Abstract
Background
Novel ways of determining cardiovascular risk are needed as a consequence of population ageing and the increased prevalence of chronic kidney disease (CKD), both of which favour vascular calcification. Since the formation of arterial calcium deposits has a genetic component, single nucleotide polymorphisms (SNPs) could predict cardiovascular events.
Methods
A selection of 1927 CKD patients and controls recruited by the NEFRONA study were genotyped for 60 SNPs from 22 candidate genes. A calcium score was calculated from the echogenicity of arterial atherosclerotic plaques and the presence of cardiovascular events during a 4-year period was recorded. Association of SNPs with the calcium score was identified by multiple linear regression models and their capacity to predict events was assessed by means of Cox proportional hazards regression and receiver operating characteristics curves.
Results
Two variants, rs2296241 of CYP24A1 and rs495392 of KL, were associated with the calcium score. Despite this, only heterozygotes for rs495392 had a lower risk of suffering an event compared with homozygotes for the major allele {hazard ratio (HR) 0.67 [95% confidence interval (CI) 0.48−0.93]}. Of note, the calcium score was associated with an increased risk of cardiovascular events [HR 1.71 (95% CI 1.35−2.17)]. The addition of the rs495392 genotype to classical cardiovascular risk factors did not increase the predictive power [area under the curve (AUC) 71.3 (95% CI 61.1−85.5) versus 71.4 (61.5−81.4)].
Conclusions
Polymorphisms of CYP24A1 and KL are associated with the extent of calcification but do not predict cardiovascular events. However, the echogenic determination of the extent of calcium deposits seems a promising non-irradiating method for the scoring of calcification in high-risk populations.
Keywords: calcium score, cardiovascular risk, klotho, single nucleotide polymorphism, vitamin D 24-hydroxylase
KEY LEARNING POINTS
What is already known about this subject?
Vascular calcification contributes to an increased cardiovascular risk.
Chronic kidney disease (CKD) patients develop extensive ectopic calcifications and have a remarkably high cardiovascular risk.
Genetic variants may determine a person’s susceptibility to vascular calcification and consequently to cardiovascular disease.
What this study adds?
CYP24A1 rs2296241 and KL rs495392 polymorphisms are associated with the extent of vascular calcification independent of a person’s age, sex, blood pressure status or CKD stage.
A non-invasive and non-irradiating ultrasound-based calcium score is useful to determine the extent of vascular calcification in CKD patients.
What impact this may have on practice or policy?
Replication of the association of rs2296241 and rs495392 with vascular calcification in different populations could strengthen the value of these polymorphisms as novel prognostic markers that would allow the implementation of specific strategies in patients with risk genotypes early in CKD to prevent calcification.
Understanding the effect of rs2296241 and rs495392 on the expression or function of vitamin D 24-hydroxylase and Klotho in vascular calcification could turn them into potential therapeutic targets.
This calcium score can be used in the clinic to quantify the extent of vascular calcification and in future genetic association studies designed to uncover new genetic determinants of calcification and cardiovascular disease.
INTRODUCTION
Current demographic changes suggest that a transition to an aged society will take place in the near future. In Spain, for instance, people >65 years of age are expected to account for 25% of the population by the year 2033 [1]. Since age is an independent risk factor for cardiovascular diseases, these have become the most frequent cause of death worldwide. Reduced kidney function is also known to have a negative impact on the circulatory system and, in fact, chronic kidney disease (CKD) patients exhibit what some authors consider a ‘premature ageing’ phenotype [2]. As a consequence, the occurrence of cardiovascular events in these individuals is more frequent than the incidence of renal replacement therapy [3], and cardiovascular mortality in the last stage of the disease is 10–20 times higher than in the general population [4]. Also, CKD prevalence has increased largely due to population ageing, currently affecting between 11 and 13% of the world's population [5], and the possibility of these figures becoming even larger raises important concerns.
Vascular calcification is a notable contributor to cardiovascular disease and a manifestation of vascular ageing [6]. The presence of calcification in any arterial wall is indeed associated with a higher risk of mortality and cardiovascular events [7] and, in particular, scoring of coronary artery calcification has been shown to improve the prediction of cardiovascular risk in individuals with CKD [8, 9]. Although CKD patients can present calcification scores up to 5 times higher than age-matched controls with coronary heart disease [10], some people suffering from CKD do not develop extensive calcifications. In fact, it has been reported that patients entering haemodialysis with low or absent coronary artery calcification show minimal progression after >2 years [11].
