Abstract
Iron deficiency (ID) is highly prevalent in kidney transplant recipients (KTRs) and has been independently associated with an excess mortality risk in this population. Several causes lead to ID in KTRs, including inflammation, medication and an increased iron need after transplantation. Although many studies in other populations indicate a pivotal role for iron as a regulator of the immune system, little is known about the impact of ID on the immune system in KTRs. Moreover, clinical trials in patients with chronic kidney disease or heart failure have shown that correction of ID, with or without anaemia, improves exercise capacity and quality of life, and may improve survival. ID could therefore be a modifiable risk factor to improve graft and patient outcomes in KTRs; prospective studies are warranted to substantiate this hypothesis.
Keywords: fibroblast growth factor-23, heart failure, immunity, iron, kidney transplantation
INTRODUCTION
Iron deficiency anaemia (IDA) affects approximately one billion individuals globally and has a particularly high prevalence among patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1], including kidney transplant recipients (KTRs) [2]. The presence of iron deficiency (ID) after kidney transplantation is strongly associated with an increased mortality risk [2, 3]. Interestingly, this association is independent of co-existing anaemia, suggesting a specific pathogenic role for ID in kidney transplantation [2]. Although the potential mechanisms driving the association between ID and mortality have not been fully elucidated, ID has been implicated in both immunological and non-immunological pathological processes. In this review, we will discuss the definition, prevalence and clinical impact of ID after kidney transplantation, address potential underlying pathophysiological pathways and propose areas for future study.
ID IN KTRs—DEFINITIONS, EPIDEMIOLOGY AND AETIOLOGY
Definition and prevalence of ID
Although an iron staining of bone marrow is the gold standard method to assess iron status, a serum ferritin level of <30 μg/L is a widely accepted alternative definition of ID [4]. However, because ferritin is an acute-phase protein, its concentration is increased in most chronic diseases as a result of inflammation, possibly masking co-existing ID. Therefore, transferrin saturation (TSAT) is more reliable in the context of chronic disease [4]. Most studies in patients with low-grade inflammation, including KTRs, use ID definitions based on the combination of ferritin concentration and TSAT [2, 5–8]. The prevalence of ID after kidney transplantation varies depending on the definition used and the time after kidney transplantation. In a cohort of 700 stable KTRs who were at least 1 year after transplantation [median time: 5.4 years, interquartile range (IQR) = 1.9–12.0 years], the prevalence of ID defined as a ferritin concentration <300 μg/L and TSAT <20% was 30% [2]. Other cohort studies, all with a median time after transplantation of at least 4 years, found prevalences between 6% and 47% [9–13].
A longitudinal study suggested that patients with pre-transplant ID remained iron-deficient after transplantation, and ferritin levels tended to decrease in the first months after transplantation. Other studies support the observation that ferritin levels and TSAT tend to decrease after transplantation, as haemoglobin (Hb) rises [8, 14, 15]. The reduction in ferritin levels after transplantation is more prominent when ferritin levels are initially high [13, 16]. This observation suggests that the decrease in ferritin levels is not purely resulting from progressive ID but from an abatement of inflammation as well.
Potential mechanisms of ID in KTRs
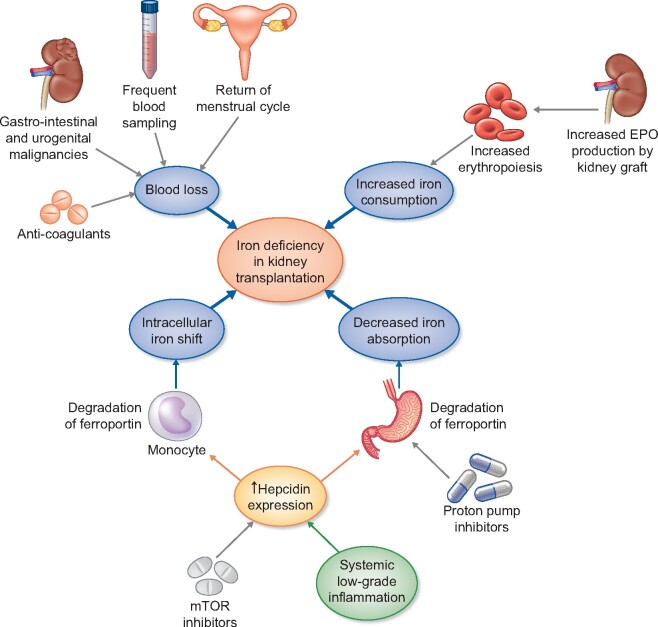
The aetiology of ID after kidney transplantation is multifactorial, as depicted in Figure 1.
FIGURE 1.
Causes of ID in KTRs. In KTRs, low-grade inflammation and mTOR inhibitors promote hepcidin upregulation. Hepcidin suppresses iron uptake from the gut by inhibiting iron exporter ferroportin on enterocytes. Hepcidin also reduces available iron by inhibiting iron export from monocytes. Meanwhile, iron usage/consumption is increased in KTRs: renewed EPO production promotes erythropoiesis. Usage of anticoagulant medication, frequent blood sampling and in some cases gastro-intestinal and urogenital malignancies result in blood loss. Female KTRs of reproductive age often have a return of their menstrual cycle, another cause of blood loss. Finally, PPIs decrease dietary iron uptake.
Inflammation
Inflammation induces hepcidin expression in the liver through cytokines including interleukin (IL)-6 and bone morphogenetic protein (BMP) [17]. In particular, BMP6, a modulator of the renal response to injury, is a major hepcidin-inducing factor through stimulation of hepatocellular Suppressor against Mothers Against Decapentaplegic (SMAD) production [4]. Hepcidin subsequently degrades the iron-exporter ferroportin in enterocytes, leading to a decreased absorption of dietary non-haem iron from the duodenum [18]. Hepcidin also decreases the bioavailability of iron by augmenting its storage in macrophages through systemic degradation of ferroportin. The absorption and handling of iron are comprehensively described elsewhere [4]. Although hepcidin is positively correlated with acute-phase protein ferritin, its correlation with TSAT is inverse in line with the presumed role of inflammation driving ID in these patients [19–21].
Medication
Medication, including anticoagulants, proton pump inhibitors (PPIs) and immunosuppressive drugs, form another major factor influencing iron status in KTRs. Anticoagulant use frequently causes chronic (microscopic) gastro-intestinal blood loss, resulting in ID. The use of PPIs has also been associated with an increased risk of ID in several populations, including KTRs [22, 23]. Mechanistically, it has been suggested that PPIs reduce iron absorption by increasing the gastric pH, thereby inhibiting the reduction of ferric iron [Fe(III)] to ferrous iron [Fe(II)], in turn precluding absorption by enterocytes. The effects of immunosuppressive medication on iron status are not fully understood. Mammalian target of rapamycin inhibitors (mTORis) seem to promote ID. In mice, the mTORi sirolimus and the calcineurin inhibitor (CNI) tacrolimus stimulated hepcidin expression [24]. In humans, mTORi use has been associated with both anaemia and functional ID [25]. Prospective studies showed that a switch from a ciclosporin- to a sirolimus-based immunosuppressive regimen led to a decline in TSAT, while in patients with a ciclosporin dose reduction in TSAT remained stable [26]. In a study where KTRs were switched from a CNI and/or mycophenolic acid (MPA)-based regimen to an everolimus-based immunosuppressive regimen, TSAT also decreased significantly [27].
