Abstract
Background
Contemporaneous data are required for women with chronic kidney disease (CKD) Stages 3–5 to inform pre-pregnancy counselling and institute appropriate antenatal surveillance.
Methods
A retrospective cohort study in women with CKD Stages 3–5 after 20 weeks’ gestation was undertaken in six UK tertiary renal centres in the UK between 2003 and 2017. Factors predicting adverse outcomes and the impact of pregnancy in accelerating the need for renal replacement therapy (RRT) were assessed.
Results
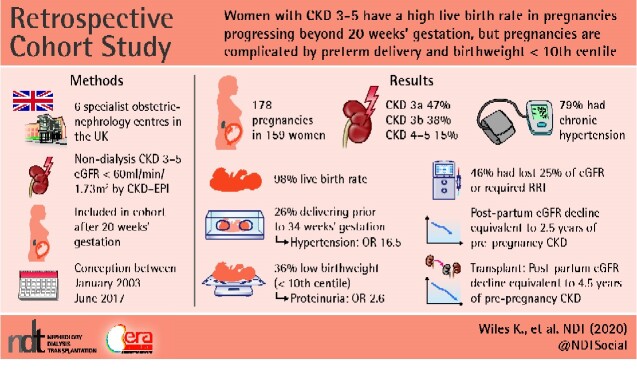
There were 178 pregnancies in 159 women, including 43 women with renal transplants. The live birth rate was 98%, but 56% of babies were born preterm (before 37 weeks’ gestation). Chronic hypertension was the strongest predictor of delivery before 34 weeks’ gestation. Of 121 women with known pre-pregnancy hypertension status, the incidence of delivery before 34 weeks was 32% (31/96) in women with confirmed chronic hypertension compared with 0% (0/25) in normotensive women. The risk of delivery before 34 weeks doubled in women with chronic hypertension from 20% [95% confidence interval (CI) 9–36%] to 40% (95% CI 26–56%) if the gestational fall in serum creatinine was <10% of pre-pregnancy concentrations. Women with a urinary protein:creatinine ratio >100 mg/mmol prior to pregnancy or before 20 weeks’ gestation had an increased risk for birthweight below the 10th centile (odds ratio 2.57, 95% CI 1.20–5.53). There was a measurable drop in estimated glomerular filtration rate (eGFR) between pre-pregnancy and post-partum values (4.5 mL/min/1.73 m2), which was greater than the annual decline in eGFR prior to pregnancy (1.8 mL/min/1.73 m2/year). The effect of pregnancy was, therefore, equivalent to 1.7, 2.1 and 4.9 years of pre-pregnancy renal disease in CKD Stages 3a, 3b and 4–5, respectively. The pregnancy-associated decline in renal function was greater in women with chronic hypertension and in those with a gestational fall in serum creatinine of <10% of pre-pregnancy concentrations. At 1 year post-partum, 46% (58/126) of women had lost ≥25% of their pre-pregnancy eGFR or required RRT. Most women with renal transplants had CKD Stage 3 and more stable renal function prior to pregnancy. Renal transplantation was not independently associated with adverse obstetric or renal outcomes.
Conclusions
Contemporary pregnancies in women with CKD Stages 3–5 are complicated by preterm delivery, low birthweight and loss of maternal renal function. Chronic hypertension, pre- or early pregnancy proteinuria and a gestational fall in serum creatinine of <10% of pre-pregnancy values are more important predictors of adverse obstetric and renal outcome than CKD Stages 3–5. Pregnancy in women with CKD Stages 3–5 advances the need for dialysis or transplantation by 2.5 years.
Keywords: chronic renal insufficiency, hypertension, pregnancy, proteinuria
Graphical Abstract
Graphical Abstract.
Key Learning Points
What is already known about this subject?
pre-pregnancy chronic kidney disease (CKD) Stages 3–5 are associated with an increased risk of adverse pregnancy outcomes in small and historical cohorts; and
the interplay between chronic hypertension, proteinuria and excretory renal function is unknown.
What this study adds?
chronic hypertension is the strongest predictor of delivery before 34 weeks’ gestation and pre- or early pregnancy proteinuria increases the risk of birthweight below the 10th centile;
the pregnancy-associated decline in renal function is greater in women with chronic hypertension and in those with a gestational fall in serum creatinine that is <10% of pre-pregnancy concentrations; and
the effect of pregnancy is equivalent to 1.7, 2.1 and 4.9 years of pre-pregnancy renal disease in CKD Stages 3a, 3b and 4–5, respectively.
What impact this may have on practice or policy?
this novel information can be used to inform pre-pregnancy counselling, antenatal surveillance and the postnatal management of women with CKD Stages 3–5, including appropriate preparation for transplantation and dialysis in the post-partum period.
INTRODUCTION
Chronic kidney disease (CKD) is estimated to complicate 3% of pregnancies, with 1 in 750 pregnancies in women with CKD Stages 3–5 [1]. CKD is a risk factor for adverse outcomes including preterm delivery, fetal growth restriction and a decline in maternal renal function [2–5]. However, the interplay between hypothesized predictors of adverse pregnancy outcomes including chronic hypertension [6, 7], proteinuria [3, 8] and advanced renal disease [3, 5] remains unclear, limiting the information available for contemporary risk counselling prior to pregnancy [1]. In view of the predicted rise in CKD prevalence in women of reproductive age as a consequence of population trends in obesity [9] and diabetes [10], the impact of CKD on pregnancy is increasingly relevant to contemporary practice.
Although all stages of CKD have been shown to increase the risk of adverse pregnancy outcomes compared with women without CKD [5], the incidence of adverse outcomes in women with moderate to severe CKD is derived from small [5, 11] and historical [2, 3, 12] cohorts and by non-systematic review of published data [4]. The largest published cohort, which included 82 pregnancies from 1971 to 1993, predates the use of estimated glomerular filtration rate (eGFR) and does not reflect contemporary standards of obstetric and renal care [2]. The impact of pregnancy upon maternal CKD remains unclear, with published data both suggesting [2, 12] and refuting [5, 11] an association between CKD stage and loss of maternal renal function. These studies are limited by small numbers and do not examine the trajectory of renal function decline prior to pregnancy. Thus, there is a need for larger, contemporaneous datasets in women with clinically significant CKD using pre-pregnancy staging of CKD for risk stratification and an analysis of renal function prior to as well as following pregnancy, to inform pre-pregnancy counselling and appropriate surveillance during pregnancy and in the post-partum period.