Individual susceptibility to calcification could be due to genetic factors, since several genome-wide association studies have uncovered variants associated with calcification of the arteries [12–15], and it has been estimated that nearly half of the variation in abdominal aortic calcium deposits is due to heritable factors [16]. This suggests that genetic variants could be used as a cardiovascular risk prediction tool for a high-risk population, such as individuals with CKD. Therefore, in this study we have tried to associate a group of single nucleotide polymorphisms (SNPs) in genes implicated in the pathogenesis of calcification with the extent of arterial calcium deposits measured with ultrasound techniques in a Spanish population of individuals at different stages of CKD.
MATERIALS AND METHODS
Study population
The study population was individuals recruited by the NEFRONA project. This project was designed to assess the usefulness of imaging techniques and circulating biomarkers in the prediction of cardiovascular disease in patients with CKD [17]. Briefly, it included 3004 CKD patients at different stages (CKD Stages 2 and 3, Stages 4 and 5 and dialysis) as well as healthy controls from 81 Spanish hospitals. The study was conducted in accordance with the Declaration of Helsinki and the human sample collection protocol was approved by the ethics committee of each hospital. All patients signed an informed consent form before enrolment. Diagnosis and staging of patients was based on current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. Cardiovascular risk was defined as the probability of experiencing any cardiovascular event during a 4-year follow-up, so the occurrence of both fatal and non-fatal events of this type in all the study participants was recorded during such period. Recorded events included myocardial infarction, unstable angina, sudden death, transient ischaemic attack, cerebrovascular accident, congestive heart failure, arrhythmia, mesenteric infarction, peripheral artery disease, amputation due to vascular disease and aortic aneurism. Only subjects with available data on their genotype for the studied SNPs and the extent of their arterial calcium deposits were considered for the present study. Additionally, since the proportion of non-white individuals was insufficient for analysis, these were not included in the study.
Arterial calcium quantification
A continuous vascular calcium score based on relative calcium area was calculated from the echogenicity of atherosclerotic plaques detected by B-mode ultrasonography in the carotid and femoral arteries. The presence of atheromatous plaques was defined as an intima-media thickness (IMT) >1.5 mm protruding into the lumen, according to the American Society of Echocardiography (ASE) Consensus Statement [18] and the Mannheim IMT Consensus [19] in 10 different arterial territories [17]. The area of the plaque was determined as previously described [20]. Briefly, measurements were made in magnified longitudinal views of each plaque. The plane in which the measurement of each plaque was made was chosen by panning around the artery until the view showing the largest extent of that plaque was found. The area of calcium was determined based on the echogenicity with HemoDyn 4M software [21].
Genotyping
DNA samples from the NEFRONA population stored in the Spanish Renal Research Network (REDinREN) Biobank were genotyped at Centro Nacional de Genotipado-Plataforma de Recursos Biomoleculares y Bioinformáticos (CEGEN-PRB3) from the Instituto de Salud Carlos III (University of Santiago de Compostela node). Genotypes for 60 SNPs from 22 genes encoding cytokines, extracellular matrix proteins, members of the receptor activator of nuclear factor (NF)-κB/receptor activator of NF-κB ligand/osteoprotegerin (RANK/RANKL/OPG) axis and vitamin D metabolism proteins, among others (Supplementary data, Table S1), were determined following a previously described protocol [22].
Statistical analysis
Hardy–Weinberg equilibrium was evaluated by means of a chi-squared test. Association of SNPs with the extent of arterial calcification measured as the calcium score was identified by univariate linear regression models. Multiple linear regression with backward elimination was used for the selection of an appropriate SNP-based model adjusted by confounding variables. The calcium score variable was logarithmically transformed to ensure the normality of the residuals in the regression models, where it was used as a dependent variable. Cox proportional hazards multiple regression models were applied for the prediction of cardiovascular risk. Finally, receiver operating characteristics (ROC) curves were calculated to determine the predictive power of those polymorphisms influencing cardiovascular risk. All statistical analyses were performed using R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) [23].
RESULTS
Population characteristics
A total of 1927 individuals were considered. Differences between CKD and control groups were statistically significant in age; sex; smoking status; presence of hypertension, diabetes and hyperlipidaemia; pulse pressure; calcium score and cardiovascular events (Table 1).
Table 1.