Malignancies
KTRs are at increased risk of gastro-intestinal cancers, such as colon carcinoma or intestinal post-transplant lymphoproliferative disorder, which may manifest as ID [28]. Thus, each patient with ID should be verified for the presence of alarm symptoms such as weight loss or rectal blood loss. Also, deep ID accompanied by low mean corpuscular volume or co-existing anaemia should trigger gastro-intestinal work-up. The isolated presence of ID without alarm symptoms, microcytosis or anaemia, which occurs in a considerable group of patients, seems insufficient to justify gastro-intestinal screening [29]. Urinary tract malignancies such as renal cell carcinoma have a much higher prevalence in KTRs as well, and may induce ID through erythrocyturia [28].
Other factors
Blood loss during transplant surgery and frequent blood sampling after transplantation may contribute to ID, especially in the early post-transplant phase [30]. Return of the menstruation cycle after successful transplantation could be another contributor to progressive ID [31]. Finally, the increase of serum erythropoietin (EPO) concentrations after kidney transplantation may cause a relative shortage of iron. Use of EPO-stimulating agents before kidney transplantation is associated with a less pronounced ferritin decrease after transplantation [14].
ID IN KTRs—DEFINITIONS, EPIDEMIOLOGY AND AETIOLOGY
In KTRs, ID has been strongly and independently associated with a higher mortality risk in two studies of KTRs with relatively good graft function [estimated glomerular filtration rate (eGFR) 52 ± 20 mL/min and 53 ± 19 mL/min, respectively; Table 1] [2, 3]. Some but not all studies suggest that iron status may also influence kidney damage and graft outcomes [3, 34]. Recently, studies in non-transplant populations suggested that peri-operative ID is an important prognostic factor, and that it might be beneficial to correct non-anaemic ID prior to surgery [36–39]. Whether this also applies to KTRs has not been studied so far. Although the aetiologies that may underlie the observed adverse outcomes have not been elucidated, several mechanisms could be involved.
Table 1.
Overview of studies addressing the relationship of ID and supplementation with clinical outcomes in KTRs
| References | PMID | N | Design | Primary findings |
|---|---|---|---|---|
| Cardiovascular disease/all-cause mortality | ||||
| Eisenga et al. [2] | 27516242 | 700 | Cohort study | Independent association of ID with all-cause mortality (fully adjusted HR = 1.77, 95% CI 1.13–2.78; P = 0.01) Higher NTproBNP concentrations in patients with non-anaemic ID [350 (IQR = 127–1069) pg/mL] than in patients without ID [159 (IQR = 72–393) pg/mL] |
| Winkelmayer et al. [3] | 15575912 | 438 | Cohort study | Independent association of %HRBC, an indicator of iron status and metabolic iron utilization, >10% with all-cause mortality (fully adjusted HR = 1.20, 95% CI 1.12–3.79; P = 0.02). |
| Infectious diseases | ||||
| Mudge et al. [32] | 22290270 | 102 | RCT | Single-dose IV iron polymaltose versus daily oral ferrous sulphate. No difference in infection risk (20% in IV arm versus 24% in oral arm; P = 0.62) |
| Fernandez-Ruiz et al. [33] | 24011120 | 228 | Cohort study | Post-transplant ferritin >500 μg/L associated with any infection (P = 0.006) or bacterial infection (P = 0.02) during the first year No association between TSAT and infection risk during the first year |
| Vaugier et al. [34] | 28784700 | 169 | Cohort study | No difference in BK virus infection between high- (>600 μg/L) and low (<600 μg/L) ferritin groups (10% versus 15%, respectively, in the high quartile; Chi-squared test; P = 0.40) |
| Fernández-Ruiz et al. [35] | 29120522 | 91 | Cohort study | Independent association of high hepcidin-25 (≥72.5 ng/mL) with overall (HR = 3.86, 95% CI 1.49–9.96; P = 0.005) and opportunistic infection (HR = 4.32, 95% CI 1.18–15.75; P = 0.027). |
Cardiac effects of ID
Given the associations of ID with all-cause mortality in KTRs (Table 1), and since cardiovascular disease is the most common cause of death in KTRs, it seems plausible that ID has adverse effects on the cardiovascular system in KTRs, as shown in other populations. No studies have so far directly assessed the association between ID and fatal or non-fatal cardiovascular outcomes in KTRs. However, it has been shown that ferritin and EPO are inversely correlated, possibly because ID promotes resistance to endogenous EPO, and that a higher EPO level is associated with a higher risk of both cardiovascular and all-cause mortality in KTRs [40]. Moreover, ID might contribute to the development of heart failure (HF), a major cause of morbidity and mortality in KTRs [41]. Although systolic heart function usually improves after transplantation, diastolic dysfunction (HF with preserved ejection fraction) tends to remain [42]. There is also an elevated incidence of incident HF in KTRs [43], which is strongly associated with anaemia both in KTRs and in the general population [43, 44]. To our knowledge, it is unknown whether ID is associated with incident HF in KTRs, although it has been described that N-terminal prohormone of brain natriuretic peptide (NTproBNP) levels are much higher in KTRs with ID compared with iron-sufficient KTRs (Table 1) [2].
Bound to Hb and myoglobin, respectively, iron has a pivotal role in oxygen transport through the body and oxygen storage in myocytes. Iron is also directly involved in various steps of cellular energy metabolism. It is an essential component of aconitase and succinate dehydrogenase, catalyst enzymes of the Krebs cycle [4]. In ID, decreased intracellular oxygen availability and impaired function of the Krebs cycle force the cell towards anaerobic glycolysis. Since muscle tissue is highly dependent on aerobic glucose metabolism, it is likely that ID compromises cardiac and skeletal muscle cell function. In vitro, ID impairs mitochondrial respiration and cardiomyocyte contractility [45, 46]. In animal models, a low-iron diet caused structural cardiac defects, cardiomyocyte hypertrophy and reduced left ventricular ejection fraction (LVEF) [47, 48].
Multiple studies have reported strong associations between ID and decreased exercise tolerance in patients with chronic heart failure (CHF) with either reduced or preserved left LVEF, which occur independently of Hb concentrations [49, 50].
Since 2007, six randomized controlled trials (RCTs) have addressed the effects of intravenous (IV) iron supplementation in iron-deficient patients with CHF; most of them also had mildly to moderately impaired kidney function (Table 2). IV iron supplementation resulted in an improved quality of life and exercise capacity and reduced the incidence of acute HF compared with placebo or standard treatment. Interestingly, ID correction also had significant effects in non-anaemic patients in most trials. In a meta-analysis of four RCTs, IV administration of ferric(III)carboxymaltose (FCM) significantly reduced cardiovascular mortality [51]. Evaluation of iron status and correction of ID are now integrated with the management of CHF patients according to guidelines of the European Society of Cardiology [52]. Meanwhile, several large trials in acute and chronic HF are ongoing to clarify the effects of ID correction on clinical outcomes [53]. Given the high prevalence and impact of HF in KTRs, the role of ID and the therapeutic value of iron supplementation in this population should be elucidated.
