Abstract
Acinetobacter baumannii is an opportunistic bacterial pathogen that causes severe infections in immunocompromised patients. The emergence of multi- and pan-drug resistant strains of A. baumannii from clinical sources has confounded treatment and enhanced morbidity and mortality associated with these infections. One way that A. baumannii circumnavigates environmental and antimicrobial challenge is by forming tertiary architectural structures of cells known as biofilms. Biofilm-inhibiting molecules could be deployed as a potential chemotherapeutic strategy to inhibit or disrupt A. baumannii biofilms and mitigate adverse outcomes due to infection. Lactoferrin is an innate immune glycoprotein produced in high concentrations in both human and bovine milk which has previously been shown to have antibacterial and anti-biofilm activities. We sought to test lactoferrin against a bank of clinical isolates of A. baumannii to determine changes in bacterial growth or biofilm formation. Our results indicate that human lactoferrin has slightly more potent antibacterial activities than bovine lactoferrin against certain strains of A. baumannii and that these effects are associated with anatomical site of isolation. Additionally, we have shown that both bovine and human lactoferrin can inhibit A. baumannii biofilm formation and that these effects are associated with anatomical site of isolation and whether the strain forms robust or weak biofilms.
Keywords: Acinetobacter baumannii, biofilm, antimicrobial peptides, glycoproteins, innate immunity
Acinetobacter baumannii is a gram-negative bacterium which causes opportunistic and nosocomial infections in immunocompromised hosts and military personnel with battlefield wounds1. A. baumannii causes a wide variety of diseases including sepsis, meningitis, skin and soft-tissue infections, and pneumonia2. Specifically, hospital-acquired pneumonia is one of the most common clinical manifestations of A. baumannii infections. It occurs commonly in patients who are mechanically ventilated in intensive care unit facilities and is, therefore, frequently referred to as “ventilator-associated pneumonia” (VAP3). A recent meta-analysis of 126 studies across 29 countries determined that A. baumannii is associated with 80% of all hospital-acquired pneumonia and VAP cases worldwide, indicating that A. baumannii is a huge global burden on hospitalized and ventilated patients4. The emergence of SARS-CoV-2 and the resultant CoVID-19 pandemic has increased the risk of hospitalized patients developing VAP caused by opportunistic bacterial pathogens including A. baumannii 5–9. The absence of a commercially available vaccine combined with emerging multi- and pan-drug resistant strains are contributing factors which hamper effective control of this infectious pathogen10. The limited options of antibiotic chemotherapeutics for these infections, coupled with the shortage of antibiotics in the development pipeline, have prompted the Centers for Disease Control and Prevention to label A. baumannii an urgent threat; the highest threat designation for bacterial pathogens11. Thus, new antimicrobial strategies are needed against A. baumannii and targeting pathways essential for bacterial virulence or survival in the host or hospital environments could prove useful for the chemotherapeutic strategies of the future.
Almost all bacterial pathogens require nutrient iron to survive and proliferate. As such, approaches to sequester or limit iron from entering the bacterial cell are promising therapeutic strategies. We recently reported that lactoferrin, an iron-binding glycoprotein that is highly abundant in innate immune cells such as neutrophils and human milk, has antimicrobial and anti-biofilm activities against the gram-positive pathogen, Streptococcus agalactiae or Group B Streptococcus (GBS)12. Lactoferrin is a 76 to 80 kDa glycosylated protein comprised of two lobes designated the N-lobe (amino acids 1–333) and the C-lobe (amino acids 345–692)13. Each lobe consists of two subdomains, (designated N1 and N2 in the N-lobe and C1, C2 in the C-lobe), harboring one iron binding site, and one glycosylation site within each lobe. The degree of glycosylation of the protein varies and therefore the molecular weight of lactoferrin varies between 76 and 80 kDa. Additionally, the stability of lactoferrin varies with glycosylation state. Each lactoferrin molecule can reversibly bind one iron molecule in each of two discrete binding sites. The binding sites are comprised of two tyrosine residues, one histidine residue and one aspartic acid residue coordinated with two carbonate or bicarbonate ions. Lactoferrin’s binding affinity for iron is 300 times higher than that of transferrin, making it one of the innate immune system’s most effective iron chelators14. Because of this, we postulated that lactoferrin could be an effective antimicrobial strategy against A. baumannii, including strains which are multi-drug resistant.
Results
Analyses of bacterial growth in increasing concentrations of bovine or human lactoferrin.
Lactoferrin has antimicrobial activity against a variety of microbial pathogens, thus its potential as a chemotherapeutic has been proposed, especially against bacteria which are resistant to multiple classes of antibiotics15–17. Bovine lactoferrin has been extensively studied due to the abundance of bovine milk as a purification source, however, bovine lactoferrin and human lactoferrin have structural and functional differences which could contribute to variations in antimicrobial activity. To this end, we sought to test the antimicrobial activity of freshly purified lactoferrin from human milk and compare this to commercially available lactoferrin purified from bovine milk against a bank of strains of A. baumannii from diverse anatomical sources with varying antibiotic susceptibility profiles (Table 1). Analyses of A. baumannii growth in response to exposure to increasing concentrations of bovine or human lactoferrin revealed a dose-dependent antimicrobial effect that varies across strains and lactoferrin isoforms (Figure 1). In A. baumannii strains isolated from wounds, human lactoferrin significantly inhibited bacterial growth at concentrations of 250 μg/mL and above (***P<0.001 and ****P<0.0001, respectively, one-way ANOVA, Figure 1A). Similarly, bovine lactoferrin inhibited the growth of wound-isolated A. baumannii at concentrations of 250 μg/mL and above (*P<0.05 and ****P<0.0001, respectively, one-way ANOVA, Figure 1B). In A. baumannii strains isolated from sputum collected from patients with respiratory infections, human lactoferrin significantly inhibited bacterial growth at concentrations of 250 μg/mL and above (P<0.001 and P<0.0001, respectively, one-way ANOVA, Figure 1C). Conversely, these strains were less susceptible to bovine lactoferrin’s antimicrobial activity, which inhibited growth at higher concentrations than the human isoform, 500 μg/mL and above (****P<0.0001, one-way ANOVA, Figure 1D). A. baumannii isolates derived from blood were highly susceptible to the antimicrobial activity of human lactoferrin, with concentrations as low as 62.5 μg/mL inhibiting bacterial growth significantly (P<0.05, one-way ANOVA, Figure 1E). Conversely, inhibition of blood isolates of A. baumannii by bovine lactoferrin required concentrations of 250 μg/mL and above (P<0.01 and P<0.0001, respectively, one-way ANOVA, Figure 1F). Isolates of A. baumannii derived from urinary tract or abdominal cavity infections were highly susceptible to human and bovine lactoferrin, with growth inhibition observed at 62.5 μg/mL (P<0.05 and P<0.01, respectively, one-way ANOVA) 125 μg/mL (P<0.01 and P<0.05, respectively, one-way ANOVA) and above (P<0.0001, one-way ANOVA, Figure 1G–H). Interestingly, supplementation with 250 μM ferric chloride in the media or saturation of the lactoferrin preparations to the holo-isoform abrogated the antimicrobial activity of both human and bovine lactoferrin against A. baumannii (Figure S1 and Figure S2), underscoring the important role of lactoferrin-dependent iron chelation in this phenotype.
Table 1.
