Abstract
Background and Aims
The three relict genera Pherosphaera, Microcachrys and Saxegothaea in Podocarpaceae produce quite distinct seed cone types in comparison with other genera and do not form a clade along with Acmopyle. The detailed seed cone morpho-anatomy of these three relict genera and affinities with other podocarps are poorly known. This study aims to understand the seed cone morpho-anatomy and affinities among these three disjunct relict genera and with other podocarps.
Methods
We comparatively analysed the seed cone morpho-anatomical traits of the three podocarps genera and used ancestral state reconstruction to understand the evolution of these traits.
Key Results
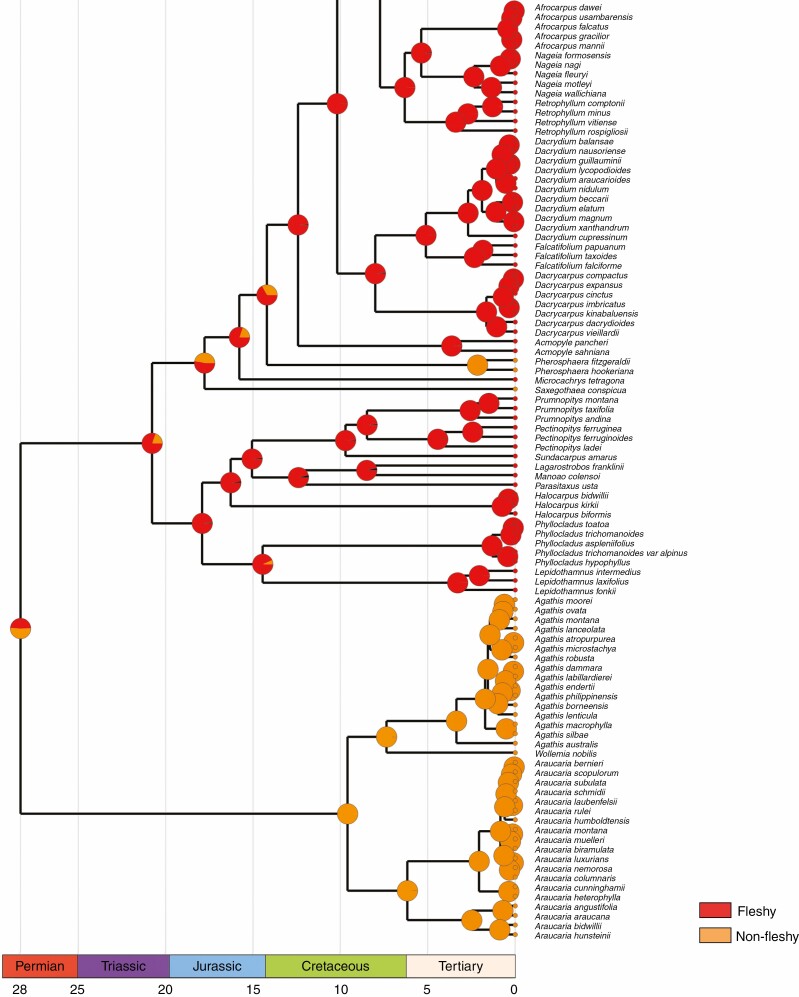
We described the seed cone morpho-anatomical structures of the three relict genera in detail. The three genera produce aggregated multiovulate cones. Both Microcachrys and Saxegothaea have an asymmetrical free cup-like epimatium. Both species of Pherosphaera lack an epimatium. The ancestral state reconstruction implies that the presence of an epimatium is an ancestral trait in podocarps and is independently lost in Pherosphaera and Phyllocladus. The seed cones are fleshy in Microcachrys and non-fleshy in Saxegothaea and Pherosphaera. The seed cone macrofossils of both extinct and living podocarps also show the presence of an epimatium and fleshiness in podocarps.
Conclusions
Altogether, the morpho-anatomy suggests that Pherosphaera, Microcachrys and Saxegothaea present affinities with each other and other podocarps, but the reconstruction of the ancestral seed cone in Podcarpaceae is quite complex due to multiple convergent evolutions of several structures. These structures (e.g. epimatium, aril and receptaculum) are of low taxonomic value but of great evolutionary and ecological significance, and are responsive adaptations to ever-changing environmental conditions.
Keywords: Conifers, evolution, endemism, Gondwana, histology, seed dispersal, Podocarpaceae, reproductive biology
Introduction
The Podocarpaceae today is one of the dominant conifer families, with 20 genera and >200 species, mostly in the Southern Hemisphere. Based on recent phylogenetic analyses, three major clades have been recognized – the Prumnopityoid clade, the Podocarpoid clade and the Dacrydioid clade. However, there is also a paraphyletic group of four genera outside these clades that comprise distinct lineages. These genera include three with compound seed cones – Saxegothaea, Microcachrys and Pherosphaera, and one with 1–2 ovules – Acmopyle, like other podocarps (Leslie et al., 2017; Andruchow-Colombo et al., 2019). The Podocarpoid and Dacrydioid clades contain most of the extant species and appear to have radiated during the Cenozoic, based on their current habitat preferences and the inferred molecular divergence dates. In contrast, members of the paraphyletic group of genera have restricted distribution and, along with some members of the Prumnopityoid clade, appear to reflect deeper divergences (Knopf et al., 2012; Leslie et al., 2017). These genera now have both restricted and disjunct distributions. Saxegothaea has one extant species in southern Chile and southern Argentina; Microcachrys contains one extant species in the alpine vegetation of central and western Tasmania, Australia; and Pherosphaera has two extant species, one in montane areas of Tasmania and the other on sandstone ledges of waterfalls in the Blue Mountains, New South Wales, Australia (Carpenter et al., 2011).
Reproductive structures and traits are important for determining the evolutionary relationships of conifers and other groups, and they also provide insight into ecological interactions and adaptation to environmental changes (Yang et al., 2015; Leslie et al., 2018; Klaus and Matzke, 2020). Female reproductive structures are a critical component of any species and they are often strongly influenced by ecological interactions, including pollination, protection of the developing seeds and seed dispersal (Tomlinson et al., 1991; Contreras et al., 2017). In the conifers, seed cones have performed these multiple functions through time, and the adaptations at any one time can probably be co-opted or changed (within developmental limitations) to work as well as possible for the next required function (Leslie, 2011). Some conifer families have responded to changing environments through time by diversifying their seed cones (Yang et al., 2015). For example, two of the currently dominant conifer families, Pinaceae and Podocarpaceae, have had distinctly different outcomes in seed cone evolution, i.e. hard scaly cones and fleshy cones, respectively (Contreras et al., 2017).
These relict genera are considered to be surviving lineages from the Palaeozoic and Mesozoic eras (Doyle and Looby, 1939; Doyle, 1945; Axsmith et al., 1998; Cantrill and Falcon-Lang, 2001). However, tracing the evolutionary history of these three extant genera is difficult because the morphological changes that have accumulated through time are represented by only a small number of extinct species in the fossil record (Looby and Doyle, 1939). These three relict Podocarpaceae genera have had a great deal of uncertainty in their relationship with other podocarp genera and in the interpretation of their origin. This is exemplified by the fact that they have demonstrated instability within various phylogenetic analyses. This morphological distinctiveness of these genera led some taxonomists to place them within their own families (e.g. Saxegothaeaceae, Pherospheraceae; Gaussen, 1974; Doweld and Reveal, 1999). The seed cone diversity in Podocarpaceae suggests several different evolutionary pathways to produce cones with different morpho-anatomical functional traits and structures (Tomlinson et al., 1991; Tomlinson, 1992; Restemeyer, 2002). No other conifer family shows such diversity in seed cone functional traits and structures. There is also a remarkable difference in seed cone morphology between the Podocarpaceae and their nearest living relative, the Araucariaceae, and the fossil record provides no clear evidence for the early evolutionary history of seed cones in the Podocarpaceae. Although morphological aspects of conifers have been considered in general in previous studies (Tomlinson et al., 1991; Tomlinson, 1992; Restemeyer, 2002; Contreras et al., 2017; Leslie et al., 2018; Herting et al., 2020; Klaus and Matzke, 2020), there remains a lack of comprehensive studies on the detailed morpho-anatomy, functional structures and trait evolution of seed cones of the Podocarpaceae. The absence of such studies has resulted in some confusing interpretations and terminology for functional structures and traits. The focus of our research has been to investigate the detailed seed cone morpho-anatomy of functional structures and trait evolution of Podocarpaceae. This study is focused on evaluating the evolution and loss of seed cone functional structures in these three palaeoendemic genera (Saxegothaea, Microcachrys and Pherosphaera) to establish their relationship with other podocarps. We assessed the detailed seed cone morpho-anatomy for consistent affinities of functional structures and traits among these three disjunct relict genera and with other podocarps.