Table 2.
Overview of RCT addressing the effect of IV iron on clinical outcomes in patients with chronic HF and ID
| Study | PMID | Year | Criteria for anaemia/iron status | Interventions (N) | Mean kidney function at baseline | Follow-up (weeks) | Outcome (intervention versus control) |
|---|---|---|---|---|---|---|---|
| Toblli et al. | 17950147 | 2007 | Hb <12.5 g/dL; TSAT <20%; Ferritin <100 μg/L | IV ISC (n = 20) IV saline (n = 20) | CrCl 39.8 ± 10.1 (ISC) CrCl 37.7 ± 10.2 mL/min (placebo) | 24 | NTproBNP↓ (P < 0.01) LVEF↑ (P < 0.01) HRQoL↑ (P < 0.01) 6 MWT↑ (P < 0.01) |
| FERRIC-HF | 18191732 | 2008 | Ferritin <100 μg/L or 100–300 μg/L with TSAT <20% | IV ISC (n = 24) Standard care (n = 11) | sCr 109 ± 42 µmol/L (ISC) sCr 104 ± 39 µmol/L (standard care) | 18 | pVO2↑ (NS, P = 0.08) NYHA↓ (P = 0.007) GAF↑ (P = 0.002) HRQoL (NS, P = 0.07) |
| FAIR-HF (+ post hoc studies) | 19920054 22297124 25683972 | 2009, 2015 | Ferritin <100 μg/L or 100–300 μg/L with TSAT <20% | IV FCM (n = 304) IV saline (n = 155) | eGFR 63.8 ± 21.2 mL/min (FCM) eGFR 64.8 ± 25.3 mL/min (placebo) | 26 | GAF↑ (P < 0.001) NYHA↓ (P < 0.001) 6 MWT↑ HRQoL↑ (P < 0.001) eGFR↑ (P = 0.04) |
| IRON-HF | 23680589 | 2013 | Hb 9–12 g/dL; ferritin <500 μg/L; TSAT <20% | IV ISC (n = 10) IV saline (n = 6) Oral FS (n = 7) | sCr 97 ± 27 µmol/L (total cohort) | 12 | pVO2 (NS) NYHA (NS) eGFR (NS) |
| CONFIRM-HF | 25176939 | 2015 | Hb <15 g/dL; Ferritin <100 μg/L or 100–300 μg/L with TSAT<20% | IV FCM (n = 150) IV saline (n = 151) | eGFR 66.4 ± 21.7 mL/min (FCM) eGFR 63.5 ± 20.9 mL/min (placebo) | 52 | 6 MWT↑ (P = 0.002) NYHA↓ (P < 0.001) GAF↑ (P = 0.001) HRQoL↑ (P < 0.05) Acute HF↓ (P = 0.009) |
| EFFECT-HF | 28701470 | 2017 | Hb <15 g/dL; Ferritin <100 μg/L or 100–300 μg/L with TSAT <20% | IV FCM (n = 86) Standard care (n = 86) | eGFR 52 ± 13 mL/min (FCM) eGFR 51 ± 12 mL/min (placebo) | 24 | pVO2↑ (P = 0.02)a GAF↑ (P < 0.05) NYHA↓ (P < 0.05) NTproBNP↓ (NS) |
Only significant after imputation.
FS: ferrous sulphate; VO2: peak VO2; HRQoL: health-related quality of life; NYHA: New York Heart Association Class; NTproBNP: N-terminal prohormone of brain natriuretic peptide; 6 MWT: six-minute walk test; GAF: Global Assessment of Functioning; NS: non-significant; CrCl: creatinine clearance; sCr: serum creatinine.
ID, fibroblast growth factor 23 and mortality risk
Emerging data, both in the general population and in KTRs, show that ID is associated with elevated fibroblast growth factor 23 (FGF23) levels and suggest that the association between ID and increased mortality in KTRs is at least partly mediated by FGF23 [54, 55].
FGF23 is a phosphaturic hormone secreted by osteocytes. FGF23 reduces phosphate reabsorption from the proximal tubule of the kidney and suppresses 1,25-dihydroxyvitamin D levels [56]. In CKD, FGF23 increases progressively and there may be a 1000-fold increase in ESRD. After kidney transplantation, FGF23 levels decrease but often remain elevated during the first weeks to months, and sometimes even years after transplantation, contributing to a tendency to hypophosphataemia [57–59].
FGF23 has been independently associated with an increased risk of cardiovascular and all-cause mortality and allograft loss in KTRs [60, 61]. It is likely that off-target effects of high FGF23 levels underlie these associations, as several animal studies have shown that intact FGF23 causes left ventricular hypertrophy [62]. Further mechanisms by which FGF23 may lead to adverse outcomes include over-stimulation of the renin–angiotensin–aldosterone system, volume overload via effects on renal sodium handling [63–65] and promotion of inflammation [66]. Although studies report inconsistent effects of FGF23 on vascular calcification in other populations, FGF23 was an independent predictor of vascular stiffness in KTRs [67].
More studies are needed to elucidate the role of FGF23 as intermediate between ID and adverse outcomes, particularly in the KTR population.
Iron and infection
Bacteria need iron to thrive, and compete to acquire it [68]. Some pathogenic bacteria, including Enterobacteria, Pseudomonas and Neisseria species, have adapted to iron scarcity and can express siderophores, compounds with a high affinity for iron, to obtain iron from the environment [68, 69]. At the same time, ID may directly affect the immune system, as discussed in more detail below [70]. In KTRs, this is of particular relevance because in these patients the balance between suppression of the allo-immune response and the risk of infection resulting from immunosuppressive therapy is narrow. An overview of studies addressing the association between ID and infection or the effect of iron therapy on incidence of infections in KTRs is provided in Table 1.
Clinical studies confirm that ID can protect against bacterial and parasitic infections [71], and that iron overload is associated with worse prognosis in patients suffering from bacteraemia, sepsis, tuberculosis and Human Immunodeficiency Virus (HIV) [72–74]. In KTRs, a ferritin concentration of >500 μg/L in the first weeks after transplantation has been associated with a higher risk of infection (26% versus 41%) [33]. In the same study, TSAT was not associated with the risk of infection, which suggests that inflammation rather than ID may have been the driving factor for higher ferritin levels [35].
In contrast, other studies suggest that ID can increase susceptibility to bacterial infection. In a general population cohort of 61 852 people, a lower TSAT was associated with a higher risk of bacteraemia, even after correction for chronic diseases [75]. Less is known about the effect of ID on viruses. Cytomegalovirus (CMV) replication in vascular endothelial cells is reduced after iron chelation in vitro, which may be relevant to KTRs as primo CMV infection and CMV reactivation are common in these patients [76].
