Abstract
Background and Aims
Colour pattern is a key cue of bee attraction selectively driving the appeal of pollinators. It comprises the main colour of the flower with extra fine patterns, indicating a reward focal point such as nectar, nectaries, pollen, stamens and floral guides. Such advertising of floral traits guides visitation by the insects, ensuring precision in pollen gathering and deposition. The study, focused in the Southwest Australian Floristic Region, aimed to spot bee colour patterns that are usual and unusual, missing, accomplished by mimicry of pollen and anthers, and overlapping between mimic-model species in floral mimicry cases.
Methods
Floral colour patterns were examined by false colour photography in 55 flower species of multiple highly diverse natural plant communities in south-west Australia. False colour photography is a method to transform a UV photograph and a colour photograph into a false colour photograph based on the trichromatic vision of bees. This method is particularly effective for rapid screening of large numbers of flowers for the presence of fine-scale bee-sensitive structures and surface roughness that are not detectable using standard spectrophotometry.
Key Results
Bee- and bird-pollinated flowers showed the expected but also some remarkable and unusual previously undetected floral colour pattern syndromes. Typical colour patterns include cases of pollen and flower mimicry and UV-absorbing targets. Among the atypical floral colour patterns are unusual white and UV-reflecting flowers of bee-pollinated plants, bicoloured floral guides, consistently occurring in Fabaceae spp., and flowers displaying a selective attractiveness to birds only. In the orchid genera (Diuris and Thelymitra) that employ floral mimicry of model species, we revealed a surprising mimicry phenomenon of anthers mimicked in turn by model species.
Conclusion
The study demonstrates the applicability of ‘bee view’ colour imaging for deciphering pollinator cues in a biodiverse flora with potential to be applied to other eco regions. The technique provides an exciting opportunity for indexing floral traits on a biome scale to establish pollination drivers of ecological and evolutionary relevance.
Keywords: False colour photography, floral colour pattern, floral guide, mimicry, bee-pollinated flowers, bird-pollinated flowers, bull’s eye, pollen, orchid pollination
INTRODUCTION
Flower colour is a key feature of pollinator attraction selectively driving the appeal of pollinator types (Lunau and Maier, 1995; van der Kooi et al., 2019). Nectar- and pollen-robbing bees exert selective pressure on bird-pollinated flowers that forge the development of bee exclusion syndromes (Lunau et al., 2011). In such cases, many Neotropical bird-pollinated flowers display flower colours inconspicuous for bees as UV-absorbing red and UV-reflecting white colours (Lunau et al., 2011; de Camargo et al., 2019), or UV-absorbing yellow flowers with no floral colour patterns (Papiorek et al., 2016). For example, Faboideae with red flowers and an elongated keel are often pollinated by birds (Feinsinger et al., 1979; Bruneau, 1997; Agostini et al., 2006; Popic et al., 2016) rather than bees.
Colours are also among the most conspicuous aspects of flowers that provide the primary lure or means to deceive pollinators (Heuschen et al., 2005; Lunau and Wester, 2017; Wester and Lunau, 2017; van der Kooi et al., 2019). The importance of colour in pollinator attraction has been shown in food-deceptive plants, where pollination attraction relies on colour similarity to model plants (Kunze and Gumbert, 2001; Gigord et al., 2002; Johnson et al., 2003). For instance, colour exhibited by model flowers is used as a principal advertising signal in orchids that employ floral mimicry (Jersáková et al., 2012). Colour is also expected to be a determinant for understanding pollinator attraction where co-occurring species rely on the same pollinator guilds. In a study on sympatric and co-flowering Faboideae species in south-west Australia (Scaccabarozzi et al., 2020a), floral morphological features and nectar (sugar composition) were not linked to pollinator type, suggesting that colour might be crucial for promoting pollinator segregation.
Colour pattern plays a fundamental role in close-range positioning of pollinators as they approach a floral resource (Lunau and Maier, 1995; An et al., 2018). Patterning can comprise the main colour of the flower, with supplemental fine-scale patterns indicating a reward focal point (Heuschen et al., 2005) such as nectar (Johnson et al., 2006), pollen (Lunau, 2000), nectaries, stamens and floral guides. Such precision in floral guidance facilitates visitation effectiveness by focusing the insect visitor on the pollination apparatus (Wilmsen et al., 2017) and/or achievement of precision in depositing pollen (Koch et al., 2017). Floral morphological mimics of nectar guides and pollen-bearing anthers are commonly employed by deceptive flowering plants such as the majority of deceptive orchids (Ackerman, 1986) to attract their pollinators (Jersáková et al., 2012).
Among flowers that hide anthers inside the corolla (Lamiaceae and species traditionally included in the Scrophulariaceae such as Orobanchaceae, Plantaginaceae and Phrymaceae), yellow and UV-absorbing pollen- and anther-mimicking floral guides are particularly common and are thought to replace the signalling function of anthers and pollen (Osche, 1983; Lunau et al., 2017). Papilionaceous flowers seem to be an exception, with only a few cases of pollen- and anther-mimicking floral guides described (Lunau, 2000). Thus, different colours in standard, wings and keel, and an extreme three-dimensional morphology might render floral guides superfluous in assigning a landing place (Lunau et al., 2017). However, Australian Fabaceae may be adopting additional pollination attraction syndromes by displaying floral guides that resemble pollen and anther mimics (Lunau, 2007); however, there are no systematic studies on colour pattern in these species.
The absorption of UV light represents a key component of pollen- and anther-mimicking floral guides that mimic the UV absorption of actual pollen and anthers caused by protective flavonoid pigments (Osche, 1983; Lunau, 2000). There have been some attempts to visualize flower colours and floral colour patterns as they are perceived by bees (Loew and Lythgoe, 1985; Vorobyev et al., 1997; Williams and Dyer, 2007). Minute, rough or convoluted structures and organs of flowers such as filaments, hairs, anthers, pollen grains, protuberances, stigmas and floral guides are not suitable for spectrophotometric analysis. As a result, such structures have received little attention in comparison with petaloid/tepaloid colour displays. In these cases, false colour photography reveals these components of the floral colour pattern that are functional to pollinators while enabling analysis in the UV spectrum perceived by invertebrates and birds. As bees are the most important pollinator group globally, false colour photography enables a bee’s eye view by merging a UV image with the blue and green channel of a colour photograph to create a false colour photograph covering bee-visible wavelengths (Verhoeven et al., 2018). However, traditional UV photography is limited by exposure time, flower movement and technical constraints (Williams and Williams, 1993). This has been overcome with improved imaging resolution capacity through digital photography (Garcia et al., 2014) and digital photography calibration (van den Berg et al., 2020).
The Southwest Australian Floristic Region (SWAFR) is a globally significant biodiversity hotspot with >8000 plant species and a large proportion of endemics (Myers et al., 2000; Hopper and Gioia, 2004; FloraBase data). Such plant richness with high species turnover occurs as a result of a combination of highly infertile soils, climatic stability (lack of recent glaciation) and geographic isolation (Hopper and Gioia, 2004; Hopper, 2009) along with diverse pollination systems (Phillips et al., 2010) which is the underpinning reason for the region being chosen for this large-scale study. Characterized by a Mediterranean climate, the SWAFR hosts uniquely stable and old landscapes (Cowling et al., 1996; Dallman, 1998; Anand and Paine, 2002) that support a wide variety of nutritional and pollinator-specialized plants (Hopper and Gioia, 2004; Lambers et al., 2011). Importantly, with just 30 % of the hotspot remaining uncleared, one of the aspects of major concern in flora conservation is the specificity of pollination syndromes. Thus, the region represents a priority area of risk for managing and mitigating the inexorable declining biodiversity. The large diversity of pollination systems in the SWAFR, include pollination by birds, mammals (i.e. the honey possum and the Western Pygmy-possum) and insects, and deceptive pollination syndromes in orchids (Hopper, 1980; Brown et al., 1997; Phillips et al., 2010). The majority of pollinating insects are Hymenoptera in the Colletidae, Halictidae and Thynnidae, but also Coleoptera and Diptera (Brown et al., 1997; Houston, 2000, 2018).
