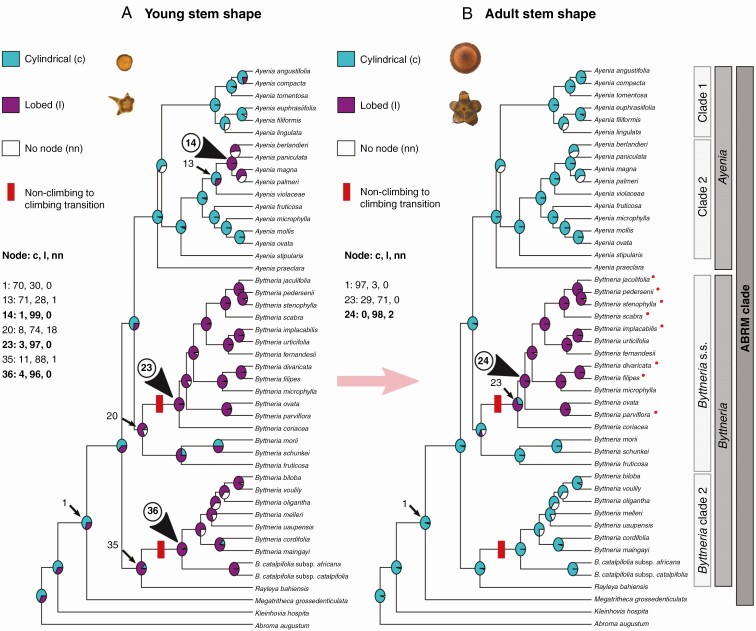
Young stem shape (Fig. 5A)
Fig. 5.
Ancestral character state reconstruction of (A) young and (B) adult stem shape within ABRM. Probabilities of occurrence are expressed in pie charts on nodes. Turquoise blue filling represents regular, cylindrical stems; magenta represents non-cylindrical, lobed stems; and white represents the node no-occurrence probabilities. Young lobed stems appeared at least three times within ABRM (nodes 14, 23 and 36, arrowheads) and only within Byttneria s.s. (node 24, arrowhead) were lobed stems conserved in adult stems. A red dash indicates the location on the phylogeny where there was the transition from non-climbing to climbing plants. Red dots beside species indicate those with polymorphic adult stems.
Values at the ABRM clade ancestral node are contradictory [Fig. 5A, node 1, 70 PP cylindrical with BI, 71 % (AsymmMk) and 85 % (Mk1) lobed with ML]. If cylindrical, young lobed stems probably appeared at least three times independently within the ABRM clade, once in Ayenia [Fig. 5A, node 14, 99 PP with BI, 98 % (AsymmMk) and 99 % (Mk1) with ML] and twice within Byttneria, once in Byttneria s.s. [Fig. 5A, node 23, 97 PP with BI, 98 %(AsymmMk) and 100 % (Mk1) with ML] and once at node 36 [Fig. 5A, 96 PP with BI, 98 % (AsymmMk) and 100 % (Mk1) with ML] or at node 35 [Fig. 5A, 88 PP with BI, 88 % (AsymmMk) and 98 % (Mk1) with ML], a clade that includes Byttneria clade 2 and Rayleya. Within Ayenia there are four species with young lobed stems (A. berlandieri, A. paniculata, A. magna and A. palmeri), usually three-lobed. In contrast, within both Byttneria s.s. and Byttneria clade 2, although B. scabra has typically more than five lobes, four- to five-lobed species prevail, including Rayleya bahiensis.
Adult stem shape ( Fig. 5B)
The ancestral state at the ABRM node was of cylindrical adult stems [Fig. 5B, node 1, 97 PP with BI, 100 % (AsymmMk and Mk1) with ML], which means that during development the lobed contour exhibited by young stems was lost in the adult stems of several lineages. Within the three clades which have young lobed stems, only within Byttneria s.s. lobed stems were conserved in the adult stems [Fig. 5B, node 24, 98 PP lobed with BI, 99 % (AsymmMk) and 98 % (Mk1) lobed with ML]. Byttneria s.s. contains 12 predominantly lobed species, mostly four- to five-lobed, eight of which conserve cylindrical contours in some portions of the plant body, and therefore the same individual can have both lobed and cylindrical adult stems (Fig. 5B, species indicated with red dots; Supplementary Data Table S3). Within the subclade consisting of erect or rarely decumbent shrubs and subshrubs in B. jaculifolia, B. pedersenii, B. stenophylla and B. scabra, cylindrical stems are prevalent near basal portions. In scandent or leaning shrubs (e.g. B. divaricata, B. filipes), we observed lobed stems along the main axis and cylindrical stems in some ramifications, indicating some level of polymophism.
Prickles ( Fig. 6)
Our results indicate that the ancestral ABRM clade state was unarmed, without prickles [Fig. 6, node 1, 84 PP with BI, 100 % (AsymmMk and Mk1) with ML]. Prickles evolved only once within Byttneria s.s. [Fig. 6, node 23, 100 PP with BI, 99 % (AsymmMk and Mk1) with ML] and were lost in one subclade of subshrubs formed by B. jaculifolia, B. pedersenii and B. stenophylla [Fig. 6, node 31, 100 PP with BI, 99 % (AsymmMk) and 100 % (Mk1) with ML].