A. baumannii clinical strains, isolation source, antibiotic resistance, and effect of bovine or human lactoferrin on growth or biofilm
| Strain ID | Source | AntibioticR profile | Growth MIC (Bovine Lf) | Growth MIC (Human Lf) | Biofilm Reduction (Bovine Lf) | Biofilm Reduction (Human Lf) |
|---|---|---|---|---|---|---|
| 4 | bronchial wash | A/SI, AK, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TEI, TIM | 62.5 μg/mL * | 62.5 μg/mL * | — | — |
| 5 | abdominal cavity | — | 62.5 μg/mL ** | 62.5 μg/mL**** | 41% * | 62% * |
| 11 | urine | A/SI, CAX, CAZ, CFT, CP, LVX, MERI, PI, T/S, TE, TIM | 250 μg/mL * | 125 μg/mL ** | — | — |
| 13 | blood | A/SI, AK, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TEI, TIM, TO | 125 μg/mL * | 125 μg/mL *** | 77% * | 75% * |
| 15 | blood | A/S, AKI, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TIM, TO | 250 μg/mL *** | 62.5 μg/mL**** | 72% * | 68% * |
| 16 | sputum | CAX, CAZ, CFT, CP, CPEI, GM, LVX, PI, T/S, TEI, TIMI, TOI | 250 μg/mL * | 125 μg/mL * | 32% * | — |
| 17 | blood | AKI, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TEI, TIM, TO | 62.5 μg/mL * | 62.5 μg/mL ** | 50% * | 70% * |
| 19 | wound | CAXI, CFTI | <1000 μg/mL | <1000 μg/mL | — | 41% * |
| 31 | sputum | A/S, AK, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TE, TIM, TO | 250 μg/mL * | 125 μg/mL ** | 53% * | — |
| 32 | Foley catheter | A/SI, CAX, CAZ, CFT, CP, CPE, GM, LVX, MER, PI, T/S, TEI, TIM, TO | 750 μg/mL**** | 250 μg/mL ** | — | — |
| 35 | sputum | — | 750 μg/mL ** | 125 μg/mL * | 67% * | 76% * |
| 37 | wound | A/SI, AK, CAX, CAZ, CFT, CP, CPE, GM, LVXI, MER, PI, T/S, TEI, TIM, TO | 250 μg/mL * | 750 μg/mL ** | — | 68% * |
| 38 | sputum | A/SI, CAX, CAZ, CFT, CP, CPE, GM, LVXI, MER, PI, T/S, TIM, TO | 750 μg/mL *** | 750 μg/mL *** | — | 34% * |
| 79 | sputum | — | <1000 μg/mL | <1000 μg/mL | — | 38% * |
| 81 | wound | — | 62.5 μg/mL ** | 125 μg/mL * | — | — |
| 101 | wound | — | 250 μg/mL * | 125 μg/mL * | 42% * | 70% * |
| 112 | sputum | A/S, CAX, CAZ, CFT, CP, CPE, GM, LVX, T/S, TE, TO | 750 μg/mL *** | 250 μg/mL **** | 56% * | 84% * |
Key terms and symbols. A. baumannii strain identification (Strain ID). Source of isolation from clinical patient (Source). Antibiotic abbreviations are as follows: Ampicillin-Sulbactam (A/S), amikacin (AK), ceftriaxone (CAX), ceftazidime (CAZ), cefotaxime (CFT), ciprofloxacin (CP), cefepime (CPE), gentamicin (GM), levofloxacin (LVX), meropenem (MER), piperacillin (PI), trimethoprim-sulfamethoxazole (T/S), tetracycline (TE), ticarcillin-K clavulanate (TIM), and tobramycin (TO). Intermediate resistance is designated with a superscript (I). Minimum inhibitory concentration (MIC) was defined as the lowest concentration at which statistically significant inhibition of growth was observed, and biofilm reduction was defined as statistically significant inhibition of bacterial biofilm, determined by one-way one-way ANOVA compared to bacterial grown in medium alone (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). For MIC analyses, bacteria were grown in increasing concentrations of either bovine lactoferrin (Bovine Lf) or human lactoferrin (Human Lf). For biofilm assays, bacteria were grown in medium alone or medium supplemented with 125 μg/mL Bovine Lf or Human Lf and percent calculated is the mean reduction in biofilm formation compared to cultures grown in medium alone.
Figure 1.
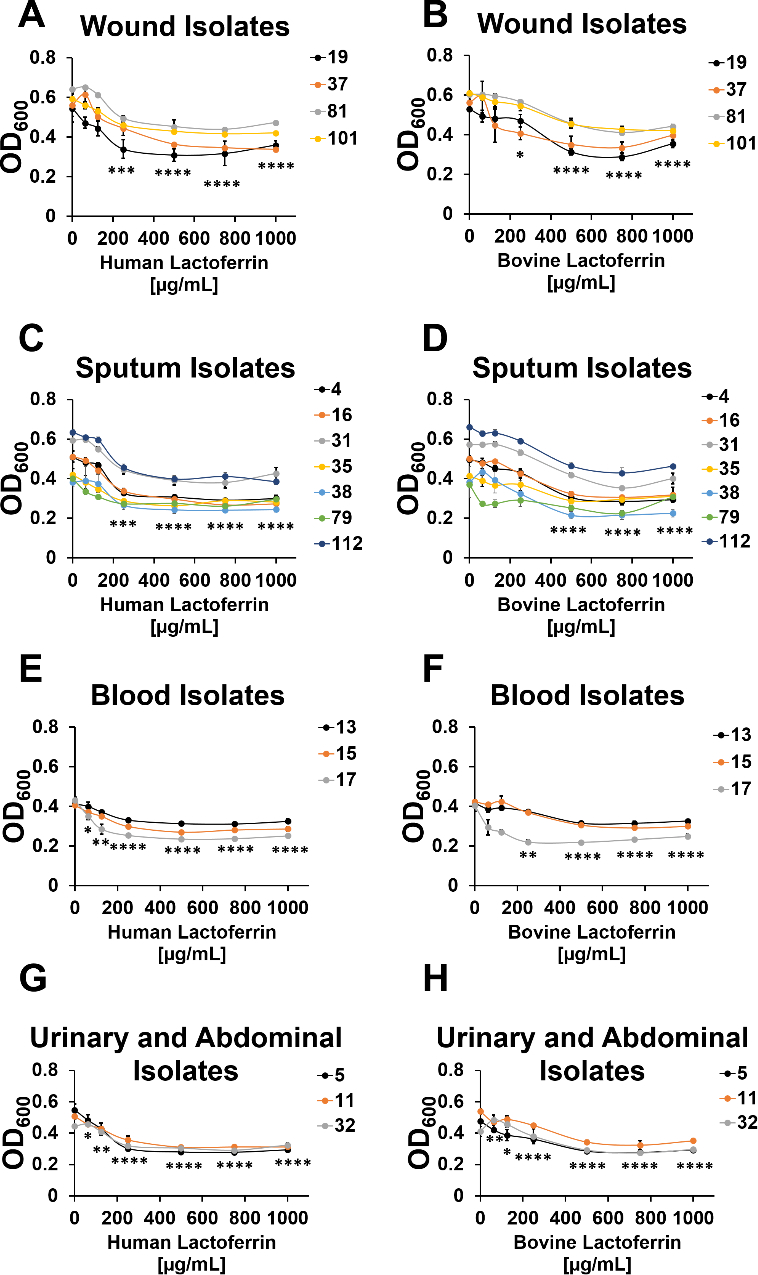
Analyses of bacterial growth in increasing concentrations of bovine or human lactoferrin. Bacterial growth was assessed at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were grown in increasing concentrations of human (A, C, G, E) or bovine lactoferrin (B, D, F, H). A. baumannii strains tested were isolated from wounds (A-B), sputum (C-D), blood (E-F), or urinary tract or abdominal cavity infections (G-H). Significant inhibition of bacterial growth compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n=3.
Analysis of the effect of bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii
Previous work from our lab and others has shown that in addition to antimicrobial activity, lactoferrin also has anti-biofilm activity against a wide range of pathogens and commensals18–21. Additionally, our previous work demonstrated that clinical A. baumannii strains isolated from different anatomical sites exhibited significant differences in biofilm formation22. We hypothesized that these differences could contribute to differential responses in lactoferrin’s ability to alter A. baumannii biofilm formation. To test this, we grew A. baumannii strains from the aforementioned bank of clinical isolates statically to promote bacterial biofilm formation. Strains were grown overnight in medium alone or medium supplemented with 125 μg/mL of lactoferrin isolated from either human or bovine milk. This concentration was selected because it is within the physiological range encountered in vivo. Our growth data suggest this concentration is sufficient to perturb bacterial growth for many strains tested (11/17) but the inhibition of growth was less than 50% (and often less than 25%). The following day, biofilms were quantified by colorimetric assay. Results indicate that 13 out of 17 strains exhibited significant reduction in biofilms when exposed to either human or bovine lactoferrin, and the level of this inhibition varied across strain types (Table 1).
Among A. baumannii isolates derived from wound infections, 75% of strains tested showed significant inhibition of biofilm formation by either human or bovine lactoferrin (Figure 2). Strains 19, 37, and 101 exhibited 41%, 68%, and 70% decrease in biofilm formation, respectively, when exposed to human lactoferrin, results that were statistically significant (*P<0.05, one-way ANOVA). Only one isolate, strain 101, exhibited a significant decrease (42%) in biofilm formation when exposed to bovine lactoferrin (*P<0.05, one-way ANOVA). Interestingly, only one strain isolated from wounds (strain 81, one of the weakest biofilm-forming strains) did not exhibit significant changes in biofilm formation in response to lactoferrin.
Figure 2.
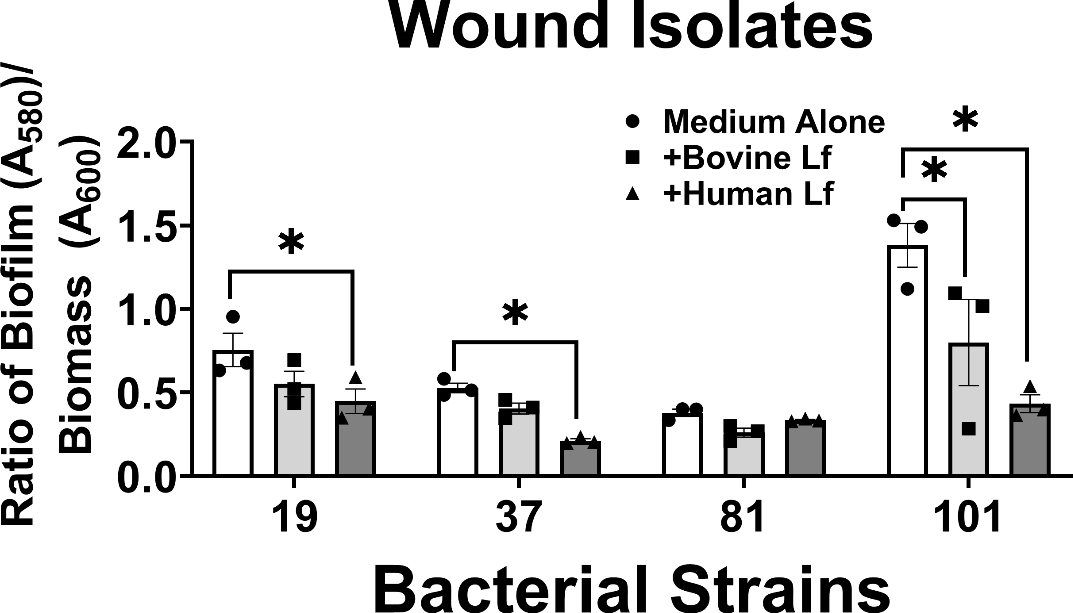
Analysis of the effect of 125 μg/mL bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii isolated from wounds (strains 19, 37, 81, and 101). Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, n=3.