MATERIALS AND METHODS
General morphology and distribution
Pherosphaera has occasionally monoecious individuals, while both species are generally dioecious evergreen shrubs. Archer (1850) established the genus Pherosphaera, including two species (P. hookeriana and P. fitzgeraldii); they occur in mountainous areas with high rainfall and close to running water. Pherosphaera hookeriana W. Archer bis ex Hooker is the type species of the genus and is distributed in montane areas of Tasmania (Australia), where it reproduces asexually by root suckers as well as by sexual reproduction (Worth et al., 2018). Pherosphaera fitzgeraldii (F. Mueller) F. Mueller ex Hooker f. is a critically endangered species distributed on sandstone ledges of waterfalls in the Blue Mountains, New South Wales (Australia). It is an evergreen shrub with spirally arranged, scaly leaves. The pollen grain of Pherosphaera ranges in size from 30 to 45 µm and has three smooth sacci (Elliott, 1948b).
Microcachrys tetragona is a monotypic paleoendemic species, restricted to alpine vegetation in central and western Tasmania, Australia (Carpenter et al., 2011). It is a highly branched, low-growing shrub with leaves in opposite pairs. Hooker (1845) described this monotypic genus as having trisaccate pollen grains (Lawson, 1923a,b). Seed cones of Microcachrys are fleshy and bright red. Saxegothaea conspicua Lindley (Mañio) is a small- to medium-sized tree (Restemeyer, 2002), native to Southern Chile and Southern Argentina.
Seed cone collection
Seed cones at different developmental stages were collected from the Australian National Botanic Gardens, Canberra, Mount Lofty Botanical Garden, South Australia and The Tasmanian Arboretum, Devonport, Tasmania, Australia. For this study, we used living seed cones (ten seed cone replicates for each species) and we have stored four specimens of each species in the plant spirit collection of the University of Adelaide, Australia. The spirit collection voucher numbers are (1) UOA-SC219-22 for Saxegothaea conspicua, (2) UOA-SC223-226 for Microcachrys tetragona, (3) UOA-SC227-230 for Pherosphaera fitzgeraldii and (4) UOA-SC231-234 for Pherosphaera hookeriana. We also collected complete plant specimens which are lodged in the State Herbarium of South Australia (AD).
Taxon processing and sectioning
Whole seed cones, plus longitudinal and cross-sections of the seed cones were photographed with a Nikon-SMZ25 stereomicroscope. Specimens were fixed in 200 mL of FAA (100 mL of 95 % ethanol + 80 mL of dH2O + 20 mL of 37 % formaldehyde solution) immediately after collection. For the histology, seed cones were fixed for 48–72 h and then processed for a 48 h cycle on a Sakura Tissue-Tek VIP6 Vacuum Infiltration Tissue Processor. They were then embedded in paraffin wax (Sakura Tissue Tek embedding centre). Longitudinal and cross-sections of 8–10 µm thickness were subsequently produced using a Leica RM 2235 Rotary Microtome and stained with H & E (DAKO Cover stainer) and Toluidine blue. The slides were observed under light microscopy and photographed at various magnifications.
Scanning electron microscopy
For scanning electron microscopy (SEM) the seed cones were fixed for 3 d in the fixative [4.0 % paraformaldehyde/1.25 % glutaraldehyde in phosphate-buffered saline (PBS) solution]. The samples were then washed twice for 5 min in PBS and 4 % sucrose. The samples were dehydrated twice in 70 % ethanol for 8 h each time, twice in 90 % ethanol for 8 h each time and twice in 100 % ethanol for 8 h each time. The dehydrated samples were placed in 100 % ethanol and hexamethyldisilazane (HMDS) (1:1) for 1 h. The HMDS was then pipetted out and the samples were air dried. The samples were mounted on an aluminium stub with sticky tabs and coated with platinum. These specimens were observed using a scanning electron microscope (Model Philip XL-30) installed at Adelaide Microscopy in the University of Adelaide.
Terminology used
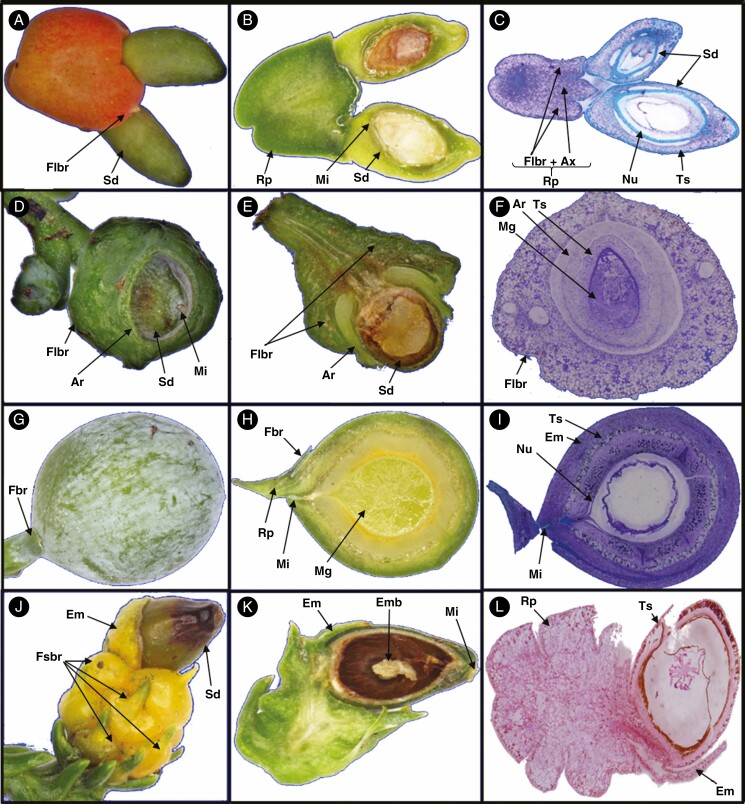
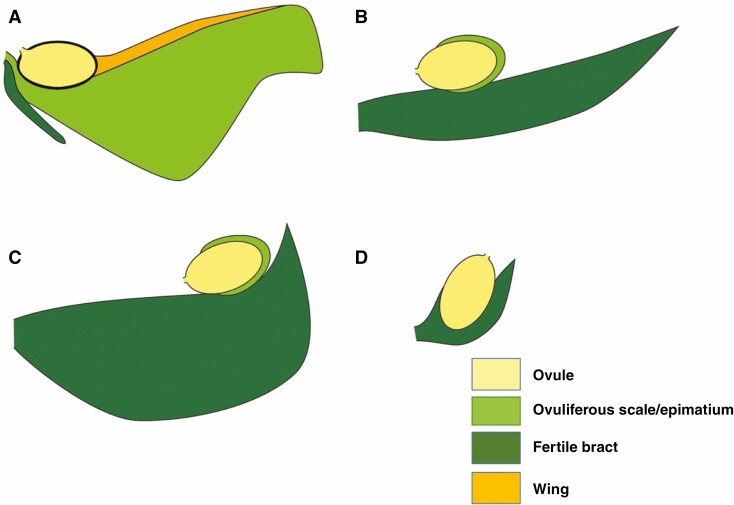
Some of the important terms used are defined here and illustrated from taxa across the Podocarpaceae. Receptaculum/podocarpium: a fleshy structure formed when the peduncle or cone axis fuses with the bracts of the seed cone into a fleshy structure (Tomlinson et al., 1991; Restemeyer, 2002). The receptacle can be formed by both fertile and sterile bracts. The receptacle is usually brightly coloured. In Fig. 1A–C, the receptaculum of Podocarpus totara is formed by fertile bracts. Aril: usually a fleshy or papery structure originating at the base of the ovule from an outgrowth of the funicle, not the testa or integument. Phyllocladus hypophyllus has two fleshy bracts; one is fertile and one is sterile to form a ring-like structure (Fig. 1D–F). The aril is free or in some cases fused with the seed (Dörken et al., 2019). Epimatium: a fleshy or non-fleshy structure in Podocarpaceae considered as homologous to the ovuliferous scale (Figs 1G–L and 4E–H). The epimatium is usually brightly coloured (Dörken et al., 2019). Ovuliferous/seed scale: a modified shoot that bears the ovule (Florin, 1954; Leslie and Losada, 2019). Herting et al. (2020) propose based on ontogenetic studies that seed scale is a modified funiculus. Fertile bract: a modified or specialized leaf that subtends the fertile scale/epimatium (Fig. 1G–H). Fleshy sterile bracts: bracts modified into a swollen and fleshy structure (Fig. 1J). Cone axis: axis of the cone. Micropyle: opening in the integument of an ovule (Figs 1B, D, H, K and 4A–F). Testa: outer layers of the seed (seed coat) protecting the embryo. The testa can sometimes be sub-divided into the exotesta, mesotesta and endotesta (Fig. 1C, F, I, L). Nucellus: the megasporangium of seed plants, often visible as the layer of cells in the central part of an ovule, surrounding the megagametophyte (Fig. 4H).
