Abstract
Low-intensity pulsed ultrasound (LIPUS) is a promising therapy that has been increasingly explored in basic research and clinical applications. LIPUS is an appealing therapeutic option as it is a noninvasive treatment that has many advantages, including no risk of infection or tissue damage and no known adverse reactions. LIPUS has been shown to have many benefits including promotion of tissue healing, angiogenesis, and tissue regeneration; inhibition of inflammation and pain relief; and stimulation of cell proliferation and differentiation. The biophysical mechanisms of LIPUS remain unclear and the studies are ongoing. In recent years, more and more research has focused on the relationship between LIPUS and stem/progenitor cells. A comprehensive search of the PubMed and Embase databases to July 2020 was performed. LIPUS has many effects on stem cells. Studies show that LIPUS can stimulate stem cells in vitro; promote stem cell proliferation, differentiation, and migration; maintain stem cell activity; alleviate the problems of insufficient seed cell source, differentiation, and maturation; and circumvent the low efficiency of stem cell transplantation. The mechanisms involved in the effects of LIPUS are not fully understood, but the effects demonstrated in studies thus far have been favorable. Much additional research is needed before LIPUS can progress from basic science research to large-scale clinical dissemination and application.
Keywords: cellular signaling pathway, low-intensity pulsed ultrasound, mechanism, mechanotransduction, stem cells
INTRODUCTION
Stem cells are widely used in tissue engineering as they are capable of self-replication and differentiation into multiple cell types.1 The proliferation ability and the differentiation properties of the stem cells are directly related to the age of the donor.2,3,4 Various methods have been established to promote stem cell proliferation and differentiation, and many growth factors have been identified that are involved in this process.5,6,7,8 However, off-target effects from growth factors are extensive. Traditional Chinese medicine has been used to stimulate the proliferation of stem cells, but the medicinal composition is complex and the mechanisms are far from well-being understood.9,10 Other methods that have been utilized to promote stem cell proliferation include transfection of specific growth factor genes,11 application of extracellular matrix,12 exposure to electromagnetic fields,13 and mechanical stimulation.14 Very recently, many studies have demonstrated that low-intensity pulsed ultrasound (LIPUS) promotes stem cell proliferation and differentiation.15,16,17,18,19,20
LIPUS is a promising therapy that is already widely used for various clinical applications and in basic science research.15,21,22,23 Unlike kilohertz ultrasound, LIPUS uses a frequency in the low megahertz range (1–3 MHz) and is more widely used as a therapy to stimulate tissue repair and regeneration, in particular, for bone fracture healing.24,25,26 LIPUS delivers low-intensity acoustic pressure waves that produce microbiomechanical interactions with the cells to elicit intracellular biological effects. This ultimately results in tissue repair and regeneration, a process named “mechanotransduction.” Accumulating evidence indicates that LIPUS is effective to stimulate osteoblasts, to promote bone formation,24 and to activate other stem/progenitor cells. LIPUS enhances viability, proliferation, and multilineage differentiation in a variety of postnatal mesenchymal stem cells (MSCs), including adipose-derived stem cells (ADSCs), bone marrow mesenchymal stem cells (BMSCs), periodontal ligament-derived stem cells (PDLSCs), and human umbilical cord-derived MSCs.27,28,29,30,31
BIOLOGICAL EFFECT OF LIPUS ON STEM/PROGENITOR CELLS
Studies demonstrate that the biological effects of LIPUS on the stem cells result from the physical impact of the ultrasonic waves. When ultrasonic waves propagate in a medium, the particles of the medium reciprocate near its equilibrium position, causing rhythmic and density changes in the medium. This change creates a pressure change that can produce a fine massaging effect on the cells. This fine massaging effect on the cells can change the volume of the cells and the permeability of the cell membrane, promoting the exchange of metabolites and thereby regulating the function of the cells. Related studies have found that when low-intensity ultrasound with different irradiation intensities, different frequencies, and different irradiation times is used, the effect of promoting stem cell proliferation is different. The expression of B lymphoma Mo-MLV insertion region homolog (Bmi-1) gene has a great effect on the self-proliferation efficiency of stem cells. Studies have confirmed that low-intensity ultrasound can effectively increase Bmi-1 gene expression and thus regulate cell proliferation at the molecular level.
Inducing stem cells to differentiate into specific tissues is another function of LIPUS. It has been found in research on cartilage differentiation that low-intensity ultrasound can increase the transforming growth factor β (TGF-β)-mediated proteoglycan deposition of the human MSCs. LIPUS has also been shown to stimulate human MSCs to efficiently differentiate into cartilage32 and rabbit MSCs to differentiate into cartilage.28 LIPUS can be combined with fibrin-hyaluronic acid (fibrin-HA) to differentiate MSCs into high-quality cartilage.33 In addition, LIPUS also produces biological effects through stem cell migration and activity.
LIPUS ACTIVATES STEM/PROGENITOR CELLS
LIPUS can act on a variety of stem cells, including BMSCs, ADSCs, and spermatogonia, promoting the activation, proliferation, and differentiation of these stem cells (Table 1).20,34,35,36,37,38,39,40,41,42,43,44,45,46 Scientists, clinicians, and patients alike hope that these effects will have therapeutic applications in a variety of disease states.47
Table 1.
