Abstract
Chemotherapy is a potential cause of focal and diffuse hepatobiliary lesions. Many of these lesions may be demonstrated on imaging, especially computed tomography and MRI. Some of these lesions, especially those of steatosis and sinusoidal obstruction syndrome, are associated with a worse prognosis and risk of hepatic failure in the context of surgical management. Notably, some chemotherapy-induced hepatic alterations, such as sinusoidal obstruction syndrome, pseudocirrhosis and focal hepatopathies, may be mistakenly interpreted as signs of cancer progression, misguiding the therapeutic planning for patients receiving chemotherapy.
Keywords: : adverse effects, chemotherapy, hepatology, imaging, liver, magnetic resonance, oncology
Practice points.
Chemotherapeutic agents induce toxicity in multiple organs, including the liver. Many of these hepatic alterations can be detected on imaging, especially MRI.
Knowledge about these lesions can reduce the risk of erroneously diagnosing them as neoplastic progression or relapse.
Steatosis/steatohepatitis is mainly related to the use of irinotecan, tamoxifen and 5-fluorouracil, among other agents, and is associated with an increased risk of postoperative hepatic failure.
Sinusoidal obstruction syndrome occurs when there is damage to the hepatic sinusoidal endothelium, usually after the administration of oxaliplatin, cyclophosphamide and vincristine. This condition is associated with an increased risk of bleeding and hepatic failure.
Pseudocirrhosis presents with a strikingly nodular appearance and is usually observed in patients undergoing treatment for breast cancer, with or without hepatic metastases. The condition may be complicated by portal hypertension in some patients.
Focal nodular hyperplasia is thought to result from microvascular alterations in the liver and is related to oxaliplatin use. Oncologists must be aware of this focal lesion, as it may be falsely considered as hypervascular metastasis. MRI with hepatospecific contrast media provides crucial information for the differential diagnosis of focal nodular hyperplasia.
