Abstract
Objective
This study intends to investigate the prognostic risk factors of bloodstream infection in Beijing.
Methods
This study is a clinical retrospective study. Four hundred forty-six patients with community-onset bloodstream infections (COBSI), admitted to the emergency department and inpatient department of Beijing Jishuitan Hospital from January 1, 2015, to December 31, 2019, were selected as the main research objects. According to whether the patient survives for 100 days or not, 363 cases were in the survival group, and 83 cases were in the death group. By analyzing the clinical data of the two groups of patients, the epidemiology, clinical characteristics, bacterial resistance, and risk factors affecting the prognosis of the patients were analyzed.
Results
A total of 446 pathogenic bacteria were isolated in this study, including 324 Gram-negative (G-) bacteria (72.6%), 121 Gram-positive (G+) bacteria (27.1%). The results of the study showed that there were significant differences in MDR, initial antibiotic use, solid tumor, CKD, septic shock, acute liver injury, AKI, central venous catheter, urinary catheter, blood replacement therapy, invasive operation, and use of three or more antibiotics between the two groups (p<0.05). The multiple logistic regression analysis showed that solid tumors (OR=3.339, 95% CI: (1.441, 7.734), p=0.005), combined septic shock (OR=20.729, 95% CI: (10.235, 41.982), p<0.001), indwelling catheters (OR=3.556, 95% CI: (1.538, 8.222), p=0.003) and continuous venovenous hemofiltration (CVVH, OR=19.548, 95% CI: (8.724, 35.641), p=0.003) are independent risk factors affecting the prognosis of COBSI patients. Appropriate initial antibiotic therapy is a protective factor affecting the prognosis of COBSI patients.
Conclusion
Solid tumors, combined septic shock, indwelling catheters, CVVH are independent risk factors affecting the prognosis of COBSI patients.
Keywords: Community-onset bloodstream infection (COBSI), China, Beijing, Retrospective study, Epidemiology
Introduction
Bloodstream infections have high morbidity and fatality rates worldwide. Bloodstream infections are divided into community-onset bloodstream infections and hospital-acquired bloodstream infections according to the location of the disease.1 The bloodstream infection in the community has the characteristics of rapid onset, dangerous conditions, and a high fatality rate. Therefore, early and appropriate antimicrobial treatment is essential to reduce the mortality rate, especially in patients with sepsis or septic shock.4 Therefore, being familiar with the pathogen distribution and prognosis-related risk factors of COBSI in the region is helpful for early clinical empirical treatment of COBSI, assessing the prognosis of the disease, and reducing the mortality of COBSI patients.
At present, the prognostic risk factors of community-onset bloodstream infections in Beijing, the capital of China, are not yet clear. Therefore, we designed a retrospective study from 2015 to 2019 to explore the epidemiology, clinical characteristics, bacterial resistance of Beijing COBSI patients and risk factors affecting the prognosis of patients and provide a reference for clinical diagnosis and treatment.
Methods
Subjects
This study is a clinical retrospective study, and selected community-onset bloodstream infection (COBSI) patients who attended the emergency department and inpatient department of Beijing Jishuitan Hospital from January 1, 2015, to December 31, 2019, as the main research objects. Analyze the clinical data of the patients to analyze the patient’s epidemiology, clinical characteristics, bacterial resistance, and risk factors affecting the patient’s prognosis. This study complies with the “Declaration of Helsinki of the World Medical Association.” Since this study is a clinical retrospective study and does not require any patient-related interventions or experiments, the ethics committee of this hospital has reviewed it without informed consent.
COBSI diagnostic criteria
Bloodstream infection (BSI) refers to a severe systemic infection syndrome in which pathogenic microorganisms such as bacteria and fungi invade the blood circulatory system and multiply in the blood, causing serious collective damage and systemic poisoning symptoms. BSI is divided into community-acquired and hospital-acquired according to the location of the disease.5 Community-onset bloodstream infection (COBSI) refers to BSI, present at the time of admission or occurred within 48 hours of admission. The initial antibiotic treatment is reasonable: if the patient’s antibacterial drugs include one or more drugs that have antibacterial effects on pathogens (judging by the results of in vitro susceptibility tests), and the methods and dosages used are in line with the existing drug use guidelines, it is considered to have received the adequate initial treatment.6
Inclusion, exclusion criteria, and endpoint
Inclusion criteria: (1) patients with a clear diagnosis of COBSI; (2) age ≥18 years; (3) bloodstream infections that existed at the time of admission or occurred within 48 hours of admission
The primary endpoint of this study was patient death.
