Abstract
Objectives
This study investigated the relationship between 28-day mortality in patients with COVID-19 pneumonia and the CURB-65 score, platelet count (PLT), mean platelet volume (MPV), and MPV/PLT ratio (MPR).
Methods
A total of 247 patients with COVID-19 pneumonia who presented to the emergency department between March 15, 2020 and May 15, 2020 were retrospectively analyzed. The age, gender, clinical presentation, history of chronic disease, thoracic computed tomography findings, MPV, PLT, MPR, CURB-65 scores, and 28-day mortality of patients were recorded.
Results
The patients had a mean age of 51 years (IQR: 39–63 years) and 55.5% were females. The most common symptom was cough (30.4% of patients). The most common comorbidity was hypertension (13.4%), 49.8% of the cases showed intermediate involvement, and 7.7% of patients died within the first 28 days. The mean MPV was 9.71 ± 1.15, the mean PLT was 226.68 ± 83.82, and the mean MPR was 0.056 ± 0.12. There were significant correlations of 28-day mortality with the CURB-65 score, MPV, and MPR levels (p = 0.000, p = 0.034, and p = 0.034, respectively). No significant correlation was found between the PLT count and 28-day mortality (p = 0.105).
Conclusions
In addition to the CURB-65 score, MPV and MPR values can be used to predict 28-day mortality in patients with COVID-19 pneumonia.
Keywords: COVID-19, Emergency department, Pneumonia, Platelet, Mean platelet volume
1. Introduction
COVID-19 was declared as a pandemic by the World Health Organization (WHO) [1]. The main presenting symptoms of COVID-19 are fever, cough and shortness of breath [2]. The clinical course and causative microorganisms are different between COVID-19 pneumonia and community-acquired pneumonia (CAP). COVID-19 pneumonia has a viral origin and can rapidly cause acute respiratory distress syndrome.
Although polymerase chain reaction (PCR) is the standard diagnostic test for COVID-19, laboratory findings are important for confirming the diagnosis. Since COVID-19 is a dynamic disease that can lead to unexpected outcomes, laboratory findings are critical to assess the progression of COVID-19 and guide treatment interventions [3]. As such, it may be helpful that parameters checked upon admission have predictive value for COVID-19 disease progression and mortality.
During the progression of COVID-19, some blood parameters have been reported to decrease significantly, while others increase significantly [4]. The laboratory abnormalities predominantly found included hypoalbuminemia, elevated inflammatory markers, such as C reactive protein, lactate dehydrogenase, and erythrocyte sedimentation rate, among others. The frequency of lymphopenia found suggests that COVID-19 might act on lymphocytes, especially T lymphocytes, as does SARS-CoV, may be including.
Görelik et al. demonstrated that the increase in mean platelet volume (MPV) strongly predicts in-hospital and long-term mortality [5]. Gölcük et al. also found that MPV is valuable for predicting mortality and disease severity at admission in patients with CAP [6]. COVID-19 pneumonia differs from CAP in terms of its features, but MPV remains a useful prognostic indicator for critically ill COVID-19 patients [7]. However, some studies showed a negative correlation between the MPV and platelet count (PLT) in severe COVID-19 patients [8].
It has also been reported that the combination of MPV and PLT may be clinically more significant than either parameter individually [8,9]. Inexpensive, accessible, and widely used laboratory tests of the severity of COVID-19 are important. MPV and PLT counts are widely and routinely used in clinical practice. The CURB-65 score can also be easily calculated in the emergency department. To our knowledge, there are no studies investigating the diagnostic value of the CURB-65 score, PLT, or MPV/PLT ratio (MPR) for COVID-19-associated mortality. Therefore, this study investigated whether the CURB-65 score, PLT count, MPV, and MPR are associated with 28-day mortality in COVID-19 pneumonia.
