Abstract
Fragile X syndrome (FXS) is the leading known inherited cause for intellectual disability. Due to mutations in the FMR1 gene, affected individuals are at risk for serious cognitive and behavioral symptoms and developmental disability. Clinical presentation varies considerably, and investigation of genetic factors not directly related to FMR1 may help better understand variability. The present study examined the BclI polymorphism of the glucocorticoid receptor gene NR3C1 in 43 individuals with FXS (28 females, age 16 to 25). Females with FXS who presented with one or more G alleles demonstrated attenuated symptoms of anxiety/depression (p=0.038) and externalizing behaviors (p=0.042) relative to individuals with the C/C allele. In the combined sample (males and females) structural neuroimaging data differentiated individuals with a G allele from those with the C/C genotype (p<0.001). Key components of anxiety/fear neurocircuitry (amygdala, insula) contributed more (relative to other regions) to the model differentiating groups. These results indicate that GR polymorphisms are associated with an altered pattern of behavioral and brain development in FXS. This information is important for understanding and treating mood disorders and altered brain development among individuals with FXS. With further research, these findings could be informative for understanding anxiety and mood disorders more broadly.
Keywords: BclI polymorphism, FMR1, structural MRI, multivariate pattern classification, anxiety
Introduction
Fragile X syndrome (FXS) is considered a model system for examining gene-brain-environment interactions due to the well-established genetic and neurobiological basis for this condition. FXS, is caused by a single gene mutation on the long arm of the X chromosome. This mutation results in expansion of CGG repeats and reduced production of the FMR1 gene protein product - the fragile X mental retardation protein (FMRP). FMRP is critical for several functions involved with synaptic plasticity and dendritic pruning(Cea-Del Rio et al., 2020; Feuge et al., 2019; Swanger and Bassell, 2011).
Due to reduced production of FMRP, individuals with FXS are at risk for a range of cognitive(Quintin et al., 2015), behavioral(Klaiman et al., 2014) and neurobiological outcomes(Alnæs et al., 2014). Some hallmark features include weaknesses in executive function, emotion regulation, attention deficit/hyperactivity and social cognition. However, clinical presentations are variable, and heterogeneity originates from several sources. First, females with FXS tend to be less affected than males and females present with higher profiles of cognitive and adaptive functioning (Bartholomay et al., 2019). Yet females present with a characteristic set of socioemotional challenges including anxiety and depression(Bailey et al., 2008). Within females, variability has been partly attributed to genetic factors related to the FMR1 mutation. For instance, altered levels of FMRP, caused by downstream effects of the FMR1 mutation, are related to social withdrawal and anxious/depressed behavior(D Hessl et al., 2001). Higher levels of FMRP are associated with more typical patterns of brain activation among females(Bruno et al., 2014). Within each sex, a distinct set of non-genetic factors, including characteristics of the home and educational environment are associated with behavioral outcomes(D. Hessl et al., 2001). However, genetic characteristics not directly related to FMR1 have received relatively little attention in this context. If identified, gene-gene interactions involving FMR1 could help predict outcomes, identify subtypes, and aid in designing/testing targeted therapies.
Genes regulating glucocorticoid function are especially relevant because FMRP regulates proteins involved with glucocorticoid feedback including annexin-(Sun et al., 2001) and glucocorticoid receptor (GR) α(Miyashiro et al., 2003). The FMR1 knockout mouse demonstrates elevated glucocorticoid levels in association with stress(Markham et al., 2006). Indeed, additional research points to aberrant hypothalamic-pituitary-adrenal (HPA) axis functioning. In particular, diurnal salivary cortisol levels are higher in children with FXS relative to levels in unaffected siblings, and levels of cortisol were significantly associated with behavioral problems in individuals with FXS(Hessl et al., 2002). Further, abnormal cortisol levels are related to social behavior(Hall et al., 2006; Hessl et al., 2006), suggesting perturbation of the cortisol regulatory system may represent a parallel mechanism implicated in the FXS phenotype.
To probe the role of GR function in FXS, we examined the BclI polymorphism of GR gene NR3C1 (rs41423247) in adolescents and young adults. The adolescent/young adult period represents a critical time in brain development (Casey et al., 2019) especially with respect to stress pathway development influenced by GR expression (Sinclair et al., 2011). The BclI restriction site polymorphism is a C/G substitution in intron 2. Prior research suggests that the G allele confers greater sensitivity to cortisol feedback relative to the C allele(Wüst et al., 2004). We hypothesized that the presence of the G allele (and, greater sensitivity to cortisol feedback) may be associated with developmental outcomes in individuals with FXS. Since aberrant cortisol regulation has been previously linked to behavioral outcomes(Hall et al., 2006; Hessl et al., 2006, 2002), we examined whether the presence of the G allele was associated with adaptive behavior and socio-emotional functioning. Altered brain structure has been linked to specific behaviors in individuals with FXS(Wolff et al., 2013). Therefore, we examined whether the presence of the G allele was associated with altered brain structure using multivariate pattern classification. Given that our primary hypotheses were related to the presence of the G allele in the context of FXS in general we include both male and female participants.
Methods
Participants included 43 individuals with FXS (28 females) age 16 to 25 years (mean=21.44, Table 1). Participant ethnicities included 39 Caucasian, 1 Pacific Islander and 3 mixed-race. Participants were recruited across the United States and Canada through advertisements, referrals and word of mouth. Research was conducted at Stanford University Medical Center. Participants and/or their caregivers gave written informed consent according to the Declaration of Helsinki. Assent was obtained from all particpants under the age of 18. Stanford University’s Institutional Review Board approved all protocols. Genetic diagnoses were confirmed via Southern blot DNA analysis (>200 CGG repeats on the FMR1 gene and evidence of aberrant methylation). Blood was drawn from each individual to estimate FMRP percentage. Lymphocytes were distinguished from other blood cell types by morphology; 200 lymphocytes were scored and the percentage of lymphocytes containing FMRP was assessed by immunostaining techniques(Willemsen et al., 1997) (Kimball Genetics, Denver). FMR1 gene activation ratio (fraction of cells with FMR1 gene active) was also available for 23 females based on the southern blot DNA analysis. We performed correlation analysis between FMRP and activation ratio to further validate the FMRP measure (r=0.721, p <0.001). Eighteen individuals were taking psychoactive medications (Table 1).
Table 1.