We report on neonatal and maternal outcomes of pregnancies in women with CKD Stages 3–5 (eGFR <60 mL/min/1.73 m2) who were not receiving dialysis at conception. The aims of this study were to investigate the impact of CKD Stages 3–5 on pregnancy, to delineate risk factors for adverse maternal and neonatal outcomes and to define the impact of pregnancy on maternal renal function by examination of renal disease trajectory before and after pregnancy in women with and without renal transplants.
MATERIALS AND METHODS
Study design
This retrospective cohort study included women from six specialist obstetric–nephrology centres in the UK to which women with CKD Stages 3–5 in those regions are referred in pregnancy (Supplementary data, Table S1). Women were included if they conceived a pregnancy between January 2003 and June 2017 and had an eGFR <60 mL/min/1.73 m2, calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [13] or a creatinine >125 µmol/L prior to 20 weeks’ gestation in the absence of a precipitant for kidney injury. Women on dialysis at the time of conception were excluded, as were those with spontaneous fetal loss prior to 20 weeks’ gestation. Conception date was calculated as 280 days before the estimated date of delivery by dating scan or from the last menstrual period if scan details were not available.
Demographic and clinical variables were documented including age, ethnicity, disease aetiology, proteinuria, renal replacement requirements (transplant and/or dialysis), and maternal and neonatal pregnancy outcomes. As obstetrics and renal medicine were not co-located at every site, reporting of the following data was incomplete and could not be included: immunosuppression dosing and concentrations, disease flare/recurrence, rejection episodes and donor characteristics.
Serum creatinine concentrations were recorded at up to nine time points: 12–24 months prior to conception, 6–12 months prior to conception, closest value prior to conception, early pregnancy, pre-delivery, first post-partum value, 3–6 months post-partum, 1 year post-partum and latest value post-partum. For women who had more than one baby during the study period, post-partum creatinine values were recorded up until the next calculated date of conception. All recorded creatinine concentrations were representative values selected by consultant nephrologists, with consideration of previous values where available and avoidance of extreme values. Creatinine concentrations were not recorded once renal replacement therapy (RRT) was commenced.
A diagnosis of chronic hypertension was assigned if there was a requirement for antihypertensive treatment prior to pregnancy, a diastolic blood pressure >85 mmHg before 16 weeks’ gestation [14] or a documented diagnosis in the hospital record. A diagnosis of chronic hypertension was not made if there was isolated use of renin–angiotensin blockade, unless confirmed by clinical information.
Birthweight was converted to a gestation-related optimal weight centile (Centile calculator, Version 8.0.3) [15]. For twin pregnancies, the larger birthweight was used in the summary outcome data.
A diagnosis of pre-eclampsia could not be reliably made due to inter-clinician variation in diagnosis and documentation in patients with renal disease and changing definitions over the study period.
Statistical analysis
Kruskal–Wallis and Chi-squared tests were used to compare demographic and outcome data across CKD stages as appropriate. Age, Black ethnicity (Black African or Black Carribbean) [16], chronic hypertension, pre-pregnancy CKD stage, proteinuria, renal transplantation, glomerulonephritis and a fall in gestational creatinine compared with pre-pregnancy values [17] were tested as predictors of adverse outcomes. A proteinuria threshold of protein:creatinine ratio (PCR) >100 mg/mmol pre-pregnancy, or in early pregnancy if no pre-pregnancy value was available, was used [3]. Adverse renal outcomes were defined at 1 year post-partum as either a 25% loss in eGFR compared with pre-pregnancy or progression to renal replacement.
Simple and multivariable logistic regression were used to determine demographic and clinical predictors of clinically important adverse obstetric outcomes, defined as gestational age <34 weeks [18] and birthweight <10th centile [19]. Risk estimates were generated using probabilities and exact binomial confidence intervals (CIs).
The trajectory of change in maternal renal function in relation to pregnancy was examined using regression modelling of pre- and post-partum CKD-EPI eGFR values, using a maximum likelihood model. Models included the overall trend in eGFR, the step loss in eGFR in relation to pregnancy and the trajectory of eGFR decline both pre- and post-partum. The optimum model was selected using information criterion comparison and linear regression. eGFR modelling was used to generate a measure of eGFR decline between pre- and post-pregnancy values according to pre-pregnancy CKD stage, converted to years of background disease based on the calculated pre-pregnancy trajectory of eGFR. To assess that this trajectory modelling was not distorted by single unconfirmed creatinine concentrations or by outlying data, the trajectory of renal decline was additionally examined in women with at least two creatinine measures both prior to, and following, pregnancy. Linear regression with case-wise deletion was used to examine the impact of clinical variables on the step change in eGFR decline between pre- and post-pregnancy values, adjusted for pre-pregnancy CKD stage.
Multifetal pregnancies were excluded from logistic and linear regression analyses and the regression modelling of maternal renal function. Statistical analyses were performed using GraphPad Prism 8 and Stata 15.1.
Ethics
The direct-care clinical team collated anonymized, retrospective data collected as part of usual patient care (Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust, Imperial Healthcare NHS Trust, King’s College Hospital NHS Foundation Trust and Nottingham University Hospitals NHS Trust). Imperial Healthcare NHS Trust and University Hospitals of Leicester NHS Trust collected data as part of registered clinical audits. Women who conceived after February 2014 at Guy’s and St Thomas’ NHS Foundation Trust, Imperial Healthcare NHS Trust and King’s College Hospital NHS Foundation Trust gave consent for access to their medical records, approved by the Research Ethics Service and the Health Research Authority (15/WA/0009).
RESULTS
There were 178 pregnancies in 159 women, including two twin pregnancies and 19 sibling infant pairs (Table 1). Median pre-pregnancy creatinine was 140 µmol/L [interquartile range (IQR) 123–167 µmol/L, range 104–457 µmol/L]. Pre-pregnancy CKD stage was 3a in 79 (47%) women, 3b in 63 (38%) women and 4–5 in 25 (15%) women. Pre-pregnancy CKD stage was unclassified for 11 women. In 10 women there was no pre-pregnancy value available but creatinine concentrations in pregnancy were >125 µmol/L (median 163 µmol/L, IQR 145–190). One pregnancy was included in a woman diagnosed with Stage 3a CKD prior to her first pregnancy, but without a serum creatinine measurement being done in the period between her pregnancies. The most common renal diagnoses were glomerulonephritis in 49 (28%) and reflux nephropathy in 47 (26%) pregnancies.
Table 1.