Bacterial identification and drug sensitivity test
This study used BD BACTEC TM FX 40 automatic blood culture instrument and matching blood culture flasks for blood culture. 20 ml of blood was collected from adults and injected into aerobic and anaerobic culture flasks for incubation in automatic blood culture bottles. If there is a positive alarm, transfer the blood culture specimen to the culture medium to continue the culture, and the BD Phoenix™-100 automatic bacterial identification/drug susceptibility system identifies strains.
Data collection
The demographic and clinical data of this study were collected from the electronic case system, including age, gender, diagnosis, comorbid diseases (diabetes, solid tumors, cardiac insufficiency, renal insufficiency, lung disease, etc.), invasive operations (deep venous catheterization, indwelling urinary catheter, etc.), antibiotic use before infection, acute complications (septic shock, acute renal insufficiency, acute cardiac insufficiency, etc.), laboratory indicators (white blood cells, hemoglobin, albumin, etc.), number of days in hospital and treatment outcome. In addition, the severity of the disease was evaluated by APACHEII score and PITT score, and the comorbidity was evaluated by Charlson score. All cases were followed up for 100 days, and the patients were divided into survival group and death group according to whether they were alive or not for 100 days.
Statistical Analysis
This study uses Excel software to organize the data and SPSS 20.0 statistical software to process the data. The mean ± standard deviation describes the measurement data conforming to the normal distribution (χ̄ ± s), and the measurement data of the non-normal distribution is represented by M (IQR). Count data is expressed in percentage (%). The comparison between the two groups is performed by t-test; the comparison of count data between groups is performed by χ2 Test or Fisher’s exact probability method, and P < 0.05 is considered statistically significant; different scoring systems predict prognosis using receiver operating characteristic curve (ROC) to calculate and compare the area under the curve (AUC) and cut-off values, etc.; Kaplan-Meier survival analysis method is used to perform univariate analysis of the relationship between initial antibiotic use and prognosis; binary logistic regression is used to calculate independent risk factors affecting prognosis.
Results
Basic clinical features
From 2015.1.1 to 2019.12.31, this study included a total of 453 patients who were diagnosed with COBSI by blood culture. Excluding 4 cases of repeated culture strains and 3 cases of contaminated strains from the same patient, finally, 446 were included in the analysis, including 252 males and 194 females. According to 100-day survival or not, patients were divided into survival group and death group, of which 363 cases were in the survival group, and 83 cases were in the death group. The results of the study showed that there were significant differences in MDR, initial antibiotic use, solid tumor, CKD, septic shock, acute liver injury, AKI, central venous catheter, urinary catheter, blood replacement therapy, invasive operation, and use of three or more antibiotics between the two groups (p<0.05). See Table 1 to Table 2 for details.
Table 1.
Demographic and clinical characteristics of the study cohort.
| Variables | 100-days outcome | ||||
|---|---|---|---|---|---|
|
| |||||
| All 446 |
Death 83 (18.6%) |
