Abstract
Background:
Young women aged 15–24 years are disproportionately affected by the HIV epidemic. Two phase III trials of a vaginal ring containing 25-mg dapivirine demonstrated HIV-1 risk reduction in adult women older than 21 years but not in those aged 18–21 years. Lack of protection was correlated with low adherence.
Methods:
In this phase-IIa, randomized, double-blind, placebo-controlled, US, multicenter trial of the dapivirine ring in sexually active females, aged 15–17 years, participants were randomized 3:1 to a dapivirine or placebo ring to be inserted monthly for 6 months (NCT02028338). Primary safety end points included grade 2 product related adverse events and any grade 3 and higher adverse events. Adherence to ring use was assessed by plasma dapivirine concentrations, residual levels in used rings, and self-report. A plasma dapivirine concentration of >95 pg/mL was used to define short-term adherence; a residual ring level of <23.5 mg was used to define long-term adherence. Acceptability was assessed through computer-assisted self-interviews.
Results:
Ninety-six participants were enrolled across 6 US sites. The median age was 16.0 years. There were no differences in safety outcomes between treatment arms. Adherence to the dapivirine ring was demonstrated by both plasma measurements (87%) and residual drug levels in rings (95%). Forty-two percent (95% confidence interval: 32 to 52) of participants reported that they never removed the ring. Participants noted no discomfort due to the ring at 87% of visits and “liking” the ring at 93% of visits.
Conclusion:
The dapivirine vaginal ring, a promising topical microbicide, was well tolerated and acceptable in young US adolescents.
Keywords: HIV-1 prevention, adolescents, microbicides
INTRODUCTION
Young women are disproportionately affected by the HIV epidemic. In eastern and southern Africa, the epicenter of the epidemic, 26% of all new infections are among women aged 15–24 years despite making up only 10% of the population.1 An investigational product, the dapivirine vaginal ring is a promising HIV-1 prevention product designed specifically for women. In 2 large, randomized, placebo-controlled, clinical trials, the monthly dapivirine ring reduced the risk of HIV-1 acquisition by 27% and 31% across the study populations of African women aged 18–45 years.2,3 However, further analysis indicated suboptimal adherence in the younger population, defined by both objective biological markers (plasma drug levels) and residual drug levels in used rings. Higher adherence to the dapivirine ring, as measured by residual drug in the used ring, was associated with a 65% [95% confidence interval (CI), 23 to 84; P = 0.009] reduction in HIV-1 risk, whereas partial adherence was not significantly associated with HIV-1 protection (relative risk reduction, 35%; 95% CI, 10 to 61; P = 0.12).2
The Microbicide Trials Network (MTN) and the Adolescent Trials Network for HIV/AIDS Interventions conducted a phase-IIa study of the dapivirine vaginal ring in low-risk young women to assess the safety and acceptability of the vaginal ring in a US adolescent population.
METHODS
Between June 2014 and July 2016, healthy, nonpregnant, HIV-1–seronegative adolescents, aged 15–17 years, were enrolled across 6 research sites in the United States (NCT02028338). The primary study objective was to assess the safety in this population of dapivirine (25 mg) administered via a silicone vaginal ring, when inserted once every 4 weeks for 24 weeks. The study protocol was approved by the Prevention Sciences Review Committee of the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health and ethics review committees at each of the study sites. The MTN-023 study was designed to contribute to the Food and Drug Administration licensure packet allowing for eventual licensing of the dapivirine ring with an adolescent indication.
Informed written guardian consent and adolescent assent were required and obtained. Key inclusion criteria included HIV negative status, effective contraception, Tanner stage 4/5, and a history of penile–vaginal intercourse. Key exclusion criteria included more than 3 partners in the 3 months before enrollment, diagnosis of a sexually transmitted infection in the 60 days before enrollment, abnormal baseline laboratory results, and grade 2 or higher pelvic examination finding per the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, dated December 2004 (Clarification dated August 2009) and Addendum 1 (Female Genital Grading Table for Use in Microbicide Studies).