Maternal characteristics of pregnant women with CKD prior to conception
| CKD stage pre-pregnancy (CKD-EPI eGFR mL/min/1.73 m2) |
|||||
|---|---|---|---|---|---|
| Alla (<60) | CKD 3a (45–59) | CKD 3b (30–44) | CKD 4–5 (<30) | P-value across CKD stages | |
| n (%) | 178 | 79 (47) | 63 (38) | 25 (15) | |
| Age at conception, years | 32.8 (30.1–35.9) | 33.2 (30.7–36.4) | 33.1 (30.5–37.5) | 32.5 (27.4–35.3) | 0.2526 |
| Black ethnicity, n/N (%) | 31/176 (18) | 17/78 (22) | 5/62 (8) | 8/25 (32) | 0.8713 |
| Body mass index, kg/m2 | 26.2 (22.3–30.9) | 26.4 (22.4–32.0) | 24.9 (21.6–31.5) | 25.5 (22.6–27.9) | 0.2706 |
| Nulliparity, n/N (%) | 61/155 (39) | 31/75 (41) | 18/50 (36) | 8/21 (38) | 0.6913 |
| Chronic hypertension, n/N (%) | 96/121 (79) | 39/55 (71) | 33/38 (87) | 16/17 (94) | 0.0159 |
| Pre-pregnancy uPCRb, mg/mmol | 63 (18–215) | 29 (13–100) | 54 (19–172) | 188 (87–341) | 0.0006 |
| Functioning renal transplant, % | 43 (24) | 23 (29) | 16 (25) | 3 (12) | 0.1117 |
| Aetiology of CKD, % | |||||
| Glomerulonephritis | 49 (28) | 25 (32) | 16 (25) | 8 (32) | 0.0744 |
| Reflux nephropathy | 47 (26) | 19 (24) | 19 (30) | 8 (32) | 0.3512 |
| Diabetic nephropathy | 16 (9) | 5 (6) | 7 (11) | 2 (8) | 0.5537 |
| Congenital/rare cause | 14 (8) | 7 (9) | 3 (5) | 1 (4) | 0.2897 |
| Polycystic kidney disease | 8 (4) | 5 (6) | 2 (3) | 0 (0) | 0.1421 |
| Other | 18 (10) | 8 (10) | 4 (6) | 4 (16) | 0.6682 |
| Unknown | 26 (31) | 10 (13) | 12 (19) | 2 (8) | 0.9415 |
Values are median ± IQR unless specified. If information was not available, then a different denominator is shown.
Includes 11 pregnancies unclassified by pre-pregnancy eGFR: serum creatinine in pregnancy >125 µmol/L (n = 10) and known CKD Stage 3a prior to a previous pregnancy with no inter-partum measure (n = 1).
Pre-pregnancy (n = 102) or early pregnancy (n = 38).
There were no measurable differences in age, ethnicity, body mass index, nulliparity, renal disease aetiology or renal transplantation between women of different CKD stages. The presence or absence of chronic hypertension was confirmed in 121 (68%) of pregnancies. In the remainder of women, there was insufficient documentation, including whether angiotensin receptor blockade was for blood pressure control or nephroprotection in the context of proteinuria. The prevalence of chronic hypertension was lower in women with CKD Stage 3a compared with women with Stages 3b and 4–5.
Pre-pregnancy quantification of proteinuria was available in 102 (57%) women, with early pregnancy values available for an additional 38 women (median gestation 13.4 weeks, IQR 9.0–17.6 weeks). Pre-pregnancy proteinuria was significantly higher in women with Stages 4 and 5 CKD compared with Stage 3 CKD. Both pre-pregnancy and early (<20 weeks’ gestation) pregnancy quantification of proteinuria were available for 75 women. The median increase in proteinuria in pregnancy was 23% (IQR 26–97%). Documentation of pre-pregnancy use and the discontinuation of angiotensin blockade in relation to pregnancy were insufficient for analysis.
Obstetric outcomes
Live birth rate was 98% (174/178), with 56% (99/178) of babies born preterm (<37 weeks’ gestation) and 26% (47/178) delivering prior to 34 weeks’ gestation. Gestational age and birthweight were lower, and the incidence of small-for-gestational-age babies (<10th and <3rd centile) higher, with increasing stage of CKD. Overall, 35% (58/167) of babies required neonatal unit admission, with higher admission rates in the infants of women with CKD Stage 3b or more. More than half of women (57%, 98/172) had a caesarean delivery with no marked differences across CKD stages (Table 2).
Table 2.
Obstetric and renal outcomes of women with CKD
| CKD stage pre-pregnancy (CKD-EPI eGFR mL/min/1.73 m2) |
|||||
|---|---|---|---|---|---|
| Alla (<60) | CKD 3a (45–59) | CKD 3b (30–44) | CKD 4– 5 (<30) | P-value across CKD stages | |
| n | 178 | 79 | 63 | 25 | |
| Gestation at delivery, weeks | 36.3 (33.3–37.7) | 37.4 (34.7–38.1) | 35.9 (33.4–37.1) | 34.7 (32.4–36.2) | 0.0003 |
| Delivery <37 weeks (%) | 99 (56) | 32 (41) | 39 (62) | 22 (88) | <0.0001 |
| Delivery <34 weeks (%) | 47 (26) | 16 (20) | 16 (25) | 10 (40) | 0.0628 |
| Caesarean delivery (%) | 98/172 (57) | 40/78 (51) | 39/60 (65) | 14/24 (58) | 0.2566 |
| Birthweight, g | 2495 (1856–2952) | 2750 (2200–3120) | 2490 (1860–2912) | 1872 (1290–2474) | <0.0001 |
| Birthweight <10th centile (%) | 57/158 (36) | 17/72 (24) | 17/53 (32) | 14/22 (64) | 0.0013 |
| Birthweight <3rd centile (%) | 34/158 (22) | 9/72 (13) | 11/53 (21) | 9/22 (41) | 0.0049 |
| Neonatal unit admission (%) | 58/167 (35) | 18/75 (24) | 24/59 (41) | 11/23 (48) | 0.0139 |
| Fall in serum creatinine in pregnancy <10% of pre-pregnancy creatinine (%) | 86/162 (53) | 36/76 (47) | 33/62 (53) | 14/24 (58) | 0.0454 |
| 25% fall in eGFR or RRT at 1 year post-partum (%) | 58/126 (46) | 17/49 (34) | 17/48 (35) | 19/22 (86) | 0.0003 |
Values are median ± IQR unless specified. If information was not available, then a different denominator is shown.