Fig. 1.
Demonstrating different morpho-anatomical structures in Podocarpaceae. (A–C) Podocarpus totara, (D–F) Phyllocladus hypophyllus, (G–I) Afrocarpus falcatus and (J–L) Dacrydium cupressinum.
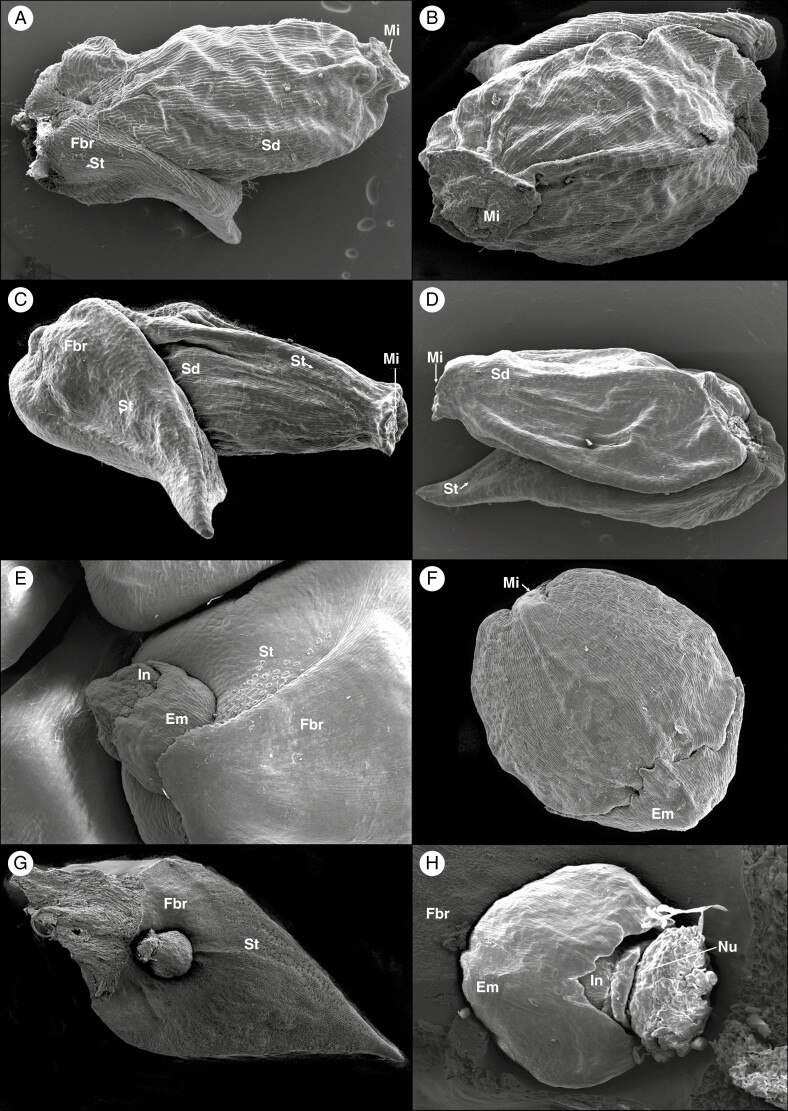
Fig. 4.
SEM micrographs of Pherosphaera fitzgeraldii shows seeds with a fertile bract (A and B); P. hookeriana shows seeds with a fertile bract (C and D); Microcachrys tetragona shows the developing ovule (E) and seed cone with epimatium (F); and Saxegothaea conspicua showing developing ovule-post pollination with epimatium, nucellus and fertile bract (G and H).
Measurements and character mapping
Different morpho-anatomical and embryological characters were recorded (Table 1). The anatomical layers were measured from mature seed cones. The measurements were taken using ImageJ 1.8.0_112 software. For ancestral reconstruction, we used the dated phylogeny of based on three chloroplast (rbcL, matK, trnL–trnF) and three nuclear genes (NEEDLY, PHYP and ITS2). The characters were mapped for their evolution using RASP 4.2-BayesTraits (Yu et al., 2020) and Mesquite 3.6 (Maddison and Maddison, 2019) with the maximum likelihood (ML) and Markov chain Monte Carlo (MCMC) reconstruction method.
Table 1.
Seed cone morpho-anatomical qualitative and quantitative characters of the three paleoendemic Podocarps
| Characters | Pherosphaera hookeriana | Pherosphaera fitzgeraldi | Microcachrys tetragona | Saxegothaea conspicua |
|---|---|---|---|---|
| Reproductive cycle | 1 year | 1 year | 1 year | 1 year |
| Cone shape | Ovoid | Ovoid | Ovoid–globose | Ovoid–globose |
| Cone size (mm) | 2–4 × 1–2.5 | 2–4.5 × 1.5–2.5 | 2.5–8 × 2–5 | 10–20 × 4–8 |
| Colour | Dry brownish | Dry brownish | Bright red | Dry brownish |
| Number of seeds per cone | 2–5 | 2–8 | 10–28 | 2–6 |
| Seed size (mm) | 1.2–1.8 × 0.4–0.8 | 1–2.5 × 0.5–0.8 | 1–2.5 × 1–2 | 3–4 × 2–3 |
| Seed shape | Ovoid | Ovoid | Ovoid | Ovoid |
| Seed surface | Rugose | Rugose | Smooth | Smooth |
| Seed colour | Shiny dark brown | Dark brown | Dark brown | Dark brown |
| Ovule | Orthotropous | Orthotropous | Anatropous | Anatropous |
| Free nuclei | 16 | 16 | 40–64 | 16 |
| Aril | Absent | Absent | Absent | Absent |
| Epidermal layers | 1 | 1 | 1 | 1 |
| Epidermal cell shape | round- isodiametric | round- isodiametric | round-rectangular | rectangular |
| Testa layers of cells | 8–14 | 8–12 | 4–9 | 4–7 |
| Nucellus | 2–4 | 4–6 | 3–5 | 3–6 |
| Embryo shape | Straight | Straight | Straight | Straight |
| Embryo size (mm) | 0.3–0.5 × 0.1–0.2 | 0.2–0.6 × 0.1–0.3 | 0.3–0.5 × 0.1–0.2 | 0.2–0.4 × 0.1–0.2 |
| Fertile bracts | 2–5 | 2–8 | 10–28 | 2–6 |
| Sterile bracts | 5–8 | 4–8 | 12–30 | 15–20 |
| Sclereid cells in testa | Present | Present | Present | Present |
| Sclereids in fertile bract | Present | Present | Present | Present |
| Resin canals in fertile bract | Present | Present | Absent | Present |
| Stomata on bracts | Present | Present | Present | Present |
| Fleshy bracts | Absent | Absent | Present | Present |
| Epimatium | Absent | Absent | Present | Present |
| Epimatium colour | – | – | Bright red | Yellowish |
| Dispersal | Barochory | Barochory | Zoochory | Barochory |
RESULTS
Pherosphaera seed cone morpho-anatomy
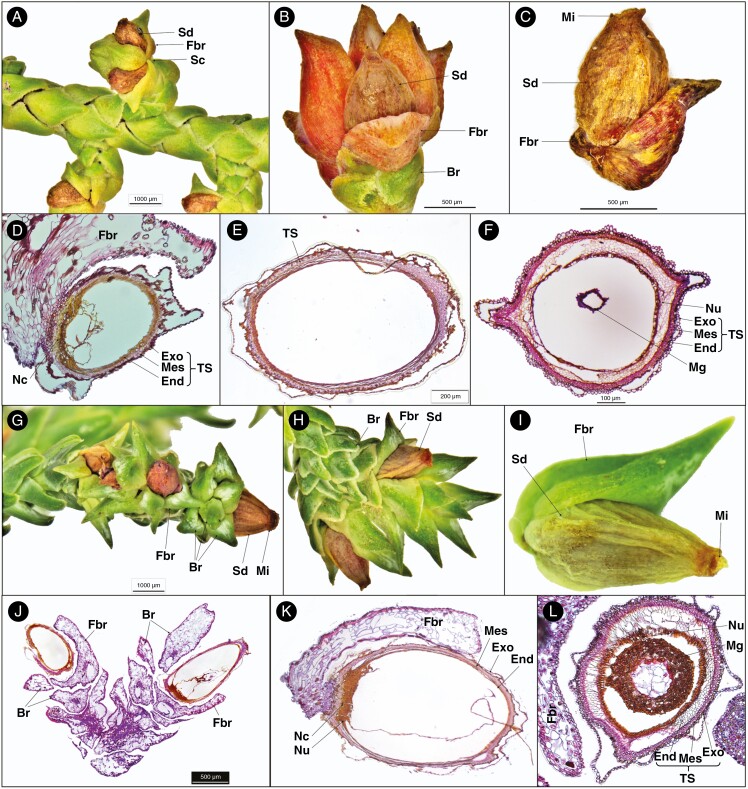
Pherosphaera hookeriana produces non-fleshy multiovulate cones occurring at the tip of the very short lateral shoots (Figs 2A–C and 4C, D). The seed cones are pink-reddish, ovoid-shaped, 2–4 mm long and 1–2.5 mm wide. The seed cones consist of 2–5 seeds, each with a fertile bract, and additionally the cone has 5–8 ovate concave sterile bracts. The seed size is about 1.2–1.8 × 0.4–0.8 mm. The ovules are erect.