Influence of low-intensity pulsed ultrasound on various stem cells
| Cell type | Study | Year of publication | Physiological effects of LIPUS |
|---|---|---|---|
| BMSCs | Li et al.44
Costa et al.43 |
2018 2018 |
LIPUS yielded favorable cell viability and improved MSC proliferation, osteoblast differentiation, and cytoskeletal modifications |
| ASCs | Sena et al.34 | 2005 | LIPUS enhanced ASC adipogenesis and osteogenesis of mASCs, LIPUS promoted mineralized nodule formation |
| SSCs | Mohaqiq et al.45
Bozkurt et al.41 |
2018 2016 |
LIUPS promoted SSC proliferation, colonization, and survival rates |
| Neural stem cells | Lv et al.40
Xia et al.20 Xia et al.42 |
2013 2019 2017 |
LIPUS promoted proliferation, cell viability, cytoskeleton morphological changes of iPSCs-NCSCs, neural differentiation of neural stem cells, and regeneration of damaged peripheral nerve. LIPUS has different effects on gene expression of neural crest stem cells |
| Muscle stem cells | Chan et al.37
Nedach et al.36 |
2010 2008 |
LIPUS promoted regeneration of muscle fibers and promoted C2C12 cells to differentiate into osteoblasts and chondrocytes |
| HSPCs | Liu et al.46
Xu et al.39 |
2019 2012 |
LIPUS improved the microenvironment, accelerated construction of bone marrow cells, and increased quantity and quality of red blood cells, white blood cells, and platelets in the peripheral blood |
| MSCs | Al-Daghreer et al.38
Ikai et al.35 |
2012 2008 |
LIPUS may improve the osteogenic commitment of hMSCs in vitro, enhance their osteogenic differentiation, promote dentinogenesis, and accelerate periodontal tissue healing and dental implant osseointegration |
LIPUS: low-intensity pulsed ultrasound; BMSCs: bone marrow mesenchymal stem cells; ASCs: adipose tissue derived stem cells; mASCs: mouse ASCs; SSCs: spermatogonial stem cells; HSPCs: hematopoietic stem/progenitor cells; iPSCs-NCSCs: induced pluripotent stem cell-derived neural crest stem cells; MSCs: mesenchymal stem cells; hMSCs: human MSCs
LIPUS and BMSCs
BMSCs are pluripotent progenitors with self-renewal capacity, long-term viability, and differentiation potential of different cell types.48 Li et al.44 treated human bone marrow mesenchymal stem cells (hBMSCs) with hepatocyte growth factor (HGF) with and without LIPUS treatment; then, cell viability and stem cell surface markers were analyzed. The results indicate that LIPUS treatment under appropriate conditions produces favorable cell viability and promotes stem cell differentiation. At the messenger ribonucleic acid (mRNA) and protein levels, the expression of alpha fetoprotein (AFP), cytokeratin 18 (CK18), albumin (ALB), and glycogen content was significantly increased in the LIPUS treatment group as compared to that in the HGF group and the control group (all P < 0.05). Furthermore, Wnt1, β-catenin, cellular myelocytomatosis (c-Myc), and cyclin D1 were all significantly increased after LIPUS. The activation of the Wnt/β-catenin signaling pathway was involved in this process.44
LIPUS can enhance BMSC proliferation, osteoblast differentiation, and cytoskeletal modifications.43,49,50 Studies have also investigated the effects of combined application of LIPUS and microRNA (miRNA) on in vitro human mesenchymal stem cells (hMSCs) models. In one study,51 the authors identified miR-31-5p as a LIPUS-induced miRNA and investigated its role through in vitro studies of gain and loss of function. The results of this study highlighted that LIPUS induced a hypoxia adaptive cell response. MiR-31-5p gain- and loss-of-function studies demonstrated that miR-31-5p overexpression was able to induce hypoxic adaptive and cytoskeletal responses. Moreover, the cotreatment with LIPUS and miR-31-5p inhibitor abolished the hypoxic responses, including angiogenesis and the expression of the Rho family proteins. In the osteoblast differentiation process or stem cells niche maintenance, the role of miRNAs, such as MiR-675-5p, was hypothesized to be a trigger of complex molecular mechanisms that could promote osteoblast differentiation of hMSCs during hypoxic conditions to result in bone formation.51 This occurs through increasing hypoxia-inducible factor 1α (HIF-1α) response and activation of Wnt/β-catenin signaling.
LIPUS and adipose tissue derived stem cells (ASCs)
In recent years, adipose tissue has received more and more attention as a source of stem cells due to its multilineage potential, self-renewal ability, and long-term viability. ADSCs can differentiate into cells of adipogenic,52 chondrogenic,53 myogenic,54 osteogenic,55 hepatic,56 and neurogenic57 lineages. ADSCs are readily available, being relatively easy to isolate during elective surgery to remove excess adipose tissue.58 ADSCs can be differentiated into adipocytes using standard cocktail differentiation medium (DM) containing dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), insulin, and indomethacin.59 Thus, differentiation of ADSCs provides an excellent model to investigate effects of LIPUS on differentiation ADSCs in vitro.
Many studies60,61 have focused on BMSCs treated with LIPUS, while reports on ADSCs treated with LIPUS are few. Duty ratio is the ratio of pulse duration to pulse total cycle, in a string of a square wave. Yue et al.49 investigated the osteogenic potential of ADSCs treated by LIPUS at different duty cycle and time points. They demonstrated that LIPUS enhanced osteogenesis of murine ADSCs (mADSCs), especially at the duty ratio of 20%. Upregulation of genes including runt-related transcription factor 2 (Runx2), osterix (Osx), and osteopontin (OPN) also occurred at duty ratios of 20% and 50%. The degree of upregulation by duty cycle of 20% was higher than that of 50%.49
Currently, the mechanisms of LIPUS on osteogenic differentiation of human ADSCs (hADSCs) are not well understood. Sena et al.34 explored whether LIPUS promotes proliferation and osteogenic differentiation of hADSCs. These authors used LIPUS stimulation to isolate hADSCs and induce osteogenesis. Their findings demonstrated that LIPUS stimulation increased alkaline phosphatase (ALP) activity in the hADSCs, promoted mineralized nodule formation, and upregulated the expression of Runx2, OPN, and osteocalcin (OCN). These findings suggest that LIPUS treatment enhances the osteogenic differentiation of hADSCs and that the LIPUS-mediated mechanism of osteogenic differentiation may be via the upregulation of heat shock protein 70 (HSP70) and HSP90 expression and activation of bone morphogenic protein (BMP) signaling pathway. With these findings in mind, LIPUS may have broad clinical applications for the proliferation and differentiation potential of ADSCs.
In another study,62 mADSCs were treated with LIPUS for either 3 days or 5 days immediately after adipogenic induction or delayed treatment for 2 days after induction. Expression of adipogenic genes peroxisome proliferator-activated receptor C1 (PPAR-c1) and aminopeptidase (APN) was examined by real-time polymerase chain reaction (PCR). Immunofluorescence (IF) staining was performed to test for PPAR-c at the protein level. The data revealed that specific patterns of LIPUS upregulated levels of all PPAR-c1 mRNA, APN mRNA, and PPAR-c protein.62 In conclusion, in culture medium containing adipogenic reagents, LIPUS enhanced ADSC adipogenesis. Clinically, this is germane to the treatment of obesity and insulin resistance; both are significant and pervasive medical conditions.
LIPUS and spermatogonia stem cells (SSCs)
In addition to the application of fracture treatment, LIPUS is also used in the treatment of soft tissue.63 SSCs are the foundation of spermatogenesis. In the experimental study of Mohaqiq et al.45, the SSCs were stimulated by LIPUS for 5 days, followed by the assessment of expression of integrin-α6 (Itga6), integrin-β1 (Itgβ1), and octamer-binding transcription factor (Oct4) on day 21 by quantitative reverse transcriptase-PCR (qRT-PCR). To investigate the proliferation rate and colonization of SSCs in different groups, the total number of counted cells and colonies and the analysis of the respective diameters were performed on days 7, 14, and 21.45 The authors demonstrated a novel, LIPUS-mediated effect on SSCs proliferation, colonization, and survival rates during 21 days in culture. They determined that LIPUS stimulation increased Itgα6 and Itgβ1 expression; these two genes play critical roles in SSC proliferation and differentiation. Hence, LIPUS stimulation could be a strategy to improve the efficiency, outcomes, and fate of stem cell transplantation, gene therapies, and stem cell enrichment.41
In another study, Moghaddam et al.64 found that LIPUS is more effective at enhancing the proliferation rate of the SSCs during 7 days of culture. In contrast, they found that higher mechanical index has a harmful effect on the SSCs. This research reveals that LIPUS may have novel applications for the proliferation and differentiation of SSCs and may be a potential treatment option for oligospermia/male infertility patients.
