Abstract
Lower urinary tract symptoms suggestive of benign prostate hyperplasia (LUTS/BPH) and depression are both increasing in Chinese aging males. However, the relationship still remains unknown. To explore their relationship, a retrospective cohort study based on propensity score matching (PSM) was conducted by analyzing the China Health and Retirement Longitudinal Study dataset. After data cleaning, a total of 5125 participants were enrolled and subjected to PSM; 1351 pairs were matched and followed for 2 years. Further logistic regression and restricted cubic spline (RCS) were performed to evaluate, model and visualize the relationship between depression and LUTS/BPH. Moreover, subgroup analyses and sensitivity analyses were adopted to verify the robustness of the conclusions. Before PSM, depressive patients showed higher odds of LUTS/BPH in all three models adjusting for different covariates (P < 0.001). After PSM, univariate logistic regression revealed that depressive patients had higher risks for LUTS/BPH than participants in the control group (odds ratio [OR] = 2.10, P < 0.001). The RCS results indicated a nonlinear (P < 0.05) and inverted U-shaped relationship between depression and LUTS/BPH. In the subgroup analyses, no increased risks were found among participants who were not married or cohabitating, received an education, had an abnormal body mass index (<18.5 kg m−2 and ≥28 kg m−2), slept more than 6 h, did not smoke, and drank less than once a month (all P > 0.05). The results of sensitivity analyses indicated identical increased risks of LUTS/BPH in all four models (all P < 0.001). In conclusion, depression enhances the risks of LUTS/BPH in aging males.
Keywords: aging males, benign prostatic hyperplasia, depression, lower urinary tract symptoms, propensity score matching
INTRODUCTION
Characterized by the unregulated enlargement of the prostate gland, benign prostatic hyperplasia (BPH) is becoming increasingly common. This enlargement could lead to compression of the urethra and bladder outlet obstruction.1 Sometimes, BPH remains asymptomatic and is not detected until health examination in the hospital, but it is the leading cause of lower urinary tract symptoms (LUTS), which present as increased urinary frequency and urgency, nocturia, urge incontinence, weak stream, etc.2 These miserable LUTS heavily burden these patients, and there is an urgent need for efficient methods to prevent and treat them.
With the aging of the population, the prevalence of LUTS/BPH has rapidly increased. In China, a study enrolling 8563 participants reported a LUTS/BPH prevalence of 11.97%. This number increased to 22.70% for men aged 70 years and older.3 It was estimated that a symptom-free man of 46 years had a 45% chance of developing LUTS/BPH over the coming 30 years, if he survived.4 In long-term studies, risk factors such as age, metabolic syndrome, obesity, and diet were identified,5,6 which enable early prevention.7 However, among all the risk factors, the role of depression is not clarified.
In modern society, depression has emerged as a common condition. The lifetime risk of depression ranges from 15% to 18%,8 and 80% of depressive patients will experience at least one recurrence in their lifetime.9 Both diseases are commonly observed in males; however, their relationship still remains unclear. Adverse impacts of LUTS/BPH may contribute to depression,10,11 and conversely, depression may activate the occurrence of LUTS/BPH by way of systemic inflammation.12 Limited studies have revealed some clues. Xiong et al.3 performed a cross-sectional study, in which participants assessed as having depression had a higher prevalence of LUTS/BPH than nondepressive participants. Notwithstanding, there is insufficient evidence to explain the relationship between LUTS/BPH and depression, which is what we aimed to do in the present study. To perform the present study, a dataset downloaded from the China Health and Retirement Longitudinal Study (CHARLS) was subjected to the relevant analyses.
PATIENTS AND METHODS
Data cleaning and study samples
To evaluate the risks of LUTS/BPH in depressive patients, a representative dataset from the CHARLS was downloaded and subjected to further data cleaning and analyses. This dataset was collected by well-trained researchers in 28 provinces of China using the probabilities proportional to size (PPS) sampling method. Specific descriptions regarding the CHARLS could be accessed via the official study website (http://charls.pku.edu.cn/) or publications.13 This study was reviewed and approved by the ethics committee of Peking University (IRB 00001052-11014; Beijing, China). Written and oral informed consent was obtained from all participants before their enrollment in this study.
In the present study, a retrospective cohort from March 2011 to August 2013 was constructed with the aid of propensity score matching (PSM) to balance the covariates between groups. In this dataset, participants were followed from 2011 to 2013. In 2011, a total of 17 705 participants were recruited. After removing the missing values and patients who had LUTS/BPH in 2011 (the data cleaning flowchart in Supplemental Figure 1 (1.1MB, tif) ), 5160 participants were remained. To balance the covariates in the two groups, the remaining study participants were subjected to PSM using 1:1 nearest neighbor matching with a caliper of 0.02. Finally, in 2011, 1380 participants who did not have depression were successfully matched with 1380 participants who had depression. The two matched groups were followed for 2 years, and their LUTS/BPH status was assessed in 2013.