The primary end point was the proportion of participants with any grade 3 or higher adverse events (AE) and grade 2 AEs related to study product use. The secondary objectives were to assess the acceptability of the ring, evaluate adherence to the ring, and measure systemic and local dapivirine exposure. Secondary end points included participants’ self-report on multiple components of acceptability via attitudinal questions, participants’ reported number of ring removals and expulsions, and dapivirine concentrations measured in plasma and vaginal fluids.
At enrollment, participants were assigned in a 3:1 ratio to receive either a silicone elastomer vaginal matrix ring containing 25 mg of dapivirine or an indistinguishable placebo vaginal ring, using a fixed-size block randomization, stratified by site. Participants were taught how to insert and remove the ring and were counseled to retain it in place for the entire month.
Participants returned for a safety assessment visit 2 weeks after enrollment and then monthly thereafter. Monthly follow-up study procedures included safety monitoring via interview and physical examination, individualized adherence counseling, ring removals based on participant recall, and assessments of sexual behavior; acceptability was assessed at the enrollment, 3 month, and the termination visits. At each visit, a new vaginal ring was provided, and the previous month’s ring was collected. A urine pregnancy test was collected monthly, and product was held in the setting of a positive result. Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas testing were performed at the screening and termination visits and when clinically indicated. All participants received a package of HIV-1 prevention services at each study visit, including risk reduction counseling, invitation to counsel and test partners, and free condoms. Oral pre-exposure prophylaxis was not offered to this low risk cohort.
Plasma and vaginal fluid samples (collected on Dacron swabs) were collected at 2, 4, 12, and 24 week visits and analyzed for dapivirine quantification using validated ultra-performance liquid chromatographic–tandem mass spectrometric assays (Clinical Pharmacology Analytical Laboratory, Johns Hopkins University School of Medicine), with lower limit of quantification being 20 pg/mL and 0.25 ng/swab, respectively.4,5 Prior pharmacokinetic studies have described the rapid rise of dapivirine plasma concentrations after ring insertion.6 To distinguish cases in which the ring was removed and reinserted immediately before a clinic visit, an adherence threshold of >95 pg/mL, which correlates with >8 hours of continuous use, was used to define short-term adherence to the ring.6 Over the course of a month, the dapivirine vaginal ring is designed to slowly release dapivirine; hence, the residual drug level left in the ring can provide information about ring use over the preceding month. In this study, testing for residual dapivirine in returned, used rings was performed monthly using acetone extraction and high-pressure liquid chromatography (Parexel, Bloemfontein, South Africa). Participants were defined as long-term adherent if the returned ring contained <23.5 mg of dapivirine.7
The study was conducted by the MTN and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. The International Partnership for Microbicides (Silver Spring, MD) developed and supplied the study rings and served as regulatory sponsor for the trial.
A sample size of 72 would provide 90% power to detect safety end point rates greater than 30%. A sample size of 96 was selected to ensure adequate numbers of participants even if retention decreased and only 75% of participants were evaluable.
All participants randomized into the clinical trial were included in the primary analysis. To assess safety, the number and percentages of participants who experienced each safety end point were tabulated by treatment group. Exact 95% binomial CIs were calculated for each safety end point for each treatment group and the 2-sided Fisher exact test was used to test for differences in event rates between the 2 treatment groups with a significance level of 0.05.
Summary statistics were calculated for acceptability questions and self-reported ring use. For the pharmacokinetic analysis, a population sample mean and SD of the dapivirine concentrations were calculated.
RESULTS
Of 128 young women screened for the study, 96 were enrolled. Seventy-three were assigned to the dapivirine vaginal ring and 23 to the placebo ring (Fig. 1). The 2 groups were similar with respect to demographic variables; the median age was 16.0 years. Nearly half of the participants identified as black or African American (49%); 25% identified as white.