LIPUS and neural stem cells
The treatment of lengthy peripheral nerve defects is challenging, and there is much ongoing research into therapies to accelerate nerve regeneration. Traditional treatments attempting to bridge peripheral nerve defects have had unsatisfactory results.40 As such, there is an imperative need to develop new strategies that can replace autologous grafts. Combination therapy with growth factors, nutritional factors, and physical stimulation with LIPUS is a new approach to promote functional recovery and nerve regeneration after peripheral nerve injury.17,20
Previous study have shown that LIPUS promotes cell proliferation, cell viability, and neural differentiation of induced pluripotent stem cell-derived neural crest stem cells (iPSCs-NCSCs) and improves the regeneration of damaged peripheral nerve.40 However, the molecular mechanisms involved in the proliferation and differentiation of iPSCs-NCSCs after LIPUS treatment are still unclear. Xia et al.20 further explored the mechanical signal transduction pathway of LIPUS in this setting. The authors found that LIPUS may facilitate the proliferation, differentiation, and cytoskeleton morphological changes of iPSCs-NCSCs through the focal adhesion kinase-extracellular signal- regulated kinase (FAK-ERK) signal pathway.
To further explore the underlying mechanisms of LIPUS treatment of iPSCs-NCSCs, Xia et al.20 reported the gene expression profiling analysis of iPSCs-NCSCs before and after LIPUS treatment using the RNA-sequencing (RNA-Seq) method. These authors found that the expression of 76 genes of iPSCs-NCSCs cultured in a serum-free neural induction medium and 21 genes of iPSCs-NCSCs cultured in a neuronal differentiation medium changed significantly after LIPUS treatment.42
Retinal ganglion cells (RGCs) are central nervous system neuronal cells that have been utilized as a classic model to evaluate the protective effects of LIPUS after trauma-induced retinal injury. Research has shown that LIPUS contributes to RGCs survival and protects RGC against apoptosis via yes-associated protein (YAP) activation, nuclear translocation, caspase-3 cleavage, and cyclin E1 activation.65,66
LIPUS and muscle stem cells
In 2010, Chan et al.37 confirmed that LIPUS can promote muscle fiber regeneration after a muscle tear injury in a murine model and has better physiological properties, especially this promotion effect is more significant before the fourth week after surgery. This study utilized C2C12 cells which are subclones of C2 myoblasts that can differentiate in culture. These cells display many characteristics of the skeletal muscle, and they represent a reliable model for studying muscle tissue under a variety of normal and pathological conditions.36,67 Puts et al.68 reported that intracellular activator protein 1 (AP-1) increased when C2C12 cells were exposed to LIPUS at 3.6 MHz and that C2C12 cells proliferated fastest. Salgarella et al.69 found that the optimal LIPUS frequency to promote proliferation of C2C12 cells is 3 MHz and 1 W cm−2, which may maximize the activity of AP-1 and interact with Ras homolog family member A (RhoA), Rho-associated protein kinase (ROCK) ERK pathways. These authors also determined that the optimal LIPUS frequency to induce skeletal muscle differentiation is 1 MHz and 500 mW cm−2, which enhances the development of myotubes.1,36,70 The application of LIPUS on muscle stem cells holds highly potential for the treatment of muscle diseases in the clinic.
LIPUS and hematopoietic stem cells
In one study employing a rabbit model of anaplastic anemia (AA), LIPUS improved the hematopoietic microenvironment, accelerated the production of bone marrow cells, and increased the quantity and quality of red blood cells, white blood cells, and platelets in the peripheral blood.46 This suggests that LIPUS may be used as a new therapeutic strategy for AA in the future. In another study, LIPUS applied for 10 min daily for 4 days promoted the viability, proliferation, and differentiation of hematopoietic stem/progenitor cells (HSPCs) derived from fresh and frozen peripheral blood leukocyte isolates and cord blood. This study revealed that LIPUS not only enhanced the proliferation of fresh HSPCs but also maintained the activity of cryopreserved HSPCs in vitro. In addition, LIPUS also enhanced burst-forming unit-erythroid colony formation.39 At present, there are few reports on the clinical application of LIPUS and its therapeutic effects on blood diseases and anemia, so this field is more worth exploring.
LIPUS and other MSCs
Periodontal disease results in the loss of dental support tissue and impairs healing. Studies have shown that both the structure and the function of damaged periodontal tissue can be restored by tissue engineering techniques based on stem cell transplantation,71 biomaterial grafts,72 and growth factor gene delivery.73 Several studies have also suggested that LIPUS might be clinically beneficial in promoting dental tissue regeneration. Indeed, in vitro and in vivo studies indicated that the exposure of dental tissues to LIPUS may promote dentinogenesis, accelerated periodontal tissue healing, and dental implant osseointegration.35,38,74
Enhancement of fracture healing is critical for the recovery and return of function after fracture. LIPUS has been proven to be effective in enhancing fracture healing by providing local acoustic mechanical stimulation. Several clinical trials have demonstrated accelerated healing after LIPUS in tibial fracture,75 complex fractures,76 and nonunion.77,78
In another study, the authors investigated the effects of combined treatment of exogenous MSCs and LIPUS on fracture healing by comparing LIPUS-MSC, MSC, and control groups. Radiography and quantitative callus width/area demonstrated that the MSC-LIPUS group had the best healing, followed by the MSC group, then the control group with the poorest healing.79 There were significant differences among each at different time points. Micro-computerized tomography (CT) data supported that MSC-LIPUS had the highest bone volume/tissue volume. Additional studies suggest that LIPUS improves the osteogenic commitment of hMSCs in vitro and enhances their osteogenic differentiation.43 These findings all indicate that the combined treatment of MSCs and LIPUS is beneficial to fracture healing.79
LIPUS is often used as an adjunct to surgery.80 It is especially valuable for high-risk patients who are not recommended for surgery, such as patients with extreme hypertension, shock, metabolic acidosis, and multiple organ failure.81 In clinical studies, LIPUS has achieved positive results in the treatment of new fractures82 and nonunion.83 Compared with general treatment, LIPUS speeds up the repair of radius and tibia in conservative treatment.8 From a long-term perspective, the clinical application of LIPUS will bring benefits to orthopedic patients.
MOLECULAR MECHANISM OF LIPUS IN ACTIVATING STEM/PROGENITOR CELLS: CELLULAR SIGNALING PATHWAYS
The molecular mechanisms of mechanotransduction of LIPUS have been studied extensively since 2007. The related molecular mechanisms of LIPUS on stem cells are more complicated. Very recently, several novel technologies have been used in this field. In 2017, Kipniss et al.84 developed a “ChaCha” system based on clustered regularly interspaced short palindromic repeats (CRISPR) technology. This development will significantly accelerate the exploration of the molecular mechanisms of LIPUS. Thus far, scientists have identified 10 cellular signaling pathways that are regulated by LIPUS. These include YAP signaling pathway, Piezo signaling pathway, angiotensin signaling pathway, TGF-β1 signaling pathway, and mitogen-activated protein kinase (MAPK), ERK, and protein kinase B (Akt) signaling pathways. Further research has focused on several major signaling pathways (Figure 1). In addition, studies reveal that stem cell signaling pathways respond to LIPUS differently dependent on the energy level applied (Table 2).15,42,85,86,87,88,89,90
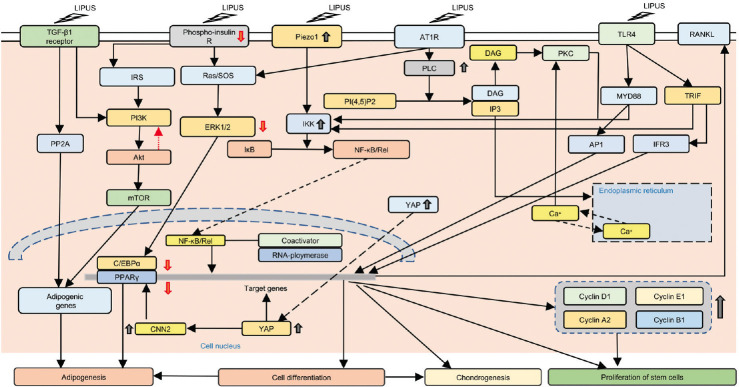
Figure 1.
