Abstract
Aims:
Bevacizumab (B) in association with systemic chemotherapy is commonly used for the treatment of colorectal cancer liver metastases. The aim of this study was to monitor tumor response, overall survival (OS) and progression-free survival (PFS) of patients with colorectal cancer liver metastases treated with transarterial chemoembolization (TACE) + B compared with TACE alone and to correlate the results with KRAS mutational status.
Patients & methods:
This was an observational multicentric case–control study (NCT03732235) on the efficacy and safety of B administered after TACE.
Results:
The disease control rate was significantly higher for the TACE + B than the TACE alone group (p < 0.001). KRAS wild-type patients had a significantly better disease control rate than those with KRAS mutations in the TACE + B group. Median OS and PFS were similar for the TACE + B and TACE groups, whereas median time to progression was significantly higher for the TACE + B group (p < 0.01).
Conclusion:
The combination of TACE with B may improve tumor response and delay disease progression.
Keywords: : angiogenesis, bevacizumab, colorectal carcinoma, DC beads radiopaque, irinotecan, liver metastases, polyethylene glycol microspheres, transarterial chemoembolization
Colorectal cancer liver metastasis (CRC-LM) is present in about 25% of patients diagnosed with colorectal cancer [1]. The gold standard treatment for CRC-LM is surgery; however, surgical resection is feasible in a limited number of cases, with a median survival at 5 years of 25–37% and frequent liver recurrence (70–80%) [2].
Locoregional therapies increase survival, resectability and quality of life for unresectable CRC-LM patients [3,4]. Transarterial chemoembolization (TACE) with irinotecan drug-eluting microspheres (DEBIRI), in particular, results in median overall survival (OS) of 18 months (range: 7.3–25 months) and 33 months when DEBIRI is combined with the FOLFOX-6 regimen, with median progression-free survival (PFS) of 6.7 months (range: 4–11 months) and 10.8 months when DEBIRI is combined with FOLFOX-6, and median overall response rate of 62% (range: 10–78%) [5–9].
TACE acts in several ways: it increases local drug concentration by delivering toxic drug to the tumor and blocks the tumor blood supply by occluding tumor feeding arteries, resulting in ischemia, hypoxia and necrosis of the tumor tissue [6]. Hypoxia stimulates angiogenesis via VEGF expression that is activated by HIF-1-α/β, resulting in neovascularization and an increased oxygen supply in the tumor tissue [10,11]. Similarly, TACE-induced hypoxia could trigger such a pathway in CRC-LM, as has been shown for TACE–lipiodol in patients with hepatocellular carcinoma [12].
Bevacizumab has been the first available antiangiogenic therapy. It binds to VEGF and prevents its interaction with its receptor, hence inhibiting the activation of VEGF signaling pathways and consequent neovascularization [13]. Bevacizumab in addition to chemotherapy (irinotecan, fluorouracil and leucovorin) is used for treatment of metastatic CRC, significantly increasing survival of patients compared with chemotherapy alone (10.6 vs 6.2 months; hazard ratio: 0.66; p < 0.001) [13]. For this reason bevacizumab is considered the first targeted therapy for patients with metastatic CRC [14]. Bevacizumab is used also in combination with TACE for CRC-LM therapy, with a median OS of 12 months (range: 7–18 months) versus 5.7 months (range: 1.5–7.7 months) for TACE alone [15].
The aim of this study was to monitor tumor response, OS and PFS of CRC-LM patients treated with TACE + bevacizumab compared with TACE alone and to correlate the results with KRAS mutational status.
Patients & methods
Study & patients selection
This was an observational, prospective, multicentric, case–control study on the efficacy and safety of bevacizumab administered after TACE. This study was approved by the local Institutional Review Board. The study started in January 2014 and was concluded in February 2020. A total of 121 consecutive patients with unresectable liver limited metastasis from CRC were screened. Of these, 29 were excluded for metastasis beyond liver, ten for other health issues, and six withdrew their consent; thus 76 consecutive CRC-LM patients were enrolled in the case–control study. Primary objectives of the study were to compare survival and time to progression; secondary objectives were tumor response and safety. All statistical analyses were based on KRAS status.
Inclusion criteria were: histological confirmation of CRC-LM; primary tumor was adenocarcinoma of colon or rectum; >18-years old; unresectable disease; refractory to >1 line of chemotherapy; at least 1 month from the end of previous systemic chemotherapy; KRAS status determined; routine blood biochemistry assays at normal levels; life expectancy >3 months; and informed consent signed.
Exclusion criteria were: contraindication to angiographic catheterization; extensive extrahepatic disease; pregnancy or breastfeeding; other severe clinical contraindications.
TACE procedures
A diagnostic angiography was performed before TACE in order to monitor tumor arterial perfusion. Each patient received two TACE procedures, on days 0 and 30 (range: 28–37 days) according to the recognized technical recommendations [16]. TACE treatment was lobar. In patients with bilobar metastases, the first TACE was administered in the most affected lobe, and the contralateral lobe with minor lesions was treated at the second TACE.