Survive 363 (81.4%) |
χ 2 /Z | p-value | |
| Patients variables | |||||
| Sex | 0.580 | 0.446 | |||
| Male | 252 (56.5%) | 50 (60.2%) | 161 (44.4%) | ||
| Female | 194 (43.5%) | 33 (39.8%) | 202 (55.6%) | ||
| Age (years) median (IQR) | 77 (17) | 78 (13) | 77 (17) | −0.746 | 0.456 |
| Charlson | 3 (2) | 4 (2) | 2 (3) | −6.477 | 0.000 |
| Comorbidities | |||||
| Cardiovascular disease | 226 (50.7%) | 47 (56.6%) | 179 (49.3%) | 1.446 | 0.229 |
| Cerebrovascular disease | 179 (40.1%) | 39 (47%) | 140 (38.6%) | 1.994 | 0.158 |
| DM | 198 (44.4%) | 42 (40.6%) | 156 (43.0%) | 1.592 | 0.207 |
| COPD | 50 (11.2%) | 10 (12.0%) | 40 (11.0%) | 0.072 | 0.789 |
| Haematological Malignancies | 10 (2.2%) | 2 (2.4%) | 8 (2.2%) | 0.000 | 1.000 |
| Solid tumors | 68 (15.2%) | 20 (24.1%) | 48 (13.2%) | 6.181 | 0.013 |
| Chronic Hepatitis | 30 (6.7%) | 7 (8.4%) | 23 (6.3%) | 0.474 | 0.491 |
| Chronic kidney disease | 74 (16.6%) | 24 (28.9%) | 50 (13.8%) | 11.191 | 0.001 |
| Gastrointestinal disease | 22 (4.9%) | 2 (2.4%) | 20 (5.5%) | 0.802 | 0.370 |
| Dementia | 134 (30.0%) | 29 (34.9%) | 105 (28.9%) | 1.163 | 0.281 |
| Autoimmune disease | 18 (4.0%) | 1 (1.2%) | 17 (4.7%) | 2.11 | 0.253 |
| SOT | 2 (0.4%) | 0 (0%) | 2 (0.6%) | 1.000 | |
| Others | 6 (1.3%) | 0 (0%) | 6 (1.7%) | 0.599 | |
| Acute comorbidities | |||||
| Septic shock | 74 (16.6%) | 50 (60.2%) | 24 (6.6%) | 140.395 | 0.000 |
| Acute kidney failure | 7 (1.6%) | 7 (8.4%) | 0 (0%) | 0.000 | |
| Acute Hepatic Failure | 36 (8.2%) | 13 (15.7%) | 23 (6.3%) | 7.919 | 0.005 |
| Others | 6 (1.3%) | 2 (2.4%) | 4 (1.1%) | 0.164 | 0.686 |
| Pre-infection Variables | |||||
| Central venous catheter | 11 (2.5%) | 6 (7.2%) | 5 (1.4%) | 9.616 | 0.002 |
| Urinary catheter | 56 (12.6%) | 23 (27.7%) | 33 (9.1%) | 21.332 | 0.000 |
| CVVH | 7 (1.6%) | 4 (4.8%) | 3 (0.8%) | 4.624 | 0.031 |
| Invasive procedures | 6 (1.3%) | 4 (4.8%) | 2 (0.6%) | 6.336 | 0.012 |
| Steroid therapy | 9 (2.0%) | 1 (1.2%) | 8 (2.2%) | 0.023 | 0.880 |
| Immunosuppressive therapy | 14 (3.1%) | 1 (1.2%) | 13 (3.6%) | 0.595 | 0.441 |
| Long-term use of antibiotics | 12 (2.7%) | 4 (4.8%) | 8 (2.2%) | 0.907 | 0.341 |
| Transplantation | 2 (0.4%) | 0 (0%) | 2 (0.6%) | 1.000 | |
| MDR | 103 (23.1%) | 12 (14.5%) | 91 (25.1%) | 4.283 | 0.039 |
| APACHE II | 14 (9) | 25 (12) | 12 (7) | −11.457 | 0.000 |
| PBS | 2 (3) | 6 (2) | 1 (2) | −12.298 | 0.000 |
| Treatment variables | |||||
| Cephalosporins | 190 (42.6%) | 31 (37.3%) | 159 (43.8%) | 1.150 | 0.284 |
| Macrolides | 16 (3.6%) | 1 (1.2%) | 15 (4.1%) | 0.941 | 0.332 |
| Fluoroquinolones | 68 (15.2%) | 8 (9.6%) | 60 (16.5%) | 2.482 | 0.115 |
| Aminoglycosides | 9 (2.0%) | 0 (0%) | 9 (2.5%) | 0.220 | |
| Carbapenems | 193 (43.3%) | 51 (61.4%) | 142 (39.1%) | 13.719 | 0.000 |
| Others | 100 (22.4%) | 23 (27.7%) | 77 (21.2%) | 1.640 | 0.200 |
| Adequate empiric antibiotic treatment | 250 (56.6%) | 37 (44.6%) | 213 (59.3%) | 5.972 | 0.015 |
| Post-antibiogram therapy | |||||
| Monotherapy | 306 (68.6%) | 52 (62.7%) | 254 (70.0%) | 1.682 | 0.195 |
| Two-drug combinations | 121 (27.1%) | 23 (27.7%) | 98 (27.0%) | 0.017 | 0.895 |
| Combinations with ≥ three drugs | 16 (3.6%) | 6 (7.2%) | 10 (2.8%) | 3.910 | 0.048 |
| Hospital stays | 9 (12) | 15 (23) | 9 (10) | −2.929 | 0.003 |
Note: DM, Diabetes Mellitus; COPD, chronic obstructive pulmonary disease; SOT, solid organ transplantation; CVVH, continuous veno-venous hemofiltration; MDR, multidrug-resistant strains; PBS, Pitt Bacteremia Score.
Table 2.
