Abstract
The COVID-19 pandemic has once again brought to the forefront the existence of a tight link between the coagulation/fibrinolytic system and the immunologic processes. Tissue-type plasminogen activator (tPA) is a serine protease with a key role in fibrinolysis by converting plasminogen into plasmin that can finally degrade fibrin clots. tPA is released in the blood by endothelial cells and hepatocytes but is also produced by various types of immune cells including T cells and monocytes. Beyond its role on hemostasis, tPA is also a potent modulator of inflammation and is involved in the regulation of several inflammatory diseases. Here, after a brief description of tPA structure, we review its new functions in adaptive immunity focusing on T cells and antigen presenting cells. We intend to synthesize the recent knowledge on proteolysis- and receptor-mediated effects of tPA on immune response in physiological and pathological context.
Keywords: Tissue-type plasminogen activator, Plasminogen activation system, Plasmin, T cells, Antigen presenting cells, Macrophages, Immunopathology
1. Introduction
Host defense against pathogens requires the activation and recruitment of specialized immune cells. Firstly, innate immune response is quickly triggered in the tissues by the direct recognition of a broad range of conserved microbial molecules named “pathogen-associated molecular patterns” (PAMPs) that are recognized by specific pattern-recognition receptors (PRR) mainly expressed by innate cells. The toll-like receptor (TLR) family represents the prototype of PRR and is able to recognize various bacterial, fungal or viral products. Recognition of microbial structure by TLR triggers the expression of pro-inflammatory cytokines and chemokines that drive the recruitment of innate cells (neutrophils and macrophages) involved in the immediate clearance of pathogens by phagocytosis. As antigen-presenting cells (APCs), macrophages and dendritic cells (DCs) that express a high level of costimulatory molecules participate in the activation of the adaptive immunity by interacting with lymphocytes. The two types of lymphocytes of the adaptive immunity are T and B cells, both of which harboring a distinct antigen-specific receptor: T cell receptor (TCR) and B cell receptor (BCR), respectively. In the secondary lymphoid organs, APCs process and present antigens associated with major histocompatibility complex (MHC) molecules on their surfaces to CD4+ helper T (Th) cells or CD8+ cytotoxic T lymphocytes (CTLs). The immune homeostasis is tightly regulated by cell–cell interactions and cytokine signals. Th1 cells secrete mainly IFN-γ and play important roles in the protection against intracellular pathogens, while Th2 cells secrete IL-4 and are involved in the response against helminths. T follicular helper cells (Tfh) cells express IL-21 and play a major role in T cell-dependent B cell responses. Th17 cells produce high amounts of IL-17 and contribute to inflammation by regulating neutrophils. B cells are directly activated by antigen binding and differentiate into antibody-secreting plasma cells.
For several years now, links between hemostasis, especially fibrinolysis, and immune processes are well-established [1], [2]. Plasminogen (PLG) activator system consists of a group of proteases and protease inhibitors regulating the conversion of the zymogen PLG into the active serine protease plasmin, the principal enzymatic mediator of fibrinolysis. Plasminogen (PLG) activator system plays also a role in wound healing, extracellular matrix (ECM) degradation and cell migration [2]. The plasminogen activators – both tissue-type (tPA) and urokinase-type (uPA) – and their main inhibitor, plasminogen activator inhibitor-1 (PAI-1), play a key role in the regulation of fibrinolysis.
In addition to its role in the degradation of fibrin, tPA also modulates inflammatory response to tissue injury in various disease conditions such as ischemic brain injury and chronic kidney disease [3], [4]. For instance, in cerebral ischemic models, tPA promotes microglial activation and the recruitment of neutrophils [5], [6]. In renal inflammation model, tPA stimulates the NF-kb pathway in macrophages leading to their infiltration and activation in kidney parenchyma [7]. More recently, a new role of tPA in the adaptive component of the immune response was brought out in experimental autoimmune encephalomyelitis (EAE), the commonly used model of multiple sclerosis; and in experimental autoimmune myasthenia gravis (EAMG), two mouse models of T-cell and B-cell-mediated autoimmune diseases, respectively [8], [9], [10].
tPA acts via its protease activity but also as a cytokine and binds cell surface receptor including the Lipoprotein Receptor-related Protein 1 (LRP1), Annexin A2 and the N-Methyl-D-Aspartate receptor [1], [3].
In this review, we recapitulate the role of tPA in some aspects of the inflammatory response –migration of immune cells and activation of mononuclear phagocyte cells – and highlight some emerging concepts that expand its role to the adjustment of the adaptive immunity, especially T cell functions and interactions with APCs. We also emphasize the role of tPA in several immune diseases.
2. Background on tPA structure and regulation
Historically, tPA is described as a serine protease produced and released by endothelial cells in the blood stream [11]. Endothelial cells are described as the major source of tPA but the distribution of the serine protease is more extended and includes brain cells, hepatocytes, smooth muscle cells, epithelial cells and immune cells such as macrophages, mastocytes or lymphocytes [12], [13], [14], [15], [16], [17], [18], [19]. In human, tPA is composed of 527 amino acids for a molecular weight of 69 kDa and is encoded by the Plasminogen Activator Tissue type (PLAT) gene on chromosome 8 [20]. tPA is secreted as preproprotein (single chain molecule) and proteolytically processed by plasmin or trypsin to generate heavy and light chains (two-chain molecule). tPA is a major modulator of the thrombolytic pathway. Its activation leads to the generation of plasmin, an enzyme that ensures the degradation of fibrin, the cleavage of ECM components and the activation of matrix metalloproteinases (MMPs) [21], [22].
tPA is composed of five different domains, four of which are localized in the heavy chain. The finger domain, also called fibronectin domain is responsible for the binding of fibrin and enables the formation of a ternary complex with PLG [23], [24]. This domain is also involved in the interaction with the Lipoprotein Receptor-related Protein 1 (LRP1), resulting, for instance, in the clearance of tPA by hepatic cells or astrocytes [25], or its transcytosis by endothelial cells [26]. The finger domain is also known to bind the membrane receptor Annexin A2, playing a role in microglial activation, cell migration and neoangiogenesis [27], [28]. The Epidermal Growth Factor (EGF)-like domain has structural homologies with the growth factor EGF and induces signaling through binding to EGF receptor (EGFR) [29], [30]. The tPA-EGFR interaction results in cell proliferation and mobility, and inhibits apoptosis [30], [31]. The function of the Kringle 1 domain is poorly understood. It was shown that glycosylation in Asp117 increases tPA affinity to the mannose receptor and its uptake by endothelial cells [32]. The Kringle 2 domain presents a functional lysine-binding site (LBS) that supports the interaction between tPA and PLG [33]. Kringle 2 favors the activation of PLG by stabilizing the ternary complex comprising tPA, PLG and fibrin, initiated by the finger domain. Interaction of the Kringle 2 domain of tPA with Platelet-Derived Growth Factor C (PDGF-C) is needed for its cleavage into the active form PDGF-CC [34]. Moreover, the Kringle 2 domain is also involved in the binding to the GluN1 subunit of NMDAR promoting NMDA-induced calcium influx and neurotoxicity [35], [36], [37]. The last domain of tPA is the catalytic domain which ensures the conversion of PLG into plasmin, thanks to a catalytic triad on His322, Asp371 and Ser478 [20].
tPA activity is tightly controlled by serine protease inhibitors, also called serpins. Four serpins are known to inhibit tPA: PLG activator inhibitor-1 (PAI-1), PAI-2, neuroserpin (NS) and protease nexin-1 (PN-1). Many cell types can produce these serpins, including vascular endothelial cells (PAI-1 and PN-1), keratinocytes (PAI-2), and neurons (NS) [38], [39], [40]. tPA inhibitors are also present in monocyte/macrophages but their expression in other immune cells is not well documented [41], [42], [43].