Fig. 2.
Seed cone morpho-anatomical features of Pherosphaera hookeriana (A–F) and P. fitzgeraldii (G–L). (A) Seed cone branch with seed cones (Sc), seed (Sd) and fertile bract (Fbr); (B) seed cone with the seed, fertile bract and bract; (C) seed and fertile bract; (D) longitudinal section of a cone scale seed unit showing fertile bract, nucellar cap (Nc) and layers of testa (TS), i.e. exotesta (Exo), mesotesta (Mes) and endotesta (End); (E) longitudinal section of a single seed cone showing the testa; (F) seed cone cross-section showing layers of the testa, i.e. exotesta, mesotesta and endotesta; (G) seed cone branch showing seed, fertile bract, micropyle (Mi) and bract (Br); (H) seed cone showing seed (Sd), fertile bract and bract; (I) single seed cone showing seed, fertile bract and micropyle; (J) longitudinal section of seed cone branch cone showing seed, fertile bract and bract; (K) longitudinal section of a single seed cone showing fertile bract, nucellus (Nu), nucellar cap and layers of testa, i.e. exotesta, mesotesta and endotesta; and (L) seed cone cross-section showing fertile bract, nucellus, megagametophyte (Mg) and layers of testa, i.e. exotesta, mesotesta and endotesta.
The transverse section of the seed shows the following three major anatomical zones (Fig. 2D–F). (1) Testa: 8–14 compact layers of rounded and isodiametric cells forming the protective cover of the seed. The testa is differentiated into the exotesta made up of two layers of rounded cells forming the outer zone of the testa, the mesotesta which is a sclerified layer consisting of 3–5 layers of dense rounded cells forming the hard protective cover of the ovule, and the endotesta with 5–7 non-lignified layers of parenchymatous cells surrounding the nucellus. (2) Nucellus: 2–4 layers of small dense round cells. (3) Megagametophyte: 10–16 dense layers surrounding the straight embryo. The fertile bract that subtends the ovule has a single enlarged resin duct with a vascular bundle.
Pherosphaera fitzgeraldii produces non-fleshy multiovulate cones. There are usually 2–8 seeds per seed cone, each positioned on a fertile bract, similar to P. hookeriana (Fig. 4A–D). The seed size is 1–2.5 × 0.5–0.8 mm. The ovules are erect (Figs 2G–L and 4A, B). The P. fitzgeraldii mature seed cone consists of three anatomical zones, similar to P. hookeriana (Table 1).
The fertile bract attached to the ovule has a single enlarged resin duct with a vascular bundle in both species. Both aril and epimatium are absent in Pherosphaera. The outer layer of the exotesta is free at some points from the inner layer.
Microcachrys seed cone morpho-anatomy
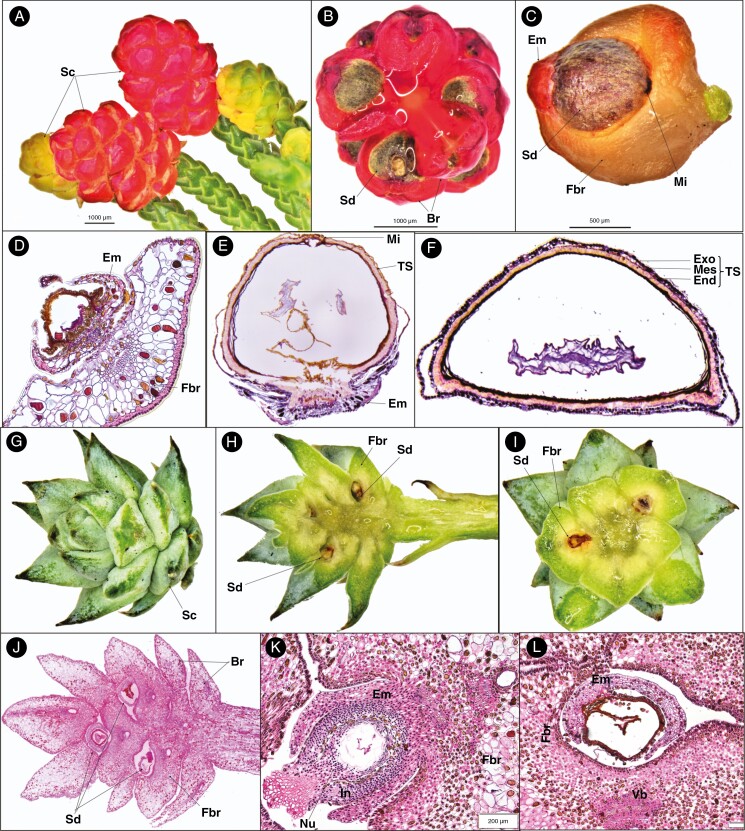
Microcachrys tetragona produces bright red, fleshy, multiovulate cones at the tip of short branches (Fig. 3A). The cones are ovoid to globose, 2.5–8 mm long and 2–5 mm wide. Seeds are ovoid, 10–28 in number, each one surrounded by a fleshy epimatium and fertile bract (Fig. 3A–D).
Fig. 3.
Seed cone morpho-anatomical features of Microcachrys (A–F) and Saxegothaea (G–L) showing: (A) fleshy red and young yellowish seed cones; (B) seed cone cross-section view showing seed (Sd) and bract (Br); (C) seed cone with epimatium (Em), seed (Sd), fertile bract (Fbr) and micropyle (Mi); (D) young ovule development (post-pollination) shows fertile bract, epimatium and ovule (Ov); (E) longitudinal section of single seed cone showing testa (TS), micropyle (Mi) and epimatium; (F) seed cone cross-section showing the different layer of testa, i.e. exotesta (Exo), mesotesta (Mes) and endotesta (End); (G) seed cone (Sc); (H) seed cone longitudinal section showing fertile bract (Fbr) and seed (Sd); (I) cross-sections showing the seed cone with the fertile bract and seed; (J) longitudinal section of seed cone showing fertile bract, seed and bract; (K) longitudinal section of developing ovule (post-pollination) showing epimatium, nucellus (Nu), fertile bract and integument (In); and (L) cross-section of developing ovule (post-pollination) showing epimatium, fertile bract and vascular bundle.
The mature ovules are inverted and are positioned on a fleshy epimatium and bract (Fig. 3C–E). The fertile bract is at a right angle to the cone axis. The seed size is 1–2.5 × 1–2 mm. Four major anatomical regions/zones were observed in the mature seed cone (Fig. 3F). (1) Epimatium: 4–8 layers of small and large cells, covering half of the ovule initially and later covering the lower part of the mature seed (Figs 3C–E and 4E, F). (2) Testa: 4–6 compact layers of small and large cells. The testa is differentiated into the exotesta consisting of two layers of rounded cells forming the outer zone of the testa, the mesotesta which is a sclerified layer consisting of 2–3 layers of dense elongated cells forming the hard protective cover of the ovule, surrounding the nucellus and the endotesta made up of 2–3 layers of dense cells. The outer layer of the exotesta is free at some points from the inner layer similar to Pherosphaera. (3) Nucellus: a protective cover of the embryo consisting of 3–5 layers of dense, smaller cells. A double-layered megaspore membrane is also present. (4) Megagametophyte: 5–8 layers of large cells surrounding the straight embryo. The fertile fleshy bract anatomy shows an outer cuticle layer and a double-layered hypodermis of sclereid cells, an enlarged vascular bundle and two enlarged resin ducts.
Saxegothaea seed cone morpho-anatomy
Saxegothaea conspicua is another genus in the Podocarpaceae that produces non-fleshy multiovulate cones on the terminal branches. The seed cones are ovoid to globose in shape, 10–20 mm long and 4–8 mm wide, with 2–6 seeds per cone, each surrounded by a triangular fertile bract (Fig. 3G–J). Seeds are ovoid, 3–4 mm long and 2–3 mm wide.