Includes 11 pregnancies unclassified by pre-pregnancy eGFR: serum creatinine in pregnancy >125 µmol/L (n = 10) and known CKD Stage 3a prior to a previous pregnancy with no inter-partum measure (n = 1).
Chronic hypertension was the strongest predictor of delivery before 34 weeks, with 32% (31/96) of women with chronic hypertension delivering before 34 weeks’ gestation (Table 3). In contrast, there were no deliveries prior to 34 weeks in normotensive women (0/25). In women with chronic hypertension, the risk of delivery before 34 weeks doubled from 20% to 40% if the gestational fall in serum creatinine concentration was <10% of pre-pregnancy concentrations (Table 4). A urinary PCR (uPCR) >100 mg/mmol prior to pregnancy or before 20 weeks’ gestation increased the risk of a birthweight below 10th centile (odds ratio = 2.57; 95% CI 1.20–5.53, P = 0.016; Table 5). Chronic hypertension and pre-existing proteinuria were stronger determinants of the risk of adverse pregnancy outcomes than CKD Stages 3–5. Maternal age, Black ethnicity and glomerulonephritis were not independently associated with adverse obstetric outcomes (Tables 3 and 5).
Table 3.
Odds ratios of delivery prior to 34 weeks in women with CKD
| Variable | Unadjusted odds ratio in all women (n = 121)a (95% CI) | P-value | Unadjusted odds ratio in women with chronic hypertension (n = 96)a (95% CI) | P-value |
|---|---|---|---|---|
| Chronic hypertension | 16.45 (2.74–∞)b | <0.001 | ||
| Gestational fall in serum creatinine <10%c | 2.67 (1.00–7.09) | 0.049 | ||
| uPCR+ >100 mg/mmold | 2.22 (0.86–5.74) | 0.101 | ||
| CKD Stages 4 and 5e | 1.89 (0.62–5.78) | 0.267 | ||
| Maternal age | 0.97 (0.89–1.05) | 0.430 | ||
| Renal transplantation | 1.36 (0.52–3.59) | 0.529 | ||
| Black ethnicity | 0.80 (0.28–2.31) | 0.680 | ||
| CKD Stage 3be | 0.96 (0.36–2.61) | 0.943 | ||
| Glomerulonephritis | 1.00 (0.40–2.49) | 0.996 |
The presence (n = 96) or absence (n = 25) of chronic hypertension was confirmed in 121 women. The absence of chronic hypertension predicted delivery after 34 weeks (i.e. all women without chronic hypertension delivered after 34 weeks). In women with confirmed chronic hypertension, only one variable was significant; therefore, adjusted analysis was not undertaken.
Median unbiased estimate of odds ratio by exact logistic regression.
Compared with pre-pregnancy values.
Pre-pregnancy (n = 102) or early pregnancy (n = 38).
Pre-pregnancy CKD stage by CKD-EPI, compared with Stage 3a.
Table 4.
Probability of delivery prior to 34 weeks in women with CKD, according to presence or absence of chronic hypertension and a gestational fall in serum creatinine concentrations
| Chronic hypertension | Gestational fall in serum creatinine, % | Number of pregnancies (n = 110)a | Delivery <34 weeks’ gestation (%) (95% CI) |
|---|---|---|---|
| No | Any | 25 | 0 (0–14)b |
| Yes | >10 | 40 | 20 (9–36) |
| <10 | 45 | 40 (26–56) |
All pregnancies confirmed to be normotensive prior to pregnancy (n = 25) and pregnancies with confirmed chronic hypertension plus pre-pregnancy and gestational serum creatinine concentrations (n = 85).
One sided 97.5% CI.
Table 5.
Odds ratios of birthweight <10th centile in women with CKD
| Variable | Unadjusted odds ratio (95% CI) | P-value | Adjusted odds ratioa (95% CI) | P-value | Final modelb (95% CI) | P-value |
|---|---|---|---|---|---|---|
| uPCR+ >100 mg/mmolc | 3.00 (1.45–6.21) | 0.003 | 1.71 (0.70–4.17) | 0.238 | 2.57 (1.20–5.53) | 0.016 |
| CKD Stages 4 and 5d | 3.39 (1.35–8.52) | 0.009 | 4.03 (1.24–13.09) | 0.020 | 1.81 (0.63–5.24) | 0.271 |
| Gestational fall in serum creatinine <10%e | 2.18 (1.08–4.41) | 0.029 | 1.26 (0.54–2.94) | 0.599 | ||
| Black race | 1.88 (0.87–4.07) | 0.111 | ||||
| Chronic hypertension | 1.65 (0.63–4.35) | 0.309 | ||||
| Glomerulonephritis | 1.40 (0.71–2.79) | 0.335 | ||||
| Maternal age | 0.98 (0.91–1.04) | 0.450 | ||||
| Renal transplantation | 0.82 (0.39–1.72) | 0.604 | ||||
| CKD Stage 3 bd | 0.85 (0.42–1.74) | 0.662 |
Adjusted for all factors found to be significant in the unadjusted analysis.
Based on significance in the adjusted model.
Pre-pregnancy (n = 102) or early pregnancy (n = 38).
Pre-pregnancy CKD stage by CKD-EPI, compared with Stage 3a.
Compared with pre-pregnancy values.
Renal outcomes
In over half of women (86/162, 53%), the gestational fall in serum creatinine was <10% of pre-pregnancy serum creatinine concentration. RRT was initiated during pregnancy in five (3%) women, including one woman with CKD Stage 5 prior to conception (eGFR 13 mL/min/1.73 m2), three women with CKD Stage 4 (eGFR 21–27 mL/min/1.73 m2) and one woman with CKD Stage 3a (eGFR 48 mL/min/1.73 m2) complicated by heavy pre-pregnancy proteinuria (uPCR 519 mg/mmol) and refractory antenatal hypertension.
Post-partum serum creatinine concentrations beyond 4-week post-partum were available for 90% (160/178) of women. Median (IQR) follow-up was 23.5 months (13.5–26.0 months, range 43 days to 6 years). At 1 year post-partum, 46% (58/126) of women had lost 25% of their eGFR compared with pre-pregnancy values or required RRT. This composite outcome was more common in women with pre-pregnancy CKD Stages 4 and 5 compared with other stages.