Descriptive statistics for C/C and G groups
| C/C | C/G or G/G | |||||
|---|---|---|---|---|---|---|
| N (female) | 20 (13) | 23 (15) | ||||
| Medication (any) | 6 | 12 | ||||
| Atypical antipsychotic | 2 | 4 | ||||
| SSRI | 4 | 7 | ||||
| Stimulants | 2 | 5 | ||||
| Other | 3 | 2 | ||||
| Mean | SD | Mean | SD | P-value | Effect size | |
| Age | 21.72 | 2.88 | 21.21 | 3.09 | >0.10 | d = 0.176 |
| FMRP% | 36.05 | 24.79 | 38.26 | 26.28 | >0.10 | d = 0.088 |
| Full Scale IQ | 74 | 23 | 72 | 18 | >0.10 | d = 0.118 |
| Vineland Adaptive Behavior | 62.20 | 17.88 | 63.57 | 20.89 | >0.10 | |
| Child Behavior Checklist | ||||||
| Total | 37 | 22 | 35 | 30 | >0.10 | |
| Internalizing | 12 | 9 | 12 | 10 | >0.10 | |
| Anxious/Depressed | 7 | 5 | 5 | 5 | >0.10 | |
| Anxious/Withdrawn | 4 | 3 | 4 | 3 | >0.10 | |
| Somatic Complaints | 2 | 2 | 3 | 3 | >0.10 | |
| Social Problems | 4 | 3 | 5 | 4 | >0.10 | |
| Thought Problems | 2 | 2 | 2 | 3 | >0.10 | |
| Attention Problems | 7 | 4 | 6 | 5 | >0.10 | |
| Externalizing | 9 | 8 | 8 | 8 | >0.10 | |
| Rule Breaking Behavior | 2 | 2 | 2 | 2 | >0.10 | |
| Aggressive Behavior | 7 | 7 | 6 | 7 | >0.10 | |
N is presented for medication usage. Other medications include neuroleptics, and other drugs known to affect neurological functioning. Chi square indicated no significant group differences in the number of individuals taking medications overall or within each class (all p’s>0.10). FMRP = Fragile X mental retardation protein. Full Scale IQ is based on the Wechsler Abbreviated Scale for Intelligence (age >/=17 years) or the Wechsler Intelligence Scale for Children (age <17 years). Vineland Adaptive Behavior composite standard scores are reported. Raw scores are reported for the Child Behavior Checklist.
Saliva samples were collected using DNA Genotek’s Oragene collection kits in order to assess BclI polymorphisms via PCR analysis. DNA was purified and extracted according to standardized protocols (dnagenotek.com). The following primers were used for amplification: 5′-TGC TGC CTT ATT TGT AAA TTC GT-3′ and 5′-AAG CTT AAC AAT TTT GGC CAT C-3′.(Bachmann et al., 2005) Following BclI digestion overnight fragments were separated on 2% agarose gels (CC - two bands (117 and 222 bp); CG - three bands (117, 222 and 335 bp); GG - undigested (335bp).
We divided participants into two groups based on the presence or absence of the G allele. Twenty individuals (13 females) presented with the C/C version of the polymorphism (hereafter referred to as the C/C group), and 23 individuals (15 females) presented with one or more G alleles (C/G (N=19) or G/G (N=4), hereafter referred to as the G group). All cognitive and behavioral assessments and imaging analyses were conducted by individuals blind to BclI polymorphism status.
All participants were free from MRI contraindications, met screening including the ability to hold still and minimal sensitivity to loud noises, and were trained to hold motionless in the scanner(Barnea-Goraly et al., 2014). Participants were part of an ongoing longitudinal investigation(Bray et al., 2011; Gothelf et al., 2008). The present study represents the first examination of GR polymorphisms in this population.
General intellectual functioning (IQ) was assessed via the Wechsler Abbreviated Scale for Intelligence(Wechsler, 1997) (age >/=17 years) or the Wechsler Intelligence Scale for Children(Wechsler, 1991) (age <17 years). Behavior was assessed using the Vineland Adaptive Behavior Scales, 2nd Edition(Sparrow et al., 2005) caregiver interview and the Child Behavior Checklist (CBCL)(Achenbach, 1991). Although the CBCL was designed for children it was used for all individuals to maintain consistency within the current study and with prior measures in the larger longitudinal study. Females completed self-report measures including the Beck Anxiety Inventory (BAI)(Beck et al., 1988), the Multidimensional Anxiety Scale for Children (MASC)(March et al., 1997) and the Beck Depression Inventory - II (BDI)(Beck et al., 1996).
Statistical analyses of behavioral data
Primary analyses were conducted on a combined sample of males and females. Given the aforementioned sex-differences associated with FXS and sex differences associated with HPA axis reactivity(Kajantie and Phillips, 2006), subsequent analyses were conducted within the female subset of our sample. The male subset was too small to allow for statistical comparisons. Group differences (C/C group vs. G group) were examined for each measure using ANCOVA. There was a wide range of IQ scores within each group and IQ was controlled in group comparisons of behavior. Raw CBCL scores were used as many individuals were outside of the assessment’s age range (6–18 years) and T scores (based on age) would be inappropriate. Analyses of CBCL raw scores were controlled for age in addition to IQ. Exploratory correlations between behavioral measures and levels of FMRP among females within each group were performed. Due to the limited range of FMRP among males, associations with FMRP were not explored (male FMRP=2–20% with the exception of one male with FMRP=60%; female FMRP=14.5–90%).
Image acquisition, processing and statistical analyses
High-resolution T1-weighted anatomical images were acquired at 3 Tesla (echo time=6ms; repetition time=35ms; flip angle=45°; field of view=24cm; slice thickness=1.5mm, 124 coronal slices; matrix=256×192; acquired resolution=0.94×1.25×1.5mm). One of two custom single-channel quadrature head coils were used (one head coil was decommissioned during the study). The number of participants scanned with each head coil did not differ between groups (X2=0.818, df=1, p>0.10), and head coil type was used as a covariate in imaging analyses. FreeSurfer 5 (http://surfer.nmr.mgh.harvard.edu/) was used to delineate 86 grey matter regions (68 cortical, 16 subcortical, 2 cerebellum, Appendix), and compute measures of regional thickness/volume. FreeSurfer is a surface based segmentation pipeline that preserves anatomical variation at the individual level and provides reliable segmentation of cortical, subcortical and cerebellar structures(Dale et al., 1999). FreeSurfer derived surfaces were examined and adjusted by editors with inter-rater reliability ≥ 0.95 (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial) until the surfaces satisfactorily delineated both the grey/white boundary and pial surface. Image quality requirements included lack of artifacts induced by subject motion, blood flow, or wraparound and approximately 19% of scans in the longitudinal study were unusable due to such artifacts.
Freesurfer cortical thickness and subcortical/cerebellar volume metrics were used as data elements (Bray et al., 2011; Bruno et al., 2017; Gothelf et al., 2008; Saggar et al., 2015). Cortical thickness metrics may represent a superior endophenotype for neurogenetic syndromes when compared to cortical volume.(Panizzon et al., 2009) Thus, all cortical structures were summarized according to thickness. Subcortical and cerebellar structures were summarized according to volume, the only available metric in the FreeSurfer parcellation.
Regional measures were normalized to remove effects of overall brain size, head coil type, sex and age using residuals from linear regression. Due to the different scale for cortical thickness (~2–4 mm) and subcortical/cerebellar volumes (several hundred mm3) additional normalization included dividing the value for each region by the absolute value of the maximum residual for that region.(Bruno et al., 2017) The resulting values were between −1 and 1 for each of 86 regions.