LIPUS acts on stem cells through several signaling pathways. LIPUS can reduce the level of YAP phosphorylation, increase the active YAP in the cell, and promote the proliferation of C2C12 cells. LIPUS can promote the transfer of YAP to the nucleus of 3T3-L1 cells, resulting in the upregulation of CCN2 in the cells and reducing the expression of PPAR genes, which hinders the generation of mature fat cells. In addition, LIPUS reduces insulin signaling pathways by inhibiting insulin receptor phosphorylation, ERK1/2, and Akt, thereby inhibiting adipocyte differentiation. LIPUS can reduce the effect of the AT1-PLCβ pathway on the NF-κB translocation in the nucleus, while increasing the expression of Piezo1, activating the NF-κB signal through the piezoelectric 1-dependent pathway, increasing the expression of RANKL, promoting bone matrix formation, and enhancing osteogenesis. LIPUS can inhibit the activation of TLR4 channels by LPS and promote the expression of cyclin and the proliferation of hADMSCs. LIPUS: low-intensity pulsed ultrasound; AT1: angiotensin II type 1; AT1R: angiotensin II receptor type 1; TLR4: toll-like receptor 4; RANKL: receptor activator of nuclear factor kappa-B ligand; IRS: insulin receptor substrate; SOS: son of sevenless; PLC: phospholipase C; DAG: diacylglycerol; PKC: protein kinase C; MYD88: myeloid differentiation primary response 88; TRIF: TIR-domain-containing adapter-inducing interferon-β; IP3: inositol trisphosphate; PI3K: phosphoinositide 3-kinases; Akt: protein kinase B; ERK: extracellular signal-regulated kinases; IKK: IκB kinase; PP2A: protein phosphatase 2; mTOR: mechanistic target of rapamycin; YAP: yes-associated protein; PPARγ: peroxisome proliferator-activated receptor gamma; CNN2: calponin 2; AP1: activator protein 1; IFR3: interferon regulatory factor 3; NF-κB: nuclear factor kappa B.
Table 2.
Cellular signaling pathways of various stem cell types regulated by different energy levels of LIPUS
| Cellular signaling pathway | Stem cell type | Study | Year of publication | LIPUS energy level (mW cm−2) | Frequency (MHz) | Treatment time (min) | Equipment |
|---|---|---|---|---|---|---|---|
| TGF-β1 | BMSCs | Xia et al.87 | 2017 | 20–50 | 3 | 20 | The LIPUS exposure device (HT2009–1, ITO Corporation Ltd., Tokyo, Japan) |
| ERK and Akt | ADSCs BMSCs |
Ling et al.86
Xie et al.15 |
2017 2019 |
30 50–60 |
0.25 1.5 |
30 5 |
The LIPUS exposure device (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) consists of an array of six transducers (34.8 mm in diameter) |
| Angiotensin | Osteoblast | Bandow et al.85 | 2007 | 30 | 1.5 | 20 | An array of 6 PZT-4 (lead-zirconate titanate) transducers (2.5 cm in diameter) fixed with a locking device |
| YAP | C2C12 MSCs |
Puts et al.90
Nishida et al.91 |
2018 2020 |
44.5 60 |
3.6 3.0 |
5 20 |
ST-SONIC apparatus (ITO Corporation Ltd.) |
| MAPK | MSCs | Gao et al.88 | 2017 | 250–750 | 1 | 5 | A calibrated therapeutic ultrasound device (DuoSon, SRA Developments, Ashburton, UK) |
| Piezo | HEK Dental stem cells |
Gao et al.88
Prieto et al.89 |
2017 2018 |
250–750 50 000–90 000 |
1 43 |
5 0–0.012 |
A modified Axioskop-2 microscope (Zeiss Microscopes, Jena, Germany) with a 40× W N-Achroplan objective (Zeiss Microscopes) |
LIPUS: low-intensity pulsed ultrasound; TGF-β1: transforming growth factor β1; ERK: extracellular signal-regulated kinase; Akt: protein kinase B; YAP: yes-associated protein; MAPK: mitogen-activated protein kinase; BMSCs: bone marrow mesenchymal stem cells; ADSCs: adipose-derived stem cells; MSCs: mesenchymal stem cells; HEK: human embryonic kidney
YAP signaling pathway
YAP plays a role in mechanical transduction in determining the fate of cells, such as C2C12 mesenchymal precursors. Recent studies have shown that LIPUS reduces the level of serine 127 phosphorylated YAP, resulting in higher levels of active YAP in the nucleus. This enhances the expression of YAP target genes associated with actin nucleation and stability, cytokinesis, and cell cycle progression. Thus, LIPUS enhances the proliferation of C2C12 cells via the YAP signaling pathway. Silencing YAP expression, however, eliminates the beneficial effects of LIPUS. Overall, studies reveal that exposure to LIPUS modulates the function of YAP and improves cell proliferation potential, which is essential for the process of tissue regeneration.90
Recently, studies have also shown that LIPUS promotes the translocation of YAP to the nucleus of 3T3-L1 cells, resulting in the upregulation of the CCN family protein 2 (CCN2), a cellular communication network factor. Forced expression of CCN2 in 3T3-L1 cells reduces the expression of PPARγ gene but does not increase the expression of alkaline phosphatase and osterix genes. Finally, CCN2 gene silencing in C3H10T1/2 cells eliminates the effect of LIPUS on PPARγ and CCAAT/enhancer binding protein α (C/EBPα) gene expression.91
Piezo signaling pathway
Piezoelectric channels Piezo1 and Piezo2 are mechanically sensitive membrane ion channels. The expression and the role of Piezo1 and Piezo2 in the dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs) after LIPUS treatment have been studied. Piezo1 and Piezo2 are present in both tooth cell types. LIPUS significantly increased the level of piezoelectric proteins in DPSC 24 h after treatment. No significant effect was observed in PDLSC after LIPUS. Treatment with Ruthenium red (RR; a polycationic pore blocker) significantly inhibited LIPUS-stimulated DPSC proliferation but not PDLSC proliferation. Within 24 h after LIPUS, the ERK1/2 MAPK signaling in DPSC was activated, and the phosphorylated c-Jun N-terminal kinase (p-JNK) and p38 MAPK in the PDLSC were increased. RR affects MAPK signaling in both tooth cell types, with the most significant effect on ERK1/2 MAPK phosphorylation levels. RR’s significant inhibition of LIPUS-induced stimulation of ERK1/2 activation in the DPSCs suggests that the DPSC cellular response to LIPUS involves piezoelectric-mediated modulation of ERK1/2 MAPK signaling. In conclusion, this study is the first to support the role of piezoelectric ion channels in transducing LIPUS responses in dental stem cells.88
Another author reported on an experimental system that can perform patch-clamp recording in the presence of LIPUS at a frequency of 43 MHz, a known frequency that stimulates nerve activity.89 The patch-clamp recordings reveal that cell membrane stress caused by sound waves in Piezo1 channel is activated by a continuous wave ultrasound of 43 MHz at 50 W cm−2 or 90 W cm−2. The NaV1.2 channel is not affected by this mechanism at these intensities, but ultrasound-induced heating can accelerate its kinetics.