This study was multicenter, and interventional radiologists used the type of microspheres that were available in their hospital. For this reason, DC Beads™ with diameter of 70–150 μm (Biocompatibles UK Ltd, Farnham, UK) were used in 21 patients; LifePearl® 100 μm (Terumo Europe NV, Leuven, Belgium) in 40 patients; and DC Beads LUMI™ 70–150 μm (Boston Scientific S.p.A., Milan, Italy) in 15 cases. These types of microspheres have similar efficacy [6], hence this should not affect the results of the study.
Irinotecan at a dosage of 100 mg was used to load the TACE microspheres, which were diluted in 5 ml of non-ionic contrast solution and 5 ml of distilled water. The irinotecan-loaded microspheres were slowly infused at a rate of 1 ml/min for a mean of 12 min.
Bevacizumab (Avastin®; Roche, Basel, Switzerland) at a dosage of 5 mg/kg was administered intravenously to the patients in the TACE + B group, starting from 15 days after the first TACE and then every 2 weeks for eight cycles [15]. The bevacizumab administration was started 15 days after TACE in order not to add the side effects of the two treatments. Although TACE was well tolerated, in our experience, it was decided to avoid patients being burdened with too many side effects that might result in suspending the treatment.
Outcome measures
The following parameters were compared at baseline and at 1, 3 and 6 months after first TACE: performance status; tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9); tumor size assessment with modified response evaluation criteria in solid tumors from abdominal and pelvic computed tomographic imaging. OS, PFS and time to progression (TTP) were computed using the Kaplan–Meier method. Follow ups for study objective assessments were made at 1, 3 and 6 months after the start of treatment, then every 3 months until progression. At the 1-month follow up all the patients were assessed, while at 3 and 6 months assessments were available for 71 (93%) and 62 (82%) patients, respectively.
KRAS status was recorded and the mutational status of codons 12 and 13 of KRAS was examined: DNA was extracted from formalin-fixed paraffin-embedded tissues, and PCR was used for the amplification of KRAS codons and analysis of mutation presence as previously reported [18].
The intensity and type of adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [17].
Statistical analysis
Continuous data were reported as median and proportions were expressed as percentages for the description of sample characteristics. The chi-square and Student t tests (p < 0.05) were used to examine continuous variables for the assessment of differences in tumor response and OS, PFS and TTP values. Comparisons were made between TACE and TACE + B groups at the different time points (1, 3 and 6 months) and between wild-type (WT) and mutated KRAS groups. OS, PFS and TTP were analyzed with the Kaplan–Meier method, and significance was established as p < 0.05 with the log rank test between the different study groups.
Results
The study included 76 patients with unresectable CRC-LMs limited to the liver that were nonresponsive to systemic therapy. Thirty-eight were treated with TACE alone and 38 with TACE + B. Fifty-two (68%) patients were male and 24 (32%) female; patient and tumor characteristics are shown in Table 1. Patients were refractory to at least one line of chemotherapy; all first-line chemotherapy was FOLFOX and second line was FOLFIRI. Two cycles of TACE were administered to 63 (83%) of patients, and 22 (29%) of patients received three TACE treatments.
Table 1. . Sample characteristics.
| Characteristic | Value |
|---|---|
| Gender | |
| Male | 52 (68%) |
| Female | 24 (32%) |
| Treatment | |
| TACE + B | 38 (50%) |
| TACE | 38 (50%) |
| Age (years) | |
| Median (range) | 70 (45–87) |
| Tumor size (mm) | |
| Median (range) | 40 (5–110) |
| Tumor nodules, n (%) | |
| 1–2 | 8 (11%) |
| 3–4 | 23 (30%) |
| >5 | 45 (59%) |
| Lobar side | |
| Right | 21 (28%) |
| Left | 21 (28%) |
| Bilobar | 34 (44%) |
| Time from diagnosis to live metastases | |
| Median (range), years | 0.6 (0–2.22) |
| Synchronous | 20 (26%) |
| Metachronous | 56 (74%) |
| Previous chemotherapy lines (n) | |
| 1 | 16 (21%) |
| 2 | 27 (36%) |
| 3 | 20 (26%) |
| 4 | 13 (17%) |
| Previous surgery | |
| Primitive tumor surgery | 48 (63%) |
| Metastasectomy | 5 (7%) |
| None | 23 (30%) |
| Liver involvement | |
| <50% | 46 (61%) |
| >50% | 30 (39%) |
| KRAS status | |
| Mutated | 35/76 (55%) 25/38 (66%) TACE + B 10/38 (26%) TACE |
| Wild-type | 31/76 (45%) 13/38 (32%) TACE + B 28/38 (74%) TACE |
| Performance status median (range) | 0 (0–2) |
| Baseline | |
| 0 | 51 (67%) |
| 1 | 23 (30%) |
| 2 | 2 (3%) |
| 1 month | |
| 0 | 42 (55%) |
| 1 | 26 (34%) |
| 2 | 8 (11%) |
| 3 months | |
| 0 | 34 (44%) |
| 1 | 27 (36%) |
| 2 | 15 (20%) |
| 6 months | |
| 0 | 30 (39%) |
| 1 | 28 (37%) |
| 2 | 18 (24%) |
B: Bevacizumab; TACE: Transarterial chemoembolization.