The Laboratory indexes of the patients in the study cohort
| Variables | 100-days outcome | ||||
|---|---|---|---|---|---|
|
| |||||
| All | Death | Survive | t/Z | p-value | |
| Laboratory index | |||||
| T (°C) | 39 (0.8) | 38.8 (0.8) | 39.1 (0.7) | −0.155 | 0.877 |
| MAP (mmHg) | 80±20 | 57 (16) | 83 (23) | −8.960 | 0.000 |
| HR (bpm) | 102 (31) | 119±28 | 100 (30) | −5.960 | 0.000 |
| RR (bpm) | 22 (11) | 25 (9) | 20 (9) | −6.293 | 0.000 |
| PCT (ng/mL) | 4.1 (12.0) | 4.4 (10.6) | 3.1 (12.1) | −1.665 | 0.096 |
| CRP (mmol/L) | 97.3 (82.5) | 120.1 (93.5) | 89.1 (82.2) | −3.163 | 0.002 |
| WBC (×109/L) | 12.0 (11.1) | 11.2 (12.9) | 13.2 (12.5) | −0.634 | 0.526 |
| Hb (g/L) | 116.6±28.1 | 112.9±32.9 | 117.8±26.3 | 1.840 | 0.078 |
| HCT (%) | 34.0±11.4 | 33.8±9.3 | 34.0 (11.3) | −1.546 | 0.122 |
| Plt (×109/L) | 181.0 (108.5) | 165.0 (108.0) | 181.5 (120.8) | −2.781 | 0.005 |
| N% | 90.2 (7.1) | 91.1 (4.9) | 90.3 (9.1) | −0.270 | 0.788 |
| FIB (g/L) | 454.5±185.5 | 437.0±184.8 | 460.2±186.8 | −0.064 | 0.955 |
| D-D (mg/L) | 4.70 (12.0) | 9.23 (14.2) | 4.53 (9.3) | −4.471 | 0.000 |
| BNP (pg/mL) | 2082 (4502) | 3184 (1190) | 1450 (3966) | −3.994 | 0.000 |
| cTn I (ng/mL) | 0.04 (0.16) | 0.9 (0.68) | 0.03 (0.08) | −5.789 | 0.000 |
| ALT (U/L) | 18 (24) | 19 (18) | 18 (25) | −0.309 | 0.757 |
| AST (U/L) | 28 (43) | 29 (33) | 27 (48) | −1.320 | 0.187 |
| ALB (g/L) | 34.1±7.9 | 29.9±3.9 | 35.5±5.3 | 7.697 | 0.000 |
| TBIL () | 16.1 (13.7) | 13.5 (14.8) | 17.1 (13.9) | −0.072 | 0.942 |
| GLU (mmol/L) | 8.4 (5.2) | 8.3 (6.1) | 8.4 (3.9) | −0.116 | 0.908 |
| UREA (mmol/L) | 9.4 (5.3) | 12.6 (11.4) | 8.1 (5.2) | −5.140 | 0.000 |
| CREA (μm/L) | 84.1 (49.3) | 122.2 (288.5) | 84.4 (49.0) | −1.982 | 0.047 |
| Ca (mmol/L) | 2.1 (0.2) | 2.1±0.2 | 2.1 (0.2) | −4.232 | 0.000 |
| K (mmol/L) | 4.0 (0.7) | 4.0±0.7 | 3.9±0.5 | −1.994 | 0.048 |
| Na (mmol/L) | 135 (8) | 136 (13) | 135 (7) | −1.447 | 0.148 |
| PH | 7.42 (0) | 7.39 (0) | 7.44 (0) | −3.180 | 0.001 |
| PO2 (mmHg) | 77.2 (42.6) | 90.1 (57.3) | 76.1 (33.1) | −0.914 | 0.361 |
| LA (mmol/L) | 2.0 (2.5) | 2.6 (3.4) | 1.7 (2.2) | −2.461 | 0.014 |
| N (×109/L) | 10.8 (10.9) | 10.5 (11.4) | 11.2 (10.6) | −0.909 | 0.363 |
| L (×109/L) | 0.7 (0.8) | 13.2 (1.9) | 0.8 (0.9) | −0.955 | 0.340 |
| RDW-cv (%) | 13.7 (1.6) | 14.1 (0.7) | 13.2 (1.9) | −2.988 | 0.003 |
| NLR (%) | 13.5 (17.8) | 23.1 (47.7) | 12.2 (16.4) | −1.467 | 0.142 |
| PLR (%) | 251.7 (352.4) | 296.3 (261.7) | 205.1 (224.1) | −0.215 | 0.830 |