Character correlations within the ABRM clade with an emphasis on Byttneria
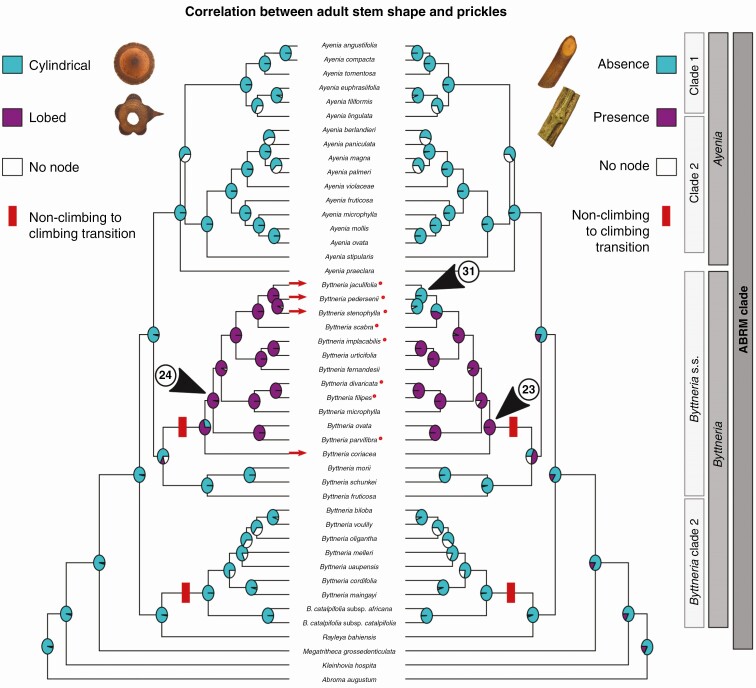
In general, the climbing growth form is present in numerous species of Byttneria (Fig. 4A) but with different climbing mechanisms (Fig. 4B). Within Byttneria clade 2 the species have unarmed, twining cylindrical stems, such as B. catalpifolia and B. biloba, while in Byttneria s.s., climbers have armed, lobed adult stems, such as B. divaricata and B. filipes (Figs 2, 5 and 6). Lobed adult stem distribution was shown to be correlated with the presence of prickles, while cylindrical adult stem distribution coincides with the absence of prickles (Fig. 7; see values obtained in the Pagel94 tests in Supplementary Data Table S5, P < 0.05).
Fig. 7.
Correlated ancestral character state reconstruction of adult stem shape and presence of prickles. Turquoise blue circles represent cylindrical, unarmed stems. Magenta filled circles represent lobed, armed stems. Lobed adult stems and presence of prickles are correlated (Pagel94 P < 0.05). Species indicated with a red dot are polymorphic and can have both cylindrical and lobed adult stems. Species indicated with red arrows have cylindrical adult stems and prickles (B. coriacea) or lobed adult stems with no prickles (e.g. B. jaculifolia).
Some exceptions exist; B. coriacea is a climbing species (scandent or leaning shrub) with prickles in spite of their cylindrical adult stems. Other species such as B. jaculifolia, B. pedersenii and B. stenophylla are unarmed and have lobed adult stems along most of the plant body, with a cylindrical adult stem near basal parts (Fig. 7, red arrows).
The following gives the ontogeny of lobed stems in B. divaricata and B. filipes, both species from the Byttneria s.s. clade (Fig. 2D, E).
Byttneria lobed stem ontogeny
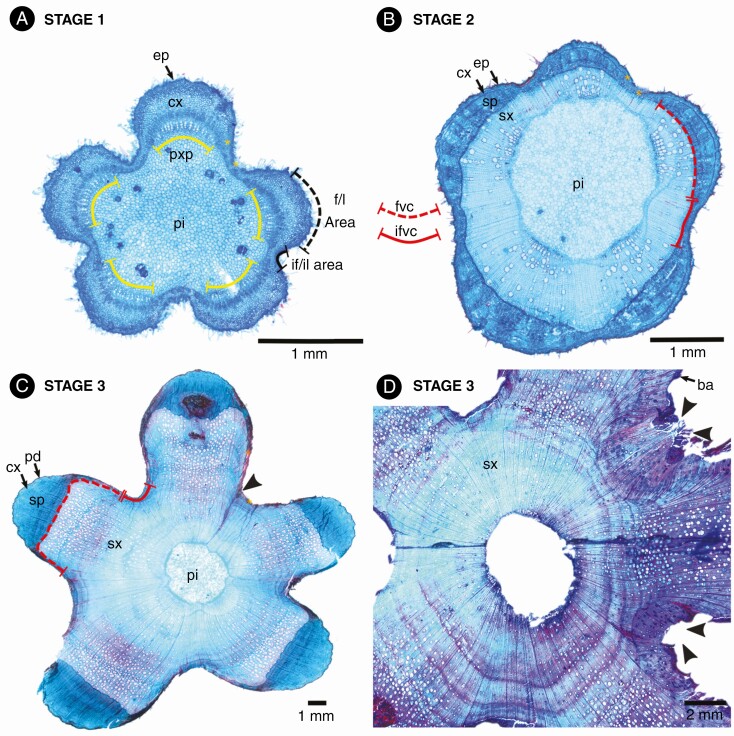
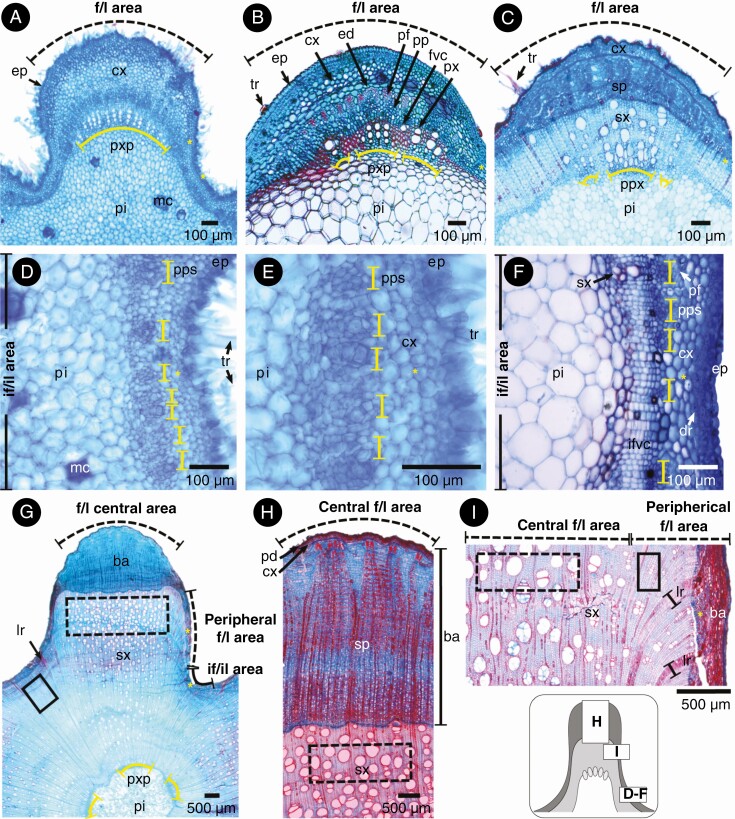
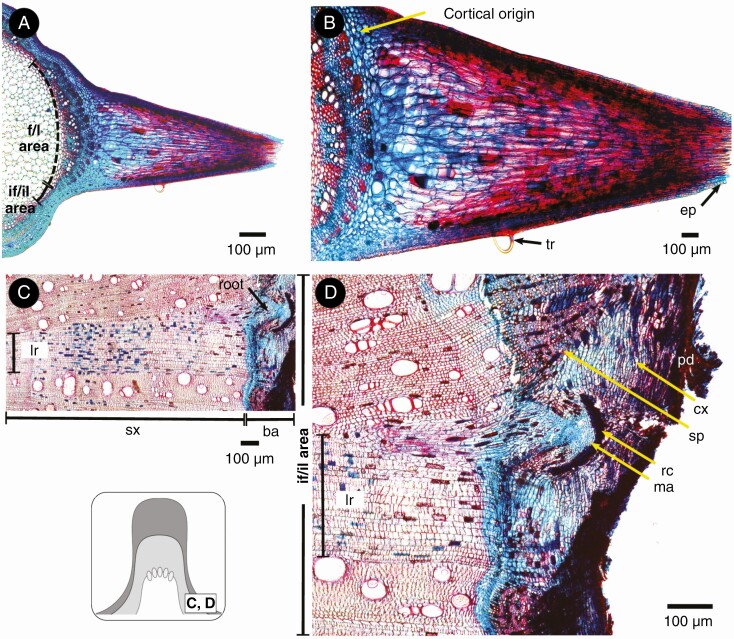
Here we depict the ontogeny of two Byttneria s.s. lobed species as a model. The stem of B. divaricata and B. filipes is five-lobed since its developmental onset at the shoot apex (stage 1, Figs 8A and 9A, B). There are five vascular bundles which coincide with the five lobular areas (Figs 8A and 9A, B, yellow brackets). There is a greater proportion of cortical cells within the central lobular areas when compared to peripheral lobular and interlobular areas (Figs 8A and A, B, D–F, yellow asterisks). Within interlobular areas there are also many isolated primary phloem strands (Fig. 9D–F, yellow brackets). Later, a single, continuous, concentric cambium becomes established, but after the onset of its activity there is a marked differential cell production rate between the lobular/fascicular area (derived from procambium) and its interlobular/interfascicular area (originated by interfascicular parenchyma) (stage 2, Figs 8B and 9C, yellow asterisks). Hence, the five stem lobes coincide with the five vascular bundles of the lobular/fascicular areas of the primary vascular system, while the five interlobes