The use of chemotherapeutic agents in the oncologic setting is related to a better prognosis, increased resectability when used in neoadjuvant therapy and improved overall survival. However, this widely used therapeutic option is also related to toxicity in multiple organs, including liver, pancreas, and gastrointestinal and genitourinary tracts, negatively affecting outcomes for some patients. Some hepatobiliary alterations are considered peculiar based on imaging findings, primary neoplastic site and chemotherapy regimen. For example, sinusoidal obstruction syndrome (SOS) presents with a diffuse heterogeneous reticular pattern in the hepatobiliary phase of MRI. Regarding the primary neoplastic site, pseudocirrhosis usually occurs in patients with metastatic breast cancer.
Steatosis/steatohepatitis
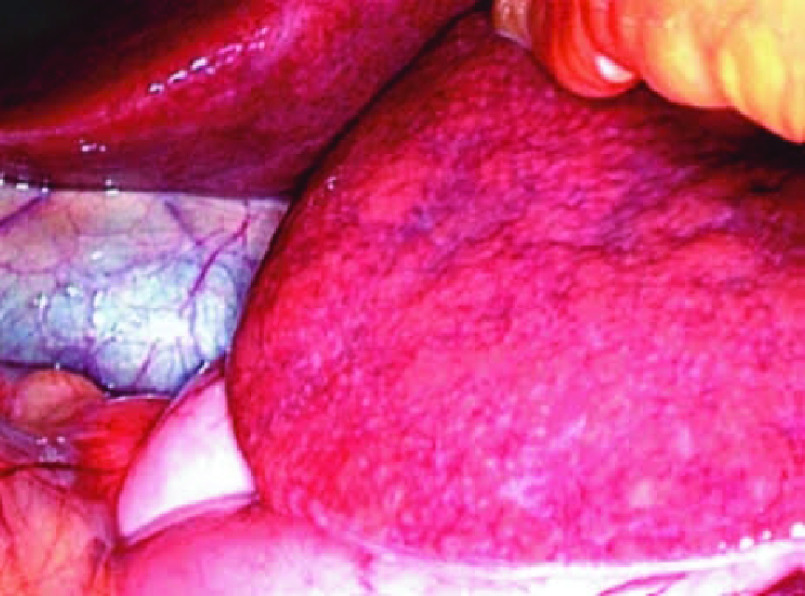
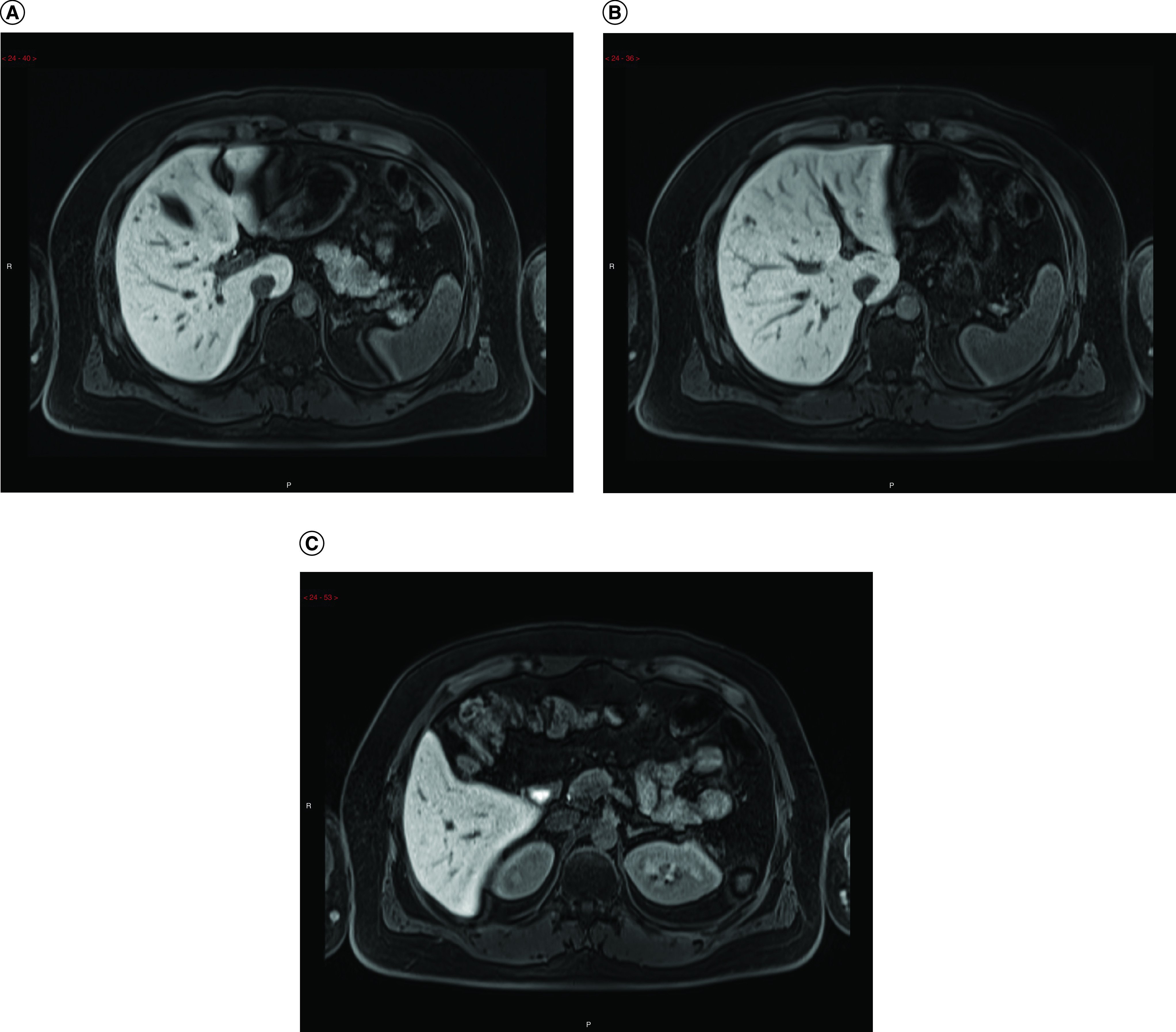
Steatosis, which refers to an increased amount of fat in the live parenchyma, shows a peak incidence 3 to 12 months after the initiation of chemotherapy and usually regresses after treatment completion. Steatosis is particularly associated with the use of irinotecan, tamoxifen, 5-fluorouracil, doxifluridine, gemcitabine, methotrexate and platinum-based drugs [1–4]. Oxidative stress, mitochondrial injury and subsequent lipid accumulation are the key processes underlying the pathophysiology of this alteration [3]. The lesion distribution pattern may be diffuse, focal, multifocal or perivascular. Intraoperatively, a steatotic liver has a yellowish appearance (Figure 1). Although hyperechogenicity on ultrasound may suggest steatosis, this evaluation is subjective, with a positive predictive value of approximately 62–77%. On computed tomography (CT), a reduction in parenchymal attenuation in relation to the spleen is suggestive of steatosis. MRI has superior reliability in detecting and determining the amount of fat in the liver during the initial phases using in-phase and out-phase gradient-echo sequences (Figure 2), spectroscopy and elastography [3,5–7]. Possible complications include chronic cirrhosis and increased mortality in patients undergoing surgical resection [1,8].
