ABSTRACT
Influenza remains one of the most contagious infectious diseases. Approximately, 25 to 50 million people suffer from influenza-like illness in the United States annually, leading to almost 1 million hospitalizations. Globally, the World Health Organization (WHO) estimates 250,000 to 500,000 mortalities associated with secondary respiratory complications due to influenza virus infection every year. Currently, seasonal vaccination represents the best countermeasure to prevent influenza virus spread and transmission in the general population. However, presently licensed influenza vaccines are about 60% effective on average, and their effectiveness varies from season to season and among age groups, as well as between different influenza subtypes within a single season. The hemagglutination inhibition (HAI) assay represents the gold standard method for measuring the functional antibody response elicited following standard-of-care vaccination, along with evaluating the efficacy of under-development influenza vaccines in both animal models and clinical trial settings. However, using the classical HAI approach, it is not possible to dissect the complexities of variable epitope recognition within a polyclonal antibody response. In this paper, we describe a straightforward competitive HAI-based method using a combination of influenza virus and recombinant hemagglutinin (HA) proteins to dissect the HAI functional activity of HA-specific antibody populations in a single assay format.
IMPORTANCE The hemagglutination inhibition (HAI) assay is a well-established and reproducible method that quantifies functional antibody activity against influenza viruses and, in particular, the capability of an antibody formulation to inhibit the binding of hemagglutinin (HA) to sialic acid. However, the HAI assay does not provide full insights on the breadth and epitope recognition of the antibody formulation, especially in the context of polyclonal sera, where multiple antibody specificities contribute to the overall observed functional activity. In this report we introduce the use of Y98F point-mutated recombinant HA (HAΔSA) proteins, which lack sialic acid binding activity, in the context of the HAI assay as a means to absorb out certain HA-directed (i.e., strain-specific or cross-reactive) antibody populations. This modification to the classical HAI assay, referred to as the competitive HAI assay, represents a new tool to dissect the magnitude and breadth of polyclonal antibodies elicited through vaccination or natural infection.
KEYWORDS: hemagglutination inhibition assay (HAI), monoclonal antibodies (mAbs), polyclonal antibodies, serum, influenza virus, influenza vaccine, antibody, HAI assay, hemagglutinin
INTRODUCTION
Influenza is a highly contagious viral respiratory disease that affects millions of people worldwide each year, with more than 800,000 reported hospitalizations in the United States during the 2018–2019 season due to influenza infection (1). Annual vaccination is recommended by the World Health Organization (WHO) to reduce influenza severity and limit transmission through elicitation of antibodies targeting mainly the hemagglutinin (HA) glycoprotein of the influenza virus (2). The hemagglutination inhibition (HAI) assay is a well-established and reproducible method that quantifies functional antibody activity against influenza viruses. In particular, using the HAI assay, it is possible to assess the capability of a polyclonal or monoclonal antibody (MAb) formulation to inhibit the binding of HA to sialic acid, which ultimately reflects the antibody-neutralizing potential. For this reason, the HAI assay still represents the gold standard for measuring vaccine-elicited immune protection against influenza virus, as recommended by the WHO (3).
However, other immunological evaluations are needed to better dissect and analyze the immune response elicited following influenza virus infection or vaccination, such as the magnitude, breadth, and durability of the antibody response (4). In the context of evaluating the effectiveness of next-generation influenza vaccines, characterizing the breadth of an elicited immune response is pivotal both for immunogen down-selection and assessment of their protective potential.
Recombinantly expressed soluble hemagglutinin (rHA) proteins engineered to encode a single point mutation (Y98F) that possess ablated sialic acid binding (HAΔSA) have been described previously and serve as useful tools for identifying HA-specific B cell populations (e.g., plasmablasts or memory B cells) in the context of influenza infection or vaccination (5–9). Unlike wild-type expressed rHA that do not carry this point mutation, rHAΔSA proteins lack sialic acid binding activity. Consequently, rHAΔSA proteins lack any agglutination capacity, and this attribute renders them suitable for usage in assays where this advantageous property circumvents nonspecific binding to the cells, such as in the case of flow cytometric-based assays or HAI (5, 6).
Importantly, the structural conformation of rHAΔSA proteins is nearly indistinguishable from that of their unmutated equivalent rHAs (5, 6, 10). In this context, the rHAΔSA proteins are also ideally suited for absorbing HA-specific antibody reactivity without introducing interference in the readout of the classical HAI assay. To this end, we describe here a straightforward modification of the classical HAI assay, referred to as the competitive HAI assay, that leverages rHAΔSA proteins for dissecting the contribution of cross-reactive versus strain-specific antibody populations within the overall HAI activity profile elicited through vaccination or natural infection.
RESULTS
HAI reduction is dependent on concentration of rHAΔSA.