RRT was initiated in 7% (9/134) of women within the first post-partum year. These women had a median pre-pregnancy eGFR of 24 mL/min/1.73 m2 (range 19–50 mL/min/1.73 m2). Median time to renal replacement was 39 weeks post-partum (range 3–51 weeks). Haemodialysis was commenced in three women, peritoneal dialysis in five women and one woman received a pre-emptive renal transplant in the first post-partum year. A further 19 women with a median pre-pregnancy eGFR of 41 mL/min/1.73 m2 (range 24–50 mL/min/1.73 m2) required renal replacement beyond the first year after delivery. Haemodialysis was commenced in 10 women, peritoneal dialysis was initiated in 4 women and 5 women received pre-emptive renal transplants at a median (IQR) of 3.0 (2.0–4.8) years post-partum.
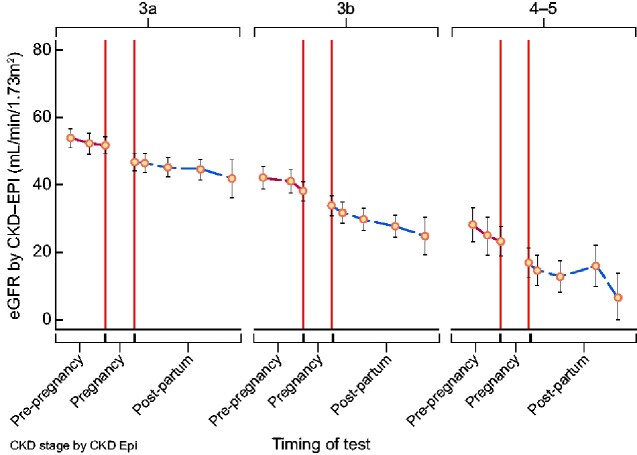
A total of 761 eGFR values were available from the 165 singleton pregnancies with pre-pregnancy eGFR data (mean 4.6 values per woman, range 1–7; Figure 1). The optimum statistical model of eGFR included the pre-pregnancy trend in eGFR and the step-decline between pre-pregnant and post-partum values, with adjustment for pre-pregnancy CKD stage (Supplementary data, Table S2). The inclusion of the post-partum eGFR did not improve the model, with no measurable difference between pre-pregnancy and post-partum eGFR trajectory (Supplementary data, Table S3).
FIGURE 1.
eGFR prior to and following pregnancy according to CKD stage: post-partum decline was not measurably different from pre-pregnancy decline and measurement of post-partum renal function did not improve a statistical model including pre-pregnancy data and the step in renal function between pre-pregnancy and post-partum values.
The step-decline between eGFR values taken before conception and post-partum was significant. Across the whole cohort, the mean decline in eGFR prior to pregnancy was 1.8 mL/min/1.73 m2/year. The step-decline in eGFR between pre-pregnancy and post-partum values was 4.5 mL/min/1.73 m2, therefore equivalent to 2.5 years of renal disease prior to pregnancy. This effect differed according to pre-pregnancy CKD stage with pregnancy calculated to be the equivalent of 1.7, 2.1 and 4.9 years of pre-pregnancy renal disease in CKD Stages 3a, 3b and 4–5, respectively (Table 6). An overall decline in renal function in relation to pregnancy was confirmed in women with at least two pre-pregnancy and two post-partum eGFR values, with 80% (86/108) of women demonstrating a step-decline in eGFR (Figure 2). Where women had more than one pregnancy, median step-decline in eGFR in the first pregnancy was 6 mL/min/1.73 m2.
Table 6.
Loss of eGFR between pre-pregnancy and post-pregnancy values according to CKD stage
| Pre-pregnancy CKD stage | Number of pregnancies | Number of eGFR values | Pre-pregnancy decline in eGFR mL/min/ 1.73 m2/year (95% CI) | eGFR loss in pregnancy mL/min/ 1.73 m2 (95% CI) | Years of pre-pregnancy disease equivalent to eGFR loss in pregnancy (95% CI) |
|---|---|---|---|---|---|
| All (3–5) | 165a | 761 | 1.8 (0.9–2.6) | 4.5 (2.0–7.1) |
|
| Transplants (3–5)b | 42 | 229 | 0.9 (0.7–2.4) | 3.7 (0.7–8.1) |
|
| Native CKD (3–5) | 123 | 532 | 2.1 (1.0–3.1) | 5.3 (2.2–8.3) | 2.5 (0.0–5.2) |
| 3a | 79 | 360 | 1.7 (0.3–3.0) | 2.9 (0.0–7.0) | 1.7 (0.0–5.5) |
| 3b | 61c | 283 | 2.2 (0.9–3.5) | 4.6 (0.7–8.5) | 2.1 (0.0–4.9) |
| 4–5 | 25 | 118 | 1.6 (0.0–3.3) | 7.9 (3.2–12.6) | 4.9d |
Multifetal pregnancies (n = 2) excluded.
CKD stage in women with transplants: 23 women with Stage 3a, 16 women with Stage 3b and 3 women with Stages 4–5.
Two twin pregnancies excluded.
No CI possible as the estimate for pre-pregnancy decline in women with CKD Stages 4 and 5 includes zero.
FIGURE 2.