Despite the unique floral diversity, the extraordinary colour variation and often bizarre pollination syndromes displayed by flowering plants in the SWAFR, few studies have investigated the colour pattern in flowering plants at a large scale. To date, targeted studies on pollination strategies have revealed the primary role of colour in mimicry strategies based on food deception in a restricted number of orchid species (Indsto et al., 2006; Gaskett et al., 2017; Scaccabarozzi et al., 2018, 2020b) that occur in both eastern and south-western Australia. Dyer et al. (2012), in a comprehensive study of >100 Australian flowering plants, measured colour reflectance and concluded that hymenopteran pollinators were potential major drivers of flower colour evolution in Australian angiosperms. They also found that overall, flower reflectance spectra complied with those of Northern Hemisphere plants.
The aim of this study was to employ false colour imaging to undertake the first comprehensive screening of flower colour patterns perceivable by bees in indicative floral groups in the hyperdiverse shrublands and forests of the south-west Australian biodiversity hotspot. Specifically, the study aimed to resolve: (1) usual (such as classical UV ‘bull’s eyes’ on flowers) and unusual colour patterns in comparison with the usual colour palettes preferred by bee pollinators (Lunau et al., 2016); (2) the supposed absence of colour pattern in bird-pollinated flowers (Papiorek et al., 2016); (3) floral colour patterns accomplished by mimicry of pollen and anthers (Lunau, 2007); and (4) the colour pattern overlap between mimic and model species in floral mimicry cases. Numerous examples of pollen and anther mimicry have been demonstrated in many floristic regions except Australia (Lunau et al., 2017). The highly diverse flora in Western Australia made possible a comparative study of pollen and anther mimicry including models and mimics, with a large portion of Fabaceae, which in other parts of the world rarely display pollen- and anther-mimicking structures.
MATERIALS AND METHODS
Study area and period
Study sites were established in key biodiverse locations in the Mediterranean south-west of Australia in late spring 2018. Sites included the bushland areas of Kings Park and Botanic Garden (Perth), the Margaret River Region and Stirling Ranges National Park, and opportunistic sampling between these widely dispersed sites. From extensive sampling, 55 species of flowering plants were selected to exemplify pollen mimicry and the diversity of colour patterns in bird- and insect-pollinated species. Timing of in situ image acquisition corresponded to the peak flowering season for the majority of the plant species in the biogeographic region. According to the Interim Biogeographic Regionalisation for Australia (IBRA; Thackway and Cresswell, 1995), sampling sites encompassed target species predicting pollination features included in the study aims in the most representative plant communities of the floristic region (SWAFR): the Swan coastal plain (SWP), Northern and Southern Jarrah forest (NJF, SJF), Warren (W), Mallee (M), Esperance plains (EP) and Sandplains and saltlakes (S) (Supplementary data Table S1). The focal species included both native flowering plants and selected naturalized species, as there are >1200 naturalized invasive species (indicated hereafter by *), with many originating from the climatically similar Mediterranean region of South Africa, California and the Mediterranean Basin. Species were identified by K.D., Michael Crisp, Eng Pin Tay, or using the online portal FloraBase supplemented by reference to Barrett and Tay (2016).
Optics and acquisition of photographs
The camera was mounted on a tripod so that two photographs – colour and UV photographs – were taken of a given flower from the exact same position and with a minimum time period between the two image:. The aperture was adapted to ambient light, but was always identical for the colour and UV photographs. To capture photographs in bee view, we used a modified Panasonic GH-1 camera linked to a UV-transmittant Ultra-Achromatic-Takumar 1:4.5/85 mm lens made of fused quartz. The low-pass filter of this camera was removed in order to increase sensitivity to UV light. Since the modified camera is sensitive to UV and infrared (IR) light, we used a UV-/IR-Cut filter (Baader) transmitting light between 400 and 700 nm to capture a normal reference picture. The UV photograph used a filter transmitting only UV light (Baader). The white balance was set differently for the colour photograph and for the UV photograph using a white Teflon disc, which strongly reflected UV and visible light.
As standard procedure, multiple flowers of the same species (at least four) were visualized through digital photography, to verify if the flower colour pattern was consistent among different plants. If the colour pattern was uniform among individuals, the best flowers were selected for imaging. Alternatively, when the pattern was indistinct via digital visualization, we photographed several flowers of multiple individuals of the same species and subsequently selected the most representative and clearest image.
Image assemblage in bee view
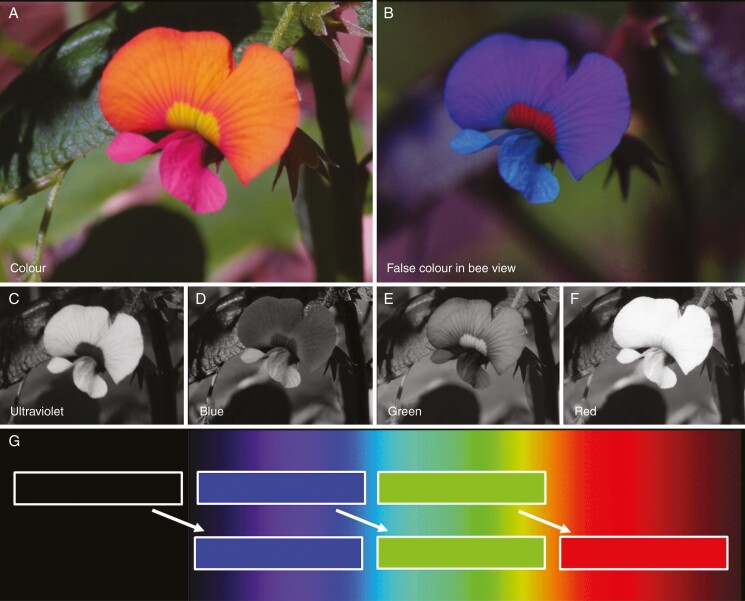
For reassembling the false colour photograph in bee view, both the colour and UV photograph were split into the three colour channels, i.e. blue, green and red. From the colour photograph, the red channel was discarded, and from the UV photograph the green and the red channels were discarded (Fig. 1). The blue channel of the UV photograph and the blue and green channels of the colour photograph were used as blue, green and red output channels of the merged false colour photograph in bee view employing the public domain computer program ImageJ (http://imagej.nih.gov/ij). Thus, UV is represented as blue in the false colour photograph, blue as green and green as red, while red is discarded. Subjective matching of colours was done using a colour hexagon (Chittka, 1992; Supplementary data Fig. S1). It has been shown that this simplified method reveals similar results compared with the method in which three photographs are taken using three different filters, with each matching the spectral sensitivity of the photoreceptor types in honey-bees (Verhoeven et al., 2018).
Fig. 1.
False colour photography explained at Chorizema cordatum. (A) Colour photograph; (B) false colour photograph in bee view; (C) blue channel of the UV photograph; (D–F) blue, green and red channel of the colour photograph; (G) merging of the false colour photograph with the light spectrum in the background.
The false colour technique in bee view is a robust method to obtain detailed information of flower colour patterns under different ambient light conditions such as full sunshine, overcast and forest shade (Verhoeven et al., 2018; Lunau et al., 2020). However, exposure for UV photographs might last several seconds under unfavourable light conditions. An issue arose when imaging was undertaken in windy conditions where the flower was repositioned while filters were exchanged. This was easily addressed by affixing the flower stem to a small stick. Small details such as pollen grains are visible on false colour photographs in bee view, but need exact congruence of the colour and the UV photograph before merging. In the case of a mismatch between the two images, computer programs such as the public domain program GIMP can be used to correct for the displacement and to enable superimposition of the two photographs. The immediate play-back function of the camera enables same-time retaking of images if misalignment or lighting had changed between imaging sessions.