The results revealed that in A. baumannii isolates derived from sputum collected from patients suffering from respiratory tract infections, 86% of strains tested (6/7 strains) showed significant inhibition of biofilm formation by either human or bovine lactoferrin (Figure 3). Strains 16, 31, 35, and 112 exhibited 32%, 53%, 67%, and 56% decrease in biofilm formation, respectively, when exposed to bovine lactoferrin; results that were statistically significant (*P<0.05, one-way ANOVA). Strains 35, 38, 79, and 112 exhibited 76%, 34%, 38%, and 84% decrease in biofilm formation, respectively, when exposed to human lactoferrin; results that were statistically significant (*P<0.05, one-way ANOVA). Interestingly, only one strain isolated from sputum (strain 4, one of the weaker biofilm-forming strains) did not exhibit significant changes in biofilm formation in response to lactoferrin.
Figure 3.
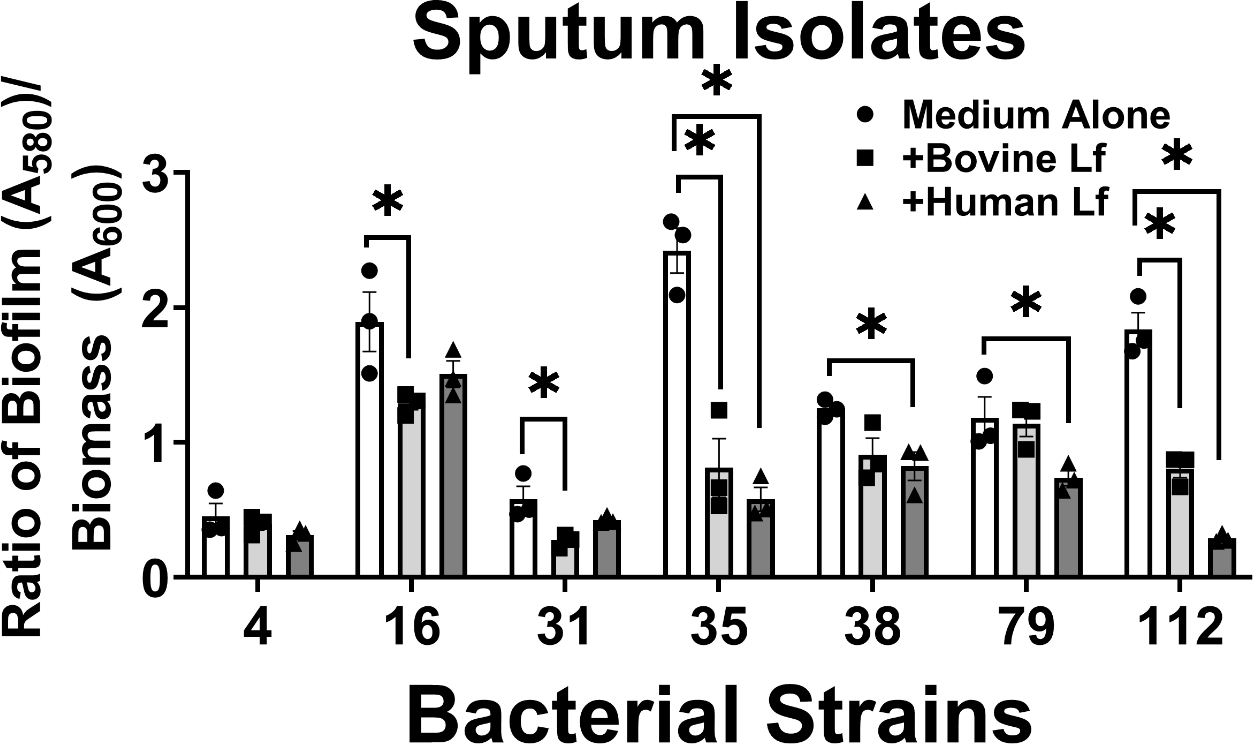
Analysis of the effect of bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii isolated from sputum (strains 4, 16, 31, 35, 38, 79, and 112). Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, n=3.
Among A. baumannii isolates derived from patient blood samples, 100% of strains tested (3/3 strains) showed significant inhibition of biofilm formation by both human and bovine lactoferrin (Figure 4). Strains 13, 15, and 17 exhibited 75%, 72%, and 50% decrease in biofilm formation, respectively, when exposed to bovine lactoferrin and 77%, 68%, and 70% decrease in biofilm formation, respectively, when exposed to human lactoferrin; results which were statistically significant (*P<0.05, one-way ANOVA). It is pertinent to note that all three strains isolated from patient blood are strong biofilm formers in vitro, a phenotype consistent with our previously published results22.
Figure 4.
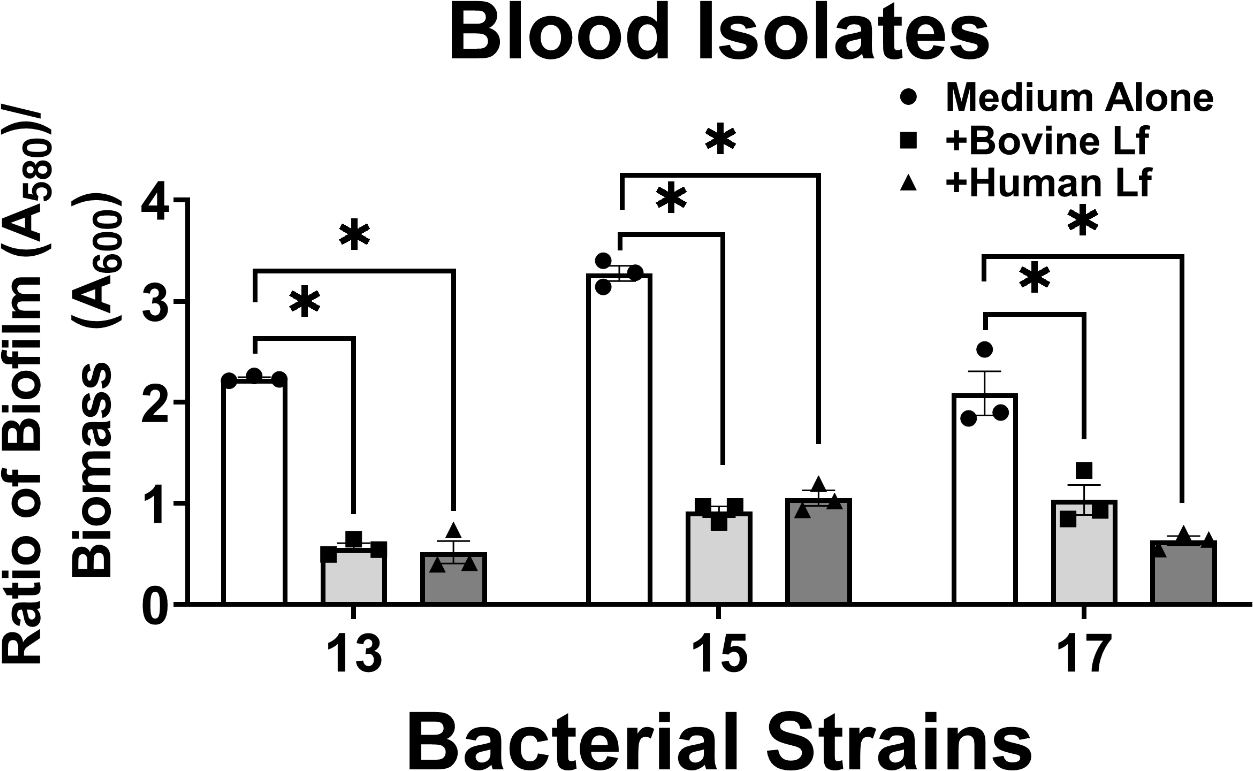
Analysis of the effect of bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii isolated from blood (strains 13, 15, and 17). Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, n=3.
Among A. baumannii isolates derived from urinary tract or abdominal cavity infections, 33% of strains tested (1/3 strains) showed significant inhibition of biofilm formation by lactoferrin (Figure 5). Strain 5 exhibited 41% and 62% decrease in biofilm formation when exposed to bovine or human lactoferrin, respectively; results which were statistically significant (*P<0.05, one-way ANOVA). It is noteworthy that the two strains in this cohort which were not affected by lactoferrin exposure were observed to be weak biofilm formers in vitro, a phenotype consistent with our previously published results22.
Figure 5.