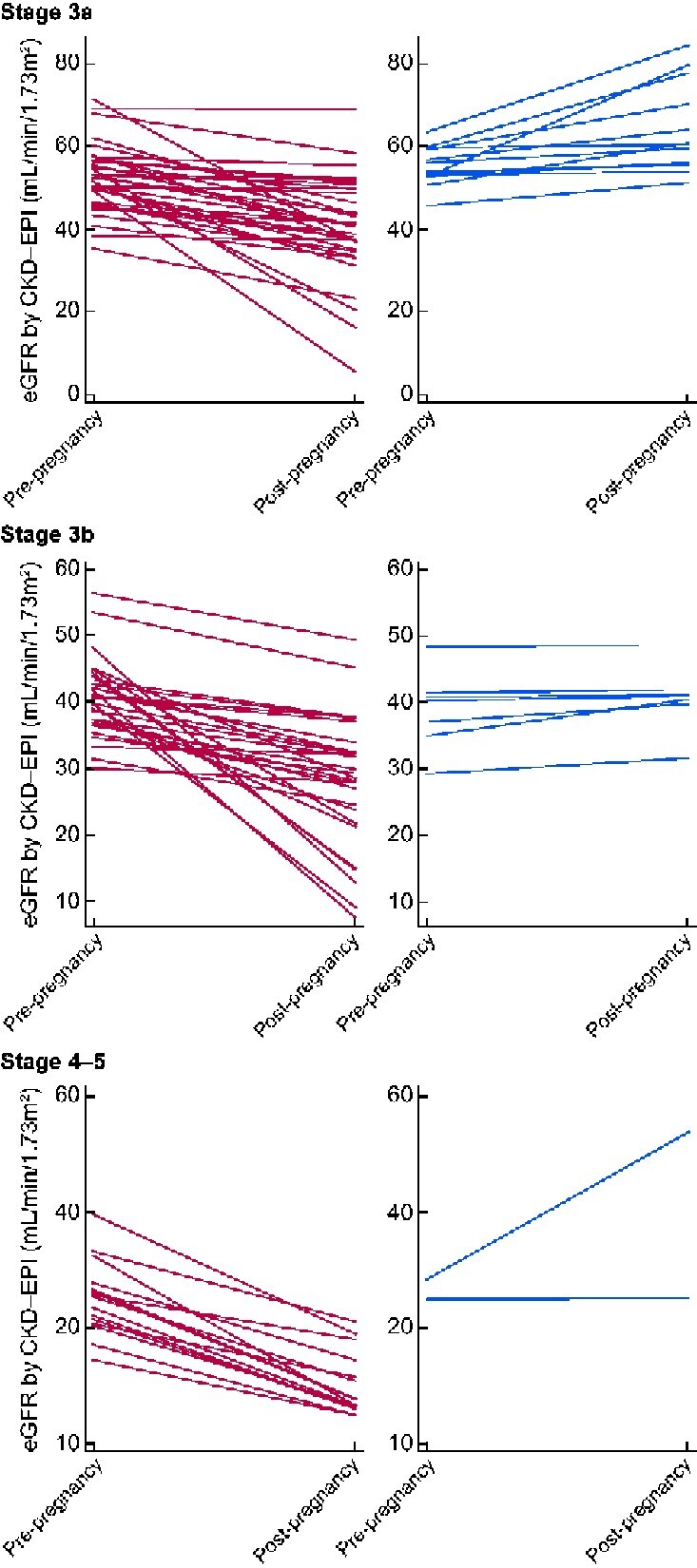
The trend in eGFR between pre-pregnancy and post-partum values according to CKD stage in women with at least two values prior to and two values after pregnancy. Stage 3a: fall in eGFR in 36 women (red) and stable eGFR in 13 women (blue). Stage 3b: fall in eGFR in 34 women (red) and stable eGFR in 7 women (blue). Stages 4 and 5: fall in eGFR in 16 women (red) and stable eGFR in 2 women (blue).
Examination of pre-pregnancy variables showed that chronic hypertension and uPCR of >100 mg/mmol were significant determinants of the step-decline between pre-pregnant and post-partum eGFR. Adjusted analysis including pre-pregnancy and post-pregnancy variables showed that chronic hypertension remained significant, though the measurable effect of proteinuria was lost when pregnancy outcomes were known. A gestational fall in serum creatinine <10% was a more significant predictor of renal function decline in pregnancy than delivery <34 weeks and birthweight less than the 10th centile. Maternal age, black ethnicity and a diagnosis of glomerulonephritis had no measurable effect on the step-decline in eGFR in relation to pregnancy (Table 7).
Table 7.
Predictors of the step-decline in eGFR in relation to pregnancy
| Variable | Single linear regression coefficient (95% CI) | P-value | Multiple linear regression of pre-pregnancy variables (95% CI) | P-value | Multiple linear regression of all variables (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Pre-pregnancy variables | ||||||
| Chronic hypertension | −8.31 (−11.89 to −4.73) | <0.001 | −6.63 (−10.53 to −2.72) | 0.001 | −5.67 (−9.52 to −1.82) | 0.004 |
| uPCR+ >100 mg/mmola | −8.72 (−11.27 to −6.19) | <0.001 | −6.78 (−10.16 to –3.40) | <0.001 | −2.43 (−5.64 to 0.77) | 0.138 |
| Renal transplantation | 3.72 (1.26 to 6.18) | 0.003 | −1.03 (−4.38 to 2.32) | 0.547 | ||
| Ethnicity: Black versus non-Black | −2.57 (−5.48 to 1.56) | 0.084 | ||||
| Glomerulonephritis | −1.26 (−3.78 to 1.26) | 0.328 | ||||
| Maternal age | 0.11 (−0.14 to 0.36) | 0.387 | ||||
| Post-pregnancy variables | ||||||
| Gestational creatinine fall <10%b | −8.04 (−10.52 to −5.56) | <0.001 | −5.57 (−8.61 to −2.53) | <0.001 | ||
| Preterm delivery <34 weeks | −8.04 (−10.52 to −5.56) | <0.001 | −1.27 (−5.21 to 2.66) | 0.527 | ||
| Birthweight <10th centile | −4.29 (−6.78 to −1.81) | 0.001 | −0.94 (−4.25 to 2.37) | 0.576 | ||
Step-decline is between eGFR pre-pregnancy and eGFR post-partum. Post-pregnancy variables are only known once the pregnancy has delivered. The coefficient is a measure of the difference in eGFR between pre-pregnancy and post-partum eGFR values that can be attributed to that variable.
Pre-pregnancy (n = 123) or early pregnancy (n = 50).
Compared with pre-pregnancy values.
Transplant outcomes
There were 43 (24%) women with a functioning renal transplant including 2 women with combined kidney–pancreas transplants. The median (IQR) time between transplantation and pregnancy was 6.0 years (4.3–8.5). Pre-pregnancy CKD stage was available for 42/43 women. Most women with renal transplants had CKD Stages 3a (23/42, 55%) and 3b (16/42, 38%), with few women with CKD Stages 4 and 5 (3/42, 7%). Median (IQR) pre-pregnancy uPCR was 19 mg/mmol (12–41 mg/mmol). The majority (24/30, 80%) had chronic hypertension. The fall in serum creatinine concentrations in pregnancy was <10% compared with pre-pregnancy values in just under half of women (20/42, 47%). Women with renal transplants had a smaller decline in eGFR (3.7 mL/min/1.73 m2) between pre- and post-pregnancy concentrations, compared with women without transplants (5.3 mL/min/1.73 m2). However, this loss was converted into a higher number of equivalent years of pre-pregnancy disease (4.3 versus 2.5 years), as women with renal transplants also had more stable renal function prior to pregnancy (decline of 0.9 mL/min/1.73 m2/year) compared with women with native CKD (decline of 2.1 mL/min/1.73 m2/year; Table 6). Of the women with a minimum of two eGFR values before and after pregnancy, 35% (12/34) showed stable kidney function between pre- and post-partum eGFR values, and 65% (22/34) showed a step-decline in renal function in relation to pregnancy. At 1 year post-partum, 22% (8/36) had lost ≥25% of their pre-pregnancy eGFR, including one woman with pre-pregnancy CKD Stage 4 who commenced haemodialysis within the first post-partum year. Renal transplantation was not independently associated with delivery prior to 34 weeks’ gestation (Table 3), birthweight <10th centile (Table 5) or a decline in kidney function in association with pregnancy (Table 7).