Interpretation of false colour images
To interpret the merged false colour photographs in bee view requires an understanding of colour vision in humans. Humans possess three colour photoreceptors: cones maximally sensitive in the blue, green and red range wavelengths. Based on the input to the three types of cones, humans perceive four unique hues due to two opponent colour mechanisms, which are the red–green and the blue–yellow mechanisms, whereby the perception of yellow requires the excitation of the green and the red cones (Supplementary data Fig. S1). That is why green and yellow colour hues might be similar to bees, since both are based on reflection in the green range of wavelength and differ in regard to the reflection in the red range of wavelength that is invisible for bees. In false colour photographs, these colours are presented as red (Supplementary data Fig. S2). Likewise, white colour hues appear white for bees only if they reflect UV light, but bee-bluegreen if they absorb UV light. In false colour photographs, the bee-white colour hue is presented as white, whereas the bee-bluegreen colour hue is represented as yellow (Supplementary data Fig. S2).
RESULTS
We examined the captured and transformed images for a total of 55 flowering plant species belonging to four plant communities and 16 families from the SWAFR, in an assemblage of target species spanning predicted pollination traits (Supplementary data Table S1).
Usual and unusual colour patterns
The UV bull’s eye of flowers and inflorescences, i.e. a floral colour pattern consisting of a UV-absorbing centre and a UV-reflecting periphery, was found in flowers that appear uniformly yellow to the human eye. To clarify, we use the term ‘UV bull’s eye’ that was coined by Silberglied (1979) in order to describe a common colour pattern of flowers and inflorescences. Specifically, it has been demonstrated that the UV-absorbing central areas of UV bull’s eyes are stamen mimicking (Lunau, 2007).
The central UV-absorbing part can exhibit pollen and anthers as in Patersonia umbrosa var. xanthina (Fig. 2A), anthers and basal parts of petals as in Hibbertia aurea (Fig. 2B), filamental hairs in Tricoryne elatior (Fig. 2C) or laminar floral guides as in Labichea lanceolata (Fig. 2D). Inflorescences also display a UV bull’s eye due to absorption of UV light in the basal parts of the petals of the ray florets as in Arctotheca calendula* (Fig. 2E). Some flowers display a similar colour in anthers and stigma (P. umbrosa var. xanthina, H. aurea and T. elatior; Fig. 2A–C), while others do not (L. lanceolata,Fig. 2D; Patersonia occidentalis and Agrostocrinum scabrum; Supplementary data Fig. S3A, B).
Fig. 2.
Colour (left), UV (middle) and false colour photograph (right) of UV bull’s eyes on yellow flowers (UV-absorbing parts in bracts). (A) Patersonia umbrosa var. xanthina F.Muell. (stamens, style); (B) Hibbertia aurea Steud. (stamens, basal parts of petals); (C) Tricoryne elatior R.Br. (filmental hairs); (D) Labichea lanceolata Benth. (floral guide); (E) Arctotheca calendula (L.) K.Lewin (basal parts of petals).
Not all yellow flowers display a UV bull’s eye; the variation includes totally UV-absorbing yellow flowers as in the invasive species Lotus subbiflorus* (Fig. 3A) and UV-absorbing areas on the standard or on the wings of legumes such as Latrobea (Fig. 3B) and Gompholobium scabrum (Supplementary data Fig. S4F).
Fig. 3.
Colour (left), UV (middle) and false colour photograph (right) showing the diversity of coloration in Fabaceae flowers. (A) Lotus subbiflorus Lag.; (B) Latrobea sp.; (C) Chorizema rhombeum R.Br. cf.; (D) Mirbelia dilatata R.Br.; (E) Hardenbergia comptoniana (Andrews) Benth.; (F) Jacksonia sericea Benth.; (G) Gastrolobium capitatum (Benth.) G.Chandler & Crisp cf; (H) Daviesia cordata Sm.; (I) Daviesia divaricata Benth.; (J) Gastrolobium sp.; (K) Kennedia lateritia F.Muell.
Unusually, most legume flowers display bull’s eye colour patterns comprising uniform and highly variable components. The uniform component is a colour patch at the base of the standard petal contrasting against the overall standard petal colour. Variable components are contrasting colours framing the colour patch at the base of the standard petal and the colour of wings and keel petals. The papilionaceous-like flowers of Comesperma virgatum (Polygalaceae) (Fig. 4G) share morphological features and colour pattern with pea flowers.
Fig. 4.
Colour (left), UV (middle) and false colour photograph (right) showing the diversity of colour pattern. (A) Thysanotus banksii R.Br.; (B) Dianella revoluta R.Br.; (C) Thelymitra crinita Lindl.; (D) Hybanthus calycinus (Ging.) F.Muell.; (E) Isotoma hypercrateriformis (R.Br.) Druce; (F) Stylidium schoenoides DC.; (G) Comesperma virgatum Labill.; (H) Caladenia flava R.Br.; (I) Diuris setacea R.Br.; (J) Dampiera linearis R.Br. cf; (K) Velleia trinervis Labill.
The colour pattern in white flowers of the insect-pollinated Stylidium schoenoides (Fig. 4F) is remarkable not only for its small size, but also for the rare bee-white basic colour, that appears white on the false colour photographs due to reflection of UV, blue and green light.
The orchid Caladenia flava (Fig. 4H) shows a diffuse colour pattern with continuous rather than sharp differences in the intensity of the UV reflection on the petals. Further examples of remarkable colour patterns include Waitzia sp. (Supplementary data Fig. S5C), Caladenia infundibularis (Supplementary data Fig. S5D) and Wahlenbergia capensis* (Supplementary data Fig. S5E).
Stamen-mimicking structures
In many flowers, the UV-absorbing centre part mimics visual features of pollen or anthers. In some flowers, the visually displayed pollen and anthers are yellow and UV absorbing. False colour rendering shows that these structures provide a strong visual contrast within the floral colour pattern that might enhance bee visibility of these floral guides.
For instance, all pea flowers included in the study exhibited a yellow colour patch at the base of the standard that mimics the yellow and UV-absorbing colour of pollen and anthers. In some species, such as Chorizema rhombeum, the contrast between the yellow and UV-absorbing anther-mimicking colour patch at the base of the standard petal is accentuated by a yellow UV-reflecting area (Fig. 3C). Alternatively, Mirbelia dilatata has a simple white UV-absorbing colour patch (Fig. 3D).
Unusually among legumes, Hardenbergia comptoniana and Hovea pungens possess a green colour patch (Fig. 3E). This patch has the same colour as the yellow colour patches when seen in ‘bee view’ through false colour rendering. Quantitative data analysis using RGB code values support this interpretation (Supplementary Method description S1). In other species, additional differently coloured areas and variation in the shape and size of the yellow, UV-absorbing colour patch produce a more complex colour pattern. Yellow, orange and red pea flowers similarly display a yellow UV-absorbing colour patch in the basal area of the standard petals, framed by a bee-UV area in Jacksonia sericea (Fig. 3F), Gastrolobium capitatum (Fig. 3G) and Daviesia divaricata (Fig. 3I), without frame but constrasting coloured wings in Daviesia cordata (Fig. 3H), and Gastrolobium sp. (Fig. 3J), or no other colour as in Kennedia lateritia (Fig. 3K). More examples of species that further increase the diversity of floral colour patterns in Fabaceae include Jacksonia sternbergiana (Supplementary data Fig. S4A), Daviesia inflata (Supplementary data Fig. S4B), Chorizema diversifolium (Supplementary data Fig. S4C), Hovea elliptica (Supplementary data Fig. S1D), Hovea pungens (Supplementary data Fig. S4E) and two colour morphs of Hovea trisperma (Supplementary data Fig. S5A, B), demonstrating that the UV pattern is independent of the pattern in the human-visible spectrum range.