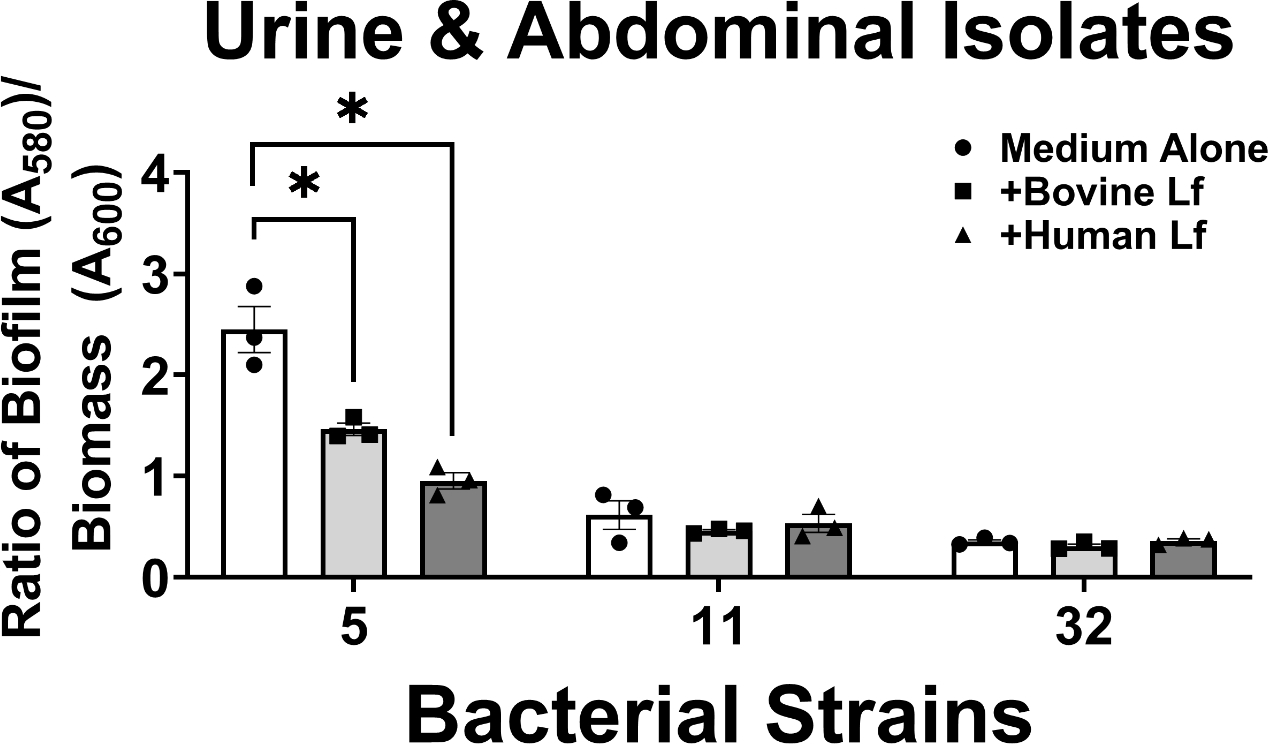
Analysis of the effect of bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii isolated from urinary tract or abdominal cavity infections (strains 5, 11, and 32). Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, n=3.
In order to determine if the iron-chelation activity of lactoferrin was also critical for the observed perturbations of A. baumannii biofilm formation, strains were grown in medium supplemented with 250 μM ferric chloride, an excess concentration that was sufficient to overcome the chelation activity. Interestingly, the addition of 250 μM ferric chloride to the media or saturation of the lactoferrin molecule with iron to create holo-isoforms abrogated the anti-biofilm activity of both human and bovine lactoferrin (Figure S2 and Figure S3), again, highlighting the important role of lactoferrin-dependent iron chelation in lactoferrin’s anti-biofilm activity.
Analysis of the effect of bovine or human lactoferrin on A. baumannii strains with respect to anatomical isolation site.
Because the incidence of susceptibility to lactoferrin-dependent inhibition of biofilm formation across A. baumannii clinical isolates varied across strain types, we hypothesized that isolation source could be a potential predictor for susceptibility to lactoferrin-association biofilm inhibition. To test this, we analyzed the effect of lactoferrin (either human or bovine) on biofilm formation based on anatomical site of isolation of the strain of A. baumannii. Results indicated that blood isolates exhibit the largest decrease in biofilm formation when exposed to bovine or human lactoferrin (66% and 81%, respectively), followed by sputum isolates which exhibit the second largest decrease in biofilm formation when exposed to bovine or human lactoferrin (42% and 52%, respectively) (Figure 6). Among urine and abdominal infection isolates, only human lactoferrin significantly inhibited biofilm formation by 46%, a result that was not observed with bovine lactoferrin. Collectively, biofilm formation by wound isolates was not significantly impacted by either human or bovine lactoferrin.
Figure 6.
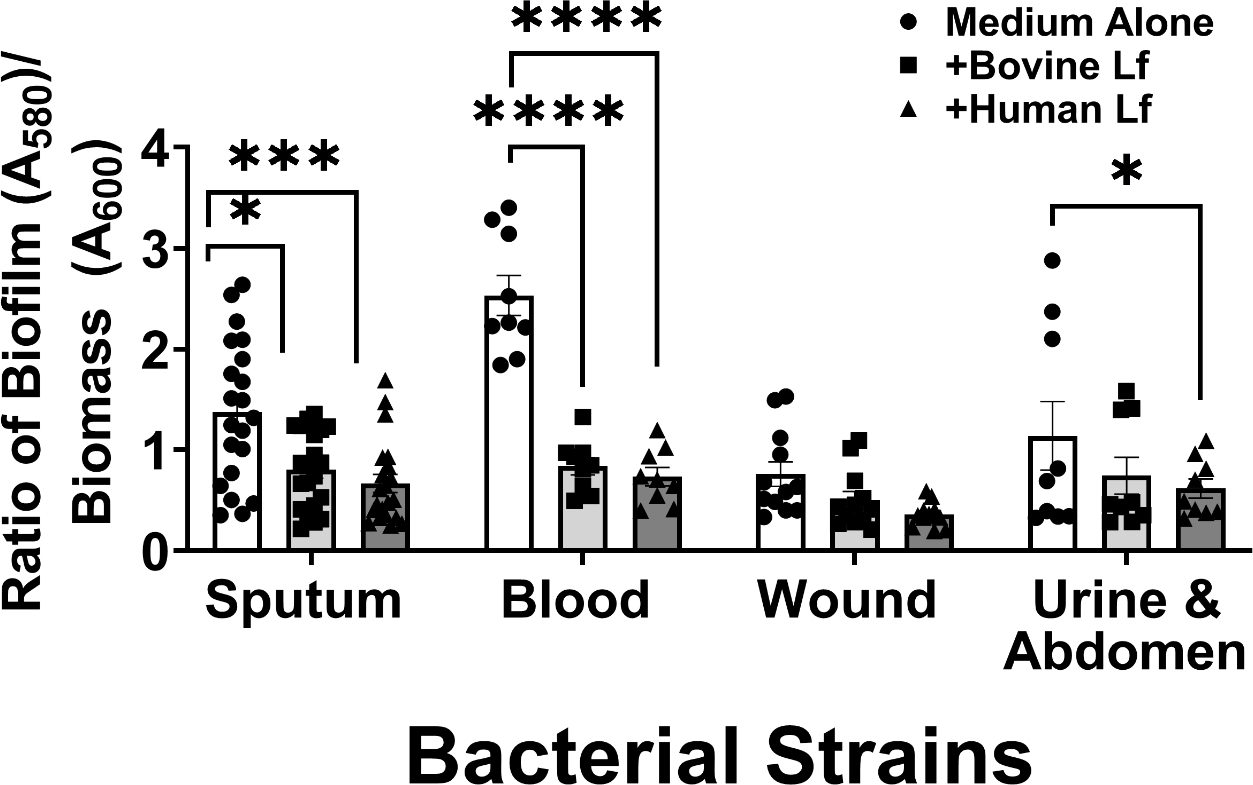
Analysis of the effect of bovine or human lactoferrin on biofilm formation by clinical strains of A. baumannii based on isolation source (sputum, blood, wound, urinary tract & abdominal infections). Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, n=3.
Analysis of the effect of bovine or human lactoferrin on high vs. low biofilm forming strains of A. baumannii
As we observed that strains which formed more biofilm were more likely to experience biofilm inhibition by lactoferrin, we hypothesized that strong biofilm formation could be a potential predictor for susceptibility to lactoferrin-associated inhibition of biofilm. To test this, we calculated the geometric mean of biofilm formed by all clinical isolates in medium alone and imposed this as a cutoff (geometric mean across all strains ratio of biofilm OD560/biomass OD600 = 1.0) to delineate between high and low biofilm formers. Upon binning the strain specific biofilm values into these two designations, we applied a one-way ANOVA to ensure that the phenotypes observed across these designations were significantly different in medium alone (P<0.0001). We then analyzed the effect of either human or bovine lactoferrin on clinical isolates of A. baumannii based on these criteria. Results in Figure 7 indicate that high biofilm forming strains of A. baumannii exhibit a 55% decrease in biofilm formation in response to exposure to bovine lactoferrin (P<0.0001, one-way ANOVA) and a 74% decrease in biofilm formation in response to exposure to human lactoferrin (P<0.0001, one-way ANOVA). Low biofilm forming strains of A. baumannii do not exhibit significant changes in biofilm formation in response to either human or bovine lactoferrin.