IRON AND ALLOGRAFT OUTCOMES
Patient data on iron status in relation to kidney allograft outcomes are scarce. A retrospective cohort study in 169 KTRs showed that a higher ferritin concentration was associated with better graft function and graft survival [34]. In contrast, a cohort study in 438 KTRs found no association between the percentage of hypochromic red blood cells (HRBCs) and graft failure, although there was a trend towards greater graft survival among KTR who received iron therapy at baseline [hazard ratio (HR) = 0.51, 95% confidence interval (CI) 0.24–1.09; P = 0.08] [3]. In a mouse heart transplant model, ID decreased allograft survival due to more severe rejection [77]. In contrast, a prolonged pancreatic islet or heart allograft survival was observed in rodents following either anti-transferrin receptor (TfR) antibody treatment or iron chelation therapy [78–80]. While clinical data on the effect of ID on kidney allograft outcomes are limited, more is known on the impact of iron (deficiency) on the immune system in general.
IRON AND THE IMMUNE SYSTEM
Cellular immunity
Acute cellular rejection, mainly orchestrated by T-lymphocytes, is one of the major threats for kidney allograft survival. Although data on the role of iron in kidney transplantation specifically are scarce, iron seems to play an important role in immune cell function. T-cell activation leads to increased cytokine production and IL-2 receptor stimulation; both processes depend on iron [70, 81–83]. The T-cell receptor is co-expressed with both CD28 and the TfR [70], a transmembrane protein that facilitates the uptake of transferrin-bound iron from the circulation into the T cell. In addition to reducing TfR stimulation, ID also decreased the expression of the co-stimulatory molecule CD28 on thymocytes and splenocytes in mice [84].
ID affects T-cell proliferation as well, since iron is an essential cofactor in various steps in DNA synthesis [82, 85]. Both TfR upregulation and iron abundance have been associated with increased cell cycle progression, while ID decreased lymphocyte proliferation in mice and humans [86–89]. T-cell differentiation and maturation also require iron [89–91]. Decreased T-lymphocyte counts, CD4+ concentrations and CD4+/CD8+ ratios have been observed in some, but not all studies in iron-deficient patients [89, 90, 92–94].
ID may impair T-cell function through decreased production of IL-2, interferon-γ, tumour necrosis factor (TNF)-α, IL-10, IL-6 or IL-4, as observed in the majority of studies in mice and humans with ID [83, 88, 92, 95, 96]. In the context of acute vascular rejection, ID may also affect the influx of T cells in the endothelium by influencing the expression of endothelial adhesion molecules such as endothelial–leucocyte adhesion molecule-1 and intercellular adhesion molecule-1 [76].
Overall, most studies seem to indicate that iron is important for T-cell proliferation and function. This underlines the relevance of future studies addressing the clinical impact of ID and iron supplementation on cellular immunity in kidney transplantation.
Humoral immunity
T-helper cells may activate B-lymphocytes, triggering the production of immunoglobulins against Humane Leukocyte Antigen (HLA) molecules, endothelial cell antigens and ABO blood group antigens that may in turn activate the complement system and drive antibody-mediated rejection [97]. Until recently, there was little evidence of any impact of ID on B-lymphocytes [98, 99]. Yet, a very recent study revealed an important role for iron in T-cell independent B-cell activation and in B-cell proliferation, and documented impaired antibody responses during ID in mice and humans [100]. Although previous studies showed conflicting data on the association between iron status and immunoglobin concentrations, the recent work suggests that ID influences not only T-cell- but also B-cell-mediated immunity [100].
Innate immunity
The innate immune system can escalate organ graft rejection through activation of T-lymphocytes and by acting directly on the kidney transplant. Activated by foreign proteins through Toll-like receptors, macrophages promote rejection [97]. Macrophages have an important role in iron storage and recycling as well [4]. However, iron-overload in macrophages attenuates their anti-pathogenic and pro-inflammatory functions [34, 72]. Importantly, macrophage function also depends on iron and iron-containing haemoproteins [81, 101, 102]. Iron is involved in macrophage activation and differentiation, as well as prostaglandin synthesis and killing capacity [101]. Finally, ID decreases the expression of Major Histocompatibility Complex (MHC) Class I molecules and thereby may enhance recognition and activation of Natural Killer (NK) cells by macrophages [103]. Hence, alterations in iron metabolism may affect all these facets of macrophage biology. This is supported by the observation that monocyte and macrophage phagocytic capacity and oxidative burst activity, or release of reactive oxygen species after activation, is impaired in children with IDA [92]. Iron-depleted macrophages had a reduced expression of IL-1β and TNF-α in response to a pro-inflammatory stimulus [102]. After induction of toxic nephritis, characterized by macrophage infiltration, iron-deficient rats showed less proteinuria and better kidney function [102].
Ischaemia and reperfusion during kidney transplantation lead to a sterile inflammatory response driving renal fibrosis: ischaemia–reperfusion injury (IRI). Granulocytes and neutrophils in particular are involved in IRI but also attract T-lymphocytes, promoting cellular rejection [104]. In granulocytes, IDA impairs the oxidative burst and pathogen killing capacity [89, 92]. Together, these findings point towards an important role for iron in innate immunity, and suggest that ID could impair the inflammatory response.
IRI and iron homoeostasis are closely linked. In a mouse model, renal IRI results in an iron shift from the liver and macrophages towards the kidneys and circulation, through the induction of the iron exporter ferroportin [105]. Hepcidin treatment, decreasing iron availability, reduced IRI, oxidative stress, renal epithelial cell apoptosis, acute tubular necrosis, neutrophil infiltration and inflammation, and improved renal function [105]. These results suggest that low iron concentrations may protect against IRI. This is supported by the observation that iron chelation during organ preservation reduces IRI in several animal models of heart, kidney or liver allograft transplantation [106–108].
In contrast, a protective effect of high iron concentrations has been proposed by others [34, 109]. Increased intra-renal iron concentrations in ferroportin knock-out mice provided protection against IRI [109]. Vaugier et al. also found a protective effect of iron against IRI [34]. Mice with iron overload (hfe−/−) were less susceptible to IRI compared with wild-type mice. This protective effect of iron was attributed to a decreased recruitment of inflammatory macrophages, together with impaired macrophage responsiveness to stimulation by Toll-like receptor agonists and increased activation of the antioxidant response [34].
In conclusion, iron is pivotal for the proliferation, activation and function of T- and B-lymphocytes and macrophages. In the context of organ preservation before transplantation, ID and iron overload both appear to reduce IRI. How these observations ultimately impact clinical outcomes after kidney transplantation remain unclear, since only observational data on clinical outcomes are available.