The flowers of Thysanotus and Dichopogon are the assumed models of some Thelymitra orchid species (Bernhardt and Burns-Balogh, 1986; Dafni and Calder, 1987). The similarity of the overall flower colour in bee view between the nectarless orchids and nectar-producing model flowers of Thysanotus banksii (Fig. 4A) was confirmed using false colour photographs. Both the blue-flowering sun orchids, Thelymitra crinita (Fig. 4C) and Thelymitra vulgaris (Supplementary data Fig. S3E), are visually similar, and in turn resemble the lily Dianella revoluta (Fig. 4B). The yellowish and brown-spotted flowers of T. benthamiana (Supplementary data Fig. S3F) look very different from the blue species of Thelymitra (Fig. 4C; Supplementary data Fig S3E). However, all three Thelymitra orchids possess yellow and UV-absorbing stamen-mimicking structures in the centre of the flowers. Many flowers in the Goodeniaceae display false stamens, for example as three-dimensional structures as in Dampiera linearis (Fig. 4J) and in Scaevola crassifolia (Supplementary data Fig. S3C), or a simple colour patch as in Velleia trinervis (Fig. 4K) and Scaevola striata (Supplementary data Fig. S3D). The shape and colour of some flowers of the Goodeniaceae family are similar to flowers of other families such as Hybanthus calycinus (Violaceae; Fig. 4D) and Isotoma hypercrateriformis (Campanulaceae) (Fig. 4E). In all these species, the stamen mimics mark the entrance to the floral tube either as simple yellow and UV-absorbing colour patches or, likewise, coloured three-dimensional structures.
The orchid Diuris setacea (Fig. 4I) displays an unusual colour pattern typical of Diuris orchids with their yellow and UV-absorbing anther-mimicking central floral guide, with these orchids described as specialized mimics of certain pea flowers (Scaccabarozzi et al., 2018, 2020b) that matched the colour patterns of some of the yellow pea flowers in this study.
Missing or fading colour patterns
Some flowers lack colourful contrasts for bees; among them are red flowers that absorb UV light and thus are bee-black. Flowers that appear red to humans appear black on the false colour photographs due to absorption of UV, blue and green light. These flowers are bee-black, such as the typical ornithophilous flowers of Verticordia mitchelliana (Fig. 5A) and Lechenaultia formosa (Fig. 5B). Fabaceous red bird-pollinated flowers possess either a small and concealed colour pattern as in Gastrolobium celsianum (Fig. 5C) and Gastrolobium rubrum (Supplementary data Fig. S6A), an inconspicuous colour pattern for bees as in Kennedia rubicunda (Fig. 5D), an invisible colour pattern for bees as in Swainsona formosa (Fig. 5E) or seemingly relict colour patterning as in Kennedia prostrata (Fig. 5F), in which the yellow colour spot is reddish like the corolla. Other bird-pollinated flowers are inconspicuous to bees, such as Gastrolobium rubrum (Supplementary data Fig. S6A), Nematolepsis phebalioides (Supplementary data Fig. S2B), Macropidia fuliginosa (Supplementary data Fig. S6C), Anigozanthos manglesii (Supplementary data Fig. S6D) and Darwinia carnea (Supplementary data Fig. S6E), and exhibit an extraordinary range of colours (from green, yellow, red to black flowers).
Fig. 5.
Colour (left), UV (middle) and false colour photograph (right) of bird-pollinated flowers. (A) Verticordia mitchelliana C.A.Gardner; (B) Lechenaultia formosa R.Br.; (C) Gastrolobium celsianum (Lemaire) G.Chandler & Crisp; (D) Kennedia rubicunda (Schneev.) Vent.; (E) Swainsona formosa (G.Don) Joy Thomps.; (F) Kennedia prostrata R.Br.
DISCUSSION
Through examining the false colour imaging of 55 flowering plants of the SWAFR, we reveal common and striking unusual colour patterns in the bee view, and we confirm the widespread UV bull’s eye as a colour pattern and type of anther mimicry. Among our target species, some colour patterns do not meet the sensory requirements of bee vision, whilst in some others colour patterns were totally missing. A curious phenomenon of floral mimicry has been detected in the orchid genera studied that employ floral mimicry of model species, plus anther mimicry in the models. In addition, resolution of multifaceted colour patterns of Fabaceae aids in the recognition of major colour schemes depending on specialization level of pollinators, clarifying phenomena such as convergence of floral traits and floral mimicry in phylogenetically diverse target species.
Colour pattern through stamen and nectar mimicry
Almost all kinds of pollen, anther and stamen mimics have a yellow and UV-absorbing colour hue like model flowers, i.e. anthers and pollen grains. Pollen and anther colours are caused by protective flavonoid pigments which shield against UV radiation (Jansen et al., 1998). The yellow colour caused by these flavonoid pigments is a side effect of pollen protection; even wind-pollinated species such as gymnosperms that do not need to attract pollinators developed these colours prior to the evolution of pollination by animals (Osche, 1979; Lunau, 2007).
The UV bull’s eye is a common and spectacular kind of anther mimicry. The UV bull’s eye occurs in flowers or inflorescences with a UV-absorbing centre and a UV-reflecting periphery, whilst the same flowers appear uniformly yellow to the human eye (Utech and Kawano, 1975; Lunau et al., 2016). However, the key feature eliciting bumble-bee response to flowers is the bee-subjective colour contrast between the UV-absorbing centre and UV-reflecting periphery, along with the higher spectral purity of the flower related to the surroundings. Similarly, bumble-bees respond to the higher spectral purity of pollen, anthers or a floral guide related to the corolla. As a behavioural response, bumble-bees respond by touching the tips of their antennae onto the bull’s eye area of flowers (Lunau et al., 1996) as a method of probing for possible food rewards.
Since the UV contrast per se is not the key feature of UV bull’s eyes eliciting behavioural responses in bees, other reasons might explain the wide occurrence of the phenomenon. One possible explanation is that non-bee pollinators such as abundant Eristalis hoverflies innately respond to the UV-absorbing yellow colour hue at the centre of the bull’s eye by extending their proboscis for probing (Lunau and Wacht, 1994). In fact, hoverflies do not extend the proboscis towards any other colour other than UV-absorbing yellow (An et al., 2018). This plausible explanation seems to be supported by false colour imaging findings: some bluish pea bee-pollinated flowers, such as Hardenbergia comptoniana (Fig. 3E) and Hovea pungens (Supplementary data Fig. S4E), possess green floral guides at the same position on the standard petal, where other pea flowers have yellow and UV-absorbing floral guides. The false colour photographs indicate that their green colour appears similar to bees to yellow and UV-absorbing colours. This is due to the green and yellow floral guides reflecting in the green range of wavelengths and that the additional reflection in the red range of wavelengths in the yellow floral guides is invisible to bees. Hovea elliptica (Supplementary data Fig. S4D) and Hovea trisperma (Supplementary data Fig. S5A, B) display white floral guides in a similar position on the standard with a similar shape, where other pea flowers have yellow and UV-absorbing floral guides. These floral guides absorb UV light and thus are bee-bluegreen and might elicit similar responses in bees.
The anthers of the buzz-pollinated Dianella revoluta (Duncan et al., 2004) is an outstanding example of colouring precision to focus pollinator activity. In D. revoluta, the flowers have a visually conspicuous yellow and UV-absorbing basal part and a visually inconspicuous bee-black distal part (Fig. 5B). Approaching bees are likely to be guided towards the more conspicuous part of the anthers (Lunau, 2007), aiding the bees in obtaining the reward and pollen transfer from flower to bee.