Figure 1. . Yellow liver.
Intraoperative photograph of a steatotic liver.
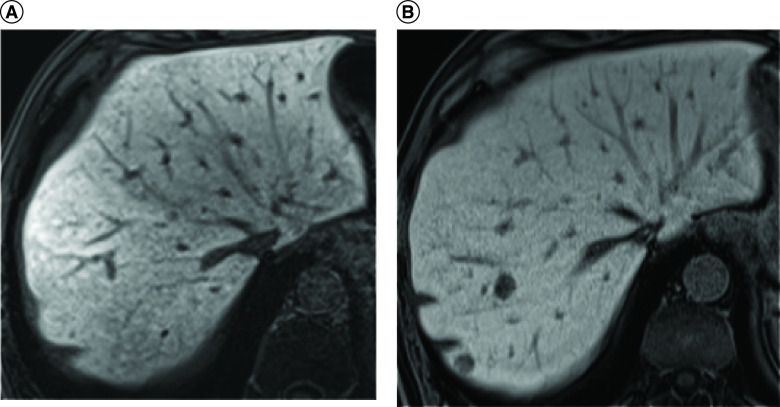
Figure 2. . Axial T1-weighted gradient echo.
MRI demonstrate loss of signal in the opposing-phase MRI predominantly in the left hepatic lobe after taxol and anastrozole use. (A) In phase. (B) Opposing phase.
Sinusoidal obstruction syndrome
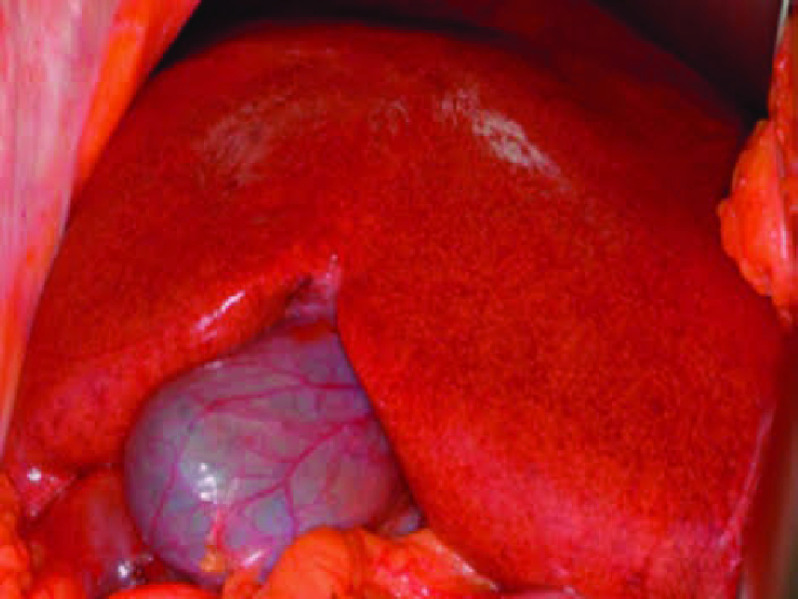
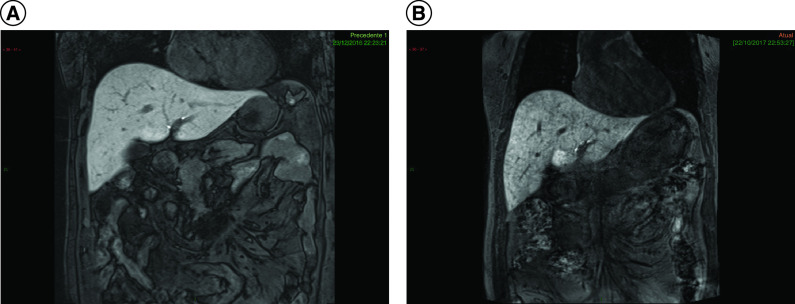
Previously called veno-occlusive disease, this disorder may present with acute (1–3 weeks), subacute and chronic phases. Chemotherapeutic regimens including oxaliplatin, cisplatin, cyclophosphamide and vincristine are associated with this condition. This pathology may occur in up to 10–60% of patients after chemotherapy for bone marrow transplant; it usually occurs 35 days after treatment initiation but may also occur later. Drugs inflict sinusoidal endothelial damage, with venular obstruction and hepatic congestion conferring a bluish appearance on the liver (Figure 3). Importantly, in contrast to Budd–Chiari syndrome, this condition is characterized by the patency of large veins (the hepatic veins and inferior vena cava). Clinically, patients with SOS present with jaundice, hepatomegaly, weight gain, abdominal pain and encephalopathy [3,4]. Chronic SOS may progress to cirrhosis. Nonspecific