We used support vector machine learning (SVM)(Christopher J.C. Burges (Bell Laboratories, 1998) to assess differences in the overall pattern of structural brain variation between C/C and G groups. SVM is a flexible technique that automatically classifies data elements based on linear separation in multidimensional space and can accommodate any type of high-dimensional data. Data elements included metrics from 86 Freesurfer-derived brain regions (Appendix). A set of linearly uncorrelated components was extracted from these elements via principle component analysis. All principal components were input as features in the subsequent SVM. A leave-one-out cross validation was performed to test classification accuracy. Features for n-1 participants comprised a dataset that was used to train a linear support vector pattern classifier (with a fixed regularization parameter C=1) to correctly identify region of interest variation patterns of the nth participant and was repeated n times. Recursive feature elimination (RFE) was used to reduce the number of features and avoid overfitting. During RFE, the bottom 30% of features, based on the absolute value of their weights, were excluded in an iterative process until model performance started degrading. The RFE procedure was done within each cross-validation set (i.e. nested RFE) to avoid bias in classification accuracy. Prediction accuracy, sensitivity, specificity and positive predictive values were calculated. Permutation testing (n=1000) was used to estimate p values for each metric. We also examined the weights of each region’s contribution to the classification algorithm to identify regions that were more important for distinguishing between groups. An arbitrary threshold (weight2 >/= 0.1) was used to indicate the 25 regions with highest contribution to the classification. Separate SVM analysis was performed within the female subset of participants (N=28) and was similar to the primary analysis. We considered this analysis exploratory due to the small sample size and model parameters differed from the primary analysis as noted. Principle component analysis was used to extract an optimal set of linearly uncorrelated components that accounted for 80% of the variance. Recursive feature elimination was used to exclude the bottom 10% of features, based on the absolute value of their weights, in an iterative process until model performance started degrading.
Results
There were no group differences in the number of individuals taking psychoactive medication overall or within each class (within the whole sample or females only, all p>0.10, Table 1, Table 2). The C/C and G groups did not differ on age, ethnicity, IQ, FMRP% or Vineland scores (Table 1, all p’s>0.10). Three individuals from the C/C group (1 male and 2 female) and two females from the G group were missing CBCL scores. These individuals were excluded from the CBCL analysis. There were no significant group differences in CBCL total, Internalizing Behavior, Externalizing Behavior or individual subscale scores (all p’s >0.10).
Table 2.
Descriptive statistics for females across C/C and G groups
| C/C | C/G or G/G | |||||
|---|---|---|---|---|---|---|
| N | 13 | 15 | ||||
| Ethnicity (N) | ||||||
| Caucasian | 17 | 22 | ||||
| Pacific Islander | 1 | 0 | ||||
| Mixed Race | 2 | 1 | ||||
| Medication (any) | 5 | 8 | ||||
| Atypical antipsychotic | 2 | 2 | ||||
| SSRI | 4 | 4 | ||||
| Stimulants | 2 | 3 | ||||
| Other | 2 | 2 | ||||
| Mean | SD | Mean | SD | P-value | Effect size | |
| Age | 21.82 | 2.65 | 21.05 | 3.46 | >0.10 | d = 0.260 |
| FMRP% | 46.16 | 22.48 | 51.37 | 19.25 | >0.10 | d = 0.261 |
| Full Scale IQ | 83 | 23 | 78 | 19 | >0.10 | d = 0.265 |
| Vineland Adaptive Behavior | 65.31 | 16.87 | 66.73 | 23.71 | >0.10 | |
| Child Behavior Checklist Total | 40 | 25 | 32 | 35 | >0.10 | |
| Internalizing | 15 | 10 | 12 | 12 | >0.10 | |
| Anxious/Depressed* | 8 | 6 | 5 | 6 | 0.038 | |
| Anxious/Withdrawn | 5 | 3 | 4 | 4 | >0.10 | |
| Somatic Complaints | 3 | 3 | 4 | 4 | >0.10 | |
| Social Problems | 5 | 3 | 5 | 4 | >0.10 | |
| Thought Problems | 2 | 2 | 2 | 3 | >0.10 | |
| Attention Problems | 7 | 4 | 5 | 6 | >0.10 | |
| Externalizing* | 10 | 9 | 6 | 8 | 0.042 | |
| Rule Breaking Behavior | 2 | 2 | 1 | 2 | 0.084 | |
| Aggressive Behavior | 8 | 8 | 5 | 7 | >0.10 | |
N is presented for medication usage. Other medications include neuroleptics, and other drugs known to affect neurological functioning. Chi square indicated no significant group differences in the number of individuals taking medications overall or within each class (all p’s>0.10). FMRP = Fragile X mental retardation protein. Full Scale IQ is based on the Wechsler Abbreviated Scale for Intelligence (age >/=17 years) or the Wechsler Intelligence Scale for Children (age <17 years). Vineland Adaptive Behavior composite standard scores are reported.
Significant group difference and large effect size.
Group comparisons for IQ, Vineland, FMRP and CBCL, were repeated within the female subset of participants (Table 2). We found significantly higher scores for the C/C group on the CBCL anxiety/depression subscale (F(1,20)=4.92, p=0.038, ) and the externalizing subscale (F(1,20)=4.73, p=0.042, ). One female participant in the C/C and one in the G group demonstrated inconsistent responses on the MASC (inconsistency index>/=10) and these scores were excluded from analysis. Scores in the C/C group were higher for the MASC Tense/Restless subscale of the physical symptom domain (Table 3, F(1,23)=5.82, p=0.024, ) but there were no significant group differences for other scales or the overall total anxiety scale (all p’s >0.10). There were no significant group differences in BDI or BAI scores (all p’s >0.10). All other group comparisons within females were not significant (all p’s >0.10, with one exception: p=0.084 for the CBCL rule breaking behavior subscale).
Table 3.
Anxiety and mood scales for female participants across C/C and G groups
| C/C | C/G or G/G | P-value | Effect size () | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Beck Anxiety Inventory | 11 | 12 | 7 | 8 | >0.10 | 0.050 |
| Beck Depression Inventory | 12 | 14 | 6 | 6 | >0.10 | 0.117 |
| Multidimensional Anxiety Scale | ||||||
| Physical Symptoms Scale | 46 | 15 | 40 | 7 | >0.10 | 0.082 |
| Tense* | 49 | 14 | 39 | 6 | 0.024 | 0.201 |
| Somatic | 44 | 15 | 43 | 10 | >0.10 | 0.005 |
| Harm Avoidance Scale | 50 | 11 | 52 | 13 | >0.10 | 0.006 |
| Perfectionism | 52 | 8 | 51 | 14 | >0.10 | 0.001 |
| Anxious coping | 49 | 13 | 52 | 12 | >0.10 | 0.018 |
| Social Anxiety Scale | 52 | 13 | 50 | 10 | >0.10 | 0.010 |
| Humiliation | 52 | 12 | 49 | 13 | >0.10 | 0.008 |
| Performance Fears | 51 | 12 | 51 | 9 | >0.10 | 0.001 |
| Separation/Panic Scale | 56 | 17 | 57 | 13 | >0.10 | 0.003 |
| Total Anxiety Scale | 50 | 18 | 48 | 11 | >0.10 | 0.016 |
| Anxiety Disorders Index | 48 | 15 | 52 | 14 | >0.10 | 0.014 |
Raw scores are reported for the Child Behavior Checklist. Raw scores are reported for the Beck Anxiety Inventory and Beck Depression Inventory. T scores are reported for the Multidimensional Anxiety Scale.