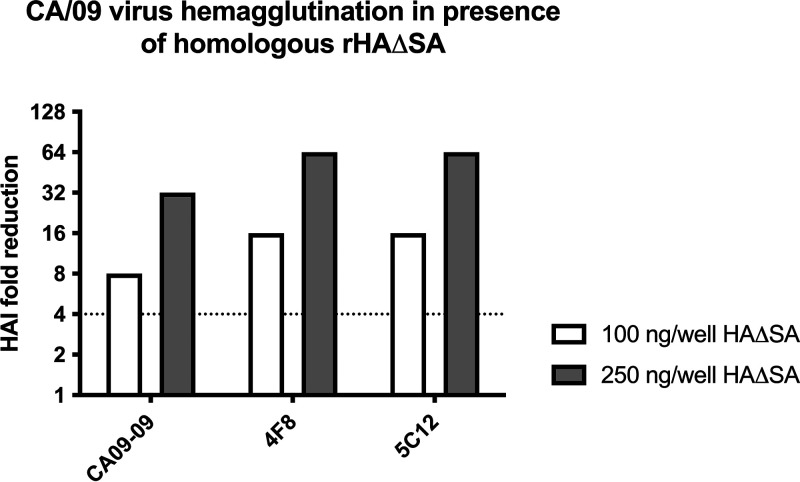
To identify a sufficient concentration of rHAΔSA protein for usage in a competitive HAI assay, two concentrations of rHAΔSA protein from A/California/04/2009 (CA/09) were evaluated for their capacity to reduce the effective HAI titer. Specifically, CA/09 rHAΔSA was tested at 4 or 10 μg/ml corresponding to 100 and 250 ng of rHAΔSA per well. As shown in Fig. 1, 4 μg/ml of rHAΔSA was sufficient to demonstrate a considerable increase (≥8-fold HAI reduction) in the HAI concentration of these MAbs. In particular, an 8-fold and 32-fold increase for the CA09-09 MAb HAI concentration was observed using 100 ng and 250 ng/well of rHAΔSA, respectively. The same rHAΔSA dose-dependent trend was also observed using the 4F8 and 5C12 MAbs and yielded a 16-fold and 64-fold increase in the MAb HAI concentrations using 100 ng and 250 ng/well of rHAΔSA, respectively.
FIG 1.
Competitive HAI assay using CA/09-specific mouse MAbs. The extent of HAI titer reduction through competitive HAI using different CA/09-specific mouse MAbs (CA09-09, 4F8, and 5C12) following preabsorption with different amounts (100 and 250 ng/well) of CA/09 rHAΔSA protein. The dotted line represents the 4-fold change in the HAI titer.
Homologous HAΔSA reduces HAI activity from influenza-infected ferrets.
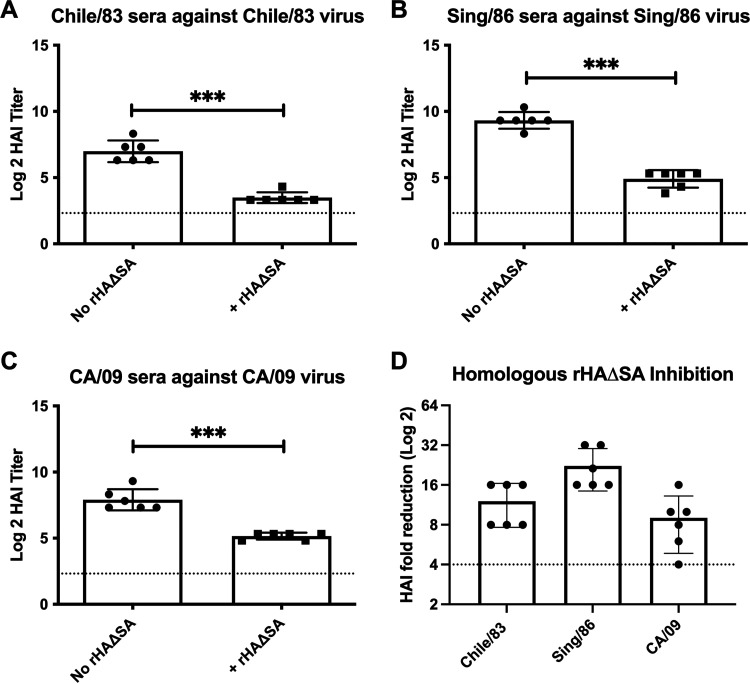
In order to initially validate the competitive HAI assay approach, we utilized rHAΔSA proteins representing seasonal or pandemic H1N1 influenza viral strains and assessed their capacity to absorb HAI activity present in antisera elicited in ferrets infected with H1N1 viral strains (11). As shown in Fig. 2, antisera collected from ferrets infected with influenza viruses representing seasonal H1N1 (A/Chile/1/1983 [Chile/83], A/Singapore/6/1986 [Sing/86]) or pandemic H1N1 (A/California/07/2009 [CA/09]) exhibited robust HAI activity against the homologous virus strain in the absence of HAΔSA protein (Fig. 2A to C). However, a significant reduction in the HAI titer was consistently observed in antisera collected from individual ferrets infected with the respective Chile/83, Sing/86, and CA/09 influenza viruses when matching (homologous) rHAΔSA protein was introduced into the assay (Fig. 2A to D).