Figure 7.
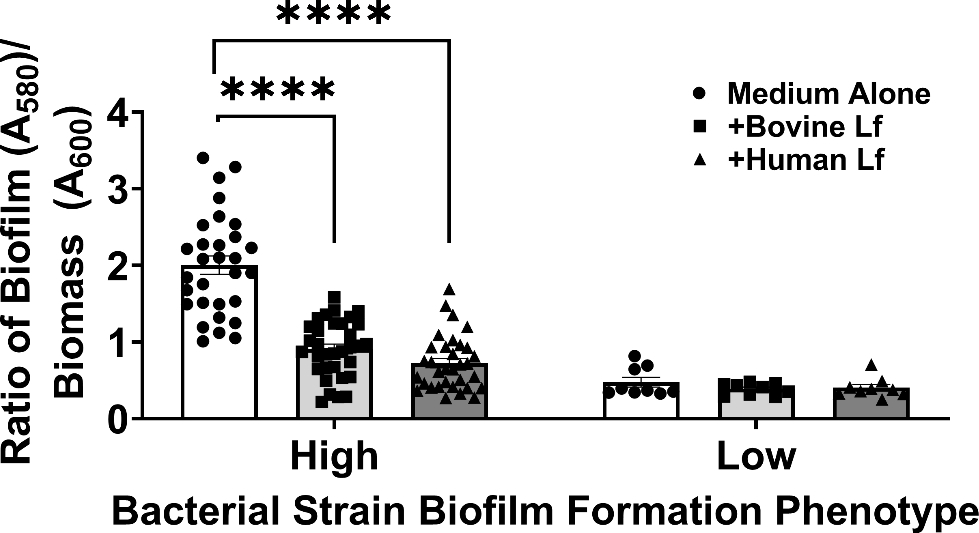
Analysis of the effect of bovine or human lactoferrin on high vs. low biofilm forming strains of A. baumannii. Strains with biofilm quantification values above the geometric mean (1.0) were designated “High” biofilm formers. Strains with biofilm quantification values below the geometric mean (1.0) were designated “Low” biofilm formers. Bacterial biomass was measured at 24 hours post-inoculation by spectrophotometric measurement of optical density at 600 nm (OD600). Cultures were decanted, washed, and adherent biofilms were stained with crystal violet. Biofilm was quantified by solubilizing crystal violet in an 80%/20% ethanol: acetone solution and evaluation at 560 nm (OD560). Cultures were grown in medium alone (Medium Alone, designated by white bars and circle points) or medium supplemented with 125 μg/mL of either bovine lactoferrin (Bovine Lf, designated by light gray bars and square points) or human lactoferrin (Human Lf, designated by dark gray bars and triangle points). Points indicate individual experiments, bars indicate mean +/− SEM. Significant inhibition of bacterial biofilm compared to medium alone negative control was determined by one-way ANOVA with Tukey’s post-hoc test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n=3.
Analysis of iron associated with human and bovine lactoferrin samples
Because our previous work has demonstrated that the antimicrobial activity of lactoferrin is associated with its capacity to bind and chelate iron, we hypothesized that differences in lactoferrin-associated iron might contribute to differential antimicrobial activities between human and bovine lactoferrin in our studies. To test this, purified lactoferrin from human breast milk, as well as commercially-available bovine milk lactoferrin were subjected to ICP-MS analysis to enumerate the iron ions associated with 100 μg of sample (Figure 8). Freshly purified fractions of lactoferrin from expressed human breast milk (Human Lf) exhibited a mean value of 12 parts per billion (PPB) of iron (Fe) ions, while commercially-sourced bovine lactoferrin (Bovine Lf) preparations exhibited a mean value of 88 PPB of Fe ions, results that were statistically significant (P<0.05, Student’s t test with Welch’s correction). Additionally, iron-saturation to yield holo-isoforms resulted in enhanced iron associated with human lactoferrin (P<0.0001, One Way ANOVA) and bovine lactoferrin (P=0.046, unpaired Student’s t test). Together, these results indicate that commercially-sourced bovine lactoferrin is significantly more iron-saturated than the freshly purified human breast milk isoform which is largely in the apo- isoform.
Figure 8. ICP-MS elemental analysis of lactoferrin preparations.
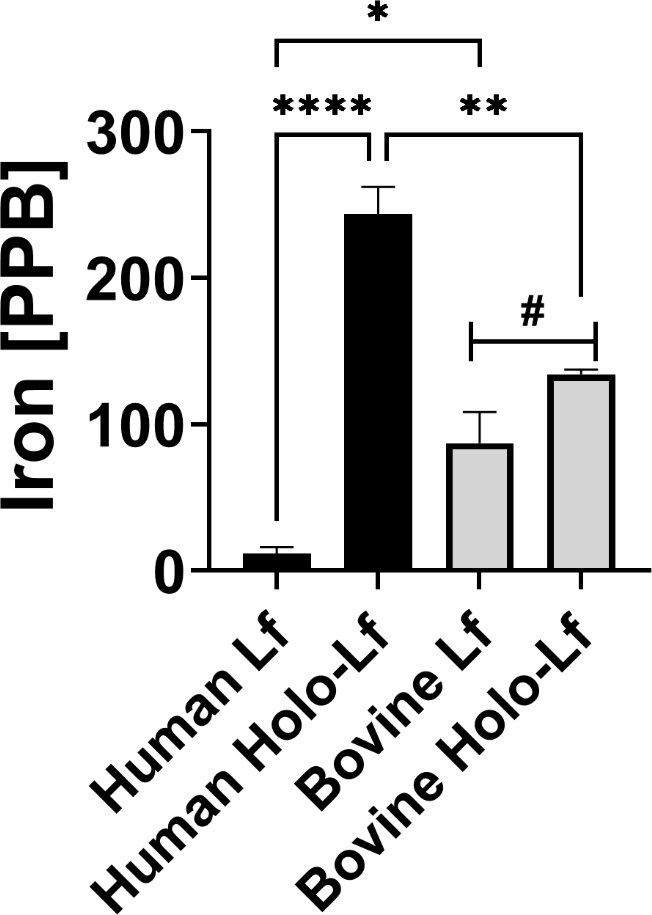
Freshly purified fractions of lactoferrin from expressed human breast milk (Human Lf, black bars), or commercially-sourced bovine lactoferrin preparations (Bovine Lf, grey bars), as well as iron-saturated isoforms of each respective preparation (holo-Lf) were subjected to ICP-MS elemental analysis to enumerate the iron (Fe) ions associated with 100 μg of sample. Bars indicate mean values of at least three biological replicates +/−SEM. *P<0.0001, **P<0.001, *P<0.05, one-way ANOVA with Tukey’s post-hoc test, #P<0.05, unpaired Student’s t test.
Discussion
A survey of clinical isolates of A. baumannii from Nashville, Tennessee revealed that antibiotic resistance to the following drugs was prevalent in most strains: ampicillin-sulbactam, amikacin, ceftriaxone, ceftazidime, cefotaxime, ciprofloxacin, cefepime, gentamicin, levofloxacin, meropenem, piperacillin, trimethoprim-sulfamethoxazole, ticarcillin- K clavulanate, tetracycline, and tobramycin22. A. baumannii isolated from wounds exhibited higher motility than strains isolated from blood, urine or Foley catheter, or sputum/bronchial wash. Blood-isolated A. baumannii strains formed significantly more biofilm than isolates from wounds, sputum or bronchial wash samples22. An inverse relationship between motility and biofilm formation was observed in the cohort of 17 clinical isolates of A. baumannii tested in this study22. Motility was also inversely correlated with induction of hemolysis22. An inverse correlation was observed between hemolysis and resistance to ticarcillin-k clavulanate, meropenem, and piperacillin22. An inverse correlation was also observed between motility and resistance to ampicillin-sulbactam, ceftriaxone, cefotaxime, ceftazidime, ciprofloxacin, or levofloxacin22. Together, this work demonstrated that strain-dependent variations in biofilm and motility are associated with anatomical site of isolation, and that there are cross-relationships between the regulation of virulence and antimicrobial resistance pathways22. To build on this study, we sought to determine if the antimicrobial glycoprotein lactoferrin could have utility in altering A. baumannii growth or biofilm formation. Specifically, the purpose of the current study was to analyze the utility of either bovine or human lactoferrin against a diverse collection of clinical strains of A. baumannii from varying isolation sources. We hypothesized that individual strain virulence phenotypes (such as biofilm formation) or anatomical site of isolation might contribute to the efficacy of lactoferrin as an antimicrobial strategy.