Definition of depression and LUTS/BPH
In this dataset, depression was assessed by an effective epidemiological questionnaire, the Center for Epidemiological Studies Depression Scale-10 (CESD-10); participants were defined as depressive patients if their total scores were ≥10.14 This questionnaire is widely used in surveying depressive participants and has been demonstrated to be effective in previous studies.15 Furthermore, males were asked, “Have you ever been diagnosed with a prostate illness, such as prostate hyperplasia, excluding prostatic cancer?” Related symptoms of prostatic hyperplasia, including dysuria, intermittent urinary stream, frequent urination at night, splitting or spraying of the urinary stream, and slow urinary stream, were explained to all the participants. Prostate hyperplasia often leads to LUTS such as those stated above, but it is not absolute. In this dataset, symptoms were mainly assessed based on participant self-report. Hence, the term LUTS/BPH was defined here, which was also commonly used in previous studies.3,4
Covariates
In the present study, age, marital status, educational levels, body mass index (BMI), abdominal obesity, sleep duration, afternoon napping, smoking, alcohol consumption and hypertension, and hukou (a kind of household registration in China) were included as covariates. Hukou, like ID cards, was divided into agricultural hukou, nonagricultural hukou, and unified residence hukou in China. In general, participants with an agricultural hukou mainly settled in rural areas and had a relatively lower income than participants with a nonagricultural hukou who mainly lived in urban areas. Age was stratified into 40–50 years, 51–60 years, 61–70 years, and ≥71 years.3 Marital status was categorized into two groups. The first group included married individuals and individuals living together (cohabitating). The other group included the separated, divorced, widowed, never married groups, as well as individuals who were married but did not live together. Educational levels were grouped into five strata: illiterate, did not finish primary school but capable of reading or writing, private primary school, elementary school, and middle school. BMI was divided into four groups: <18.5 kg m−2, ≥18.5 and <24 kg m−2, ≥24 and <28 kg m−2, and ≥28 kg m−2 according to Zhang et al.’s study.16 Abdominal obesity was defined as waist circumference >90 cm for males.17 Sleeping time was categorized into three groups: <6 h, 6–8 h, and >8 h. Afternoon napping was categorized into two groups: yes and no. The smoking status consisted of yes, no, and quit. Alcohol consumption was categorized as once a month, less than once a month, and never. Hypertension was defined as systolic pressure ≥140 mmHg or diastolic pressure ≥90 mmHg according to the definition of the World Health Organization (WHO).18
Statistical analyses
In the present study, categorical data are shown as proportions (%), and continuous data are displayed as the mean ± standard deviation (s.d.). Differences in baseline characteristics in 2011 were evaluated by Student’s t-test for continuous data and the Chi-square test for categorical data. Furthermore, to assess the odds ratios (ORs) for the association between LUTS/BPH and depression, univariate logistic regression and multivariate logistic regression were adopted. After evaluating the baseline characteristics, missing values of covariates were interpolated using multivariate imputation by chained equations based on random forest methods. Then, PSM was employed to balance the covariates between the depression group and the nondepression group. Nearest neighbor matching with a caliper of 0.02 and 1:1 no-replacement matching was selected. After PSM, univariate logistic regression was selected to assess the OR values for the two matched groups. A restricted cubic spline was adopted to flexibly model and visualize the relationship between depression and LUTS/BPH with three knots. Finally, missing values were dropped, and the models were subjected to sensitivity analyses. All analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), and the figures were made using R version 3.6.3 or R version 4.0.2. P < 0.05 (two-sided) was considered statistically significant.