coincide with the five interlobular/interfascicular areas. Sometimes, primary xylem parenchyma cells between files of primary xylem vessels dilate and what appeared to be a single vascular bundle in later stages may resemble three or more bundles close together (Fig. 9B, C). The lobes persist and become increasingly pronounced while secondary growth advances (stage 3, Figs 8C, D and 9G-I). In the interlobular areas and peripheral lobular areas, the production of secondary phloem drastically drops and later practically ceases (Fig. 9G, I, yellow asterisks). Not only is the rate of secondary xylem and phloem different between the lobes and interlobes, but so too are the xylem and phloem cell type features and abundance of both areas. For instance, in B. divaricata the tangential diameter of vessels is markedly greater within the central lobular areas (106.07 ± 33.22 μm) when compared to the interlobular areas (29.29 ± 11.36 μm) (P < 0.01), while there are more vessels within the interlobular areas (95 ± 34.13/mm2) than within the central lobular areas (38.2 ± 10.42/mm2) (P < 0.01). Rays become much wider in the transition from lobular to interlobular areas (Figs 9I and 10C, D). The pith slightly retains the stem’s lobed outline, being five-lobed (Fig. 8). At node height, it is common that two of the initial five lobes merge into four lobes exactly where a leaf and its axillary branch departs, the merged lobes separate and reappear right after the node, with signs of this fusion still noticeable in some portions (Figs 2D, E and 8C, D, black arrowheads). Whenever prickles and lobes are present, prickles grow exclusively on top of lobes (e.g. B. divaricata, B. filipes) (Figs 2D, E and 10A). In young stems, prickles consist of an epidermal outgrowth filled with elongated cells of cortical parenchyma (Fig. 10A, B). In contrast, adventitious roots grow only from the interfascicular/interlobular areas or close to peripheral fascicular/lobular areas (Figs 2E and 10C, D). These roots originate from vascular cambium within wide rays, either from the middle or the margins of a ray, displacing the secondary phloem and cortex as they break through the bark (Fig. 10C, D).
Fig. 8.
The three stages in the ontogeny of Byttneria divaricata lobed stems. (A) Stage 1, stem while in primary growth. Lobed stems have this shape since primary growth and vascular bundles (marked by protoxylem poles in yellow brackets) match the central areas of each lobe. (B) Stage 2, onset of secondary growth. A single continuous vascular cambium becomes established. (C, D) Stage 3, advanced secondary growth; (C) note the pith remains lobed. f/l area = fascicular/lobular area, if/il area = interfascicular/interlobular area, fvc = fascicular vascular cambium, ifvc = interfascicular vascular cambium, ep = epidermis, cx = cortex, pxp = protoxylem pole, pi = pith, sp = secondary phloem, sx = secondary xylem. Yellow asterisks indicate peripheral fascicular/lobular areas and interfascicular/interlobular areas, and black arrowheads show remnants of lobe fusions.