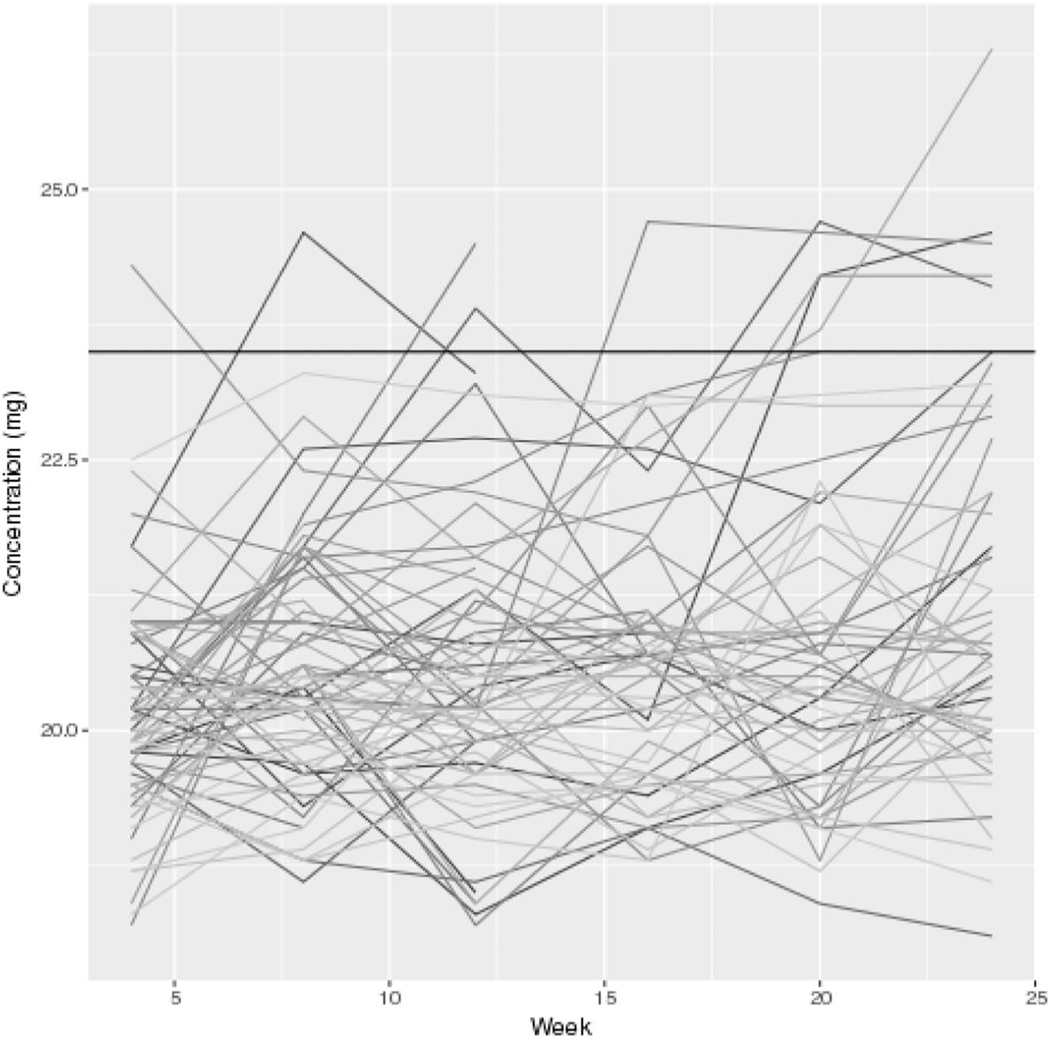
FIGURE 1.
Residual drug level in returned rings for each participant through the course of study participation. The solid line represents 23.5 mg.
A total of 91 of 96 participants (95%) completed the clinical trial as per protocol. Of the 5 participants who did not complete the study, 1 was lost to follow-up and 4 declined further participation. Retention during the follow-up visits was comparable between the 2 treatment groups and between the research centers.
There were 10 clinician-initiated product holds in 9 participants during the study. Four holds were because of AEs, 3 as a result of pregnancy in young women using combined hormonal contraception, and 3 as a result of other reasons. The 4 AEs that prompted temporary study product hold were intrauterine device expulsion, trichomoniasis, and 2 episodes of pelvic inflammatory disease. All were deemed unrelated to study product use.
There were no statistically significant differences in the frequency of the primary safety end points between the study arms (Table 1). Overall, 295 AEs were reported for 85 participants (88.5%): 222 AEs were reported for 63 participants (86.3%) in the dapivirine arm, and 73 AEs were reported for 22 participants (95.7%) in the placebo arm. Across the entire study, there were 3 grade 3 events: marijuana toxicity, incarcerated hernia, and increased blood pressure. All 3 occurred in the dapivirine group and were judged to be unrelated to study product. Incident sexually transmitted infections occurred at a similar rate in the 2 study arms. There were no HIV seroconversions.
