Abstract
Beta-D-glucan (BDG) testing can expedite the diagnosis of invasive fungal infections in immunocompromised hosts. Elevated BDG levels have been reported in both in-vitro studies assessing cross-reactivity with Nocardia spp. and published cases of patients with nocardiosis, but there is little data on this association in solid organ transplantation (SOT) recipients. To explore this association, we conducted a case series of SOT recipients with culture-proven nocardiosis and BDG testing who received their care at our institution between 2016 and 2021. We found thirteen cases of nocardiosis in SOT recipients, of which three cases met our case definition of an elevated BDG. Their clinical courses are detailed in the present report. We found that BDG may be elevated in SOT with nocardiosis with no identified cause of false positive BDG, though a causal association cannot be determined. Future prospective studies that better evaluate the association between nocardiosis and BDG are warranted, as are studies that better characterize the possible variability in reactivity amongst Nocardia spp.
Keywords: Nocardiosis, Solid organ transplantation, Beta-D-glucan
Introduction
Nocardia spp. are well-recognized pathogens that can lead to a range of infectious syndromes in immunocompetent [1] or immunocompromised [2] hosts, including solid organ transplant (SOT) recipients [3]. The diagnosis of nocardial infections rests on a combination of host, clinical, and radiographic factors along with the isolation of Nocardia spp. in culture from a suspected site of involvement [3]. A definitive diagnosis of nocardiosis often requires tissue sampling, a process that can prove challenging.
Invasive fungal infections (IFI) present an analogous dilemma. Antigen assays have resultantly been developed to assist in the diagnosis of several IFI including cryptococcosis [4] and endemic mycoses [5]. In particular, the beta-D-glucan (BDG) assay detects a component of the cell wall of most fungi [6] and can be useful in a variety of clinical contexts [7], [8], [9]. In a large meta-analysis, the sensitivity and specificity of BDG for IFI were 76.8% and 85.3%, respectively [9].
Since the clinical presentations of IFI and nocardiosis are often overlapping, patients diagnosed with Nocardia infection may undergo testing with BDG for work-up of IFI. Interestingly, elevated BDG levels (>60 pg/mL) have been reported in both in-vitro studies assessing cross-reactivity with Nocardia spp. [10] and published cases of patients with nocardiosis (Table 1) [10], [11], [12]. To our knowledge, BDG testing has not been previously described in the setting of nocardiosis in SOT recipients. To further explore this association, we conducted a case series of SOT recipients with culture-proven nocardiosis and BDG testing who received their care at our institution between 2016 and 2021.
Table 1.
Summary of reported cases with elevated beta-D-glucan in association with nocardiosis.
| Age/sex | Relevant comorbidities | Immunosuppression | Antimicrobial prophylaxis | Diagnosis | Species | Diagnostic methods | Negative fungal diagnostics | Peak BDG level | Potential BDG confounders | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 65/F | Autoimmune hemolytic anemia | Not reported | None | CNS | Nocardia abscessus | Histopathology, 16S ribosomal DNA sequencing | Serum cryptococcal antigen, serum galactomannan level, fungal CSF culture | >523 pg/mL (CSF); serum BDG negative | None | Not reported | [10] |
| 86/F | Colorectal cancer, diabetes mellitus | 6-month dexamethasone (4 mg/day) course | None | Pulmonary | Nocardia nova | Cytology, 16S rRNA sequencing | Aspergillus antigen, Aspergillus precipitating antibody, fungal sputum culture | ≥300 pg/mL | None | Clinical improvement then death from underlying malignancy | [11] |
| 73/M | Cryptogenic organizing pneumonia | 10-month steroid and immunosuppressive regimen | None | Disseminated (pulmonary and CNS) | Nocardia farcinica | Histopathology, 16S rRNA sequencing | Serum cryptococcal antigen | 94.7 pg/mL | Intravenous ampicillin-sulbactam use, serum galactomannan level elevated | Clinical improvement then death from aspiration pneumonia | [12] |
Abbreviations: BDG, beta-D-glucan; CNS, central nervous system; CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; rRNA, ribosomal ribonucleic acid.