2. Material and methods
A retrospective analysis of 7.138 patients who presented to the emergency department of the Bursa Yüksek Ihtisas Training and Research Hospital between March 15, 2020 and May 15, 2020 was conducted. Of these patients, 5.517 with a negative PCR, and 1.621 with a positive PCR but no evidence of COVID-19 pneumonia were excluded from the study. Accordingly, a total of 247 patients were included. Approval was obtained from the ethics committee of the hospital during the planning stage of the study (2011-KAEK-25 2020/05-20).
Patient information was gathered from the hospital automation system and patient files. The assessment of the patients and files were made by 2 emergency medicine specialists and 2 emergency medical assistants who completed 2 years of training.
State chart abstractors were trained in chart abstraction prior to data collection, and the abstractors were not aware of the study objectives. The abstractors used the hospital information management system to obtain data, which were collected on data abstraction forms. Abstractor performance was monitored. However, interobserver reliability was not assessed.The study was carried out in accordance with the principles of the Declaration of Helsinki. All data pertaining to the study were obtained from electronic hospital records. (Hospital Information Management System).
The sampling method was described as retrospective study. Age, gender, clinical presentation, chronic disease history, computerize tomography (CT) findings, and 28-day mortality were recorded. COVID-19 pneumonia imaging findings of patients with CT were evaluated by the radiologist according to the Expert Consensus Statement on the Reporting of COVID-19-Related Chest CT Findings of the North American Society of Radiology [10].
MPV, PLT and MPR were calculated according to hemogram examinations performed during the first evaluation in the emergency department. CURB 65 was calculated the patients age, vital signs (respiratory rate and blood pressure), biochemistry analysis (blood urea nitrogen) and confusion. Missing data were excluded. The final diagnosis and hospitalization information were recorded, and the 28-day mortality rate was calculated.
Inclusion and exclusion criterias:
Patients over 18 years of age and with a positive PCR, and evidence of COVID-19 pneumonia were included the study.
Patients under 18 years of age, and those who were pregnant, had a negative PCR result, or had no signs of pneumonia on CT data were excluded from the study.
2.1. Statistical analysis
Data were analyzed using SPSS 22.0 software for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics are expressed as means ± standard deviation or median values, while categorical variables are expressed as numbers and percentages (%). The Kolmogorov-Smirnov test was used to analyze the normality of the distribution of the data. The Mann-Whitney U and Kruskal-Wallis tests were used to evaluate significant differences in continuous numerical variables between the groups, because the assumptions of parametric tests were not met. Spearman correlation analysis was used to investigate the relationships of the CURB-65 score with the other parameters. The Chi-square test and Fisher's exact test were used to determine the relationships of categorical variables. Variables considered useful for predicting mortality were included in a logistic regression model using the “enter” method. A receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic value of the MPR value for predicting 28-day mortality. A p-value < 0.05 was considered statistically significant. The 95% confidence intervals (CIs) were also calculated.
3. Results
A total of 247 patients with positive PCR results for COVID-19 were included in the study. The median patient age was 51 years (IQR: 39–63). There were 137 (55.5%) females in the study. Cough was the most common symptom (n = 75, 30.4%). Of the 247 patients, 74 (30%) had comorbidities, among which hypertension (HT) was the most common (n = 33, 13.4%). CT revealed intermediate involvement in 123 (49.8%) of patients with pneumonia. While 15 patients (6.1%) died within the first 14 days, 19 (7.7%) died within the first 28 days. Patients vital signs, clinical and demographic data are shown in Table 1, Table 2 .
Table 1.