Yellow and UV-absorbing floral attractants are very common in bee-pollinated flowering plants in Western Australia (Dyer, 1996) and are documented in most of the flowers of this study that support pollen and stamen mimicry in bee-pollinated plants as a global phenomenon. Pollen-, anther- and stamen-mimicking floral guides are a key attractant used to elicit the first physical contact of approaching bees with a flower (Lunau et al., 1996; Wilmsen et al., 2017).
Some stamen-mimicking structures manifest intriguing combinations of structure and colour pattern. For example, the three-dimensional protuberances on flowers of the Goodeniaceae as stamen mimics is compelling but requires confirmation from behavioural observations to demonstrate how these tactile stimuli are interpreted by flower visitors.
In European flowers, stamen mimicry in flowers of the Fabaceae family is rare, but is found in Colutea arborescens which displays an anther-mimicking colour patch on the standard, and Lotus purpureus with a stamen-mimicking floral guide on the wings (Lunau, 2000, 2007). In contrast, in this study, flowers of the Fabaceae in south-west Australia mostly display a yellow, UV-absorbing and anther-mimicking colour patch on the standard petals, suggesting that stamen and pollen mimicry has facilitated the diversification of the extraordinary number of legumes in the SWAFR.
Unusual colour patterns of bee-pollinated flowers
A surprising finding in this study was the presence of melittophilous UV-reflecting white flowers in all the white-flowered species investigated. The human-white flowers of Stylidium schoenoides reflect UV light with varied intensity and appear to show up as different colours to bees (Fig. 4F). Normally, white flowers that are pollinated by bees are strongly UV-absorbing and thus appear colourful to bees (Kevan et al., 1996). The large white-flowered trigger plant Stylidium schoenoides (Stylidiaceae) strongly reflects UV light and deploys a robust trigger-like column to catapult pollen onto the visiting insect. Curiously, Stylidium species are known to be pollinated by a variety of solitary bees and bombyliid flies (Armbruster et al., 1994; Brown et al., 1997; S. Armbruster for S. schoenoides, pers. comm.). Given the trigger mechanism, the pollen is placed in different parts on the insect body in order to avoid hybridization and pollen wastage (Armbruster et al., 1994; Brown et al., 1997). Through colour manipulation experiments, it would be interesting to test the role of UV-reflecting white flowers on the attraction of solitary bees or flies. In fact, Stylidium has inflorescences or solitary flowers (Western Australia Herbarium, 1998), often with small flowers, and thus being ‘conspicuous’ at distance may be critical for preferential pollination attraction, particularly in the highly floriferous and pollinator competitive environment of the SWAFR. Further investigations are certainly needed to explore this unexpected colour pattern in this genus. In contrast to south-west Australia, the alpine flora of New Zealand is dominated by white flowers, but these absorb UV light (Bischoff et al., 2013) and thus appear bee-bluegreen to the pollinating bees. Also in the Australian Alps, white flowers are UV absorbing (Inouye and Pyke, 1988). Dalrymple et al. (2020) provide evidence that vegetation cover influences brightness of flower colours, possibly explaining the bright colour of Stylidium schoenoides which is a key understorey species. Beyond pollinator assemblages, abiotic factors such as precipitation and solar radiation were shown to drive the evolution of coloration patterns of flowering plants across Australia (Dalrymple et al., 2020). In light of this, it would be of relevance to correlate the colour schemes noticed in the SWAFR with multiple biotic and abiotic predictors of flower coloration.
In many flowers, the yellow and UV-absorbing floral guides are framed by a contrasting red colour that reflects UV light. This yellow and red pattern appears red and blue in the false colour photographs and thus represents bee-green and bee-UV colour hues. The differently coloured frame displaying a bee-UV colour hue is a phenomenon that has not been reported previously. Interestingly, in European legumes, a similar framed pattern is visible, but is smaller in size in Colutea arborescens and bee-black in Lotus purpureus.
Identifying bird-pollinated flowers and missing colour patterns
The method employed here uniquely revealed the ability to distinguish between bird and insect pollination syndromes of species within the same habitat. Red and UV-absorbing flowers appear black to bees and therefore are inconspicuous to bees. Lunau et al. (2011) found that in Neotropical bird-pollinated flowers, UV-reflecting white flowers and UV-absorbing red flowers, i.e. bee-white and bee-black colours, respectively, assist in devaluing the floral resources for bees due to their low bee-subjective colour saturation. The role of red coloration in the SWAFR may operate in a similar way for native bees; however, introduced honey-bees have learnt to rob many red and red-tubular bird-pollinated flowers (Phillips et al., 2014), most probably based on structural rather than colour cues. The entirely bee-black and bird-pollinated Sturt’s Desert Pea (Swainsona formosa) (Fig. 5E), arguably one of the world’s most striking and spectacular flowering plants (Jusaitis, 1994), displays a colour pattern selectively conspicuous to birds.
Some bee-pollinated legumes display no colour pattern at all such as in Lotus subbiflorus* native in Europe and North Africa (Barrett and Tay, 2016) (Fig. 3A). Why does the signalling pattern differ in flowers of the Fabaceae from south-west Australia andthose from central Europe? One possible answer is that strong three-dimensional properties of the flowers might replace the colour pattern and indicate a landing site that physically guides the flower visitors. Another possibility is that nectarless flowers might direct the attention of visitors towards another part of the flower to improve pollination effectiveness, with pollen-, anther- and stamen-mimicking floral guides known to be used to promote landing of approaching bees (Lunau et al., 1996; Wilmsen et al., 2017). In addition, the wings and standard petals of the yellow-flowered legumes might display different colours in order to provide easily detactable cues for flower discrimination and orientation of pollinating bees.
The case in the case: double floral mimicry
Floral mimicry is a widespread and common occurrence in the SWAFR, particularly in orchids. Western Australian Diuris orchids represent one of the few cases where an explanatory pollination mechanism by native bees (Trichocolletes, Colletidae) operates via mimicry of food-rewarding pea plants (Scaccabarozzi et al., 2018, 2020b). Diuris orchid flowers (Fig. 4I) are surprising matches for the colour pattern of a variety of native legume species. In the cases examined in this study, all peas exhibited a central area on the standard that acted as a floral guide through UV absorption, with the peripheral area being UV-reflecting. Whereas in pea plants the UV-absorbing area is located at the base of the standard petal, the matching pattern on the orchids is strategically placed on the labellum and lateral labellum lobes that align the visiting insect with the stigmatic surface.
Surprisingly, from the false colour photography, the model pea flowers exihibit yellow and UV-absorbing stamen-mimicking floral guides reproduced in the Diuris flowers, by advertising false signals (Fig. 4I). Diuris also employ physical mimicry of the keel of legumes to complete the mimicry syndrome. Similarly, blue sun orchids thought to engage in guild mimicry include Thelymitraixioides Smith ex Sw. (Sydes and Calder, 1993) and T. megcalyptra Fitzg. (syn. T. nuda R.Br.; Bernhardt and Burns-Balogh, 1986). Jones (2006) suggested that T. crinita (Lindl.) is a Batesian mimic of Orthorosanthus laxus (Endl.; Iridaceae) and ultimately corroborated the mimicry hypothesis based on visual and olfactory cues for the species T. macrophylla (Lindl.).
Tepals of the strikingly coloured blue sun orchids (T. vulgaris and T. crinita) (Supplementary data Figs S3E and 4C) show a uniform and similar colour, except for the UV-absorbing column that houses the pollinia and stigma. The pattern is surprisingly reproduced by the sympatric non-orchid, Dianella revoluta (Fig. 4B), which has not been previously described as a potential model and includes anther-mimicking structures.
Similar to what was first observed in the European orchid Cephalanthera longifolia, the mimicry in orchids includes pollen imitation (or pseudopollen; van der Pijl and Dodson, 1966; Dafni and Ivri, 1981). Alternatively, the self-pollinated T. benthamiana (Brundrett, 2019) shows an unusual colour pattern (Supplementary data Fig. S3F) within the genus, characterized by an inconspicuous colour for bees, but pollen mimicry appears to be consistent as UV-absorbing spots on the tepals may resemble pollen.
