Abstract
Purpose of review
Acute central and peripheral nervous system injury may occur in association with coronavirus disease 2019 (COVID-19) caused by infection with the severe acute respiratory syndrome coronavirus 2 virus. This review will assist readers to recognize neurologic manifestations associated with COVID-19 including common and life-threatening symptoms and diagnostic testing. We will also review current recommendations for treatment of neurologic injury associated with COVID-19 infection in children.
Recent findings
Data from systematic reviews and prospectively collected cohorts of children with COVID-19 are beginning to characterize the breadth of neurologic manifestations associated with COVID-19 in the acute infectious and postinfectious periods. Among hospitalized children in particular, neurologic symptoms are common. Life threatening conditions including encephalitis, myelitis, stroke, and demyelinating syndromes have been reported. Within the pediatric population, age, and preexisting neurologic conditions appear to be important factors in determining likely phenotypes. Treatment at this time is based on careful neuromonitoring, supportive care, and neuromodulatory therapies as indicated.
Summary
Neurologic symptoms are common in children with COVID-19 and may be life threatening. The pathophysiology, therapeutic options, and long-term outcomes from COVID-19 associated neurologic injury are currently being investigated.
Keywords: acute neurologic injury, coronavirus disease 2019, neurocritical care, pediatrics
INTRODUCTION
Acute central and peripheral nervous system injury may occur in children and adults during or after infection with the causative virus of coronavirus disease 2019 (COVID-19). The purpose of this review is to provide updates on diagnosis, evaluation and treatment of neurologic manifestations associated with COVID-19 in children and to provide insight into directions for future research on this topic.
Box 1.
no caption available
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2), the virus causing COVID-19, has infected over 4 million children in the United States per the American Academy of Pediatrics (AAP) [1▪]. As adult vaccination rates have risen, children now account for over 20% of new cases reported [2▪]. Among pediatric patients, the case fatality rate for COVID-19 remains low but morbidity associated with the disease may be significant. Neurologic symptoms associated with COVID-19 have been actively investigated in adult and pediatric patients following documentation of headache, ageusia, anosmia, cerebrovascular disease, and encephalopathy in the initial reports of SARS-CoV-2 outbreak in Wuhan, China [3▪,4▪▪]. Approximately 18 months into the pandemic, COVID-19 associated central and peripheral nervous system disease has been reported in patients with acute infection, during the postinfectious period, and following asymptomatic infection [5▪▪]. In this review, we will discuss keys for recognition of COVID-19 associated neurological disease in children as well as current approaches to management.
Early recognition of central and peripheral nervous system injury associated with COVID-19 during the acute phase of illness, as will be discussed in this review, may facilitate appropriate long-term monitoring and care to prevent or mitigate chronic neurologic deficits. The long-term impact of COVID-19 on the emotional and physical health of adults, termed long COVID or postacute sequelae of COVID-19 (PACS), is only beginning to be reported in pediatric populations [6,7▪,8].
RECOGNITION OF ACUTE CORONAVIRUS DISEASE 2019 ASSOCIATED NEUROLOGICAL DISEASE
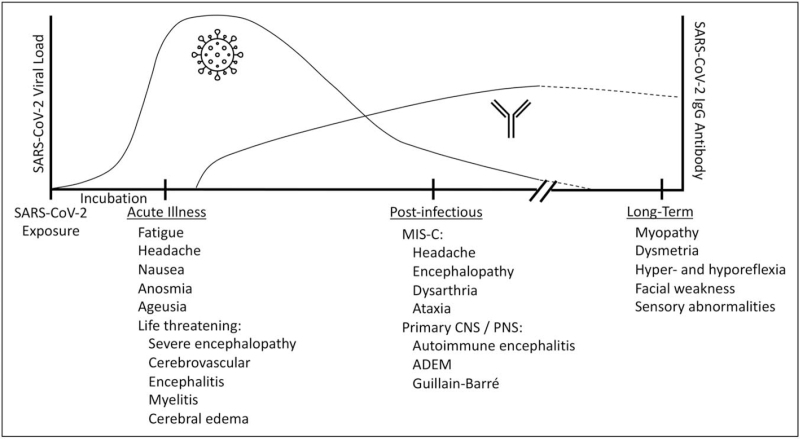
Given the breadth of neurologic symptoms reported in patients meeting the World Health Organization (WHO) criteria for probable / possible SARS-CoV-2 infection [9], this review will be divided into a recognition of COVID-19 associated neurologic disease during the period of active infection and neurologic disease in the immediate postinfectious period (Fig. 1). We will review common and life-threatening presentations, associated symptoms, physical exam findings, and diagnostic studies.