Clinical and demographic data of the patients.
| n | % | ||
|---|---|---|---|
| Gender | Female | 137 | 55.5 |
| Male | 110 | 44.5 | |
| Fever | No | 181 | 73.3 |
| Yes | 66 | 26.7 | |
| Cough | No | 172 | 69.6 |
| Yes | 75 | 30.4 | |
| Sore throat | No | 234 | 94.7 |
| Yes | 13 | 5.3 | |
| Diarrhea | No | 232 | 93.9 |
| Yes | 15 | 6.1 | |
| Weakness | No | 233 | 94.3 |
| Yes | 14 | 5.7 | |
| Dyspnoea | No | 221 | 89.5 |
| Yes | 26 | 10.5 | |
| Loss of smell-taste | No | 219 | 88.7 |
| Yes | 28 | 11.3 | |
| Joint pain | No | 206 | 83.4 |
| Yes | 41 | 16.6 | |
| Comorbidities | No | 173 | 70 |
| Yes | 74 | 30 | |
| HT | No | 214 | 86.6 |
| Yes | 33 | 13.4 | |
| DM | No | 224 | 90.7 |
| Yes | 23 | 9.3 | |
| COPD /ASTHMA | No | 227 | 91.9 |
| Yes | 20 | 8.1 | |
| CAD | No | 236 | 95.5 |
| Yes | 11 | 4.5 | |
| Malignancy | Yok | 241 | 97.6 |
| Var | 6 | 2.4 | |
| Pneumonia | Atypical | 29 | 11.7 |
| Intermediate | 123 | 49.8 | |
| Typical | 95 | 38.5 | |
| 28-day mortality | Yes | 19 | 7.7 |
| No | 228 | 92.3 | |
| Total | 247 | 100 | |
HT: Hypertension, DM: Diabetes Mellitus, COPD: Chronic Obstructive Pulmonary Disease, CAD: Coronary Artery Disease.
Table 2.
Age, clinical and laboratory data.
| Age, Median (IQR 25–75) | 51 (36–93) |
| Respiration Rate, Median (IQR 25–75) | 16 (14–20) |
| Temperature, Mean ± Standard Deviation | 36.05 ± 0.74 |
| Diastolic Blood Pressure, Median (IQR 25–75) | 106(90–122) |
| Systolic Blood Pressure, Median (IQR 25–75) | 154 (136–158) |
| SPO2 Mean ± Standard Deviation | 92.52 ± 5.65 |
SPO2: Oxygen saturation.
The average MPV of patients was 9.71 ± 1.15 fL, the mean platelet level was 226.68 ± 83.82 μL, and the mean MPR was 0.056 ± 0.12 fL/μL.
The Mann-Whitney U test was performed to investigate whether there were relationships among the CURB-65 score, MPV, PLT, and MPR levels in patients who died within 28 days. The CURB-65 score, MPV, and MPR were significantly different between patients who died within 28 days and those who did not (p = 0.000, p = 0.034, and p = 0.034, respectively) [Table 3 ].
Table 3.
28-day mortality analysis of variables.
| 28-day mortality | n | Median (IQR: 25–75) | p value | |
|---|---|---|---|---|
| CURB-65 | No | 228 | 0 (0–1.0) | <0.001⁎ |
| Yes | 19 | 5.0(4.0–5.0) | ||
| Total | 247 | 0 (0–1.0) | ||
| MPV | No | 228 | 9.50 (8.33–10.30 | <0.05⁎ |
| Yes | 19 | 10.10(9.30–11.20) | ||
| Total | 247 | 9.50(8.90–10.30) | ||
| Platelet | No | 228 | 211,500(174250–262,000) | >0.05⁎ |
| Yes | 19 | 192,000(124000-262,000) | ||
| Total | 247 | 208,000(172000–262,000) | ||
| MPR | No | 228 | 0.45 (0.35–0.57) | <0.05⁎ |
| Yes | 19 | 0.61(0.39–0.87) | ||
| Total | 247 | 0.46(0.35–0.58) |
Mann Whitney U test, MPV:Mean Platelet Volume, MPR:Mean Platelet Volume/Platelet Count Ratio.
In a logistic regression analysis, the presence of a comorbidity was a significant predictor of 28-day mortality (Exp beta = 0.173; (95% CI 0.063–0.473, p = 0.001) [Table 4 ]. No significant correlation was found between the CURB-65 score and MPR (p > 0.05) according to the Spearman's rho test.