Case reports
We retrospectively reviewed the electronic medical records of SOT recipients with positive Nocardia cultures treated at Yale New Haven Hospital between October 2016 and March 2021. Cases were identified by querying our institution’s laboratory information system. For purposes of our study, we defined cases as (1) SOT recipients who had (2) a positive culture for Nocardia spp, (3) a clinical syndrome compatible with nocardiosis, and (4) underwent testing with the Fungitell® BDG assay (Associates of Cape Cod, MA, USA) as part of their workup. An elevated BDG was defined as> 60 pg/mL [13]. The Yale University Institutional Review Board approved this study (HIC#2000023859).
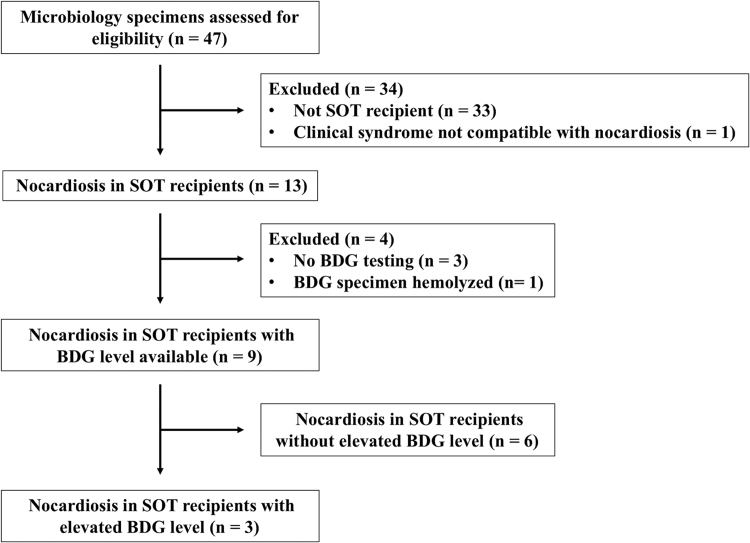
We identified thirteen cases of microbiologically-proven nocardiosis in SOT recipients (Fig. 1). Ten of thirteen cases underwent BDG testing during their clinical courses, and BDG results were available for nine cases. Three of nine cases had elevated BDG (>60 pg/mL) (Table 2), and their clinical courses are detailed below.
Case 1
– Nocardia nova/africana
Fig. 1.
Selection of nocardiosis cases in solid organ transplant recipients with beta-D-glucan testing. Abbreviations: BDG, beta-D-glucan; SOT, solid organ transplant.
Table 2.
Yale New Haven Hospital nocardiosis cases in solid organ transplant recipients who underwent beta-D-glucan testing.
| Age/sex | Relevant comorbidities | Immunosuppression | SOT induction therapy | Antimicrobial prophylaxis | Time since SOT | Diagnosis | Species | Diagnostic methods | Negative fungal diagnostics | Peak BDG level | Potential BDG confounders | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases with elevated BDG | ||||||||||||
| 51/M | Kidney transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | Alemtuzumab | Atovaquone, acyclovir | 2 months | Pulmonary | Nocardia nova/africana | BAL culture | Fungal BAL culture, Pneumocystis jirovecii BAL PCR, galactomannan BAL testing, serum cryptococcal antigen, urine histoplasma antigen | >500 pg/mL | None | Clinical improvement |
| 64/M | Liver transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | N/A | None | 2 years | Disseminated (skin abscess and CNS) | Nocardia anaemiae/N. pseudovaccinii | Abscess I&D and nocardia culture | Fungal wound culture, fungal blood culture, fungal CSF culture, urine histoplasma antigen, serum cryptococcal antigen, serum coccidioides antigen, toxoplasmosis PCR | >500 pg/mL | None | Deceased due to shock and decompensated cirrhosis |
| 62/Fa | Kidney-pancreas transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | N/A | None | 11 years | Disseminated (pulmonary and cardiac) | Nocardia abscessus | BAL culture | Fungal BAL culture, fungal blood culture, urine histoplasma antigen, serum cryptococcal antigen, serum coccidioides antigen | >500 pg/mL | IVIG (1 week prior to initial BDG; BDG 5 weeks after last IVIG dose was 331 pg/mL) | Deceased due to cardiogenic shock after mitral valve repair |
| Cases with normal BDG | ||||||||||||
| 52/Ma | Kidney transplant | Mycophenolate mofetil, prednisone (5 mg/daily), belatacept monthly, eculizumab biweekly | Thymoglobulin | Atovaquone, valganciclovir | 10 weeks | Disseminated (CNS, pulmonary, and skin) | Nocardia farcinica | Blood and deep wound cultures | Serum cryptococcal antigen | 46 pg/mL | None | Clinical improvement |
| 57/F | Heart transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | Basiliximab | Atovaquone, acyclovir | 2 months | Skin abscess | Nocardia farcinica | Abscess I&D and nocardia culture | N/A | <31 pg/mL | None | Clinical Improvement |
| 53/F | Kidney transplant | Tacrolimus, mycophenolate mofetil, prednisone (3 mg/daily) | N/A | None | 17 years | Disseminated (CNS, pulmonary and skin) | Nocardia spp. | Abscess I&D and nocardia culture | N/A | <31 pg/mL | None | Clinical Improvement |