Fig. 9.
Details of the ontogeny of lobed stems. (A, C–E, G–I) Byttneria divaricata; (B, F) B. filipes. (A, B) Primary growth in the fascicular/lobular (f/l) areas; note vascular bundles (indicated by protoxylem poles in yellow brackets) match the central f/l area, and less cortex is present in peripheral f/l areas and interfascicular/interlobular (i/l) areas (yellow asterisks) with respect to central f/l areas. (B) Incipient secondary growth. (C) Initial secondary growth in f/l areas; next to the pith note the protoxylem poles (yellow brackets) showing that a single vascular bundle might fragment in later stages given dilatation of the the primary xylem parenchyma. (D–F) Primary growth in if/il areas; solitary primary phloem strands (yellow brackets). (E) Close up of the phloem strands; (F) incipient secondary growth, with secondary xylem differentiation. (G–I) Mature secondary growth; secondary phloem production almost ceases within the peripheral f/l area and within if/il areas (yellow asterisks). Note in the central f/l area (dashed lines rectangles), tangential diameter of vessels is markedly greater with respect to peripheral f/l areas and if/il areas (solid lines rectangles). Greyscale diagram shows the position of D–F, H and I within a lobed stem. f/l area = fascicular/lobular area, if/il area = interfascicular/interlobular area, tr = trichome, ep = epidermis, dr = druse, cx = cortex (which includes ed = endodermis), pf = pericyclic fibres, pp = primary phloem, pps = primary phloem strands, ifvc = interfascicular vascular cambium, px = primary xylem, pxp = protoxylem poles, pi = pith, mc = mucilaginous cells, ba = bark (pd = peridermis + cx = cortex + sp = secondary phloem), sx = secondary xylem, lr = ’limiting ray’.
Fig. 10.
Prickles and adventitious roots in Byttneria filipes. (A) Prickle on an f/l area. (B) Prickles are formed by elongated cortical cells covered by an epidermal layer. (C) Root growing from a limiting ray within the if/il area. (D) Root emerging from the ray margins, displacing both secondary phloem and cortex as it breaks through the bark. Greyscale diagram shows the position of C and D within a lobed stem. f/l area = fascicular/lobular area, if/il area = interfascicular/interlobular area, tr = trichome, ep = epidermis, cx = cortex, ba = bark (pd = peridermis + cx = cortex + sp = secondary phloem), sx = secondary xylem, lr = limiting ray’, ma = meristematic activity, rc = root cap.
DISCUSSION
Non-cylindrical stems have evolved multiple times within climbing plants and are a common climbing mechanism, aiding the climbers in anchoring to other plants or surfaces in search of light. Here we show for the first time how lobed stems with prickles and adventitious roots are formed in Byttneria correlated with the primary vascular system and leaf position and how they correlate to plant growth, being an effective novel mechanism for climbing, which has also independently evolved in other woody plant lineages. First, we explore the ontogeny of these stems and then their distribution and possible functions in the genus.
Ontogenetically, most lobed stems initiate their growth cylindrically and lobulation is acquired only after secondary growth is well established, by differential activity of sectors of the vascular cambium (Carlquist 2001; Angyalossy et al., 2015). This has been shown, for instance, in Grewia caffra, another climbing Malvaceae (Gama and Oskolski, 2021). However, differently from Grewia caffra and other lobed stem plants, in Byttneria s.s. the entire stem is already lobed in the primary body and the distribution of vascular bundles corresponds to that of the fascicular/lobular areas, therefore being a rare case where the primary vascular system correlates directly with the adult stem shape. Similar examples have been cited before only for a few species of Sapindaceae, which are distantly related (Schenck, 1893; Tamaio and Angyalossy, 2009; Lopes et al., 2017; Chery et al., 2020b). Each of the vascular bundles of Byttneria is immediately aligned with the distinctive lobes in the stem, while the interlobes completely lack vascular bundles, having just a few isolated primary phloem strands, similarly to other Malvaceae (Butterfield, 1976; Gama and Oskolski, 2021). Subsequently, there is an increased cambial activity within fascicular lobular areas, and a decreased activity within interfascicular interlobular areas. This differential activity increases the lobed stem shape. Although the cambium is known for having a double origin, i.e. procambium and interfascicular parenchyma (sometimes referred to as pericyclic parenchyma), it is unusual that the cambium shows a differential activity between both areas, especially in stems. However, this differential activity is not exclusive to Byttneria but is occasionally also seen in other groups. In Piperales, for instance, the interfascicular cambium produces only wide and large rays, while the fascicular areas produce the axial elements (Carlquist, 1990; Isnard et al., 2012; Trueba et al., 2015). The resulting stems are marked by wide interfascicular rays clearly separating the fascicular areas. Another example is found in species of Thunbergia (Acanthaceae), where interxylary phloem islands are produced exclusively by the interfascicular cambium (Angyalossy et al., 2015). These results are remarkable examples of the fine developmental control behind numerous cambial variants and how fascicular and interfascicular cambial areas might reflect in their products their different origins.