IRON SUPPLEMENTATION IN KTRs
ID can be treated with either oral or IV iron preparations. In the context of CHF and CKD, IV iron supplementation has a superior efficacy to correct iron parameters, compared with oral preparations [5, 110, 111]. A likely explanation for this phenomenon is that hepcidin, which is increased by inflammation, prevents intestinal iron absorption. Moreover, oral iron supplementation is associated with side effects such as abdominal pain, obstipation or diarrhoea, and compliance is notoriously poor [112]. Furthermore, different studies have demonstrated that oral iron supplements change the gut microbiome in favour of Bacteroides and Enterobacteria at the expense of symbiotic Bifidobacteria and Lactobacilli [68]. Lactobacilli are among the few species that do not rely on iron availability. Human microbiota have a major interaction with the immune system and recent studies in kidney transplantation suggest an important effect of the host microbiota profile on diarrhoea, graft survival, the incidence of infections and metabolism of immunosuppressive medication [113–115]. Vice versa, immunosuppression affects the microbiome. In the first months after kidney transplantation, the microbiota profile shifts in favour of pathogenic bacteria such as Escherichia, Salmonella, Yersinia, Campylobacter and Pseudomonas, while the diversity is significantly reduced [113, 116]. The impact of iron on the microbiota after transplantation has not been studied systematically. However, because of overgrowth of the pathogenic species that are known to express siderophores and need iron at the expense of iron-independent Lactobacilli, it could be speculated that intra-intestinal iron supplementation has a detrimental effect on the microbiota in KTRs and that abundance of intra-intestinal iron increases the risk of enteritis or abdominal sepsis. In a small RCT assessing the effects of oral iron supplementation in recently transplanted KTRs, there was no sign of increased infection risk [117].
The unfavourable effects of oral iron supplements can be avoided by IV iron administration. Although a single dose of oral iron sulphate (210 mg daily) may be as effective as a single dose of 500 mg IV iron polymaltose in patients with anaemia, IV iron supplementation may be more effective when given repeatedly [32, 118, 119]. FCM and iron sucrose (ISC) injections have been shown to be effective and safe in anaemic or iron-deficient KTRs [118, 120]. IV iron supplementation compared with oral treatment did not increase the risk of infection in a study of 102 KTRs [32]. There was a non-significant trend towards less gastro-intestinal side effects in the intravenously treated group [32].
A potential concern with the IV administration of iron in KTRs is the worsening of hypophosphataemia. Since ID is associated with increased FGF23 concentrations, it might be expected that iron supplementation reduces FGF23 and restores phosphate homoeostasis. Surprisingly, some IV iron preparations, such as iron polymaltose and FCM, are known to induce an acute rise in intact FGF23 and, as a result, a decrease in phosphate levels [121–125]. In a small cohort of 23 KTRs who had received up to 1000 mg FCM, mean serum phosphate concentrations decreased by 0.27 mmol/L on average, although only one patient needed short-term phosphate supplementation [123]. The relationship between use of different IV iron preparations and occurrence of hypophosphataemia needs to be delineated in more detail in future studies.
CONCLUSIONS AND FUTURE DIRECTIONS
ID is highly prevalent among KTRs and is an independent risk factor for premature mortality in this population. Potential mechanisms include direct effects on cardiac and skeletal muscle metabolism. Iron status also influences the immune system at various levels, but whether this impacts the risk of infection or rejection remains unclear. Iron supplementation might influence phosphate homoeostasis and the microbiome in KTRs, and therefore studies addressing the efficacy and safety of supplementation are needed. Iron supplementation in iron-deficient KTRs without overt anaemia is currently not recommended by guidelines, in the absence of supporting evidence.
The established beneficial effects of ID correction in CHF patients and ESRD patients, as recently demonstrated in the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial, warrant prospective studies to demonstrate the clinical effects of iron supplementation in KTRs [6]. A randomized, controlled clinical trial to investigate the effect of FCM versus placebo on exercise capacity and quality of life in KTRs, and to explore its effects on phosphate metabolism, among others, is currently ongoing (EFFECT-KTx, ClinicalTrials.gov NCT03769441). More studies are required to establish which is the optimal ID definition in KTRs, to further clarify its impact on morbidity and mortality, and to define optimal ID management strategies in KTRs.
FUNDING
This work has been supported by the Dutch Kidney Foundation (grant no 17OKG18). M.F.E. has received consulting fees from Vifor Pharma. D.A.H. has received lecture and consulting fees from Astellas Pharma and Chiesi Farmaceutici SpA, as well as grant support from Astellas Pharma, Chiesi Farmaceutici and Novartis. M.H.d.B. has received consulting fees from Amgen, Astra Zeneca, Bayer, Kyowa Kirin, Pharmacosmos, Sanofi Genzyme and Vifor Fresenius Medical Care Renal Pharma (all to employer).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kassebaum NJ, Jasrasaria R, Naghavi M. et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eisenga MF, Minović I, Berger SP. et al. Iron deficiency, anemia, and mortality in renal transplant recipients. Transpl Int 2016; 29: 1176–1183 [DOI] [PubMed] [Google Scholar]
- 3. Winkelmayer WC, Lorenz M, Kramar R. et al. Percentage of hypochromic red blood cells is an independent risk factor for mortality in kidney transplant recipients. Am J Transplant 2004; 4: 2075–2081 [DOI] [PubMed] [Google Scholar]
- 4. Camaschella C. Iron deficiency. Blood 2019; 133: 30–39 [DOI] [PubMed] [Google Scholar]
- 5. Macdougall IC, Bock AH, Carrera F. et al.; on behalf of the FIND-CKD Study Investigators. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macdougall IC, White C, Anker SD. et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Filippatos G, Colet JC. et al.; for the FAIR-HF Trial Investigators. The impact of intravenous ferric carboxymaltose on renal function: an analysis of the FAIR-HF study. Eur J Heart Fail 2015; 17: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeong JC, Ro H, Yang J. et al. Characteristics of anemia and iron deficiency after kidney transplant. Transpl Proc 2019; 51: 1406–1409 [DOI] [PubMed] [Google Scholar]
- 9. Lorenz M, Kletzmayr J, Perschl A. et al. Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 2002; 13: 794–797 [DOI] [PubMed] [Google Scholar]
- 10. Molnar MZ, Czira M, Ambrus C. et al. Anemia is associated with mortality in kidney-transplanted patients - A prospective cohort study. Am J Transplant 2007; 7: 818–824 [DOI] [PubMed] [Google Scholar]