At 1 and 3 months post-treatment, the disease control rate (DCR) was significantly higher for the TACE + B group than the TACE group – 100 versus 84% (p < 0.05) at 1 month and 97 versus 76% at 3 months (p < 0.001) – whereas at 6 months after therapy, no statistically significant difference (72 vs 63%; p = 0.174) was observed (Table 2).
Table 2. . Disease control rate.
p < 0.05.
B: Bevacizumab; DCR: Disease control rate; TACE: Transarterial chemoembolization.
KRAS WT patients had a significantly better tumor response than those with KRAS mutated (mut): TACE + B (WT) versus TACE + B (mut) at 3 months, 100 versus 96% (p < 0.05) and at 6 months, 91 versus 76% (p < 0.001). Additionally, TACE (WT) had a DCR of 65% at 3 months, significantly higher than that of TACE mut (22%; p < 0.001) (Table 3).
Table 3. . Tumor response.
| TACE + B (WT) | TACE + B (mut) | TACE (WT) | TACE (mut) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 1 month | 3 months | 6 months | 1 month | 3 months | 6 months | 1 month | 3 months | 6 months | |
| CR | 33 | 42 | 36 | 0 | 4 | 4 | 0 | 8 | 11 | 0 | 11 | 25 |
| PR | 50 | 33 | 27 | 84 | 64 | 36 | 41 | 15 | 28 | 30 | 11 | 25 |
| SD | 17 | 25 | 0 | 16 | 28 | 36 | 41 | 42 | 17 | 30 | 0 | 0 |
| PD | 0 | 0 | 9 | 0 | 4 | 24 | 19 | 35 | 44 | 40 | 78 | 50 |
| DCR | 100 | 100 | 91 | 100 | 96 | 76 | 82 | 65 | 56 | 60 | 22 | 50 |
| p-values | 1 month | 3 months | 6 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TACE-B (WT) vs TACE (WT) | < 0.001† | < 0.001† | < 0.001† | |||||||||
| TACE-B (mut) vs TACE (mut) | < 0.001† | < 0.001† | 0.031905† | |||||||||
| TACE-B (WT) vs TACE-B (mut) | // | 0.043352† | 0.00427† | |||||||||
| TACE (WT) vs TACE (mut) | 0.000607 | < 0.001† | 0.395292 |
p < 0.05.
B: Bevacizumab; CR: Complete response; DCR: Disease control rate; mut: Mutated; PD: Progressive disease; PR: Partial response; SD: Stable disease; TACE: Transarterial chemoembolization; WT: KRAS wild-type.
Concerning the levels of tumor markers CEA and CA19-9, the comparison between TACE + B and TACE showed a significant difference only in CA19-9 at 3 months: 85 kU/l (range: 40–150 kU/l) versus 206 kU/l (range: 25–925 kU/l), respectively (p < 0.05) (Table 4). The comparison between TACE + B at diagnosis versus after treatment showed a significant reduction of CA19-9 at each time point (p < 0.05), whereas CEA levels did not differ significantly after treatment (Table 4). The opposite situation was observed for the comparison of TACE at diagnosis versus after treatment, which showed a significant decrease only in CEA level after treatment (p < 0.05) at each time point (Table 4). The analysis of CEA and CA19-9 levels between KRAS WT and mutants did not show any significant difference (p < 0.05).
Table 4. . Tumor biomarkers assessment.
| Whole sample | TACE + B | TACE | p-value (TACE + B vs TACE) | p-value TACE + B diagnosis vs after treatment | p-value TACE diagnosis vs after treatment | ||
|---|---|---|---|---|---|---|---|
| CEA (U/ml) | Baseline | 250 (8.5–932) | 298 (150–438) | 250 (8.5–932) | 0.203014 | ||
| Median (range) | 1 month | 197 (4.9–800) | 238.5 (132–157) | 208.5 (4.9–800) | 0.221778 | 0.066655 | 0.000107† |
| 3 months | 180 (38–736) | 182 (110–269) | 196 (38–736) | 0.093944 | 0.074979 | 0.000496† | |
| 6 months | 96 (5.1–436) | 80 (40–96) | 110 (5.1–436) | 0.121733 | 0.057249 | 0.022245† | |
| CA19-9 (kU/l) | Baseline | 230 (24–436) | 197 (70–348) | 182 (24–436) | 0.388423 | ||
| Median (range) | 1 month | 187.5 (17–378) | 147 (70–259) | 178 (17–378) | 0.471428 | 0.027662† | 0.10505 |
| 3 months | 150 (25–925) | 85 (40–150) | 206 (25–925) | 0.0456† | 0.034715† | 0.325877 | |
| 6 months | 93 (20–1259) | 40 (25–278) | 90 (20–1259) | 0.17702 | 0.022483† | 0.3444 |
p < 0.05.