FIG 2.
Competitive HAI assay using sera from preimmune animals infected with seasonal and pandemic H1N1 virus strains. (A to C) The extent of HAI titer reduction through competitive HAI. HAI titer of antisera from ferret infected with H1N1 seasonal (Chile/83 and Sing/86) and pandemic (CA/09) influenza virus strains following preabsorption with the corresponding rHAΔSA protein representing seasonal (Chile/83 and Sing/86) or pandemic (CA/09) virus strains. Differences in HAI titer were analyzed using a paired t test (***, P < 0.0001). Dotted lines represent the 1:40 HAI titer threshold. (D) The fold change in HAI activity for the same serum samples following preabsorption with rHAΔSA protein representing seasonal (Chile/83 and Sing/86) or pandemic (CA/09) virus strains. The dotted line represents the 4-fold change in the HAI titer.
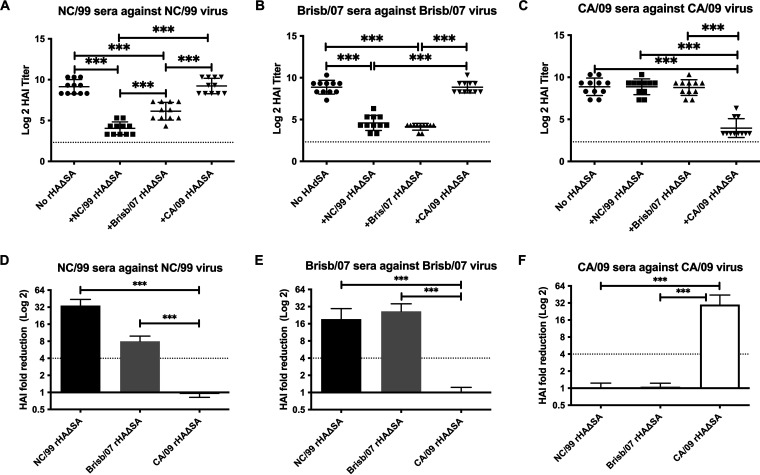
rHAΔSA from seasonal or pandemic influenza virus strains reduces HAI activity from HA-VLP vaccinated mice.
To further validate the competitive HAI assay approach, we utilized rHAΔSA proteins representing seasonal or pandemic H1N1 influenza viral strains and assessed their capacity to absorb HAI activity present in antiserum elicited in BALB/c mice vaccinated with viral-like particles (VLP) (12). As shown in Fig. 3, antisera from mice immunized with VLP representing seasonal H1N1 (A/New Caledonia/20/1999 [NC/99], A/Brisbane/59/2007 [Brisb/07]) or pandemic H1N1 (CA/09) viruses exhibited robust HAI activity against the homologous virus strain in the absence of the HAΔSA protein. However, a significant reduction in HAI titer was consistently observed in antisera collected from individual mice vaccinated with the respective NC/99, Brisb/07, or CA/09 VLP vaccines when matching (homologous) HAΔSA protein was introduced into the assay. Importantly, no reduction in the HAI titer against NC/99 or Brisb/07 viral strains was detected when pandemic CA/09 HAΔSA protein was introduced into assays (Fig. 3A, B, D and E). Likewise, there was no reduction in HAI titer against the CA/09 virus following introduction of NC/99 or Brisb/07 HAΔSA protein into the assay (Fig. 3C and F).
FIG 3.
Competitive HAI assay using sera from mice vaccinated with VLP-HA from seasonal and pandemic H1N1 strains. (A to C) The extent of HAI titer reduction through competitive HAI. HAI titer of antisera from BALB/c mice immunized with H1N1 seasonal (NC/99 and Brisb/07) and pandemic (CA/09) influenza virus strains following preabsorption with rHAΔSA protein representing seasonal (NC/99 and Brisb/07) or pandemic (CA/09) virus strains. Differences in HAI titer were analyzed using a one-way analysis of variance (ANOVA) (***, P < 0.0001). The dotted lines represent the 1:40 HAI titer threshold. (D to F) The extent of antibody HAI fold reduction following preabsorption with rHAΔSA proteins. The dotted lines represent the 4-fold change in the HAI titer.
rHAΔSA reduces HAI activity of HA-specific MAbs.