| 66/M | Kidney transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | Alemtuzumab | None | 3 years | CNS | Nocardia farcinica | Brain abscess drainage with tissue culture | N/A | <31 pg/mL | None | Clinical stability at follow up (2 months after discharge) |
| 50/M | Kidney transplant | Belatacept, mycophenolate mofetil, prednisone (5 mg/daily) | Alemtuzumab | Valganciclovir, atovaquone | 8 months | Disseminated (CNS, pulmonary) | Nocardia nova complex | Brain abscess drainage with tissue culture | N/A | <31 pg/mL | None | Clinical and radiologic improvement |
| 65/M | Kidney transplant | Tacrolimus, mycophenolate mofetil, prednisone (5 mg/daily) | Alemtuzumab | None | 1 year | Pulmonary | Nocardia nova complex | BAL culture | N/A | <31 pg/mL | None | Clinical and radiologic improvement |
Abbreviations: BAL, bronchoalveolar lavage; BDG, beta-D-glucan; CNS, central nervous system; CSF, cerebrospinal fluid; I&D, incision and drainage; IVIG, intravenous immunoglobulin; N/A, not applicable; PCR, polymerase chain reaction; SOT, solid organ transplantation.
Although these patients were on hemodialysis at the time of admission, our institution uses non-cellulose membranes. As a result, this was not deemed to confound the BDG level.
A 51-year-old man with a history of focal segmental glomerulosclerosis underwent kidney transplantation with alemtuzumab induction. His post-transplant course was complicated by acute cellular rejection (three weeks post-transplantation) treated with anti-thymocyte globulin and methylprednisolone. His immunosuppressive regimen consisted of tacrolimus, mycophenolate mofetil (MMF), and prednisone (5 mg/day). Post-transplant prophylaxis included atovaquone and acyclovir.
Two months post-transplantation, he presented with acute-onset pleuritic chest pain, productive cough, and fever at an outside hospital. A chest radiograph demonstrated right middle lobe consolidation consistent with pneumonia. He was given ceftriaxone and azithromycin. Due to fever (38.9 °C) on hospital day (HD) 2, antimicrobial therapy was broadened to cefepime, doxycycline, micafungin, and vancomycin. On HD3, serum BDG drawn on day of admission resulted as>500 pg/mL (reference range<60 pg/mL). On HD4, a computed tomography (CT) scan of the chest revealed a large cavitary pulmonary lesion, several pulmonary nodules, and bilateral ground-glass opacities. On HD7, bronchoscopy was performed, and bronchoalveolar lavage (BAL) cultures grew Nocardia nova/africana prompting a switch in antimicrobial therapy to trimethoprim-sulfamethoxazole (TMP-SMX) and meropenem. Subsequently, magnetic resonance imaging (MRI) of the brain was obtained and ruled out central nervous system involvement. BAL studies including fungal culture, bacterial and mycobacterial culture, Pneumocystis jirovecii polymerase chain reaction (PCR), and galactomannan testing were negative. In addition, serum cryptococcal antigen and urine Histoplasma antigen were negative. After review of the cumulative data, the elevated BDG was attributed to nocardiosis.
Approximately one-month post-hospital discharge, his regimen was switched to TMP-SMX and ceftriaxone when susceptibility results were made available. He completed 10-weeks of combination therapy before transitioning to TMP-SMX monotherapy. One month after transitioning, his symptoms improved, and imaging with a CT scan of the chest demonstrated a stable cavitary lesion in the left upper lobe with improvement in the left lower lobe nodule.
Case 2
- Nocardia abscessus
A 62-year-old woman with a history of pancreas and kidney transplant due to type 1 diabetes mellitus (eleven years prior) presented with lower extremity edema, fatigue, and dysphagia. At time of presentation, she was on MMF, prednisone (5 mg/day), and tacrolimus. Her post-transplant course had been complicated by renal graft failure requiring hemodialysis.