FIGURE 1.
Central and peripheral neurologic manifestations associated with COVID-19. Neurologic manifestations that have been reported at acute, postinfectious, and late timepoints in children with COVID-19 are shown in relation to typical viral load and antibody response. ADEM, acute disseminated encephalomyelitis; CNS, COVID-19, coronavirus disease 2019; central nervous system; MIS-C, multisystem inflammatory syndrome in children; PNS, peripheral nervous system.
Recognition of COVID-19 associated neurologic disease requires maintaining a high index of suspicion, particularly in patients presenting in or traveling from highly endemic regions. The WHO and the Centers for Disease Control and Prevention (CDC) maintain COVID-19 tracking dashboards that include the number of positive cases, hospitalizations, mortalities, and vaccinations administered that will be useful to assess likelihood of infection. Direct person-to-person respiratory transmission remains the most common mode of SARS-Co-V-2 infection [10▪]. Critical to assessing the likelihood of infection is the proximity of the patient to an infected individual, the duration of the exposure, and the ventilation of the space (e.g. indoor vs. outdoor). A recent investigation using an insurance database compared COVID-19 infection rates amongst households with or without a birthday in the preceding 2 weeks discovered a 31% increase above county-level prevalence in households after a child's birthday [11]. Because COVID-19 associated neurologic disease has been reported with both acute and chronic infection, close contacts over the prior weeks may be relevant.
Clinical findings
The Overcoming COVID-19 US public health surveillance registry of children and adolescents hospitalized with COVID-19-related complications was utilized to investigate COVID-19 associated neurological disease. Overall, 22% of hospitalized children had neurologic involvement with 12% of those patients developing life-threatening neurologic conditions [12▪▪]. The overall incidence is consistent with data from a systematic review of 3700 children [5▪▪] which included both hospitalized and nonhospitalized patients, and reported an incidence of 17% of cases with neurologic manifestations; 1% of which were deemed life-threatening.
Generalized neurologic complaints, including headache, fatigue, and nausea/vomiting are the most common neurological manifestations in children with COVID-19. Within pediatric cohorts age appears to be strongly associated with the neurologic presentation. Among hospitalized patients, fatigue and headache are reported 2–4 times as common in children 5–20 years old compared to children <5 years old. Anosmia and ageusia are reported approximately 10× more commonly in older children [5▪▪]. Importantly, these neurologic symptoms may be underreported in the youngest patients.
One third of children hospitalized with COVID-19 require critical care [13]. Among hospitalized children with COVID-19 associated neurologic disease 12% were determined through careful case review to have life-threatening neurologic involvement [12▪▪]. Of the patients with life-threatening illness, 25% died and 40% were discharged with new neurologic deficits. Similar to the finding that preexisting conditions are a risk factor for severe COVID-19, a preexisting neurologic disorder appears to be a strong risk factor for neurologic manifestations associated with COVID-19.
In critically ill patients, severe encephalopathy is the most common finding [12▪▪]. This may be related in part to multiorgan dysfunction, sedative medications, hypoxemia, hypotension, and metabolic disturbances. Delirium screening has become a routine part of pediatric neurocritical care and although reports in pediatric patients are limited, adults with COVID-19 requiring intensive care have high incidence of delirium [14▪]. Other life-threatening conditions reported during SARS-CoV-2 infection include: ischemic or hemorrhagic stroke, acute CNS infection/acute disseminated encephalomyelitis (ADEM), and fulminant cerebral edema. In children <5 years old hospitalized with COVID-19, seizure or status epilepticus was almost as common a presenting symptom as encephalopathy [12▪▪].