Table 1.
Summary of Adverse Events (AEs) and Serious Adverse Events (SAE) by Severity
| No. (%) of participants with AEs | Dapivirine Ring (N = 73), n (%) | Placebo Ring (N = 23), n (%) | All Participants (N = 96), n (%) |
|---|---|---|---|
| At least one AE | 63 (86.3) | 22 (95.7) | 85 (88.5) |
| Primary end point | |||
| Grade 2 related (%, 95% CI)* | 8 (11, 5 to 20) | 2 (9, 1 to 28) | |
| Grade 3 or higher (%, 95% CI)* | 3 (4, 1 to 12) | 0 (0 to 15) | |
| By severity (max severity of any AE) | |||
| Grade 1: mild | 16 (21.9) | 10 (43.5) | 26 (27.1) |
| Grade 2: moderate | 44 (60.3) | 12 (52.2) | 56 (58.3) |
| Grade 3: severe | 3 (4.1) | 0 | 3 (3.1) |
| Grade 4: potentially life threatening | 0 | 0 | 0 |
| Grade 5: death | 0 | 0 | 0 |
| At least one product-related AE | 23 (31.5) | 10 (43.5) | 33 (34.4) |
| At least one AE leading to permanent discontinuation | 0 | 0 | 0 |
| At least one SAE | 2 (2.7) | 0 | 2 (2.1) |
| SAE by criterion | |||
| At least one product-related SAE | 0 | 0 | 0 |
| Resulted in death | 0 | 0 | 0 |
| Was life-threatening | 0 | 0 | 0 |
| Required inpatient hospitalization or prolongation of existing hospitalization | 1 (1.4) | 0 | 1 (1.0) |
| Required procedure or surgery | 1 (1.4) | 0 | 1 (1.0) |
Fisher exact test P value = 1.00.
The mean plasma dapivirine concentration at 1 and 6 months was 255 pg/mL and 243 pg/mL, respectively. Mean vaginal fluid concentrations at the same time points were 45 pg/mL and 52 pg/mL. Concentrations were consistent with product adherence for the duration of study participation.
By self-report, 42% (40 of 96) participants reported that they were 100% adherent to study product use; that is, the ring was only removed for study procedures. The most commonly reported reason for the ring being out during the preceding month was because of sexual activity. The majority of ring removals and expulsions were short in duration; in 91% of monthly reports, the pause in ring use was for less than 12 hours.
Dapivirine plasma drug concentrations and residual levels in the used rings confirmed study product adherence. Eighty-seven percent of plasma samples had levels greater than 95 pg/mL, suggestive of adherence to study product in the day before visit. Ninety-five percent of returned, used vaginal rings had levels less than 23.5 mg, indicative of adherence over the past month. The spaghetti plot presented in Figure 1 shows the actual residual drug level at each visit for participants randomized to the dapivirine group.
Participants found the ring easy to use and minimally impactful in their daily activities and sex. The most frequently cited worry that participants reported regarding ring use was that the primary sex partner might feel it during sexual intercourse (28%).
DISCUSSION
In this study, a monthly vaginal ring containing dapivirine was found to be well tolerated and acceptable in a US adolescent population. Furthermore, based on self-report and supported by dapivirine plasma concentrations and residual levels in used rings, a majority of the participants demonstrated consistent use of the vaginal ring. These results are novel in 2 respects: this is the first microbicide trial performed in an adolescent population as young as 15 years and the first trial to suggest high levels of adherence to ring use among young women using a microbicide product. The ring was well tolerated in this population. The rate of AEs between the placebo and dapivirine vaginal ring arms was comparable.