imaging findings include hepatosplenomegaly, ascites, gallbladder wall thickening and portosystemic shunts. The presence of portal hypertension signs in surgical patients is of significance, as these signs are potentially related to a worse prognosis due to an increased bleeding risk or hepatic failure after the procedure [4,9]. On contrast-enhanced CT, heterogeneous enhancement is observed, which is attributed to perfusion disturbances. It is important to note the presence of ascites in the context of SOS; otherwise, it may be mistaken for disease recurrence or progression [8]. A diffuse reticular pattern in the hepatobiliary phase of gadoxetic acid-enhanced MRI in postchemotherapy patients is a characteristic feature of SOS (Figures 4 & 5) [4].
Figure 3. . Blue liver.
Intraoperative photograph of a liver with sinusoidal obstruction syndrome.
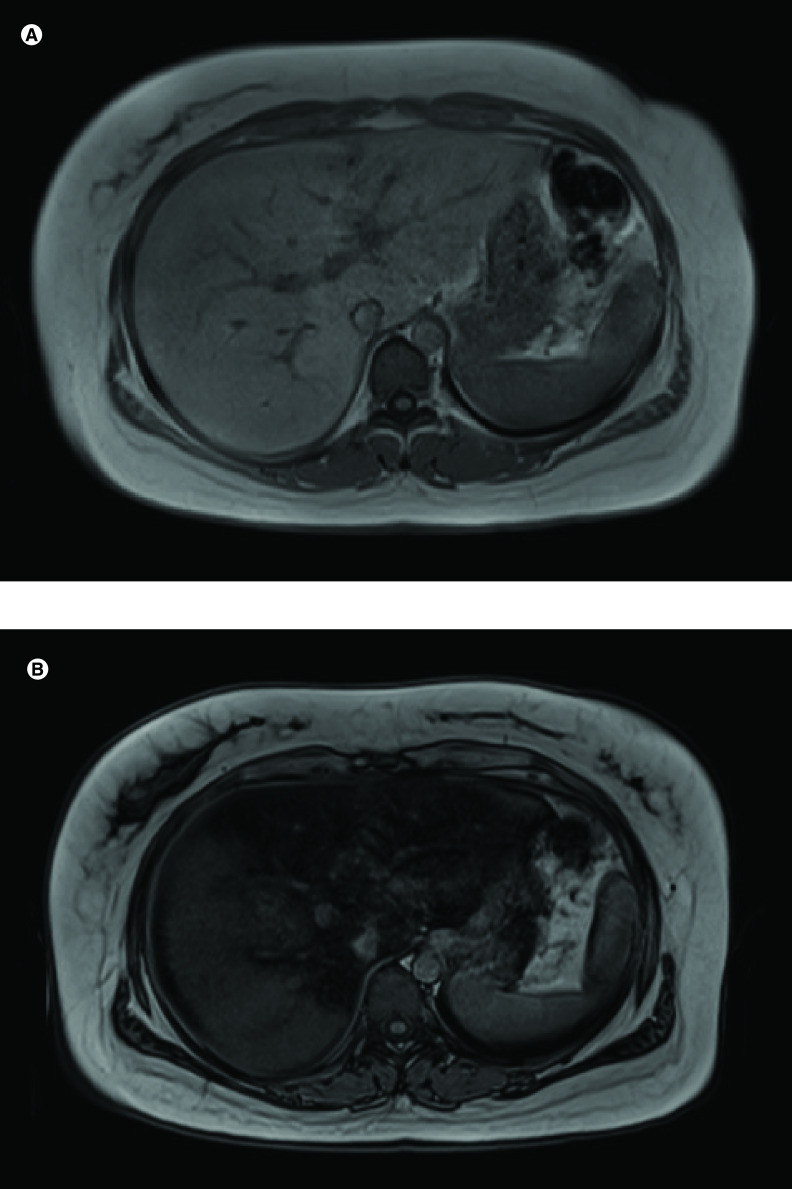
Figure 4. . Axial T1-weighted MRI obtained using hepatospecific contrast media in the hepatobiliary phase.
(A) Before chemotherapy. (B) After chemotherapy with the FOLFOX regimen in a patient with colon cancer; demonstrates a diffuse heterogeneous reticular pattern. Regarded to be the characteristic feature of sinusoidal obstructive syndrome.
Figure 5. . Coronal T1-weighted MRI obtained using hepatospecific contrast media in the hepatobiliary phase.