NOTE: T: temperature; MAP: mean arterial pressure; HR: heart rate; RR: respiratory rate; PCT: procalcitonin; CRP: C-reactive protein; WBC: white blood cell; Hb: Hemoglobin; HCT: hematocrit; Plt: blood platelet; FIB: Fibrinogen; BNP: B-type natriuretic peptide; cTn I: I Cardiac troponin I; ALT: glutamate-pyruvate transaminase; AST: Glutamic-oxal(o)acetic transaminase; ALB: albumin; TBIL: total bilirubin; GLU: Blood glucose; CREA: creatinine; PO2: oxygen partial pressure; LA: Lactic acid; N: Absolute neutrophil count; L: Absolute lymphocyte count; RDW: Red Cell volume Distribution; NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio
Etiological characteristics
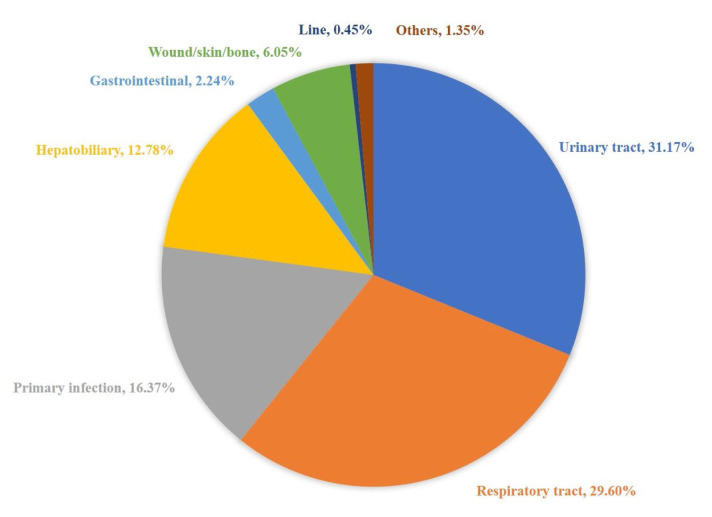
A total of 446 pathogenic bacteria were isolated in this study, including 324 Gram-negative (G-) bacteria (72.6%), 121 Gram-positive (G+) bacteria (27.1%), and one fungus (0.2%). G-bacteria are mainly Escherichia coli 205 strains and Klebsiella pneumoniae 59 strains, and the detection rates were 46.0% and 13.2%, respectively. The detection rates of ESBL production were 36.1% of Escherichia coli and 16.9% of Klebsiella pneumoniae. The G+ bacteria were mainly 71 strains of Staphylococcus and 36 strains of Streptococcus, and the detection rates were 15.9% and 8.1%, respectively. The detection rate of multidrug-resistant strains (MDR) in G+ bacteria was 15.7%, and the specific source of infection is shown in Figure 1. Among them, urinary system infections were the most common, accounting for 31.2%, and lower respiratory tract infections followed, accounting for 29.6%. Primary infections accounted for 16.4%.
Figure 1.
Source of bloodstream infection.
Prognostic risk factors for COBSI
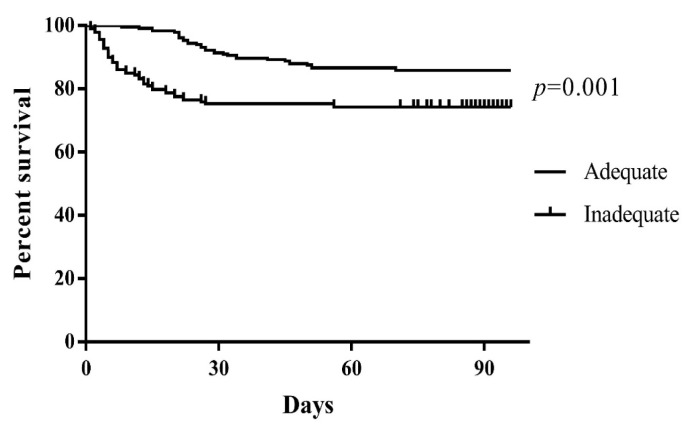
The 12 statistically significant variables (MDR, initial antibiotic use, solid tumor, CKD, septic shock, acute liver injury, AKI, central venous catheter, urinary catheter, blood replacement therapy, invasive operation, and use of three or more antibiotics) in the above single factor analysis were included as covariables. Multivariate logistics regression analysis was performed on the results, and the results are shown in Table 3. Solid tumors (OR=3.339, 95% CI: (1.441, 7.734), p=0.005), combined septic shock (OR=20.729, 95% CI: (10.235, 41.982), p<0.001), indwelling catheters (OR=3.556, 95% CI: (1.538, 8.222), p=0.003) and CVVH (OR=19.548, 95% CI: (8.724, 35.641), p=0.003) are independent risk factors that affect the prognosis. Reasonable initial antibiotic therapy is a protective factor for prognosis. Of the 446 COBSI patients enrolled, all received antibiotic therapy, of which 250 patients had reasonable initial treatment. The K-M survival curve is shown in Figure 2.