B: Bevacizumab; TACE: Transarterial chemoembolization.
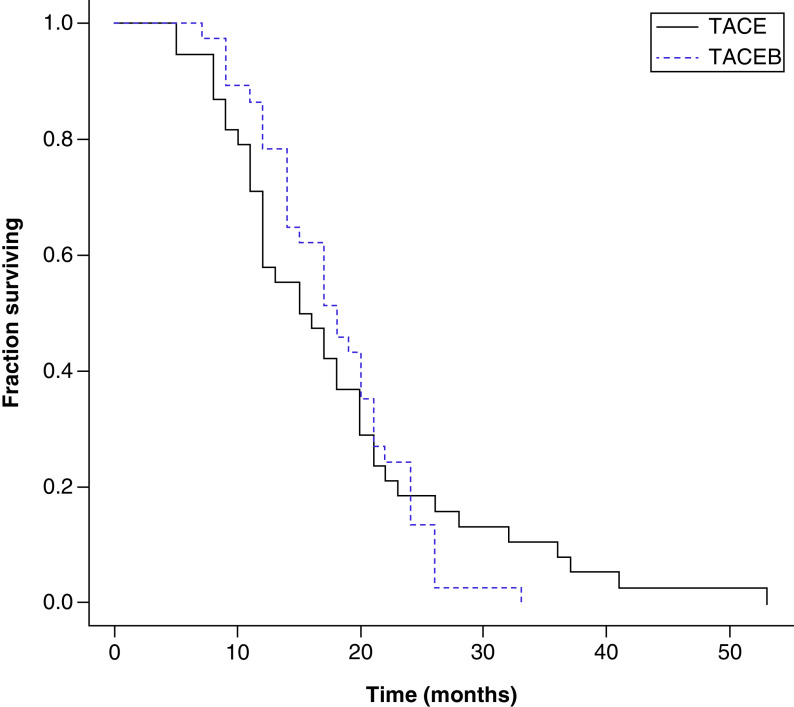
OS and PFS did not differ significantly between the TACE + B and TACE groups (Figure 1). Median OS was 18 months (range: 7–26 months) versus 15.8 months (range: 5–52 months), respectively, for the TACE + B and TACE groups, while median PFS was 13 months (range: 3–24 months) versus 11.15 months (range: 4–51 months), respectively. However, the median TTP was significantly higher for the TACE + B group than the TACE group, at 8 months (range: 3–15 months) versus 2.08 months (range: 1.03–11 months) for the TTP group (p < 0.001) (Table 5). No patient was operated on due to a downsizing of metastasis in both arms.
Figure 1. . Kaplan–Meier curves of overall survival for transarterial chemoembolization (TACE) and TACE + bevacizumab groups.
Log rank test = 0.011119; p-value: 0.916022.
B: Bevacizumab; TACE: Transarterial chemoembolization.
Table 5. . OS, PFS and TTP.
| TACE-B | TACE | TACE + B (WT) | TACE + B (mut) | TACE (WT) | TACE (mut) | p-value (TACE + B vs TACE) | p-value (TACE + B WT vs mut) | p-value (TACE WT vs mut) | p-value (TACE + B WT vs TACE WT) | p-value (TACE + B mut vs TACE mut) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from metastasis diagnosis | 18 (7–26) | 15.8 (5–52) | 14.5 (12–33) | 19 (7–26) | 27.7 (9–52.73) | 15.03 (13.1–21.2) | 0.25 | 0.348499 | 0.111447 | 0.309137 | 0.048317† |
| TTP | 10 (3–15) | 2.08 (1.03–11) | 8 (3–24) | 10 (3–24) | 5.06 (1.3–9) | 1.7 (1.1–8) | <0.001† | 0.399375 | 0.215221 | 0.013926† | <0.001† |
| PFS | 13 (3–24) | 11.15 (4–51) | 12 (3–28) | 14 (3–24) | 22.63 (3–51.4) | 10.83 (3.8–23) | 0.3123 | 0.295387 | 0.222201 | 0.226051 | 0.280808 |
| OS from primitive tumor | 21.73 (13.5–27) | 27.8 (3–58) | 13.53 (4–23) | 23.7 (9–52) | 32.26 (15.5–58) | 25.76 (12.1–61) | 0.09 | 0.222201 | 0.127852 | 0.127852 | 0.284394 |
p < 0.05.
B: Bevacizumab; mut: Mutated; OS: Overall survival; PFS: Progression-free survival; TACE: Transarterial chemoembolization; TTP: Time to progression; WT: Wild-type.