3. Role of the plasminogen activation system in migration and adhesion of immune cells
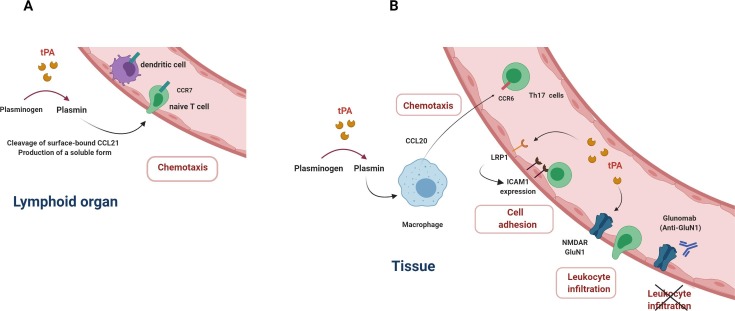
In order to carry out immunosurveillance against microorganisms, naive T cells circulate between secondary lymphoid organs (SLOs) and the blood via the lymph, while DCs traffic from tissues to the draining lymph nodes to present antigens. Antigen presentation requires the expression of specific adhesion molecules and chemokines to control the homing of the immune cells. For instance, CCR7 expression on naïve T cells and DCs is required for their migration towards SLOs [44], [45]. Interestingly, plasmin – locally generated via the activation of PLG by tPA at the surface of T cells or DCs cleaves the chemokine CCL21 (C—C motif Chemokine ligand 21), one of the two ligands of CCR7, to generate a soluble form with chemotactic activity [46] (Fig. 1 A). In addition, plasmin processes CCL21 bound at the surface of APCs and thereby prevents the tethering of T cells to APCs, that potentially limits the priming of T cells or their retention in SLOs [47]. Interestingly, in vitro studies indicate that plasmin also induces the release of CCL20 by human macrophages and DCs. Furthermore, CCL20 produced by macrophages promotes the chemotaxis of Th17 cells which expressed CCR6, the cognate receptor of CCL20 [48] (Fig. 1 B). So, in a context where PLG activation is enhanced, excessive plasmin generation could favor the recruitment of pathogenic Th17 cells in tissues and consequently support chronic inflammation or autoimmune diseases. In addition, plasmin also promotes the chemotaxis of human DCs in transwell migration assay by acting on Annexin A2, triggering ERK1/2 and AKT2 signaling pathways [49].
Fig. 1.
Effects of the plasminogen activation system on migration and adhesion of immune cells. tPA is involved in the recruitment of immune cells by promoting the release of chemokines (A and B). tPA increases the expression of the adhesion molecule ICAM1 on endothelial cells by acting on the LRP1 receptor (C). tPA-NMDAR binding on endothelial cells facilitates diapedesis of leucocytes (D). The monoclonal antibody Glunomab limits the leucocyte infiltration by blocking the interaction between tPA and NMDAR.
Complementary to these effects on chemotaxis, tPA has also a role in immune cell motility. Adhesion of macrophages to fibrin in vitro is dependent on the interaction of tPA with the integrin MAC1 (CD11b/CD18) complexed with the receptor LRP1. The tPA inhibitor PAI-1 is necessary to macrophage migration by promoting MAC1 internalization and cell detachment [50]. tPA regulates the adhesion of T cells to brain endothelial cells and increases T cell migration in vitro [51]. To this end, tPA binds to the cell membrane receptor LRP1 on endothelial cells via a “cytokine-like” action to drive the up-regulation of the adhesion molecule intercellular adhesion molecules-1 (ICAM-1) (Fig. 1 C). Of note, tPA effect is also observed in vivo since the intravenous administration of tPA enhances ICAM-1 expression on brain endothelial cells and is associated with a worsening of EAE, induced by immunization with a myelin antigen such as myelin oligodendrocyte glycoprotein (MOG) [51]. These data indicate a contribution of tPA in T-cell mediated-immunopathology and neuroinflammation. In line with this, tPA regulates the transmigration of monocytes across endothelial cells by acting on the GluN1 subunit of NMDAR [52], [53] expressed on endothelial cells [54], [55] (Fig. 1 D). This action leads to signaling events including the activation of the ERK1/2 pathway and the Rho/ROCK pathway, which drives the increase in endothelial permeability through the phosphorylation of myosin light chain [55]. Our group has demonstrated that blocking the action of tPA on GluN1 by using a monoclonal antibody (mAb, Glunomab®) reduces the in vitro transmigration of human T lymphocytes and monocytes [54]. Accordingly, in vivo, Glunomab® limits the severity of EAE disease by drastically reducing the leukocyte infiltration, including T cells, and the subsequent neural tissue inflammation and demyelination. This blockade of tPA action on endothelial GluN1 by Glunomab® occurs without modification of T cell activation measured with CD25 and CD69 markers.
4. The role of tPA in T cell activity
The role of tPA in the T cell-mediated EAE model is still a matter of debate. Previous reports demonstrate an increase in tPA activity in EAE [56], [57]. In addition, expression of tPA and its inhibitor PA1 transcripts are found in the sites of tissue damage and neuroinflammation suggesting an involvement of this serine protease in the EAE physiopathology [58]. Initially, Lu et al described that tPA-/- developed a more severe form of EAE but with a delayed onset in the disease [57]. Although most studies corroborated this finding and showed a protective role of tPA [8], [56], [59], others reported a detrimental function of tPA in the pathogenesis of EAE with either tPA deficiency which results in a less severe form of EAE or tPA administration that increases the severity of EAE [9], [51]. These inconsistencies between several studies may be attributed to the differences in the experimental protocols including myelin antigen doses, sex and age of the animals or the number of immunizations/boosts. Moreover, variations in mouse housing or environmental factors such seasonal parameters may also influence the course of EAE. Since tPA also acts on neurological parameters such as blood brain barrier permeability, microglial activation or intra parenchymal fibrinolysis, the discrepant results observed in the studies may be the result of a differential range of immunological and non-immunological functions of tPA.