Angiotensin signaling pathway
LIPUS therapy is a method of applying mechanical stress to tissue. LIPUS is used clinically to promote tissue regeneration. In 2007, a team from Japan applied LIPUS to different stages of osteoblast maturation and analyzed the resultant chemokine and cytokine expression. Compared with immature osteoblasts, mature osteoblasts express high levels of nuclear factor κB ligand (RANKL), monocyte chemotactic protein (MCP)-1, and macrophage inflammatory protein (MIP). Interestingly, protein and mRNA expression of the mechanical receptor angiotensin II type 1 (AT1), which is a known mechanical receptor in cardiomyocytes, was detected in osteoblasts, and the expression levels increased significantly during cell maturation. In addition, LIPUS-induced ERK phosphorylation and RANKL/chemokine expression were abolished by specific AT1 inhibitors. Therefore, AT1, as a mechanoreceptor of osteoblasts, may play an important role in bone metabolism.85
In 2020, another group studied the effects of LIPUS on the differentiation of adipocytes. These authors demonstrated that LIPUS inhibited the gene expression of angiotensinogen (AGT) produced by mature adipocytes, as well as angiotensin-converting enzyme 1 (ACE1) and blood vessel gene expression of angiotensin.91 Studies have shown that LIPUS inhibits fat formation by inhibiting insulin signaling and decreases PPARγ expression by increasing the production of CCN2, resulting in a decrease in mature adipocytes.
TGF-β1 signaling pathway
Previous studies have shown that the biological activity of stem cells can be activated by mechanical stress. In 2017, Xia’s team found that LIPUS stimulates the expression of β1 integrin in the BMSCs and that TGF-β1, collagen 2 (COL2), aggrecan, and SRY-box transcription factor 9 (SOX9) increased significantly.87 These results indicate that the influence of LIPUS on cartilage differentiation of BMSCs may be caused by integrin-mediated mechanical transduction pathway. They also found that after LIPUS stimulation, the expression of phosphorylated mechanistic target of rapamycin (p-mTOR) in the BMSCs increased significantly, leading to speculation that LIPUS may promote the phosphorylation of mTOR in the BMSC, thereby promoting the adaptation to moderate mechanical stress. The inhibitory effect of mTOR affects the cartilage differentiation of BMSCs and the therapeutic effect of LIPUS. When the mTOR inhibitor rapamycin was used, the expression of COL2, aggrecan, and SOX9 in the BMSCs was significantly reduced, indicating that mTOR may be a positive factor for BMSCs cartilage differentiation. In addition, LIPUS stimulation after rapamycin treatment had no significant effect on the expression of COL2, aggrecan, SOX9, or COL1 in the BMSCs. Therefore, mTOR is a positive factor in LIPUS-stimulated BMSC cartilage differentiation.87
MAPK signaling pathway
Recently, a study showed that LIPUS promotes the proliferation of MSCs in a strength- and cell-specific manner by activating a unique MAPK pathway.88 MSCs separated from tooth tissues were treated with 1 MHz LIPUS at 250 mW cm−2 or 750 mW cm−2 for 5 min or 20 min, respectively. After 24 h of incubation after a single LIPUS treatment, cell proliferation was assessed by 5-bromo-2-deoxyuridine (BrdU) staining. Up to 4 h after treatment, total and activated p38, ERK1/2, and JNK MAPK signaling proteins were measured using specific enzyme-linked immunosorbent assay (ELISA). Selective MAPK inhibitors PD98059 (ERK1/2), SB203580 (p38), and SP600125 (JNK) were used to determine the activation of specific MAPK pathways. The results showed that after LIPUS treatment, the proliferation of all MSC types increased significantly. In MSC, JNK MAPK signaling was activated immediately after LIPUS, and phosphorylation of p38 MAPK increased significantly in these cells 4 h after exposure.88
This effect on MAPK is related to endoplasmic reticulum (ER) stress-related markers including activation of transcription factor 4 (ATF-4) and phosphorylated eukaryotic initiation factor 2α (eIF2α). Finally, inhibition of p38 reduces LIPUS-induced increases in phosphorylation of eIF2α and ATF-4 levels. Taken together, these results indicate that LIPUS promotes human endothelial cell apoptosis and inhibits angiogenesis by activating p38 MAPK-mediated ER stress signals.92
ERK and Akt signaling pathway
The effects and mechanisms of LIPUS on the proliferation of human amniotic mesenchymal stem cells (hADMSCs) were studied. The authors demonstrated that LIPUS promotes the proliferation of hADMSCs. Cell cycle analysis showed that LIPUS promotes cells from G0/G1 phase to S phase and G2/M phase. Western blot results indicated that LIPUS promotes the phosphorylation and activation of ERK1/2 and Akt and significantly upregulates the expression of cyclin D1, cyclin E1, cyclin A2, and cyclin B1. ERK1/2 inhibitor (U0126) and phosphoinositide 3-kinases (PI3K) inhibitor (LY294002) significantly reduced LIPUS-induced phosphorylation of ERK1/2 and Akt, respectively, thereby reducing LIPUS-induced proliferation of hADMSC. In conclusion, LIPUS promotes the proliferation of hADMSCs, and ERK1/2 and PI3K-Akt signaling pathways may play an important role in this process.86
In another study, the effects and mechanisms of LIPUS on hBMSCs were studied.15 The authors found that LIPUS promotes the proliferation of hBMSC when the exposure time is 5 min or 10 min per day. LIPUS increased the phosphorylation of PI3K/AKt and significantly upregulated the expression of cyclin D1. These effects were inhibited when cells were treated with PI3K inhibitors (LY294002), thereby reducing LIPUS-mediated proliferation. This indicates that LIPUS may provoke the proliferation of hBMSCs by activating PI3K/AKt signaling pathway and by increasing expression of cyclin D. LIPUS intensity of 50 mW cm−2 or 60 mW cm−2 and an exposure time of 5 min were determined to be the ideal LIPUS exposure parameters to stimulate hBMSC proliferation.
APPLICATION OF LIPUS IN UROLOGY
According to statistics, 2%–14% of men worldwide have chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) symptoms. These patients have no evidence of infection but have chronic pelvic pain.93 Karpukhin et al.94 first applied ultrasound therapy for CP in 1977. Additional studies by Li and other investigators found that transabdominal ultrasound treatment has a positive effect on CP, especially to relieve prostate pain.95 According to a literature review, rectal and abdominal LIPUS treatments are effective in improving clinical symptoms of CP/CPPS.96
In recent years, LIPUS has been demonstrated to have benefits on diverse pathological processes of human body. Several studies have focused on whether LIPUS has potential to improve erectile function. In a study by Lei et al.97 in 2015, LIPUS was found to improve erectile function and to reverse pathologic changes in penile tissue of streptozotocin (STZ)-induced type I diabetic erectile dysfunction (ED) rats. The content of endothelial and smooth muscle, the ratio of collagen I/collagen III, the number of elastic fibers, and the expression of endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) all increased.97 This suggests that LIPUS has potential for the treatment of ED and that its clinical effects need to be further verified.