Table 4.
Logistic regression analysis of variables.
| Variables | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I.for EXP(B) |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Gender | −0.768 | 0.508 | 2.283 | 1 | 0.131 | 0.464 | 0.171 | 1.256 | |
| Comorbidity | −1.756 | 0.518 | 11.466 | 1 | 0.001 | 0.173 | 0.063 | 0.477 | |
| Constant | −1.199 | 0.367 | 10.699 | 1 | 0.001 | 0.301 | |||
The CURB-65 score, MPV, PLT, and MPR did not differ by the type of pneumonia (p > 0.05) according to the Kruskal-Wallis test.
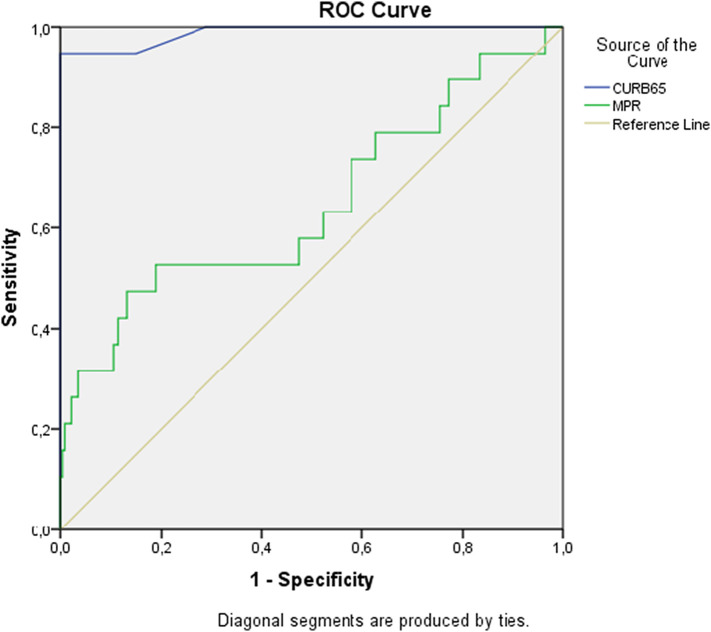
In the ROC curve analysis to identify predictors of 28-day mortality, the area under the curve (AUC) for the CURB-65 score was 0.988 (95% CI 0.966–1.000, p < 0.001) while that for the MPR was 0.647 (95% CI 0.496–0.797, p < 0.05) [Fig. 1 ].
Fig. 1.
Area under curve (AUC) values of mean platelet volume/platelet ratio to prediction mortality.
When the cutoff value of the CURB-65 score for 28-day mortality was 2.5, it had a sensitivity of 94.7% and specificity of 95.7%. When the cutoff value was 3.5, the score had a sensitivity of 94.7% and specificity of 100.0%. A cutoff value of the MPR for 28-day mortality of 0.042 had a sensitivity of 73.7% and specificity of 42.1%. When the cutoff value was 0.048, the sensitivity was 52.6% and the specificity was 52.6% [Table 5 ].
Table 5.
ROC analysis according to CURB-65 and MPR at the diagnosis of mortality.
| AUC(95% CI) | p | Risk Fact | Cut off | Sensitivity % | Specificity % | |
|---|---|---|---|---|---|---|
| 28-day mortality | 0.988 (0.966–1.000) | 0.000 | CURB65 | 2.5 | 94.7 | 95.2 |
| 3.5 | 94.7 | 100.0 | ||||
| 0.647 (0.496–0.797) | 0.034 | MPR | 0.042 | 73.7 | 42.1 | |
| 0.048 | 52.6 | 52.6 | ||||
| 0.060 | 47.4 | 81.1 |
The Chi-square test and Fisher's exact test showed significant associations of HT, diabetes mellitus, and chronic renal failure with 28-day mortality (p < 0.05, p < 0.05, and p < 0.05, respectively) [Table 6 ].
Table 6.