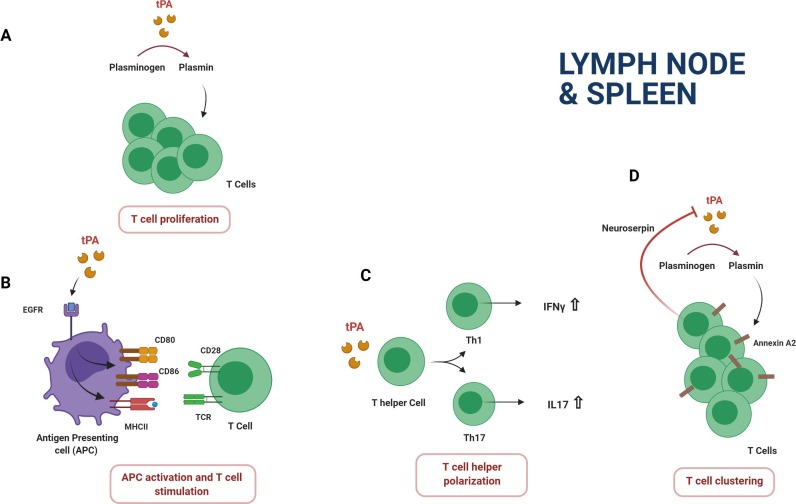
Recent data from our lab and Brenner‘s team have highlighted a novel role of tPA in T cell biology. In vitro, T cells from tPA -/- mice have a defect in proliferation following polyclonal or antigen-specific stimulation [9], [10]. This effect concerns both CD4+ and CD8+ T cells and was reversed by addition of exogenous tPA. Conversely, tPA increases the expansion and activation status of T cells from wild type (WT) splenocytes in culture. tPA induces the same effect on purified T cells stimulated by anti-CD3/CD28 monoclonal antibodies (Abs) indicating that APCs are dispensable to drive tPA-induced proliferation (Fig. 2 A). We have demonstrated that stimulating action elicited by tPA on T cells implies its proteolytic properties and plasmin generation but not enhancement of NMDAR functions. The molecular target of plasmin is currently unknown but the matrix metalloproteinase 2 and 9 constitutes strong candidates. Indeed, these proteases are produced as inactive zymogens, pro-MMPs, which are activated by plasmin. Using pharmacological blockade approach and experiments with genetically-deficient cells, Benson et al show that MMP2 and 9 expressed by CD4+ and CD8+ T cells contribute to their proliferation and activation after polyclonal or antigen-specific stimulation [60].
Fig. 2.
Effects of the plasminogen activation system on T cell functions. tPA increases MHC-II and costimulatory CD80 and CD86 molecule expression on antigen presenting cells (dendritic cells and macrophages) by the binding of the EGF-R. T cell activation and proliferation is enhanced by tPA through its proteolytic action (A). tPA increases the production of the proinflammatory cytokines IFN-γ and IL-17 (B). tPA contributes to the T cell clustering by a plasmin dependent mechanism. This effect is reversed by neuroserpin (C).
Nevertheless, an indirect effect of tPA over T cell activity cannot be discarded. In fact, it has been observed a reduced proliferation of WT T cells in vitro following co-culture with MOG-loaded APCs from tPA -/- EAE mice pointing out a activating role of the serine protease in antigen presentation [8]. At least in part, this function of tPA is protease-dependent since T cell reactivity is reduced after treatment of EAE mice with mutant tPA without catalytic activity or PAI-1 inhibitor [8]. In addition, we also showed that initial PA pretreatment of splenocytes containing APCs, but depleted of T cells, potentiated antigen specific proliferation of T cells that have been added subsequently to the co-culture [9]. Since APC-mediated expansion of T cells importantly depends on the maturation state of APCs, we recently investigated the impact of tPA on the phenotype and functions of macrophages and DCs in the T-cell mediated EAE model [9]. tPA polarizes splenic macrophages and DCs from EAE mice towards a more mature phenotype by increasing MHC-II and the costimulatory molecules CD80 and CD86 expression. In addition, splenic APCs from EAE mice incubated with tPA have an increased capacity to stimulate encephalitogenic CD4+ T cells. Interestingly, these properties of tPA on APCs involve the EGF-R and are independent of both proteolytic activity and NMDAR activation (Fig. 2 B). Furthermore, it has been described a reduction of CD4 + CD25 + Foxp3 + regulatory T cells (Tregs) among splenocytes of tPA -/- mice [8], [10]. This reduction was more pronounced in EAE and EAMG tPA -/- mice indicating a potential role of tPA in the autoimmune process through an involvement of Tregs. Conversely, WT mice treated with PAI-1 inhibitor exhibited more Tregs.
Importantly, tPA also modulates the release of cytokines by immune cells. In vitro, tPA increases IL-6 and IL-10 secretion by activated splenocytes via a plasmin-dependent mechanism [9]. In addition, we also described that CD4+ T cells from tPA-/- EAE mice produced less IFN-γ than cells from WT EAE mice. Others showed that splenocytes from tPA-/- mice produce less IFN-γ, IL-17 and IL-10 than splenocytes from WT mice following antigen-specific stimulation [8] (Fig. 2 C). Consistent with these data, it has been reported that splenocytes from mice deficient for the tPA inhibitor PAI-1 release more IFN- γ and IL-6 in response to the staphylococcal enterotoxin B superantigen [61]. Thus, tPA promotes proinflammatory Th1 and Th17 phenotypes but also increases the secretion of IL-10, mainly known as an immunoregulatory mediator. So, given the role of tPA in T cell activation, Treg biology and in the production of cytokines with pro-and anti-inflammatory properties, further studies are needed to conclude on the impact of tPA on the outcome of a specific immune response context in vivo [62].
Recent studies showed a role of the tPA inhibitor NS in T cell activation. Indeed, NS is expressed by resting T cells with a vesicular subcellular localization and is translocated to the immune synapse when T cells contact APCs. This suggests that NS may modulate signaling between T cells and APCs by regulating extracellular proteolysis [63]. Loef et al have investigated the role of tPA and its inhibitor NS in the T cell clustering, a phase that influences the T cell fate, especially after initial priming of T cells by APCs [19], [64], [65]. Authors report that inhibition of NS expression increases homotypic interactions and proliferation of activated T cells [19] (Fig. 2 C). They also demonstrated that cell clustering is a plasmin-dependent mechanism that is mediated by cleaving Annexin A2 receptor and associated to modifications of the actin cytoskeleton through the activation of ROCK II pathway and myosin ATPase. Overall, the studies converge towards a role of tPA in favor of T cell activation. Further studies are needed to consolidate our knowledge about the role of tPA in T cell migration and activation and, more broadly, to determine potential involvement of tPA in the regulation of other T cell functions such as development and differentiation. The final challenge is to determine how tPA could impact T cell mediated immunity and immunopathology in vivo.
