Abstract
To evaluate outcomes between extraperitoneal robotic single-port radical prostatectomy (epR-spRP) and extraperitoneal robotic multiport radical prostatectomy (epR-mpRP) performed with the da Vinci Si Surgical System, comparison was performed between 30 single-port (SP group) and 26 multiport (MP group) cases. Comparisons included operative time, estimated blood loss (EBL), hospital stay, peritoneal violation, pain scores, scar satisfaction, continence, and erectile function. The median operation time and EBL were not different between the two groups. In the SP group, the median operation time of the first 10 patients was obviously longer than that of the latter 20 patients (P < 0.001). The median postoperative hospital stay in the SP group was shorter than that in the MP group (P < 0.001). The rate of peritoneal damage in the SP group was less than that in the MP group (P = 0.017). The pain score and overall need for pain medications in the SP group were lower than those in the MP group (P < 0.001 and P = 0.015, respectively). Patients in the SP group were more satisfied with their scars than those in the MP group 3 months postoperatively (P = 0.007). At 3 months, the cancer control, recovery of erectile function, and urinary continence rates were similar between the two groups. It is safe and feasible to perform epR-spRP using the da Vinci Si surgical system. Therefore, epR-spRP can be a treatment option for localized prostate cancer. Although epR-spRP still has a learning curve, it has advantages for postoperative pain and self-assessed cosmesis. In the absence of the single-port robotic surgery platform, we can still provide minimally invasive surgery for patients.
Keywords: extraperitoneal approach, minimally invasive surgery, prostate cancer, robotic radical prostatectomy, single-port
INTRODUCTION
Robot-assisted radical prostatectomy (RARP) is a currently accepted standard of care for the surgical management of patients with localized prostate cancer.1,2,3 RARP has become the predominant surgical modality to manage localized prostate cancer in the US.4 In recent years, the number of RARP cases has been increasing in China. RARP and open radical prostatectomy can offer comparable oncological and functional outcomes, but the robotic approach offers potential advantages, including decreased blood loss, a shorter hospital stay, and a shorter recovery period.5,6,7 Traditional robotic radical prostatectomy often requires 5–6 puncture points, which is controversial with respect to obtaining better cosmesis and reducing surgical trauma and postoperative pain.8,9 With the development of technology, it is a challenge for urologists to balance the maximization of oncological results and the minimization of the surgery-related impact on patients’ quality of life.10
The role of laparoendoscopic single-site surgery (LESS) is to reduce trauma to the abdominal wall, minimize postoperative pain, and accelerate postoperative recovery.11 However, the inherent limitations of parallel instrumentation in LESS have limited further development of this technique.11,12 White et al.13 showed that the application of robotics can improve the outcomes of LESS radical prostatectomy. However, due to the challenges associated with ergonomics and intracorporeal suturing, robotic single-port radical prostatectomy is not widely used. Only a few centers have successfully performed single-port radical prostatectomy with the da Vinci Si surgical system worldwide.13,14,15 Currently, studies of robotic single-port radical prostatectomy, mainly via the transperitoneal approach13,14,16 and the extraperitoneal approach, have been rare. A systematic review and meta-analysis of data comparing transperitoneal and extraperitoneal RARP showed similar oncological and functional outcomes and a shorter operation time with the extraperitoneal approach.17,18 The extraperitoneal approach avoids the peritoneal cavity, has little effect on intestinal function, and does not require Trendelenburg position; thus, it shortens postoperative convalescence.19 Therefore, whether extraperitoneal robotic single-port radical prostatectomy (epR-spRP) will benefit to patients is unclear.
In this study, we describe our epR-spRP technique with the da Vinci Si Surgical System and present our preliminary clinical experience. Furthermore, we compared the perioperative and short-term postoperative outcomes of epR-spRP and extraperitoneal robotic multiport radical prostatectomy (epR-mpRP).
PATIENTS AND METHODS
Following approval from the Institutional Review Board and Medical Ethics Committee of Shanghai Changzheng Hospital (Shanghai, China), data were collected from April 2019 to March 2020. A total of 56 consecutive patients diagnosed with localized prostate cancer based on clinical characteristics, prostate biopsy results, prostate-specific antigen (PSA) levels, and imaging studies were included at Changzheng Hospital in Shanghai, China. These patients underwent extraperitoneal RARP with the da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Thirty patients underwent single-port surgery (SP group), and 26 patients underwent multiport surgery (MP group). Exclusion criteria included a previous infraumbilical midline incision, body mass index (BMI) >40 kg m−2, preoperative PSA level >20 ng ml−1, biopsy Gleason score >7, prior prostate treatment, or preoperative evidence of extraprostatic disease. After a comprehensive discussion, informed consent was obtained, and patients undergoing single-port surgery were advised to receive additional assistant ports as needed during the operation.
Data including perioperative information regarding age, BMI, previous abdominal surgeries, operation time (measured from skin incision to skin closure), estimated blood loss (EBL), conversion rate, peritoneal rupture, postoperative pain score, hospital stay, complications, and final incision satisfaction were collected. Complications were assessed intraoperatively or postoperatively using the Clavien–Dindo classification system20 and were classified as major (grade ≥III) or minor (grade ≤II). Pain was assessed using a visual analog pain scale (VAPS).21 Pathology data, including the final pathological stage, positive surgical margins (PSM), and lymph node invasion, were also recorded. All of the patients were followed up regularly to monitor the state of urinary continence (pads daily), erectile function (International Index of Erectile Function, IIEF-5), and biochemical recurrence (PSA >0.2 ng ml−1 twice in a row). No use or the use of no more than one urine pad per day was considered urinary continence.