A CT scan of the chest was ordered to work-up the dysphagia and revealed mediastinal lymphadenopathy. Due to concern for a bacterial infection, piperacillin-tazobactam was empirically started. Blood cultures, sputum culture, Coccidioides serology, serum galactomannan, Histoplasma urine antigen, and BDG were negative. On HD3 she developed worsening respiratory status and left thigh pain. Creatine kinase was found to be elevated (4552 U/L). An MRI of the left femur revealed left thigh myositis, and a left thigh muscle biopsy revealed necrotizing fibers and non-specific acute inflammation with negative stains and cultures for microorganisms. Due to progressive respiratory decline, bronchoscopy was performed, and BAL cultures grew Nocardia abscessus. Additionally, intravenous immunoglobulin (IVIG) was administered due to concern for a paraneoplastic or progressive neurological process.
One-week post-IVIG, serum BDG measured>500 pg/mL. Antimicrobial therapy was empirically switched to TMP-SMX and meropenem. Further imaging revealed a right upper lobe pulmonary mass likely due to nocardiosis. Five weeks after the last dose of IVIG, serum BDG remained elevated at 331 pg/mL. Two months into her hospital stay, she died from cardiogenic shock after undergoing mitral valve surgical repair.
Case 3
– Nocardia anaemiae/Nocardia pseudovaccinii
A 64-year-old man underwent liver transplantation for alcoholic cirrhosis, without the need for induction immunosuppression, and was on maintenance MMF, prednisone (5 mg), and tacrolimus. Notably, his post-transplant course was complicated by acute cellular rejection 1 month after transplant which was treated with methylprednisolone. Two years post-transplantation, he presented with failure to thrive, diarrhea, and a perineal rash involving the penis and gluteal folds.
The rash was due to Herpes simplex virus 2 (HSV-2) as determined by HSV-2 PCR of a skin swab from the affected area. He underwent sigmoidoscopy as part of his work-up for diarrhea. Cytomegalovirus (CMV) immunostaining of biopsied rectal tissue was positive and confirmed the diagnosis of CMV proctitis. Valganciclovir therapy was initiated. On HD8, he developed subcutaneous abscesses in the left calf and left elbow. Wound cultures obtained by incision and drainage of multiple abscesses grew Nocardia spp. identified as either Nocardia anaemiae or Nocardia pseudovaccinii by 16S rRNA sequencing. An MRI of the brain was obtained due to concern for disseminated nocardiosis and revealed multiple non-specific hypodensities concerning for abscesses. A transthoracic echocardiogram was negative for vegetations. He was placed on a treatment dose of TMP-SMX and meropenem. He later developed worsening liver function which peaked on HD25 with elevated aspartate aminotransferase 907 U/L (reference range 11–33 U/L), alanine aminotransferase 996 U/L (reference range 6–34 U/L), and alkaline phosphatase 1766 U/L (reference range 9–122 U/L). Liver biopsy was performed and revealed cholestatic hepatitis and bile duct injury consistent with drug-induced livery injury. TMP-SMX was considered a possible cause and switched to linezolid. He developed cytopenias on HD37 attributed to linezolid, so it was substituted with moxifloxacin and minocycline.
On HD51, he had a witnessed tonic-clonic seizure, and a repeat MRI revealed parenchymal enhancement suggestive of worsening nocardiosis or a new infectious process. A lumbar puncture was performed, and cerebrospinal fluid (CSF) studies revealed 0 nucleated cells/uL (reference range<6 cells/uL), glucose 81 mg/dL (reference range 40–70 mg/dL), and protein 69.2 mg/dL (reference range 15–45 mg/dL). CSF bacterial cultures were negative. A serum BDG was obtained to assess for a fungal infection due to unclear etiology of the MRI findings and returned elevated at>500 pg/mL. The patient was empirically started on anidulafungin; however, blood cultures, galactomannan, and CSF fungal and mycobacterial cultures were negative. On HD70, anidulafungin was discontinued, and the elevated BDG was attributed to nocardiosis. He was discharged with a plan to complete 6 months of therapy for nocardiosis. Unfortunately, he died 3 months later due to toxic metabolic encephalopathy complicated by aspiration pneumonia and acute renal failure.