Relationship between comorbidities and 28-day mortality.
| 28-day mortality |
Total | p value | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| HT | No | n | 201 | 13 | 214 | <0.05# |
| % | 88.2% | 68.4% | 86.6% | |||
| Yes | n | 27 | 6 | 33 | ||
| % | 11.8% | 31.6% | 13.4% | |||
| DM | No | n | 211 | 13 | 224 | <0.05# |
| % | 92.5% | 68.4% | 90.7% | |||
| Yes | n | 17 | 6 | 23 | ||
| % | 7.5% | 31.6% | 9.3% | |||
| COPD /ASTHMA | No | n | 210 | 17 | 227 | >0.05# |
| % | 92.1% | 89.5% | 91.9% | |||
| Yes | n | 18 | 2 | 20 | ||
| % | 7.9% | 10.5% | 8.1% | |||
| CAD | No | n | 219 | 17 | 236 | >0.05⁎ |
| % | 96.1% | 89.5% | 95.5% | |||
| Yes | n | 9 | 2 | 11 | ||
| % | 3.9% | 10.5% | 4.5% | |||
| CRF | No | n | 226 | 17 | 243 | <0.05⁎ |
| % | 99.1% | 89.5% | 98.4% | |||
| Yes | n | 2 | 2 | 4 | ||
| % | 0.9% | 10.5% | 1.6% | |||
| Malignancy | No | n | 224 | 17 | 241 | >0.05⁎ |
| % | 98.2% | 89.5% | 97.6% | |||
| Yes | n | 4 | 2 | 6 | ||
| % | 1.8% | 10.5% | 2.4% | |||
| Total | n | 228 | 19 | 247 | ||
| % | 92.3% | 7.7% | 100.0% | |||
Chi-square test.
Fisher's Exact test, HT: Hypertension, DM: Diabetes Mellitus, CRF: Chronic Renal Failure COPD: Chronic Obstructive Pulmonary Disease, CAD: Coronary Artery Disease.
4. Discussion
Since coronavirus disease 2019 (COVID-19), a life-threatening infectious disease sustained by the severe respiratory syndrome coronavirus 2 (SARS-CoV-2), is frequently complicated by thrombotic episodes, both venous and arterial [11]. We provide here an updated analysis of current scientific literature data exploring the association between CURB-65 score, PLT count, MPV, and MPR and 28-day mortality rate in patients with COVID-19.
The clinical symptoms of COVID-19 usually include fever, weakness, and dry cough [12]. Similar to other investigations, we found that the most common symptoms were cough and intermediate involvement [13].
Prior reports show comorbidities (HT, DM, COPD/Astma, CAD, Malignancy) were detected in 32% [14] of the elderly patients with COVID-19, and 26% [13] of those with underlying chronic diseases who died due to COVID-19 pneumonia.
We observed a 30% rate of comorbidities; hypertension was detected in 13.4% of patients, and thus represents an important risk factor for 28-day mortality caused by COVID-19 pneumonia. The presence of a comorbidity (HT, DM, COPD/Astma, CAD, Malignancy) was also an independent risk factor. However, while advanced age was an important risk factor for mortality in other studies, it was not an independent risk factor in this study [13].
Platelet activation in viral pneumonia may cause lung damage by stimulating the respiratory inflammatory response [15]. The tendency toward temporarily lower PLT in patients with COVID-19 may indicate worsening of the thrombotic state, where a lower PLT is associated with increased mortality [16]. In a study conducted in Wuhan, China, thrombocytopenia on presentation to hospital in patients with COVID-19 was associated with a 4.24-fold increase in the risk of mortality [17].
Some studies found associations of thrombocytopenia with the severity of COVID-19 and associated mortality. It has been reported that as the PLT decreases, the mortality rate increases [18]. We did not find any relationship between PLT and mortality. Similarly, other studies have reported normal PLT in many patients at the time of hospitalization [19].