Characteristics of the population
| Variable | CKD stage |
|||||
|---|---|---|---|---|---|---|
| Total | Control | CKD 2–3 | CKD 4–5 | Dialysis | P-value | |
| (N = 1928) | (n = 275) | (n = 657) | (n = 536) | (n = 460) | ||
| Age (years), mean (SD) | 61.9 (9.5) | 59.6 (8.6) | 64.5 (7.6) | 62.85 (9.0) | 58.3 (11.3) | <0.001 |
| Sex, n (%) | ||||||
| Male | 1295 (67.2) | 183 (66.5) | 474 (72.1) | 342 (63.8) | 296 (64.3) | 0.008 |
| Female | 633 (32.8) | 92 (33.5) | 183 (27.9) | 194 (36.2) | 164 (35.7) | |
| Smoking status, n (%) | ||||||
| Non-smoker | 724 (37.6) | 83 (30.2) | 245 (37.3) | 214 (39.9) | 182 (39.6) | 0.037 |
| Current smoker | 1204 (62.4) | 192 (69.8) | 412 (62.7) | 322 (60.1) | 278 (60.4) | |
| Hypertension, n (%) | ||||||
| No | 265 (13.7) | 144 (52.4) | 49 (7.5) | 16 (3.0) | 56 (12.2) | <0.001 |
| Yes | 1663 (86.3) | 131 (47.6) | 608 (92.5) | 520 (97.0) | 404 (87.8) | |
| Pulse pressure (mmHg), mean (SD) | 62.9 (18.5) | 56.5 (13.9) | 64.1 (17.3) | 67.1 (20.1) | 60.2 (19.0) | <0.001 |
| Hyperlipidaemia, n (%) | ||||||
| No | 645 (33.5) | 148 (53.8) | 161 (24.5) | 142 (26.5) | 194 (42.2) | <0.001 |
| Yes | 1283 (66.5) | 127 (46.2) | 496 (75.5) | 394 (73.5) | 266 (57.8) | |
| Diabetes, n (%) | ||||||
| No | 1389 (72.0) | 231 (84.0) | 446 (67.9) | 351 (65.5) | 361 (78.5) | <0.001 |
| Yes | 539 (28.0) | 44 (16.0) | 211 (32.1) | 185 (34.5) | 99 (21.5) | |
| Calcium score, median (IQR) | 0.22 (0.08–0.48) | 0.16 (0.05–0.32) | 0.22 (0.08–0.48) | 0.24 (0.09–0.47) | 0.26 (0.10–0.59) | <0.001 |
| Cardiovascular events, n (%) | ||||||
| No | 1740 (90.2) | 269 (97.8) | 599 (91.2) | 472 (88.1) | 400 (87.0) | <0.001 |
| Yes | 188 (9.8) | 6 (2.2) | 58 (8.8) | 64 (11.9) | 60 (13.0) | |
IQR, interquartile range; SD, standard deviation.
Association of individual SNPs with calcium score
All the evaluated SNPs met the Hardy–Weinberg equilibrium. Univariate linear regression models showed a significant association with the calcium score for six SNPs: rs11568820 of VDR, rs2248359 and rs2296241 of CYP24A1, rs3102735 of TNFRSF11B and rs385564 and rs495392 of KL. Moreover, the association of these SNPs, except rs11568820 of VDR, remained significant after adjusting for potentially confounding variables in multiple linear regression models (Table 2).
Table 2.
Multiple linear regression models for each SNP and the calcium score
| Gene | Polymorphism | Genotype | n | Estimate (95% CI) | P-valuea |
|---|---|---|---|---|---|
| VDR | rs11568820 | AA | 813 | Reference | |
| AG | 536 | −0.05 (−0.19–0.08) | 0.428 | ||
| GG | 91 | 0.21 (−0.06–0.48) | 0.124 | ||
| CYP24A1 | rs2248359 | CC | 367 | Reference | |
| CT | 472 | −0.21 (−0.38 to −0.05) | 0.010 | ||
| TT | 148 | −0.19 (−0.42–0.04) | 0.110 | ||
| CYP24A1 | rs2296241 | AA | 263 | Reference | |
| AG | 505 | 0.14 (−0.04–0.32) | 0.126 | ||
| GG | 220 | 0.36 (0.14–0.58) | 0.001 | ||
| TNFRSF11B | rs3102735 | TT | 1391 | Reference | |
| TC | 397 | −0.05 (−0.19–0.08) | 0.464 | ||
| CC | 29 | 0.48 (0.03–0.93) | 0.036 | ||
| KL | rs385564 | GG | 895 | Reference | |
| GC | 748 | 0.03 (−0.08–0.15) | 0.563 | ||
| CC | 164 | 0.23 (0.03–0.44) | 0.025 | ||
| KL | rs4945392 | GG | 987 | Reference | |
| GT | 694 | −0.06 (−0.18–0.06) | 0.326 | ||
| TT | 135 | −0.28 (−0.49 to −0.06) | 0.014 |
Adjusted by age, sex, hypertension, diabetes and CKD stage.