Cerebrovascular complications of COVID-19 have been reported in 1–5% of hospitalized adults [15]. The International Pediatric Stroke Study Group (IPSS) recently reported 8 cases in children associated with SARS-CoV-2 detected by PCR [16▪]. The Overcoming COVID-19 US public health surveillance registry of children and adolescents hospitalized with COVID-19-related complications reported 12 ischemic or hemorrhagic strokes in hospitalized children [12▪▪]. The IPSS has not detected an increase in the incidence of neonatal or pediatric stroke, however the survey was performed over a relatively short time given the low baseline stroke risk in children and many patients were not tested for SARS-CoV-2. The Pediatric COVID Brain Imaging Group (PECOBIG) has published a case series of patients with abnormal imaging obtained within 3 months of COVID-19 diagnosis and reported that 18% of the patients in this group had evidence of thromboembolic events or vasculopathy. Cerebral microvascular injury has also been reported in adult patients with severe COVID-19 [17▪].
Diagnostic studies
Diagnosis of SARS-CoV-2 infection is primarily made by detection of viral RNA by reverse-transcription polymerase chain reaction (RT-PCR) in respiratory secretions. The accuracy of the test is highly dependent on the sample quality and test characteristics. However, RT-PCR is generally both highly accurate and highly sensitive, particularly in comparison to other methods of detection. Case reports of children with life-threatening neurologic injury and detection of SARS-CoV-2 by RT-PCR in cerebrospinal fluid (CSF) have been published [18▪,19]. A systematic review which included both adult and pediatric patients identified 321 patients with CNS symptoms and CSF testing for SARS-CoV-2 by RT-PCR. Of these patients, only 6% had detectable SARS-CoV-2 RNA.
Characteristic laboratory features of SARS-CoV-2 include elevated C-reactive protein, elevated serum ferritin, elevated aminotransaminase levels, elevated lactate dehydrogenase levels, and lymphopenia [20▪▪]. These findings, although not unique to COVID-19, should prompt specific testing for SARS-CoV-2 if it has not already been performed. In adult patients with neurologic symptoms, the use of brain specific serum biomarkers has been investigated including neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) [21▪], markers of neuronal/axonal and astrocytic injury respectively. NfL and GFAP have been found to be elevated in the blood, particularly in severe COVID-19, relative to controls [22,23▪]. Geis et al. investigated NfL levels in children with asymptomatic to moderate COVID-19, 30% of whom had neurologic symptoms, and observed no significant change. It is unclear whether the lack of detection was related to more mild disease, differences in the pathobiology of COVID-19 between children and adults, or other factors. Brain specific biomarkers are not widely available and require further investigation before their use to recognize COVID-19 associated neurologic disease.
RECOGNITION OF POST-INFECTIOUS CORONAVIRUS DISEASE 2019 ASSOCIATED NEUROLOGICAL DISEASE
Hyperinflammatory shock, resembling the clinical presentation of children with severe Kawasaki's disease, was initially reported in previously healthy children with known exposure to COVID-19 [24▪]. Now called multisystem inflammatory syndrome in children (MIS-C), this condition was also a signal that children could present with postinfectious complications of COVID-19. Acute neurologic disease in the postinfectious period of COVID-19 can broadly be characterized as those occurring in association with MIS-C and those occurring with more isolated central or peripheral nervous system involvement. Many of these conditions are life threatening and require the application of neurocritical care.
Multisystem inflammatory syndrome in children neurologic disease
Diagnostic criteria for MIS-C and guidance for management of children with MIS-C have been published by an expert panel convened by the American College of Rheumatology [25▪]. Although SARS-CoV-2 RNA may still be detected by PCR in 13–69% of MIS-C cases, detection of immunoglobulin G (IgG) antibody to the SARS-CoV-2 nucleocaspid or spike protein will be positive in 90–95% of cases [26]. Based on the diagnostic criteria used to diagnose MIS-C (cardiovascular involvement, Kawasaki disease criteria, or broader MIS-C criteria) neurologic involvement is reported in 5–55% of cases of MIS-C [26].