- 11. Przybylowski P, Malyszko J, Glowinska I. et al. Prevalence of iron deficiency in heart and kidney allograft recipients. Trans Proc 2011; 43: 3885–3887 [DOI] [PubMed] [Google Scholar]
- 12. Molnar MZ, Mucsi I, Macdougall IC. et al. Prevalence and management of anaemia in renal transplant recipients: data from ten European centres. Nephron Clin Pract 2011; 117: c127–c134 [DOI] [PubMed] [Google Scholar]
- 13. Allegra V, Mengozzi G, Martimbianco L. et al. Long-term monitoring of iron stores in renal transplant recipients. Nephron 1990; 55: 440–441 [DOI] [PubMed] [Google Scholar]
- 14. Tornero F, Prats D, Alvarez-Sala JL. et al. Iron deficiency anemia after successful renal transplantation. J Urol 1993; 149: 1398–1400 [DOI] [PubMed] [Google Scholar]
- 15. Lataste A, Renoult E, Lederlin P. et al. Analysis of serum ferritin changes after kidney transplantation: a prospective study of 123 cases. Transplant Proc 1994; 26: 2001–2005 [PubMed] [Google Scholar]
- 16. Teruel JL, Lamas S, Vila T. et al. Serum ferritin levels after renal transplantation: a prospective study. Nephron 1989; 51: 462–465 [DOI] [PubMed] [Google Scholar]
- 17. Eisenga MF, Dullaart RPF, Berger SP. et al. Association of hepcidin-25 with survival after kidney transplantation. Eur J Clin Invest 2016; 46: 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz AJ, Das NK, Ramakrishnan SK. et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest 2018; 129: 336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malyszko J, Malyszko JS, Mysliwiec M.. A possible role of hepcidin in the pathogenesis of anemia among kidney allograft recipients. Transplant Proc 2009; 41: 3056–3059 [DOI] [PubMed] [Google Scholar]
- 20. Han W, Bai XJ, Han LL. et al. The relationship between serum fibroblast growth factor 23, Klotho, and lumbar spine bone mineral density in northern Chinese postmenopausal women. Menopause 2019; 26: 546–553 [DOI] [PubMed] [Google Scholar]
- 21. Malyszko J, Malyszko JS, Pawlak K. et al. Hepcidin, iron status, and renal function in chronic renal failure, kidney transplantation, and hemodialysis. Am J Hematol 2006; 81: 832–837 [DOI] [PubMed] [Google Scholar]
- 22. Lam JR, Schneider JL, Quesenberry CP. et al. Proton pump inhibitor and histamine-2 receptor antagonist use and iron deficiency. Gastroenterology 2017; 152: 821–829 [DOI] [PubMed] [Google Scholar]
- 23. Douwes RM, Gomes-Neto AW, Eisenga MF. et al. Chronic use of proton-pump inhibitors and iron status in renal transplant recipients. J Clin Med 2019; 8: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colucci S, Pagani A, Pettinato M. et al. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood 2017; 130: 2111–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Przybylowski P, Malyszko JS, MacDougall IC. et al. Iron metabolism, hepcidin, and anemia in orthotopic heart transplantation recipients treated with mammalian target of rapamycin. Transplant Proc 2013; 45: 387–390 [DOI] [PubMed] [Google Scholar]
- 26. Maiorano A, Stallone G, Schena A. et al. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 2006; 82: 908–912 [DOI] [PubMed] [Google Scholar]
- 27. Sánchez Fructuoso A, Calvo N, Moreno MA. et al. Study of anemia after late introduction of everolimus in the immunosuppressive treatment of renal transplant patients. Transplant Proc 2007; 39: 2242–2244 [DOI] [PubMed] [Google Scholar]
- 28. Au E, Wong G, Chapman JR.. Cancer in kidney transplant recipients. Nat Rev Nephrol 2018; 14: 508–520 [DOI] [PubMed] [Google Scholar]
- 29. García García de Paredes A, Teruel Sánchez-Vegazo C, Hernanz Ruiz N. et al. Do patients with iron deficiency without anemia benefit from an endoscopic examination? J Dig Dis 2017; 18: 416–424 [DOI] [PubMed] [Google Scholar]
- 30. Zheng S, Coyne DW, Joist H. et al. Iron deficiency anemia and iron losses after renal transplantation. Transpl Int 2009; 22: 434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim JH, Chun CJ, Kang CM. et al. Kidney transplantation and menstrual changes. Transpl Proc 1998; 30: 3057–3059 [DOI] [PubMed] [Google Scholar]
- 32. Mudge DW, Tan KS, Miles R. et al. A randomized controlled trial of intravenous or oral iron for posttransplant anemia in kidney transplantation. Transplantation 2012; 93: 822–826 [DOI] [PubMed] [Google Scholar]
- 33. Fernández-Ruiz M, López-Medrano F, Andrés A. et al. Serum iron parameters in the early post-transplant period and infection risk in kidney transplant recipients. Transpl Infect Dis 2013; 15: 600–611 [DOI] [PubMed] [Google Scholar]
- 34. Vaugier C, Amano MT, Chemouny JM. et al. Serum iron protects from renal postischemic injury. J Am Soc Nephrol 2017; 28: 3605–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernández-Ruiz M, Parra P, Ruiz-Merlo T. et al. Association between baseline serum hepcidin levels and infection in kidney transplant recipients: potential role for iron overload. Transpl Infect Dis 2018; 20: e12807. [DOI] [PubMed] [Google Scholar]
- 36. Rössler J, Schoenrath F, Seifert B. et al. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. Br J Anaesth 2020; 124: 25–34 [DOI] [PubMed] [Google Scholar]
- 37. Spahn DR, Schoenrath F, Spahn GH. et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019; 393: 2201–2212 [DOI] [PubMed] [Google Scholar]
- 38. Miles LF, Sandhu RNS, Grobler AC. et al. Associations between non-anaemic iron deficiency and outcomes following surgery for colorectal cancer: an exploratory study of outcomes relevant to prospective observational studies. Anaesth Intensive Care 2019; 47: 152–159 [DOI] [PubMed] [Google Scholar]
- 39. Triphaus C, Judd L, Glaser P. et al. Effectiveness of preoperative iron supplementation in major surgical patients with iron deficiency. Ann Surg 2019; 1. Doi: 10.1097/SLA.0000000000003643 [DOI] [PubMed] [Google Scholar]
- 40. Sinkeler SJ, Zelle DM, Homan Van Der Heide JJ. et al. Endogenous plasma erythropoietin, cardiovascular mortality and all-cause mortality in renal transplant recipients. Am J Transplant 2012; 12: 485–491 [DOI] [PubMed] [Google Scholar]
- 41. Rangaswami J, Mathew RO, Parasuraman R. et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant 2019; 34: 760–773 [DOI] [PubMed] [Google Scholar]
- 42. Xu B, Harb S, Hawwa N. et al. Impact of end-stage renal disease on left and right ventricular mechanics: does kidney transplantation reverse the abnormalities? JACC Cardiovasc Imaging 2017; 10: 1081–1083 [DOI] [PubMed] [Google Scholar]
- 43. Lentine KL, Schnitzler MA, Abbott KC. et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis 2005; 46: 720–733 [DOI] [PubMed] [Google Scholar]
- 44. Klip IT, Postmus D, Voors AA. et al. Hemoglobin levels and new-onset heart failure in the community. Am Heart J 2015; 169: 94–101 [DOI] [PubMed] [Google Scholar]
- 45. Hoes MF, Grote Beverborg N, Kijlstra JD. et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Banasiak W, Josiak K, Kasztura M. et al. Iron depletion affects genes encoding mitochondrial electron transport chain and genes of non oxidative metabolism, pyruvate kinase and lactate dehydrogenase, in primary human cardiac myocytes cultured upon mechanical stretch. Cells 2018; 7: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrak J, Havlenova T, Krijt M. et al. Myocardial iron homeostasis and hepcidin expression in a rat model of heart failure at different levels of dietary iron intake. Biochim Biophys Acta Gen Subj 2019; 1863: 703–713 [DOI] [PubMed] [Google Scholar]