Thus, false colour patterns in both Thelymitra and Diuris provide additional information on the reproductive ecology of orchids. The imagery revealed finer details of mimicry syndromes (pollen lures for example) that in combination with other cues (i.e. scent and floral guides) ensure pollination. However, confirmation of floral mimicry requires extensive observational studies and testing of multiple criteria (Roy and Widmer, 1999; Johnson and Schiestl, 2016), with false colour photography providing an effective and rapid means for preliminary assessment of floral mimicry.
Common and dissimilar colour schemes in pea plants
Pea plants represent one of the most important shrub components of the understorey vegetation in the SWAFR (Scaccabarozzi et al., 2020a), with a wide diversity of floral colours (sky blue, intense purples, vibrant reds and combinations of colours) and patterns. Pea plants also have a high number of sympatric taxa displaying an abundance of flowers, often with overlapping flowering periods (Western Australian Herbarium, 1998). Globally, pea plants are predominantly pollinated by bees (Green and Bohart, 1975; Arroyo, 1981; Aronne et al., 2012; Carleial et al., 2015), with Trichocolletes and Leioproctus among major genera pollinating SWAFR pea plants (Scaccabarozzi et al., 2020a). However, in the SWAFR, beetles have also been observed to be the principal visitor of Bossiaea, Isotropis and Viminaria species (Scaccabarozzi et al., 2020a).
Here we found that pea plants that attracted beetles are characterized by a predominance of UV-reflecting yellow petals, with a minor component of red coloration, i.e. on keel–wing petals or at the junction with the standard.
Overall, four major colour schemes in pea plants can been distinguished here: (1) bicoloured, encompassing the commonly known ‘egg and bacon pea plants’, because of the yellow, red and brown coloration as seen by humans; (2) pink; (3) violet; and (4) red. The first group includes pea plants characterized by both specialized and generalized interactions (Gastrolobium, Jacksonia and Daviesia; Fig. 3; Supplementary data Fig. S4) (Scaccabarozzi et al., 2020a) with their pollinators, whereas the third group includes pea plants (Hardenbergia and Hovea; Fig. 3E, Supplementary data Fig. S4D, E) displaying more specialized interactions. Mirbelia dilatata (Fig. 3D) in the second group has been shown to share a specialized interaction similar to Hardenbergia (Scaccabarozzi et al., 2020a). The final group corresponds to red flowers that are likely to be bird pollinated (Phillips et al., 2014). Thus, this visual classification provides an interpretive key for linking colour patterns to the level of bee pollinator specialization, supporting previous studies conducted in eastern Australia where different bee species perceive colour according to their particular sensory capabilities (Shrestha et al., 2019).
Moreover, ‘egg and bacon’ pea plants deviate from traditional colour patterns of Australian flora species (Shrestha et al., 2020) and with spectral properties in the UV sector of the bee hexagon (Chittka, 1992; Scaccabarozzi et al., 2018, 2020b), again suggesting a customized adaptation to local pollinators’ sensory ecology. Convergence of floral traits in ‘egg and bacon pea plants’ in the SWAFR can be a result of Müllerian mimicry, where a range of species sharing a similar phenotype receive an advantage by a facilitation effect (de Camargo et al., 2019). Such convergence might be an adaptive response to enhance the contrast with the background as a key selection pressure (Shrestha et al., 2019). In fact, unrelated genera displaying this coloration suggest that colour traits are independently derived. Since the prevalent ‘egg and bacon pea plants’ in the SWAFR bloom predominantly over spring (with flowering shifts along latitude gradients), the coloration maximizes the contrast with the floriferous background or renders flowers distinguishable when in sympatry with similarly coloured species (see argument in Arnold and Chittka, 2019).
Interestingly, beetles were observed to be aggressive and forceful visitors of ‘egg and bacon pea plants’ (Isotropis cuneifolia, Bossiaea aquifolium, B. linophylla and Viminaria juncea), often pushing between floral parts in somewhat random movements and exhibiting mating rituals on the pea flower (Scaccabarozzi et al., 2020a). Similarly to what has been observed in the Mediterranean red poppy, Papaver rhoeas, beetles moving rapidly within and between flowers in search of mates often remain in situ for several minutes (D. Scaccabarozzi, pers. obs.), suggesting that visits are also driven by rendezvous cues (Keasar et al., 2010).
Unusually, the wing petals in the majority of south-western Australian pea plant flowers are oriented horizontally. This orientation may act as a landing platform for insects such as in the beetle-pollinated Isotropis cuneifolia (Scaccabarozzi et al., 2020a), and operate in a similar way to the Mediterranean red poppy for hosting beetles during mating rituals and pollen consumption.
Conclusion
False colour photography provides a rapid, accurate and didactically convincing means to identify classical floral colour patterns such as the UV bull’s eye, widespread phenomena such as stamen mimicry, and rare colour patterns. As a digital, field-robust and rapid in situ method, colour patterns are immediately available for interpretation, smoothly enabling deployment of the technique across many plant groups in a biodiverse biome. Besides facilitating data collection, false colour photography resolves plant–pollinator interactions and ecological specialization by revealing usual and unusual colour patterns, and fine details of both floral structures (filamental hairs, anthers, stigmata) and floral guides (anther and pollen mimics) that are not accessible to spectrophotometry and may play a crucial role in insect attraction.
Interpretation of false colour patterns in the food-deceptive orchid genera Thelymitra and Diuris highlighted the potential mechanisms and the pollination strategies employed by the orchid species for their reproduction. The method was able to predict likely associated pollinator types such as birds and bees, although such predictions do require field validations based on observations and or exclusion experiments. The technique provides an exciting new opportunity for indexing floral traits on a biome scale and to more broadly understand pollination drivers of ecological, evolutionary relevance with important application in conservation policy and management.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: list of species in the SWAFR indicating plant family, plant community, photo acquisition site, colour pattern, life form and number of figure. Method description S1: quantitative colour analysis using RGB code values. Figure S1: colour hexagon for bee colour vision. Figure S2: colour names of some selected colour hues and the corresponding representation in false colour photos in bee view. Figure S3: colour, UV and false colour photo of bee-pollinated flowers. Figure S4: colour, UV and false colour photo showing the diversity of coloration in Fabaceae flowers. Figure S5: colour, UV and false colour photo of flowers with an unusual colour pattern. Figure S6: colour, UV and false colour photo of bird-pollinated flowers.
ACKNOWLEDGEMENTS
We thank Michael Crisp and Eng Pin Tay for their help with photographic identification of flowering plant species, and Anya Dawson and Andrew Brown for advice on location of field sites. This research was undertaken on Noongar tribal lands, and we acknowledge their continued cultural and spiritual association with the landscapes, biodiversity and waters of the south-west of Australia.
FUNDING
K.D. is supported by the Australian Government through the Australian Research 388 Council Industrial Transformation Training Centre for Mine Site Restoration (Project Number 389 ICI150100041).