The ovules are inverted, with each one positioned on a fertile bract. The seed size is 3–4 × 2–3 mm. Four major anatomical zones were observed in the mature cone (Fig. 3K, L). (1) Epimatium: 6–8 layers of round and rectangular cells, covering half the ovule (Figs 3K and 4G, H). (2) Testa: 3–4 layers of small rounded, elongated and rectangular cells. The testa is differentiated into the exotesta, a single or double layer of rounded cells forming the outer zone of the testa, and the endotesta, a sclerified layer consisting of 3–5 layers of dense elongated cells forming the hard protective cover of the ovule, surrounding the nucellus. (3) Nucellus: the protective cover of the embryo which consists of 3–6 layers of small cells. A double-layered megaspore membrane was also observed, similar to that in Microcachrys. (4) Megagametophyte: 4–6 layers of large cells surrounding the straight embryo. The ovule is attached directly to one fertile bract with three vascular bundles (two small on the upper side towards the ovule and one large on the lower side of the ovule) and one large resin duct.
Comparison of seed cone morpho-anatomical characters
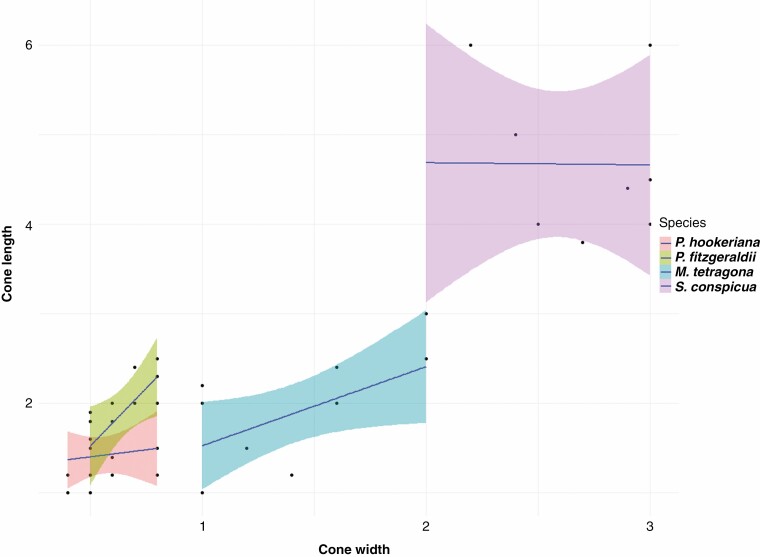
All three genera produce multiovulate cones. However, the seed cones of Pherosphaera and Saxegothaea are non-fleshy, while Microcachrys has fleshy seed cones. An aril is absent in all three genera. An epimatium is present in both Saxegothaea and Microcachrys, but absent in Pherosphaera. The ovule is erect in both species of Pherosphaera and inverted in Saxegothaea and Microcachrys. The shape of the seed cone is ovoid in Pherosphaera, and ovoid to globose in Microcachrys and Saxegothaea. Microcachrys has 10–28 seeds per cone, Pherosphaera has 2–8 and Saxegothaea has 2–6. The seed surface is rugose in Pherosphaera and smooth in both Saxegothaea and Microcachrys (Fig. 4). The testa morphology and anatomy are similar in all three genera. Fleshy bracts are present in Microcachrys but absent in both Saxegothaea and Pherosphaera. Stomata were observed on the fertile bracts of all species. All genera have a straight embryo. The seed cones of Pherosphaera and Microcachrys are smaller than those of Saxegothaea (Fig. 5).
Fig. 5.
Seed cone size (length × width, mm) based on ten replicas, showing individual cone size range and comparison with other species. Pherosphaera hookeriana and P. fitzgeraldii have smaller seed cone sizes as compared with Microcachrys tetragona and Saxegothaea conspicua.
Discussion
Seed cone reproductive and morpho-anatomical comparisons
Multiovulate cones.
Pherosphaera, Saxegothaea and Microcachrys all produce aggregated multiovulate cones, and this distinguishes them from other podocarp genera. Non-aggregated multiovulate cones are also present in other genera of Podocarpaceae, e.g. Phyllocladus, Lagarostrobos and Prumnopitys. Production of multiovulate cones is also common in other conifers (Tomlinson, 1992, 1994). The seed cone and seed size in these aggregated seed cones are smaller in comparison with non-aggregated and solitary seed cones (Supplementary data Fig. S1). The ovules of Saxegothaea and Microcachrys are inverted, while Pherosphaera has erect ovules. Inverted ovules are common in other conifers (Stiles, 1912; Elliott, 1948a; Herting et al., 2020). These relict genera have several fertile bracts, each with a single ovule arranged in a small compact strobilus, and this clearly distinguishes them from all other genera. However, despite the wide variation among Podocarpaceae cone morphology, all have a single ovule per bract–scale complex (Tomlinson et al., 1991; Contreras et al., 2017).
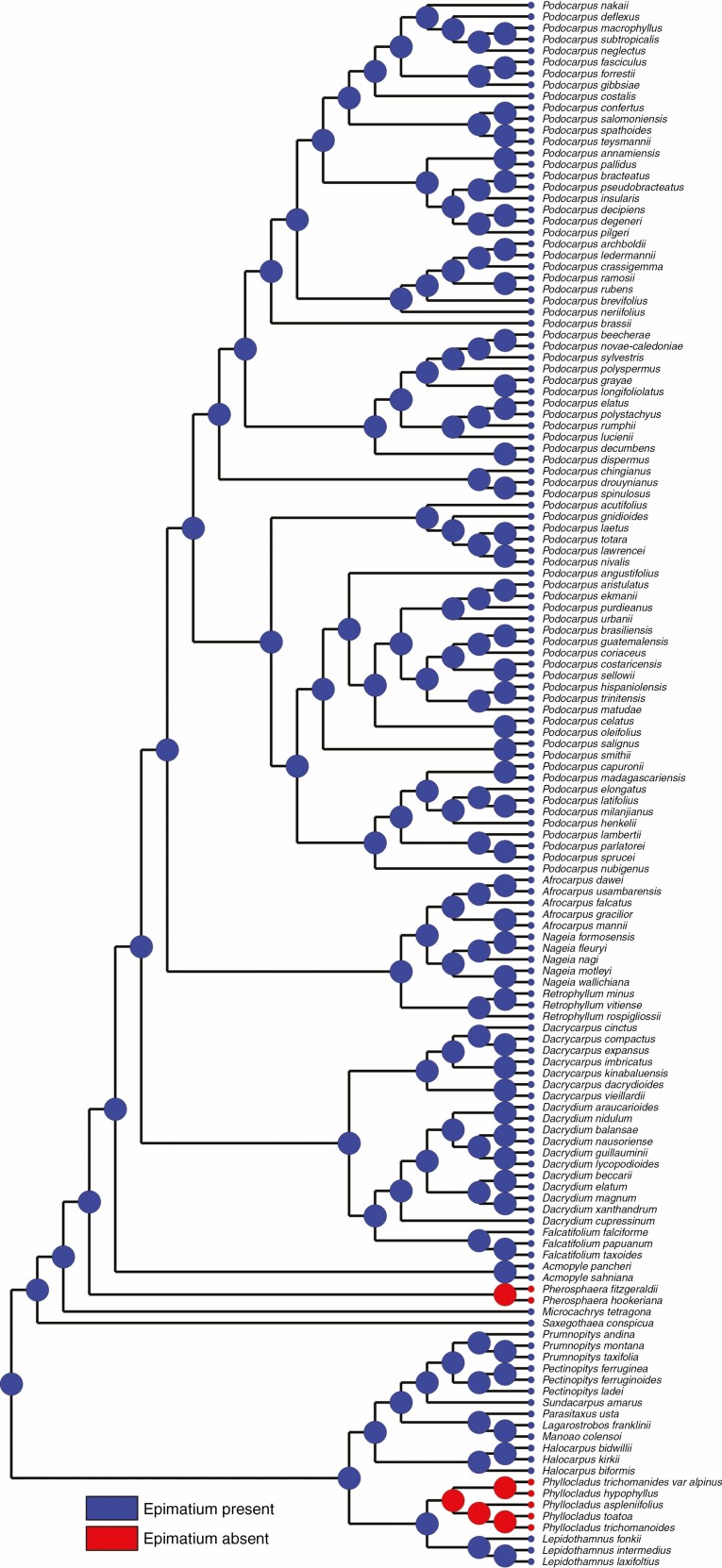
Epimatium traits.