Table 3.
Multivariate analysis of risk factors for mortality in the study cohort.
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| All patients (n = 446) | ||
| Adequate empiric antibiotic treatment | 0.457 (0.235–0.887) | 0.021 |
| Solid tumors | 3.339 (1.441–7.734) | 0.005 |
| Septic shock | 20.729 (10.235–41.982) | 0.000 |
| Urinary catheter | 3.556 (1.538–8.222) | 0.003 |
| CVVH | 19.548 (8.724–35.641) | 0.003 |
Figure 2.
Kaplan Meier survival curves of patients treated with adequate empiric antibiotics (flat line) vs. Those patients treated with inadequate (dots line).
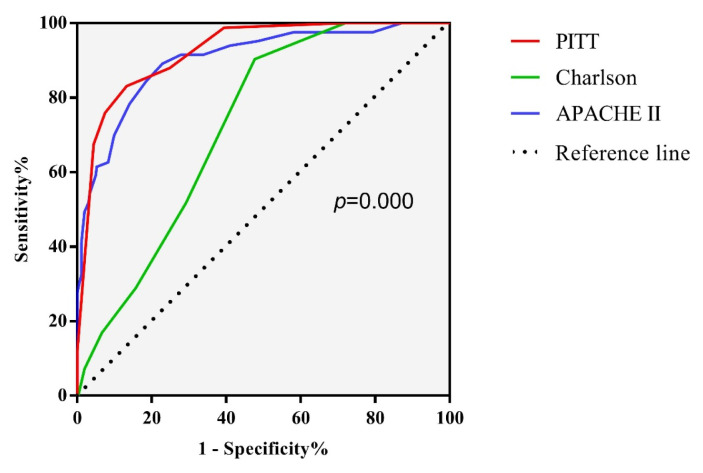
Different scoring systems predict prognosis using ROC curve calculations and compare the area under the curve (AUC) and cut-off values. The results are shown in Table 4 and Figure 3. Charlson score, APACHE II score, and PITT score have good predictive values for the 100-day prognosis of COBSI patients. Among them, the area under the curve of the PITT score is 0.925, and the cut-off value is 3.5, which has a better predictive value.
Table 4.
Comparison of auc for 4 groups to predict 100-day mortality.
| Cut-off point | Sensitivity | Specificity | AUC | P | 95% CI | |
|---|---|---|---|---|---|---|
| APACHE II | 16.50 | 0.892 | 0.771 | 0.902 | 0.000 | 0.864–0.940 |
| Charlson | 2.50 | 0.904 | 0.523 | 0.724 | 0.000 | 0.675–0.774 |
| PITT | 3.50 | 0.831 | 0.868 | 0.925 | 0.000 | 0.896–0.953 |
Figure 3.
Comparison of C-statistic for 4 groups to predict a 100-day mortality.
Discussion
This study analyzed the 100-day prognostic risk factors of the selected cases and showed that the COBSI 100-day all-cause mortality rate was 18.6%, similar to previous studies’ results.7 This study shows that solid tumors, combined with septic shock, indwelling catheters, and CVVH are independent risk factors affecting the prognosis of COBSI patients. Charlson score, APACHE II score, and PITT score have good predictive values for the prognosis of COBSI patients. The PITT score is better than the Charlson and APACHE II scores and has a better predictive value. In addition, appropriate initial antibiotic therapy is a protective factor affecting the prognosis of COBSI patients.
In this study, urinary system infections accounted for the largest proportion of primary infections of COBSI, which was similar to the results of Mehl et al.8 The second and third most common infections in this study were lower respiratory tract and primary infections. The reason was related to the distribution of patients admitted to the emergency department of tertiary hospitals in this region. The median age of patients in this study was 77 years old, and the elderly accounted for a large proportion. The elderly have decreased immune function and insufficient ability to resist infection, which is important in the onset of urinary system infections. In addition, older adults often cause incomplete or complete urinary tract obstruction due to prostate hyperplasia, bladder neck obstruction, urinary calculus, and other reasons, resulting in urinary system infections.