In contrast to prior microbicide trials that found low adherence to the vaginal ring among the youngest women, in our trial, more than 95% of the participants used the ring consistently based on residual ring measurements. There are many differences between our cohort and the young women of ASPIRE and The Ring Study, which might explain the difference in adherence rates, not least of which are cultural norms and expectations. However, detailing exactly which differences might drive such a difference is difficult. The adolescents in our trial required parental permission to participate, and this disclosure may have provided adherence support for the adolescent outside of the research site. Several secondary analyses of vaginal gel microbicide trials have shown that partner disclosure of gel use was associated with significantly higher adherence rates.8,9
Our study has several strengths. With high study retention and frequent follow-up, the primary results of this trial contribute to an important body of safety data including women who became pregnant during ring use.10 In addition, this study showed that adolescents found the ring acceptable and were adherent when provided with counseling and support. An important limitation is the generalizability of study results to high-risk young women in resource-limited settings.
Young women are at the center of the HIV epidemic in sub-Saharan Africa. Prevention options are urgently needed to control the epidemic,11 and including adolescents in biomedical interventional trials is imperative.12 MTN-023/IPM 030 demonstrated that such research is feasible. The present study along with an ongoing study in Africa (Clinical Trials NCT03593655) will provide safety data to support the use of the dapivirine ring in adolescents and provide unfettered access to this promising prevention method.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of the investigators and staff at the Adolescent Medicine Trials, Units, and Microbicide Trials Network sites who participated in this research: Montefiore Medical Center (D.F., MD; Helen Nazario, DNP); St. Jude Children’s Research Hospital (Aditya Gaur, MD; Mary Dillard, BSN, ACRN, CCRC); University of Colorado (D.R., MD; Kalina Miller BA, RN-BSN); Fenway Community Health Center (K.H.M., MD; Julian Dormitzer, RN, BSN); University of Alabama (C.H., MD; Faye Heard, MPH); University of Pittsburgh (K.E.B., MD; Ingrid Macio, PA). The authors especially thank the young women and guardians who gave their time to participate in this research.
The MTN-023/IPM 030 study was designed and implemented by the Microbicide Trials Network (MTN) in conjunction with the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the US National Institutes of Health. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD 040533). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were developed and supplied by the International Partnership for Microbicides (IPM).
D.R. has program grant from Gilead to routinize HIV testing in adolescents. K.H.M. has unrestricted research grants from Gilead. S.L.H. serves as a consultant to Merck. K.E.S. currently serves as the Global Director of Scientific Affairs for HIV in Merck Research Labs. During the conduct of the study, K.E.S. was at the Sidney Kimmel Medical College of Thomas Jefferson University where she is still on faculty. A.N. is a full-time employee of the International Partnerships for Microbicides, the developer of the dapivirine vaginal ring.
MTN-023/IPM 030 Team members listed in the Acknowledgements.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.UNAIDS. UNAIDS data. 2017. Available at https://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed November 12, 2019.
- 2.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New Engl J Med. 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375: 2133–2143. [DOI] [PubMed] [Google Scholar]
- 4.Parsons TL, Emory JF, Seserko LA, et al. Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J Pharm Biomed Anal. 2014;98:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seserko LA, Emory JF, Hendrix CW, et al. The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis. 2013;5: 2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel A, Haazen W, Nuttall J, et al. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS. 2014;28:1479–1487. [DOI] [PubMed] [Google Scholar]
- 7.Nel AKM, Kamupira M, Woodsong C, et al. Safety, Acceptability, and Pharmacokinetic Assessment (Adherence) of Monthly Dapivirine Vaginal Microbicide Rings (Ring-004) for HIV Prevention. Presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI); March 5–8, 2012; Seattle, WA. Abstract. [Google Scholar]
- 8.Mngadi KT, Maarschalk S, Grobler AC, et al. Disclosure of microbicide gel use to sexual partners: influence on adherence in the CAPRISA 004 trial. AIDS Behav. 2014;18:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Succop SM, MacQueen KM, van Loggerenberg F, et al. Trial participation disclosure and gel use behavior in the CAPRISA 004 tenofovir gel trial. AIDS Care. 2014;26:1521–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makanani B, Balkus JE, Jiao Y, et al. Pregnancy and infant outcomes among women using the dapivirine vaginal ring in early pregnancy. J Acquir Immune Defic Syndr. 2018;79:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc. 2015;18(2 suppl 1):19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy BJ, Kapogiannis BG, Lally MA, et al. Youth-specific considerations in the development of preexposure prophylaxis, microbicide, and vaccine research trials. J Acquir Immune Defic Syndr. 2010;54(suppl 1): S31–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]