Armamentarium of instruments
The da Vinci Si Surgical System (Intuitive Surgical) was used to perform RP. For the SP group, the scope holder arm and two primary robotic arms were used, while the fourth secondary arm was not used. An 8-cm quadri-channel laparoscopic port (Lagis Inc., Taichung, China) was used to perform epR-spRP. This port consists of one 12-mm port, two 8-mm ports, and an insufflation cannula through which CO2 pneumoperitoneum was achieved and set at 12–14 mmHg. For the MP group, the scope holder arm, the two primary robotic arms, and two 12-mm assistant ports were used.
Surgical technique
To achieve an extraperitoneal approach, patients were placed in a low lithotomy position with no steep Trendelenburg position. Prophylactic single-dose intravenous antibiotics (e.g., cephalosporins) and subcutaneous prophylactic heparin were administered prior to surgery. Lymphadenectomy was performed before completing vesicourethral anastomosis if the Gleason score was >6 and/or if the PSA level was >10 ng ml−1. Low-risk patients underwent intrafascial nerve-sparing RP. The operation was performed in a manner similar to that of extraperitoneal RARP, as previously reported.9 We also made slight modifications for epR-spRP with the da Vinci Si System. All of the patients underwent epR-spRP by two high-volume surgeons who were experienced and beyond their learning curves with extraperitoneal multiport robotic surgery.
epR-spRP approach
A single 4- to 5-cm transverse incision was made at the upper 3 transverse fingers of the pubic symphysis (Figure 1a and Supplementary Figure 1a (310.9KB, tif) ). The skin, subcutaneous fat, and abdominal external oblique aponeurosis were carefully dissected layer by layer (Supplementary Figure 1b (310.9KB, tif) ). The rectus abdominis muscle was bluntly divided at the midline, and the retropubic anterior bladder space was fully dissociated. The port was placed (Figure 2a), and the scope holder arm (30° upward) and the two primary robotic arms were installed (Figure 2b). A drainage tube was routinely placed, and a postoperative image of the incision after epR-spRP is shown in Figure 2c. Representative images of epR-spRP are shown in Figure 3. The periurethral structure was reconstructed as much as possible to improve urinary continence (Figure 3k). To increase the working space, we used a posterior peritoneal suspension stitch, and the thread was fixed outside the abdominal wall with straight forceps (Figure 3l). Damage to the inferior epigastric vessels was avoided.
Figure 1.
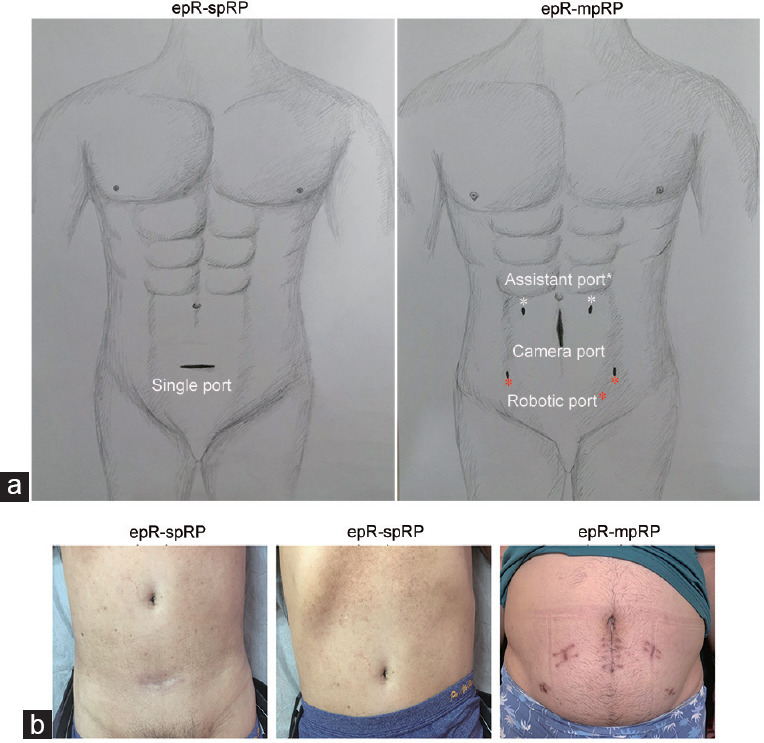
Position of the incision and images of the incision after 3 months. (a) The epR-spRP incision was made at the upper 3 transverse fingers of the pubic symphysis. The epR-mpRP incisions include an incision for placing the camera port, two incisions for placing the robotic arm ports, and two incisions for placing the assistant ports. Dr. Qi-Wei Yang provided the drawing. (b) Images of the incision 3 months after epR-spRP; the incisions were completely obscured by underwear. Images of the incisions after epR-mpRP, which were obvious. epR-spRP: extraperitoneal robotic single-port radical prostatectomy; epR-mpRP: extraperitoneal robotic multiport radical prostatectomy.
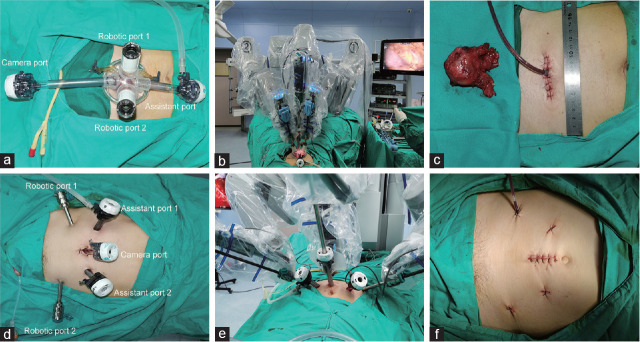
Figure 2.