Significant group difference and large effect size.
Within females in the G group, there was a significant negative relationship between FMRP and anxiety as measured by the MASC (physical symptoms subscale rs=−0.552, p=0.041, somatic symptoms subscale rs=−0.609, p=0.021, Figure 1). There was a trend for a negative relationship between FMRP and BAI scores (rs=−0.495, p=0.061). There were no significant relationships between FMRP and anxiety/depression measures among females in the C/C group (all p’s>0.10). No other associations with FMRP were significant within either group of females. Correlation strength (following Fisher’s r to Z and subsequent comparison(Myers and Sirois, 2006)) between FMRP and the MASC physical symptom scale was significantly greater among females in the G group relative to females in the C/C group (Z=1.97, p=0.024). There was a trend for increased correlation strength between FMRP and the MASC somatic subscale (MASC somatic Z=1.43, p=0.076) and between FMRP and the BAI (z=1.55, p=0.06) among females in the G group.
Figure 1. Correlations with FMRP and anxiety measures.
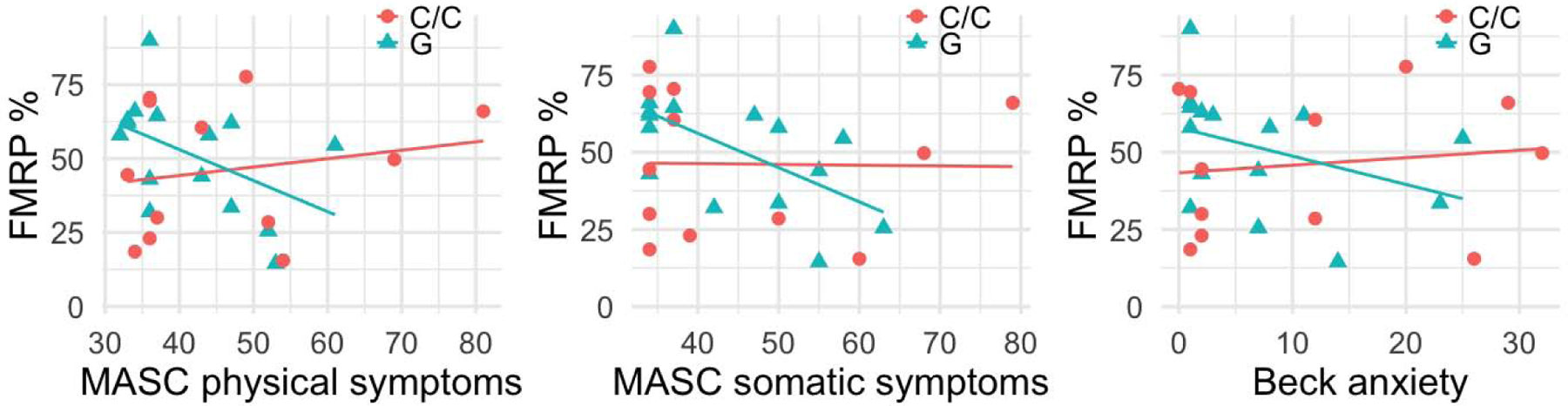
Relationships between anxiety measures and fragile X mental retardation protein (FMRP) for females are shown for each group (G group vs C/C group). FMRP is shown as a percentage. MASC = multidimensional anxiety scale for children, T scores are depicted. Beck anxiety = the Beck anxiety inventory, raw scores are depicted. See Supplement for color version of this figure.
The SVM pattern classification was able to discriminate between C/C and G groups using a leave one out approach. RFE indicated the model with 14 features was optimal with classification accuracy of 88.4% (p<0.001), sensitivity of 85.0% (p=0.001), specificity of 91.3% (p<0.001), positive predictive value of 90.7% (p<0.001) and negative predicative value of 85.9% (p>0.10). Regions with the highest contribution to the classification accuracy included bilateral subcortical, frontal, temporal and parietal regions (Figure 2, Table 4). We also performed post-hoc univariate between-group comparisons for each of these regions controlling for overall brain size, head coil type, sex and age using ANCOVA (Table 4). RFE among female participants indicated the model with 9 features was optimal with classification accuracy of 67.9% (p=0.046), sensitivity of 61.5% (p>0.10), specificity of 73.3% (p<0.026), positive predictive value of 69.8% (p=0.029) and negative predictive value of 65.6% (p=0.078). Regions with the highest contribution to the classification accuracy included bilateral subcortical, frontal, temporal parietal and occipital regions (Table 5).
Figure 2. Brain regions highlighted in the support vector machine learning model.