They demonstrate onset of a diffuse heterogeneous reticular pattern after chemotherapy with the FOLFOX regimen for 8 months.
Pseudocirrhosis
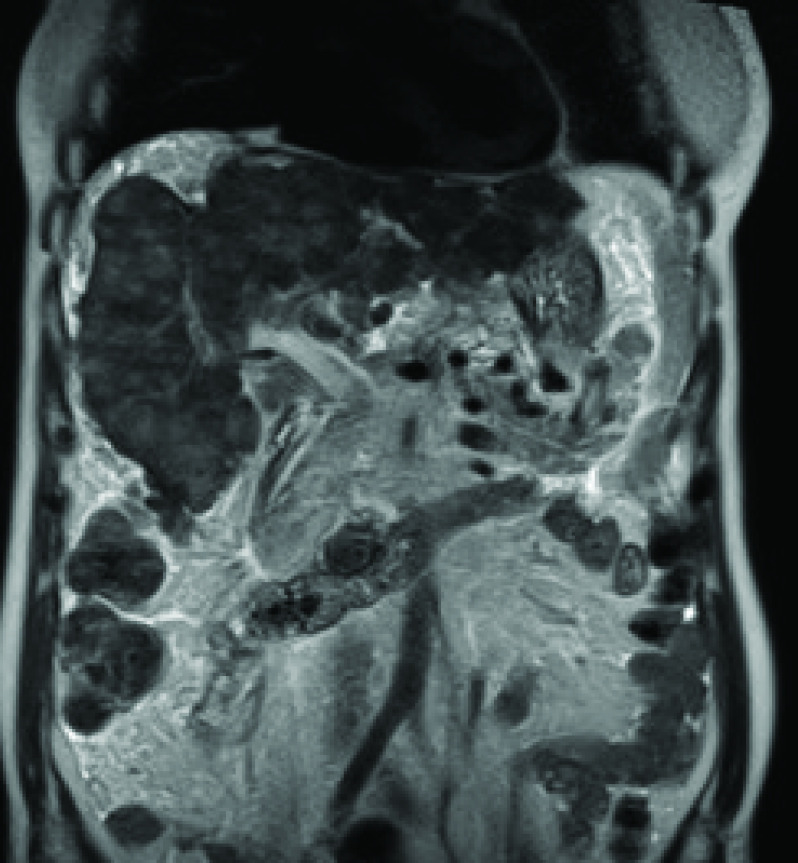
Pseudocirrhosis is characterized by diffuse liver nodularity mimicking cirrhosis; however, histopathologically, pseudocirrhosis appears as nodular regenerative hyperplasia without significant fibrosis [10]. Pseudocirrhosis is usually seen in patients with hepatic metastasis, although it may occur in a liver without subjacent disease [4]. It is typically related to breast cancer in 50–75% of patients [1,9]. However, it can also occur in patients with primary cancers of the colon and pancreas, those with carcinoid tumors and those with Hodgkin lymphoma [1,4,9]. Rapid progression (usually 1 to 3 months after chemotherapy initiation), in contrast to true cirrhosis, is usually diagnostic of pesudocirrhosis. Tamoxifen and capecitabine are some of the offending drugs. Imaging findings include diffuse nodularity, capsular retraction, reduced liver volume and caudate lobe enlargement (Figure 6). Portal hypertension signs, including portosystemic shunts (in 9% of the cases), may be present [1,8,9].
Figure 6. . Coronal T2-weighted MRI.
It demonstrates a diffuse nodular pattern with capsular retraction and hepatic parenchymal distortion.