Stress urinary incontinence (SUI) is related to pelvic floor weakness and impaired urethral sphincter. The histopathology of SUI includes loss of muscle cells, replacement of muscle tissue with fibrous tissue, and aging of the sphincter complex.98 Recently, stem cell-based therapy has been utilized for urinary sphincter deficiency and for nerve regeneration to treat SUI in many studies.98 Very recently, using a rat model of SUI, scientists found that LIPUS restored the leak point pressure and bladder capacity and activated satellite cell myodifferentiation.99 These findings suggest that LIPUS may be a promising therapy for human SUI in the future.
PERSPECTIVES
LIPUS has many effects on stem cells. Studies show that LIPUS can stimulate stem cells in vitro; promote stem cell proliferation, differentiation, and migration; maintain stem cell activity; alleviate the problems of insufficient seed cell source, differentiation, and maturation; and circumvent the low efficiency of stem cell transplantation. The mechanisms involved in the effects of LIPUS are not fully understood, but the effects demonstrated in studies thus far have been favorable. Much additional research is needed before LIPUS can progress from basic science research to large-scale clinical dissemination and application.
AUTHOR CONTRIBUTIONS
TFL, GL, ZL, and SJX generated the concept. YT, YG, and GL collected the information and drafted the manuscript. GL, ABRM, and TFL reviewed and edited the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This article was supported by China Scholarship Council (No. 201808420351).
REFERENCES
- 1.Abrunhosa VM, Soares CP, Batista Possidonio AC, Alvarenga AV, Costa-Felix RP, et al. Induction of skeletal muscle differentiation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 2014;40:504–12. doi: 10.1016/j.ultrasmedbio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175–86. doi: 10.1007/s00277-012-1438-x. [DOI] [PubMed] [Google Scholar]
- 4.Charif N, Li YY, Targa L, Zhang L, Ye JS, et al. Aging of bone marrow mesenchymal stromal/stem cells: implications on autologous regenerative medicine. Biomed Mater Eng. 2017;28:S57–63. doi: 10.3233/BME-171624. [DOI] [PubMed] [Google Scholar]
- 5.Jin X, Zhang Z, Lu Y, Fan Z. Suppression of long non-coding RNA LET potentiates bone marrow-derived mesenchymal stem cells (BMSCs) proliferation by up-regulating TGF-β1. J Cell Biochem. 2018;119:2843–50. doi: 10.1002/jcb.26459. [DOI] [PubMed] [Google Scholar]
- 6.Peng W, Zhang J, Zhang H, Liu G, Dong W, et al. Effects of lentiviral transfection containing bFGF gene on the biological characteristics of rabbit BMSCs. J Cell Biochem. 2018;119:8389–97. doi: 10.1002/jcb.27034. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HN, Hou XK, Tang TT, Leng P. [Experimental research on human insulin-like growth factor I gene transfect the cultured bone marrow mesenchymal stem cells] Zhonghua Wai Ke Za Zhi. 2005;43:263–7. [Article in Chinese] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhang S, Zeng D, Xia L, Lamichhane A, et al. rhPDGF-BB promotes proliferation and osteogenic differentiation of bone marrow stromal cells from streptozotocin-induced diabetic rats through ERK pathway. Biomed Res Int. 2014;2014:637415. doi: 10.1155/2014/637415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, Zhou W, Liu S, Chen P, Wu H. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int J Clin Exp Med. 2015;8:7125–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Cai B, Zhang AG, Zhang X, Ge WJ, Dai GD, et al. Promoting effects on proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by four “Kidney-Tonifying” traditional Chinese herbs. Biomed Res Int 2015. 2015 doi: 10.1155/2015/792161. 792161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin WP, Chen XW, Zhang LQ, Wu CY, Huang ZD, et al. Effect of neuroglobin genetically modified bone marrow mesenchymal stem cells transplantation on spinal cord injury in rabbits. PLoS One. 2013;8:e63444. doi: 10.1371/journal.pone.0063444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Qiu T, Zhang P, Wang X, Yin Y, et al. IKVAV regulates ERK1/2 and Akt signalling pathways in BMMSC population growth and proliferation. Cell Prolif. 2014;47:133–45. doi: 10.1111/cpr.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Yu J, Yang Y, Tang X, Zhao D, et al. Effect of 1 mT sinusoidal electromagnetic fields on proliferation and osteogenic differentiation of rat bone marrow mesenchymal stromal cells. Bioelectromagnetics. 2013;34:453–64. doi: 10.1002/bem.21791. [DOI] [PubMed] [Google Scholar]
- 14.Song G, Ju Y, Soyama H, Ohashi T, Sato M. Regulation of cyclic longitudinal mechanical stretch on proliferation of human bone marrow mesenchymal stem cells. Mol Cell Biomech. 2007;4:201–10. [PubMed] [Google Scholar]
- 15.Xie S, Jiang X, Wang R, Xie S, Hua Y, et al. Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating PI3K/AKt signaling pathways. J Cell Biochem. 2019;120:15823–33. doi: 10.1002/jcb.28853. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Gao Q, Zhu S, Wu Q, Zhu R, et al. Low-intensity pulsed ultrasound regulates proliferation and differentiation of neural stem cells through notch signaling pathway. Biochem Biophys Res Commun. 2020;526:793–8. doi: 10.1016/j.bbrc.2020.03.142. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Motoyoshi M, Shinoda M, Iwata K, Shimizu N. Low-intensity pulsed ultrasound accelerates nerve regeneration following inferior alveolar nerve transection in rats. Eur J Oral Sci. 2016;124:246–50. doi: 10.1111/eos.12271. [DOI] [PubMed] [Google Scholar]
- 18.Maung WM, Nakata H, Miura M, Miyasaka M, Kim YK, et al. Low-intensity pulsed ultrasound stimulates osteogenic differentiation of periosteal cells in vitro. Tissue Eng Part A. 2021;27:63–73. doi: 10.1089/ten.TEA.2019.0331. [DOI] [PubMed] [Google Scholar]
- 19.Amini A, Chien S, Bayat M. Impact of ultrasound therapy on stem cell differentiation - a systematic review. Curr Stem Cell Res Ther. 2020;15:462–72. doi: 10.2174/1574888X15666200225124934. [DOI] [PubMed] [Google Scholar]
- 20.Xia B, Chen G, Zou Y, Yang L, Pan J, et al. Low-intensity pulsed ultrasound combination with induced pluripotent stem cells-derived neural crest stem cells and growth differentiation factor 5 promotes sciatic nerve regeneration and functional recovery. J Tissue Eng Regen Med. 2019;13:625–36. doi: 10.1002/term.2823. [DOI] [PubMed] [Google Scholar]
- 21.Xin Z, Lin G, Lei H, Lue TF, Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5:255–66. doi: 10.21037/tau.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Savchenko O, Li Y, Qi S, Yang T, et al. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng. 2019;66:2704–18. doi: 10.1109/TBME.2018.2889669. [DOI] [PubMed] [Google Scholar]
- 23.Elvey MH, Miller R, Khor KS, Protopapa E, Horwitz MD, et al. The use of low-intensity pulsed ultrasound in hand and wrist nonunions. J Plast Surg Hand Surg. 2020;54:101–6. doi: 10.1080/2000656X.2019.1693393. [DOI] [PubMed] [Google Scholar]