Neuroimaging is a key diagnostic tool for detection of COVID-19 associated central and peripheral neurologic pathology. The American Society of Pediatric Neurology Pediatric COVID Brain Imaging Group solicited cases of COVID-19 in children with abnormal neuroimaging. Findings in children with MIS-C have included diffusion restriction or T2-weighted signal abnormality in the splenium of the corpus callosum, microhemorrhages, subcortical white matter changes, leptomeningeal enhancement, cerebral edema, and myelitis [12▪▪,27▪,28▪▪].
Electroencephalography (EEG) may detect seizures and encephalopathic features such as diffuse slowing [12▪▪,29▪]. Nerve conduction and electromyelography studies, when performed, may show myopathic or neuropathic changes.
Primary central and/or peripheral nervous system involvement
Guillain-Barré syndrome (GBS) was reported in four children in the overcoming COVID-19 public health registry [12▪▪] with numerous other case reports in pediatric patients [30▪,31,32]. Cases of GBS in adult patients presenting in the first six months of the pandemic was reviewed by Uncini et al.[33▪] who, in general, found fairly characteristic presentations of GBS and GBS variants including weakness, hypoareflexia, sensory disturbances, and albumin-cytological dissociation in the majority of patients. Forty percentage of patients required intensive care and respiratory failure occurred in a third of patients. In patients with SARS-CoV-2 testing of CSF performed, all were negative. One child with GBS and SARS-CoV-2 RNA detected in CSF has been reported [31]. Abnormal enhancement of cranial and spinal nerves is a common finding in children with COVID-19 and abnormal neuroimaging [28▪▪]. However, a review of the National Immunoglobulin Database found that the incidence of GBS in the United Kingdom fell during the pandemic. This could be due to social distancing and other prevention measures that dramatically reduced spread of other viral infections [34▪].
Several case reports of anti-NMDA receptor encephalitis in pediatric patients have been reported in younger males with COVID-19 [35▪,36▪], which is in contrast to the typical epidemiology of anti-NMDA receptor encephalitis in pediatric patients. ADEM may affect children with acute COVID-19, MIS-C, or as an isolated finding in the postinfectious period [5▪▪,12▪▪,28▪▪]. Myelitis (focal or long segment) may also present without abnormalities in the brain or brainstem [28▪▪].
TREATMENT
The treatment of COVID-19 associated neurologic injury in children is guided by the following: supportive care, neurocritical care for patients with life threatening conditions, considered use of immunomodulatory therapies, and rehabilitation services.
For conditions such as agnosia and anosmia, which resolve over several weeks in the vast majority of cases, reassurance to the child and caregivers may be most beneficial. Nutritional intake and weight should be monitored. Olfactory training is available and may be beneficial. Inhaled corticosteroids have been trialed in adults without an improvement in time to resolution of symptoms [37].
For MIS-C and severe neurologic presentations associated with COVID-19 there are no trials comparing therapies in children. Intravenous immunoglobulin (IVIG) at a dose of 2 g/kg, corticosteroids, and low-dose aspirin are considered first-line therapies for MIS-C based on expert consensus and evidence from patients with Kawasaki's disease [25▪]. Anakinra is recommended as a second-line agent for IVIG- and/or steroid-refractory MIS-C. Generally, at this time, there is insufficient evidence to support changing treatment approaches for conditions such as pediatric stroke, GBS, ADEM, or cerebral edema in the setting of COVID-19. Case reports are available which suggest increased steroid responsiveness of these conditions. For example, a child diagnosed with anti-NMDA receptor encephalitis had no clinical response to IVIG or plasmapheresis but showed rapid improvement following treatment with high-dose methylprednisolone [36▪]. Our approach is to provide standard neurocritical care for children with COVID-19 and life-threatening neurologic manifestations, with early consideration of corticosteroids and other immunomodulatory therapies while monitoring closely for secondary infections.
Early consultation of rehabilitation services, including physical therapy, occupational therapy, and speech therapy may be beneficial for patients with COVID-19 and neurologic deficits. Rehabilitation services can typically be safely applied in critically ill children [38]. Given persistent deficits that may be present at 6 weeks and 6 months after initial presentation [27▪] referral for inpatient or outpatient rehabilitation after recovery from acute illness may also be warranted.