5. Role of tPA in an B cell-mediated autoimmune model
The role of tPA in the humoral response has been scarcely investigated in a mouse model of myasthenia gravis (MG). MG is an autoimmune disease mainly mediated by anti-acetylcholine receptor (AchR) autoAbs and is characterized by muscle weakness and fatigue in patients. In EAMG, tPA-/- mice showed a higher production of anti-AchR-autoAbs associated to increased levels of B-cell activating factor (BAFF), a molecule promoting B cell survival. This suggests that tPA may impede the development of antibody response, hypothetically by targeting directly B cells and/or indirectly anti-AchR Th cells [10]. Nevertheless, in this first study, disease score severity in EAMG is weak and associated with low level of autoAbs measured lately after immunization. Thus, understanding of the functions of tPA in B cell-mediated immunity requires other investigations.
6. Effects of tPA on mononuclear phagocyte cells
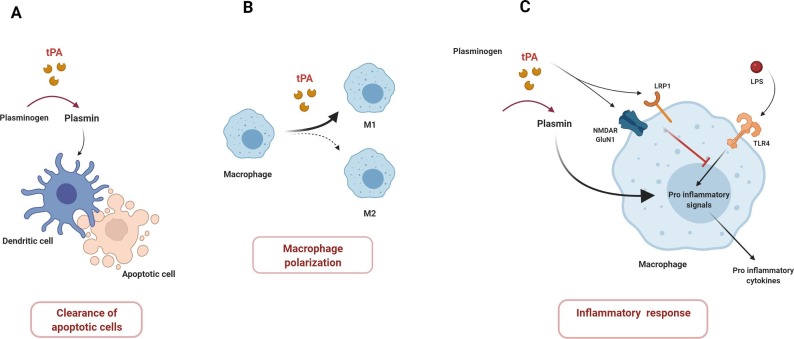
Monocytes/macrophages and DCs provide a critical link between innate and adaptive immunity and are key regulators of protective immune responses and tolerance to self-antigens. The efficient clearance of necrotic cells is an essential process for the prevention of autoimmune diseases such as systemic lupus erythematosus. Notably, works of Medcalf ‘s team identified a critical role of the PLG activator system in the phagocytosis of dead cells by DCs in homeostatic context [66]. In vitro, tPA and PLG increase the phagocytosis of dead cells by human DCs through a plasmin-dependent mechanism (Fig. 3 A). Moreover, plasmin tends to maintain DCs into an immature phenotype, with a higher production of TGF-β and lower ability to elicit allogeneic response. This suggests that plasmin promotes immune tolerance by facilitating phagocytic function. Conversely, in another study, plasmin-stimulated human DCs trigger Th1 polarization of CD4+ T cells in vitro [49]. DCs are highly plastic cells and the variability in their isolation and culture conditions may affect their phenotypes and functions and so explain the divergent effects of plasmin in these two studies. Nevertheless, it would be especially relevant to determine the putative immunomodulatory role of plasmin in response to sterile tissue injury (e.g. stroke) versus infection disease settings in which DAMPs and PAMPs modulate the DCs functions and so impact adaptive immunity. Otherwise, it should be noticed that inflammatory cytokines and PAMPs are known to enhance tPA expression, which could lead to an increase of plasmin generation and so modulate tolerogenic or immunogenic status of DCs [67].
Fig. 3.
Effects of the plasminogen activation system on mononuclear phagocyte cells. tPA promotes the clearance of apoptotic cells by dendritic cells (A). tPA is involved in the polarization of macrophages (B). tPA may have opposite effects on macrophage activation. tPA, as a plasmin activating protease, has a proinflammatory effects whereas tPA exerts anti-inflammatory effects via the binding to the NMDAR and LRP1 receptors.
Although DCs are considered the best professional APCs, it should be kept in mind that, in addition to their innate effector functions, macrophages are also key operators of adaptive immunity and immune tolerance. Generally, macrophages form a heterogeneous cell population and can be experimentally polarized into two major subtypes termed M1 and M2, although intermediate phenotypes also exist. M1 macrophages are involved in pro-inflammatory activities and are classically activated upon IFN-γ or LPS stimulation whereas alternatively activated M2 macrophages promote immunoregulation and tissue repair and are activated upon IL-4 and IL-13 stimulation [68]. Macrophages have the ability to polarize CD4+ Th cells and to contribute to leukocyte recruitment at the site of infections via chemokine production [69]. For example, in the context of mycobacterial infection, a subset of macrophages drives Th17 polarization to the detriment of Th1 and Th2 cytokine production by T cells [70].
In a model of renal injury, tPA skews the polarity of macrophages and promotes phenotype and survival of M1 macrophages [71], [72] (Fig. 3 B) This shift of polarity requires the Annexin A2 receptor and the activation of the NF-κB pathway in macrophages. Conversely, PLG/plasmin injection in the pleural cavity induces the infiltration of macrophages with increased expression of the M2 markers CD206, arginase-1, and decreased the expression of the M1 marker, inducible nitric oxide synthase (iNOS) [73]. These M2 polarized macrophages contribute to the resolution of the inflammation by phagocyting apoptotic neutrophils.
Several reports point out an intriguing and complex role of the PLG activation system in the regulation of the inflammatory responses mediated by macrophages following PAMP stimulation [1]. In this context, opposite effects of tPA on inflammatory response are driven either by its cytokine or proteolytic functions. Indeed, by studying the role of LRP1 in inflammation, Mantuano et al showed that LRP1-deficient mice have an exacerbated response to LPS, a ligand of TLR4, with macrophages spontaneously exhibiting a proinflammatory phenotype [74]. Interestingly, tPA, which can act as a ligand of LRP1, inhibits the inflammatory profile of WT macrophages in presence of LPS [74]. This effect of tPA is mediated by NMDAR expressed by macrophages, likely in cooperation with LRP1, and does not require a protease activity [75]. Enzymatically inactive tPA also antagonizes the response of macrophages stimulated by TLR2 or TLR9 agonists but fails to alter nucleotide-binding oligomerization domain-1 (NOD1) and NOD2 receptor signaling [76].
Paradoxically, tPA, through the generation of plasmin, has pro-inflammatory properties. In human monocytes, plasmin directly induces the release of the cytokines IL-1 and TNF via the activation of the NF-κB pathway as well as the upregulation of the chemokine monocyte chemoattractant protein-1 (MCP-1) and the costimulatory molecule CD40 through p38 MAPK and Janus kinase (JAK)/STAT pathways [77], [78]. Additionally, Ward et al have shown that LPS-induced response in macrophages is enhanced by plasmin [79]. In a mouse model of macrophage activation syndrome (MAS), a disease characterized by an uncontrolled activation and proliferation of T lymphocytes and macrophages causing multiorgan dysfunctions, plasmin potentiates the effect of TLR9 agonist and contributes to the increase of monocyte/macrophage infiltration and to the cytokine storm [80]. Of note, macrophages express the PLG receptor Plg-Rkt, associated with urokinase receptor uPAR [81]. It was shown that tPA could interact with Plg-Rkt to promote PLG activation and local plasmin generation. This pathway could increase the proinflammatory effects induced by plasmin [82]. So, these different studies highlight diverse and sometimes opposite effects of tPA versus plasmin in activation of macrophages by LPS in vitro. This inspired a recent study that demonstrated that tPA alone inhibits the macrophage response to LPS by a cytokine-like effect; while tPA increases the proinflammatory phenotype of macrophages via the generation of plasmin (Fig. 3 C) [83].