The emergence of multi- and pan-drug resistant A. baumannii strains, in particular strains resistant to carbapenems, pose a serious threat to hospitalized patients23. The need for novel treatment options is urgently required, and antimicrobial peptides and glycoproteins with selective toxicity against antibiotic-resistant bacteria are promising candidates. Lactoferrin, an antimicrobial glycoprotein that is abundant in human and bovine milk, innate immune cell granules, and mucus, has been shown to have wide antimicrobial activity against a variety of bacteria, fungi, parasites, and viruses, including the novel coronavirus SARS-CoV-224. Seminal work by Dijkshoorn et al., has shown that the lactoferrin-derived peptide hLF(1–11) was highly bactericidal against five multi-drug resistant strains of A. baumannii in vitro, leading to a 3–4 log reduction in bacterial viability25. Similarly, results published by de Léséleuc and colleagues has revealed that bovine and murine sera have antimicrobial effects which are bypassed by some A. baumannii strains via a heme oxygenase gene which facilitates heme consumption as an iron source to circumnavigate the potential iron restriction within sera (likely contributed by transferrin and lactoferrin)26. Mahdi and colleagues further refined these studies by purifying lactoferrin and lactoperoxidase from camel colostrum and applying this to multi-drug resistant A. baumannii. Their results indicate that camel colostrum lactoferrin can exert an antimicrobial effect against A. baumannii in a dose-dependent fashion, and that the combination of lactoferrin and lactoperoxidase can ameliorate A. baumannii infections in vivo27. Additional work by Kamoshido et al. revealed that susceptibility to colistin was inversely related to resistance to lactoferrin in A. baumannii. Specifically, loss of lipopolysaccharide (LPS) production due to colistin resistance selection enhanced the susceptibility of the type strain A. baumannii 19606T to human lactoferrin, implicating LPS as a potential barrier against lactoferrin challenge in this specific strain which survived 1000 μg/mL lactoferrin challenge28. Our work expands on these studies, leveraging lactoferrin from milk sources such as human milk and the more abundant bovine milk and a larger bank of A. baumannii isolates from diverse anatomical isolates and antimicrobial susceptibility profiles. Our work indicates that 15 out of 17 strains were susceptible to growth inhibition by either bovine or human lactoferrin, highlighting the broad antimicrobial activity that these molecules exert against clinical strains of A. baumannii. Human lactoferrin had a lower MIC than bovine lactoferrin for 8 strains of A. baumannii, 6 of which were MDR, in our study. Conversely, bovine lactoferrin had a lower MIC for 2 strains (one MDR and one susceptible strain) compared to human lactoferrin, underscoring strain variability with respect to susceptibility to these isoforms of lactoferrin.
In addition to its ability to inhibit growth, lactoferrin has been demonstrated to possess anti-biofilm activities against a wide range of commensal and pathogenic microorganisms. Lactoferrin has been shown to inhibit bacterial biofilms in the opportunistic pathogen Pseudomonas aeruginosa at concentrations well beneath those that inhibit bacterial growth18. Human lactoferrin has potent anti-biofilm and anti-adhesion properties against intestinal symbionts such as Bacteriodes fragilis and Bacteriodes thetaiotamicron19. Additionally, bovine lactoferrin, and its cognate peptides which can be generated by hydrolysis, have been established as an effective anti-biofilm molecule against several bacteria including Actinobacillus pleuropneumoniae, Listeria monocytogenes, Escherichia coli, P. aeruginosa, Pseudomonas fluorescens, and Streptococcus mutans20,21,29–31. We are the first to demonstrate the anti-biofilm activity of human and bovine lactoferrins against clinical A. baumannii strains, and the first to report strain heterogeneity in this pathogen’s response to both human and bovine isoforms of this important antimicrobial glycoprotein.
Human and bovine lactoferrin possess very high sequence similarity, however, differences between these isoforms within in vitro and in vivo studies have been observed. A recent study by Rosa and colleagues has attributed differences in function and efficacy of human and bovine lactoferrin to physico-chemical properties such as glycosylation state, thermostability, resistance to proteolysis, iron-saturation of the molecule, and cross-metalation by aluminum, copper, magnesium, manganese, and zinc, which are chelated by lactoferrin at lower affinities32. Indeed, our results mirror those previously described as we observed that the commercially available bovine lactoferrin we utilized had overall lower antimicrobial and antibiofilm activity against the bank of 17 strains of A. baumannii we tested, compared to the freshly purified human milk lactoferrin samples. Elemental analyses revealed that the bovine isoform was partially saturated with iron; which could ultimately hamper iron-chelation-dependent antimicrobial activity. Conversely, our fresh preparations of human milk lactoferrin were comprised of apo-isoforms of the protein which likely contributed to the heightened antimicrobial activity this glycoprotein has against A. baumannii.
The utility of antimicrobial peptides as an alternative class of antibiotics is an area of increasing interest. Unlike antibiotics currently in use, bacteria are less effective at developing resistance to antimicrobial peptides; a result that is likely due to co-evolutionary forces at play during the host-pathogen interaction33,34. To that end, lactoferrin and its cognate peptides such as lactoferricin have been used as effective therapies to treat urinary tract infections, as an oral treatment for irritable bowel syndrome due to its normalizing effects on the gut microbiome35–39. Additionally, lactoferrin antimicrobial peptides have been shown to have utility against four strains of MDR A. baumannii in an experimental infection in mice, with treatment leading to a 2–3 log reduction in bacterial burden in vivo25. Our work refines the existing literature to provide a clear rationale for the potential utility of apo-lactoferrin against a broad bank of A. baumannii strains from diverse anatomical isolation sources.
Conclusions
In conclusion, we report that physiologically relevant concentrations of either human or bovine lactoferrin have the capacity to inhibit the growth of most clinical isolates of A. baumannii tested. Additionally, both human and bovine lactoferrin can inhibit biofilm formation by a variety of A. baumannii strains at concentrations well beneath MIC50 for growth. While human lactoferrin tended to show greater statistically significant differences from control than bovine, there were no statistically significant differences between results obtained from the two forms. Furthermore, we report that freshly isolated human milk lactoferrin exhibits antimicrobial and anti-biofilm effects and that these effects vary across A. baumannii isolates from diverse anatomical origin and varying phenotypes with respect to antibiotic susceptibilities. Specifically, human lactoferrin significantly inhibited biofilm formation from strains isolated from sputum, blood, urinary tract or abdominal infections, whereas bovine lactoferrin was only able to significantly inhibit biofilm formation in strains isolated from sputum or blood. Interestingly, wound-isolated A. baumannii strains’ biofilm was not significantly impacted by human or bovine lactoferrin and were previously identified as being weak biofilm formers22. Analysis of low versus high biofilm forming phenotypes revealed that this was the strongest predictor for the efficacy of lactoferrin in inhibiting biofilms, indicating cellular processes required for development of robust biofilm formation are likely the target of the antimicrobial activity of lactoferrin. Our work underscores the potential utility of the antimicrobial glycoprotein lactoferrin against A. baumannii as the age of antibiotics continues to wane.
Methods
Bacterial strains and culture conditions
Seventeen clinical isolates of A. baumannii were utilized in this study (Table 1). These strains were selected from diverse anatomical origin and from a wide range of disease presentations including urinary tract, respiratory, wound, intra-abdominal infections, and bacteremia. They represented a wide range of quantitative biofilm formation phenotypes as well as susceptibility or resistance to antibiotics. Antimicrobial susceptibility to ampicillin-sulbactam (A/S), amikacin (AK), ceftriaxone (CAX), ceftazidime (CAZ), cefotaxime (CFT), ciprofloxacin (CP), cefepime (CPE), gentamicin (GM), levofloxacin (LVX), meropenem (MER), piperacillin (PI), trimethoprim-sulfamethoxazole (T/S), tetracycline (TE), ticarcillin-K clavulanate (TIM), and tobramycin (TO) was determined as previously described22. All isolates were grown overnight in Luria-Bertani (LB) broth (ThermoFisher) at 37 °C in room air under shaking conditions 180 rpm to an optical density of 600 nm (OD600) between 0.8–1.0.
Purification of lactoferrin from human milk
Expressed human breast milk was gathered by Dr. J. Hendrik Weitkamp from the Vanderbilt Department of Pediatrics, under a collection protocol approved by the Vanderbilt University Institutional Review Board (IRB #100897) from healthy donors between 3 days and 3 months post-partum and purified as previously described40. Lactoferrin was purified from human milk as previously described12. Briefly, de-identified human milk samples were centrifuged at 8000 g for 45 minutes to separate milk fats from the soluble fraction. Following centrifugation, the upper fat layer was removed, and proteins were precipitated from the soluble fraction by the addition of ammonium sulfate and incubation at 4°C overnight. Precipitated proteins were fractionated by ion-exchange chromatography. The purity and identity of the fractions were determined by high resolution mass spectrometry analysis. Fractions containing greater than 99% lactoferrin were combined and used in the subsequent assays. Holo-isoforms of both bovine and human lactoferrin were prepared by saturating lactoferrin samples with ferric chloride and dialyzing to remove unbound iron as previously described24.
Bacterial growth curve assays
A. baumannii biofilms were cultured and analyzed as previously described41,42 with some modifications. Briefly, overnight cultures were diluted ten-fold in fresh media comprised of 50% LB broth and 50% calprotectin buffer with increasing concentrations (0, 62.5 μM, 125 μM, 250 μM, 500 μM, 750 μM, 1000 μM) of human or commercially available bovine lactoferrin (Friesland Campina) alone or in medium supplemented with 250 μM ferric chloride. Cultures were incubated at 37 °C for 24 h in shaking conditions (180 rpm). The OD600 was measured to quantify the density of the bacterial cells in each culture. Minimum inhibitory concentration (MIC) was determined as the concentration at which statistically significant inhibition of growth was observed.