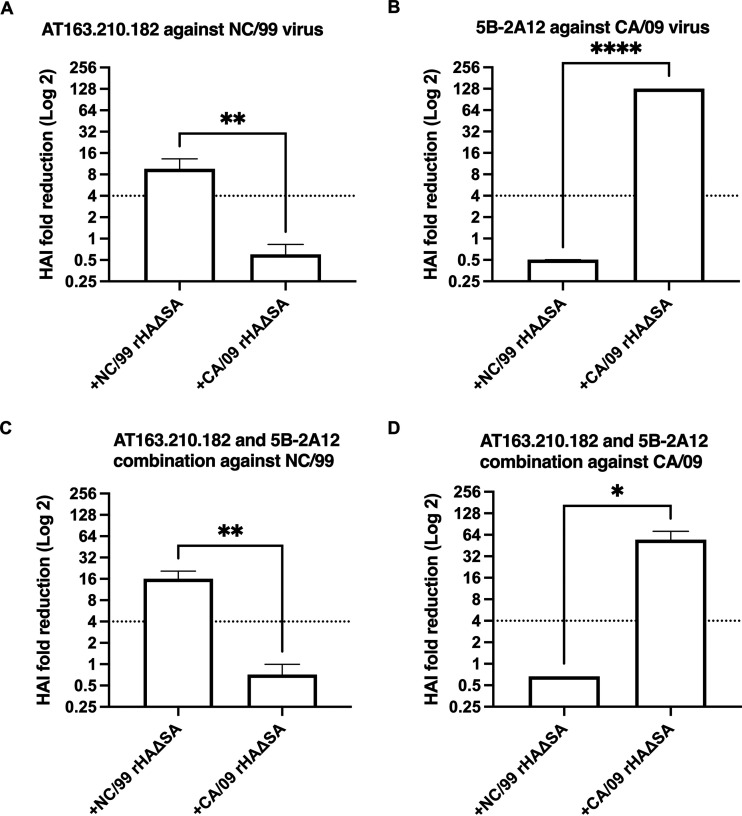
In addition to evaluating polyclonal antibody populations, we also tested MAbs previously generated from seasonal (Brisb/07) or pandemic (CA/09) H1N1 vaccinated mice (10). In particular, the HAI activity of these MAbs used alone or in combination was evaluated in the presence/absence of corresponding rHAΔSA. As shown in Fig. 4, we observed a significant increase in the HAI titer of seasonal (NC/99)-specific MAb AT163.210.182 alone or in combination with the CA/09-specific MAb 5B-2A12 in the presence of NC/99 rHAΔSA protein (Fig. 4A and C). Similarly, an increase in the HAI titer of MAb 5B-2A12 alone or in combination with MAb AT163.210.182 was observed against the pandemic CA/09 virus in the presence of homologous CA/09 rHAΔSA protein (Fig. 4B and D).
FIG 4.
Competitive HAI assay using H1N1-specific mouse MAbs against seasonal and pandemic H1N1 virus strains. (A to D) HAI fold reduction of MAb AT163.210.182 (A), MAb 5B-2A12 (B), or a 1:1 combination of MAb AT163.210.182 and MAb 5B-2A12 (C and D) against H1N1 seasonal (NC/99) and pandemic (CA/09) influenza virus strains following preabsorption with rHAΔSA protein representing homologous seasonal (NC/99) or pandemic (CA/09) virus strains. Differences in HAI titer were analyzed using a t test (*, P < 0.05; **, P < 0.01; ****, P < 0.0001). Dotted line represents the 4-fold change in the HAI titer.
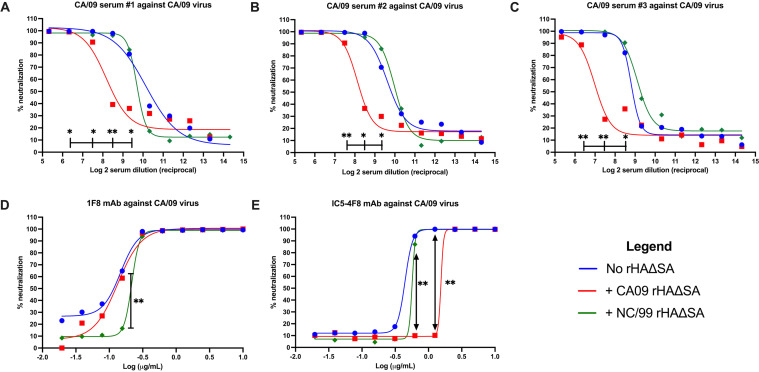
rHAΔSA reduces the neutralization of HA-specific polyclonal and MAbs.
As a further tactic to validate our functional antibody depletion approach, we tested the ability of rHAΔSA protein to decrease the neutralization potency of polyclonal antibodies or MAbs in a focus reduction assay (FRA)-based neutralization assay. As shown in Fig. 5, and similar to the competitive HAI assay, CA/09 rHAΔSA protein decreased the neutralizing potency of CA/09-elicited antisera against the homologous CA/09 virus. In particular, a significant reduction in the 50% inhibitory concentration (IC50) of CA/09-elicited antisera, from ∼1:500 to 1:1,000 to ∼1:130 to 1:300, was observed in the absence or presence of the CA/09 rHAΔSA, respectively (Fig. 5A to C). This reduction corresponded to a decrease in neutralizing activity from ∼100% to ∼40% in all CA/09-elicited antisera in the 1:180 to 1:360 dilution range (Fig. 5A to C). Importantly, no reduction in the IC50 and the overall serum neutralizing potency was observed when NC/99 rHAΔSA protein was introduced in the assay (Fig. 5A to C).
FIG 5.