CONCLUSION
Neurologic manifestations are common in pediatric patients after infection with SARS-CoV-2. The Global Consortium Study of Neurologic Dysfunction in COVID-19 (GCS-NeuroCOVID)-Pediatrics [39▪], Collaboration to Assess Risk and Identify long-term outcomes (CARING) for Children with COVID, and Researching COVID to Enhance Recovery (RECOVER) are a few of the investigative groups working to understand the epidemiology, phenotypes, pathophysiology, and long-term impact of COVID-19 in children and their families including detailed neuropsychological evaluations. As we continue to learn more about the impact of SARS-CoV-2 on the neurologic system of children, earlier recognition and treatment options to reduce neurologic morbidity may be developed.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪. Critical Updates on COVID-19. Available at: http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/. [Google Scholar]; Updates and interim guidance on COVID-19 provided by the American Academy of Pediatrics.
- 2▪. Children and COVID-19: state-level data report. Available at: http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. [Google Scholar]; Report on COVID-19 cases in children by the American Academy of Pediatrics on data available as of July 15, 2021.
- 3▪.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the initial manuscripts that brought worldwide attention to the COVID-19 outbreak in Wuhan, China.
- 4▪▪.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]; The initial report on neurological manifestations of adult patients with COVID-19. More than one-third of patients had neurological symptoms.
- 5▪▪.Panda PK, Sharawat IK, Panda P, et al. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr 2020; 67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first systematic review of neurological manifestations associated with COVID-19 in children. 17% of children had neurologic symptoms with 1% of children having severe symptoms.
- 6.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110:2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr 2021; 110:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]; Case series of five children with potential long COVID. The children had symptoms fatigue and none had fully returned to school after 6 months.
- 8.Hageman JR. Long COVID-19 or post-acute sequelae of SARS-CoV-2 infection in children, adolescents, and young adults. Pediatr Ann 2021; 50:e232–e233. [DOI] [PubMed] [Google Scholar]
- 9. WHO COVID-19 Case definition. Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. [Google Scholar]
- 10▪.Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 2021; 174:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review on the transmission of SARS-CoV-2 including suggestions for evidence-based policies and practices to reduce transmission.
- 11.Whaley CM, Cantor J, Pera M, Jena AB. Assessing the association between social gatherings and COVID-19 risk using birthdays. JAMA Intern Med 2021; 181:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.LaRovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 2021; 78:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]; Case series from 61 hospitals in the Overcoming COVID-19 public health registry. Of 1695 patients included, 22% had neurologic involvement. Of the patients with neurologic manifestations, 12% developed life threatening neurologic illness.
- 13.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med 2021; 9:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]; An adult study of patients with COVID-19 admitted to intensive care units found that delirium was common and associated with benzodiazepine use and lack of family visitation.
- 15.Majmundar N, Ducruet A, Prakash T, et al. Incidence, pathophysiology, and impact of coronavirus disease 2019 (COVID-19) on acute ischemic stroke. World Neurosurg 2020; 142:523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Beslow LA, Linds AB, Fox CK, et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol 2021; 89:657–665. [DOI] [PubMed] [Google Scholar]; International survey of pediatric stroke centers reported the incidence of SARS-CoV-2 positivity in children with acute ischemic stroke.
- 17▪.Lee M-H, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med 2021; 384:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neuropathology of patients who died from COVID-19 demonstrated microvascular injury and perivascular inflammatory cell infiltration.
- 18▪.Cheraghali F, Tahamtan A, Hosseini SA, et al. Detection of SARS-CoV-2 from cerebrospinal fluid in a 34-month-old child with encephalitis. Front Pediatr 2021; 9:565778. [DOI] [PMC free article] [PubMed] [Google Scholar]; A rare presentation from adults or children with central nervous system injury associated and SARS-CoV-2 detection by PCR in the CSF.