A decrease in the PLT increases MPV. Güçlü et al. found that a 1-unit increase in MPV between the first and third days of hospitalization secondary to COVID-19 increased mortality by 1.76 times. In addition to lung capacity, the MPV value could be used as an auxiliary marker for predicting mortality in COVID-19 patients [20]. An increase in MPV is also related to worse prognoses in patients with chronic inflammatory disease, severe pneumonia, and septic shock [21]. In patients with severe CAP, an increase in MPV after hospitalization was found to predict mortality [22]. In this study, the MPV value was a significant predictor of 28-day mortality in COVID-19 pneumonia patients.
In patients with CAP, mortality increases as the CURB-65 score increases [23]. In this study, we evaluated the correlation between the MPV and CURB-65 score. We also examined whether the accuracy of predictions of 28-day mortality in COVID-19 pneumonia patients could be improved by combining the MPV and CURB-65 score. Similar to studies of CAP patients, our results confirmed that the CURB-65 score is an independent predictor of mortality in patients with COVID-19 pneumonia [24].
The pathophysiological mechanism underlying the association of the MPR with COVID-19 prognosis is unclear but may involve the following. First, under inflammatory conditions, platelet production increases due to the increased thrombopoietin synthesis mediated by cytokines [25]. Second, the MPV reflects the metabolism, proliferation, and platelet production of megakaryocytes in the bone marrow [26]. Poor prognosis in patients with a low PLT count and high MPV may be associated with an increased risk of oxidative stress, thrombosis, and apoptosis in active platelets [27]. In many diseases, an increase in MPR is associated with an unfavorable prognosis, such as post-ischemic stroke, pneumonia, sepsis, critical illness, febrile epilepsy, and malignant tumors [28].
In severe COVID-19 patients, the MPV is negatively correlated with the PLT. Therefore, the use of the MPR is highly recommended as an indicator of platelet function [29]. Ranias et al. showed that an increase in the MPR in pneumonia patients with ischemic stroke can predict 30-day mortality [30]. In our study, we also found that an increase in MPR predicted a poor prognosis, and mortality, in patients with COVID-19 pneumonia.
5. Conclusion
We determined that CURB-65 score, PLT count, MPV and MPR may be associated with 28-day mortality in COVID-19 pneumonia patients.
Declarations
Ethical committee approval was obtained from Yuksek Ihtisas Education and Research Hospital Ethical committee during the study planning phase.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interest
There is no financial and non-financial competing interest.
There is no conflict of interest between authors.
Acknowledgment
We would like to thank Melih Yuksel for his contribution.
References
- 1.WHO/Europe Coronavirus disease (COVID-19) outbreak- WHO announces COVID-19 out break a pandemic https://www.euro.who.int/en/health_topics/health_emergencies/coronavirus_covid19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic.
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer D.G., Germano B.C.C., Mendes A.S., Victor L.S., Cavalcanti G.B., Jr., Oliveira L.S. Laboratory parameters in COVID 19. Clin Case Stud. 2020;2:36. [Google Scholar]
- 4.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic creview and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelik O., Tzur I., Barchel D., Sarafian D.A., Swarka M., Beberashvili I., et al. BMC Pulm Med. 2017;17:137. doi: 10.1186/s12890-017-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golcuk Y., Golcuk B., Bilge A., Irik M., Dikmen O. Combination of mean platelet volüme and the CURB-65 score better predicts 28-day mortality in patients with community-acquired pneumonia. Am J Emerg Med. 2015;33:648–652. doi: 10.1016/j.ajem.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Shin D.H., Rhee S.Y., Jeon H.J., Park J.Y., Kang S.W., Oh J. An in crease in mean platelet volume/platelet countratio is associated with vascular Access failure in hemodialysis patients. PLoS One. 2017;12(1):e170357. doi: 10.1371/journal.pone.0170357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Li Y., Sheng X., Wang F., Cheng D., Jian G., et al. Combination of mean platelet volume/platelet countratio and the APACHE II score better predicts the short-term outcome in patients with acute kidney injury receiving continuous renal replacement therapy. Kidney Blood Press Res. 2018;43(2):479–489. doi: 10.1159/000488694. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F., Chen Z., Wang P., Hu X., Gao Y., He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. TumourBiol. 2016;37(7):9323–9331. doi: 10.1007/s13277-015-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson S., Kay F.U., Abbara S., Bhalla S., Chung J.H., Chung M., et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G., Sanchis-Gomar F., Favaloro E.J., Lavie C.J., Henry B.M. Coronavirus disease 2019-associated coagulopathy. Mayo Clin Proc. 2021;96(01):203–217. doi: 10.1016/j.mayocp.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Bing X., ZaZhi X., The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . 41(2) 2020. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China; pp. 145–151. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lê V.B., Schneider J.G., Boergeling Y., Berri F., Ducatez M., Guerin J.L., et al. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am J Respir Crit Care Med. 2015;191(7):804–819. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 16.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 May;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S., et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020 May;31(4):490–496. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. ClinChimActa. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 20.Güçlü E., Kocayiğit H., Okan H., Erkorkmaz U., Yürümez Y., Yaylacı S., et al. Effect of COVID-19 on platelet count and its indices. Rev Assoc Med Bras. 2020 Aug;66(8):1122–1127. doi: 10.1590/1806-9282.66.8.1122. [DOI] [PubMed] [Google Scholar]
- 21.Mirsaeidi M., Peyrani P., Aliberti S., Filardo G., Bordon J., Blasi F., et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137(2):416–420. doi: 10.1378/chest.09-0998. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Li Y., Zeng Y., Tian Y., Wen Y., Wang Z. High mean platelet volume associates with in-hospital mortality in severe pneumonia patients. Mediators Inflamm. 2020;2020:8720535. doi: 10.1155/2020/8720535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man S.Y., Lee N., Ip M., Antonia G.E., Chau S.S., Mak P., et al. Prospective comparison of three predictive rules for assessing severity of community acquired pneumonia in Hong Kong. Thorax. 2007;62:348–353. doi: 10.1136/thx.2006.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.H., Chung H.J., Kim K., Jo Y.H., Rhee J.E., Kim Y.J., et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med. 2013;31:72–79. doi: 10.1016/j.ajem.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gorelik O., Izhakian S., Barchel D., Sarafian D.A., Tzur I., Swarka M., et al. Prognostic significance of platelet count changes during hospitalization for community-acquired pneumonia. Platelets. 2017;28(4):380–386. doi: 10.1080/09537104.2016.1219032. [DOI] [PubMed] [Google Scholar]
- 26.Pulavendran S., Rudd J.M., Maram P., Thomas P.G., Akhilesh R., Malayer J.R., et al. Combination therapy targeting platelet activation and virus replication protects mice against lethal influenza pneumonia. Am J Respir Cell Mol Biol. 2019;61(6):689–701. doi: 10.1165/rcmb.2018-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu S.G., Becker R.C., Berger P.B., Bhatt D.L., Eikelboom J.W., Konkle B., et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Garcia A.C., Arachchillage D.R.J., Kempny A., Gonzalez R.A., Garcia A.M., Uebing A., et al. Platelet count and mean platelet volume predict outcome in adults with Eisenmenger syndrome. Heart. 2018;104(1):45–50. doi: 10.1136/heartjnl-2016-311144. [DOI] [PubMed] [Google Scholar]
- 29.Shin D.H., Rhee S.Y., Jeon H.J., Park J.Y., Kang S.W., Oh J. An increase in mean platelet volume/platelet count ratio is associatedwith vascular access failure in hemodialysis patients. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nageeb R.S., Abozaid M., Nageeb G.S., Omran A.A. Mean platelet volume to platelet count ratio as a laboratory indicator of mortality. Egypt J Neurol Psychiatry Neurosurg. 2018;54:27. doi: 10.1186/s41983-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.