Competitive neutralization (FRA) assay using H1N1-specific antisera and mouse and human MAbs against the H1N1 CA/09 pandemic virus strains. (A to C) The extent of HAI titer reduction through competitive HAI using antisera from H1N1 CA/09 HA vaccinated mice. (D and E) IC50 value of MAb 1F8 (D) and MAb IC5-4F8 (E) against H1N1 pandemic (CA/09) influenza virus strains following preabsorption with rHAΔSA protein representing seasonal (NC/99) and pandemic (CA/09) virus strains. Differences in the neutralizing activity at different dilutions (sera) or concentrations (MAbs) were analyzed using a one-way ANOVA (*, P < 0.05; **, P < 0.01).
Similarly, a decrease in the IC50 of broadly neutralizing HA-head-directed MAb 1F8 was observed in the presence of NC/99 rHAΔSA protein from ∼0.14 to 0.22 μg/ml (Fig. 5D). In particular, a significant decrease in neutralizing activity from ∼65% to ∼15% was observed using 0.156 μg/ml of MAb 1F8 (Fig. 5A to C). No decrease in the IC50 and overall 1F8 neutralizing potency was observed when CA/09 rHAΔSA protein was introduced in the assay (Fig. 5A to C). Lastly, a decrease in the IC50 of MAb IC5-4F8, from ∼0.44 to 0.57 and 1.54 μg/ml, was observed in the presence of the NC/99 or the CA/09 rHAΔSA proteins, respectively (Fig. 5E).
DISCUSSION
The detailed analysis of antibody responses elicited through influenza vaccination is pivotal to determining vaccine effectiveness of current, as well as next-generation, vaccines. In this context, the HAI assay still represents the gold standard methodology for assessing vaccine effectiveness. This assay is also fundamental to preliminary evaluations of the breadth and neutralizing potential of head-directed MAbs, as well as polyclonal antibody responses against HA in general.
In this communication, we introduced rHAΔSA protein into the HAI assay as a strategy to further dissect HA-specific polyclonal or MAb reactivity profiles. Specifically, we aimed to establish an assay platform capable of revealing whether functional antibody activity was composed of broadly reactive antibodies targeting shared epitopes, or rather, whether functional antibody activity was composed of nonoverlapping, narrowly reactive specificities.
In this context, the utility of the competitive HAI approach is highlighted by the observation that in addition to homologous protein, introduction of heterologous rHAΔSA protein was also capable of reducing functional antibody activity. Such was clearly the case for NC/99 and Brisb/07 rHAΔSA proteins, which exhibited reciprocal inhibition in the mismatched (heterologous) assays (Fig. 2A, B, D, and E). Collectively, these results support the antigenic relatedness of NC/99 and Brisb/07 viruses and also reveal that HAI activity in both of the VLP-vaccinated cohorts was directed toward epitopes partially shared between these respective viruses.
Shared epitope recognition was also confirmed using MAbs. As an example, the neutralizing activity of broadly reactive HA head-directed MAb 1F8 against the CA/09 pandemic influenza virus was reduced in the presence of seasonal NC/99 rHAΔSA protein. Importantly, MAb 1F8 was isolated from a mouse immunized with a computationally optimized broadly reactive antigen (COBRA) next-generation influenza vaccine and exhibits broad reactivity and potent functional activity (10, 13–15). Similarly, we observed a decrease in the HAI activity of Brisb/07-elicited MAb AT163.210.182 in the presence of NC/99 rHAΔSA protein.
However, as a further confirmation of the antigenic uniqueness of seasonal (NC/99) and pandemic (CA/09) rHAΔSA and the nonoverlapping epitope recognition profiles of MAb AT163.210.182 (seasonal-specific MAb) and MAb 5B-2A12 (pandemic-specific MAb), HAI activity of these MAbs was unaffected when they were combined. This was confirmed by the comparable HAI activity exhibited by MAb AT163.210.182 and MAb 5B-2A12 when tested alone or in combination against the NC/99 and CA/09 virus and in the presence of the corresponding rHAΔSA proteins.
Collectively, this paper details the specificity of the competitive HAI approach and hints at its utility for dissecting complex HAI profiles. Further, we suggest that the competitive HAI approach is a viable strategy for selection of universal influenza immunogen candidates that elicit broadly reactive functional antibody reactivity.
MATERIALS AND METHODS
Recombinant HAΔSA proteins.
Genes encoding the HAΔSA were cloned into the pcDNA3.1/Zeo (+) vector as previously described (6). In detail, the encoding genes consisting of the extracellular domain of HA possessing the Y98F mutation to attenuate sialic acid binding (HAΔSA) were truncated at the 3′ end of HA genes starting from amino acid 521 (H1N1 A/California/04/09 HA numbering) and modified to include a FoldOn of T4 fibritin trimerization domain to promote its expression as a soluble trimeric protein, an AviTag sequence, and a hexahistidine affinity tag, as previously described (6, 16). The different rHAΔSA proteins were expressed through a transient transfection of the EXPI293F cells (Thermo Fisher Scientific, Waltham, MA) with the vectors encoding the different HAΔSA and following previously described methods. Alternatively, HA proteins were expressed through the generation of stable transfected cells supplemented with 100 μg/ml of Zeocin (InvivoGen, San Diego, CA), as previously described (6, 16). Recombinant HAΔSA proteins were then purified through the ÄKTA Pure system using HisTrap columns (GE Healthcare, Chicago, IL) through immobilized metal affinity chromatography (IMAC), as previously described (6).