Port and robotic arm placement. (a) Port placement in epR-spRP. (b) The scope holder arm and the two primary robotic arms were used in epR-spRP. (c) Postoperative image of the incision after epR-spRP. (d) Port placement in epR-mpRP. (e) Robotic arm installation in epR-mpRP. (f) The incision after epR-mpRP. epR-spRP: extraperitoneal robotic single-port radical prostatectomy; epR-mpRP: extraperitoneal robotic multiport radical prostatectomy.
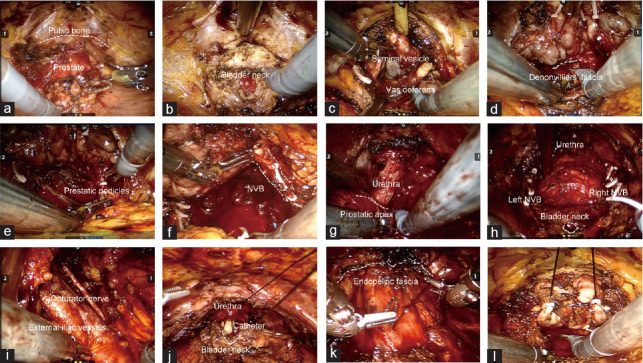
Figure 3.
Representative images of epR-spRP. (a) The preperitoneal working space was enlarged. (b) The bladder neck was dissected. (c) The seminal vesicle and vas deferens were dissected. (d) The prostate and rectal space were separated. (e) The prostatic pedicles were dissected. (f) The neurovascular bundles were spared. (g) The prostatic apex and urethra were dissected. (h) The prostate was resected. (i) Lymphadenectomy. (j) Vesicourethral anastomosis. (k) The periurethral structure was reconstructed. (l) Posterior peritoneal suspension was used. epR-spRP: extraperitoneal robotic single-port radical prostatectomy; NVB: neurovascular bundle.
During single-port surgery, assistants should be careful not to blur the vision of the operator caused by drastic changes in pneumoperitoneum pressure and to avoid air leakage caused by damage to the port when changing the instrument. It is undeniable that, because there is no instrument that allows for wrist movement at the end, the scope holder arm and robotic arms should be moved according to the situation when changing the surgical field of vision. The 30° upward angle of view was used throughout the single-port operation.
epR-mpRP approach
A 3-cm midline longitudinal skin incision was made below the umbilicus to place the 12-mm camera port. The anterior rectus sheath was divided at the midline, and a finger was used to progress the preperitoneal space into the pubic bone at the midline. A self-made airbag was introduced into the anterior bladder space under the pubic bone. An adequate working space was then achieved by inflating the airbag with 600–800 ml of air. Two 8-mm robotic arm ports were placed 8 cm from the camera port. Two 12-mm ports were placed above the robotic arm ports and the midpoint of the camera port connection for assistance. The assistant ports were placed 8 cm from the camera port and robotic arm ports. The incisions and port placement images used for epR-mpRP are shown in Figure 1a and 2d–2f, respectively.
Statistical analyses
Normally distributed data were analyzed with the one-sample Kolmogorov–Smirnov test. Univariate analysis was performed using parametric (Student’s t-test) and non-parametric (Mann–Whitney U test) tests for continuous variables and the Chi-square test for categorical variables, as appropriate. Statistical significance was set at P < 0.05.
RESULTS
Description of baseline features
There was no difference in age, BMI, preoperative PSA level, or clinical stage between the two groups. No metastases were found by MRI or bone scintigraphy before the operation. Fifteen patients (50.0%) in the SP group and 12 patients (46.2%) in the MP group had an IIEF-5 score >21. According to the D’Amico risk classification of prostate cancer,22 there were 8 medium-risk patients (26.7%) in the SP group and 6 medium-risk patients (23.1%) in the MP group (P = 0.757), and all of the others were low-risk patients. Six patients (20.0%) in the SP group had a history of abdominal surgery: 3 with a history of appendectomy, 1 with traumatic splenectomy, and 2 with herniorrhaphy. Five patients (19.2%) in the MP group had a history of abdominal surgery: 4 with a history of appendectomy and 1 with herniorrhaphy. The baseline characteristics of patients during the perioperative period are shown in Table 1.
Table 1.