RESULTS
Baseline characteristics of the participants in 2011
In total, 5125 participants were included. Subjects were divided into a nondepressive group and a depressive group with 3690 and 1435 participants, respectively. Before PSM, all the covariates in Table 1 were statistically unbalanced (P < 0.05), except hypertension (P = 0.889). After PSM, 1380 pairs were successfully matched with 11 balanced covariates. The baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristic of participants in 2011
| Characteristic | Nondepression cases | Depression cases | Overall cases | χ2/t | P |
|---|---|---|---|---|---|
| Total, n (%) | 3690 (72.0) | 1435 (28.0) | 5125 (100.0) | - | - |
| Age (year), n (%) | 29.9832 | <0.001 | |||
| 40–50 | 790 (21.4) | 238 (16.6) | 1028 (20.1) | ||
| 51–60 | 1463 (39.7) | 526 (36.7) | 1989 (38.8) | ||
| 61–70 | 1001 (27.1) | 471 (32.8) | 1472 (28.7) | ||
| ≥71 | 434 (11.8) | 200 (13.9) | 634 (12.4) | ||
| Marital status, n (%) | 65.2937 | <0.001 | |||
| Married/cohabitated | 3356 (91.0) | 1191 (83.0) | 4547 (88.7) | ||
| Others | 334 (9.1) | 244 (17.0) | 578 (11.3) | ||
| Educational levels, n (%) | 140.2274 | <0.001 | |||
| Illiterate | 1016 (27.5) | 568 (39.6) | 1584 (30.9) | ||
| Did not finish primary school but capable of reading or writing | 955 (25.9) | 434 (30.2) | 1389 (27.1) | ||
| Primary private school | 1066 (28.9) | 306 (21.3) | 1372 (26.8) | ||
| Elementary school | 394 (10.7) | 99 (6.9) | 493 (9.6) | ||
| Middle school | 258 (7.0) | 28 (2.0) | 286 (5.6) | ||
| Hukou, n (%) | 64.8906 | <0.001 | |||
| Agricultural hukou | 2804 (76.0) | 1237 (86.2) | 4041 (78.9) | ||
| Nonagricultural hukou | 860 (23.3) | 190 (13.2) | 1050 (20.6) | ||
| Unified residence hukou | 24 (0.7) | 8 (0.6) | 32 (0.6) | ||
| BMI (kg m−2), n (%) | 62.1680 | <0.001 | |||
| <18.5 | 168 (5.3) | 124 (10.0) | 292 (6.6) | ||
| ≥18.5 and <24 | 1842 (57.7) | 779 (63.0) | 2621 (59.2) | ||
| ≥24 and <28 | 886 (27.7) | 255 (20.6) | 1141 (25.8) | ||
| ≥28 | 298 (9.3) | 78 (6.3) | 376 (8.5) | ||
| Abdominal obesity, n (%) | 28.6472 | <0.001 | |||
| Yes | 976 (30.3) | 278 (22.3) | 1254 (28.1) | ||
| No | 2241 (69.7) | 968 (77.7) | 3209 (71.9) | ||
| Sleeping time (h),n (%) | 156.8409 | <0.001 | |||
| <6 | 1554 (42.2) | 879 (61.5) | 2433 (47.6) | ||
| 6–8 | 1806 (49.1) | 447 (31.3) | 2253 (44.1) | ||
| >8 | 321 (8.7) | 104 (7.3) | 425 (8.3) | ||
| Afternoon napping, n (%) | 12.7493 | <0.001 | |||
| Yes | 2228 (60.4) | 788 (54.9) | 3016 (58.9) | ||
| No | 1462 (39.6) | 647 (45.1) | 2109 (41.2) | ||
| Smoking, n (%) | 12.4786 | 0.003 | |||
| Yes | 2124 (57.6) | 896 (62.4) | 3020 (58.9) | ||
| No | 992 (26.9) | 321 (22.4) | 1313 (25.6) | ||
| Quitted | 574 (15.6) | 218 (15.2) | 792 (15.5) | ||
| Alcohol consumption, n (%) | 29.1653 | <0.001 | |||
| Never | 1498 (40.6) | 701 (48.9) | 2199 (42.9) | ||
| Less than once a month | 416 (11.3) | 131 (9.1) | 547 (10.7) | ||
| More than once a month | 1776 (48.1) | 603 (42.0) | 2379 (46.4) | ||
| Systolic pressure (mmHg), mean±s.d. | 131.01±21.93 | 129.45±22.40 | 130.57±22.07 | 2.1048 | 0.0354 |
| Diastolic pressure (mmHg), mean±s.d. | 76.88±12.37 | 75.50±12.80 | 76.49±12.51 | 3.3029 | 0.0010 |
| Hypertension, n (%) | 0.0196 | 0.889 | |||
| Yes | 1236 (37.6) | 486 (37.8) | 1722 (37.6) | ||
| No | 2054 (62.4) | 800 (62.2) | 2854 (62.4) | ||
| LUTS/BPH cases in 2013, n (%) | 17.1483 | <0.001 | |||
| Yes | 166 (4.5) | 106 (7.4) | 272 (5.3) | ||