- 48. Chung YJ, Luo A, Park KC. et al. Iron-deficiency anemia reduces cardiac contraction by downregulating RyR2 channels and suppressing SERCA pump activity. JCI Insight 2019; 4: e125618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jankowska EA, Rozentryt P, Witkowska A. et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906 [DOI] [PubMed] [Google Scholar]
- 50. Ebner N, Jankowska EA, Ponikowski P. et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. Int J Cardiol 2016; 205: 6–12 [DOI] [PubMed] [Google Scholar]
- 51. Anker SD, Kirwan BA, van Veldhuisen DJ. et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20: 125–133 [DOI] [PubMed] [Google Scholar]
- 52. McDonagh T, Damy T, Doehner W. et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mordi IR, Tee A, Lang CC.. Iron therapy in heart failure: ready for primetime? Card Fail Rev 2018; 4: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eisenga MF, van Londen M, Leaf DE. et al. C-terminal fibroblast growth factor 23, iron deficiency, and mortality in renal transplant recipients. J Am Soc Nephrol 2017; 28: 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eisenga MF, De Jong MA, Van der Meer P. et al. Iron deficiency, elevated erythropoietin, fibroblast growth factor 23, and mortality in the general population of the Netherlands: a cohort study. PLoS Med 2019; 16: e1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vervloet MG. Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 2019; 15: 109–120 [DOI] [PubMed] [Google Scholar]
- 57. Evenepoel P, Naesens M, Claes K. et al. Tertiary “hyperphosphatoninism” accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 2007; 7: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 58. Bhan I, Shah A, Holmes J. et al. Post-transplant hypophosphatemia: tertiary “Hyper-Phosphatoninism”? Kidney Int 2006; 70: 1486–1494 [DOI] [PubMed] [Google Scholar]
- 59. Wolf M, Weir MR, Kopyt N. et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 2016; 100: 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baia LC, Humalda JK, Vervloet MG. et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8: 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf M, Molnar MZ, Amaral AP. et al. Elevated fbroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011; 22: 956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Borst MH, Vervloet MG, ter Wee PM. et al. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Jong MA, Mirkovic K, Mencke R. et al. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant 2016; 32: 73–80 [DOI] [PubMed] [Google Scholar]
- 65. Mhatre KN, Wakula P, Klein O. et al. Crosstalk between FGF23- and angiotensin II-mediated Ca2+ signaling in pathological cardiac hypertrophy. Cell Mol Life Sci 2018; 75: 4403–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh S, Grabner A, Yanucil C. et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016; 90: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu YC, Tsai JP, Wang LH. et al. Positive correlation of serum fibroblast growth factor 23 with peripheral arterial stiffness in kidney transplantation patients. Clin Chim Acta 2020; 505: 9–14 [DOI] [PubMed] [Google Scholar]
- 68. Yilmaz B, Li H.. Gut Microbiota and iron: the crucial actors in health and disease. Pharmaceuticals 2018; 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dickson K, Liu S, Zhou J. et al. Selective sensitivity of the gut microbiome to iron chelators in polybacterial abdominal sepsis. Med Hypotheses 2018; 120: 68–71 [DOI] [PubMed] [Google Scholar]
- 70. Schaefer B, Effenberger M, Zoller H.. Iron metabolism in transplantation. Transpl Int 2014; 27: 1109–1117 [DOI] [PubMed] [Google Scholar]
- 71. Gwamaka M, Kurtis JD, Sorensen BE. et al. Iron deficiency protects against severe plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012; 54: 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest 2002; 32: 70–78 [DOI] [PubMed] [Google Scholar]
- 73. Lan P, Pan KH, Wang SJ. et al. High serum iron level is associated with increased mortality in patients with sepsis. Sci Rep 2018; 8: 11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Atamna A, Hamud H, Daud W. et al. Chronic use of oral iron supplements is associated with poor clinical outcomes in patients with gram-negative bacteremia. Eur J Clin Microbiol Infect Dis 2019; 38: 689–693 [DOI] [PubMed] [Google Scholar]
- 75. Mohus RM, Paulsen J, Gustad L. et al. Association of iron status with the risk of bloodstream infections: results from the prospective population-based HUNT Study in Norway. Intensive Care Med 2018; 44: 1276–1283 [DOI] [PubMed] [Google Scholar]
- 76. Cinatl J, Scholz M, Weber B. et al. Effects of desferrioxamine on human cytomegalovirus replication and expression of HLA antigens and adhesion molecules in human vascular endothelial cells. Transpl Immunol 1995; 3: 313–320 [DOI] [PubMed] [Google Scholar]
- 77. Resch T, Ashraf MI, Ritschl PV. et al. Disturbances in iron homeostasis result in accelerated rejection after experimental heart transplantation. J Heart Lung Transplant 2017; 36: 732–743 [DOI] [PubMed] [Google Scholar]
- 78. Rastellini C, Braun M, Li X. et al. Prolongation of pancreatic islet graft survival by blocking transferrin receptor (CD71). Trans Proc 2001; 33: 518–519 [DOI] [PubMed] [Google Scholar]
- 79. Woodward JE, Bayer AL, Chavin KD. et al. Anti-transferrin receptor monoclonal antibody: a novel immunosuppressant. Transplantation 1998; 65: 6–9 [DOI] [PubMed] [Google Scholar]
- 80. Whitley WD, Hancock WW, Kupiec-Weglinski JW. et al. Iron chelation suppresses mononuclear cell activation, modifies lymphocyte migration patters, and prolongs rat cardiac allograft survival in rats. Transplantation 1993; 56: 1182–1188 [DOI] [PubMed] [Google Scholar]
- 81. Cronin SJF, Woolf CJ, Weiss G. et al. The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci 2019; 6: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yarosz EL, Ye C, Kumar A. et al. Cutting edge: activation-induced iron flux controls CD4 T cell proliferation by promoting poroper IL-2R signaling and mitochondrial function. J Immunol 2020; 204: 1708–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bergman M, Bessler H, Salman H. et al. In vitro cytokine production in patients with iron deficiency anemia. Clin Immunol 2004; 113: 340–344 [DOI] [PubMed] [Google Scholar]
- 84. Kuvibidila SR, Porretta C.. Iron deficiency and in vitro iron chelation reduce the expression of cluster of differentiation molecule (CD)28 but not CD3 receptors on murine thymocytes and spleen cells. Br J Nutr 2003; 90: 179–189 [DOI] [PubMed] [Google Scholar]
- 85. Puig S, Ramos-Alonso L, Romero AM. et al. The elemental role of iron in DNA synthesis and repair. Metallomics 2017; 9: 1483–1500 [DOI] [PubMed] [Google Scholar]
- 86. Laskey J, Webb I, Schulman HM. et al. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp Cell Res 1988; 176: 87–95 [DOI] [PubMed] [Google Scholar]