The fact that in Byttneria s.s. two of the five lobes commonly merge at the nodes, separating again at internodes, suggests that there is a direct influence of the leaves in the stem architecture. Leaf phyllotaxis was also related to the lobes in Grewia, given that lobes in them grow opposite to the distichous plane, at 45° to this plane (Gama and Oskolski, 2021). Merging of cylinders is seen also in Serjania (Sapindaceae) at the node areas, and was also related to the primary vasculature (Johnson and Truscott, 1956). Some control of auxin, which is produced by young leaves, is therefore expected, but more studies are needed to describe this mechanism in detail. It is common to find a correlation between the leaves and the architecture of cambial variants (Pace et al., 2011; Cabanillas et al., 2017; Cabanillas, 2019). In Bignoniaceae, the phloem wedges typical of the lianas of this family appear in alternation with the decussate leaves, growing in a spiral across the stem length (Pace et al., 2009, 2011). In Callaeum (Malpighiaceae), the phloem wedges are instead always under the leaves, and are the first step in the formation of fissured stems in this group (Cabanillas et al., 2017).
In Byttneria the lobulation comes accompanied by prickles, which grow exclusively on top of the lobes and have an epidermis plus cortex origin. Since these prickles are only found upon the lobes, they are also in opposition to the vascular bundles. These prickles contribute greatly to the anchorage of branches upon supports. In addition, exclusively in the interlobes, adventitious roots are formed from wide rays, which also may help anchor these plants when touching a surface and probably contribute to water absorption, since many of the species in Byttneria s.s. grow on river margins (e.g. B. filipes), and these roots sprout during periods of inundation (Cristóbal, 1976), a phenomenon seen in many plants that share the same habitat (Steffens and Rasmussen, 2016). In organs in secondary growth, it is common that new roots are formed from the cambium in front of the rays (e.g. Bannan, 1941; Fink, 1982), a situation also seen here. The rays become larger in the limits between lobular and interlobular areas, which may favour the formation of stem-borne roots in these areas. However, the formation of these wider rays is probably an architectural response to non-cylindrical stems, based on the disparity in cambial activity rates between lobular and interlobular areas.
Evolution of lobed stems within the ABRM clade of Malvaceae is probably a novel mode of stem climbing
Lobed stems in adult plants have appeared at least twice in Malvaceae, in climbing Grewia (Gama, 2020; Gama and Oskolski, 2021) and here in the large clade of Byttneria s.s. We here categorized climbers of the ABRM clade following Cristóbal (1976) as: (1) unarmed lianas and (2) scandent or leaning shrubs armed with prickles upon lobes (Cristóbal, 1976). At present, Byttneria is not monophyletic, with species falling within two different lineages of the ABRM clade (Whitlock and Hale, 2011; Sharber, 2018). Mapping growth form onto the ABR (Ayenia, Byttneria, Rayleya) phylogeny, it was found that climbers evolved at least twice within different clades, with transitions as (1) trees and shrubs to unarmed lianas, and (2) trees and shrubs to semi-scandent leaners (Whitlock and Hale, 2011). Our work corroborates their results, with two independent origins for climbing plants in ABRM, but taking a step forward by incorporating the relationship of lobed stems with the emergence of different climbing mechanisms.
Young lobed stems appeared at least three times independently within the ABRM clade. Their presence in the adult stems has, however, been conserved only within Byttneria s.s. where they are suggested to have co-evolved with prickles, as a novel climbing mechanism within the tribe. All climbers within Byttneria s.s. are armed with prickles as a means to either climb or lean, and almost all of them have adult lobed stems. Pagel94 tests indicate a strong correlation between the evolution of prickles and lobes in Byttneria s.s. The presence of lobed stems with prickles can be considered a synapomorphy to Byttneria s.s., while the twining stems can be considered a synapomorphy of Byttneria clade 2, and used to support the delimitation of two new genera to these taxa.
The presence of spiny structures and non-cylindrical stems aiding plants to climb have appeared in multiple independent lineages, such as Schnella (previously scandent Bauhinia) (Fabaceae), Machaerium (Fabaceae), and even in certain climbing cacti such as Selenicereus (Fisher and Blanco, 2014; Dias-Leme et al., 2020; Soffiatti and Rowe, 2020). The mechanism for climbing combining star-shaped stems, spines and roots is so effective that it has been suggested in biomimetics in the creation of prospective climbing robots (Soffiatti and Rowe, 2020). However, the correlation between climbing mechanism and cambial variant exhibited by a plant cannot be generalized; for instance, in studies based on climbing plants from American, African and Asian tropical regions, this correlation was not observed (Lehnebach, 2012).
It has been proposed that the climbing growth form was lost once in the node that leads to Byttneria section species in the ABR group (Whitlock and Hale, 2011). Our results corroborate this proposal, with the climbing growth form being lost once in the Byttneria s.s clade within ABRM, forming a clade of shrubs composed of B. scabra, B. jaculifolia, B. pedernesii and B. stenophylla. They have lobed young stems, but cylindrical adult stems near basal parts. Prickles, in addition, were lost in a lineage represented by three species, B. jaculifolia, B. pedernesii and B. stenophylla. Finally, non-climbers (small trees, shrubs and subshrubs) within Byttneria s.s., namely B. fruticosa, B. morii and B. schunkei, are polymorphic and may or may not have lobed young stems, but are unarmed and have adult cylindrical stems. These results in non-climbers (shrubby to small trees) reinforce the suggestion that lobes and prickles are correlated with the climbing growth form as a novel mechanism for these plants to reach light.
CONCLUSIONS
In the ABRM clade, climbers are present in two clades, Byttneria s.s. and Byttneria clade 2, representing two independent origins with two different climbing mechanisms: lobed adult stems armed with prickles, and adult cylindrical twining stems, respectively. These features can be considered synapomorphies to these two clades, and support the delimitation of two newly circumscribed genera. We conclude that in Byttneria the secondary body shape reflects the primary body shape of lobed stems, and lobes coincide with the distribution of vascular bundles in the primary plant body, indicating a differential activity between the fascicular and interfascicular cambium within the stem. Prickles in Byttneria have an epidermal and cortical origin, while adventitious roots are related to the wide rays typical of the limits between the lobular and interlobular areas and both are also related to a more efficient climbing mechanism.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Summary of woody plants with non-cylindrical stems. Table S2: Herbaria consulted. Table S3: Character states for the ABRM clade and outgroups. Table S4: Ancestral character state reconstruction probabilities. Table S5: Values obtained in Pagel94 tests between adult stem shape and prickles.