- 19.Powers KT, Santoro JD. Metabolic stroke-like episode in a child with FARS2 mutation and SARS-CoV-2 positive cerebrospinal fluid. Mol Genet Metab Rep 2021; 27:100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Irfan O, Muttalib F, Tang K, et al. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child 2021; 106:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review of COVID-19 in children comparing hospitalization rates and mortality between high- and low-income countries. Children from low-income countries were less likely to be admitted to an ICU and had a higher rate of death.
- 21▪.DeKosky ST, Kochanek PM, Valadka AB, et al. Blood biomarkers for detection of brain injury in COVID-19 patients. J Neurotrauma 2021; 38:1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Position paper describing rationale for use of blood based biomarkers for detection of neurologic injury in patients with COVID-19.
- 22.Ameres M, Brandstetter S, Toncheva AA, et al. Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol 2020; 267:3476–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Kanberg N, Ashton NJ, Andersson L-M, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020; 95:e1754–e1759. [DOI] [PubMed] [Google Scholar]; Study of blood biomarkers GFAP and NfL in patients with mild, moderate, and severe COVID-19. Patients with severe COVID-19 had elevation of both biomarkers compared to healthy controls.
- 24▪.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first descriptions of MIS-C in children with known exposure to COVID-19.
- 25▪.Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol 2020; 72:1791–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multidisciplinary expert panel review and recommendations for management of children with MIS-C.
- 26.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr 2020; 226:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Penner J, Abdel-Mannan O, Grant K, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 2021; 5:473–482. [DOI] [PubMed] [Google Scholar]; Single center study of children with MIS-C with neurologic exam performed at 6 months after hospitalization.
- 28▪▪.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health 2021; 5:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]; Largest study of neuroimaging in children with SARS-CoV-2 infection and neurologic manifestations. Abnormal imaging findings included features of ADEM, cranial nerve enhancement, splenial lesions, and myositis.
- 29▪.Abdel-Mannan O, Eyre M, Lobel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol 2020; 77:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neuroimaging case series in children with MIS-C. All four patients with neurologic symptoms had lesions in the splenium.
- 30▪.Frank CHM, Almeida TVR, Marques EA, et al. Guillain-Barre syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr 2020; doi:10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]; First reported case of GBS in a patient with SARS-CoV-2 infection.
- 31.Araújo NM, Ferreira LC, Dantas DP, et al. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain-Barré syndrome. Pediatr Infect Dis J 2021; 40:e274–e276. [DOI] [PubMed] [Google Scholar]
- 32.Khalifa M, Zakaria F, Ragab Y, et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatr Infect Dis Soc 2020; 9:510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Uncini A, Vallat JM, Jacobs BC. Guillain-Barre syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry 2020; 91:1105–1110. [DOI] [PubMed] [Google Scholar]; Systematic review of adults with GBS associated with SARS-CoV-2 infection.
- 34▪.Pelletier JH, Rakkar J, Au AK, et al. Trends in US pediatric hospital admissions in 2020 compared with the decade before the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2037227. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cross-sectional study of 49 pediatric hospitals in the U.S. describing changes in admission patterns during the pandemic.
- 35▪.Burr T, Barton C, Doll E, et al. N-Methyl-d-aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol 2021; 114:75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of anti-NMDA receptor encephalitis in a child with COVID-19.
- 36▪.Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Childs Nerv Syst 2021; 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Case report of a 7-year-old child with anti-NMDA receptor encephalitis associated with COVID-19 infection who responded to treatment with corticosteroids.
- 37.Abdelalim AA, Mohamady AA, Elsayed RA, et al. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol 2021; 42:102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink EL, Beers SR, Houtrow AJ, et al. Early protocolized versus usual care rehabilitation for pediatric neurocritical care patients: a randomized controlled trial. Pediatr Crit Care Med 2019; 20:549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Schober ME, Robertson CL, Wainwright MS, et al. COVID-19 and the pediatric nervous system: global collaboration to meet a global need. Neurocrit Care 2021; 1–8. doi:10.1007/s12028-021-01269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of a global collaborative group investigating early and late neurologic dysfunction in children hospitalized with SARS-CoV-2.