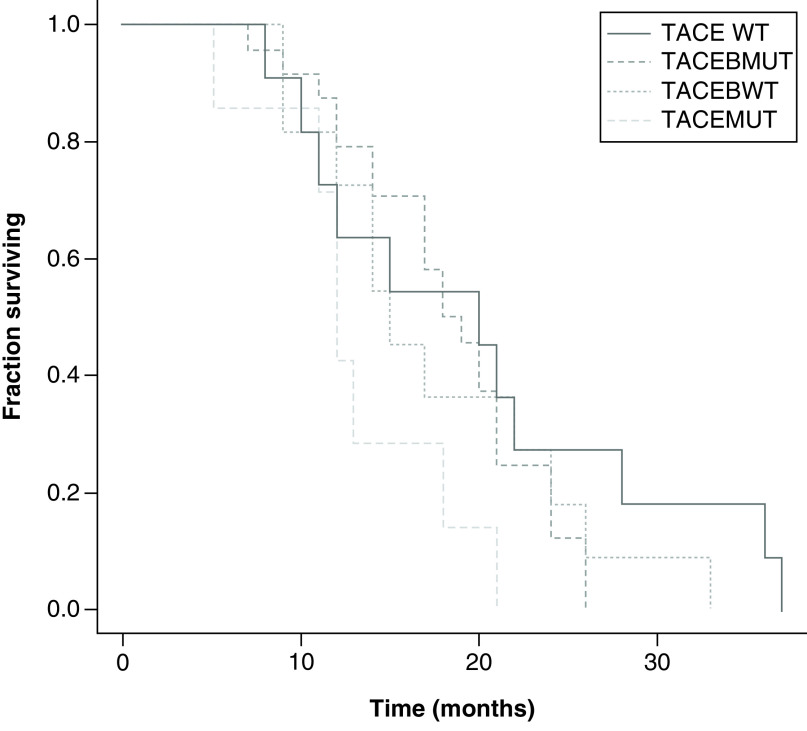
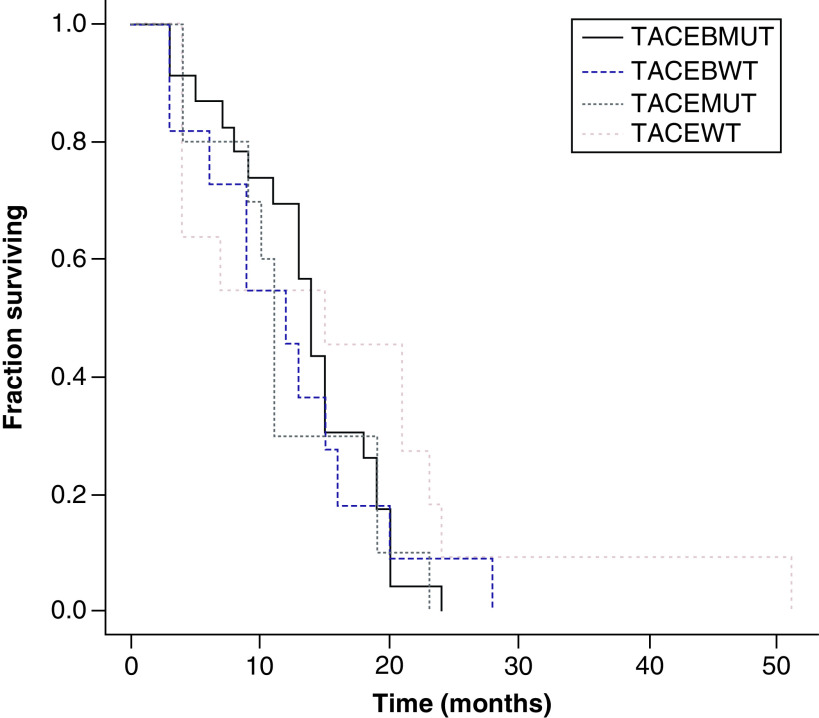
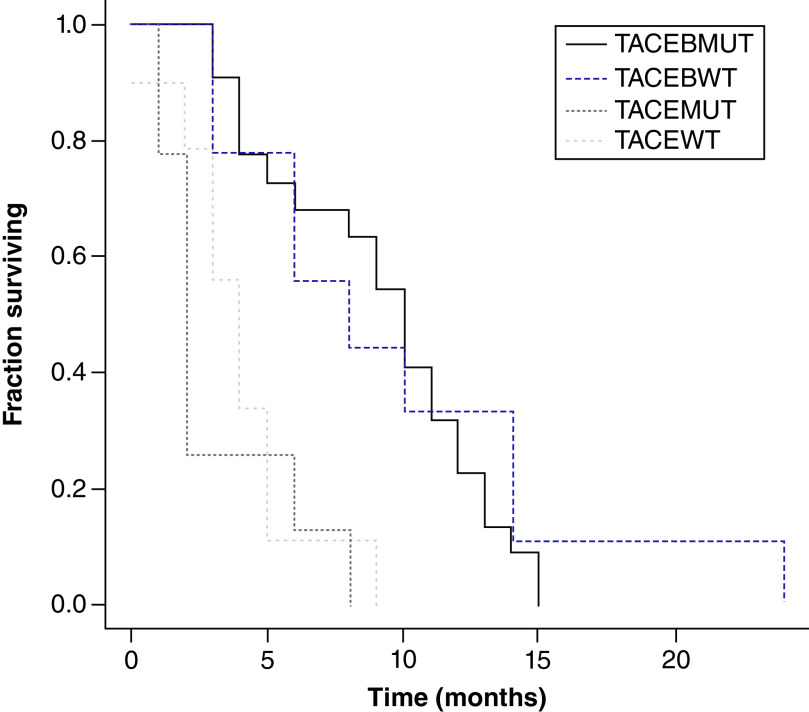
In relation to OS, PFS and TTP, there was no statistically significant difference between KRAS WT and mutant in either the TACE + B or the TACE groups. However, for TTP the TACE + B WT (8 months; range: 3–24 months) and mutant (10 months; range: 3–24 months) had TTP greater than the TACE WT (5.06 months; range: 1.3–9 months) and TACE mutant groups (1.7 months; range: 1.1–8 months; p < 0.01) (Figures 2–4 & Table 5). OS was greater in the TACE + B mutant versus TACE mutant group, at 19 months (range: 7–26 months) versus 15.03 months (range: 13.1–21.2 months; p < 0.05) (Table 5).