A free asymmetrical cup-shaped epimatium is present in Saxegothaea and Microcachrys but absent in Pherosphaera (Supplementary data Fig. S2). The epimatium is fleshy in Microcachrys but not in Saxegothaea (Fig. 3). A similar well-developed epimatium with an asymmetrical morphology is present in other podocarps, e.g. Lepidothamnus, Dacrydium, Falcatifolium, Manoao and Lagarostrobos. The ancestral state reconstruction implies that the presence of epimatium is an ancestral trait in podocarps (Fig. 6). According to Elliott (1948b), the absence of an epimatium could be the result of an evolutionary reduction responsible for the production of erect ovules in Pherosphaera. The function of the epimatium has been hypothesized as allowing the production of an inverted ovule, which is vital for pollen scavenging (Tomlinson, 1992). An epimatium is considered equivalent to the ovuliferous scale in conifers (Tomlinson, 1992) and, for example, Herting and Stützel (2020) reported that the epimatium development in the Podocarpaceae resembles the development of the ovuliferous scale in the Araucariaceae. A general comparison of the ovule position, epimatium and attachment of the fertile bract in seed cones of Saxegothaea, Microcachrys and Pherosphaera with Pinus is shown in Fig. 7.
Fig. 6.
Character mapping of the presence of an epimatium in different genera of Podocarpaceae using maximum likelihood.
Fig. 7.
Diagrammatic representation of a single seed cone scale from Pinus (A), Pherosphaera (B), Saxegothaea (C) and Microcachrys (D). Pinus is used to represent a typical coniferous cone scale arrangement. The ovule with micropyle is shown in yellow, the bract scale is in dark green and the ovuliferous scale and epimatium is in light green. In (B) (Saxegothaea) and (C) (Microcachrys), the epimatium is interpreted as a homologous structure to ovuliferous scale in other conifers. In (D) (Pherosphaera), the epimatium has disappeared completely.
Megagametophyte and embryo traits.
The number of archegonia present varies from two in Pherosphaera to three in Saxegothaea, and five or six in Microcachrys. Similar variation has been observed previously in the number of archegonia for different Podocarpaceae, e.g. Phyllocladus has one, Podocarpus has 11 and Microcachrys has 5–6 (Lawson, 1923a; Quinn, 1986). However, Wilson and Owens (1999) reported that Podocarpus totara usually has 4–6, but occasionally only two archegonia per ovule. Twenty archegonia per ovule are reported in both Podocarpus nivalis and Afrocarpus falcatus (Boyle and Doyle, 1953; Osborn, 1960). A gametophyte with a few large archegonia is an ancestral state that is absent from Pherosphaera, and the archegonial complex in Microcachrys is regarded as a derived condition (Lawson, 1923a, b; Taylor et al., 2005). In Saxegothaea, archegonia are enclosed by thick layers of female gametophyte with a small depression over the neck (Looby and Doyle, 1939), and this condition was also observed in Pherosphaera hookeriana by Elliott (1948a). Similarly, the presence of several relict nuclei in the Pherosphaera proembryo was reported by Elliott (1948b), and the current study endorses those findings. Similarly, we observed two-celled and hence binucleate embryos in Pherosphaera and Saxegothaea, and Doyle and Looby (1939) reported two-celled units in Saxegothaea as a result of the direct division of the proembryo original cell nucleus with subsequent wall formation. The Podocarpaceae typically has a binucleate, single-celled embryonic condition (Elliott, 1948a). Lawson (1923b) suggested that two-celled units are derived in Podocarpaceae; however, given that such units occur in all other conifers (Elliot, 1948a), this is probably the ancestral condition, and the single-celled state in Podocarpaceae is derived.
Testa.
In the three relict genera, the testa is papery and can be differentiated into an exotesta, mesotesta and endotesta (Figs 2 and 3). The outer testa is fleshy in some podocarp genera (e.g. Retrophyllum, Afrocarpus, Prumnopitys and Pectinopitys) and papery in other genera (e.g. Dacrydium, Dacrycarpus and Falcatifolium). The podocarp genera and species with fleshy outer testa have hard or woody endotesta.
Bract traits.
Similarities were observed in the anatomy of the fertile and sterile bracts of the seed cone of Pherosphaera, Saxegothaea and Microcachrys, showing that the fleshy or swollen bracts of the latter two genera result simply from the accumulation of water, rather than more complex anatomical modifications. Restemeyer (2002) also reported that in Microcachrys the desiccated leaves change into fleshy cone bracts. Several resin ducts were observed in the seed cone bracts of fleshy and swollen bracts. Thomson (1909) also reported similarities in the bract–scale complexes of Saxegothaea and Microcachrys.
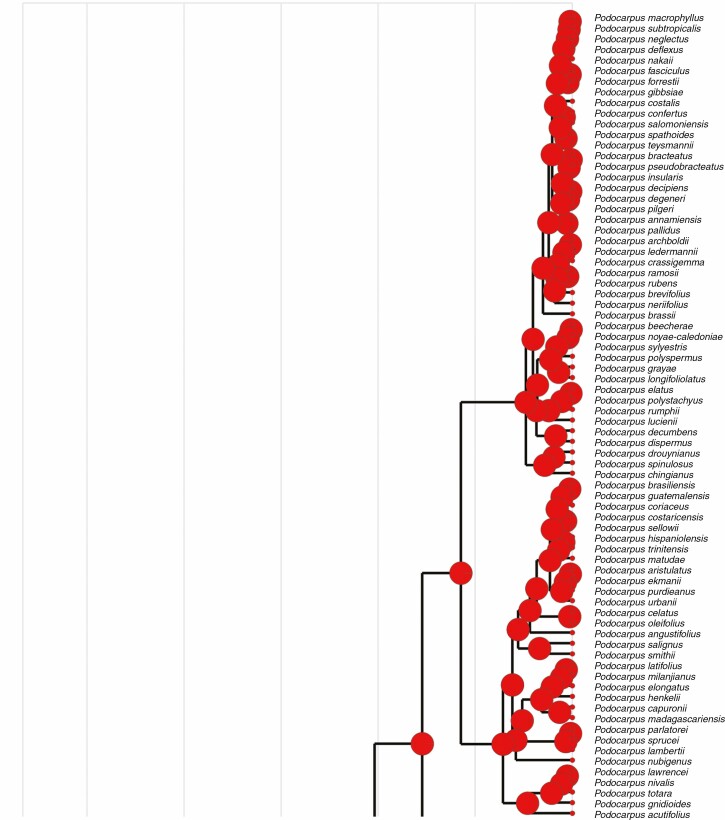
Fleshiness and ancestral seed cones
The ancestral state reconstruction indicates that different functional structures and traits evolved multiple times in Podocarpaceae. The ancestral state reconstruction of fleshiness in podocarps suggests that it is an ancestral trait, and Saxegothaea and Pherosphaera have evolved non-fleshy seed cones independently (Fig. 8). Based on statistical approaches, Klaus and Matzke (2020) suggested that the ancestral seed cones in Podocarpaceae were non-fleshy, and the earliest transition from non-fleshy into fleshy seed cones (e.g. at the base of the clade that includes Podocarpus, Afrocarpus, Nageia, Retrophyllum, Dacrydium, Falcatifolium and Dacrycarpus) occurred in the Late Cretaceous (approx. 82.4 million years ago), and these taxa are mainly bird dispersed. The ancestral state reconstruction of Herting et al. (2020) also implies that the ancestral seed cones in Podocarpaceae were fleshy. However, several fleshy structures occur across the clades and genera. These fleshy and non-fleshy structures shift multiple times, and more extensive studies are required in the Prumnopityoid, Podocarpoid and Dacrydioid clades.
Fig. 8.
Character mapping of seed cone morphology in different genera of Podocarpaceae using maximum likelihood.
Comparisons with other taxa
Stiles (1912) considered Pherosphaera to be closely related to Phyllocladus (which has a simple fleshy bract and aril) as both have erect ovules, and Doyle and Looby (1939) considered Pherosphaera to be an advanced derivative of Phyllocladus. However, embryology, morpho-anatomy and phylogenetics do not support a close relationship between Pherosphaera and Phyllocladus (e.g. polyembryony is distinctly different in these two genera, the presence of an aril, independent loss of the epimatium and Phyllocladus clade formation with other podocarps, namely the Prumnopityoid clade) (Elliott, 1948b; Leslie et al., 2018).