| No | 3524 (95.5) | 1329 (92.6) | 4853 (94.7) |
Discrepancies between depression and nondepression groups were tested according to their data types. Continuous data were analyzed by student’s t-test and categorical data were analyzed by Chi-square test. BMI: body mass index; -: no data; LUTS: lower urinary tract symptom; BPH: benign prostate hyperplasia; s.d.: standard deviation
Confirm the results of propensity scores matching
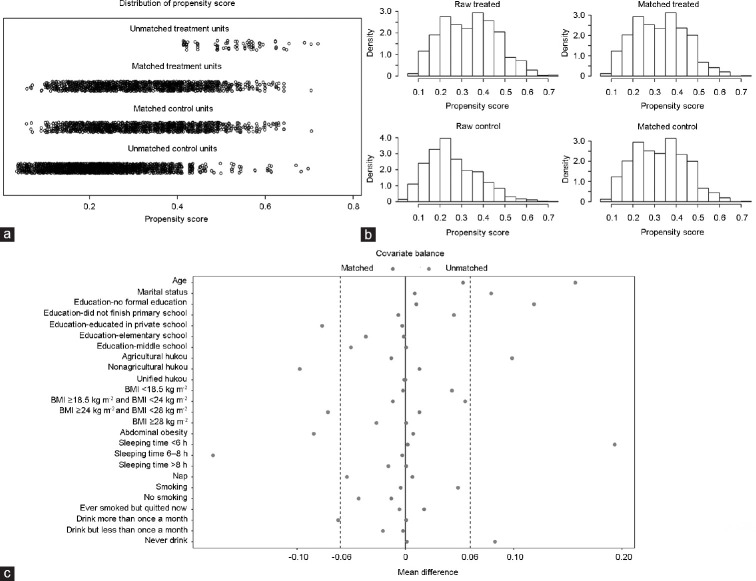
To demonstrate that the covariates reached an equilibrium, jitter plots, histograms of propensity scores, and love plots are shown in Figure 1a–1c, respectively. After PSM, the distribution of the propensity scores became symmetrical (Figure 1a). Figure 1b reveals that before PSM, the distributions of the propensity scores in the depressive group and nondepressive group were obviously different, while after PSM, they were nearly the same. Mean differences before PSM and after PSM were displayed in Figure 1c, which disclosed that after PSM, all the mean differences in the matched covariates were less than 6.0%. In conclusion, all covariates were well balanced by PSM in this study.
Figure 1.
Propensity scores matching. (a) The distribution of propensity scores in the control and treatment groups. (b) Histogram of propensity scores in the control and treatment groups. (c) The mean differences between the depression group and the nondepression group. BMI: body mass index.
Regression between depression and LUTS/BPH
To assess the ORs between depression and LUTS/BPH, four models were built (Figure 2). Before PSM and missing value interpolation, model 1, model 2, and model 3 were constructed. In model 1, univariate logistic regression was adopted, revealing that the depression group had a 1.69-time higher risk of LUTS/BPH than the nondepression group (95% confidence interval [CI]: 1.32–2.18, P < 0.001). In model 2, which was adjusted for educational levels, marital status, and hukou, the depression group still had a 2.01-time higher risk of LUTS/BPH than the nondepression group (95% CI: 1.55–2.61, P < 0.001). In model 3, which was adjusted for all the covariates, an OR value of 2.02 was estimated (95% CI: 1.50–2.72, P < 0.001). After PSM, model 4 was built using univariate logistic regression, and it revealed an OR value of 2.10 (95% CI: 1.48–2.98, P < 0.001).
Figure 2.
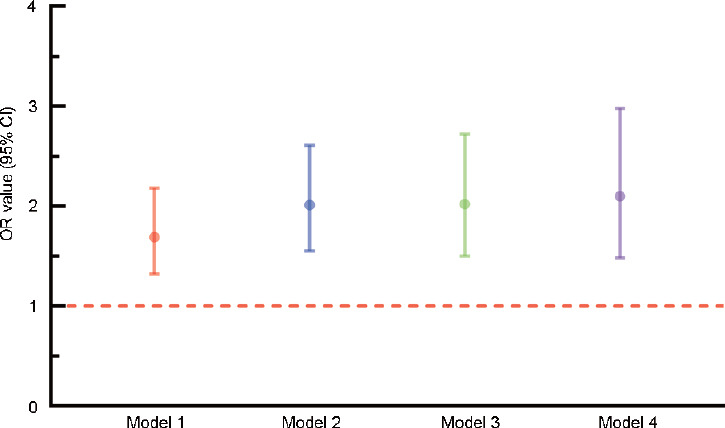
ORs in four different models. Model 1: adjusted for no other variables. Model 2: adjusted for educational level, marital status, and hukou. Model 3: adjusted for the model 2 variables plus age, BMI, abdominal obesity, sleeping time, afternoon napping, smoking, alcohol consumption, and hypertension. Model 4: adjusted for no other variables. CI: confidence interval; OR: odds ratio; BMI: body mass index.
Relationship between depression and LUTS/BPH
To further model and visualize the relationship between depression and LUTS/BPH, restricted cubic spline regression was selected. Participants with CESD-10 questionnaire scores = 10 were treated as the reference group. As shown in Figure 3, an inverted U-shaped relationship between depression and LUTS/BPH was detected. With the increases in CESD-10 scores, the ORs for LUTS/BPH increased. When the score was greater than 13, the ORs for LUTS/BPH began to fall. Notably, the relationship between depression and LUTS/BPH is nonlinear (nonlinearity P = 0.0159).
Figure 3.