Figure 2. . Kaplan–Meier curves of overall survival for transarterial chemoembolization (TACE) with KRAS wild-type (WT) and mutant and TACE + bevacizumab with KRAS WT and mutant groups.
Log rank test = 3.140685; p-value: 0.370439.
B: Bevacizumab; MUT: Mutated; TACE: Transarterial chemoembolization; WT: Wild-type.
Figure 3. . Kaplan–Meier curves of progression-free survival for transarterial chemoembolization (TACE) with KRAS wild-type (WT) and mutant and TACE + bevacizumab with KRAS WT and mutant groups.
Log rank test = 2.538308; p-value: 0.468408.
B: Bevacizumab; MUT: Mutated; TACE: Transarterial chemoembolization; WT: Wild-type.
Figure 4. . Kaplan–Meier curves of time to progression for transarterial chemoembolization (TACE) with KRAS wild-type (WT) and mutant and TACE + bevacizumab with KRAS WT and mutant groups.
Log rank test = 27.088651; p-value: 0.000006.
B: Bevacizumab; MUT: Mutated; TACE: Transarterial chemoembolization; WT: Wld-type.
Tolerability
No complications were observed during TACE in either the TACE + B or the TACE group. Most TACE-related adverse events were correlated with post-embolic syndrome and were of mild or moderate intensity in both TACE + B and TACE groups. The most frequently observed TACE-related adverse events were pain (grade: 2), observed in 24 (32%) patients and resolved in 2 days (range: 1–5 days), and transaminase rise, which was observed in 52 (68%) patients and was of grade 2–3 intensity.
Concerning the bevacizumab-related adverse events of the TACE + B group, 12 patients (32%) among 38 treated with bevacizumab at 5.0 mg/kg every 2 weeks presented with proteinuria (grade: 2), 10 (26%) with increased blood pressure (grade: 2) and 20 (53%) with skin rash (grade: 2). No thromboembolic effects or bleeding were observed. No significant changes were reported in the ECG or in the echocardiographic study of the heart in either the TACE + B or the TACE group.
Discussion
To our knowledge, this study is the first trial to evaluate, on the basis of KRAS mutational status, the treatment effect of TACE alone or in combination with bevacizumab for second- and third-line treatment in patients with liver-limited metastatic colorectal cancer. The results demonstrate that the antiangiogenic agent is a tolerable adjunct to TACE, and set the stage for larger clinical trials examining this approach.
The statistically significant improvement in PFS observed in the TACE + B arm should be interpreted with caution, due to the still limited sample size. There was no statistically significant difference in OS between the two treatment arms, with a median survival of 18 months (range: 7–26 months) for the TACE + B arm and 15.8 months (range: 5–52 months) for the TACE arm. Any perceived difference in OS cannot be attributed to bevacizumab toxicity, because there were no bevacizumab-related deaths. The TACE + B group had longer TTP than with TACE alone: 8 months (range: 3–15 months) versus 2.08 months (range: 1.03–11 months; p < 0.001). However, whether the activity of concurrent systemic bevacizumab and hepatic artery therapy delays the progression of extrahepatic metastases or reduces the intrahepatic progression of disease cannot be definitively proven in our study. PFS did not differ significantly between the two groups, at 13 months (range: 3–24) versus 11.15 months (range: 4–51). PFS and OS of the group receiving TACE alone were in agreement with previously published studies of TACE that reported ranges of 5–8.1 and 14–16.8 months, respectively [19–21].
TACE + B resulted in better DCR at 1 (100 vs 84%) and 3 (96 vs 76%) months (p < 0.001). These results suggested that TACE + B was effective for the treatment of CRC-LM. TACE + B had the synergistic activity of irinotecan and bevacizumab, increasing DCR in comparison with TACE alone, as previously noted [15,22]. These results were higher than those reported by other studies which reported disease control in 88.9, 37–41.2 and 17.6–47% of patients at 3, 6 and 12 months, respectively [21,23,24].
Concerning tumor response and trend of the markers, Scevola et al. reported a median reduction in CEA for responders of 87.5% [23]. In the present study the reduction of tumor markers appears similar for CEA in both arms but was more favorable for CA19-9, which decreased more significantly in the TACE + B group of patients compared with the TACE subset.