ACKNOWLEDGEMENTS
This work is part of the BSc thesis of L.L.M. We are indebted to the thesis committee for their invaluable comments, Teresa Terrazas, Estela Sandoval-Zapotitla, N. Ivalú Cacho and Rebeca Hernández-Gutiérrez (Institute of Biology, UNAM). We also thank the chief editor, Rowan Sage, for carefully reviewing earlier versions of the manuscript and suggesting insightful alterations; Joyce Chery (Cornell University) for her careful review and suggestions; Rosa Nejapa and Angélica Quintanar-Castillo (Institute of Biology, UNAM) for their invaluable comments and support throughout the development of this work, in the taking of pictures and helping with ancestral character state reconstructions, respectively; and Gugu Gama and Alexei Oskolski (University of Johannesburg and Komarov Institute) for sharing their work on Grewia with us, and insightful comments and discussions on Grewia and Byttneria stems, as well as for providing us with references. To people involved in the fieldwork, we thank Pedro Acevedo-Rodríguez (Smithsonian Institution) in Brazil, Rosa Nejapa (Institute of Biology, UNAM) and Pablo Cabanillas (Universidad de La Plata) in Argentina, and Hanta Razafindraibe (Missouri Botanical Garden) in Madagascar. Pedro Acevedo-Rodríguez (Smithsonian Institution) also granted permission to add some of his pictures to Fig. 2. We also thank all the people involved in the development of software we used in this work such as BayesTraits, GIMP, ImageJ, Inkscape, Mesquite and RStudio. Author contribution statements: L.L.M. and M.R.P. conceived the idea. M.R.P. collected plants in the field and analysed the herbarium plants deposited in Madagascar. L.L.M. collected the data from MEXU, virtual herbaria and literature, and performed phylogenetic comparative analysis using trees provided from the works of W.S. and B.W. L.L.M. and M.R.P. did the lab anatomical work. L.L.M. wrote the original versions of the manuscript with guidance and input from M.R.P. L.L.M. and M.R.P. analysed and interpreted the results together. All authors discussed the project at different times and critically read and approved the final version of the manuscript.
FUNDING
This work was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (UNAM-DGAPA-PAPIIT, Project IA200521, Mexico).
LITERATURE CITED
- Acevedo-Rodríguez P., et al. 2015 onwards. Lianas and climbing plants of the neotropics: cross sections of liana stems. https://naturalhistory.si.edu/research/botany/research/lianas-and-climbing-plants-neotropics/lianas-cross-sections (19 December 2020).
- Amorim AM. 2003. The anomalous-stemmed species of Heteropterys subsect. Aptychia (Malpighiaceae). Brittonia 55: 127–145. [Google Scholar]
- Angyalossy V, Angeles G, Pace MR, et al. 2012. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecology & Diversity 5: 167–182. [Google Scholar]
- Angyalossy V, Pace MR, Lima AC. 2015. Liana anatomy: a broad perspective on structural evolution of the vascular system. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE, eds. Ecology of lianas. Chichester: John Wiley & Sons, 253–287. [Google Scholar]
- Arbo MM. 1981. Anatomía de tallo y hoja de Rayleya bahiensis Cristóbal (Sterculiaceae). Bonplandia 5: 51–62. [Google Scholar]
- Bannan MW. 1941. Vascular rays and adventitious root formation in Thuja occidentalis L. American Journal of Botany 28: 457–463. [Google Scholar]
- Barbosa ACF, Costa GRO, Angyalossy V, Dos Santos TC, Pace MR. 2018. A simple and inexpensive method for sharpening permanent steel knives with sandpaper. IAWA Journal 39: 497–503. [Google Scholar]
- Barbosa ACF, Gerolamo CS, Lima AC, Angyalossy V, Pace MR. 2021. Polishing entire stems and roots using sandpaper under water: An alternative method for macroscopic analyses. Applications in Plant Sciences 9: e11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa ACF, Pace MR, Witovisk L, Angyalossy V. 2010. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA Journal 31: 373–383. [Google Scholar]
- Barnett LC, Dorr LJ. 1990. A new, arborescent species of Byttneria (Sterculiaceae) from French Guiana. Brittonia 42: 271–275. [Google Scholar]
- Basson PW, Bierhorst DW. 1967. An analysis of differential lateral growth in the stem of Bauhinia surinamensis. Bulletin of the Torrey Botanical Club 94: 404–411. [Google Scholar]
- Bayer C, Kubitzki K. 2003. Malvaceae. In: Kubitzki K, Bayer C, eds. Flowering plants · Dicotyledons: Malvales, Capparales and Non-betalain Caryophyllales. The families and genera of vascular plants, vol. 5. Berlin:Springer-Verlag, 225–311. [Google Scholar]
- Bukatsch F. 1972. Bemerkungen zur doppelfärbung astrablau-safranin. Mikrokosmos 61: 255. [Google Scholar]
- Butterfield BG. 1976. The ontogeny of the vascular cambium in Hoheria angustifolia Raoul. New Phytologist 77: 409–420. [Google Scholar]
- Caballé G. 1993. Liana structure, function and selection: a comparative study of xylem cylinders of tropical rainforest species in Africa and America. Botanical Journal of the Linnean Society 113: 41–60. [Google Scholar]