The megaspore membrane is well developed in Pherosphaera, Saxegothaea and Microcachrys (Lawson, 1923a, b). The presence of a megaspore membrane is reported in all conifers, but its appearance varies among different groups. For instance, in the Taxaceae, it is thin and inconspicuous, while in the Podocarpaceae it is thick and obvious (Fiordi et al., 1996). The similarity in the megaspore membrane between Pherosphaera and Microcachrys led Doyle (1945) to suggest that these genera are closely related. Elliott (1948b) reported that Pherosphaera is consistent with the Podocarpaceae and especially with Dacrydium in its gamete development, except that the nucellus is free from the integument in Dacrydium. One other peculiar feature of Saxegothaea is the absence of a pollination drop as the nucellus protrudes from the micropyle and forms a papillous stigma-like surface as a receptive structure, and gamete delivery to the ovule is achieved by the growth of a pollen tube (Tomlinson et al., 1991). Saxegothaea also has non-saccate pollen, similar to Araucariaceae, Larix, Pseudotsuga and Tsuga (Tomlinson, 1994). Microcachrys has a unique arrangement of seed cone bracts among podocarps, with the bracts or bract–scale complexes arranged in alternate whorls of four. This character resembles Diselma archeri (Cupressaceae) more than its closer suggested relative P. hookeriana, which has spirally arranged leaves. Seed cones of Saxegothaea show a gradual transition from foliage to sterile and fertile bracts, which is similar to some Araucariaceae species, e.g. Araucaria rulei and A. columnaris (Young, 1910). Similarly, Saxegothaea resembles Araucaria and Microcachrys in the arrangement of vascular bundles (enlargement of the vascular bundle and resin ducts) in the fertile bracts of the seed cone (Stiles, 1908). Gaussen (1974) and Woltz (1985) established Saxegothaea as a separate family, Saxegothaeaceae, due to its resemblance to Araucariaceae.
Wilde (1944) proposed that the podocarps follow two lines of evolution; one is the reduction of the fertile branch system while retaining large leaves, and the second is the retention of numerous fertile branches while the leaves are reduced (imbricate). Pherosphaera and Microcachrys fall in the second category. Retention of numerous fertile branches and reduced leaves is common to all three genera studied here. Despite these genera looking morphologically distinct from other podocarps, they share a similar seed cone reproductive development, functional structures and morpho-anatomy with each other and with other podocarps. Similarly, the ancestral state reconstructions also provide an insight on the evolution and loss of structures and traits (Contreras et al., 2017; Leslie et al., 2017).
What the fossil record tells us about the initial seed cones in Podocarpaceae
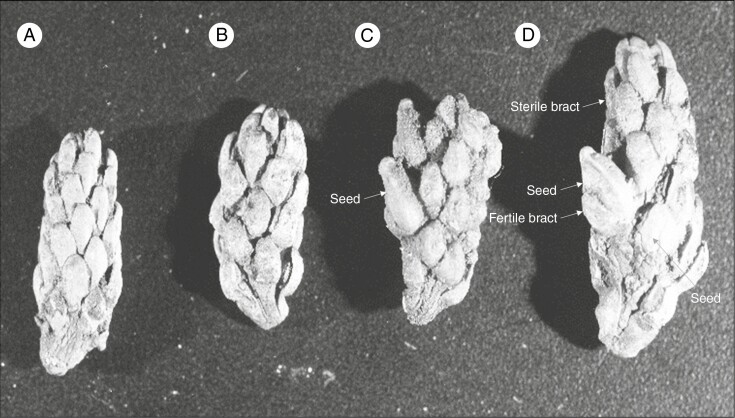
The fossil record also provides an insight into the relictual distribution of Saxegothaea, Microcachrys and Pherosphaera in the Podocarpaceae. An undescribed extinct seed cone, informally named Fecundistrobus, from the middle Cretaceous of Winton, Queensland (Peters, 1985) shows morphological similarities to Pherosphaera and Saxegothaea as the fleshy fertile scale and receptacle are absent (Fig. 9). Fecundistrobus also has sterile bracts below the fertile bracts, similar to Saxegothaea, Microcachrys and Pherosphaera, with an even distribution of ovules. Erect ovules are present in both Fecundistrobus and Pherosphaera (Peters, 1985). The fossilized seed cone of an extinct species (Friisia lusitanica) with an inverted seed and epimatium from the Cretaceous of western Portugal resembles Microcachrys (Mendes and Kvaček, 2020). Other seed cones from the Jurassic of Yorkshire (Scarburgia hallii) and the Middle Jurassic of Poland (Harrisiocarpus gucikii and H. cracoviensis) also resemble Microcachrys (Reymanówna, 1987). Mehtaia rajmahalensis (from the Jurassic of India) is another extinct genus showing similarities to Fecundistrobus and Pherosphaera (Vishnu-Mitre, 1958). Rao (1972) reported the fossil seed cone of the extinct species Nipaniostrobus sahnii from the Cretaceous Rajmahal Hills in India as showing morphological resemblance to Saxegothaea, Microcachrys and Pherosphaera. It can be inferred that podocarps once had an Indo-Australian distribution. The sterile foliage of the extinct species Microstrobos sommervillae (Pherosphaera sommervillae) from the Early Eocene Buckland sediments in south-eastern Tasmania) also supports Pherosphaera as a paleoendemic genus (Townrow, 1965).
Fig. 9.
The seed cones (A–D at different levels of maturity) of an undescribed Fecundistrobus species from Winton, Queensland show morphological similarities with Pherosphaera seed cones as having non-fleshy bracts. The photo was taken by Dr M. D. Peters and is from the collection held in the David T. Blackburn Palaeobotany Collection at the University of Adelaide. Abbreviations: Ar, aril; Ax, cone axis; Br, bract; Edo, endotesta; Em, epimatium; Ep, epidermis; Exo, exotesta; Emb, embryo; Fbr, fertile bract; Fs, fertile scale; Fsbr, fleshy sterile bract; Flbr, fleshy bract; Gm, gynophore membrane; In, integument; Mes, mesotesta; Mg, megagametophyte; Mi, micropyle; Nu, Nnucellus; Nc, nucellar cap; Ov, ovule; Rc, resin canal; Rd, resin duct; Pc, pollen cone; Po, pollen; Rp, receptaculum/podocarpium; Sc, seed cone; Sd, seed; Sl, sclereid; Slsc, fleshy sarcotesta-like seed coat; Ts, testa; Vb, vascular bundle.
Conclusions
This study provides insights into the seed cone morphology and trait evolutions in three relict genera, Pherosphaera, Microcachrys and Saxegothaea, with discrete and widely separated distributions. Despite the probable extinction of closely related sister species and genera, and the evolution of morphological structures as adaptations to changing environmental conditions, they still share basic reproductive traits with other podocarps. The detailed seed cone morpho-anatomy and ancestral state reconstruction imply that Pherosphaera, Microcachrys and Saxegothaea have evolved and lost traits. These three relict genera produce aggregate multiovulate cones. Some fossil podocarp taxa and sister family Araucariaceae also have multiovulate cones. The seed cone morpho-anatomy and reproductive biology unequivocally place these genera in the family Podocarpaceae. Evaluation of the fossils and these extant paleoendemic genera suggests that the ancestral seed cones were fleshy and non-fleshy seed cones (Saxegothaea and Pherosphaera) twice independently. However, podocarps demonstrate complex multiple convergent evolutions and are also characterized by substantial variation in seed cone structure and basic architecture, and this is reflected in the relict lineages studied here. Given their deep branches, these lineages suggest that such variation has always been characteristic of the group.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: comparison of seed cone size and seed size of Podocarpaceae. Figure S2: epimatium morphology mapping in different genera of Podocarpaceae using maximum likelihood. Figure S3: seed size shows that Saxegothaea has a comparatively larger seed size, followed by Microcachrys and Pherosphaera. Figure S4: Pherosphaera hookeriana and Pherosphaera fitzgeraldii.
ACKNOWLEDGEMENTS
We acknowledge Adelaide Microscopy, University of Adelaide, Australian National Botanic Gardens, Canberra, Mount Lofty Botanical Garden, SA and The Tasmanian Arboretum, Devonport. The authors are very grateful to Dr Veit Martin Dörken (University of Konstanz, Germany) for his help in the use of correct terminologies.