In relation to KRAS mutation status, we highlight that patients KRAS WT had better results than those with mutations. KRAS WT patients, indeed, had a significantly better DCR than those with mutations in the TACE + B group (100 vs 96 and 91 vs 76% at 3 and 6 months, respectively). Similar results were obtained in another study combining TACE and systemic cetuximab, which resulted in a DCR of 50%; patients of the KRAS WT group achieved a better objective response rate than the KRAS-mutated group: 70.8% (95% CI: 52.6–89.0) compared with 37.5% (95% CI: 13.8–61.2), respectively [25].
As concerning the toxicity, the results of this study showed that TACE + B was well tolerated; the observed toxicity levels were mild or moderate and adverse events were easily manageable, in agreement with previous studies [9,14,15,22]. TACE + B did not increase adverse events or severe adverse events, and no increased bevacizumab-associated toxicity was observed, as previously reported by Di Noia et al., in whose study grade 3 adverse events occurred in 6/40 patients (15%) [24].
The main limitations of this report were: the limited number of patients enrolled in a period of 6 years; the case–control method based on the choice of the patient that is not widely accepted in the methodology of randomized trials; and the use of different brands of microspheres because the interventional radiologists were left free to use the embolic of their choice provided they had a similar diameter and the same dose of irinotecan was loaded. The results of this study, however, have confirmed and further validated the results of other studies [9,14,15,22]. Unfortunately, the improvement in response rates did not correlate with an improvement in downsizing to resection in the TACE + B versus the TACE control arm because no patient became eligible for surgery.
Future prospective randomized clinical trials of TACE plus systemic bevacizumab in a larger number of patients are required to confirm these results. Possible challenges of future studies may be due to the clinical heterogeneity of patients with hepatic metastases, mainly because of the following issues: to determine whether it is logical and ethical to treat with TACE only patients with liver-only metastases or also patients with liver-dominant metastases and diffusion beyond the liver; to develop better diagnostic and functional methods to define the percentage of liver involvement and to recognize distant metastases; to assess the KRAS mutation status and other biological and immunological parameters that have appeared in recent years; the use of adjuvant therapy; the use of palliative therapy containing bevacizumab or other drugs as EGFR inhibitors; the most commonly accepted definitions of when to suspend systemic chemotherapy in patients with unresectable metastases; and a better balance between surgeons, medical oncologists and interventional radiologists in multidisciplinary teams.
Conclusion
This study suggests that TACE combined with bevacizumab is safe and feasible in subjects with unresectable liver-limited metastases from CRC and may potentially have more benefits in relation tumor response than TACE alone.
Future perspective
Bevacizumab is an antiangiogenic drug that is commonly used for treatment of colorectal cancer liver metastases. It is often associated with systemic chemotherapy and not frequently with TACE. The study aim was to monitor tumor response, OS and PFS after TACE. TACE combined with bevacizumab is a safe and feasible regimen in subjects with unresectable liver limited metastases from colorectal cancer and may potentially have more benefits as concerning tumor response and time to progression than TACE alone.
Summary points.
Transarterial chemoembolization (TACE) acts in several ways: it increases local drug concentration by delivering toxic drug to the tumor and blocks the tumor blood supply by occluding tumor-feeding arteries, resulting in ischemia, hypoxia and necrosis of the tumor tissue.
Hypoxia stimulates angiogenesis via VEGF expression. Bevacizumab (B) inhibits the activation of VEGF signaling pathways and for this reason is often used in association with systemic therapy and TACE for treatment of colorectal cancer liver metastases.
The disease control rate was significantly higher for the TACE + B than the TACE group, and in particular KRAS wild-type patients had a significant better disease control rate than those with KRAS mutations.
Overall survival and progression-free survival did not differ in the TACE + B and TACE groups, whereas median time to progression was significantly higher for the TACE + B group.
TACE+ B therapy was well tolerated; the observed toxicity levels were mild or moderate and adverse events were easily manageable.
The association of TACE with bevacizumab may improve tumor response and delay disease progression.
Footnotes
Author contributions
Conceptualization: G Fiorentini, C Aliberti and S Guadagni. Methodology: G Fiorentini, R Inchingolo, M Nardella, A Rebonato, C Fiorentini and R Nani. Software: D Sarti. Validation: R Inchingolo, M Nardella, A Rebonato, C Fiorentini and G Fiorentini. Formal analysis: D Sarti. Investigation: G Fiorentini and S Guadagni. Resources: G Fiorentini and S Guadagni. Data curation: D Sarti. Writing: original draft preparation, G Fiorentini and D Sarti; review and editing, G Fiorentini and D Sarti. Visualization: R Inchingolo, M Nardella, A Rebonato and C Fiorentini. Supervision: S Guadagni. Project administration: G Fiorentini.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data (NCT03732235). No individual participant data that underlies the results reported in the article will be available, and nor will information regarding the study protocol or statistical analysis, beyond what is reported in the text.