Discussion
The utility of BDG as a diagnostic tool for IFI was first explored among patients with hematological malignancies [8], [9], and the literature surrounding interpretation and applicability of BDG assays has since expanded to other populations, including SOT recipients [14]. In SOT recipients with a compatible clinical syndrome, an elevated BDG can be highly suggestive of an IFI; however, elevated BDG has also been reported in association with non-fungal organisms, particularly Nocardia species both in in-vitro studies and in case reports [10], [11], [12]. Notably, Nocardia can produce disease that is clinically indistinguishable from a fungal infection in an immunocompromised population. Despite this clinical and microbiological overlap, the diagnostic utility of the BDG assay for the diagnosis of nocardiosis has not been explored in a clinical setting. Indeed, there are no prior published reports describing elevated BDG in SOT recipients infected with Nocardia, and the American Society of Transplantation guidelines on nocardial infections did not include the BDG assay [3]. This case series builds on prior data in the non-transplant population suggesting that nocardiosis may be associated with elevated BDG and expands this observation to the setting of SOT.
In addition to cross reactivity with certain bacteria, there are other established reasons for falsely elevated BDG levels. These include hemodialysis with cellulose membranes [15], IVIG [16], various antimicrobial agents [17], and surgical gauze containing glucan [18]. Although two cases in our series were on hemodialysis at the time of admission, our institution uses non-cellulose dialysis membranes which are not known to interfere with BDG levels [19]. Therefore, hemodialysis was not deemed to be a confounder of BDG testing. Notably, Case 2 did receive IVIG one week prior to measurement of serum BDG, but the BDG level remained significantly elevated (331 pg/mL) for>5 weeks after IVIG. In an analysis of 21 pediatric patients receiving IVIG, BDG normalized in 64% and 100% of patients at one and three weeks, respectively [20]. Given the sustained positivity in our case, it is unclear whether or not IVIG played a significant role in confounding the results.
It is interesting to note that all three cases of Nocardia farcinica (maximum BDG 46 pg/mL; Table 2) at our institution did not satisfy the case definition of BDG elevation (>60 pg/mL). This is consistent with an in-vitro study performed by Sawai et al. [12] that evaluated N. farcinica isolated in a brain specimen and reported mild elevation of BDG levels to about 20 pg/mL compared to control (pure blood agar). Other published studies suggest that levels of BDG may vary across infection with different Nocardia spp. [10], [11], [12] Whether species heterogeneity is related to variations in cell-wall content or to other pathogen or host factors is unclear and deserves further investigation.
Our case series is limited by its modest size, retrospective design and descriptive format, which precludes any causal inference. Additionally, the small number of nocardiosis cases in SOT recipients with BDG results available limits our ability to characterize BDG variation across Nocardia spp. The intent of the report is not to establish a causative relationship between BDG elevation and nocardiosis. Rather, this report serves to highlight an association that is poorly known in the clinical setting of transplantation.
In light of the abovementioned results, SOT recipients with elevated BDG, no identified cause of false positive BDG, and clinical concern for IFI with a negative comprehensive workup (including microbiology, histopathology, serology and antigen testing) may merit a work-up for infection with Nocardia spp. This series has important implications for the diagnostic utility of BDG for the diagnosis of nocardiosis in SOT. Future prospective studies that better evaluate the association between nocardiosis and BDG, are warranted, as are studies that better characterize the possible variability in reactivity amongst Nocardia spp.
Ethical approval
The Yale University Institutional Review Board approved this study (HIC#2000023859).
Consent
Need for informed consent was waived by our institution’s IRB.
CRediT authorship contribution statement
Matthew Ringer: Writing – original draft, Conceptualization, Methodology. Christopher Radcliffe: Writing – original draft, Conceptualization, Methodology. Christopher A. Kerantzas: Data curation, Writing – review & editing. Maricar Malinis: Conceptualization, Methodology, Supervision, Writing – review & editing. Marwan M. Azar: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declarations of Competing Interest
None.
Acknowledgements
None.