Monoclonal and polyclonal antibodies from influenza virus-vaccinated or -infected mice and ferrets.
The mouse MAbs used in this study were previously described by our group (10) or provided by the International Reagent Resource (IRR) or BEI Resources. Polyclonal antisera elicited through vaccination of mice or infection of ferrets with seasonal or pandemic H1N1 influenza strains were previously described (11, 12).
Competitive hemagglutination inhibition (HAI) assay.
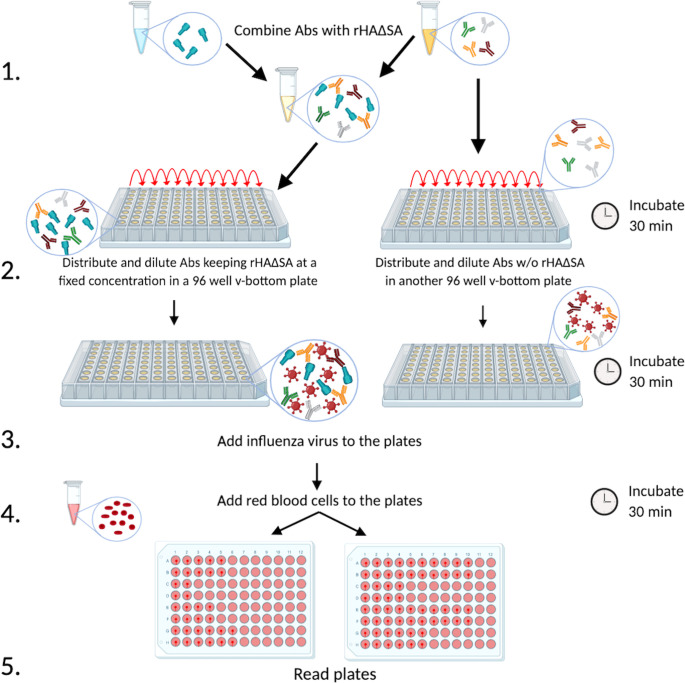
As shown in Fig. 6, the starting dilution of the purified monoclonal antibodies (MAbs) or polyclonal antibody populations to be evaluated was combined with the designated rHAΔSA to achieve a final concentration of 100 ng/well of rHAΔSA. As per WHO recommendations for when polyclonal antibodies from serum samples are used (17), serum was first treated with receptor-destroying enzyme (RDE; Denka Seiken, Japan) and then diluted 1:10 in order to avoid possible interference during the assay. Similar to the conventional HAI assay, the starting 1:10 dilution was then used in the competitive HAI assay to perform the remainder of the 2-fold serial dilutions. However, unlike the HAI assay, the serum samples were initially diluted 2-fold in phosphate-buffered saline (PBS) containing 8 μg/ml of the designated rHAΔSA to achieve a final concentration of 100 ng/well. Subsequently, additional 2-fold serial dilutions were performed in PBS containing 4 μg/ml of the designated rHAΔSA in order to keep the protein concentration fixed and corresponding to 100 ng of rHAΔSA/well. Importantly, for these reasons, we recommend evaluating serum samples exhibiting an HAI titer of ≥1:80 in order to observe a meaningful reduction in HAI activity (≥4-fold change). The antibody-rHAΔSA mixture was then incubated for 30 min at room temperature (RT) to allow for antibody absorption. Lastly, the influenza virus solution, adjusted to 8 hemagglutinating units (HAU) as per the WHO HAI protocol (17), was added, and the remainder of the assay was performed as previously described (16).
FIG 6.
Schematic representation of the R-HAI assay. Monoclonal or polyclonal antibodies from vaccinated or infected experimental animals or individuals are combined with rHAΔSA from a particular seasonal or pandemic influenza virus strain in order to absorb the antibodies specific to that HA (step 1). Subsequently, antibodies are distributed on a 96-well v-bottom plate in the presence or absence of HAΔSA (100 ng/well) (step 2), and the influenza virus of interest is added (step 3). Afterward, red blood cells are added (step 4), and the plates are read to determine HAI titer (step 5).
Competitive focus reduction assay.