Altogether, these findings indicate that PLG activation system regulates both aspects of the mononuclear phagocyte cell activation. Schematically, tPA acts as a pro-inflammatory mediator by its plasmin activating protease capability while tPA exerts anti-inflammatory effects when behaving as a cytokine. However, it must take into account that the phenotype and functions of macrophages and DCs result from dynamic processes in response to sequential signals from the local environment. In addition, analysis of phenotypic markers (eg MHC II or costimulatory molecules) do not always recapitulate the functions of myeloid cells. In this way, Menges et al have shown that injection of matured DCs in presence of TNF induced antigen-specific tolerogenic T cells in EAE model [84]. Furthermore, beyond the useful but maybe oversimplistic M1/M2 paradigm, intermediate and reversible myeloid cell phenotypes may coexist in tissues that could explain the apparently contradictory effects of tPA [85].
Some pieces of evidence show that tPA is involved in immune tolerance and adaptive immunity, mainly through the regulation of macrophages and DC activity states and maturation. Nevertheless, additional studies are needed to better understand the function(s) of the different domains of tPA in these immunological processes, and their potential impact in immunological diseases, especially in condition where PLG activation system is enhanced.
7. Conclusion and perspectives
It is well established that tPA and related proteins are involved in various inflammation regulatory processes such as innate leukocyte activation and migration to the site of inflammation; and inflammatory mediator secretion. As reviewed above, recent studies indicate that tPA also emerges as an important player of the adaptive immune response. tPA plays a role in the motility and chemotaxis of T cells and APCs as well as their migration across the endothelial barrier. Fundamentally, tPA acts as an activating factor of the adaptive immune response by mediating T cell proliferation, activation and cytokine production and by shaping the functions of macrophages and DCs.
However, the impact of tPA on numerous functions of antigen-specific lymphocytes remains to be explored including priming, differentiation in effector or regulatory cells, and generation of memory immune response. This field is difficult to investigate using a standard immunization protocol with conventional mice, since the frequency of antigen-specific lymphocytes is usually low. The use of a transgenic TCR or BCR crossed with a tPA-deficient background could provide powerful tools to circumvent this limit in vivo and in vitro. Integrating more widely the study of tPA inhibitors in the future research is also necessary to have an overview of the PLG system in adaptive immunity. The contribution of tPA in autoimmune diseases has been underinvestigated. The studies with tPA-deficient mice have shown contradictory effects in EAE model and deleterious effects in EAMG model. Extended research concerning the relationships between tPA and adaptive immunity in the context of protective immunity, or immunopathology, may lead to the development of novel tPA-based therapeutic research axes in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
All the figures were created with BioRender.com.
Funding information
This work was supported by grants from Regional Council of Normandy, the Etablissement Français du sang (EFS), the Association pour la Recherche sur la Sclérose En Plaques (ARSEP) and by the Instituto de Salud Carlos III-Spanish Ministerio de Ciencia e Innovación (PI18/00357; RD16-0015/0019, partially co-financed by F.E.D.E.R., European Union, “Una manera de hacer Europa”.
Author contributions
CS, PH, GP, DB, DC, BL-M, FD and OT substantially contributed to the conception of this work, drafted and revised the manuscript, before finally approving its submission. CS, PH, GP, DB, DC, BL-M, FD and OT agreed to be accountable for all aspects of the work.
References
- 1.Heissig B., Salama Y., Takahashi S., Osada T., Hattori K. The multifaceted role of plasminogen in inflammation. Cell. Signal. 2020;75:109761. doi: 10.1016/j.cellsig.2020.109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker S.K., Strickland S. A critical role for plasminogen in inflammation. J. Exp. Med. 2020;217 doi: 10.1084/jem.20191865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L., Hu K. Tissue plasminogen activator and inflammation: from phenotype to signaling mechanisms. Am J Clin Exp Immunol. 2014;3:30–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Mehra A., Ali C., Parcq J., Vivien D., Docagne F. The plasminogen activation system in neuroinflammation. BBA. 2016;1862(3):395–402. doi: 10.1016/j.bbadis.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C., An J., Strickland D.K., Yepes M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 2009;174(2):586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhl B., Zuchtriegel G., Puhr-Westerheide D., Praetner M., Rehberg M., Fabritius M., Hessenauer M., Holzer M., Khandoga A., Fürst R., Zahler S., Krombach F., Reichel C.A. Tissue plasminogen activator promotes postischemic neutrophil recruitment via its proteolytic and nonproteolytic properties. Arterioscler. Thromb. Vasc. Biol. 2014;34(7):1495–1504. doi: 10.1161/ATVBAHA.114.303721. [DOI] [PubMed] [Google Scholar]
- 7.Lin L., Wu C., Hu K. Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. 2012;23(8):1329–1338. doi: 10.1681/ASN.2011111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizrachi T., Gur-Wahnon D., Al-Roof Higazi A., Brenner T. Role of tissue plasminogen activator in clinical aggravation of experimental autoimmune encephalomyelitis and its therapeutic potential. Cell. Immunol. 2020;348:104040. doi: 10.1016/j.cellimm.2020.104040. [DOI] [PubMed] [Google Scholar]
- 9.Hélie P., Camacho-Toledano C., Lesec L., Seillier C., Miralles A.J., Ortega M.C., Guérit S., Lebas H., Bardou I., Vila-Del Sol V., Vivien D., Le Mauff B., Clemente D., Docagne F., Toutirais O. Tissue plasminogen activator worsens experimental autoimmune encephalomyelitis by complementary actions on lymphoid and myeloid cell responses. J. Neuroinflamm. 2021;18:52. doi: 10.1186/s12974-021-02102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gur-Wahnon D., Mizrachi T., Wald-Altman S., Al-Roof Higazi A., Brenner T. Tissue plasminogen activator involvement in experimental autoimmune myasthenia gravis: aggravation and therapeutic potential. J. Autoimmun. 2014;52:36–43. doi: 10.1016/j.jaut.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Levin E.G., del Zoppo G.J. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am. J. Pathol. 1994;144:855–861. [PMC free article] [PubMed] [Google Scholar]