In recent years, the distribution of pathogens causing BSI has changed significantly; G- bacteria gradually occupy the first place of BSI pathogenic bacteria;9 the results of this study are consistent with this conclusion. This study shows that among the pathogens of COBSI in this region, Escherichia coli has the highest detection rate, 46.0% (205 strains/446 strains), similar to the results reported in the literature in other regions in China.10–12 It is consistent with the national surveillance report of bacterial resistance in 2019, but its detection rate is higher than that of European countries.13 The most common pathogens of COBSI in developed countries are Staphylococcus aureus, Enterococcus, Pseudomonas aeruginosa, Streptococcus pneumoniae, Acinetobacter baumannii and Klebsiella pneumoniae.14–15 The most common source of infection is the urinary system, followed by the lower respiratory tract.
Patients with solid tumors often receive chemotherapy treatment, and chemotherapy drugs affect their immune system function. When such patients are combined with bloodstream infection, it seriously affects the quality of life and prognosis.16 In patients with chronic renal insufficiency, especially dialysis patients, their humoral immunity, cellular immunity, granulocyte, and macrophage functions are significantly reduced, and nutritional intake is reduced. In addition, the dialysis process will also increase the risk of infection.17 At the same time, patients with renal insufficiency often have obstacles in maintaining electrolyte balance and maintaining effective circulating blood flow, which increases the risk of death. Septic shock is a severe acute complication of bloodstream infection. When bloodstream infection develops into septic shock, the fatality rate can reach 40% to 50%.18 Indwelling a urinary catheter will cause damage to the urethral mucosa, affect the normal physiological environment of the urethra, and increase the chance and risk of infection. The above factors will affect the prognosis of bloodstream infection.
In patients with bloodstream infections, especially in critically ill patients, in addition to opening venous access as soon as possible, fluid resuscitation and removal of the source of infection, antibiotic therapy is also an important measure for the treatment of bloodstream infections.19 However, in previous studies, the results of early antibiotic treatment on the prognosis were quite different. Some studies have shown that early and appropriate antibiotic treatment does not affect the prognosis,20–21 but other studies believe that early and appropriate antibiotic treatment can significantly improve the prognosis of patients.22–25 Such opposite conclusions may be attributed to the different severity of the disease, comorbidities, immune status and pathogen distribution characteristics of the study cohort.26 Similar to the results of many previous studies, this study supports that appropriate initial antibiotic treatment is a protective factor for prognosis and that early and appropriate antibiotic treatment can significantly improve the prognosis.
In addition, an important conclusion of this study is to compare the predictive value of the Charlson score, APACHE II score, and PITT score on the prognosis of COBSI. Previous studies have confirmed that the Charlson score, PITT score, and CDS (chronic disease score) are effective tools for evaluating the prognosis of bacteremia.27 This study shows that the three scores have good predictive value for the prognosis of bloodstream infection. However, the PITT score has a better predictive value. The cut-off values of the Charlson score, APACHE II score and PITT score are 2.5, 16.5, and 3.5, respectively.
Limitations: first of all, this study is a clinical retrospective study, and the research period is short, not enough to reflect the time trend of BSI pathogens or characteristics. Secondly, the data on the source of infection included in this study are few and underrepresented. Finally, this study is a single-center clinical study with small sample size, and it is still necessary to increase the sample size and conduct a multicenter clinical study.
Conclusions
Solid tumors, combined septic shock, indwelling catheters, and CVVH are independent risk factors affecting COBSI patients’ prognosis. Appropriate initial antibiotic therapy is a protective factor affecting the prognosis of COBSI patients. Charlson score, APACHE II score, and PITT score have good predictive values for the 100-day prognosis of COBSI patients.
Footnotes
Competing interests: The authors declare no conflict of Interest.
Authors Contribution. Liu Y was conception and design of the research. Liu Y and Cui BC were writing of the manuscript. Pi CM and Yu XH were acquisition of data. Liu ZW analysis and interpretation of the data. Li X and Ma LP were statistical analysis. Liu Y and Wang C were Critical revision of the manuscript for intellectual content.