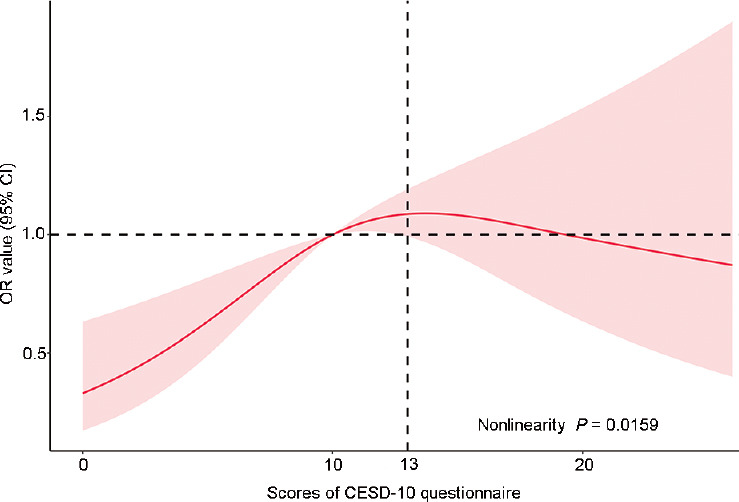
Restricted cubic spline regression between depression and LUTS/BPH. The red area was drawn using the data from the 95% CI. OR: odds ratio; CI: confidence interval; CESD-10: Center for Epidemiological Studies Depression Scale-10; LUTS/BPH: lower urinary tract symptoms suggestive of benign prostate hyperplasia.
Subgroups analyses
To further examine whether the relationship between LUTS/BPH and depression existed across subgroups, univariate logistic regression was adopted for subgroup analyses (Figure 4). No increased risks were found among participants who were not married or cohabitating (P = 0.326), received an education (P > 0.05), owned a nonagricultural hukou (P = 0.268), had an abnormal BMI (<18.5 kg m−2 and ≥28 kg m−2, P > 0.05), had abdominal obesity (P = 0.493), slept more than 6 h (P > 0.05), did not smoke (P > 0.05), and drank less than once a month (P = 0.699). However, increased risks were observed in all age groups (P < 0.05), men with an agricultural hukou (P < 0.001), participants without abdominal obesity (P < 0.001), both subjects who napped in the afternoon and those who did not (P < 0.01), and participants who did and did not have hypertension (P < 0.05).
Figure 4.
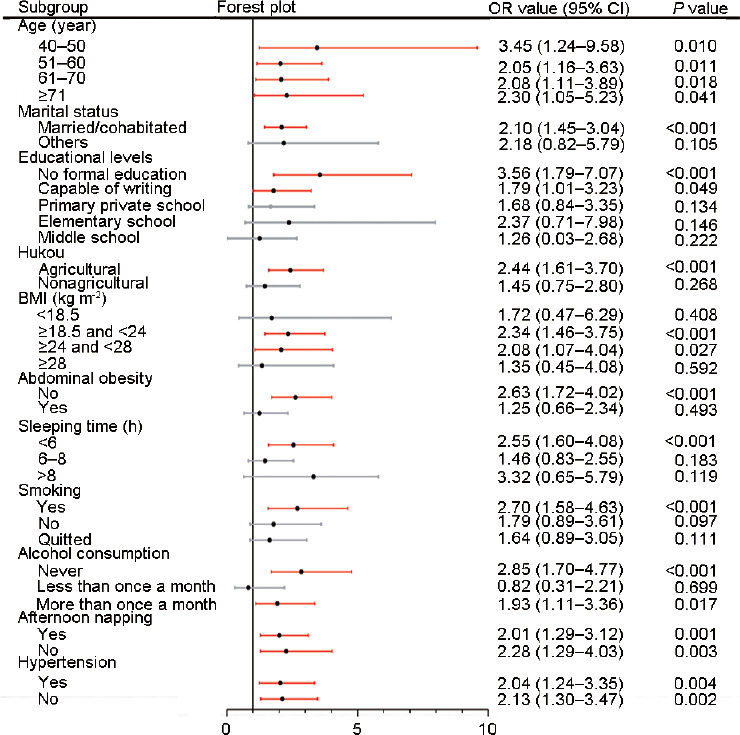
Results of subgroup analyses. Red lines indicated that the P values were significant (< 0.05). Gray lines indicated that the P values were not significant (P > 0.05). CI: confidence interval; OR: odds ratio; BMI: body mass index.
Sensitivity analysis
The analyses previously summarized before were based on interpolation of missing values using multivariate imputation by chained equations based on random forest methods. In this section, all the missing values were dropped. Then, analyses identical to those stated above were performed. After dropping the missing values, the remaining 4385 participants were subjected to univariate logistic regression and multivariate logistic regression to build model 1, model 2, and model 3 (Figure 5). In model 1, adjusted for no covariates, depressive patients had a 1.68-fold higher risk of LUTS/BPH than their counterparts (95% CI: 1.28–2.22, P < 0.001). In model 2, adjusted for educational levels, marital status, and hukou, a 1.93-fold increased risk of LUTS/BPH was detected (95% CI: 1.44–2.57, P < 0.001). In model 3, adjusted for all the covariates, the depression group had a 2.01-fold increased risk of LUTS/BPH (95% CI: 1.49–2.70, P < 0.001). Subsequently, the covariates were balanced using PSM with 1:1 no-replacement nearest neighbor matching and a caliper of 0.02. After all the covariates were balanced, univariate logistic regression was adopted to build model 4. In model 4, an OR value of 1.64 was estimated (95% CI: 1.14–2.36, P < 0.001). The results from this sensitivity analysis remained consistent with those from the previous analyses.