- 24.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384–98. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Romano CL, Romano D, Logoluso N. Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol. 2009;35:529–36. doi: 10.1016/j.ultrasmedbio.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Padilla F, Puts R, Vico L, Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54:1125–45. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Cui JH, Park K, Park SR, Min BH. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: an in vivo study. Tissue Eng. 2006;12:75–82. doi: 10.1089/ten.2006.12.75. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Choi BH, Min BH, Son YS, Park SR. Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artif Organs. 2006;30:707–15. doi: 10.1111/j.1525-1594.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang YK, Chen CS. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J Cell Mol Med. 2013;17:823–32. doi: 10.1111/jcmm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu B, Zhang Y, Zhou J, Li J, Deng F, et al. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS One. 2014;9:e95168. doi: 10.1371/journal.pone.0095168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marvel S, Okrasinski S, Bernacki SH, Loboa E, Dayton PA. The development and validation of a LIPUS system with preliminary observations of ultrasonic effects on human adult stem cells. IEEE Trans Ultrason Ferroelectr Freq Control. 2010;57:1977–84. doi: 10.1109/TUFFC.2010.1645. [DOI] [PubMed] [Google Scholar]
- 32.Ebisawa K, Hata K, Okada K, Kimata K, Ueda M, et al. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:921–9. doi: 10.1089/1076327041348437. [DOI] [PubMed] [Google Scholar]
- 33.Choi JW, Choi BH, Park SH, Pai KS, Li TZ, et al. Mechanical stimulation by ultrasound enhances chondrogenic differentiation of mesenchymal stem cells in a fibrin-hyaluronic acid hydrogel. Artif Organs. 2013;37:648–55. doi: 10.1111/aor.12041. [DOI] [PubMed] [Google Scholar]
- 34.Sena K, Leven RM, Mazhar K, Sumner DR, Virdi AS. Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med Biol. 2005;31:703–8. doi: 10.1016/j.ultrasmedbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Ikai H, Tamura T, Watanabe T, Itou M, Sugaya A, et al. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodontal Res. 2008;43:212–6. doi: 10.1111/j.1600-0765.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 36.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1191–204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- 37.Chan YS, Hsu KY, Kuo CH, Lee SD, Chen SC, et al. Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: an in vitro and in vivo study. Ultrasound Med Biol. 2010;36:743–51. doi: 10.1016/j.ultrasmedbio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Al-Daghreer S, Doschak M, Sloan AJ, Major PW, Heo G, et al. Long term effect of low intensity pulsed ultrasound on a human tooth slice organ culture. Arch Oral Biol. 2012;57:760–8. doi: 10.1016/j.archoralbio.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, et al. Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett. 2012;34:1965–73. doi: 10.1007/s10529-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 40.Lv Y, Zhao P, Chen G, Sha Y, Yang L. Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett. 2013;35:2201–12. doi: 10.1007/s10529-013-1313-4. [DOI] [PubMed] [Google Scholar]
- 41.Bozkurt A, Boecker A, Tank J, Altinova H, Deumens R, et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials. 2016;75:112–22. doi: 10.1016/j.biomaterials.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Xia B, Zou Y, Xu Z, Lv Y. Gene expression profiling analysis of the effects of low-intensity pulsed ultrasound on induced pluripotent stem cell-derived neural crest stem cells. Biotechnol Appl Biochem. 2017;64:927–37. doi: 10.1002/bab.1554. [DOI] [PubMed] [Google Scholar]
- 43.Costa V, Carina V, Fontana S, De Luca A, Monteleone F, et al. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J Cell Physiol. 2018;233:1558–73. doi: 10.1002/jcp.26058. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Liu Y, Cai Y, Li X, Bai M, et al. Ultrasound irradiation combined with hepatocyte growth factor accelerate the hepatic differentiation of human bone marrow mesenchymal stem cells. Ultrasound Med Biol. 2018;44:1044–52. doi: 10.1016/j.ultrasmedbio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Mohaqiq M, Movahedin M, Mokhtari Dizaji M, Mazaheri Z. Upregulation of integrin-α6 and integrin-β1 gene expressions in mouse spermatogonial stem cells after continues and pulsed low intensity ultrasound stimulation. Cell J. 2018;19:634–9. doi: 10.22074/cellj.2018.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Chen W, Jiang J, Zhou W, Zhang Y, et al. Treatment effect of low-intensity pulsed ultrasound on benzene- and cyclophosphamide-anduced aplastic anemia in rabbits. Phys Ther. 2019;99:1443–52. doi: 10.1093/ptj/pzz074. [DOI] [PubMed] [Google Scholar]
- 47.Burks SR, Ziadloo A, Hancock HA, Chaudhry A, Dean DD, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One. 2011;6:e24730. doi: 10.1371/journal.pone.0024730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmadi F, McLoughlin IV, Chauhan S, Ter-Haar G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog Biophys Mol Biol. 2012;108:119–38. doi: 10.1016/j.pbiomolbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Yue Y, Yang X, Wei X, Chen J, Fu N, et al. Osteogenic differentiation of adipose-derived stem cells prompted by low-intensity pulsed ultrasound. Cell Prolif. 2013;46:320–7. doi: 10.1111/cpr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–68. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 51.Peng S, Gao D, Gao C, Wei P, Niu M, et al. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (review) Mol Med Rep. 2016;14:623–9. doi: 10.3892/mmr.2016.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 53.Winter A, Breit S, Parsch D, Benz K, Steck E, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–29. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 54.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84:191–7. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 56.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–7. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 57.Huang T, He D, Kleiner G, Kuluz J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med. 2007;30(Suppl 1):S35–40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 59.Jing W, Lin Y, Wu L, Li X, Nie X, et al. Ectopic adipogenesis of preconditioned adipose-derived stromal cells in an alginate system. Cell Tissue Res. 2007;330:567–72. doi: 10.1007/s00441-007-0493-4. [DOI] [PubMed] [Google Scholar]
- 60.Ning GZ, Song WY, Xu H, Zhu RS, Wu QL, et al. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: better choice of transplantation treatment for spinal cord injury: treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci Ther. 2019;25:496–508. doi: 10.1111/cns.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sant’Anna EF, Leven RM, Virdi AS, Sumner DR. Effect of low intensity pulsed ultrasound and BMP-2 on rat bone marrow stromal cell gene expression. J Orthop Res. 2005;23:646–52. doi: 10.1016/j.orthres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Fu N, Yang X, Ba K, Fu Y, Wei X, et al. Low-intensity pulsed ultrasound induced enhanced adipogenesis of adipose-derived stem cells. Cell Prolif. 2013;46:312–9. doi: 10.1111/cpr.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oatley JM, Reeves JJ, McLean DJ. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. 2004;71:942–7. doi: 10.1095/biolreprod.104.028894. [DOI] [PubMed] [Google Scholar]