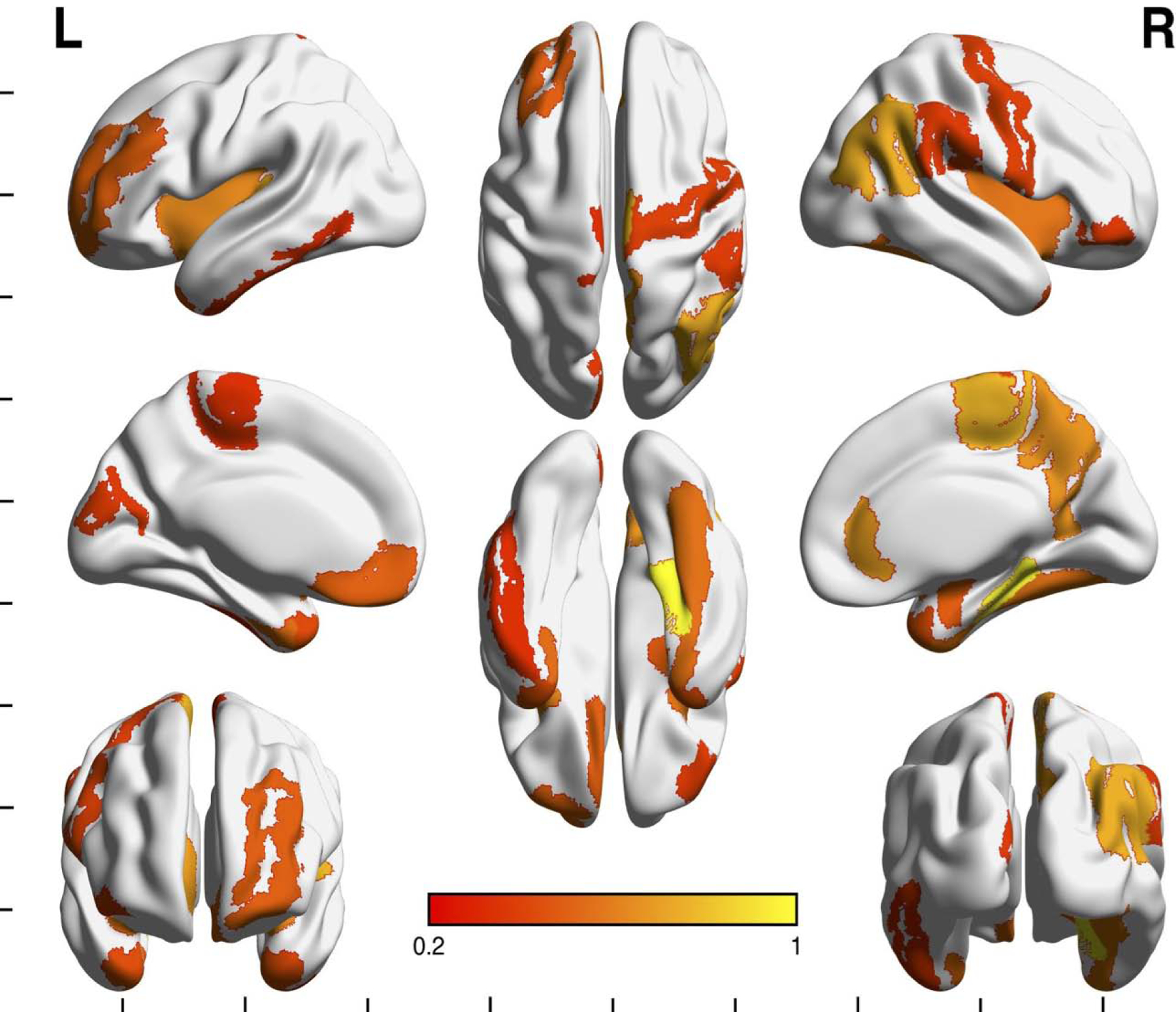
Multi view surface rendering depicting cortical regions that were highlighted in the support vector machine learning (SVM) model. Shading indicates regions contributing to the model with a squared weight >/= 0.1. Shading in bar indicates value of squared weight. Not pictured are subcortical regions (left caudate, left pallidum, right amygdala, right pallidum). See Supplement for color version of this figure.
Table 4.
Regions highlighted in support vector machine learning model and correlations with behavioral measures
| Left Hemisphere | G vs C/C | p-Val | Effect size () | Correlations in C/C group | Correlations in G group | |
|---|---|---|---|---|---|---|
| Subcortical | L caudate | ↑ | 0.095 | .074 | MASC physical (rs= 0.542, p= 0.037) and somatic (rs= 0.651, p= 0.009) | |
| L pallidum | ↓ | 0.535 | .010 | |||
| Frontal | L paracentral gyrus | ↓ | 0.667 | 0.005 | CBCL anx/dep (rs= 0.540, p= 0.046) | |
| L medial orbitofrontal gyrus | ↑ | 0.253 | 0.035 | |||
| L rostral middle frontal gyrus | ↑ | 0.088 | 0.077 | CBCL externalizing (rs= −0.730, p= 0.007) | ||
| Temporal | L insula | ↓ | 0.037 | 0.112 | ||
| L parahippocampal gyrus | ↓ | 0.116 | 0.065 | |||
| L temporal pole | ↓ | 0.946 | 0.000 | MASC physical (rs= 0.573, p= 0.026) and tense (rs= 0.640, p= 0.010) | ||
| L entorhinal | ↑ | 0.258 | 0.034 | |||
| L inferior temporal gyrus | ↑ | 0.459 | 0.015 | |||
| L transverse temporal gyrus | ↑ | 0.072 | 0.085 | |||
| Occipital | L cuneus | ↑ | 0.645 | 0.006 | ||
| Right hemisphere | ||||||
| Subcortical | R amygdala | ↓ | 0.463 | .015 | MASC physical (rs= 0.569, p= 0.042) and somatic (rs= 0.693, p= 0.009) | |
| R pallidum | ↑ | 0.035 | .114 | CBCL externalizing (rs= 0.553, p= 0.040) | ||
| Frontal | R paracentral gyrus | ↑ | 0.123 | 0.063 | ||
| R rostral anterior cingulate | ↓ | 0.161 | 0.052 | |||
| R pars orbitalis | ↑ | 0.280 | 0.031 | |||
| R precentral gyrus | ↓ | 0.833 | 0.001 | |||
| Temporal | R insula | ↓ | 0.031 | 0.120 | CBCL anx/dep (rs= 0.580, p= 0.030) | |
| R parahippocampal gyrus | ↓ | 0.005 | 0.191 | MASC somatic (rs= −0.567, p= 0.027), CBCL anx/dep (rs= −0.636, p= 0.015), externalizing (rs= −0.779, p= 0.001), | ||
| R temporal pole | ↑ | 0.423 | 0.017 | CBCL externalizing (rs= − 0.680, p= 0.007) | ||
| R fusiform gyrus | ↑ | 0.663 | 0.005 | |||
| Parietal | R inferior parietal | ↓ | 0.051 | 0.099 | BAI (rs= −0.572, p= 0.026), MASC physical (rs, −0.587, p= 0.021), somatic (rs= −0.518, p= 0.048) | |
| R supramarginal | ↓ | 0.852 | 0.001 | BAI (rs= 0.654, p= 0.015) and MASC tense (rs= 0.571, p= 0.042); | ||
| R precuneus | ↑ | 0.093 | 0.074 | CBCL externalizing (rs= 0.642, p= 0.024). | CBCL anx/dep (rs= 0.591, p= 0.026) | |
Regions were identified as having greatest contribution to the model based on a squared weight >/= 0.1. L = left, R = right. G vs. C/C indicates direction of difference between groups. ↑ indicates larger volume/thickness for the G group. ↓ indicates smaller volume/thickness for the G group. P-values and effect sizes refer to the univariate comparison between groups of for each region. CBCL = Child behavior checklist. anx/dep = anxiety/depression subscale. MASC = Multidimensional anxiety scale for children. Physical, somatic and tense indicate the subscales of the MASC. BAI = Beck anxiety inventory.
Table 5.
Regions highlighted in female support vector machine learning model
| Right Hemisphere | Left Hemisphere | |
|---|---|---|
| Subcortical | caudate | caudate |
| thalamus | amygdala | |
| ventral diencephalon | ventral diencephalon | |
| hippocampus | hippocampus | |
| Frontal | precentral gyrus | medial orbitofrontal gyrus |
| paracentral gyrus | pars opercularis | |
| Parietal | superior parietal gyrus | superior parietal gyrus |
| pericalcarine | supramarginal | |
| inferior parietal | ||
| Temporal | parahippocampal gyrus | parahippocampal gyrus |
| insula | ||
| inferior temporal gyrus | bank of the superior temporal sulcus | |
| Occipital | lingual gyrus | lingual gyrus |
| cuneus | ||
Regions were identified as having greatest contribution to the model based on a squared weight >/= 0.1.
Exploratory correlations were performed, within-group, between the thickness/volume of regions identified with the highest contributions and behavioral measures demonstrating significant group differences or relationships with FMRP. These analyses were undertaken for females only because they were based on significant results among females. Results are presented in Table 4.