Figure 5.
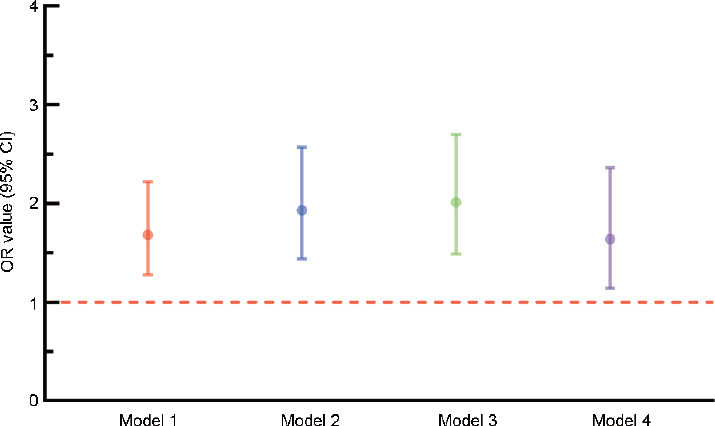
Results of the sensitivity analysis. Model 1: adjusted for no other variables. Model 2: adjusted for educational level, marital status and hukou. Model 3: adjusted for the model 2 variables plus age, BMI, abdominal obesity, sleeping time, afternoon napping, smoking, alcohol consumption and hypertension. Model 4: adjusted for no other variables. CI: confidence interval; OR: odds ratio; BMI: body mass index.
DISCUSSION
Currently, the global trend toward aging is becoming more pronounced, with an increasing proportion of the population being older.19 In the aging population, depression and LUTS/BPH are frequently concurrent; accordingly, further exploration to confirm the relationship between them is merited. The CHARLS, a follow-up dataset targeting the aging population in China, offered us an opportunity to study this relationship.
In the present study, after a 2-year follow-up, depressed participants in 2011 displayed higher risks of suffering LUTS/BPH in 2013 than the nondepressed group. Both before and after propensity score matching, this conclusion remained unchanged. In other studies, this association was also disclosed, but the direction of causality was not addressed. According to Wong et al.20, patients with moderate to severe LUTS were at risk of having clinically relevant depressive symptoms,20 which were also revealed by Coyne et al.21 and Breyer et al.22 However, virtually all these publications were based on cross-sectional studies, which can only provide clues regarding the relationship between these conditions. The direction of causation remains unclear. A prospective cohort study from Huang et al.23 demonstrated that BPH patients had a 1.87-time higher risk of suffering depression than their healthy counterparts after adjusting for some covariates. These literatures indicated that depression and LUTS/BPH could interact with each other and involved a cause-and-effect relationship on both sides.24 In this study, we showed that depression could elevate the risk of LUTS/BPH, providing novel evidence for the causal direction from depression to LUTS/BPH. Moreover, an inverted U-shaped relationship between depression and LUTS/BPH risk was detected in our study, indicating a nonlinear relationship.
Although a clear association between depression and LUTS/BPH was disclosed here, the underlying mechanism still remains unknown. This relationship may be explained by systemic inflammation. According to previous studies,25,26 a psychological stressor could activate fundamental inflammatory signaling pathways (such as nuclear factor-kappa B [NF-κB]) in human peripheral blood mononuclear cells. In male patients with major depression, this phenomenon was confirmed again by Pace et al.27 Moreover, systemic inflammation could also lead to the proliferation of epithelial and stromal prostatic cells followed by prostate hyperplasia and LUTS.28,29 Thus, systemic inflammation, a mediating role in depression and LUTS/BPH, may be responsible for the increased incidence of LUTS/BPH.
In the subgroup analyses, these risks were not identified in some subgroups. For educational levels, statistical significance was only observed in men with no formal education, which revealed that education may play a protective role. One study performed in 319 female Hispanic depressive patients showed similar findings.30 Two-thirds of their participants received less than high school education, and patients with college education reported less stigma surrounding depression than participants with lower education levels.30 Decreasing the incidence and impact of depression certainly leads to reduced LUTS/BPH risks. Interestingly, an increased risk was not observed in participants with BMI <18.5 kg m−2. The potential reason may be attributed to inflammation induced by obesity, a sort of metabolic disease, which further contributes to LUTS/BPH.31 Although some evidence of increased risks in participants with BMI ≥28 kg m−2 was revealed, the difference in risks between the two BMI groups did not reach the traditional level of statistical significance. Further cohort studies targeting obesity, depression and LUTS/BPH should be performed.