Association of a combination of SNPs with calcium score
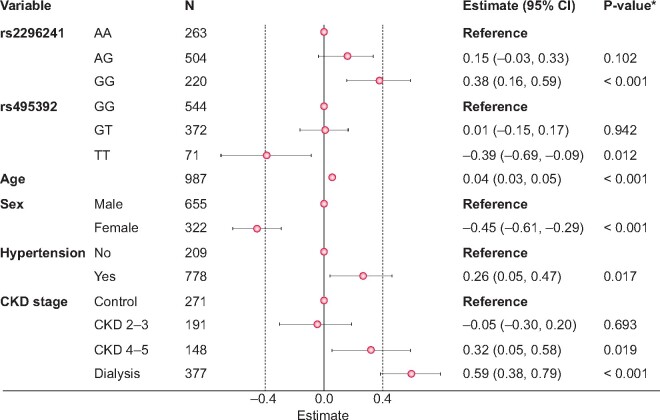
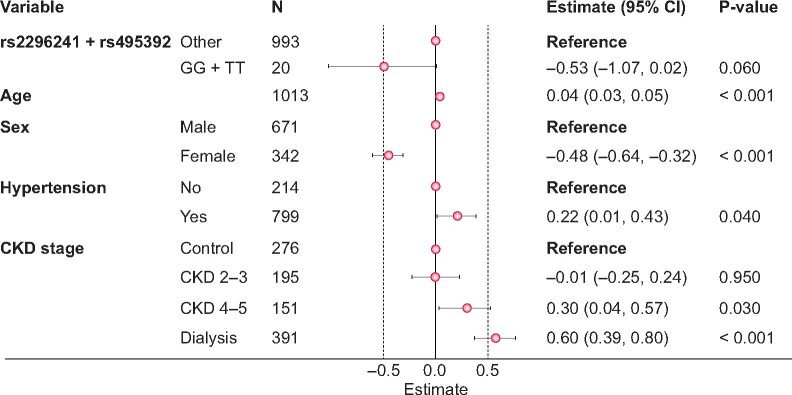
A multiple regression model including the six associated SNPs along with potentially confounding variables and the calcium score as the dependent variable was adjusted next. After eliminating SNPs with P-values >0.05, only rs2296241 of CYP24A1 and rs495392 of KL remained associated with the calcium score (Figure 1). In both cases, only homozygotes for the minor allele presented different score values and thus we next performed the analysis with grouped genotypes. We found a lower calcium score for these double homozygotes than for the rest of genotypes (0.6-fold), but without statistical significance (Figure 2).
FIGURE 1.
Multiple regression model of calcium score adjusted for six polymorphisms and confounding variables. aAdjusted by genotype for rs11568820 of VDR, rs2248359 and rs2296241 of CYP24A1, rs3102735 of TNFRSF11B and rs385564 and rs495392 of KL, as well as age, sex, hypertension, diabetes and CKD stage. Only those variables with a significant effect on the calcium score are shown.
FIGURE 2.
Multiple regression model of calcium score with grouped genotypes for rs2296241 and rs495392 polymorphisms.
Cardiovascular risk prediction by means of calcium score and associated SNPs
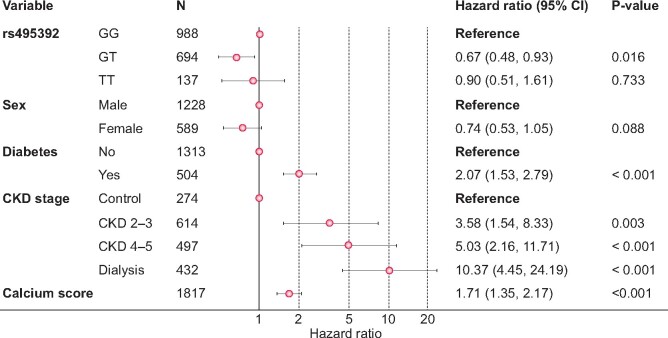
In order to evaluate the utility of both SNPs associated with the calcium score for the prediction of cardiovascular risk in CKD patients, Cox proportional hazards multiple regression models were adjusted next. Only heterozygote individuals for the rs495392 polymorphism had a significantly lower risk of having a cardiovascular event in the studied period compared with homozygotes for the major allele {hazard ratio [HR] 0.67 [95% confidence interval (CI) 0.48–0.93]} (Figure 3). However, homozygotes for the minor allele did not have a lower cardiovascular risk, suggesting that heterozygotes may have a different phenotype from both types of homozygotes.