Preoperative patient characteristics
| Characteristic | SP group (n=30) | MP group (n=26) | P |
|---|---|---|---|
| Age (year), median (IQR) | 64.5 (60.0–69.0) | 66.5 (61.0–69.3) | 0.542 |
| BMI (kg m−2), median (IQR) | 23.9 (21.7–25.3) | 23.7 (21.9–25.5) | 0.783 |
| Prior abdominal surgery, n (%) | 6 (20.0) | 5 (19.2) | 0.942 |
| PSA (ng ml−1), median (IQR) | 9.0 (7.9–9.8) | 9.4 (8.7–10.8) | 0.304 |
| cT stage, n (%) | 0.979 | ||
| cT1c | 16 (53.3) | 14 (53.8) | |
| cT2a | 10 (33.3) | 9 (34.6) | |
| cT2b | 4 (13.3) | 3 (11.5) | |
| Biopsy ISUP grade, n (%) | 0.799 | ||
| Grade group 1 | 21 (70.0) | 19 (73.1) | |
| Grade group 2 | 3 (10.0) | 3 (11.5) | |
| Grade group 3 | 6 (20.0) | 4 (15.4) | |
| D’Amico risk classification, n (%) | 0.757 | ||
| Low-risk | 22 (73.3) | 20 (76.9) | |
| Medium-risk | 8 (26.7) | 6 (23.1) | |
| IIEF-5 score, n (%) | 0.774 | ||
| >21 | 15 (50.0) | 12 (46.2) | |
| ≤21 | 15 (50.0) | 14 (53.8) |
IQR: interquartile range; BMI: body mass index; PSA: prostate-specific antigen; IIEF-5: International Index of Erectile Function; SP: single-port; MP: multiport; ISUP: International Society of Urological Pathology
Surgical outcomes
All of the patients underwent extraperitoneal RARP successfully. No patient required additional ports, and no patient in the SP group underwent epR-mpRP and open conversion. Lymph node dissection was performed in 8 patients in the SP group and 6 patients in the MP group. Overall, the median operation time was not significantly different between the SP and MP groups (142.5 min vs 134.0 min; P = 0.097). Furthermore, the median operation time of the latter 20 patients in the SP group was shorter than that of the first 10 patients (132.0 min vs 192.5 min; P < 0.001), similar to that of patients in the MP group (Figure 4). The median EBL between the two groups was not different (123.0 ml vs 120.0 ml; P = 0.166), and no patient received a perioperative blood transfusion. The median hospital stay in the SP group was significantly shorter than that in the MP group (4.5 days vs 7.0 days; P < 0.001). The nerve preservation rates and operative complications were similar between the two groups, and no major complications occurred in either group. The rate of peritoneal damage in the SP group was significantly less than that in the MP group (0 vs 19.2%; P = 0.017). Three of the five patients who experienced peritoneal damage in the MP group had a history of lower abdominal surgery. The PSM rate was not different between the SP and MP groups. The lymph nodes of all of the patients were negative. The complete surgical results and postoperative pathological stages are shown in Table 2.
Figure 4.
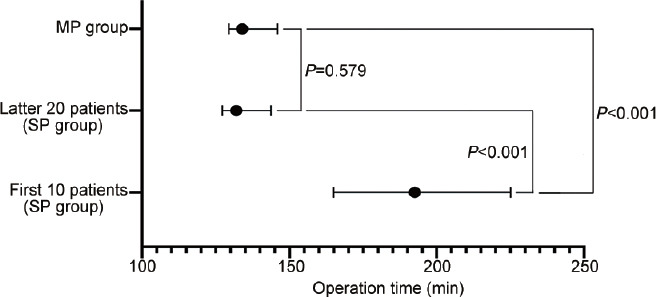
Operation time in the SP and MP groups. The median operation time of the first 10 patients in the SP group was significantly longer than that of the latter 20 patients in the SP group and that in the MP group (P< 0.001). The median operation time of the latter 20 patients in the SP group was not significantly different from that of patients in the MP group (P= 0.579). SP: single-port; MP: multiport.
Table 2.
Comparisons of intraoperative and postoperative data and complications between the single-port group and the multiport group
| Variable | SP group (n=30) | MP group (n=26) | P |
|---|---|---|---|
| Operative time (min), median (IQR) | 142.5 (129.5–180.0) | 134 (129.5–146) | 0.097 |
| EBL (ml), median (IQR) | 123.0 (115.0–160.0) | 120.0 (110.0–130.0) | 0.166 |
| Bladder catheterization (day), median (IQR) | 14 | 14 | |
| PSM, n (%) | 0 (0) | 0 (0) | |
| Hospital stay (day), median (IQR) | 4.5 (4.0–5.0) | 7.0 (6.0–8.0) | <0.001 |
| Transfusion rate, n (%) | 0 (0) | 0 (0) | |
| Peritoneum broken, n (%) | 0 (0) | 5 (19.2) | 0.017 |
| Lymphadenectomy, n (%) | 8 (26.7) | 6 (23.1) | 0.757 |
| Lymph node invasion, n (%) | 0 (0) | 0 (0) | |
| Nerve-sparing procedure, n (%) | 0.943 | ||
| Unilateral | 8 (26.7) | 6 (23.1) | |
| Bilateral | 18 (60.0) | 16 (61.5) | |
| None | 4 (13.3) | 4 (15.4) | |
| Complications, n (%) | 0.559 | ||
| Clavien I–II | 9 (30.0) | 6 (23.1) | |
| Clavien III–V | 0 (0) | 0 (0) | |
| ISUP grade after RP, n (%) | 0.827 | ||
| Grade group 1 | 12 (40.0) | 10 (38.5) | |
| Grade group 2 | 4 (13.3) | 3 (11.5) | |
| Grade group 3 | 9 (30.0) | 7 (26.9) | |
| Grade group 4 | 5 (16.7) | 6 (23.1) | |
| pT stage after RP, n (%) | 0.828 | ||
| T2a | 8 (26.7) | 9 (34.6) | |
| T2b | 12 (40.0) | 11 (42.3) | |
| T2c | 6 (20.0) | 3 (11.5) | |
| T3a | 4 (13.3) | 3 (11.5) | |
| Positive surgical margins, n (%) | 3 (10.0) | 2 (7.7) | 0.867 |
| Pain score, median (IQR) | |||
| Day of the operation | 6.0 (5.0–7.0) | 7.0 (6.8–8.0) | <0.001 |
| First postoperative day | 5.0 (4.8–6.0) | 7.0 (6.0–7.0) | <0.001 |
| Second postoperative day | 4.0 (3.0–4.3) | 4.0 (4.0–4.0) | 0.224 |
| Discharge day | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.874 |
| Need for any pain medication, n (%) | 11 (36.7) | 18 (69.2) | 0.015 |
| Need for opioids, n (%) | 2 (6.7) | 7 (26.9) | 0.04 |
| 3 months PSA <0.1 ng ml−1, n (%) | 29 (96.7) | 25 (96.2) | 0.536 |
| 3 months continence (0–1 pad per day), n (%) | 22 (73.3) | 20 (76.9) | 0.757 |
| 3 months erectile function recovery, n (%) | 10 (33.3) | 8 (30.8) | 0.838 |
| 3 months scar satisfaction, n (%) | 26 (86.7) | 14 (53.8) | 0.007 |
IQR: interquartile range; EBL: estimated blood loss; PSM: positive surgical margin; RP: radical prostatectomy; PSA: prostate-specific antigen; SP: single-port; MP: multiport; ISUP: International Society of Urological Pathology
The pain score in the SP group was much lower than that in the MP group on the day of the operation and on the first postoperative day (day of the operation: 6.0 vs 7.0; P < 0.001; first postoperative day: 5.0 vs 7.0; P < 0.001), but there was no difference in the pain score between the two groups from the second postoperative day to discharge. The overall need for pain medications and opioids in the SP group was less than that in the MP group (need for pain medications: 36.7% vs 69.2%; P = 0.015; need for opioids: 6.7% vs 26.9%; P = 0.04). Three months after the operation, the surgical scars of patients in the SP group were completely obscured by underwear (Figure 1b). The satisfaction rate with the appearance of scars in the SP group was higher than that in the MP group (86.7% vs 53.8%; P = 0.007).