For sleeping time, a trend toward higher risks of LUTS/BPH was also unveiled in patients with shorter sleep duration. This trend was also detected for afternoon napping. In general, people who sleep longer indicate higher sleep quality and lower risks of sleep-related diseases, which are closely related to depression.32,33 On the other hand, depressive patients are inherently sleep deprived;34 therefore, participants in this experiment who were short of sleep had a higher risk of depression than those who were well rested, which led to a consequent increase in the incidence of LUTS/BPH. It was also found that men who did not smoke and drank less than once a month did not have an increased risk of LUTS/BPH, revealing a potential protective role for moderate alcohol intake and not smoking cigarettes. Previous studies that aimed to evaluate this phenomenon have also produced different results, with no unifying conclusions. In a meta-analysis that included 19 studies, alcohol intake of 36 g or more per day was associated with a 35% reduction in the likelihood of BPH.35 In a prospective longitudinal cohort study, heavy smoking was reported to be significantly correlated with LUTS/BPH, while high alcohol intake was not.36 Whether protective or irrelevant, neither of these studies reported that alcohol intake increased the risk of LUTS/BPH. However, the effect of cigarette seems more complicated. Xu et al.37 concluded that there was no difference in BPH risk between current smokers, nonsmokers, and ever smokers, no matter how the smokers smoked. These inconsistent conclusions still need more persuasive evidence to clarify the relationship between smoking and LUTS/BPH risk.
The present study still has some limitations. First, although we adopted an effective method, PSM, to balance the covariates at baseline, we must admit that latent covariates not collected in the CHARLS, especially the willingness of patients to visit doctors, may bias the conclusion. Depressed patients are generally reluctant to seek therapy and undergo a medical examination, which could contribute to the worsening of the BPH condition and justify the results. Second, due to the design of our study, some potentially protective covariates in the subgroup analyses, such as education, still need further validation, and we have only provided initial clues here. Furthermore, a simplified method to define LUTS/BPH according to Zhang et al.38 method was adopted. The definition of LUTS/BPH was mainly based on self-report as opposed to more objective tests such as prostatic ultrasonography, which may bias the diagnoses.
In conclusion, depression enhances the risks of LUTS/BPH for aging males. For patients with LUTS/BPH, attention should be paid to their mental status, and if the patient is depressed at the same time, treatment targeting depression should be considered. Not being married, living alone, higher educational levels, lower BMI, longer sleeping time, smoking cessation, and moderate alcohol consumption act as potentially protective factors, but further cohort studies are needed for validation.
CONCLUSIONS
Depression enhances the risks of LUTS/BPH in aging males. Not being married, living alone, higher educational levels, lower BMI, longer sleeping time, smoking cessation, and moderate alcohol consumption act as potentially protective factors, but further cohort studies are needed for validation.
AUTHOR CONTRIBUTIONS
YX carried out the data analyses and drafted the manuscript. YCZ helped to carry out the statistical analysis and draft the manuscript. TJ participated in its design and coordination. FQ helped to revise the manuscript. JHY conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
Data cleaning flowchart. LUTS/BPH: lower urinary tract symptoms suggestive of benign prostate hyperplasia; PSM: propensity score matching.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (No. 81871147 and No. 81671453), Sichuan Science and Technology Program (2018TJPT0018), and Chengdu Science and Technology Program (No. 2019-YFYF-00087-SN). The data that support the findings of this study are available from China Health and Retirement Longitudinal Study (CHARLS) website (http://charls.pku.edu.cn/).