Discussion
The present study represents the first investigation of the GR (NR3C1) BclI polymorphism and its relationship with behavior and brain development in FXS. Findings included lower levels of anxiety and externalizing behaviors, each comparison associated with a large effect size(Cohen, 1969), among females with one or more G allele. Polymorphism status was not associated with adaptive behavior, cognition or FMRP. Within our mixed sex sample, patterns of regional grey matter thickness/volume were able to distinguish individuals with at least one G allele from those with the C/C polymorphism. Key components of anxiety/fear neuro-circuitry were highlighted as contributing more (relative to other regions) in the model differentiating between individuals with or without a G allele. These findings constitute evidence that, within individuals with FXS, the presence of one or more G alleles may be protective and is associated with altered developmental outcomes evidenced by behavior and brain. Findings are related to mood and anxiety in particular, suggesting that further understanding of GR polymorphism and interactions with FMRP will be important for identifying and treating anxiety in individuals with FXS.
We hypothesized that the presence of the G allele would have a protective effect among individuals with FXS, allowing them to compensate for aberrant glucocorticoid feedback(Miyashiro et al., 2003). Behavioral outcomes in females supported this hypothesis. Among females, the presence of the G allele was associated with lower self- and caregiver-reported anxiety symptoms. Significant differences were found for self-reported physical symptoms of anxiety (MASC) and, in particular, on the Tense/Restless subscale which includes items such as “I feel restless and on edge”, “I get shaky or jittery”, “I am jumpy”. Caregiver report also indicated lower scores in association with the G allele on the CBCL anxiety/depression subscale which includes a broad range of symptoms, e.g. “fears school”, “nervous, tense” and “feels too guilty.” Perhaps individuals with FXS are better at identifying physical symptoms of anxiety relative to social anxiety or separation/panic symptoms. A more comprehensive caregiver and/or child interview (e.g. the Anxiety Disorders Interview Schedule(Silverman et al., 2001)) and observational measures(Mian et al., 2015) will be important to further specify the interplay between genetic factors and mood symptoms.
Interestingly, females with the G allele also demonstrated significantly lower scores on the CBCL Externalizing domain. This domain is associated with problems that involve other people and their expectations and it is distinct from the Internalizing domain which captures anxious/depressed behaviors. Externalizing behavior problems may represent another domain that is protected by the presence of the G allele. Alternatively, elevated externalizing problem behaviors in individuals with the C/C polymorphism could represent downstream effects of mood (anxiety/depression) symptoms. The aforementioned behavioral results do not survive conservative correction for multiple comparisons but, because each of our comparisons were within separate behavior domains and were hypotheses-driven, multiple comparison correction may be overly conservative. These behavioral results should be considered preliminary until further replication. We also point out that effect sizes () for each significant comparison were in the “large” range (Cohen, 1969). Consideration of effect sizes in conjunction with p-values is critical to the overall interpretation of quantitative research results (Sullivan and Feinn, 2012).
Self-reports of anxiety and depression were only collected for females who have higher levels of functioning and ability to complete such measures. However, caregiver report of anxiety/depression via the CBCL was included and we found no group differences in this scale within our mixed sex sample. One limitation of the present study was the relatively small number of males participating (N=15 vs N=23 females) thus we did not undertake group comparisons (C/C group vs. G group) for the male subset of participants. Given that the present study required participants to complete an MRI scan, our sample was biased to include more females than males as males with FXS tend to present with more severe cognitive and behavioral symptoms(Bartholomay et al., 2019). One potential explanation for the significant association between the G allele and reduced mood symptoms in females is a potential interaction with GR polymorphisms and FMRP. Females have higher levels of FMRP due to the presence of a second, unaffected X chromosome(Bartholomay et al., 2019). There may be a threshold level of FMRP that is required in order for the protective effects of the G allele to be realized. This hypothesis is also supported by our correlation results demonstrating decreasing anxiety symptoms in association with increasing FMRP only among individuals with the G allele. Significant associations in this group were present with the physical symptoms and the somatic subscale of the MASC as well as a trend for a negative association between FMRP and self-report of anxiety on the Beck inventory. GR polymorphisms may indeed have significant effects on behavioral outcomes in males with FXS who have much lower levels of FMRP and studies with larger sample sizes and additional outcomes would be informative to further understand sex-specific effects. Additionally, although we have validated our FMRP measure with activation ratio in a subsample of our female participants, our quantification of FMRP in peripheral blood cells presents a challenge for interpretation. Future studies that have the potential to quantify FMRP levels directly in the brain will be important for further validation of our findings.
Extant research has demonstrated clear abnormalities in stress reactivity in FXS, which is correlated to circulating steroid levels(Hessl et al., 2002; Wisbeck et al., 2000). While a definitive mechanism of action for this observation has remained elusive, prior work shows that FMRP directly binds GRα mRNA, with resultant reductions in GRα protein in neural dendrites in FMR1 knockout mice(Miyashiro et al., 2003). The relative decrease in GRα availability in postsynaptic neurons may underlie the decreased cortisol sensitivity observed in FXS and associated impairment in the HPA feedback circuit. Here, we report a novel finding where the BclI polymorphism appears to somewhat ‘rescue’ or attenuate putative dysregulation of the glucocorticoid feedback loop, at least at the level of anxiety-related behavior. Interestingly, increased sensitivity associated with the G allele may confer a degree of protection against opposing effects on the cortisol system driven by reduced FMRP, presenting a compelling target for further investigation and possible intervention for stress-related psychiatric symptoms such as anxiety and depression.
Results also indicate that GR polymorphisms have a significant association with brain development in the full study sample (males and females). Specifically, using SVM, we demonstrated that the overall pattern of variation in thickness/volume was able to distinguish between the presence or absence of a G allele (C/C vs G groups). Within this overall significant model, regions with higher overall contribution included components of an interconnected anxiety/fear network, namely the amygdala, orbitofrontal gyrus, inferior frontal gyrus (pars orbitalis), middle frontal gyrus, anterior cingulate, parahippocampal gyrus, insula and fusiform gyrus(Banks et al., 2007; Brühl et al., 2014; Davidson, 2002; Etkin and Wager, 2007; Paulus and Stein, 2006).