References
- 1.Beaman B.L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Wilson J.W. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restrepo A., Clark N.M., Infectious Diseases Community of Practice of the American Society of Transplantation Nocardia infections in solid organ transplantation: guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clin Transpl. 2019;33(9) doi: 10.1111/ctr.13509. [DOI] [PubMed] [Google Scholar]
- 4.Powderly W.G., Cloud G.A., Dismukes W.E., Saag M.S. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1994;18(5):789–792. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]
- 5.CLSI . 2nd ed. Clinical and Laboratory Standards Institute; 2021. Principles and procedures for detection and culture of fungi in clinical specimens. CLSI guideline M54. [Google Scholar]
- 6.Obayashi T., Yoshida M., Tamura H., Aketagawa J., Tanaka S., Kawai T. Determination of plasma (1-->3)-beta-D-glucan: a new diagnostic aid to deep mycosis. J Med Vet Mycol. 1992;30(4):275–280. doi: 10.1080/02681219280000361. [DOI] [PubMed] [Google Scholar]
- 7.Karageorgopoulos D.E., Qu J.M., Korbila I.P., Zhu Y.G., Vasileiou V.A., Falagas M.E. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamoth F., Cruciani M., Mengoli C., Castagnola E., Lortholary O., Richardson M., Marchetti O., Third European Conference on Infections in Leukemia (ECIL-3) beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin Infect Dis. 2012;54(5):633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 9.Karageorgopoulos D.E., Vouloumanou E.K., Ntziora F., Michalopoulos A., Rafailidis P.I., Falagas M.E. beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 10.Koncan R., Favuzzi V., Ligozzi M., Sorrentino A., Cornaglia G., Cascio G.L. Cross-reactivity of Nocardia spp. in the fungal (1-3)-beta-d-glucan assay performed on cerebral spinal fluid. Diagn Microbiol Infect Dis. 2015;81(2):94–95. doi: 10.1016/j.diagmicrobio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Yagyu K., Nakatsuji Y., Matsushita H. Elevated serum beta-d-glucan levels in cavitary pulmonary nocardiosis. BMJ Case Rep. 2020;13(7) doi: 10.1136/bcr,2020-234738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawai T., Nakao T., Yamaguchi S., Yoshioka S., Matsuo N., Suyama N., Yanagihara K., Mukae H. Detection of high serum levels of beta-D-Glucan in disseminated nocardial infection: a case report. BMC Infect Dis. 2017;17(1):272. doi: 10.1186/s12879-017-2370-4. 017-2370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S., Hang J.P., Zhang L., Wang F., Zhang D.C., Gong F.H. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-D-glucan for invasive fungal infection: focus on cutoff levels. J Microbiol Immunol Infect. 2015;48(4):351–361. doi: 10.1016/j.jmii.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Theel E.S., Doern C.D. beta-D-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol. 2013;51(11):3478–3483. doi: 10.1128/JCM.01737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato A., Takita T., Furuhashi M., Takahashi T., Maruyama Y., Hishida A. Elevation of blood (1-->3)-beta-D-glucan concentrations in hemodialysis patients. Nephron. 2001;89(1):15–19. doi: 10.1159/000046037. [DOI] [PubMed] [Google Scholar]
- 16.Usami M., Ohata A., Horiuchi T., Nagasawa K., Wakabayashi T., Tanaka S. Positive (1-->3)-beta-D-glucan in blood components and release of (1-->3)-beta-D-glucan from depth-type membrane filters for blood processing. Transfusion. 2002;42(9):1189–1195. doi: 10.1046/j.1537-2995.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 17.Marty F.M., Lowry C.M., Lempitski S.J., Kubiak D.W., Finkelman M.A., Baden L.R. Reactivity of (1-->3)-beta-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother. 2006;50(10):3450–3453. doi: 10.1128/AAC.00658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakao A., Yasui M., Kawagoe T., Tamura H., Tanaka S., Takagi H. False-positive endotoxemia derives from gauze glucan after hepatectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology. 1997;44(17):1413–1418. [PubMed] [Google Scholar]
- 19.Kanda H., Kubo K., Hamasaki K., Kanda Y., Nakao A., Kitamura T., Fujita T., Yamamoto K., Mimura T. Influence of various hemodialysis membranes on the plasma (1-->3)-beta-D-glucan level. Kidney Int. 2001;60(1):319–323. doi: 10.1046/j.1523-1755.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 20.Egger M., Pruller F., Raggam R., Divjak M.K., Kurath-Koller S., Lackner H., Urban C., Stenger V. False positive serum levels of (1-3)-ss-D-Glucan after infusion of intravenous immunoglobulins and time to normalisation. J Infect. 2018;76(2):206–210. doi: 10.1016/j.jinf.2017.10.017. [DOI] [PubMed] [Google Scholar]