References
- 1.Laupland KB, Pasquill K, Dagasso G, et al. Population-based risk factors for community-onset bloodstream infections. Eur J Clin Microbiol Infect Dis. 2020;39(4):753–758. doi: 10.1007/s10096-019-03777-8. [DOI] [PubMed] [Google Scholar]
- 2.Yo CH, Hsein YC, Wu YL, et al. Clinical predictors and outcome impact of community-onset polymicrobial bloodstream infection. Int J Antimicrob Agents. 2019;54(6):716–722. doi: 10.1016/j.ijantimicag.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Mao S, Ge Z, Zhao H, et al. analysis on distribution and drug resistance of pathogen caused community-onset bloodstream infection. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(1):67–72. doi: 10.3760/cma.j.issn.2095-4352.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Timsit JF, Ruppe E, Barbier F, et al. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi: 10.1007/s00134-020-05950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health in China. Diagnostic criteria for nosocomial infections(proposed) Natl Med J china. 2001;81(5):314–320. [Google Scholar]
- 6.Anderson DJ, Moehring RW, Sloane R, et al. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. PLoS One. 2014;9(3):e91713. doi: 10.1371/journal.pone.0091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnan M, Gudiol C, Calatayud L, et al. Risk factors for, and clinical relevance of, faecal extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis. 2011;30(3):355–360. doi: 10.1007/s10096-010-1093-x. [DOI] [PubMed] [Google Scholar]
- 8.Mehl A, Asvold BO, Kummel A, et al. Trends in antimicrobial resistance and empiric antibiotic therapy of bloodstream infections at a general hospital in Mid-Norway: a prospective observational study [published correction appears in BMC Infect Dis. 2017 June 23;17 (1):446] BMC Infect Dis. 2017;17(1):116. doi: 10.1186/s12879-017-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZQ, Wang SD, Feng DD, et al. Epidemiological risk factors for nosocomial bloodstream infections: A four-year retrospective study in China. J Crit Care. 2019;52:92–96. doi: 10.1016/j.jcrc.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Bates DW, Pruess KE, Lee TH. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch Intern Med. 1995;155(6):593–8. doi: 10.1001/archinte.1995.00430060050006. [DOI] [PubMed] [Google Scholar]
- 11.Laupland KB, Gregson DB, Flemons WW, et al. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect. 2007;135(6):1037–42. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.liang Xin, xiao Han. Clinical characteristics and pathogen profile in 193 cases of community acquired blood stream infection. Chin J Infect Chemother. 2019;19(1):6–11. [Google Scholar]
- 13.Yan xiong, Hong zhang, Yan tian chen, et al. Investigation on the distribution of pathogenic bacteria from community and hospital acquired blood current infection and their routes of infection. Laboratory Medicine. 2014;20(10):1007–1012. [Google Scholar]
- 14.Reza MA, Cormican M. Audit of aspects of practice in relation to patients with suspected community-onset blood stream infection. Ir J Med Sci. 2017;186(4):999–1001. doi: 10.1007/s11845-017-1588-x. [DOI] [PubMed] [Google Scholar]
- 15.Laupland KB, Svenson LW, Gregson DB, et al. Long-term mortality associated with community-onset bloodstream infection. Infection. 2011;39(5):405–410. doi: 10.1007/s15010-011-0149-x. [DOI] [PubMed] [Google Scholar]
- 16.Loonen AJ, de Jager CP, Tosserams J, et al. Biomarkers and molecular Analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2014;9(1):e87315. doi: 10.1371/journal.pone.0087315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 18.Zirkel J, Klinker H, Kuhn A, et al. Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med Mycol. 2012;50(1):50–55. doi: 10.3109/13693786.2011.587211. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):1–67. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 20.Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother. 2008;52(9):3188–94. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corona A, Bertolini G, Lipman J, et al. Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC) J Antimicrob Chemother. 2010;65(6):1276–85. doi: 10.1093/jac/dkq088. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Lee CH, Chuang MC, et al. Impact of inappropriate empirical antibiotic therapy on outcome of bacteremic adults visiting the ED. Am J Emerg Med. 2012;30(8):1447–56. doi: 10.1016/j.ajem.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen HC, Lin WL, Lin CC, et al. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother. 2013;68(4):947–53. doi: 10.1093/jac/dks475. [DOI] [PubMed] [Google Scholar]
- 25.Leibovici L, Shraga I, Drucker M, et al. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244(5):379–86. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A. An alternate pathophysiologic paradigm of sepsis and septic shock: implications for optimizing antimicrobial therapy. Virulence. 2014;5(1):80–97. doi: 10.4161/viru.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaquero-Herrero MP, Ragozzino S, Castano-Romero F, et al. The Pitt Bacteremia Score, Charlson Comorbidity Index and Chronic Disease Score are useful tools for the prediction of mortality in patients with Candida bloodstream infection. Mycoses. 2017;60(10):676–685. doi: 10.1111/myc.12644. [DOI] [PubMed] [Google Scholar]