LITERATURE CITED
- Ackerman JD. 1986. Coping with the epiphytic existence: pollination strategies. Selbyana 9: 52–60. [Google Scholar]
- Agostini K, Sazima M, Sazima I. 2006. Bird pollination of explosive flowers while foraging for nectar and caterpillars. Biotropica 38: 674–678. [Google Scholar]
- An L, Neimann A, Eberling E, Algora H, Brings S, Lunau K. 2018. The yellow specialist: dronefly Eristalis tenax prefers different yellow colours for landing and proboscis extension. Journal of Experimental Biology 221: jeb184788. [DOI] [PubMed] [Google Scholar]
- Anand RR, Paine M. 2002. Regolith geology of the Yilgarn Craton, Western Australia: implications for exploration. Australian Journal of Earth Sciences 49: 3–162. [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. 1994. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75: 315–329. [Google Scholar]
- Arnold SEJ, Chittka L. 2019. Flower colour diversity seen through the eyes of pollinators. A commentary on: ‘Floral colour structure in two Australian herbaceous communities: it depends on who is looking’. Annals of Botany 124: viii–viix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronne G, Giovanetti M, De Micco V. 2012. Morphofunctional traits and pollination mechanisms of Coronilla emerus L. flowers (Fabaceae). ScientificWorldJournal 2012: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo MTK. 1981. Breeding systems and pollination biology in Leguminosae. In: Polhill RM, Raven PH, eds, Advances in legume systematics. Kew: Royal Botanic Gardens, 723–769. [Google Scholar]
- Barrett RL, Tay PE. 2016. Perth plants. A field guide to the bushland and coastal flora of Kings Park and Bold Park. Perth: CSIRO Publishing. [Google Scholar]
- van den Berg CP, Troscianko J, Endler JA, Marshall NJ, Cheney KL. 2020. Quantitative colour pattern analysis (QCPA): a comprehensive framework for the analysis of colour patterns in nature. Methods in Ecology and Evolution 11: 316–332. [Google Scholar]
- Bernhardt P, Burns-Balogh P. 1986. Floral mimesis in Thelymitra nuda (Orchidaceae). Plant Systematics and Evolution 151: 187–202. [Google Scholar]
- Bischoff M, Lord JM, Robertson AW, Dyer AG. 2013. Hymenopteran pollinators as agents of selection on flower colour in the New Zealand mountains: salient chromatic signals enhance flower discrimination. New Zealand Journal of Botany 51: 181–193. [Google Scholar]
- Brown EM, Burbidge AH, Dell J, Edinger D, Hopper SD, Wills RT. 1997. Pollination in Western Australia: a database of animals visiting flowers, Handbook No. 15. Perth: Western Australian Naturalists’ Club. [Google Scholar]
- Brundrett MC. 2019. A comprehensive study of orchid seed production relative to pollination traits, plant density and climate in an urban reserve in Western Australia. Diversity 11: 123. [Google Scholar]
- Bruneau A. 1997. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae). American Journal of Botany 8: 54–71. [Google Scholar]
- de Camargo MGG, Lunau K, Batalha MA, Brings S, de Brito VLG, Morellato LPC. 2019. How flower colour signals allure bees and hummingbirds: a community-level test of the bee avoidance hypothesis. New Phytologist 222: 1112–1122. [DOI] [PubMed] [Google Scholar]
- Carleial S, Delgado-Salinas A, Domínguez CA, Terrazas T. 2015. Reflexed flowers in Aeschynomene amorphoides (Fabaceae: Faboideae): a mechanism promoting pollination specialization? Botanical Journal of the Linnean Society 177: 657–666. [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170: 533–543. [Google Scholar]
- Cowling RM, Rundel PW, Lamont BB, Kalin Arroyo M, Arianoutsou M. 1996. Plant diversity in mediterranean-climate regions. Trends in Ecology & Evolution 11: 362–366. [DOI] [PubMed] [Google Scholar]
- Dafni A, Calder DM. 1987. Pollination by deceit and floral mimesis in Thelymitra antennifera (Orchidaceae). Plant Systematics and Evolution 158: 11–22. [Google Scholar]
- Dafni A, Ivri Y. 1981. Floral mimicry between Orchis israelitica Baumann and Dafni (Orchidaceae) and Bellevalia flexuosa Boiss. (Liliaceae). Oecologia 49: 229–232. [DOI] [PubMed] [Google Scholar]
- Dallman PR. 1998. Plant life in the world’s Mediterranean climates: California, Chile, South Africa, Australia, and the Mediterranean basin. Los Angeles: University of California Press. [Google Scholar]
- Dalrymple RL, Kemp DJ, Flores-Moreno H, et al. 2020. Macroecological patterns in flower colour are shaped by both biotic and abiotic factors. New Phytologist 228: 1972–1985. [DOI] [PubMed] [Google Scholar]
- Duncan DH, Cunningham SA, Nicotra AB. 2004. High self-pollen transfer and low fruit set in buzz-pollinated Dianella revoluta (Phormiaceae). Australian Journal of Botany 52: 185–193. [Google Scholar]
- Dyer AG. 1996. Reflection of near-ultraviolet radiation from flowers of Australian native plants. Australian Journal of Botany 44: 473–488. [Google Scholar]
- Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MG, Simonov V, Wong BB. 2012. Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proceedings of the Royal Society B: Biological Sciences 279: 3606–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinsinger P, Colwell RK, Terborgh J, Chaplin SB. 1979. Elevation and the morphology, flight energetics, and foraging ecology of tropical hummingbirds. The American Naturalist 113: 481–497. [Google Scholar]
- Garcia JE, Greentree AD, Shrestha M, Dorin A, Dyer AG. 2014. Flower colours through the lens: quantitative measurement with visible and ultraviolet digital photography. PLoS One 9: e96646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskett AC, Endler JA, Phillips RD. 2017. Convergent evolution of sexual deception via chromatic and achromatic contrast rather than colour mimicry. Evolutionary Ecology 31: 205–227. [Google Scholar]
- Gigord LD, Macnair MR, Stritesky M, Smithson A. 2002. The potential for floral mimicry in rewardless orchids: an experimental study. Proceedings of the Royal Society B: Biological Sciences 269: 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TW, Bohart GE. 1975. The pollination ecology of Astragalus cibarius and Astragalus utahensis (Leguminosae). American Journal of Botany 62: 379–386. [Google Scholar]
- Heuschen B, Gumbert A, Lunau K. 2005. A generalised mimicry system involving angiosperm flower colour, pollen and bumblebees’ innate colour preferences. Plant Systematics and Evolution 252: 121–137. [Google Scholar]
- Hopper SD. 1980. Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Australian Journal of Botany 28: 61–75. [Google Scholar]
- Hopper SD. 2009. OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322: 49–86. [Google Scholar]
- Hopper SD, Gioia P. 2004. The Southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35: 623–650. [Google Scholar]
- Houston TF. 2000. Native bees on wildflowers in western Australia – A synopsis of bee visitation of wildflowers based on the bee collection of the Western Australian Museum. Special Publication No. 2, Perth: Western Australian Insect Study Society Inc. [Google Scholar]
- Houston TF. 2018. A guide to the native bees of Australia. Melbourne: CSIRO Publishing. [Google Scholar]
- Indsto JO, Weston PH, Clements MA, Dyer AG, Batley M, Whelan RJ. 2006. Pollination of Diuris maculata (Orchidaceae) by male Trichocolletes venustus bees. Australian Journal of Botany 54: 669–679. [Google Scholar]
- Inouye DW, Pyke GH. 1988. Pollination biology in the Snowy Mountains of Australia: comparisons with montane Colorado, USA. Australian Journal of Ecology 13: 191–210. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. 1998. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends in Plant Science 3: 131–135. [Google Scholar]
- Jersáková J, Jürgens A, Šmilauer P, Johnson SD. 2012. The evolution of floral mimicry: identifying traits that visually attract pollinators. Functional Ecology 26: 1381–1389. [Google Scholar]
- Johnson SD, Schiestl FP. 2016. Floral mimicry. Oxford: Oxford University Press. [Google Scholar]
- Johnson SD, Peter CI, Nilsson LA, Ågren J. 2003. Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84: 2919–2927. [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. 2006. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 87: 2709–2716. [DOI] [PubMed] [Google Scholar]