In order to confirm the specificity of the observed HAI activity reductions and to evaluate if such reductions could also be observed in other types of neutralization assays, we introduced rHAΔSA into a focus reduction assay (FRA). Similar to the competitive HAI assay protocol described above, the starting dilution of the MAb (10 μg/ml) or RDE-treated serum polyclonal (1:40) antibody populations to be evaluated were serially diluted 2-fold in duplicate in PBS containing 2 μg/ml of rHAΔSA for a final volume of 50 μl/well. The antibody-HAΔSA mixture was then incubated for 30 min at RT to allow for antibody absorption. The FRA was then performed as previously described (10, 16). In brief, MDCK-SIAT1 cells seeded the day before at a density of 2.5 to 3 × 105 cells/ml were rinsed with 0.01 M PBS, pH 7.2 (Thermo Fisher Scientific), followed by the addition of the 2-fold serially diluted RDE-treated serum-HAΔSA mixtures. Afterward, 50 μl of virus (A/California/07/2009) diluted in virus growth medium containing 1 μg/ml l-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich, St. Louis, MO) and standardized to 1.2 × 104 focus forming units (FFU)/ml, corresponding to 600 focus-forming units/50 μl, was added to each well, including control wells. Following a 2-h incubation period at 37°C with 5% CO2, the cells in each well were then overlaid with 100 μl of equal volumes of 1.2% Avicel RC/CL (type RC581 NF; FMC Health and Nutrition, Philadelphia, PA) in 2× minimum essential medium containing 1 μg/ml TPCK-treated trypsin, 0.1% bovine serum albumin (BSA, Thermo Fisher Scientific), and penicillin/streptomycin (Thermo Fisher Scientific). Plates were incubated for 18 to 22 h at 37°C and 5% CO2. The overlays were then removed from each well, and the monolayer was washed once with PBS to remove any residual Avicel. The plates were fixed with ice-cold 4% formalin (VWR International, Radnor, PA) in PBS for 30 min at 4°C followed by a PBS wash and permeabilization using 0.5% Triton X-100 (Sigma-Aldrich) in PBS/glycine (VWR International) at RT for 20 min. Plates were washed three times with PBS/0.1% Tween 20 (PBST; VWR International) and incubated for 1 h with a MAb against influenza A nucleoprotein (IRR) in enzyme-linked immunosorbent assay (ELISA) buffer (PBS containing 10% horse serum [Thermo Fisher Scientific] and 0.1% Tween 80 [VWR International]). Following washing three times with PBST, the cells were incubated with goat anti-mouse peroxidase-labeled IgG (SeraCare, Milford, MA) in ELISA buffer for 1 h at RT. Plates were washed three times with PBST, and infectious foci (spots) were visualized using TrueBlue substrate (SeraCare) containing 0.03% H2O2 incubated at RT for 10 to 15 min. The reaction was stopped by washing five times with distilled water. Plates were dried and foci enumerated using an ImmunoSpot S6 Ultimate analyzer with BioSpot software (CTL, Shaker Heights, OH). The FRA titer was reported as the reciprocal of the highest dilution of serum corresponding to 50% foci reduction compared with the virus control minus the cell control.
ACKNOWLEDGMENTS
We thank Matin Bahmanabadi and Spencer R. Pierce for helpful technical assistance.
Mouse MAbs to recombinant H1 HA influenza A/Brisbane/59/2007 (H1N1), clone AT163.210.182 (FR-495), were obtained through the International Reagent Resource (IRR), Influenza Division, World Health Organization Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, Centers for Disease Control and Prevention (Atlanta, GA, USA).
The following reagents were obtained through BEI Resources, NIAID, NIH: monoclonal anti-influenza A virus H1 hemagglutinin (HA), A/California/04/2009 (H1N1)pdm09, clone CA09-09 (ascites, mouse), NR-28666; clone 4F8 (produced in vitro), NR-42021; clone 5C12 (produced in vitro), NR-42019; and clone IC5-4F8 (produced in vitro), NR-48783.
We declare no competing interests.
This work has been funded by the National Institute of Allergy and Infectious Diseases, a component of the NIH, Department of Health and Human Services, under the Collaborative Influenza Vaccine Innovation Centers (CIVICs) contract 75N93019C00052, and in part by the University of Georgia (UGA-001). In addition, T.M.R. is supported by the Georgia Research Alliance as an eminent scholar. G.A.K.’s contributions to the manuscript preceded his current position at Cellular Technology Limited (CTL).
Conceptualization, G.A.K. and G.A.S.; methodology, G.A.K. and G.A.S.; validation, R.A.R. and J.W.E.; formal analysis, G.A.K., G.A.S. and R.A.R.; investigation, G.A.K. and G.A.S.; data curation, G.A.K., G.A.S. and R.A.R.; writing-original draft preparation, G.A.S. and G.A.K.; writing-review and editing, G.A.K., G.A.S., and T.M.R.; supervision, G.A.S. and T.M.R.; project administration, G.A.S., G.A.K. and T.M.R.; and funding acquisition, T.M.R.
Contributor Information
Ted M. Ross, Email: tedross@uga.edu.
Kanta Subbarao, The Peter Doherty Institute for Infection and Immunity.