Three-month oncological and functional outcomes
All of the patients underwent a 3-month follow-up to assess postoperative PSA levels, and the proportion of patients with a PSA level <0.1 ng ml−1 was similar between the two groups. According to the 3-month continence results, the rates of patients who used no pad or one pad per day in the SP and MP groups were not different (73.3% vs 76.9%; P = 0.757). According to the 3-month erectile function results, 10 (33.3%) and 8 (30.8%) patients in the SP and MP groups, respectively, had erections sufficient for intercourse with or without oral phosphodiesterase inhibitors (P = 0.838). The oncological and functional outcomes are shown in Table 2.
DISCUSSION
Prostate cancer is the second most common malignant tumor among men in the world. In China, there were 144 887 new prostate cancer patients in 2017.23 An increasing number of Chinese doctors have mastered the RARP technique, and more prostate cancer patients are treated with robotic RP. Currently, we are in the precision surgery era for prostate cancer, in which oncological and functional outcomes are equally important, aiming to maximize patients’ quality of life.10,24 Therefore, the da Vinci SP Surgical System was approved for use in urology patients, allowing the introduction of an endoscopic articulating camera and three instruments through a single channel robotic port.25,26 The present analysis confirms that SP-RARP is safe and feasible.27,28,29 The advantages of single incision can translate into preservation of the patient’s body image, self-esteem, and cosmesis.10 Currently, the new single-port platform is not approved for use in China, where the da Vinci S/Si Surgical System models remain the most popular in most centers. Kaouk et al.14 and White et al.13 successfully performed transabdominal approach robotic spRP with the da Vinci Si Surgical System. Preliminary experience has demonstrated the novel and minimally invasive effect of robotic spRP. Nevertheless, we still need to explore more surgical methods that are beneficial to patients. In the present study, we successfully performed 30 epR-spRP procedures with the aid of the da Vinci Si Surgical System.
The operation time and amount of bleeding are very important when considering the feasibility and safety of an operation. Kaouk et al.30 reported that the median operation time and median EBL associated with extraperitoneal radical prostatectomy using the new single-port platform were 197.5 min and 143 ml, respectively. A recent high-volume surgical center experience showed that the average operation time and average EBL of conventional extraperitoneal robotic RP were 146 min and 100 ml, respectively.31 Ploussard et al.32 reported that the median operation time and median EBL of RARP performed using an extraperitoneal approach were 128.9 min and 515.4 ml, respectively. Our study showed that the median operation time and median EBL associated with epR-spRP were 142.5 min and 123.0 ml, respectively. The operation time and EBL associated with epR-spRP with the da Vinci Si Surgical System are acceptable. Moreover, we found that the median time for the first 10 patients in the SP group was significantly longer than that in the MP group and that for the latter 20 patients in the SP group. The median operation time for the latter 20 patients in the SP group was similar to that in the MP group. Zorn et al.33 found that the learning curve of a laparoscopically skilled surgeon for RARP with respect to 4-h safety is 25 cases. Therefore, the epR-spRP learning curve for surgeons with significant robotic experience seems to be reasonable. However, the time required to adapt to this technology might not be as long as previously thought, and similar findings have been found in studies using the new SP system.27 This finding could be related to our rich experience with single-port laparoscopic surgery.34,35
Extraperitoneal single-port RARP resembles classic open anatomic radical retropubic prostatectomy.36 Direct access to the prostate with minimal manipulation of the peritoneal cavity can prevent postoperative ileus and contribute to a rapid recovery period.30,32 Similar results were obtained in our study, and the median hospital stay in the SP group was significantly shorter than that in the MP group. With the reduction of the hospital stay and the use of robotic arms, epR-spRP could be helpful in decreasing medical expenses. Single-port surgery also provides less incision pain and better cosmetic results than multiport surgery.37 Similarly, we found that the SP group showed significant advantages in reducing postoperative pain and improving cosmetic results. In this study, we observed significant differences regarding the use of postoperative pain medications between the two groups. The overall need for analgesic medications, including opioids, was significantly lower in the SP group than in the MP group. The extraperitoneal single-port approach might also be useful for reducing the widespread use of opioids, providing a safe operation, and achieving rapid recovery for patients, thus reducing the final cost, which is the goal of surgeons.