Bacterial biofilm quantification
A. baumannii biofilms were cultured and analyzed as previously described22,43 with some modifications. Briefly, overnight cultures were diluted ten-fold in fresh media comprised of 50% LB broth and 50% calprotectin buffer alone or supplemented with 250 μM ferric chloride, then treated with 125 μg/mL of either bovine or human lactoferrin (concentrations that were subinhibitory to growth for most strains). Samples were incubated at 37 °C for 24 h in dark static conditions. The OD600 was measured to quantify the density of the bacterial cells in each culture. Biofilm formation was determined by crystal violent staining. A 1% solution (w/v) of crystal violet (Sigma-Aldrich) was used to stain bacterial cells, decanted, and then washed twice with distilled water. 200 μL of 80%/20% ethanol/acetone solution (Sigma-Aldrich) was added to each well to solubilize the crystal violet and the absorbance at 560 nm was recorded. Total biofilm (as determined by OD560 of crystal violet stain) for each isolate was normalized to its respective total mass by calculating the ratio of absorbance at 560 nm to 600 nm (biomass). Each biofilm assay consisted of 2–3 technical replicates and the assay was repeated at least three times using fresh overnight cultures. All OD560/OD600 ratios from strains grown in medium alone above 1.0, the geometric mean, were considered strong biofilm formers, while strains exhibiting values below this cutoff were considered weak biofilm formers. To validate this cutoff value, absorbance values were subjected to the D’Agostino and Pearson test of normality as previously described44 and after segregation into weak vs. strong cohorts, a one-way ANOVA was applied (P<0.0001).
Inductively coupled plasma mass spectrometry analyses
To enumerate the iron molecules associated with lactoferrin isoforms, Inductively Coupled Plasma Mass Spectrometery (ICP-MS) was performed as previously described40. Briefly, 100 μg of each lactoferrin isoform were digested in 0.9 mL 50% nitric acid (Thermo Fisher) overnight at 37ºC in sealed Teflon sample containers (VWR). The following day, samples were diluted in 9 mL of deionized water in metal-free tubes (VWR). Elemental quantification was performed using a Thermo-Element 2 HR- ICP-MS instrument.
Statistical analyses
Statistical analyses of growth were performed by Student’s t test comparing to medium alone control within discrete strain experiments and comparing between bovine and human lactoferrin. Analyses of biofilm formation were performed using one-way ANOVA with either Tukey’s or Dunnett’s post hoc correction for multiple comparisons. All reported P values are adjusted to account for multiple comparisons. P values of ≤0.05 were considered significant. All data analyzed in this work were derived from at least three biological replicates. Statistical analyses were performed using GraphPad Prism 6 or 8 software (GraphPad Prism Software Inc., La Jolla, California) or Microsoft Excel.
Supplementary Material
Acknowledgments
Funding
This work has been funded by the National Institutes of Health grant R01 HD090061 (to J.A.G.), by the Department of Veterans Affairs Office of Research BX005352 (to J.A.G.) from the National Institutes of Child Health and Human Development, MD007586 (RCMI) and MD007593 (MeTRC) from the National Institute on Minority Health and Health Disparities (NIMHD) (to D.M.) 2T32HL007411-39 (to J.L), K08AI151100 (to R.S.D.), National Science Foundation Award Numbers NSF 1547757 and NSF 1400969 (to S.M.D.), and NSF CHE-1847804 and NIH R35GM133602 (to S.D.T.). Additional support was provided by the Vanderbilt Institute for Clinical and Translational Research program supported by the National Center for Research Resources, Grant UL1 RR024975-01, and the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 which supports access to Core Facilities and Biostatistics support.
Footnotes
Ethics Approval and Consent to Participate
Ethics approval to carry out this study was provided by the Meharry Medical College Institutional Review Board (IRB 081204AAH23119) and by Vanderbilt University Medical Center (IRB 100897). The secondary use of de-identified or coded samples is not considered research involving human subjects under 45 CFR 46. Biospecimens used in this study were de-identified and need for consent was waived by the IRB in accordance with federal regulation (45 CFR 46, Department of Health and Human Services, Authority: 5 U.S.C. 301; 42 U.S.C. 289(a); 42 U.S.C. 300v-1(b)).
Supporting Information Available
Bacterial growth and biofilm assays performed in cultures supplemented with exogenous nutrient iron are available as supplemental files in “Supporting Information”. This information is available free of charge on the ACS Publications website.
References
- (1).Sheppard FR; Keiser P; Craft DW; Gage F; Robson M; Brown TS; Petersen K; Sincock S; Kasper M; Hawksworth J; Tadaki D; Davis T; Stojadinovic A; Elster E The Majority of US Combat Casualty Soft-Tissue Wounds Are Not Infected or Colonized upon Arrival or during Treatment at a Continental US Military Medical Facility. Am. J. Surg. 2010. 200(4):489–95. doi: 10.1016/j.amjsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- (2).Morris FC; Dexter C; Kostoulias X; Uddin MI; Peleg AY The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019. 10:1601. doi: 10.3389/fmicb.2019.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Garnacho-Montero J; Timsit JF Managing Acinetobacter baumannii Infections. Current Opinion in Infectious Diseases. 2019. 32(1):69–76. doi: 10.1097/QCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- (4).Mohd Sazlly Lim S; Zainal Abidin A; Liew SM; Roberts JA; Sime FB The Global Prevalence of Multidrug-Resistance among Acinetobacter baumannii Causing Hospital-Acquired and Ventilator-Associated Pneumonia and Its Associated Mortality: A Systematic Review and Meta-Analysis. Journal of Infection. 2019. 79(6):593–600. doi: 10.1016/j.jinf.2019.09.012. [DOI] [PubMed] [Google Scholar]
- (5).Chen N; Zhou M; Dong X; Qu J; Gong F; Han Y; Qiu Y; Wang J; Liu Y; Wei Y; Xia J; Yu T; Zhang X; Zhang L Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020. 395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Rawson TM; Moore LSP; Zhu N; Ranganathan N; Skolimowska K; Gilchrist M; Satta G; Cooke G; Holmes A Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2020. 71(9): 2459–2468. 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lai C-C; Wang C-Y; Hsueh P-R Co-Infections among Patients with COVID-19: The Need for Combination Therapy with Non-Anti-SARS-CoV-2 Agents? J. Microbiol. Immunol. Infect. 2020. 53(4): 505–512. 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Yu N; Li W; Kang Q; Xiong Z; Wang S; Lin X; Liu Y; Xiao J; Liu H; Deng D; Chen S; Zeng W; Feng L; Wu J Clinical Features and Obstetric and Neonatal Outcomes of Pregnant Patients with COVID-19 in Wuhan, China: A Retrospective, Single-Centre, Descriptive Study. Lancet. Infect. Dis. 2020. 20(5): 559–564. 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wang Z; Yang B; Li Q; Wen L; Zhang R Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2020. 71(15): 769–777. 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gellings PS; Wilkins AA; Morici LA Recent Advances in the Pursuit of an Effective Acinetobacter baumannii Vaccine. Pathogens. 2020. 9(12):1066. doi: 10.3390/pathogens9121066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Antibiotic Resistance Threats in the United States, 2019. 10.15620/cdc:82532: Atlanta, GA: 2019. [DOI] [Google Scholar]
- (12).Lu J; Francis JD; Guevara MA; Moore RE; Chambers SA; Doster RS; Eastman RS; Eastman AJ; Rogers LM; Noble KN; Manning SD; Damo SM; Aronoff DM; Townsend SD; Gaddy JA Antibacterial and anti-biofilm activity of the human breast milk glycoprotein lactoferrin against Group B Streptococcus. ChemBiochem. 2021. doi: 10.1002/cbic.202100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Anderson BF; Baker HM; Norris GE; Rice DW; Baker EN Structure of Human Lactoferrin: Crystallographic Structure Analysis and Refinement at 2·8 Å Resolution. J. Mol. Biol. 1989. 209(4):711–34. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- (14).Mazurier J; Spik G Comparative Study of the Iron-Binding Properties of Human Transferrins. I. Complete and Sequential Iron Saturation and Desaturation of the Lactotransferrin. BBA - Gen. Subj. 1980. 629(2):399–408. [DOI] [PubMed] [Google Scholar]