DISCUSSION
Women with CKD Stages 3–5 have a high live birth rate (98%) in pregnancies progressing beyond 20 weeks’ gestation, but pregnancies are complicated by preterm delivery (56%) and birthweight <10th centile (36%). Chronic hypertension was the strongest predictor of the risk of delivery before 34 weeks, with one-third of women with chronic hypertension delivering before 34 weeks in this cohort. The absence of a gestational fall in serum creatinine that was <10% of pre-pregnancy values was associated with a doubling of the risk of delivery before 34 weeks from 20% to 40% in women with chronic hypertension. Pre- or early pregnancy proteinuria approximating to >1 g/24 h was the strongest predictor of birthweight <10th centile. Chronic hypertension, pre- or early pregnancy proteinuria and the absence of gestational fall in serum creatinine were stronger predictors of adverse pregnancy outcome than CKD Stages 3–5. The trajectory of eGFR was not measurably different pre- and post-partum, but there was a step-decline in renal function in relation to pregnancy in 80% of women. This was equivalent to between 1.7 and 4.9 years of background renal disease depending on renal function prior to pregnancy and CKD stage. Overall, pregnancy was estimated to bring forward the need for RRT by 2.5 years. Predictors of the risk of a decline in eGFR in relation to pregnancy were chronic hypertension and a gestational fall in serum creatinine <10%.
The finding of a higher incidence of preterm delivery and low birthweight with increasing CKD stage confirms previous data [3–5]. However, CKD stage is confounded by an increased prevalence of both chronic hypertension and proteinuria, and this study is the first to demonstrate that chronic hypertension and pre- or early pregnancy proteinuria are stronger predictors of adverse obstetric outcome than CKD stage in women with CKD Stages 3–5. Overall rates of RRT in this cohort were low, with 3% of women commencing dialysis during pregnancy and 7% within the first post-partum year. However, renal replacement was not limited to women with CKD Stages 4 and 5. Although documentation of the clinical indicators for dialysis was insufficient to allow detailed analysis, hypertension and proteinuria were considered to be clinically important in those with pre-pregnancy CKD Stage 3 who progressed to dialysis during pregnancy or within the first year post-partum.
The novel conversion of renal function decline during pregnancy to 2.5 equivalent years of background renal disease is useful for both patients and clinicians in providing a tangible measure of the effect of pregnancy on renal function. The effect of pregnancy can be estimated to bring forward to need for renal replacement by 1.7, 2.1 and 4.9 years in Stages 3a, 3b and 4–5, respectively. Women with CKD Stages 4–5 and renal disease progression, which in the absence of pregnancy would be anticipated to lead to renal replacement within 5 years, should be advised that a decline in GFR is likely to precipitate the need for RRT either in pregnancy or within the immediate post-partum period.
Although the loss of eGFR in relation to pregnancy was less in women with renal transplants, this effect was confounded by the majority having CKD Stage 3a prior to pregnancy, and an apparent protective effect of renal transplantation on the decline in eGFR in relation to pregnancy was lost when data were corrected for chronic hypertension and proteinuria. Although there is a potential treatment paradox in women with renal transplants, with a lower threshold for iatrogenic preterm delivery when there is a gestational increase in serum creatinine concentration in transplants compared with native CKD, the finding that renal transplantation does not increase the risk of adverse obstetric outcomes is reassuring. The impact upon pregnancy outcomes of other factors specific to renal transplantation including episodes of rejection, presence of cytomegalovirus, immunosuppression dosing and concentrations, disease recurrence, transplant rejection and donor characteristics remain unknown, and prospective studies are warranted.
In women with normal renal function, physiological adaptation to pregnancy includes a 50% increase in creatinine clearance and a gestational fall in serum creatinine concentration [20, 21]. In the absence of published data, this has been used anecdotally as a poor prognostic indictor [17]. Our study is the first to confirm that a failure of serum creatinine concentration to fall in pregnancy by ≥10% compared with pre-pregnancy values is associated with both delivery prior to 34 weeks and a greater pregnancy-related decline in eGFR. The absence of a gestational fall in serum creatinine in pregnancy could represent impaired renal adaptation to pregnancy and/or disease progression. However, our data suggest that the gestational effect on renal function in women with CKD is more complex than a continuation of the disease course prior to pregnancy as the optimum statistical model of eGFR required both the non-pregnant trajectory in eGFR and the step-decline in eGFR between pre-pregnancy and post-partum values.
To our knowledge, this is the largest study of obstetric and renal outcomes in women with CKD Stages 3–5, surpassing recent cohorts that included 15 and 47 women [2, 5]. The largest previous study to date precedes CKD staging and included 82 pregnancies with serum creatinine concentrations >124 µmol/L, delivering between 23 and 47 years ago [2]. We therefore provide much needed contemporary data to inform pre-pregnancy counselling and the intra- and post-partum surveillance of women with moderate and severe pre-pregnancy CKD, stratified by pre-pregnancy CKD stage (CKD-EPI [22]).
A limitation of the study is the absence of pre-eclampsia coding as a modifier of both obstetric and renal outcomes. In the absence of defined diagnostic criteria (in any national or international guidance) for superimposed pre-eclampsia in women with chronic hypertension and/or pre-existing proteinuria, a reliable diagnosis of pre-eclampsia could not be determined by retrospective case-note review. Preterm delivery <34 weeks and birthweight <10th centile were used as important obstetric outcomes in this study. Although these outcomes may suggest the possibility of underlying pre-eclampsia, these surrogate markers are confounded by any pathology that impacts growth and delivery as well as differences in clinician threshold for iatrogenic preterm delivery. The potential relationship between acute kidney injury due to superimposed pre-eclampsia and loss of kidney function in relation to pregnancy could not, therefore, be determined by this study. Similarly, gestational diabetes and urinary tract infection were inconsistently coded during the study period. This study excluded spontaneous pregnancy loss prior to 20 weeks due to the possibility of miscoding error and information bias. The live birth rate of 98% therefore refers only to pregnancies that progress beyond 20 weeks’ gestation. Predictors of early pregnancy loss in women with CKD Stages 3–5 have not been identified and the effect of early pregnancy loss on long-term maternal renal function is unknown. Evidence for use of low-dose aspirin for pre-eclampsia prophylaxis coincided with the time period of this study, although use and adherence could not be confirmed in all women. There were insufficient data on the use of renin–angiotensin blockade, and whether there is benefit in the use of angiotensin-converting enzyme inhibitors in the pre- and post-partum period outside of standard indications in CKD remains unknown.