The risk of an inadvertent peritoneal puncture is not uncommon during extraperitoneal pelvic surgery with a multiport approach.38 Peritoneal damage can increase the difficulty of surgery and the recovery of intestinal function postoperatively, and there is also an increased risk of intestinal injury.9,38 However, regarding the surgical technique, compared with the multiport approach, there is only a midline entry site when the single-port approach is used.30 As the lateral ports are eliminated, the risk of an inadvertent peritoneal puncture is significantly decreased.19 In our study, there was no peritoneal damage in the SP group, even in patients with a history of lower abdominal surgery, while there was a significant increase in peritoneal damage in the MP group, especially in patients with a history of abdominal surgery. In the current study, the total complication rate of the SP group was not significantly different from that of the MP group, and there were no complications worse than Clavien–Dindo III grade. This result is similar to the reported total complications of conventional extraperitoneal robotic radical prostatectomy and extraperitoneal radical prostatectomy with the new single-port robot.19,32 We also found that single-port surgery did not affect the efficiency of nerve sparing, which could be related to the proportion of low-risk patients in our group. It should be emphasized that the above results were achieved by surgeons and surgical teams with extensive experience with the da Vinci Si Surgical System.
The PSM rate is our key concern and is directly related to the prognosis of prostate cancer.39 A large, retrospective study showed that the total PSM rate of extraperitoneal RARP was 31.3%.32 In the present study, the PSM rate in the SP group was 10.0%, which was not significantly different from that in the MP group (Table 2). At the 3-month postoperative follow-up, the proportions of patients with a PSA level <0.1 ng ml−1 in the SP and MP groups were both greater than 96%. The low PSM rate and decrease in PSA levels postoperatively could be related to the proportion of low-risk patients in our study. According to the functional follow-up results obtained 3 months postoperatively, there was no difference in the recovery rate of urinary continence between the two groups, and more than 70% of patients recovered continence 3 months postoperatively (0–1 pad per day). The recovery of urinary continence could benefit from reconstruction of the periurethral structures. Urethral anatomical reconstruction technology played an important role in the early recovery of urinary continence.40,41,42 There was no significant difference in the recovery of erectile function between the two groups, and approximately 30% of patients resumed sexual intercourse. Regarding the functional results, the continence and erectile function rates were within the range based on previous research on extraperitoneal RARP.32,43 Our study involved all patients who were followed up for an average of 3 months. Although the early oncological and functional results of the SP group were expected, their impact on long-term results cannot be concluded. The oncological and functional results after RP are greatly affected by many parameters, so it is necessary to further expand the sample size and randomization.
It remains a great challenge to achieve true single-port access in the upright environment of the robotic arm due to internal and external conflicts, a limited surgical space, and the loss of freedom. To reduce the collision of instruments, we made an incision close to the prostate (Figure 1a). This approach can also significantly reduce the risk of injury to the peritoneum and subepigastric vessels. By learning from the experience of White et al.13 and Kaouk et al.,14 we used an upward 30° camera and tissue pull technology throughout the process to reduce instrument collisions and increase the operating space. In addition, it is crucial to keep the camera far from the surgical field and further abduct the proximal robotic arms to reduce external collision and provide additional space for robotic arm movement. According to changes in the surgical site, manual adjustment of the distance between the scope holder arm and the robotic arm is also a good way to focus the operator’s field of vision regarding the key anatomical areas to compensate for the loss of visual acuity. If the resected prostate affects the surgical field of vision during a single-port operation, the port can be loosened to remove the specimen. The entire process does not waste much time. Surgical specimens are generally removed from the camera port incision during a multiport operation, and the scope holder arm must be reinstalled and sutured for fixation, which takes considerable time. Therefore, single-port surgery is advantageous in patients in whom a large prostate interferes with the visual field via the extraperitoneal approach. Doctors who are inexperienced in single-port laparoscopic radical prostatectomy can change the 0° camera angle when performing vesicourethral anastomosis to ensure smooth suturing. It is undeniable that there remains a phenomenon of “chopsticks,” which occurs during robotic single-port surgery, and collisions of instruments often occur. Console surgeons and assistants should also have extensive experience. It is not recommended for beginners to perform such operations. Although the da Vinci Si Surgical System is not designed for single-port surgery, it can provide more experience for operating a single-port robotic surgical platform in the future.
The limitations of this study should be mentioned. Overall, patients who underwent epR-spRP with the da Vinci Si Surgical System were highly screened individuals for whom a more conservative surgical approach was adopted. Therefore, these results are preliminary, and the risk of selection bias is inevitable. Because a new method has been adopted, its feasibility must be determined before a prospective, comparative analysis is performed. This study involved only a small cohort of patients and a short follow-up, weakening the conclusions of the oncological and functional evaluations. The surgeons involved have considerable robotic experience, so the bias of the learning curve is an important limitation. Additionally, the patient enrolment was consecutive, and the procedure approach was based on the surgeon’s preference. Using the da Vinci Si Surgical System for epR-spRP has strict requirements for the surgeon and assistant. The new SP platform could become preferential technology for countries/centers where this technology is available compared to single-port RARP with the da Vinci Si Surgical System.
CONCLUSIONS
It is safe and feasible to perform epR-spRP with the da Vinci Si Surgical System, and epR-spRP can be used as a treatment option for prostate cancer. In this study, compared with epR-mpRP, epR-spRP was associated with a significantly shorter postoperative hospital stay, a decreased need for postoperative pain medication, and a better cosmetic result. The rates of PSMs, major postoperative complications, and 3-month functional results in the SP group were comparable to those in the MP group. Although this study has some limitations, the preliminary results show the specific advantages of robotic single-port surgery. We encourage more experienced doctors in robotic surgery to perform this minimally invasive surgery, clearly define the limitations of the technique, and assess its potential benefits by expanding sample sizes and performing randomized trials.