REFERENCES
- 1.Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S11–8. doi: 10.1038/ijir.2008.55. [DOI] [PubMed] [Google Scholar]
- 2.Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016;2:16031. doi: 10.1038/nrdp.2016.31. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Y, Zhang Y, Li X, Qin F, Yuan J. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male. 2020 doi: 10.1080/13685538.2020.1781806. Doi: 10.1080/13685538.2020.1781806. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Verhamme KM, Dieleman JP, Bleumink GS, van der Lei J, Sturkenboom MC, et al. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care – the Triumph project. Eur Urol. 2002;42:323–8. doi: 10.1016/s0302-2838(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 5.Vignozzi L, Gacci M, Maggi M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat Rev Urol. 2016;13:108–19. doi: 10.1038/nrurol.2015.301. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Peng L, Cao D, Gou H, Li Y, et al. The association between metabolic syndrome and benign prostatic hyperplasia: a systematic review and meta-analysis. Aging Male. 2020 doi: 10.1080/13685538.2020.1771552. Doi: 10.1080/13685538.2020.1771552. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Peng B, Lei GL, Wei Q, Yang L. Study of phosphodiesterase 5 inhibitors and α-adrenoceptor antagonists used alone or in combination for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Minerva Urol Nefrol. 2020;72:13–21. doi: 10.23736/S0393-2249.19.03408-8. [DOI] [PubMed] [Google Scholar]
- 8.Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 10.Kupelian V, Wei JT, O’Leary MP, Kusek JW, Litman HJ, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 11.Coyne KS, Wein AJ, Tubaro A, Sexton CC, Thompson CL, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103(Suppl 3):4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 12.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43:61–8. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andresen EM, Byers K, Friary J, Kosloski K, Montgomery R. Performance of the 10-item Center for Epidemiologic Studies Depression scale for caregiving research. SAGE Open Med. 2013;1:2050312113514576. doi: 10.1177/2050312113514576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amtmann D, Kim J, Chung H, Bamer AM, Askew RL, et al. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol. 2014;59:220–9. doi: 10.1037/a0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Xiong Y, Dong J, Guo T, Tang X, et al. Caffeinated drinks intake, late chronotype, and increased body mass index among medical students in Chongqing, China: a multiple mediation model. Int J Environ Res Public Health. 2018;15:1721. doi: 10.3390/ijerph15081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 18.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801–12. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Y. Towards deeper research and better policy for healthy aging - using the unique data of Chinese longitudinal healthy longevity survey. China Economic J. 2012;5:131–49. doi: 10.1080/17538963.2013.764677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SY, Hong A, Leung J, Kwok T, Leung PC, et al. Lower urinary tract symptoms and depressive symptoms in elderly men. J Affect Disord. 2006;96:83–8. doi: 10.1016/j.jad.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009;103(Suppl 3):24–32. doi: 10.1111/j.1464-410X.2009.08438.x. [DOI] [PubMed] [Google Scholar]
- 22.Breyer BN, Kenfield SA, Blaschko SD, Erickson BA. The association of lower urinary tract symptoms, depression and suicidal ideation: data from the 2005-2006 and 2007-2008 National Health and Nutrition Examination Survey. J Urol. 2014;191:1333–9. doi: 10.1016/j.juro.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CY, Chiu KM, Chung SD, Keller JJ, Huang CC, et al. Increased risk of depressive disorder following the diagnosis of benign prostatic enlargement: one-year follow-up study. J Affect Disord. 2011;135:395–9. doi: 10.1016/j.jad.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Dunphy C, Laor L, Te A, Kaplan S, Chughtai B. Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Rev Urol. 2015;17:51–7. doi: 10.3909/riu0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 28.Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology. 2019;65:458–64. doi: 10.1159/000496289. [DOI] [PubMed] [Google Scholar]
- 29.Hung SF, Chung SD, Kuo HC. Increased serum C-reactive protein level is associated with increased storage lower urinary tract symptoms in men with benign prostatic hyperplasia. PLoS One. 2014;9:e85588. doi: 10.1371/journal.pone.0085588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez V, Sanchez K, Killian MO, Eghaneyan BH. Depression screening and education: an examination of mental health literacy and stigma in a sample of Hispanic women. BMC Public Health. 2018;18:646. doi: 10.1186/s12889-018-5516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2019;22:12–9. doi: 10.1080/13685538.2018.1434772. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Rawtaer I, Fam J, Jiang MJ, Feng L, et al. Sleep correlates of depression and anxiety in an elderly Asian population. Psychogeriatrics. 2016;16:191–5. doi: 10.1111/psyg.12138. [DOI] [PubMed] [Google Scholar]
- 33.Chang KJ, Son SJ, Lee Y, Back JH, Lee KS, et al. Perceived sleep quality is associated with depression in a Korean elderly population. Arch Gerontol Geriatr. 2014;59:468–73. doi: 10.1016/j.archger.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 35.Parsons JK, Im R. Alcohol consumption is associated with a decreased risk of benign prostatic hyperplasia. J Urol. 2009;182:1463–8. doi: 10.1016/j.juro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 36.Choo MS, Han JH, Shin TY, Ko K, Lee WK, et al. Alcohol, smoking, physical activity, protein, and lower urinary tract symptoms: prospective longitudinal cohort. Int Neurourol J. 2015;19:197–206. doi: 10.5213/inj.2015.19.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Fu S, Chen Y, Chen Q, Gu M, et al. Smoking habits and benign prostatic hyperplasia: a systematic review and meta-analysis of observational studies. Medicine. 2016;95:e4565. doi: 10.1097/MD.0000000000004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Zhang X, Li H, Wu F, Wang H, et al. Prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) in China: results from the China Health and Retirement Longitudinal Study. BMJ Open. 2019;9:e022792. doi: 10.1136/bmjopen-2018-022792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data cleaning flowchart. LUTS/BPH: lower urinary tract symptoms suggestive of benign prostate hyperplasia; PSM: propensity score matching.