Components of the anxiety/fear network have been previously associated with the FXS-specific neuro-phenotype and the amygdala may be especially affected. For example, the fmr1 knockout (KO) mouse shows deficits in amygdala inhibitory tone(Olmos-Serrano et al., 2010). Human neuroimaging research also indicates both structural and functional abnormalities of the amygdala(Gothelf et al., 2008; Kim et al., 2014), and anatomical differences in the insula(Cohen et al., 2011) and PFC(Bray et al., 2011). Further, our group has revealed a deficit in habituation of neural responses to facial stimuli in anterior cingulate and fusiform gyrus(Bruno et al., 2014). Thus, the presence of the G allele, and greater sensitivity to cortisol feedback, may modify the influence of FMRP on anxiety-related neural circuitry. This altered course of brain development may represent an important intermediary mechanism between the presence of the G allele and reduced anxiety/mood symptoms. In support of this hypothesis correlations among females indicated that volume or thickness of a unique subset of highlighted brain regions was related to anxiety symptoms and externalizing behaviors in each group. Specifically, within the G group, left caudate volume, and thickness of the left paracentral gyrus, left temporal pole, right insula and right precuneus were positively related to anxiety symptoms. Right parahippocampal gyrus thickness and right inferior parietal gyrus thickness were negatively related to anxiety. Finally, right temporal pole thickness was negatively related to externalizing behavior and right palladium volume was positively related to externalizing behavior. Within the C/C group right amygdala volume and right supramarginal gyrus thickness correlated positively with anxiety symptoms. Left rostral middle frontal gyrus thickness correlated negatively with externalizing behavior and right precuneus thickness correlated positively with externalizing behavior.
We also note similar brain-based classification results using SVM in a female-only model. Although the model was significant, accuracy was lower among this reduced sample (N=28). Regions highlighted by the female-only model include anxiety/fear circuitry, i.e. the amygdala, insula and orbitofrontal gyrus, which were also highlighted by the model including males and females. Unique regions were also highlighted indicating sex-specific patterns of brain morphology in association with the G allele. The subset of male participants in the present study (N=15) was not sufficient for SVM analysis. Additional studies with larger samples of each sex will be important for bearing out sex differences in association with GR polymorphisms. Consideration of additional genetic factors is also relevant. For example, brain structure has been previously linked to monoamine oxidase A promoter polymorphisms in boys with FXS(Wassink et al., 2014).
Our behavioral and brain imaging assessments were conducted in adolescence/young adulthood and we cannot specify how and when FMRP and GR polymorphisms interact to alter outcomes. FMRP is known to have long-range effects on both brain and behavior development; thus, one hypothesis is that the G allele is protective and interacts with FMRP from an early stage producing a different trajectory of brain development. Longitudinal studies will be important for understanding interactions between FMRP and GR polymorphisms across development, potentially identifying critical developmental windows when interventions may be maximally effective. Further, additional studies designed to elucidate the potential impact of GR polymorphisms in the general population as well as other clinical populations are warranted.
The present study describes the association between GR polymorphisms and both behavior and brain-based outcomes among adolescents/young adults with FXS. We found evidence for protective effects of the G allele in terms of behavioral outcomes in females. We also demonstrate an association between the G allele and altered patterns of brain structure that highlight anxiety/fear circuitry. The present findings lend understanding to the diverse clinical outcomes associated with FXS and can also help pave the way for designing targeted therapies for this population. Therapies may be targeted to individuals with specific polymorphisms at critical developmental windows and/or designed to correct aberrant development of specific aspects of anxiety/fear circuitry. Furthermore, anxiety and depression represent some of the most common psychiatric diagnoses irrespective of neurogenetic syndromes. Continued investigation of gene-gene interactions involving FMRP and GR in the context of brain and behavior developmental is of critical import for understanding and treating mood disorders for individuals with and without FXS.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability: Anonymized data will be shared following reasonable request from a qualified investigator. Requests can be made via email to the corresponding author.
Declarations of interest: none
References
- Achenbach TM, 1991. Manual for the child behavior checklist/ 4–18 and 1991 profile. University of Vermont, Department of Psychiatry, Brlington, VT. [Google Scholar]
- Alnæs D, Sneve MH, Espeseth T, Endestad T, van de Pavert SHP, Laeng B, 2014. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis 14, 1–20. 10.1167/14.4.1 [DOI] [PubMed] [Google Scholar]
- Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ, 2005. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology 30, 297–306. 10.1016/j.psyneuen.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB, 2008. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am. J. Med. Genet. Part A 146, 2060–2069. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Luan Phan K, 2007. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci 2, 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Weinzimer SA, Ruedy KJ, Mauras N, Beck RW, Marzelli MJ, Mazaika PK, Aye T, White NH, Tsalikian E, Fox L, Kollman C, Cheng P, Reiss AL, 2014. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner-the Diabetes Research in Children Network (DirecNet) experience. Pediatr. Radiol 44, 181–186. 10.1007/s00247-013-2798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay K, Lee C, Bruno J, Lightbody A, Reiss A, 2019. Closing the Gender Gap in Fragile X Syndrome: Review on Females with FXS and Preliminary Research Findings. Brain Sci. 9, 11. 10.3390/brainsci9010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G, 1996. Beck depression inventory second edition manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA, 1988. An Inventory for Measuring Clinical Anxiety: Psychometrical Properties. J. Consult. ans Clincal Psychol 56, 893–897. [DOI] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody A. a., Walter E, Chen K, Patnaik S, Reiss AL, 2011. Aberrant Frontal Lobe Maturation in Adolescents with Fragile X Syndrome is Related to Delayed Cognitive Maturation. Biol. Psychiatry 70, 852–858. 10.1016/j.biopsych.2011.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S, 2014. Neuroimaging in social anxiety disorder-A meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev 47, 260–280. 10.1016/j.neubiorev.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Bruno JL, Garrett AS, Quintin EM, Mazaika PK, Reiss AL, 2014. Aberrant face and gaze habituation in fragile X syndrome. Am. J. Psychiatry 171, 1099–1106. 10.1176/appi.ajp.2014.13111464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JL, Hosseini SMH, Saggar M, Quintin E-M, Raman MM, Reiss AL, 2017. Altered Brain Network Segregation in Fragile X Syndrome Revealed by Structural Connectomics. Cereb. Cortex 27. 10.1093/cercor/bhw055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, Cohen AO, 2019. Development of the emotional brain. Neurosci. Lett 693, 29–34. 10.1016/j.neulet.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea-Del Rio CA, Nunez-Parra A, Freedman SM, Kushner JK, Alexander AL, Restrepo D, Huntsman MM, 2020. Disrupted inhibitory plasticity and homeostasis in Fragile X syndrome. Neurobiol. Dis 142, 104959. 10.1016/j.nbd.2020.104959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher JC Burges (Bell Laboratories, L.T., 1998. A Tutorial on Support Vector Machines for Pattern Recognition. Data Min. Knowl. Discov 2, 121–167. 10.1023/A:1009715923555 [DOI] [Google Scholar]