- 64.Moghaddam ZH, Mokhtari-Dizaji M, Movahedin M, Ravari ME. Estimation of the distribution of low-intensity ultrasound mechanical index as a parameter affecting the proliferation of spermatogonia stem cells in vitro. Ultrason Sonochem. 2017;37:571–81. doi: 10.1016/j.ultsonch.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–94. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Menz MD, Oralkan O, Khuri-Yakub PT, Baccus SA. Precise neural stimulation in the retina using focused ultrasound. J Neurosci. 2013;33:4550–60. doi: 10.1523/JNEUROSCI.3521-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, et al. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem. 2004;48:223–33. [PubMed] [Google Scholar]
- 68.Puts R, Rikeit P, Ruschke K, Kadow-Romacker A, Hwang S, et al. Activation of mechanosensitive transcription factors in murine C2C12 mesenchymal precursors by focused low-intensity pulsed ultrasound (FLIPUS) IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:1505–13. doi: 10.1109/TUFFC.2016.2586972. [DOI] [PubMed] [Google Scholar]
- 69.Salgarella AR, Cafarelli A, Ricotti L, Capineri L, Dario P, et al. Optimal ultrasound exposure conditions for maximizing C2C12 muscle cell proliferation and differentiation. Ultrasound Med Biol. 2017;43:1452–65. doi: 10.1016/j.ultrasmedbio.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Ikeda K, Takayama T, Suzuki N, Shimada K, Otsuka K, et al. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci. 2006;79:1936–43. doi: 10.1016/j.lfs.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 71.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavik E, Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 73.Madonna R, De Caterina R. Stem cells and growth factor delivery systems for cardiovascular disease. J Biotechnol. 2011;154:291–7. doi: 10.1016/j.jbiotec.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Wu G, Chen L, Zhu G, Wang Y. Low-intensity ultrasound accelerates mandibular implant bone integration in dogs with mandibular osteoradionecrosis. J Surg Res. 2013;182:55–61. doi: 10.1016/j.jss.2012.03.062. [DOI] [PubMed] [Google Scholar]
- 75.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Leung KS, Lee WS, Tsui HF, Liu PP, Cheung WH. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol. 2004;30:389–95. doi: 10.1016/j.ultrasmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Nolte PA, van der Krans A, Patka P, Janssen IM, Ryaby JP, et al. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma. 2001;51:693–702. doi: 10.1097/00005373-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 79.Cheung WH, Chin WC, Wei FY, Li G, Leung KS. Applications of exogenous mesenchymal stem cells and low intensity pulsed ultrasound enhance fracture healing in rat model. Ultrasound Med Biol. 2013;39:117–25. doi: 10.1016/j.ultrasmedbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Lerner A, Stein H, Soudry M. Compound high-energy limb fractures with delayed union: our experience with adjuvant ultrasound stimulation (exogen) Ultrasonics. 2004;42:915–7. doi: 10.1016/j.ultras.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 81.Leighton R, Watson JT, Giannoudis P, Papakostidis C, Harrison A, et al. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and meta-analysis. Injury. 2017;48:1339–47. doi: 10.1016/j.injury.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 82.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961–73. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Gebauer D, Mayr E, Orthner E, Ryaby JP. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. 2005;31:1391–402. doi: 10.1016/j.ultrasmedbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Kipniss NH, Dingal P, Abbott TR, Gao Y, Wang H, et al. Engineering cell sensing and responses using a GPCR-coupled CRISPR-Cas system. Nat Commun. 2017;8:2212. doi: 10.1038/s41467-017-02075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bandow K, Nishikawa Y, Ohnishi T, Kakimoto K, Soejima K, et al. Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol. 2007;211:392–8. doi: 10.1002/jcp.20944. [DOI] [PubMed] [Google Scholar]
- 86.Ling L, Wei T, He L, Wang Y, Wang Y, et al. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50:e12383. doi: 10.1111/cpr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia P, Wang X, Qu Y, Lin Q, Cheng K, et al. TGF-beta1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8:281. doi: 10.1186/s13287-017-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Q, Cooper PR, Walmsley AD, Scheven BA. Role of Piezo channels in ultrasound-stimulated dental stem cells. J Endod. 2017;43:1130–6. doi: 10.1016/j.joen.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M. Activation of Piezo1 but not NaV1.2 channels by ultrasound at 43 MHz. Ultrasound Med Biol. 2018;44:1217–32. doi: 10.1016/j.ultrasmedbio.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puts R, Rikeit P, Ruschke K, Knaus P, Schreivogel S, et al. Functional regulation of YAP mechanosensitive transcriptional coactivator by focused low-intensity pulsed ultrasound (FLIPUS) enhances proliferation of murine mesenchymal precursors. PLoS One. 2018;13:e0206041. doi: 10.1371/journal.pone.0206041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishida T, Nagao Y, Hashitani S, Yamanaka N, Takigawa M, et al. Suppression of adipocyte differentiation by low-intensity pulsed ultrasound via inhibition of insulin signaling and promotion of CCN family protein 2. J Cell Biochem. 2020 doi: 10.1002/jcb.29680. Doi: 10.1002/jcb.29680. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 92.Su Z, Xu T, Wang Y, Guo X, Tu J, et al. Lowintensity pulsed ultrasound promotes apoptosis and inhibits angiogenesis via p38 signalingmediated endoplasmic reticulum stress in human endothelial cells. Mol Med Rep. 2019;19:4645–54. doi: 10.3892/mmr.2019.10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cohen JM, Fagin AP, Hariton E, Niska JR, Pierce MW, et al. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS One. 2012;7:e41941. doi: 10.1371/journal.pone.0041941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karpukhin VT, Nesterov NI, Roman DL. [Ultrasonic therapy of chronic prostatitis] Vopr Kurortol Fizioter Lech Fiz Kult. 1977;3:75–7. [Article in Russian] [PubMed] [Google Scholar]
- 95.Li HS, Wang B, Han L, Wang CH, Xin ZC. [Transperineal ultrasonic therapy for chronic prostatitis] Zhonghua Nan Ke Xue. 2013;19:49–53. [Article in Chinese] [PubMed] [Google Scholar]
- 96.Lin G, Reed-Maldonado AB, Lin M, Xin Z, Lue TF. Effects and Mechanisms of low-intensity pulsed ultrasound for chronic prostatitis and chronic pelvic pain syndrome. Int J Mol Sci. 2016;17:1057. doi: 10.3390/ijms17071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lei H, Xin H, Guan R, Xu Y, Li H, et al. Low-intensity pulsed ultrasound improves erectile function in Streptozotocin-induced type I diabetic rats. Urology. 2015;86:1241.e11–8. doi: 10.1016/j.urology.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 98.Klein G, Hart ML, Brinchmann JE, Rolauffs B, Stenzl A, et al. Mesenchymal stromal cells for sphincter regeneration. Adv Drug Deliv Rev. 2015;82–3:123–36. doi: 10.1016/j.addr.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 99.Yang B, Li M, Lei H, Xu Y, Li H, et al. Low intensity pulsed ultrasound influences the myogenic differentiation of muscle satellite cells in a stress urinary incontinence rat model. Urology. 2019;123:297.e1–8. doi: 10.1016/j.urology.2018.09.020. [DOI] [PubMed] [Google Scholar]