AUTHOR CONTRIBUTIONS
GQJ and ZJ Wang provided substantial contributions to collect the dataset, conceive the study idea, participate in data analysis, conduct statistical analyses, and draft the manuscript. JZS, ZQZ, ZJ Wu, LY, and BL were involved in the data collection. LHW supervised the study and drafted the manuscript. DLX was the initiator of the project and research, provided the surgical data, procured the funding, and participated in the study design and coordination. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
The incision of epR-spRP. (a) The incision position of epR-spRP. (b) The incision anatomical layers of epR-spRP. epR-spRP: extraperitoneal robotic single-port radical prostatectomy; epR-mpRP: extraperitoneal robotic multiport radical prostatectomy.
ACKNOWLEDGMENTS
We thank Dr. Qi-Wei Yang for providing a professional drawing. This study was supported by the Medical Guidance Project of Shanghai Science and Technology Committee (No. 19411967600 and No. 17411972000).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, et al. Clinically localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Xia L, Sperling CD, Taylor BL, Talwar R, Chelluri RR, et al. Associations between hospital volume and outcomes of robot-assisted radical prostatectomy. J Urol. 2020;203:926–32. doi: 10.1097/JU.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–30. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–66. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 7.Sooriakumaran P, Pini G, Nyberg T, Derogar M, Carlsson S, et al. Erectile function and oncologic outcomes following open retropubic and robot-assisted radical prostatectomy: results from the LAParoscopic Prostatectomy Robot Open Trial. Eur Urol. 2018;73:618–27. doi: 10.1016/j.eururo.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Gettman MT, Hoznek A, Salomon L, Katz R, Borkowski T, et al. Laparoscopic radical prostatectomy: description of the extraperitoneal approach using the da Vinci robotic system. J Urol. 2003;170:416–9. doi: 10.1097/01.ju.0000076015.88739.a2. [DOI] [PubMed] [Google Scholar]
- 9.Joseph JV, Rosenbaum R, Madeb R, Erturk E, Patel HR. Robotic extraperitoneal radical prostatectomy: an alternative approach. J Urol. 2006;175:945–50. doi: 10.1016/S0022-5347(05)00340-X. [DOI] [PubMed] [Google Scholar]
- 10.Checcucci E, De Cillis S, Pecoraro A, Peretti D, Volpi G, et al. Single-port robot-assisted radical prostatectomy: a systematic review and pooled analysis of the preliminary experiences. BJU Int. 2020;126:55–64. doi: 10.1111/bju.15069. [DOI] [PubMed] [Google Scholar]
- 11.Gettman MT, Box G, Averch T, Cadeddu JA, Cherullo E, et al. Consensus statement on natural orifice transluminal endoscopic surgery and single-incision laparoscopic surgery: heralding a new era in urology? Eur Urol. 2008;53:1117–20. doi: 10.1016/j.eururo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Martin OD, Azhar RA, Clavijo R, Gidelman C, Medina L, et al. Single port radical prostatectomy: current status. J Robot Surg. 2016;10:87–95. doi: 10.1007/s11701-016-0589-5. [DOI] [PubMed] [Google Scholar]
- 13.White MA, Haber GP, Autorino R, Khanna R, Forest S, et al. Robotic laparoendoscopic single-site radical prostatectomy: technique and early outcomes. Eur Urol. 2010;58:544–50. doi: 10.1016/j.eururo.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single-port transumbilical surgery in humans: initial report. BJU Int. 2009;103:366–9. doi: 10.1111/j.1464-410X.2008.07949.x. [DOI] [PubMed] [Google Scholar]
- 15.White MA, Autorino R, Spana G, Hillyer S, Stein RJ, et al. Robotic laparoendoscopic single site urological surgery: analysis of 50 consecutive cases. J Urol. 2012;187:1696–701. doi: 10.1016/j.juro.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, et al. Initial experience with da Vinci single-port robot-assisted radical prostatectomies. Eur Urol. 2020;77:373–9. doi: 10.1016/j.eururo.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Diaz RR, Cho KS, Choi YD. Meta-analysis of transperitoneal versus extraperitoneal robot-assisted radical prostatectomy for prostate cancer. J Laparoendosc Adv Surg Tech A. 2013;23:919–25. doi: 10.1089/lap.2013.0265. [DOI] [PubMed] [Google Scholar]
- 18.Akand M, Erdogru T, Avci E, Ates M. Transperitoneal versus extraperitoneal robot-assisted laparoscopic radical prostatectomy: a prospective single surgeon randomized comparative study. Int J Urol. 2015;22:916–21. doi: 10.1111/iju.12854. [DOI] [PubMed] [Google Scholar]
- 19.Kaouk J, Aminsharifi A, Wilson CA, Sawczyn G, Garisto J, et al. Extraperitoneal versus transperitoneal single port robotic radical prostatectomy: a comparative analysis of perioperative outcomes. J Urol. 2020;203:1135–40. doi: 10.1097/JU.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 21.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 23.Zhai Z, Zheng Y, Li N, Deng Y, Zhou L, et al. Incidence and disease burden of prostate cancer from 1990 to 2017: results from the Global Burden of Disease Study 2017. Cancer. 2020;126:1969–78. doi: 10.1002/cncr.32733. [DOI] [PubMed] [Google Scholar]
- 24.Checcucci E, Amparore D, De Luca S, Autorino R, Fiori C, et al. Precision prostate cancer surgery: an overview of new technologies and techniques. Minerva Urol Nefrol. 2019;71:487–501. doi: 10.23736/S0393-2249.19.03365-4. [DOI] [PubMed] [Google Scholar]