Future work includes the generation of a prediction model for women with CKD, comparable to those available to women with hypertensive disorders of pregnancy [23], to provide appropriate counselling and surveillance. Clinical indicators for the commencement of dialysis in pregnancy for the optimization of maternal and neonatal outcomes are also warranted. Research into the contribution of superimposed pre-eclampsia in mediating adverse pregnancy outcomes may be informative. Prospective studies, including predefined diagnostic criteria, in conjunction with emerging biomarkers of placental dysfunction [7], may offer valuable insight into pathophysiology of adverse outcomes to develop targeted interventions.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the National Institute for Health Research (NIHR) Rare Diseases Translational Research Collaboration as well as the Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London for funding K.W. under the terms of a doctoral research fellowship. This work was also supported by the NIHR Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
FUNDING
L.C.C. is supported by a Research Professorship from the NIHR (RP-2014-05-019). P.T.S. is partly funded by Tommy’s (Registered charity no. 1060508) and by Collaborations for Leadership in Applied Health Research and Care (CLAHRC) South London (NIHR).
AUTHORS’ CONTRIBUTIONS
L.L. conceived the study. P.W., K.W., K.B., M.H., K.B.-R., N.B., S.C., R.K., C.N.-P. and L.M.W. collected the data. K.W., P.T.S. and P.W. analysed the data. K.W. and P.W. wrote the manuscript with input from all authors. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author in accordance with the data sharing policies of King's College London and Imperial College London, with input from the investigator group where applicable, subject to submission of a suitable study protocol and analysis plan.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. K.B. has received consulting fees and lecture fees from Alexion. M.H. has received consulting fees and travel support from Vifor Fresenius and lecture fees from Merck, Sharp & Dohme (MSD) and Novartis. C.N.-P. has received consulting fees from Alliance Pharma and Union Chimique Belge (UCB) and lecture fees from Sanofi and UCB. L.L. has received lecture fees/honoraria from Alexion and Aurinia, consulting fees from Achillion, Astra-Zeneca, Aurinia, Glaxo Smith Kline (GSK), Pfizer and research grant funding from Roche.
REFERENCES
- 1. Piccoli GB, Zakharova E, Attini R. et al. Pregnancy in chronic kidney disease: need for higher awareness. A pragmatic review focused on what could be improved in the different CKD stages and phases. J Clin Med 2018; 7: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones DC, Hayslett JP.. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med 1996; 335: 226–232 [DOI] [PubMed] [Google Scholar]
- 3. Imbasciati E, Gregorini G, Cabiddu G. et al. Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis 2007; 49: 753–762 [DOI] [PubMed] [Google Scholar]
- 4. Williams D, Davison J.. Chronic kidney disease in pregnancy. BMJ 2008; 336: 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piccoli GB, Cabiddu G, Attini R. et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 2015; 26: 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bramham K, Parnell B, Nelson-Piercy C. et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014; 348: g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bramham K, Seed PT, Lightstone L. et al. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int 2016; 89: 874–885 [DOI] [PubMed] [Google Scholar]
- 8. De Castro I, Easterling TR, Bansal N. et al. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int 2017; 91: 1464–1472 [DOI] [PubMed] [Google Scholar]
- 9. MacLaughlin HL, Hall WL, Sanders TA. et al. Risk for chronic kidney disease increases with obesity: Health Survey for England 2010. Public Health Nutr 2015; 18: 3349–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu B, Bell K, Stanford A. et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns NHANES 2007-2012. BMJ Open Diabetes Res Care 2016; 4: e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Y, Liu J, Cai Q. et al. The pregnancy outcomes in patients with stage 3-4 chronic kidney disease and the effects of pregnancy in the long-term kidney function. J Nephrol 2018; 31: 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trevisan G, Ramos JG, Martins-Costa S. et al. Pregnancy in patients with chronic renal insufficiency at Hospital de Clínicas of Porto Alegre, Brazil. Ren Fail 2004; 26: 29–34 [DOI] [PubMed] [Google Scholar]
- 13. Levin A, Stevens PE.. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014; 85: 49–61 [DOI] [PubMed] [Google Scholar]
- 14. Macdonald-Wallis C, Silverwood RJ, Fraser A. et al. Gestational-age-specific reference ranges for blood pressure in pregnancy: findings from a prospective cohort. J Hypertens 2015; 33: 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perinatal institute. Gestation Network. Centile calculator 8.0.3, 2018. www.perinatal.org.uk/FetalGrowth/fetalgrowth.aspx (26 June 2019, date last accessed)
- 16. Small MJ, Allen TK, Brown HL.. Global disparities in maternal morbidity and mortality. Semin Perinatol 2017; 41: 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzpatrick A, Mohammadi F, Jesudason S.. Managing pregnancy in chronic kidney disease: improving outcomes for mother and baby. Int J Womens Health 2016; 8: 273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manuck TA, Rice MM, Bailit JL. et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016; 215: 103.e1–103.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendez-Figueroa H, Truong VT, Pedroza C. et al. Small-for-gestational-age infants among uncomplicated pregnancies at term: a secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. Am J Obstet Gynecol 2016; 215: 628.e1–628e7 [DOI] [PubMed] [Google Scholar]
- 19. Davison JM, Noble MCB.. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol 1981; 88: 10–17 [DOI] [PubMed] [Google Scholar]
- 20. Chapman AB, Abraham WT, Zamudio S. et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998; 54: 2056–2063 [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence. Chronic Kidney Disease in Adults: Assessment and Management CG182. 2015. https://www.nice.org.uk/guidance/cg182 (26 July 2019, date last accessed) [PubMed]
- 23. Ukah UV, Payne B, Karjalainen H. et al. Temporal and external validation of the fullPIERS model for the prediction of adverse maternal outcomes in women with pre-eclampsia. Pregnancy Hypertens 2019; 15: 42–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author in accordance with the data sharing policies of King's College London and Imperial College London, with input from the investigator group where applicable, subject to submission of a suitable study protocol and analysis plan.