Ethical conduct of research
This study was approved by the local Institutional Review Board. The study started in January 2014 and was concluded in February 2020. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Engstrand J, Strömberg C, Nilsson H, Freedman J, Jonas E. Synchronous and metachronous liver metastases in patients with colorectal cancer-towards a clinically relevant definition. World J. Surg. Oncol. 17(1), 228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RP, Kokudo N, Folprecht G et al. Colorectal liver metastases: a critical review of state of the art. Liver Cancer 6(1), 66–71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27(8), 1386–1422 (2016). [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN Guidelines): colon cancer, 2019. www.nccn.org/professionals/physician_gls/pdf/colon.pdf [DOI] [PubMed]
- 5.Stutz M, Mamo A, Valenti D, Hausvater A et al. Real-life report on chemoembolization using DEBIRI for liver metastases from colorectal cancer. Gastroenterol. Res. Pract. 2015, 715102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentini G, Sarti D, Nani R, Aliberti C, Fiorentini C, Guadagni S. Updates of colorectal cancer liver metastases therapy: review on DEBIRI. Hepat. Oncol. 7(1), HEP16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• More recent data on the application of DEBIRI indicating size of microspheres, doses of irinotecan, efficacy and toxicity.
- 7.Fiorentini G, Sarti D, Aliberti C, Carandina R, Mambrini A, Guadagni S. Multidisciplinary approach of colorectal cancer liver metastases. World J. Clin. Oncol. 8(3), 190–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorentini G, Aliberti C, Tilli M et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a Phase III study. Anticancer Res. 32(4), 1387–1395 (2012). [PubMed] [Google Scholar]; •• First controlled randomized trial comparing intra-arterial infusion of irinotecan-loaded drug-eluting beads versus intravenous therapy for hepatic metastases from colorectal cancer.
- 9.Martin RC, Scoggins CR, Schreeder M et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 121(20), 3649–3658 (2015). [DOI] [PubMed] [Google Scholar]; •• First randomized trial of irinotecan drug-eluting beads with concurrent chemotherapy for patients with colorectal liver-limited metastasis.
- 10.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 49(5), 523–529 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 9(6), 669–676 (2003). [DOI] [PubMed] [Google Scholar]; •• Pivotal evaluation study on VEGF and its potential activity in cancer therapy.
- 12.Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J. Clin. Med. Res. 8(4), 297–302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mody K, Baldeo C, Bekaii-Saab T. Antiangiogenic therapy in colorectal cancer. Cancer J. 24(4), 165–170 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Kabbinavar F, Hurwitz HI, Fehrenbacher L et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 21(1), 60–65 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Fiorentini G, Sarti D, Nardella M et al. Chemoembolization alone or associated with bevacizumab for therapy of colorectal cancer metastases: preliminary results of a randomized study. In Vivo 34(2), 683–686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of a randomized study of chemoembolization with bevacizumab versus chemoembolization alone.
- 16.Lencioni R, Aliberti C, de Baere T et al. Transarterial treatment of colorectal cancer liver metastases with irinotecan-loaded drug-eluting beads: technical recommendations. J. Vasc. Interv. Radiol. 25(3), 365–369 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Trotti A, Colevas AD, Setser A et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 13(3), 176–181 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga M, Kaneta T, Miwa K et al. A comparison of four methods for detecting KRAS mutations in formalin-fixed specimens from metastatic colorectal cancer patients. Oncol. Lett. 12(1), 150–156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinwande O, Dendy M, Ludwig JM, Kim HS. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: a systematic review. Surg. Oncol. 26(3), 268–275 (2017). [DOI] [PubMed] [Google Scholar]; • First report of a systemic review of DEBIRI.
- 20.Ngo A, von Stempel C, Corbo B et al. Transarterial chemoembolisation of colorectal liver metastases with irinotecan-loaded beads: a bi-institutional analysis of 125 treatments in 53 patients. Cardiovasc. Intervent. Radiol. 42(7), 979–990 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Mauri G, Varano GM, Della Vigna P et al. Transarterial embolization with small-size particles loaded with irinotecan for the treatment of colorectal liver metastases: results of the MIRACLE III study. Cardiovasc. Intervent. Radiol. 41(11), 1708–1715 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Fiorentini G, Sarti D, Aliberti C et al. Chemoembolization in conjunction with bevacizumab: preliminary results. J. Vasc. Interv. Radiol. 29(9), 1236–1239 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Scevola G, Loreni G, Rastelli M, Sposato S, Ramponi S, Miele V. Third-line treatment of colorectal liver metastases using DEBIRI chemoembolization. Med. Oncol. 34(3), 377 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Di Noia V, Basso M, Marsico V et al. DEBIRI plus capecitabine: a treatment option for refractory liver-dominant metastases from colorectal cancer. Future Oncol. 15(20), 2349–2360 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Fiorentini G, Aliberti C, Sarti D et al. Locoregional therapy and systemic cetuximab to treat colorectal liver metastases. World J. Gastrointest. Oncol. 7(6), 47–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of the combination of intra-arterial hepatic chemotherapy and cetuximab.