- 12.Louessard M., Lacroix A., Martineau M., Mondielli G., Montagne A., Lesept F., Lambolez B., Cauli B., Mothet J.-P., Vivien D., Maubert E. Tissue Plasminogen Activator Expression Is Restricted to Subsets of Excitatory Pyramidal Glutamatergic Neurons. Mol. Neurobiol. 2016;53(7):5000–5012. doi: 10.1007/s12035-015-9432-7. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson T.K., Lawrence D.A. Characterization of Tissue Plasminogen Activator Expression and Trafficking in the Adult Murine Brain. eNeuro. 2018;5 doi: 10.1523/ENEURO.0119-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Z., Nayak L., Wang W., Yurdagul A., Wang X., Cai B., Lapping S., Ozcan L., Ramakrishnan R., Pestell R.G., Jain M.K., Tabas I. An ATF6-tPA pathway in hepatocytes contributes to systemic fibrinolysis and is repressed by DACH1. Blood. 2019;133:743–753. doi: 10.1182/blood-2018-07-864843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesbauer F., Kaun C., Zorn G., Maurer G., Huber K., Wojta J. HMG CoA reductase inhibitors affect the fibrinolytic system of human vascular cells in vitro: a comparative study using different statins. Br. J. Pharmacol. 2002;135:284–292. doi: 10.1038/sj.bjp.0704454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson H.M., Haites N.E., Reid F.J., Booth N.A. Interleukin-1 beta up-regulates the plasminogen activator/plasmin system in human mesangial cells. Kidney Int. 1996;49:1097–1104. doi: 10.1038/ki.1996.159. [DOI] [PubMed] [Google Scholar]
- 17.Hart P.H., Burgess D.R., Vitti G.F., Hamilton J.A. Interleukin-4 stimulates human monocytes to produce tissue-type plasminogen activator. Blood. 1989;74:1222–1225. [PubMed] [Google Scholar]
- 18.Sillaber C., Baghestanian M., Bevec D., Willheim M., Agis H., Kapiotis S., Füreder W., Bankl H.C., Kiener H.P., Speiser W., Binder B.R., Lechner K., Valent P. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J. Immunol. 1999;162:1032–1041. [PubMed] [Google Scholar]
- 19.Loef E.J., Brooks A.E.S., Lorenz N., Birch N.P., Dunbar P.R. Neuroserpin regulates human T cell-T cell interactions and proliferation through inhibition of tissue plasminogen activator. J. Leukoc. Biol. 2020;107(1):145–158. doi: 10.1002/jlb.v107.110.1002/JLB.2A1019-098RR. [DOI] [PubMed] [Google Scholar]
- 20.Pennica D., Holmes W.E., Kohr W.J., Harkins R.N., Vehar G.A., Ward C.A., Bennett W.F., Yelverton E., Seeburg P.H., Heyneker H.L., Goeddel D.V., Collen D. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y., Nagai N., Yamakawa K., Kawakami J., Lijnen H.R., Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Lee S.-R., Arai K., Lee S.-R., Tsuji K., Rebeck G.W., Lo E.H. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003;9(10):1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 23.Hoylaerts M., Rijken D.C., Lijnen H.R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin, J Biol Chem. 1982;257(6):2912–2919. [PubMed] [Google Scholar]
- 24.Kagitani H., Tagawa M., Hatanaka K., Ikari T., Saito A., Bando H., Okada K., Matsuo O. Expression in E. coli of finger-domain lacking tissue-type plasminogen activator with high fibrin affinity. FEBS Lett. 1985;189:145–149. doi: 10.1016/0014-5793(85)80860-7. [DOI] [PubMed] [Google Scholar]
- 25.Casse F., Bardou I., Danglot L., Briens A., Montagne A., Parcq J., Alahari A., Galli T., Vivien D., Docagne F. Glutamate controls tPA recycling by astrocytes, which in turn influences glutamatergic signals. J. Neurosci. 2012;32(15):5186–5199. doi: 10.1523/JNEUROSCI.5296-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivien D., Gauberti M., Montagne A., Defer G., Touzé E. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J. Cereb. Blood Flow Metab. 2011;31(11):2119–2134. doi: 10.1038/jcbfm.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siao C.-J., Fernandez S.R., Tsirka S.E. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J. Neurosci. 2003;23(8):3234–3242. doi: 10.1523/JNEUROSCI.23-08-03234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma M., Ownbey R.T., Sharma M.C. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp. Mol. Pathol. 2010;88(2):278–286. doi: 10.1016/j.yexmp.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand T., Lesept F., Chevilley A., Lenoir S., Aimable M., Briens A., Hommet Y., Bardou I., Parcq J., Vivien D. Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR. Cell Death Dis. 2015;6(10):e1924. doi: 10.1038/cddis.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa F., Gauberti M., Parcq J., Macrez R., Hommet Y., Obiang P., Hernangómez M., Montagne A., Liot G., Guaza C., Maubert E., Ali C., Vivien D., Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. J. Exp. Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liot G., Roussel B.D., Lebeurrier N., Benchenane K., Lopez-Atalaya J.P., Vivien D., Ali C. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J. Neurochem. 2006;98(5):1458–1464. doi: 10.1111/jnc.2006.98.issue-510.1111/j.1471-4159.2006.03982.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper J., Van’t Hof A., Otter M., Biessen E.A., Rijken D.C., van Berkel T.J. Interaction of mutants of tissue-type plasminogen activator with liver cells: effect of domain deletions. Biochem. J. 1996;313(Pt 3):775–780. doi: 10.1042/bj3130775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K.S., Hong Y.-K., Lee Y., Shin J.-Y., Chang S.-I., Chung S.I., Joe Y.A. Differential inhibition of endothelial cell proliferation and migration by urokinase subdomains: amino-terminal fragment and kringle domain. Exp. Mol. Med. 2003;35(6):578–585. doi: 10.1038/emm.2003.76. [DOI] [PubMed] [Google Scholar]
- 34.Fredriksson L., Li H., Fieber C., Li X., Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23(19):3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Atalaya J.P., Roussel B.D., Levrat D., Parcq J., Nicole O., Hommet Y., Benchenane K., Castel H., Leprince J., To Van D., Bureau R., Rault S., Vaudry H., Petersen K.-U., Santos J.-D., Ali C., Vivien D. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J. Cereb. Blood Flow Metab. 2008;28(6):1212–1221. doi: 10.1038/jcbfm.2008.14. [DOI] [PubMed] [Google Scholar]