- Cabanillas PA. 2019. Actuopaleontología de lianas de la región rioplatense. PhD Thesis, Universidad Nacional de La Plata, Argentina. [Google Scholar]
- Cabanillas PA, Borniego ML, Sáenz AA. 2014. Nueva variante cambial en el género Ephedra (Ephedraceae). Boletín de la Sociedad Argentina de Botánica 49: 201–206. [Google Scholar]
- Cabanillas PA, Hurrell JA. 2012. Plantas trepadoras: tipo biológico y clasificación. Ciencias Morfológicas 14: 1–15. [Google Scholar]
- Cabanillas PA, Pace MR, Angyalossy V. 2017. Structure and ontogeny of the fissured stems of Callaeum (Malpighiaceae). IAWA Journal 38: 49–66. [Google Scholar]
- Carlquist S. 1990. Wood anatomy and relationships of Lactoridaceae. American Journal of Botany 77: 1498–1505. [Google Scholar]
- Carlquist S. 2001. Cambial variants (anomalous secondary growth). In Carlquist S, ed. Comparative wood anatomy: systematic, ecological, and evolutionary aspects of dicotyledon wood. Berlin: Springer-Verlag, 271–295. [Google Scholar]
- Carlquist S. 2007. Successive cambia revisited: ontogeny, histology, diversity, and functional significance. The Journal of the Torrey Botanical Society 134: 301–332. [Google Scholar]
- Cheek M, Dorr LJ. 2007. Flora of Tropical East Africa: Sterculiaceae. Kew: Royal Botanic Gardens, Kew. [Google Scholar]
- Chery JG, Cunha-Neto IL, Pace MR, Acevedo-Rodríguez P, Specht CD, Rothfels CJ. 2020a. Wood anatomy of the neotropical liana lineage Paullinia L. (Sapindaceae). IAWA Journal 41: 278–300. [Google Scholar]
- Chery JG, Pace MR, Acevedo-Rodríguez P, Specht CD, Rothfels CJ. 2020b. Modifications during early plant development promote the evolution of nature’s most complex woods. Current Biology 30: 237–244.e2. [DOI] [PubMed] [Google Scholar]
- Cristóbal CL. 1960. Revisión del género Ayenia (Sterculiaceae). Opera Lilloana 4: 1–230. [Google Scholar]
- Cristóbal CL. 1965. Megatritheca (Sterculiaceae), género nuevo de África tropical. Adansonia 5: 365–373. [Google Scholar]
- Cristóbal CL. 1976. Estudio taxonómico del género Byttneria Loefling (Sterculiaceae). Bonplandia (Corrientes) 4: 1–428. [Google Scholar]
- Cristóbal CL. 1981. Rayleya, nueva Sterculiaceae de Bahia–Brasil. Bonplandia (Corrientes) 5: 43–50. [Google Scholar]
- Cristóbal CL. 1985. Una nueva especie de Byttneria (Sterculiaceae) del Perú. Boletín de la Sociedad Argentina de Botánica 24: 125–129. [Google Scholar]
- Cunha Neto ILD, Martins FM, Somner GV, Tamaio N. 2017. Successive cambia in liana stems of Paullinieae and their evolutionary significance in Sapindaceae. Botanical Journal of the Linnean Society 186: 66–88. [Google Scholar]
- Darwin C. 1865. (digitally printed in 2009). On the movements and habits of climbing plants. New York: Cambridge University Press. [Google Scholar]
- Dias-Leme CL, Cunha Neto ILD, Angyalossy V. 2020. How the neotropical liana Machaerium multifoliolatum (Fabaceae) develop their distinctive flattened stems? Flora 269: 151629. [Google Scholar]
- Evert RF. 2006. Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Fink S. 1982. Adventitious root primordia – the cause of abnormally broad xylem rays in hard- and softwoods. IAWA Bulletin 3: 31–38. [Google Scholar]
- Fisher JB, Blanco MA. 2014. Gelatinous fibers and variant secondary growth related to stem undulation and contraction in a monkey ladder vine, Bauhinia glabra (Fabaceae). American Journal of Botany 101: 608–616. [DOI] [PubMed] [Google Scholar]
- Font Quer P. 2001. Diccionario de Botánica. Barcelona: Ediciones Península. [Google Scholar]
- Francis WD. 1924. The development of buttresses in Queensland trees. Proceedings of the Royal Society of Queensland. 36: 21–37. [Google Scholar]
- Gama GM. 2020. Structure, development and origin of winged stem in lianescent species of Grewia L. (Malvaceae): a new type of cambial variant. MSc Thesis, University of Johannesburg, South Africa. [Google Scholar]
- Gama G, Oskolski A. 2021. Stem anatomy of Grewia caffra (Malvaceae): an uncommon cambial variant in the order Malvales. Plant Systematics and Evolution 307. doi: 10.1007/s00606-021-01746-3 [DOI] [Google Scholar]
- IAWA Committee. 1989. IAWA list of microscopic features for hardwood identification. IAWA Bulletin 10: 219–332. [Google Scholar]
- Inkscape Developers. 2020. Inkscape. (Computer program). https://inkscape.org/ (19 December 2020).
- Isnard S, Prosperi J, Wanke S, et al. 2012. Growth form evolution in Piperales and its relevance for understanding angiosperm diversification: an integrative approach combining plant architecture, anatomy, and biomechanics. International Journal of Plant Sciences 173: 610–639. [Google Scholar]
- Jacques FM, De Franceschi D. 2007. Menispermaceae wood anatomy and cambial variants. IAWA Journal 28: 139–172. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw-Hill Book Company, Inc. [Google Scholar]
- Johnson MA, Truscott FH. 1956. On the anatomy of Serjania. I. Path of the bundles. American Journal of Botany 43: 509–518. [Google Scholar]
- Kimball S, Mattis P, The GIMP Development Team . 1995-2020. GIMP. (Computer program). https://www.gimp.org/ (19 December 2020).