- 87. Kuvibidila SR, Porretta C, Baliga BS.. Iron deficiency alters the progression of mitogen-treated murine splenic lymphocytes through the cell cycle. J Nutr 2001; 131: 2028–2033 [DOI] [PubMed] [Google Scholar]
- 88. Kuvibidila S, Yu L, Ode D. et al. Effects of iron deficiency on the secretion of interleukin-10 by mitogen-activated and non-activated murine spleen cells. J Cell Biochem 2003; 90: 278–286 [DOI] [PubMed] [Google Scholar]
- 89. Chandra RK, Saraya AK.. Impaired immunocompetence associated with iron deficiency. J Pediatr 1975; 86: 899–902 [DOI] [PubMed] [Google Scholar]
- 90. Attia MA, Essa SA, Nosair NA. et al. Effect of iron deficiency anemia and its treatment on cell mediated immunity. Indian J Hematol Blood Transfus 2009; 25: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brekelmans P, Van Soest P, Leenen PJM. et al. Inhibition of proliferation and differentiation during early T cell development by anti‐transferrin receptor antibody. Eur J Immunol 1994; 24: 2896–2902 [DOI] [PubMed] [Google Scholar]
- 92. Hassan TH, Badr MA, Karam NA. et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine (Baltimore) 2016; 95: e5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Das I, Saha K, Mukhopadhyay D. et al. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: a case control study. J Nat Sci Biol Med 2014; 5: 158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aly SS, Fayed HM, Ismail AM. et al. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr 2018; 18: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bonaccorsi-Riani E, Danger R, Lozano JJ. et al. Iron deficiency impairs intra-hepatic lymphocyte mediated immune response. PLoS One 2015; 10: e0136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kuvibidila SR, Velez M, Gardner R. et al. Iron deficiency reduces serum and in vitro secretion of interleukin-4 in mice independent of altered spleen cell proliferation. Nutr Res 2012; 32: 107–115 [DOI] [PubMed] [Google Scholar]
- 97. Nankivell BJ, Alexander SI.. Rejection of the kidney allograft. N Engl J Med 2010; 363: 1451–1462 [DOI] [PubMed] [Google Scholar]
- 98. Svoboda M, Drabek J, Krejci J. et al. Impairment of the peripheral lymphoid compartment in iron-deficient piglets. J Vet Med Series B 2004; 51: 231–237 [DOI] [PubMed] [Google Scholar]
- 99. Klecha AJ, Salgueiro J, Wald M. et al. In vivo iron and zinc deficiency diminished T- and B-selective mitogen stimulation of murine lymphoid cells through protein kinase C-mediated mechanism. Biol Trace Elem Res 2005; 104: 173–183 [DOI] [PubMed] [Google Scholar]
- 100. Jiang Y, Li C, Wu Q. et al. Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat Commun 2019; 10: 2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Soares MP, Hamza I.. Macrophages and iron metabolism. Immunity 2016; 44: 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pereira M, Chen TD, Buang N. et al. Acute iron deprivation reprograms human macrophage metabolism and reduces inflammation in vivo. Cell Rep 2019; 28: 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sottile R, Federico G, Garofalo C. et al. Iron and ferritin modulate MHC Class i expression and NK cell recognition. Front Immunol 2019; 10: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Scozzi D, Ibrahim M, Menna C. et al. The role of neutrophils in transplanted organs. Am J Transplant 2017; 17: 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Scindia Y, Dey P, Thirunagari A. et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 2015; 26: 2800–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Niu X, Huang WH, De Boer B. et al. Iron-induced oxidative rat liver injury after non-heart-beating warm ischemia is mediated by tumor necrosis factor α and prevented by deferoxamine. Liver Transpl 2014; 20: 904–911 [DOI] [PubMed] [Google Scholar]
- 107. Huang H, He Z, Roberts LJ. et al. Deferoxamine reduces cold-ischemic renal injury in a syngeneic kidney transplant model. Am J Transplant 2003; 3: 1531–1537 [DOI] [PubMed] [Google Scholar]
- 108. Deboer DA, Clark RE.. Iron chelation in myocardial preservation after ischemia-reperfusion injury: the importance of pretreatment and toxicity. Ann Thorac Surg 1992; 53: 412–418 [DOI] [PubMed] [Google Scholar]
- 109. Wang X, Zheng X, Zhang J. et al. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. Am J Physiol Physiol 2018; 315: F1042–F1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Beck-Da-Silva L, Piardi D, Soder S. et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 2013; 168: 3439–3442 [DOI] [PubMed] [Google Scholar]
- 111. Lewis GD, Malhotra R, Hernandez AF. et al.; for the NHLBI Heart Failure Clinical Research Network. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tolkien Z, Stecher L, Mander AP. et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One 2015; 10: e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fricke WF, Maddox C, Song Y. et al. Human microbiota characterization in the course of renal transplantation. Am J Transplant 2014; 14: 416–427 [DOI] [PubMed] [Google Scholar]
- 114. Lee JR, Magruder M, Zhang L. et al. Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am J Transplant 2019; 19: 488–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ardalan M, Vahed SZ.. Gut microbiota and renal transplant outcome. Biomed Pharmacother 2017; 90: 229–236 [DOI] [PubMed] [Google Scholar]
- 116. Swarte JC, Douwes RM, Hu S. et al. Characteristics and dysbiosis of the gut microbiome in renal transplant recipients. J Clin Med 2020; 9: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mudge DW, Atcheson B, Taylor PJ. et al. The effect of oral iron administration on mycophenolate mofetil absorption in renal transplant recipients: a randomized, controlled trial. Transplantation 2004; 77: 206–209 [DOI] [PubMed] [Google Scholar]
- 118. Rozen-Zvi B, Gafter-Gvili A, Zingerman B. et al. Intravenous iron supplementation after kidney transplantation. Clin Transplant 2012; 26: 608–614 [DOI] [PubMed] [Google Scholar]
- 119. Shepshelovich D, Rozen-Zvi B, Avni T. et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 677–690 [DOI] [PubMed] [Google Scholar]
- 120. Grimmelt AC, Cohen CD, Fehr T. et al. Safety and tolerability of ferric carboxymaltose (FCM) for treatment of iron deficiency in patients with chronic kidney disease and in kidney transplant recipients. Clin Nephrol 2009; 71: 125–129 [DOI] [PubMed] [Google Scholar]
- 121. Schouten BJ, Hunt PJ, Livesey JH. et al. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 2009; 94: 2332–2337 [DOI] [PubMed] [Google Scholar]
- 122. Wolf M, Koch TA, Bregman DB.. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 2013; 28: 1793–1803 [DOI] [PubMed] [Google Scholar]
- 123. Sari V, Atiqi R, Hoorn EJ. et al. Ferric carboxymaltose-induced hypophosphataemia after kidney transplantation. Neth J Med 2017; 75: 65–73 [PubMed] [Google Scholar]
- 124. Huang LL, Lee D, Troster SM. et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dial Transplant 2018; 33: 1628–1635 [DOI] [PubMed] [Google Scholar]
- 125. Wolf M, Chertow GM, Macdougall IC. et al. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018; 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]