- Jones DL. 2006. A complete guide to native orchids of Australia: including the island territories. Sydney, Australia: Reed New Holland. [Google Scholar]
- Jusaitis M. 1994. Floral development and breeding system of Swainsona formosa (Leguminosae). Hortscience 29: 117–119. [Google Scholar]
- Keasar T, Harari AR, Sabatinelli G, et al. 2010. Red anemone guild flowers as focal places for mating and feeding by Levant glaphyrid beetles. Biological Journal of the Linnean Society 99: 808–817. [Google Scholar]
- Kevan K, Giurfa M, Chittka L. 1996. Why are there so many and so few white flowers? Trends in Plant Science 1: 280–284. [Google Scholar]
- Koch L, Lunau K, Wester P. 2017. To be on the safe site – ungroomed spots on the bee’s body and their importance for pollination. PLoS One 12: e0182522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Dyer AG, Kevan PG, Lunau K. 2019. Functional significance of the optical properties of flowers for visual signalling. Annals of Botany 123: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze J, Gumbert A. 2001. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behavioral Ecology 12: 447–456. [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. 2011. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 348: 7. [Google Scholar]
- Loew ER, Lythgoe JN. 1985. The ecology of colour vision. Endeavour 9: 170–174. [DOI] [PubMed] [Google Scholar]
- Lunau K. 2000. The ecology and evolution of visual pollen signals. Plant Systematics and Evolution 222: 89–111. [Google Scholar]
- Lunau K. 2007. Stamens and mimic stamens as components of floral colour patterns. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 127: 13–41. [Google Scholar]
- Lunau K, Maier EJ. 1995. Innate colour preferences of flower visitors. Journal of Comparative Physiology A 177: 1–19. [Google Scholar]
- Lunau K, Wacht S. 1994. Optical releasers of the innate proboscis extension in the hoverfly Eristalis tenax L. (Syrphidae, Diptera). Journal of Comparative Physiology A 174: 574–579. [Google Scholar]
- Lunau K, Wester P. 2017. Mimicry and deception in pollination. In: Becard G, ed. Advances in Botanical Research, Vol. 82, How plants communicate with their biotic environment. Amsterdam: Academic Press, 259–279. [Google Scholar]
- Lunau K, Wacht S, Chittka L. 1996. Colour choices of naive bumble bees and their implications for colour perception. Journal of Comparative Physiology A 178: 477–489. [Google Scholar]
- Lunau K, Papiorek S, Eltz T, Sazima M. 2011. Avoidance of achromatic colours by bees provides a private niche for hummingbirds. Journal of Experimental Biology 214: 1607–1612. [DOI] [PubMed] [Google Scholar]
- Lunau K, Konzmann S, Bossems J, Harpke D. 2016. A matter of contrast: yellow flower colour constrains style length in Crocus species. PLoS One 11: e0154728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunau K, Konzmann S, Winter L, Kamphausen V, Ren Z-X. 2017. Pollen and stamen mimicry: the alpine flora as a case study. Arthropod-Plant Interactions 11: 427–447. [Google Scholar]
- Lunau K, Ren ZX, Fan XQ, Trunschke J, Pyke GH, Wang H. 2020. Nectar mimicry: a new phenomenon. Scientific Reports 10: 7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Osche G. 1979. Zur Evolution optischer Signale bei Blütenpflanzen. Biologie in unserer Zeit 9: 161–170. [Google Scholar]
- Osche G. 1983. Optische Signale in der Coevolution von Pflanze und Tier. Berichte der Deutschen Botanischen Gesellschaft 96: 1–27. [Google Scholar]
- Papiorek S, Junker RR, Alves-dos-Santos I, et al. 2016. Bees, birds and yellow flowers: pollinator-dependent convergent evolution of UV-patterns. Plant Biology 18: 46–55. [DOI] [PubMed] [Google Scholar]
- Phillips RD, Hopper SD, Dixon KW. 2010. Pollination ecology and the possible impacts of environmental change in the Southwest Australian biodiversity hotspot. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RD, Steinmeyer F, Menz MH, Erickson TE, Dixon KW. 2014. Changes in the composition and behaviour of a pollinator guild with plant population size and the consequences for plant fecundity. Functional Ecology 28: 846–856. [Google Scholar]
- van der Pijl L, Dodson CH. 1966. Orchid flowers: their pollination and evolution. Miami FL: University of Miami Press, 214. [Google Scholar]
- Popic TJ, Davila YC, Wardle GM. 2016. Cheater or mutualist? Novel florivory interaction between nectar‐rich Crotalaria cunninghamii and small mammals. Austral Ecology 41: 390–398. [Google Scholar]
- Roy BA, Widmer A. 1999. Floral mimicry: a fascinating yet poorly understood phenomenon. Trends in Plant Science 4: 325–330. [DOI] [PubMed] [Google Scholar]
- Scaccabarozzi D, Cozzolino S, Guzzetti L, et al. 2018. Masquerading as pea plants: behavioural and morphological evidence for mimicry of multiple models in an Australian orchid. Annals of Botany 122: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaccabarozzi D, Dixon KW, Tomlinson S, et al. 2020. a. Pronounced differences in visitation by potential pollinators to co-occurring species of Fabaceae in the southwestern Australian biodiversity hotspot. Botanical Journal of the Linnean Society 194: 308–325. [Google Scholar]
- Scaccabarozzi D, Guzzetti L, Phillips RD, et al. 2020. b. Ecological factors affecting pollination success in an orchid that mimics multiple species of pea plants (Fabaceae). Botanical Journal of the Linnean Society 194: 253–269. [Google Scholar]
- Shrestha M, Dyer AG, Garcia JE, Burd M. 2019. Floral colour structure in two Australian herbaceous communities: it depends on who is looking. Annals of Botany 124: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha M, Garcia JE, Burd M, Dyer AG. 2020. Australian native flower colours: does nectar reward drive bee pollinator flower preferences? PLoS One 15: e0226469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberglied RE. 1979. Communication in the ultraviolet. Annual Review of Ecology and Systematics 10: 373–398. [Google Scholar]
- Sydes MA, Calder DM. 1993. Comparative reproductive biology of two sun-orchids; the vulnerable Thelymitra circumsepta and the widespread T. ixioides (Orchidaceae). Australian Journal of Botany 41: 577–589. [Google Scholar]
- Thackway R, Cresswell ID. 1995. An interim biogeographic regionalisation for Australia: a framework for setting priorities in the National Reserves System Cooperative Program, Version 4.0. Canberra: Australian Nature Conservation Agency. [Google Scholar]
- Utech FH, Kawano S. 1975. Spectral polymorphisms in angiosperm flowers determined by differential ultraviolet reflectance. Botanical Magazine Tokyo 88: 9–30. [Google Scholar]
- Verhoeven C, Ren ZX, Lunau K. 2018. False colour photography: a novel digital approach to visualize the bee view of flowers. Journal of Pollination Ecology 23: 102–118. [Google Scholar]
- Vorobyev M, Gumbert A, Kunze J, Giurfa M, Menzel R. 1997. Flowers through insects eyes. Israel Journal of Plant Sciences 45: 93–101. [Google Scholar]
- Wester P, Lunau K. 2017. Plant–pollinator communication. In: Becard G, ed, Advances in Botanical Research, Vol. 82, How plants communicate with their biotic environment. Amsterdam: Academic Press, 225–257. [Google Scholar]
- Western Australian Herbarium . 1998. FloraBase – the Western Australian Flora.Department of Biodiversity, Conservation and Attractions. https://florabase.dpaw.wa.gov.au/ [Google Scholar]
- Williams AR, Williams GF. 1993. The invisible image – a tutorial on photography with invisible radiation, Part 1: Introduction and reflected ultraviolet techniques. Journal of Biological Photography 61: 115–132. [PubMed] [Google Scholar]
- Williams S, Dyer AG. 2007. A photographic simulation of insect vision. Journal of Ophthalmic Photography 29: 10–14. [Google Scholar]
- Wilmsen S, Gottlieb R, Junker RR, Lunau K. 2017. Bumblebees require visual pollen stimuli to initiate and multimodal stimuli to complete a full behavioral sequence in close-range flower orientation. Ecology and Evolution 7: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.