- Cohen J, 1969. Statistical power analysis for the behavioural sciences. Academic Press, New York. [Google Scholar]
- Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL, 2011. Insular volume reduction in fragile X syndrome. Int. J. Dev. Neurosci 29, 489–494. 10.1016/j.ijdevneu.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno MI, 1999. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, 2002. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol. Psychiatry 51, 68–80. 10.1016/S0006-3223(01)01328-2 [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuge J, Scharkowski F, Michaelsen-Preusse K, Korte M, 2019. FMRP Modulates Activity-Dependent Spine Plasticity by Binding Cofilin1 mRNA and Regulating Localization and Local Translation. Cereb. Cortex 29, 5204–5216. 10.1093/cercor/bhz059 [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro J. a., Hoeft F, Eckert M. a., Hall SS, O’Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL, 2008. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP). Ann. Neurol 63, 40–51. 10.1002/ana.21243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, Reiss A, 2006. Social Escape Behaviors in Children with Fragile X Syndrome. J. Autism Dev. Disord 36, 935–947. 10.1007/s10803-006-0132-z [DOI] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor a., Reiss a. L., 2001. The Influence of Environmental and Genetic Factors on Behavior Problems and Autistic Symptoms in Boys and Girls With Fragile X Syndrome. Pediatrics 108, e88–e88. 10.1542/peds.108.5.e88 [DOI] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, Reiss AL, 2001. The Influence of Environmental and Genetic Factors on Behavior Problems and Autistic Symptoms in Boys and Girls With Fragile X Syndrome. Pediatrics 108, e88–e88. 10.1542/peds.108.5.e88 [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL, 2002. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology 27, 855–872. 10.1016/S0306-4530(01)00087-7 [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL, 2006. Social behavior and cortisol reactivity in children with fragile X syndrome. J. Child Psychol. Psychiatry 47, 602–610. 10.1111/j.1469-7610.2005.01556.x [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kim SY, Burris J, Bassal F, Koldewyn K, Chattarji S, Tassone F, Hessl D, Rivera SM, 2014. Fear-specific amygdala function in children and adolescents on the fragile x spectrum: A dosage response of the FMR1 gene. Cereb. Cortex 24, 600–613. 10.1093/cercor/bhs341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiman C, Quintin E-M, Jo B, Lightbody AA, Hazlett HC, Piven J, Hall SS, Chromik LC, Reiss AL, 2014. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics 134, 315–24. 10.1542/peds.2013-3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK, 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 554–565. 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- Markham JA, Beckel-Mitchener AC, Estrada CM, Greenough WT, 2006. Corticosterone response to acute stress in a mouse model of Fragile X syndrome. Psychoneuroendocrinology 31, 781–785. 10.1016/j.psyneuen.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Mian ND, Carter AS, Pine DS, Wakschlag LS, Briggs-Gowan MJ, 2015. Development of a novel observational measure for anxiety in young children: The Anxiety Dimensional Observation Scale. J. Child Psychol. Psychiatry Allied Discip 56, 1017–1025. 10.1111/jcpp.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J, 2003. RNA Cargoes Associating with FMRP Reveal Deficits in Cellular Functioning in Fmr1 Null Mice. Neuron 37, 417–431. 10.1016/s0896-6273(03)00034-5 [DOI] [PubMed] [Google Scholar]
- Myers L, Sirois MJ, 2006. Spearman Correlation Coefficients, Differences between, in: Encyc. John Wiley & Sons, pp. 1–2. 10.1002/9781118445112.stat02802 [DOI] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM, 2010. Defective GABAergic Neurotransmission and Pharmacological Rescue of Neuronal Hyperexcitability in the Amygdala in a Mouse Model of Fragile X Syndrome. J. Neurosci 30, 9929–9938. 10.1523/jneurosci.1714-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS, 2009. Distinct Genetic Influences on Cortical Surface Area and Cortical Thickness. Cereb. Cortex 19, 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, 2006. An Insular View of Anxiety. Biol. Psychiatry 60, 383–387. 10.1016/j.biopsych.2006.03.042 [DOI] [PubMed] [Google Scholar]
- Quintin E-M, Jo B, Hall SS, Bruno JL, Chromik LC, Raman MM, Lightbody AA, Martin A, Reiss AL, 2015. The cognitive developmental profile associated with fragile X syndrome: A longitudinal investigation of cognitive strengths and weaknesses through childhood and adolescence. Dev. Psychopathol 8, 1–13. 10.1017/S0954579415001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar M, Hosseini SMH, Bruno JL, Quintin E, Raman MM, Kesler SR, Reiss AL, 2015. Estimating individual contribution from group-based structural correlation networks. Neuroimage 120, 274–284. 10.1016/j.neuroimage.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA, 2001. Test-retest reliability of anxiety symptoms and diagnoses with the anxiety disorders interview schedule for DSM-IV: Child and parent versions. J. Am. Acad. Child Adolesc. Psychiatry 40, 937–944. 10.1097/00004583-200108000-00016 [DOI] [PubMed] [Google Scholar]
- Sinclair D, Webster MJ, Wong J, Weickert CS, 2011. Dynamic molecular and anatomical changes in the glucocorticoid receptor in human cortical development. Mol. Psychiatry 16, 504–515. 10.1038/mp.2010.28 [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA, 2005. Vineland-II Adaptive Behavior Scales: Survey Forms Manual. NCS Pearson, Inc., Minneapolis, MN. [Google Scholar]
- Sullivan GM, Feinn R, 2012. Using Effect Size—or Why the P Value Is Not Enough. J. Grad. Med. Educ 4, 279–282. 10.4300/jgme-d-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H-T, Cohen S, Kaufmann WE, 2001. Annexin-1 is abnormally expressed in Fragile X syndrome: Two-dimensional electrophoresis study in lymphocytes. Am. J. Med. Genet 103, 81–90. [DOI] [PubMed] [Google Scholar]
- Swanger S. a, Bassell GJ, 2011. Making and breaking synapses through local mRNA regulation. Curr. Opin. Genet. Dev 21, 414–421. 10.1016/j.gde.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Davis LK, Reiss AL, Piven J, 2014. Testing for association of the monoamine oxidase A promoter polymorphism with brain structure volumes in both autism and the fragile X syndrome. J. Neurodev. Disord 6. 10.1186/1866-1955-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler adult intelligence scale—3rd Edition. Harcourt Assessment, San Antonio, TX. [Google Scholar]
- Wechsler D, 1991. WISC-III: Wechsler intelligence scale for children. third edition. Psychological Corperation, San Antonio, TX. [Google Scholar]
- Willemsen R, Smits A, Mohkamsing S, Van Beerendonk H, De Haan A, De Vries B, Van Den Ouweland A, Sistermans E, Galjaard H, Oostra B. a., 1997. Rapid antibody test for diagnosing fragile X syndrome: A validation of the technique. Hum. Genet 99, 308–311. 10.1007/s004390050363 [DOI] [PubMed] [Google Scholar]
- Wisbeck JM, Huffman LC, Freund L, Gunnar MR, Davis EP, Reiss a L., 2000. Cortisol and social stressors in children with fragile X: a pilot study. J. Dev. Behav. Pediatr 10.1097/00004703-200008000-00004 [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Hazlett HC, Lightbody AA, Reiss AL, Piven J, 2013. Repetitive and self-injurious behaviors : associations with caudate volume in autism and fragile X syndrome 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst S, van Rossum EFC, Federenko IS, Koper JW, Kumsta R, Hellhammer DH, 2004. Common Polymorphisms in the Glucocorticoid Receptor Gene Are Associated with Adrenocortical Responses to Psychosocial Stress. J. Clin. Endocrinol. Metab 89, 565–573. 10.1210/jc.2003-031148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