References
- Andruchow-Colombo A, Wilf P, Escapa IH. 2019. A South American fossil relative of Phyllocladus: Huncocladus laubenfelsii gen. et sp. nov. (Podocarpaceae), from the early Eocene of Laguna del Hunco, Patagonia, Argentina. Australian Systematic Botany 32: 290–309. [Google Scholar]
- Andruchow-Colombo W.. 1850. Note on Microcachrys, Hook. fil., and on a new allied genus of Coniferae of Van Diemen’s Land. Hooker’s Journal of Botany and Kew Garden Miscellany 2: 51. [Google Scholar]
- Axsmith BJ, Taylor TN, Taylor EL. 1998. Anatomically preserved leaves of the conifer Notophytum krauselii (Podocarpaceae) from the Triassic of Antarctica. American Journal of Botany 85: 704–713. [PubMed] [Google Scholar]
- Boyle P, Doyle J. 1953. Development in Podocarpus nivalis in relation to other podocarps: 1. Gametophytes and fertilization. Scientific Proceedings of the Royal Dublin Society 26: 179–205. [Google Scholar]
- Cantrill DJ, Falcon-Lang HJ. 2001. Cretaceous (late Albian) coniferales of Alexander Island, Antarctica. 2. Leaves, reproductive structures and roots. Review of Palaeobotany and Palynology 115: 119–145. [DOI] [PubMed] [Google Scholar]
- Carpenter RJ, Jordan GJ, Mildenhall DC, Lee DE. 2011. Leaf fossils of the ancient Tasmanian relict Microcachrys (Podocarpaceae) from New Zealand. American Journal of Botany 98: 1164–1172. [DOI] [PubMed] [Google Scholar]
- Contreras D, Duijnstee I, Ranks S, Marshall C, Looy C. 2017. Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Perspectives in Plant Ecology, Evolution and Systematics 24: 93–117. [Google Scholar]
- Dörken VM, Nimsch H, Rudall PJ. 2019. Origin of the Taxaceae aril: evolutionary implications of seed-cone teratologies in Pseudotaxus chienii. Annals of Botany 123: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doweld A, Reveal J. 1999. Validation of new suprageneric names in Pinophyta. Phytologia 84: 363–367. [Google Scholar]
- Doyle J. 1945. Developmental lines in pollination mechanisms in the Coniferales. Scientific Proceedings of the Royal Dublin Society 24: 43–62. [Google Scholar]
- Doyle J, Looby W. 1939. Embryogeny in Saxegothaea and its relation to other podocarps. Scientific Proceedings of the Royal Dublin Society 22: 127–147. [Google Scholar]
- Elliott CG. 1948a. Studies of the life histories and morphology of Tasmanian conifers. PhD thesis, University of Tasmania. [Google Scholar]
- Elliott CG. 1948b. The embryogeny of Pherosphaera hookeriana. Proceedings of the Linnean Society of New South Wales 73: 120–129. [Google Scholar]
- Fiordi AC, Lippi MM, Marini S, Tani G. 1996. Ultrastructural features of megasporogenesis in Torreya nucifera (Taxaceae). Plant Systematics and Evolution 202: 13–25. [Google Scholar]
- Florin R. 1954. The female reproductive organs of conifers and taxads. Biological Reviews 29: 367–389. [Google Scholar]
- Gaussen H. 1974. Les gymnospermes actuelles et fossiles Podocarpaces. Toulouse: Faculté des Sciences. [Google Scholar]
- Herting J, Stützel T. 2020. Morphogenesis of the seed cone of Araucaria araucana (Molina) K. Koch and the evolution of the coniferous seed scale. Flora 273: 151719. [Google Scholar]
- Herting J, Stützel T, Klaus KV. 2020. The ancestral conifer cone: what did it look like? A modern trait-evolution approach. International Journal of Plant Sciences 181: 871–886. [Google Scholar]
- Hooker JD. 1845. On the Huon Pine, and on Microcachrys, a new genus of coniferae from Tasmania: together with remarks upon the geographical distribution of that order in the southern hemisphere. The London Journal of Botany 4: 137–157. [Google Scholar]
- Klaus KV, Matzke NJ. 2020. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Systematic Biology 69: 61–75. [DOI] [PubMed] [Google Scholar]
- Knopf P, Schulz C, Little DP, Stützel T, Stevenson DW. 2012. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics 28: 271–299. [DOI] [PubMed] [Google Scholar]
- Lawson AA. 1923a. The life history of Microcachrys tetragona (Hook.). Proceedings of the Linnean Society of New South Wales 48: 177–193. [Google Scholar]
- Lawson AA. 1923b. Life history of Pherosphaera. Proceedings of the Linnean Society of New South Wales 48: 177–193. [Google Scholar]
- Leslie AB. 2011. Predation and protection in the macroevolutionary history of conifer cones. Proceedings of the Royal Society B: Biological Sciences 278: 3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AB, Losada J. 2019. Reproductive ontogeny and the evolution of morphological diversity in conifers and other plants. Integrative and Comparative Biology 59: 548–558. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu JM, Mathews S. 2017. Variation in seed size is structured by dispersal syndrome and cone morphology in conifers and other nonflowering seed plants. New Phytologist 216: 429–437. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu J, Holman G, Campbell CS, Mei W, Raubeson LR, Mathews S. 2018. An overview of extant conifer evolution from the perspective of the fossil record. American Journal of Botany 105: 1531–1544. [DOI] [PubMed] [Google Scholar]
- Looby W, Doyle J. 1939. The ovule, gametophytes and proembryo in Saxegothaea. Scientific Proceedings of the Royal Dublin Society 22: 95–117. [Google Scholar]
- Maddison W, Maddison D. 2019. Mesquite: A modular system for evolutionary analysis. Version 3. 61. 2019. [Google Scholar]
- Mendes MM, Kvaček J. 2020. Friisia lusitanica gen. et sp. nov., a new podocarpaceous ovuliferous cone from the Lower Cretaceous of Lusitanian Basin, western Portugal. Cretaceous Research 108: 104352. [Google Scholar]
- Osborn T. 1960. Some observations on the life history of Podocarpus falcatus. Australian Journal of Botany 8: 243–255. [Google Scholar]
- Peters MD. 1985. A taxonomic analysis of a middle Cretaceous megafossil plant assemblage from Queensland, Australia. PhD thesis, University of Adelaide, Australia. [Google Scholar]
- Quinn C. 1986. Embryogeny in Phyllocladus. New Zealand Journal of Botany 24: 575–579. [Google Scholar]
- Rao A. 1972. The Jurassic flora of the Rajmahal Hills. 18th Sir AC Seward Memorial Lecture. Lucknow: Birbal Sahni Institute of Palaeobotany. [Google Scholar]
- Restemeyer J. 2002. Morphologische und morphogenetische Untersuchungen zur Phylogenie und Evolution der Podocarpaceae und Phyllocladaceae. PhD thesis, Universität Bochum, Germany. [Google Scholar]
- Reymanówna M. 1987. A Jurassic podocarp from Poland. Review of Palaeobotany and Palynology 51: 133–143. [Google Scholar]
- Stiles W. 1908. The anatomy of Saxegothaea Conspicua Lindl. New Phytologist 7: 209–222. [Google Scholar]
- Stiles W. 1912. The Podocarpeae. Annals of Botany 26: 443–514. [Google Scholar]
- Taylor TN, Kerp H, Hass H. 2005. Life history biology of early land plants: deciphering the gametophyte phase. Proceedings of the National Academy of Sciences, USA 102: 5892–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RB. 1909. The megasporophyll of Saxegothaea and Microcachrys. Botanical Gazette 47: 345–354. [Google Scholar]
- Tomlinson P. 1992. Aspects of cone morphology and development in Podocarpaceae (Coniferales). International Journal of Plant Sciences 153: 572–588. [Google Scholar]
- Tomlinson P. 1994. Functional morphology of saccate pollen in conifers with special reference to Podocarpaceae. International Journal of Plant Sciences 155: 699–715. [Google Scholar]
- Tomlinson P, Braggins J, Rattenbury J. 1991. Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. American Journal of Botany 78: 1289–1303. [Google Scholar]
- Townrow JA. 1965. Notes on Tasmanian pines. I – Some lower Tertiary podocarps. In Papers and Proceedings of the Royal Society of Tasmania 99: 87–108. [Google Scholar]
- Vishnu-Mittre. 1958. Studies on the fossil flora of Nipania (Rajmahal Series) India–Pentoxyleae. Palaeobotanist 6: 31–46. [Google Scholar]
- Wilde MH. 1944. A new interpretation of coniferous cones: I. Podocarpaceae (Podocarpus). Annals of Botany 8: 1–41. [Google Scholar]
- Wilson VR, Owens JN. 1999. The reproductive biology of totara (Podocarpus totara) (Podocarpaceae). Annals of Botany 83: 401–411. [Google Scholar]
- Woltz P. 1985. Place des gymnospermes endémiques des Adnes méridionales dans la végétation du Chili. Lazaroa 8: 293–314. [Google Scholar]
- Worth JR, Marthick JR, Rossetto M, Cohen J, Bourke G, Jordan GJ. 2018. Development of 15 nuclear EST microsatellite markers for the paleoendemic conifer Pherosphaera hookeriana (Podocarpaceae). Applications in Plant Sciences 6: e01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lin L, Ferguson DK. 2015. Parallel evolution of leaf morphology in gnetophytes. Organisms Diversity & Evolution 15: 651–662. [Google Scholar]
- Young MS. 1910. The morphology of the Podocarpineae. Botanical Gazette 50: 81–100. [Google Scholar]
- Yu Y, Blair C, He X. 2020. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Molecular Biology and Evolution 37: 604–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.