FIGURE 3.
Cox proportional hazards multiple regression model.
Also, the calcium score was positively associated [HR 1.71 (95% CI 1.35–2.17)] with the likelihood of having a cardiovascular event, giving validity to this method of calcium quantification. Since previous investigations have shown that pulse pressure can predict calcifications in CKD patients [24], we checked whether our score was related to this parameter in order to further prove its validity. We found a positive correlation between both variables (Pearson’s r = 0.16, P < 0.001) and higher values of the calcium score among individuals with pulse pressure ≥60 mmHg [linear regression estimate 0.11 (95% CI 0.07–0.15), P < 0.001]. Although statistically significant, we believe the clinical relevance of this relationship is not strong, suggesting the effect of both variables may not be synonymous. Thus we next added pulse pressure as an additional variable in the Cox model featuring rs495392. However, this did not alter the association of the calcium score with an increased probability of suffering an event [HR 1.61 (95% CI 1.31–2.11)] and pulse pressure did not show any association itself [HR 1.01 (95% CI 1.00–1.02)].
Finally, ROC curves were calculated to determine the predictive power of rs495392 in the population. A model including all previously considered confounding variables (age, sex, hypertension, diabetes and CKD stage) was compared with a model including these along with genotypes for the SNP. No differences in the values of the area under each of the curves were found (Figure 4).
FIGURE 4.
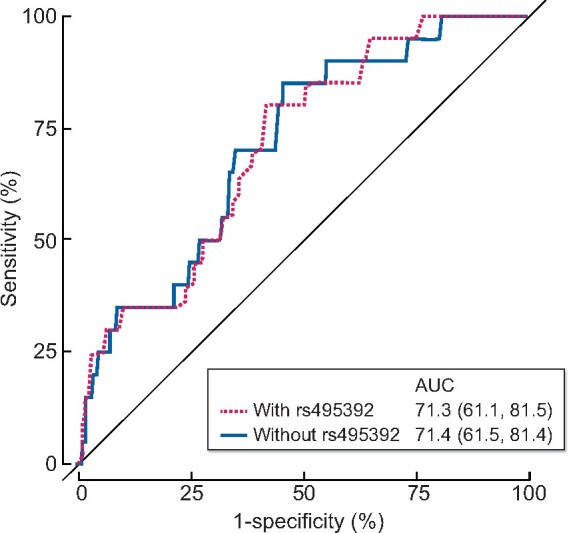
ROC curves for the validation of rs495392 polymorphism, based on classical cardiovascular risk factors used as confounding variables in the previous models.
DISCUSSION
The present study shows an association of the rs2296241 polymorphism of CYP24A1 and rs495392 of KL with the extent of vascular calcification in the carotid and femoral arteries independent from age, sex, CKD stage or blood pressure status. Despite this, the inclusion of the genotype for these SNPs as additional variables in a predictive model featuring risk factors for vascular calcification did not improve its predictive capability.
The CYP24A1 gene codes for cytochrome P450 family 24 subfamily A member 1, an enzyme that catalyses multiple reactions including 24-hydroxylation of 1,25-hydroxyvitamin D3 or calcitriol and is therefore involved in vitamin D catabolism. Genome-wide association studies had previously shown this locus to be implicated in the genetic determination of vitamin D levels [25, 26]. Also, increases in the expression of this gene occur in CKD [27] and, more specifically, the rs2248359 SNP has been associated with a greater risk of suffering the condition [22]. Regarding vascular calcification, a common variant of CYP24A1 (rs2762939) has been associated with lower amounts of coronary artery calcium as measured by electron beam computed tomography [28]. The SNP associated with arterial calcium extent in our study has been reported to be related to other pathologies like bone mineral density loss following haematopoietic cell transplantation [29]. Since no other study has yet found the rs2296241 polymorphism to be associated with vascular calcification, validation of our results in a different population would strengthen their significance. It is noteworthy, however, that inactivating mutations in the CYP24A1 gene have been described in individuals with chronic hypercalcaemia who also had vascular calcification [30].