- Kraus JE, Arduin M. 1997. Basic manual of methods in plant morphology. Seropédica, Rio de Janeiro: EDUR, UFRJ. [Google Scholar]
- Lehnebach R. 2012. Evolution et diversité des traits morpho-anatomiques chez les lianes tropicales. MSc Thesis, Université Montpellier II–Sciences et Techniques du Languedoc, France. [Google Scholar]
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50: 913–925. [DOI] [PubMed] [Google Scholar]
- Löffler B. 1914. Entwicklungsgeschichtliche und vergleichend anatomische Untersuchung des Stammes und der Uhrfederranken von Bauhinia (Phanera) spec: ein Beitrag zur Kenntnis der rankenden Lianen. Vienna: Staatsdruckerei. [Google Scholar]
- Lopes WAL, De Souza LA, De Almeida OJG. 2017. Procambial and cambial variants in Serjania and Urvillea species (Sapindaceae: Paullinieae). Journal of the Botanical Research Institute of Texas 11: 421–432. [Google Scholar]
- Maddison WP, Maddison DR. 2019. Mesquite: a modular system for evolutionary analysis. Version 3.61. (Computer program). http://www.mesquiteproject.org (19 December 2020).
- Obaton M. 1960. Les lianes ligneuses a structure anormale des forêts denses d’Afrique occidentale. Annales des Sciences Naturalles, Botanique et Biologie Végétale; sér 12, tome I: 1–220. [Google Scholar]
- Pace MR. 2015. Evolution of the vascular system in lineages that contain lianas. PhD Thesis, Universidade de São Paulo, Brazil. [Google Scholar]
- Pace MR, Angyalossy V, Acevedo-Rodríguez P, Wen J. 2018. Structure and ontogeny of successive cambia in Tetrastigma (Vitaceae), the host plant of Rafflesiaceae. Journal of Systematics and Evolution 56: 394–400. [Google Scholar]
- Pace MR, Lohmann LG, Angyalossy V. 2009. The rise and evolution of the cambial variant in Bignonieae (Bignoniaceae). Evolution & Development 11: 465–479. [DOI] [PubMed] [Google Scholar]
- Pace MR, Lohmann LG, Angyalossy V. 2011. Evolution of disparity between the regular and variant phloem in Bignonieae (Bignoniaceae). American Journal of Botany 98: 602–618. [DOI] [PubMed] [Google Scholar]
- Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London. Series B: Biological Sciences 255: 37–45. [Google Scholar]
- Pagel M, Meade A. 2019. BayesTraits V3.0.2. (Computer program). http://www.evolution.rdg.ac.uk/BayesTraitsV3.0.2/BayesTraitsV3.0.2.html (19 December 2020).
- Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics (Oxford, England) 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Radlkofer L. 1875. Monographie der Sapindaceen-Gattung Serjania I-XVIII. München: Verlag der K.B. Akademie. [Google Scholar]
- Rahman M, Hassan M, Mia M, Huq A. 2012. A synoptical account of the Sterculiaceae in Bangladesh. Bangladesh Journal of Plant Taxonomy 19: 63–78. [Google Scholar]
- Rajput KS, Nunes OM, Brandes AFN, Tamaio N. 2012. Development of successive cambia and pattern of secondary growth in the stem of the Neotropical liana Rhynchosia phaseoloides (Sw.) DC. (Fabaceae). Flora 207: 607–614. [Google Scholar]
- Rajput KS, Lekhak MM, Kapadane KK, Yadav SR. 2017. Formation of tri-lobed stem and development of successive cambia in the stems of Argyreia hookeri CB Clarke (Convolvulaceae). Flora 233: 140–149. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing (Computer program). https://www.R-project.org/ (19 December 2020). [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- RStudio Team. 2020. RStudio: integrated development environment for R. Boston, MA: RStudio, PBC (Computer program). http://www.rstudio.com/ (19 December 2020). [Google Scholar]
- Rupp P. 1964. Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate. Mikrokosmos 53: 123–128. [Google Scholar]
- Schenck H. 1893. Beiträge zur Biologie und Anatomie der Lianen, im Besonderen der in Brasilien einheimischen Arten. II Teil, Beiträge zur Anatomie der Lianen. In Schimper AFW, ed. Botanische Mitteilungen aus den Tropen. Jena: Verlag von Gustav Fisher. [Google Scholar]
- Schliep KP. 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27: 592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharber WV. 2018. Systematics, evolution, and biogeography of Ayenia (Malvaceae subfamily Byttnerioideae). PhD Thesis, University of Miami, USA. [Google Scholar]
- Soffiatti P, Rowe NP. 2020. Mechanical innovations of a climbing cactus: functional insights for a new generation of growing robots. Frontiers in Robotics and AI 7. doi: 10.3389/frobt.2020.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto P, Acevedo-Rodríguez P, Vasconcelos TN, Roque N. 2020. Towards a standardization of terminology of the climbing habit in plants. The Botanical Review 86: 180–210. [Google Scholar]
- Steffens B, Rasmussen A. 2016. The physiology of adventitious roots. Plant Physiology 170: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens PF. 2001 onwards. Angiosperm phylogeny website. Version 14, July 2017 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/ (19 December 2020).
- Tamaio N, Angyalossy V. 2009. Variação cambial em Serjania caracasana (Sapindaceae): enfoque na adequação terminológica. Rodriguésia 60: 651–666. [Google Scholar]
- Thiers B. 2016. Index Herbariorum: a global directory of public herbaria and associated staff. http://sweetgum.nybg.org/science/ih/ (19 December 2020).
- Trueba S, Rowe NP, Neinhuis C, Wanke S, Wagner ST, Isnard S. 2015. Stem anatomy and the evolution of woodiness in Piperales. International Journal of Plant Sciences 176: 468–485. [Google Scholar]
- Wagner KA. 1946. Notes on the anomalous stem structure of a species of Bauhinia. The American Midland Naturalist 36: 251–256. [Google Scholar]
- Whitlock BA, Hale AM. 2011. The phylogeny of Ayenia, Byttneria, and Rayleya (Malvaceae s.l.) and its implications for the evolution of growth forms. Systematic Botany 36: 129–136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.