- 36.Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E.T., Vivien D., Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 37.Parcq J., Bertrand T., Baron A.F., Hommet Y., Anglès-Cano E., Vivien D. Molecular requirements for safer generation of thrombolytics by bioengineering the tissue-type plasminogen activator A chain. J. Thromb. Haemost. 2013;11:539–546. doi: 10.1111/jth.12128. [DOI] [PubMed] [Google Scholar]
- 38.Handt S., Jerome W.G., Tietze L., Hantgan R.R. Plasminogen activator inhibitor-1 secretion of endothelial cells increases fibrinolytic resistance of an in vitro fibrin clot: evidence for a key role of endothelial cells in thrombolytic resistance. Blood. 1996;87:4204–4213. [PubMed] [Google Scholar]
- 39.Jang S., Yang T.H., An E.J., Yoon H.K., Sohn K.-C., Cho A.Y., Ryu E.-K., Park Y.-S., Yoon T.Y., Lee J.-H., Kim C.D. Role of plasminogen activator inhibitor-2 (PAI-2) in keratinocyte differentiation. J. Dermatol. Sci. 2010;59(1):25–30. doi: 10.1016/j.jdermsci.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Miranda E., Lomas D.A. Neuroserpin: a serpin to think about. Cell. Mol. Life Sci. 2006;63(6):709–722. doi: 10.1007/s00018-005-5077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie H., Jamieson A., Booth N.A. Regulation, location and activity of plasminogen activator inhibitor 2 (PAI-2) in peripheral blood monocytes, macrophages and foam cells. Thromb. Haemost. 1997;77(06):1168–1173. [PubMed] [Google Scholar]
- 42.Kennedy S., van Diepen A., van den Hurk C., Coates L., Woo Lee T., Ostrovsky L., Miranda E., Perez J., Davies M., Lomas D., Dunbar R., Birch N. Expression of the serine protease inhibitor neuroserpin in cells of the human myeloid lineage. Thromb. Haemost. 2007;97(03):394–399. [PubMed] [Google Scholar]
- 43.Mansilla S., Boulaftali Y., Venisse L., Arocas V., Meilhac O., Michel J.-B., Jandrot-Perrus M., Bouton M.-C. Macrophages and platelets are the major source of protease nexin-1 in human atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2008;28(10):1844–1850. doi: 10.1161/ATVBAHA.108.171389. [DOI] [PubMed] [Google Scholar]
- 44.Bromley S.K., Thomas S.Y., Luster A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-Sánchez N., Riol-Blanco L., Rodríguez-Fernández J.L. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J. Immunol. 2006;176(9):5153–5159. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz N., Loef E.J., Kelch I.D., Verdon D.J., Black M.M., Middleditch M.J., Greenwood D.R., Graham E.S., Brooks A.ES., Dunbar P.R., Birch N.P. Plasmin and regulators of plasmin activity control the migratory capacity and adhesion of human T cells and dendritic cells by regulating cleavage of the chemokine CCL21. Immunol. Cell Biol. 2016;94(10):955–963. doi: 10.1038/icb.2016.56. [DOI] [PubMed] [Google Scholar]
- 47.Friedman R.S., Jacobelli J., Krummel M.F. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 2006;7(10):1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 48.Li Q., Laumonnier Y., Syrovets T., Simmet T. Recruitment of CCR6-expressing Th17 cells by CCL20 secreted from plasmin-stimulated macrophages. Acta Biochim. Biophys. Sin. (Shanghai) 2013;45:593–600. doi: 10.1093/abbs/gmt049. [DOI] [PubMed] [Google Scholar]
- 49.Li X., Syrovets T., Genze F., Pitterle K., Oberhuber A., Orend K.-H., Simmet T. Plasmin triggers chemotaxis of monocyte-derived dendritic cells through an Akt2-dependent pathway and promotes a T-helper type-1 response. Arterioscler. Thromb. Vasc. Biol. 2010;30(3):582–590. doi: 10.1161/ATVBAHA.109.202044. [DOI] [PubMed] [Google Scholar]
- 50.Cao C., Lawrence D.A., Li Y., Von Arnim C.A.F., Herz J., Su E.J., Makarova A., Hyman B.T., Strickland D.K., Zhang L.i. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25(9):1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Zhang X., Mu L., Zhang M., Gao Z., Zhang J., Yao X., Liu C., Wang G., Wang D., Kong Q., Liu Y., Li N.a., Sun B.o., Li H. t-PA acts as a cytokine to regulate lymphocyte-endothelium adhesion in experimental autoimmune encephalomyelitis. Clin Immunol. 2014;152(1-2):90–100. doi: 10.1016/j.clim.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Reijerkerk A., Kooij G., van der Pol S.M.A., Leyen T., van het Hof B., Couraud P.-O., Vivien D., Dijkstra C.D., de Vries H.E. Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J. Immunol. 2008;181(5):3567–3574. doi: 10.4049/jimmunol.181.5.3567. [DOI] [PubMed] [Google Scholar]
- 53.Reijerkerk A., Kooij G., van der Pol S.M.A., Leyen T., Lakeman K., van Het Hof B., Vivien D., de Vries H.E. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J. Neurochem. 2010;113:447–453. doi: 10.1111/j.1471-4159.2010.06598.x. [DOI] [PubMed] [Google Scholar]
- 54.Macrez R., Ortega M.C., Bardou I., Mehra A., Fournier A., Van der Pol S.M.A., Haelewyn B., Maubert E., Lesept F., Chevilley A., de Castro F., De Vries H.E., Vivien D., Clemente D., Docagne F. Neuroendothelial NMDA receptors as therapeutic targets in experimental autoimmune encephalomyelitis. Brain. 2016;139(9):2406–2419. doi: 10.1093/brain/aww172. [DOI] [PubMed] [Google Scholar]
- 55.Mehra A., Guérit S., Macrez R., Gosselet F., Sevin E., Lebas H., Maubert E., De Vries H.E., Bardou I., Vivien D., Docagne F. Nonionotropic Action of Endothelial NMDA Receptors on Blood-Brain Barrier Permeability via Rho/ROCK-Mediated Phosphorylation of Myosin. J. Neurosci. 2020;40(8):1778–1787. doi: 10.1523/JNEUROSCI.0969-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahl L.CM., Nasa Z., Chung JieYu, Niego B., Tarlac V., Ho H., Galle A., Petratos S., Lee J.Y., Alderuccio F., Medcalf R.L., Mengod G. The Influence of Differentially Expressed Tissue-Type Plasminogen Activator in Experimental Autoimmune Encephalomyelitis: Implications for Multiple Sclerosis. PLoS One. 2016;11(7):e0158653. doi: 10.1371/journal.pone.0158653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu W., Bhasin M., Tsirka S.E. Involvement of tissue plasminogen activator in onset and effector phases of experimental allergic encephalomyelitis. J. Neurosci. 2002;22(24):10781–10789. doi: 10.1523/JNEUROSCI.22-24-10781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teesalu T., Hinkkanen A.E., Vaheri A. Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice. Am. J. Pathol. 2001;159(6):2227–2237. doi: 10.1016/S0002-9440(10)63073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.East E., Baker D., Pryce G., Lijnen H.R., Cuzner M.L., Gverić D. A role for the plasminogen activator system in inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis. Am. J. Pathol. 2005;167(2):545–554. doi: 10.1016/S0002-9440(10)62996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benson H.L., Mobashery S., Chang M., Kheradmand F., Hong J.S., Smith G.N., Shilling R.A., Wilkes D.S. Endogenous matrix metalloproteinases 2 and 9 regulate activation of CD4+ and CD8+ T cells. Am. J. Respir. Cell Mol. Biol. 2011;44(5):700–708. doi: 10.1165/rcmb.2010-0125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renckens R., Pater J.M., van der Poll T. Plasminogen activator inhibitor type-1-deficient mice have an enhanced IFN-gamma response to lipopolysaccharide and staphylococcal enterotoxin B. J. Immunol. 2006;177:8171–8176. doi: 10.4049/jimmunol.177.11.8171. [DOI] [PubMed] [Google Scholar]