- 25.Dobbs RW, Halgrimson WR, Talamini S, Vigneswaran HT, Wilson JO, et al. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol. 2020;38:897–905. doi: 10.1007/s00345-019-02898-1. [DOI] [PubMed] [Google Scholar]
- 26.Bertolo R, Garisto J, Gettman M, Kaouk J. Novel system for robotic single-port surgery: feasibility and state of the art in urology. Eur Urol Focus. 2018;4:669–73. doi: 10.1016/j.euf.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Kaouk J, Garisto J, Bertolo R. Robotic Urologic Surgical interventions performed with the single port dedicated platform: first clinical investigation. Eur Urol. 2019;75:684–91. doi: 10.1016/j.eururo.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Kaouk J, Bertolo R, Eltemamy M, Garisto J. Single-port robot-assisted radical prostatectomy: first clinical experience using the SP surgical system. Urology. 2019;124:309. doi: 10.1016/j.urology.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Dobbs RW, Halgrimson WR, Madueke I, Vigneswaran HT, Wilson JO, et al. Single-port robot-assisted laparoscopic radical prostatectomy: initial experience and technique with the da Vinci® SP platform. BJU Int. 2019;124:1022–7. doi: 10.1111/bju.14864. [DOI] [PubMed] [Google Scholar]
- 30.Kaouk J, Valero R, Sawczyn G, Garisto J. Extraperitoneal single-port robot-assisted radical prostatectomy: initial experience and description of technique. BJU Int. 2020;125:182–9. doi: 10.1111/bju.14885. [DOI] [PubMed] [Google Scholar]
- 31.Scarcia M, Zazzara M, Divenuto L, Cardo G, Portoghese F, et al. Extraperitoneal robot-assisted radical prostatectomy: a high-volume surgical center experience. Minerva Urol Nefrol. 2018;70:479–85. doi: 10.23736/S0393-2249.18.03114-4. [DOI] [PubMed] [Google Scholar]
- 32.Ploussard G, de la Taille A, Moulin M, Vordos D, Hoznek A, et al. Comparisons of the perioperative, functional, and oncologic outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014;65:610–9. doi: 10.1016/j.eururo.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 33.Zorn KC, Orvieto MA, Gong EM, Mikhail AA, Gofrit ON, et al. Robotic radical prostatectomy learning curve of a fellowship-trained laparoscopic surgeon. J Endourol. 2007;21:441–7. doi: 10.1089/end.2006.0239. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Liu B, Wu Z, Yang Q, Hehir M, et al. Transumbilical laparoendoscopic single-site surgery: more than 1-year experience in radical nephrectomy and its learning curve study. J Endourol. 2011;25:1859–65. doi: 10.1089/end.2011.0015. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Liu B, Wu Z, Yang Q, Chen W, et al. A matched-pair comparison of laparoendoscopic single-site surgery and standard laparoscopic radical nephrectomy by a single urologist. J Endourol. 2012;26:676–81. doi: 10.1089/end.2011.0161. [DOI] [PubMed] [Google Scholar]
- 36.Ragavan N, Dholakia K, Ramesh M, Stolzenburg JU. Extraperitoneal vs. transperitoneal robot-assisted laparoscopic radical prostatectomy-analysis of perioperative outcomes, a single surgeon’s experience. J Robot Surg. 2019;13:275–81. doi: 10.1007/s11701-018-0850-1. [DOI] [PubMed] [Google Scholar]
- 37.Lo IS, Lee HY, Chou YH, Huang CN, Wu WJ, et al. Robot-assisted extraperitoneal radical prostatectomy, single site plus two model. J Laparoendosc Adv Surg Tech A. 2018;28:140–4. doi: 10.1089/lap.2017.0421. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi Y, Kataoka A, Johnin K, Yoshiki T, Okada Y. Simple techniques for atraumatic peritoneal dissection from the abdominal wall and for preventing peritoneal injury during trocar placement under retroperitoneoscopy. J Urol. 2003;169:256–7. doi: 10.1016/S0022-5347(05)64080-3. [DOI] [PubMed] [Google Scholar]
- 39.Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303–13. doi: 10.1016/j.eururo.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 40.Manfredi M, Checcucci E, Fiori C, Garrou D, Aimar R, et al. Total anatomical reconstruction during robot-assisted radical prostatectomy: focus on urinary continence recovery and related complications after 1000 procedures. BJU Int. 2019;124:477–86. doi: 10.1111/bju.14716. [DOI] [PubMed] [Google Scholar]
- 41.Checcucci E, Pecoraro A, De Cillis S, Manfredi M, Amparore D, et al. The importance of anatomical reconstruction for continence recovery after robot assisted radical prostatectomy: a systematic review and pooled analysis from referral centres. Minerva Urol Nefrol. 2020;73:165–77. doi: 10.23736/S2724-6051.20.04146-6. [DOI] [PubMed] [Google Scholar]
- 42.Patel VR, Coelho RF, Palmer KJ, Rocco B. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009;56:472–8. doi: 10.1016/j.eururo.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Ploussard G, Xylinas E, Salomon L, Vordos D, Hoznek A, et al. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: experience in a high-volume laparoscopy reference centre. BJU Int. 2010;105:1155–60. doi: 10.1111/j.1464-410X.2009.09013.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The incision of epR-spRP. (a) The incision position of epR-spRP. (b) The incision anatomical layers of epR-spRP. epR-spRP: extraperitoneal robotic single-port radical prostatectomy; epR-mpRP: extraperitoneal robotic multiport radical prostatectomy.