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is a benign hepatocitary lesion with an unknown pathogenesis, although intrahepatic vascular disorders are potentially implicated. FNH may even have a pathogenesis similar to that of focal SOS [11,12]. This alteration is usually associated with oxaliplatin use, and in one study, up to 15% of patients with colorectal cancer presented with FNH [13]. The lesions may be solitary or multinodular. On CT, FNH classically presents as a homogeneous, isodense or slightly hypodense lesion in relation to the liver parenchyma. On contrast-enhanced CT, arterial hyperenhancement is observed; in cases where a central scar is evident, late enhancement may be observed. The detection and characterization of this type of lesion are essential considering its worrisome differential diagnosis with hypervascular metastasis. The most independent discriminant feature for differentiating FNH from metastases is the presence of a nonspherical shape, poorly defined margins, and especially, areas of hypercaptation in the hepatobiliary phase with hepatospecific contrast media (Figures 7 & 8). Additional features favoring FNH diagnosis include signal isointensity on T1- and T2-weighted images, absence of halo enhancement (early enhancement) and absence of restriction to water diffusion in the echo-planar sequence [11].
Figure 7. . T1-weighted MRI postcontrast with hepatospecific contrast agent in the hepatobiliary phase.
They demonstrate subtle focal enhancing lesions in a patient who received oxaliplatin.
Figure 8. . Axial MRIs of nodules.
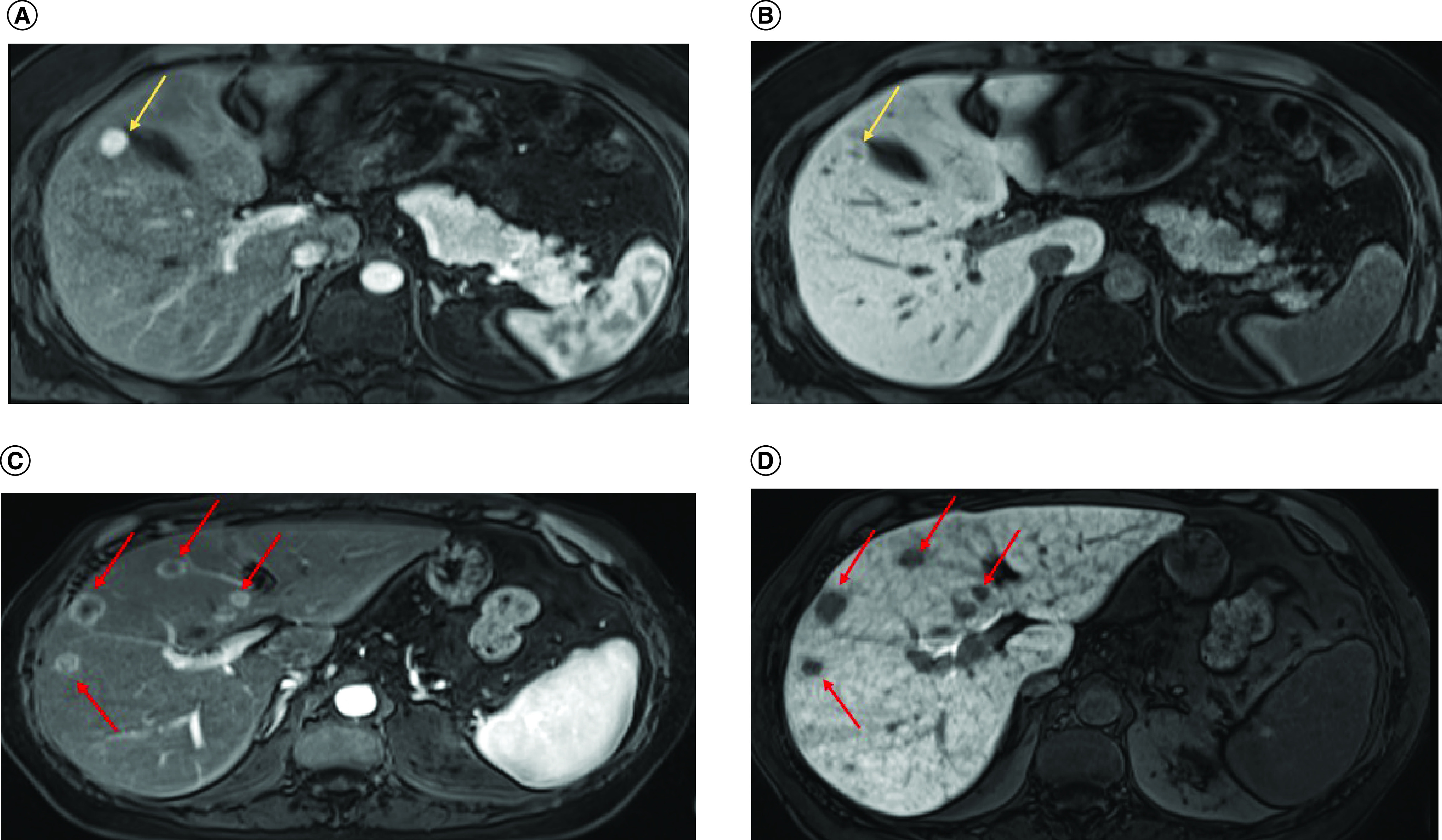
(A) Axial MRI shows a hypervascular nodule (arrow) that is predominantly hyperintense in the hepatobiliary phase (arrow in [B]). The imaging findings are consistent with FNH. (C) In another contrasting example the arrows indicate several nodules with peripheral arterial hyperenhancement (arrows) and central hypointensity in the hepatobiliary phase (arrow in [D]), suggesting metastases. The pattern of reticular enhancement associated with sinusoidal obstruction syndrome is also visible.
Conclusion
Chemotherapy is one of the main treatment modalities for neoplasia and is administered as a neoadjuvant or adjuvant therapy. Although it increases patient survival, drug-induced toxicity may hinder the continuation of a specific regimen in many cases. In this report, the authors focused on reviewing and describing different types of chemotherapy-related liver toxicities. An oncologist aware of these potential complications is better suited for optimal patient care (Table 1).
Table 1. . Take-home messages.