- 62.Bedke T., Muscate F., Soukou S., Gagliani N., Huber S. Title: IL-10-producing T cells and their dual functions. Semin. Immunol. 2019;44:101335. doi: 10.1016/j.smim.2019.101335. [DOI] [PubMed] [Google Scholar]
- 63.Lorenz N., Loef E.J., Verdon D.J., Chen C.-J., Mansell C.J., Angel C.E., Brooks A.E.S., Dunbar P.R., Birch N.P. Human T cell activation induces synaptic translocation and alters expression of the serine protease inhibitor neuroserpin and its target protease. J. Leukoc. Biol. 2015;97(4):699–710. doi: 10.1189/jlb.1A0814-392R. [DOI] [PubMed] [Google Scholar]
- 64.Krummel M.F., Mahale J.N., Uhl L.F.K., Hardison E.A., Mujal A.M., Mazet J.M., Weber R.J., Gartner Z.J., Gérard A. Paracrine costimulation of IFN-γ signaling by integrins modulates CD8 T cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2018;115(45):11585–11590. doi: 10.1073/pnas.1804556115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zumwalde N.A., Domae E., Mescher M.F., Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J. Immunol. 2013;191(7):3681–3693. doi: 10.4049/jimmunol.1201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borg R.J., Samson A.L., Au A.-L., Scholzen A., Fuchsberger M., Kong Y.Y., Freeman R., Mifsud N.A., Plebanski M., Medcalf R.L., Nishimura S.L. Dendritic Cell-Mediated Phagocytosis but Not Immune Activation Is Enhanced by Plasmin. PLoS One. 2015;10(7):e0131216. doi: 10.1371/journal.pone.0131216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kruithof E.K.O., Dunoyer-Geindre S. Human tissue-type plasminogen activator. Thromb. Haemost. 2014;112(08):243–254. doi: 10.1160/TH13-06-0517. [DOI] [PubMed] [Google Scholar]
- 68.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts C.A., Dickinson A.K., Taams L.S. The Interplay Between Monocytes/Macrophages and CD4(+) T Cell Subsets in Rheumatoid Arthritis. Front. Immunol. 2015;6:571. doi: 10.3389/fimmu.2015.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tatano Y., Shimizu T., Tomioka H. Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization. Sci. Rep. 2014;4:4146. doi: 10.1038/srep04146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin L., Hu K. Tissue-type plasminogen activator modulates macrophage M2 to M1 phenotypic change through annexin A2-mediated NF-κB pathway. Oncotarget. 2017;8:88094–88103. doi: 10.18632/oncotarget.21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin L., Jin Y., Hu K. Tissue-type plasminogen activator (tPA) promotes M1 macrophage survival through p90 ribosomal S6 kinase (RSK) and p38 mitogen-activated protein kinase (MAPK) pathway. J. Biol. Chem. 2015;290(12):7910–7917. doi: 10.1074/jbc.M114.599688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugimoto M.A., Ribeiro A.L.C., Costa B.R.C., Vago J.P., Lima K.M., Carneiro F.S., Ortiz M.M.O., Lima G.L.N., Carmo A.A.F., Rocha R.M., Perez D.A., Reis A.C., Pinho V., Miles L.A., Garcia C.C., Teixeira M.M., Sousa L.P. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood. 2017;129:2896–2907. doi: 10.1182/blood-2016-09-742825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantuano E., Brifault C., Lam M.S., Azmoon P., Gilder A.S., Gonias S.L. LDL receptor-related protein-1 regulates NFκB and microRNA-155 in macrophages to control the inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 2016;113(5):1369–1374. doi: 10.1073/pnas.1515480113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantuano E., Azmoon P., Brifault C., Banki M.A., Gilder A.S., Campana W.M., Gonias S.L. Tissue-type plasminogen activator regulates macrophage activation and innate immunity. Blood. 2017;130:1364–1374. doi: 10.1182/blood-2017-04-780205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Das L., Azmoon P., Banki M.A., Mantuano E., Gonias S.L., Furuta S. Tissue-type plasminogen activator selectively inhibits multiple toll-like receptors in CSF-1-differentiated macrophages. PLoS ONE. 2019;14(11):e0224738. doi: 10.1371/journal.pone.0224738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burysek L., Syrovets T., Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 2002;277(36):33509–33517. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- 78.Syrovets T., Jendrach M., Rohwedder A., Schüle A., Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. 2001;97:3941–3950. doi: 10.1182/blood.v97.12.3941. [DOI] [PubMed] [Google Scholar]
- 79.Ward J.R., Dower S.K., Whyte M.K.B., Buttle D.J., Sabroe I. Potentiation of TLR4 signalling by plasmin activity. Biochem. Biophys. Res. Commun. 2006;341(2):299–303. doi: 10.1016/j.bbrc.2005.12.188. [DOI] [PubMed] [Google Scholar]
- 80.Shimazu H., Munakata S., Tashiro Y., Salama Y., Dhahri D., Eiamboonsert S., Ota Y., Onoda H., Tsuda Y., Okada Y., Nakauchi H., Heissig B., Hattori K. Pharmacological targeting of plasmin prevents lethality in a murine model of macrophage activation syndrome. Blood. 2017;130:59–72. doi: 10.1182/blood-2016-09-738096. [DOI] [PubMed] [Google Scholar]
- 81.Andronicos N.M., Chen E.I., Baik N., Bai H., Parmer C.M., Kiosses W.B., Kamps M.P., Yates J.R., Parmer R.J., Miles L.A. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115:1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miles L.A., Ny L., Wilczynska M., Shen Y., Ny T., Parmer R.J. Plasminogen Receptors and Fibrinolysis. Int. J. Mol. Sci. 2021;22:1712. doi: 10.3390/ijms22041712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zalfa C., Azmoon P., Mantuano E., Gonias S.L. Tissue-type plasminogen activator neutralizes LPS but not protease-activated receptor-mediated inflammatory responses to plasmin. J. Leukoc. Biol. 2019;105(4):729–740. doi: 10.1002/JLB.3A0818-329RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Menges M., Rössner S., Voigtländer C., Schindler H., Kukutsch N.A., Bogdan C., Erb K., Schuler G., Lutz M.B. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Locati M., Curtale G., Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020;15(1):123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]