The KL gene codes for the protein Klotho, an enzyme mainly produced in the kidney whose expression is reduced in CKD patients [31]. It is involved in mineral and vitamin D metabolism and reduced production of this protein has also been associated with vascular calcification [32]. Genetic variants of the KL gene have been linked to survival in end-stage renal disease patients undergoing haemodialysis [33], as well as to several cardiovascular disorders [34–36]. In particular, the rs495392 SNP has recently been associated with the formation of atherosclerotic plaques in patients with CKD [37]. However, no study to date has linked any KL polymorphism with vascular calcification. In fact, one study reported the lack of association of several Klotho, β-Klotho and fibroblast growth factor 23 variants with valvular or coronary artery calcification [38]. For this reason, it would be interesting to replicate the association reported here in a different population. The finding of a KL mutation causative of severe tumoral calcinosis [39], which is accompanied by phosphate and vitamin D biochemical abnormalities, indicates variants of this gene can actually be associated with the development of ectopic calcification.
Our results also emphasize the role of phosphate and vitamin D in calcification, whose metabolic alterations are among those classically studied in CKD and therefore are the target of many therapies aimed at alleviating the complications of the disease. Interestingly, some authors have speculated on the possibility of Klotho also constituting a link between dysregulated mineral metabolism and cellular senescence [2]. In fact, a relationship between Klotho and some senescence pathways has already been reported [40, 41], and this process has been shown to be among those driving calcification and osteogenic differentiation of vascular smooth muscle cells [42].
Both SNPs associated with the extent of arterial calcification in our study have a rather uncertain effect on CYP24A1 and KL expression since the variant of the first gene introduces a synonymous change in its protein sequence and the second one is located in an intronic region. Consequently, the possibility of these variants being in linkage disequilibrium with another SNP not considered in the study but having a functional effect has to be taken into account. To the best of our knowledge, no functional studies have been carried out. However, a functional effect produced by these SNPs cannot be completely disregarded. For instance, synonymous genetic variants have indeed been shown to have a deep influence in translation [43], splicing [44], mRNA stability [45] and protein folding [46]. In addition, an intronic SNP of the KL gene (rs577912) has been demonstrated to produce different Klotho protein levels in lymphoblastoid cell lines depending on their genotype [33]. For these reasons, we believe that further studies aimed at determining the effect of rs2296241 and rs495392 in the expression of vitamin D 24-hydroxylase and Klotho should be encouraged in order to properly understand the mechanism behind the observed association with vascular calcification. A mechanistic understanding of the effect of these SNPs on calcification would contribute to the implementation of novel therapies for patients with risk genotypes while still in the early stages of CKD. With regards to the fact that heterozygotes for rs495392 had a significantly lower risk of having a cardiovascular event compared with both homozygotes, this could be explained under an inheritance model differing from classical dominance/recessiveness (i.e. codominance or overdominance).
It is also worth noting that ours is one of the few studies in the scientific literature searching for genetic variants associated with calcification in CKD patients. A study conducted in >1500 individuals from the Chronic Renal Insufficiency Cohort, replicated in two additional populations, found 28 SNPs in 23 loci associated with coronary artery calcium, 4 of them also significantly associated with myocardial infarction [47]. In contrast, our study was unable to identify any relationship between the polymorphisms affecting arterial calcium area and having any type of cardiovascular event. Since cardiovascular risk is a complex trait to which many different factors contribute, this suggests that the associations between SNPs and vascular calcification are perhaps indirect.
Despite this lack of improvement of predictive capability for cardiovascular events of the rs2296241 and rs495392 polymorphisms, our study provides the literature with a method for the evaluation of the extent of calcification that has proved to be useful. Some studies had predicted coronary artery calcification by detecting atherosclerotic plaque by ultrasound imaging of the carotid and femoral arteries [48, 49] and echographies had already been pointed out as a viable non-invasive way of assessing ectopic calcification in CKD, albeit semiquantitatively [50]. However, our method allows the quantification of the calcified area in the carotid and femoral arteries and, contrary to the ‘gold standard’ of computed tomography–based coronary artery calcium scores, this ultrasonography-derived measurement does not require exposure to radiation. This calcium score can therefore be used in future genetic association studies to uncover new genetic determinants of calcification and cardiovascular disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the NEFRONA team (Eva Castro, Virtudes María, Teresa Molí, Teresa Vidal, Meritxell Soria) and the Biobank of REDinREN for their inestimable support. The NEFRONA study investigator group is listed in the Supplementary Material. We also thank Juan Pérez Ortega from Fundación para la Investigación y la Innovación Biosanitaria del Principado de Asturias (FINBA) for his administrative support.