REFERENCES
- 1.Garten R, Blanton L, Elal AIA, Alabi N, Barnes J, Biggerstaff M, Brammer L, Budd AP, Burns E, Cummings CN, Davis T, Garg S, Gubareva L, Jang Y, Kniss K, Kramer N, Lindstrom S, Mustaquim D, O’Halloran A, Sessions W, Taylor C, Xu X, Dugan VG, Fry AM, Wentworth DE, Katz J, Jernigan D. 2018. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 67:634–642. 10.15585/mmwr.mm6722a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souty C, Blanchon T, Bonmarin I, Levy-Bruhl D, Behillil S, Enouf V, Valette M, Bouscambert M, Turbelin C, Capai L, Roussel V, Hanslik T, Falchi A. 2015. Early estimates of 2014/15 seasonal influenza vaccine effectiveness in preventing influenza-like illness in general practice using the screening method in France. Hum Vaccin Immunother 11:1621–1625. 10.1080/21645515.2015.1046661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter CW, Oxford JS. 1979. Determinants of immunity to influenza infection in man. Br Med Bull 35:69–75. 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 4.Cox RJ. 2013. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother 9:405–408. 10.4161/hv.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittle JR, Wheatley AK, Wu L, Lingwood D, Kanekiyo M, Ma SS, Narpala SR, Yassine HM, Frank GM, Yewdell JW, Ledgerwood JE, Wei CJ, McDermott AB, Graham BS, Koup RA, Nabel GJ. 2014. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J Virol 88:4047–4057. 10.1128/JVI.03422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ecker JW, Kirchenbaum GA, Pierce SR, Skarlupka AL, Abreu RB, Cooper RE, Taylor-Mulneix D, Ross TM, Sautto GA. 2020. High-yield expression and purification of recombinant influenza virus proteins from stably-transfected mammalian cell lines. Vaccines (Basel) 8:462. 10.3390/vaccines8030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abreu RB, Kirchenbaum GA, Clutter EF, Sautto GA, Ross TM. 2020. Preexisting subtype immunodominance shapes memory B cell recall response to influenza vaccination. JCI Insight 5:e132155. 10.1172/jci.insight.132155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeletti D, Frank GM, Yewdell JW. 2017. Flow-cytometric method measuring B cell surface immunoglobulin avidity. Methods Mol Biol 1623:181–189. 10.1007/978-1-4939-7095-7_15. [DOI] [PubMed] [Google Scholar]
- 9.Martin J, Wharton SA, Lin YP, Takemoto DK, Skehel JJ, Wiley DC, Steinhauer DA. 1998. Studies of the binding properties of influenza hemagglutinin receptor-site mutants. Virology 241:101–111. 10.1006/viro.1997.8958. [DOI] [PubMed] [Google Scholar]
- 10.Sautto GA, Kirchenbaum GA, Abreu RB, Ecker JW, Pierce SR, Kleanthous H, Ross TM. 2020. A computationally optimized broadly reactive antigen subtype-specific influenza vaccine strategy elicits unique potent broadly neutralizing antibodies against hemagglutinin. J Immunol 204:375–385. 10.4049/jimmunol.1900379. [DOI] [PubMed] [Google Scholar]
- 11.Carter DM, Darby CA, Johnson SK, Carlock MA, Kirchenbaum GA, Allen JD, Vogel TU, Delagrave S, DiNapoli J, Kleanthous H, Ross TM. 2017. Elicitation of protective antibodies against a broad panel of H1N1 viruses in ferrets preimmune to historical H1N1 influenza viruses. J Virol 91:e01283-17. 10.1128/JVI.01283-17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, Anderson SF, Strugnell T, Cortes-Garcia G, Vogel TU, Parrington M, Kleanthous H, Ross TM. 2016. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J Virol 90:4720–4734. 10.1128/JVI.03152-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sautto GA, Ecker JW, Ross TM. 2021. An H1N1 computationally optimized broadly reactive antigen elicits a neutralizing antibody response against an emerging human-infecting Eurasian avian-like swine influenza virus. J Virol 95:e0242120. 10.1128/JVI.02421-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skarlupka AL, Reneer ZB, Abreu RB, Ross TM, Sautto GA. 2020. An influenza virus hemagglutinin computationally optimized broadly reactive antigen elicits antibodies endowed with group 1 heterosubtypic breadth against swine influenza viruses. J Virol 94:e02061-19. 10.1128/JVI.02061-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarr D, Gingerich AD, Asthiwi NM, Almutairi F, Sautto GA, Ecker J, Nagy T, Kilgore MB, Chandler JD, Ross TM, Tripp RA, Rada B. 2021. Dual oxidase 1 promotes antiviral innate immunity. Proc Natl Acad Sci USA 118:e2017130118. 10.1073/pnas.2017130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sautto GA, Kirchenbaum GA, Ecker JW, Bebin-Blackwell AG, Pierce SR, Ross TM. 2018. Elicitation of broadly protective antibodies following infection with influenza viruses expressing H1N1 computationally optimized broadly reactive hemagglutinin antigens. Immunohorizons 2:226–237. 10.4049/immunohorizons.1800044. [DOI] [PubMed] [Google Scholar]
- 17.Webster R, Cox N, Stöhr K. 2002. WHO manual on animal influenza diagnosis and surveillance. WHO, Geneva, Switzerland. [Google Scholar]