| Adverse effects in the liver | Radiological findings | Common agents |
|---|---|---|
| Sinusoidal obstruction syndrome | US: Ascites, gallbladder wall thickening and hepatosplenomegaly. CT: Ascites, decreased right hepatic vein diameter (<0.45 cm), paraesophageal varices, hepatosplenomegaly and recanalization of the umbilical vein. MRI: Diffuse hypointense reticular pattern on postcontrast delayed hepatobiliary phase T1-weighted imaging. |
Oxaliplatin, 6-MP, dacarbazine, azathioprine, cyclophosphamide, fluorouracil and vincristine |
| Pseudocirrhosis | US, CT, MRI: Segmental volume loss, capsular retraction, fibrosis and caudate lobe enlargement mimicking macronodular cirrhosis on CT; occurs in patients with metastatic breast and colon cancers. | Gemcitabine, FOLFOX, oxaliplatin, irinotecan, fluorouracil and methotrexate |
| Steatosis | US: Increased echogenicity and beam attenuation. CT: Reduced liver attenuation. MRI: Reduced liver signal intensity in out-of-phase imaging observed in acutely ill patients with steatohepatitis. |
Irinotecan, oxaliplatin, cetuximab, tamoxifen and antivascular endothelial growth factor therapy |
| Focal nodular hyperplasia | MRI: Signal isointensity on T1- and T2-weighted images, absence of halo enhancement (early enhancement) and absence of restriction to water diffusion in the echo-planar imaging sequence. | Oxaliplatin-based chemotherapy |
Future perspective
Chemotherapy-related changes observed on liver imaging are increasingly being described in the literature, and they have important implications for therapeutic planning. Sensitive imaging methods help determine safe treatment regimens and enable prompt intervention for adverse effects.
Acknowledgments
The authors would like to acknowledge the kind permission to use operatory photographs taken by MG Toneto.
Footnotes
Author contributions
MD Soldatelli authored the original presentation for the Radiological Society of North America (2019) on the same subject; GB Torri was responsible manuscript submission and processing; GF Luersen gave the final manuscript review and; CLA Ghezzi accounted for cases and illustration selection.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. Informed consent was obtained for the use of patients' photographs.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Liu YI, Jha P, Wang ZJ et al. Abdominal complications of chemotherapy: findings at computed tomography. Clin. Imaging 36(1), 54–60 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Miyake K, Hayakawa K, Nishino M, Morimoto T, Mukaihara S. Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad. Radiol. 12(6), 722–727 (2005). [DOI] [PubMed] [Google Scholar]
- 3.McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. Imaging of drug-induced complications in the gastrointestinal system. Radiographics 36(1), 71–87 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Houshyar R, Bhosale P, Choi J-I, Gulati R, Lall C. Chemotherapy induced liver abnormalities: an imaging perspective. Clin. Mol. Hepatol. 20(3), 317–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report presents a comprehensive review of chemotherapy-related complications, including vascular complications such as portal vein thrombosis.
- 5.Guglielmo FF, Venkatesh SK, Mitchell DG. Liver MR elastography technique and image interpretation: pearls and pitfalls. Radiographics 39(7), 1983–2002 (2019). [DOI] [PubMed] [Google Scholar]; • Describes the peculiarities involving magnetic resonance elastography, which might help to differentiate steatosis from steatohepatitis.
- 6.Salameh N, Larrat B, Abarca-Quinones J et al. Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 253(1), 90–97 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J. Magn. Reson. Imaging 34(4), 729–749 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 258(1), 41–56 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan C, Truong MT, Sagebiel TL et al. Abdominal and pelvic complications of nonoperative oncologic therapy. Radiographics 34(4), 941–961 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Young ST, Paulson EK, Washington K, Gulliver DJ, Vredenburgh JJ, Baker ME. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. Amer. J. Roentgenol. 163(6), 1385–1388 (1994). [DOI] [PubMed] [Google Scholar]; • Some papers are more focused on specific hepatic complications of chemotherapy, such as this study by Young et al., who reported on pseudocirrhosis.
- 11.Han NY, Park BJ, Sung DJ et al. Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic acid–enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology 271(2), 416–425 (2014). [DOI] [PubMed] [Google Scholar]; • Studied the differentiation of liver metastases from areas of focal nodular hyperplasia.
- 12.Donadon M, Di Tommaso L, Roncalli M, Torzilli G. Multiple focal nodular hyperplasias induced by oxaliplatin-based chemotherapy. World J. Hepatol. 5(6), 340–344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Studied the differentiation of liver metastases from areas of focal nodular hyperplasia.
- 13.Wicherts DA, de Haas RJ, Sebagh M et al. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann. Surg. Oncol. 18(3), 659–669 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]