FUNDING
This work was financed by the Spanish National Plan for Scientific and Technical Research and Innovation 2013–2016, co-founded by European Union FEDER/ERDF funds ISCII-FEDER/ERDF (PI10/00173, PI18/00610, PI18/00694) and Thematic Network for Collaborative Research in Health (RETICS) REDinREN from ISCIII (RD16/0009/0005, RD16/0009/0011). G.S.-B. received a graduate fellowship from the Gobierno del Principado de Asturias (“Severo Ochoa” Program). I.R. is financially supported by FINBA. Funders had no role in the design, execution, interpretation or writing of the study.
AUTHORS’ CONTRIBUTIONS
J.-M.V. and I.R. contributed to the research idea and study design and were responsible for supervision or mentorship. G.S.-B. and N.M.-F. contributed to data acquisition. G.S.-B., S.C., J.-M.V. and I.R. contributed to data analysis and interpretation. V.R.-S., S.C. and G.S.-B. contributed to statistical analysis. G.S.-B. and I.R. co-wrote the manuscript. Each author contributed important intellectual content during manuscript drafting and reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Instituto Nacional de Estadística. Population Figures and Demographic Censuses. Population Projection for Spain 2018-2068. https://www.ine.es/uc/t9s3CLHx (22 June 2020, date last accessed).
- 2. Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 2013; 9: 661–670. [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Murray AM, Li S et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489–495 [DOI] [PubMed] [Google Scholar]
- 4. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32(5 Suppl 3): S112–S119 [DOI] [PubMed] [Google Scholar]
- 5. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw LJ, Raggi P, Berman DS et al. Coronary artery calcium as a measure of biologic age. Atherosclerosis 2006; 188: 112–119 [DOI] [PubMed] [Google Scholar]
- 7. Rennenberg RJ, Kessels AG, Schurgers LJ et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 2009; 5: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsushita K, Sang Y, Ballew SH et al. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol 2015; 26: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Budoff MJ, Reilly MP et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2017; 2: 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braun J, Oldendorf M, Moshage W et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 1996; 27: 394–401 [DOI] [PubMed] [Google Scholar]
- 11. Bellasi A, Kooienga L, Block GA et al. How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol 2009; 22: 255–262 [PubMed] [Google Scholar]
- 12. O'Donnell CJ, Kavousi M, Smith AV et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011; 124: 2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Setten J, Isgum I, Smolonska J et al. Genome-wide association study of coronary and aortic calcification implicates risk loci for coronary artery disease and myocardial infarction. Atherosclerosis 2013; 228: 400–405 [DOI] [PubMed] [Google Scholar]
- 14. Adams HH, Ikram MA, Vernooij MW et al. Heritability and genome-wide association analyses of intracranial carotid artery calcification: the Rotterdam study. Stroke 2016; 47: 912–917 [DOI] [PubMed] [Google Scholar]
- 15. Choi SY, Shin E, Choe EK et al. Genome-wide association study of coronary artery calcification in asymptomatic Korean populations. PLoS One 2019; 14: e0214370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Donnell CJ, Chazaro I, Wilson PWF et al. Evidence for heritability of abdominal aortic calcific deposits in the Framingham Heart Study. Circulation 2002; 106: 337–341 [DOI] [PubMed] [Google Scholar]
- 17. Junyent M, Martinez M, Borras M et al. Predicting cardiovascular disease morbidity and mortality in chronic kidney disease in Spain. The rationale and design of NEFRONA: a prospective, multicenter, observational cohort study. BMC Nephrol 2010; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stein JH, Korcarz CE, Hurst RT et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21: 93–111; quiz 189–190 [DOI] [PubMed] [Google Scholar]
- 19. Touboul PJ, Hennerici MG, Meairs S et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis 2004; 18: 346–349 [DOI] [PubMed] [Google Scholar]
- 20. Barnett PA, Spence JD, Manuck SB et al. Psychological stress and the progression of carotid artery disease. J Hypertens 1997; 15: 49–55 [DOI] [PubMed] [Google Scholar]
- 21. Craiem D, Chironi G, Graf S et al. Atheromatous plaque: quantitative analysis of the echogenicity of different layers. Rev Esp Cardiol 2009; 62: 984–991 [DOI] [PubMed] [Google Scholar]
- 22. Valls J, Cambray S, Perez-Guallar C et al. Association of candidate gene polymorphisms with chronic kidney disease: results of a case-control analysis in the Nefrona cohort. Front Genet 2019; 10: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R Core Team. R: A Languaje and Enviroment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019