- (15).Bruni N; Capucchio MT; Biasibetti E; Pessione E; Cirrincione S; Giraudo L; Corona A; Dosio F Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules. 2016. 21(6):752. doi: 10.3390/molecules21060752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).García-Montoya IA; Cendón TS; Arévalo-Gallegos S; Rascón-Cruz Q Lactoferrin a Multiple Bioactive Protein: An Overview. Biochimica et Biophysica Acta - General Subjects. 2012. 1820(3):226–36. doi: 10.1016/j.bbagen.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Campione E; Cosio T; Rosa L; Lanna C; Girolamo S Di; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. International Journal of Molecular Sciences. 2020. 21(14):4903. doi: 10.3390/ijms21144903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Singh PK; Parsek MR; Greenberg EP; Welsh MJ A Component of Innate Immunity Prevents Bacterial Biofilm Development. Nature 2002. 64:102232. doi: 10.1016/j.anaerobe.2020.102232. [DOI] [PubMed] [Google Scholar]
- (19).de Sá Almeida JS; de Oliveira Marre AT; Teixeira FL; Boente RF; Domingues RMCP; de Paula GR; Lobo LA Lactoferrin and Lactoferricin B Reduce Adhesion and Biofilm Formation in the Intestinal Symbionts Bacteroides fragilis and Bacteroides thetaiotaomicron. Anaerobe 2020. 10.1016/j.anaerobe.2020.102232. [DOI] [PubMed] [Google Scholar]
- (20).Quintieri L; Zühlke D; Fanelli F; Caputo L; Liuzzi VC; Logrieco AF; Hirschfeld C; Becher D; Riedel K Proteomic Analysis of the Food Spoiler Pseudomonas Fluorescens ITEM 17298 Reveals the Antibiofilm Activity of the Pepsin-Digested Bovine Lactoferrin. Food Microbiol. 2019. 82:177–193. doi: 10.1016/j.fm.2019.02.003. [DOI] [PubMed] [Google Scholar]
- (21).Luna-Castro S; Aguilar-Romero F; Samaniego-Barrón L; Godínez-Vargas D; De La Garza M Effect of Bovine Apo-Lactoferrin on the Growth and Virulence of Actinobacillus pleuropneumoniae. BioMetals 2014. 27(5):891–903. doi: 10.1007/s10534-014-9752-5. [DOI] [PubMed] [Google Scholar]
- (22).Boone RL; Whitehead B; Avery TM; Lu J; Francis JD; Guevara MA; Moore RE; Chambers SA; Doster RS; Manning SD; Townsend SD; Dent L, Marshall D, Gaddy JA; Damo SM Analysis of Virulence Phenotypes and Antibiotic Resistance in Clinical Strains of Acinetobacter baumannii Isolated in Nashville, Tennessee. BMC Microbiol. 2021. 21(1):21. doi: 10.1186/s12866-020-02082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Landman D; Quale JM; Mayorga D; Adedeji A; Vangala K; Ravishankar J; Flores C; Brooks S Citywide Clonal Outbreak of Multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: The Preantibiotic Era Has Returned. Arch. Intern. Med. 2002. 162(13):1515–20. [DOI] [PubMed] [Google Scholar]
- (24).Kothary V; Doster RS; Rogers LM; Kirk LA; Boyd KL; Romano-Keeler J; Haley KP; Manning SD; Aronoff DM; Gaddy JA Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Front. Cell. Infect. Microbiol. 2017. 7:19. doi: 10.3389/fcimb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dijkshoorn L; Brouwer CPJM; Bogaards SJP; Nemec A; Van Den Broek PJ; Nibbering PH The Synthetic N-Terminal Peptide of Human Lactoferrin, HLF(1–11), Is Highly Effective against Experimental Infection Caused by Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2004. 48(12):4919–21. doi: 10.1128/AAC.48.12.4919-4921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).de Léséleuc L; Harris G; KuoLee R; Xu HH; Chen W Serum Resistance, Gallium Nitrate Tolerance and Extrapulmonary Dissemination Are Linked to Heme Consumption in a Bacteremic Strain of Acinetobacter baumannii. Int. J. Med. Microbiol. 2014. 304(3–4):360–9. doi: 10.1016/j.ijmm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- (27).Mahdi L; Mahdi N; Al-kakei S; Musafer H; Al-Joofy I; Essa R; Zwain L; Salman I; Mater H; Al-Alak S; Al-Oqaili R Treatment Strategy by Lactoperoxidase and Lactoferrin Combination: Immunomodulatory and Antibacterial Activity against Multidrug-Resistant Acinetobacter baumannii. Microb. Pathog. 2018. 114:147–152. doi: 10.1016/j.micpath.2017.10.056. [DOI] [PubMed] [Google Scholar]
- (28).Kamoshida G; Akaji T; Takemoto N; Suzuki Y; Sato Y; Kai D; Hibino T; Yamaguchi D; Kikuchi-Ueda T; Nishida S; Unno Y; Tansho-Nagakawa S; Ubagai T; Miyoshi-Akiyama T; Oda M; Ono Y Lipopolysaccharide-Deficient Acinetobacter baumannii Due to Colistin Resistance Is Killed by Neutrophil-Produced Lysozyme. Front. Microbiol. 2020. 1:573. doi: 10.3389/fmicb.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).García-Borjas KA; Ceballos-Olvera I; Luna-Castro S; Peña-Avelino Y Bovine Lactoferrin Can Decrease the in Vitro Biofilm Production or Shown Synergy with Antibiotics against Listeria and Escherichia coli Isolates. Protein Pept. Lett. 2021. 28(1):101–107. doi: 10.2174/0929866527666200403111743. [DOI] [PubMed] [Google Scholar]
- (30).Ramamourthy G; Vogel HJ Antibiofilm Activity of Lactoferrin-Derived Synthetic Peptides against Pseudomonas aeruginosa PAO1. Biochem. Cell Biol. 2020. 99(1):138–148. doi: 10.1139/bcb-2020-0253. [DOI] [PubMed] [Google Scholar]
- (31).Allison LM; Walker LA; Sanders BJ; Yang Z; Eckert G; Gregory RL Effect of Human Milk and Its Components on Streptococcus mutans Biofilm Formation. J. Clin. Pediatr. Dent. 2015. 39(3):255–61. doi: 10.17796/1053-4628-39.3.255. [DOI] [PubMed] [Google Scholar]
- (32).Rosa L; Cutone A; Lepanto MS; Scotti MJ; Conte MP; Paesano R; Valenti P Physico-Chemical Properties Influence the Functions and Efficacy of Commercial Bovine Lactoferrins. BioMetals 2018. 31(3):301–312. doi: 10.1007/s10534-018-0092-8. [DOI] [PubMed] [Google Scholar]
- (33).Zasloff M Antimicrobial Peptides of Multicellular Organisms. Nature. 2002. 415(6870):389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- (34).Yen CC; Shen CJ; Hsu WH; Chang YH; Lin HT; Chen HL; Chen CM Lactoferrin: An Iron-Binding Antimicrobial Protein against Escherichia coli Infection. BioMetals 2011. 24(4):585–94. doi: 10.1007/s10534-011-9423-8. [DOI] [PubMed] [Google Scholar]
- (35).Bellamy W; Wakabayashi H; Takase M; Kawase K; Shimamura S; Tomita M Killing of Candida albicans by Lactoferricin B, a Potent Antimicrobial Peptide Derived from the N-Terminal Region of Bovine Lactoferrin. Med. Microbiol. Immunol. 1993. 182(2):97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- (36).Zagulski T; Lipiński P; Zagulska A; Jarza̧bek, Z. Antibacterial System Generated by Lactoferrin in Mice in vivo Is Primarily a Killing System. Int. J. Exp. Pathol. 1998. 79(2):117–23. doi: 10.1046/j.1365-2613.1998.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ha C; Kornbluth A Mucosal Healing in Inflammatory Bowel Disease: Where Do We Stand? Current Gastroenterology Reports. 2010. 12(6):471–8. doi: 10.1007/s11894-010-0146-8. [DOI] [PubMed] [Google Scholar]
- (38).Hu W; Zhao J; Wang J; Yu T; Wang J; Li N Transgenic Milk Containing Recombinant Human Lactoferrin Modulates the Intestinal Flora in Piglets. Biochem. Cell Biol. 2012. 90(3):485–96. doi: 10.1139/o2012-003. [DOI] [PubMed] [Google Scholar]
- (39).Ochoa TJ; Pezo A; Cruz K; Chea-Woo E; Cleary TG Clinical Studies of Lactoferrin in Children. Biochemistry and Cell Biology. 2012. 90(3):457–67. doi: 10.1139/o11-087. [DOI] [PubMed] [Google Scholar]
- (40).Craft KM; Gaddy JA; Townsend SD Human Milk Oligosaccharides (HMOs) Sensitize Group B Streptococcus to Clindamycin, Erythromycin, Gentamicin, and Minocycline on a Strain Specific Basis. ACS Chem. Biol. 2018. 13(8):2020–2026. 10.1021/acschembio.8b00661. [DOI] [PubMed] [Google Scholar]
- (41).Gaddy J; Arivett B; McConnel M; Lopez-Rojas R; Pachon J; Actis L Role of Acinetobactin-Mediated Iron Acquisition Functions in the Interaction of Acinetobacter baumannii Strain ATCC 19606T with Human Lung Epithelial Cells, Galleria mellonella Caterpillars, and Mice. Infect. Immun. 2012. 80(3):1015–24. 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Nwugo CC; Arivett BA; Zimbler DL; Gaddy JA; Richards AM; Actis LA Effect of Ethanol on Differential Protein Production and Expression of Potential Virulence Functions in the Opportunistic Pathogen Acinetobacter baumannii. PLoS One 2012. 7(12):e51936. 10.1371/journal.pone.0051936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Gaddy JA; Tomaras AP; Actis LA The Acinetobacter baumannii 19606 OmpA Protein Plays a Role in Biofilm Formation on Abiotic Surfaces and in the Interaction of This Pathogen with Eukaryotic Cells. Infect. Immun. 2009. 77(8):3150–60. 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Parker RE; Laut C; Gaddy JA; Zadoks RN; Davies HD; Manning SD Association between Genotypic Diversity and Biofilm Production in Group B Streptococcus. BMC Microbiol. 2016. 16:86. doi: 10.1186/s12866-016-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