- 24. Russo D, Morrone LF, Brancaccio S et al. Pulse pressure and presence of coronary artery calcification. Clin J Am Soc Nephrol 2009; 4: 316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang TJ, Zhang F, Richards JB et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010; 376: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang X, O’Reilly PF, Aschard H et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun 2018; 9: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petkovich M, Jones G. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens 2011; 20: 337–344 [DOI] [PubMed] [Google Scholar]
- 28. Shen H, Bielak LF, Ferguson JF et al. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol 2010; 30: 2648–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yao S, Sucheston LE, Smiley SL et al. Common genetic variants are associated with accelerated bone mineral density loss after hematopoietic cell transplantation. PLoS One 2011; 6: e25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colussi G, Ganon L, Penco S et al. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): implications for mineral metabolism changes in chronic renal failure. Nephrol Dial Transplant 2014; 29: 636–643 [DOI] [PubMed] [Google Scholar]
- 31. Koh N, Fujimori T, Nishiguchi S et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 2001; 280: 1015–1020 [DOI] [PubMed] [Google Scholar]
- 32. Navarro-Gonzalez JF, Donate-Correa J, Muros de Fuentes M et al. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 2014; 100: 34–40 [DOI] [PubMed] [Google Scholar]
- 33. Friedman DJ, Afkarian M, Tamez H et al. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res 2009; 24: 1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imamura A, Okumura K, Ogawa Y et al. Klotho gene polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospastic angina in Japanese. Clin Chim Acta 2006; 371: 66–70 [DOI] [PubMed] [Google Scholar]
- 35. Jo SH, Kim SG, Choi YJ et al. KLOTHO gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in a Korean population. Int Heart J 2009; 50: 23–32 [DOI] [PubMed] [Google Scholar]
- 36. Elghoroury EA, Fadel FI, Elshamaa MF et al. Klotho G-395A gene polymorphism: impact on progression of end-stage renal disease and development of cardiovascular complications in children on dialysis. Pediatr Nephrol 2018; 33: 1019–1027 [DOI] [PubMed] [Google Scholar]
- 37. Valdivielso JM, Bozic M, Galimudi RK et al. Association of the rs495392 Klotho polymorphism with atheromatosis progression in patients with chronic kidney disease. Nephrol Dial Transplant 2019; 34: 2079–2088 [DOI] [PubMed] [Google Scholar]
- 38. Tangri N, Alam A, Wooten EC et al. Lack of association of Klotho gene variants with valvular and vascular calcification in Caucasians: a candidate gene study of the Framingham Offspring Cohort. Nephrol Dial Transplant 2011; 26: 3998–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ichikawa S, Imel EA, Kreiter ML et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 2007; 117: 2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ikushima M, Rakugi H, Ishikawa K et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 2006; 339: 827–832 [DOI] [PubMed] [Google Scholar]
- 41. de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett 2006; 580: 5753–5758 [DOI] [PubMed] [Google Scholar]
- 42. Sanchis P, Ho CY, Liu Y et al. Arterial “inflammaging” drives vascular calcification in children on dialysis. Kidney Int 2019; 95: 958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stergachis AB, Haugen E, Shafer A et al. Exonic transcription factor binding directs codon choice and affects protein evolution. Science 2013; 342: 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eriksson M, Brown WT, Gordon LB et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 2003; 423: 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Presnyak V, Alhusaini N, Chen YH et al. Codon optimality is a major determinant of mRNA stability. Cell 2015; 160: 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pechmann S, Frydman J. Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat Struct Mol Biol 2013; 20: 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferguson JF, Matthews GJ, Townsend RR et al. Candidate gene association study of coronary artery calcification in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). J Am Coll Cardiol 2013; 62: 789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohen GI, Aboufakher R, Bess R et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging 2013; 6: 1160–1167 [DOI] [PubMed] [Google Scholar]
- 49. Laclaustra M, Casasnovas JA, Fernandez-Ortiz A et al. Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: the AWHS study. J Am Coll Cardiol 2016; 67: 1263–1274 [DOI] [PubMed] [Google Scholar]
- 50. Karohl C, D'Marco Gascon L, Raggi P. Noninvasive imaging for assessment of calcification in chronic kidney disease. Nat Rev Nephrol 2011; 7: 567–577 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.