Abstract
Background
Erythropoiesis-stimulating agents (ESAs) are currently the mainstay of treatment for anaemia of chronic kidney disease (CKD). Vadadustat is an investigational oral hypoxia-inducible factor prolyl-hydroxylase inhibitor that stimulates endogenous erythropoietin formation. The INNO2VATE programme comprises two studies designed to evaluate the safety and efficacy of vadadustat versus the ESA darbepoetin alfa in ameliorating anaemia in patients with dialysis-dependent CKD (DD-CKD). Here we describe the trial design along with patient demographics and baseline characteristics.
Methods
Two Phase 3, open-label, sponsor-blind, active-controlled trials enrolled adults with anaemia of CKD who recently initiated dialysis and had limited ESA exposure (incident DD-CKD trial) or were receiving maintenance dialysis with ESA treatment (prevalent DD-CKD trial). Study periods include correction/conversion (Weeks 0–23), maintenance (Weeks 24–52), long-term treatment (Weeks 53 to end of treatment) and safety follow-up. The primary safety endpoint is the time to the first major adverse cardiovascular event and the primary efficacy endpoint is the change in haemoglobin (baseline to Weeks 24–36).
Results
A total of 369 and 3554 patients were randomized in the incident DD-CKD and prevalent DD-CKD trials, respectively. Demographics and baseline characteristics were similar among patients in both trials and comparable to those typically observed in DD-CKD.
Conclusions
The two INNO2VATE trials will provide important information on the safety and efficacy of a novel approach for anaemia management in a diverse DD-CKD population. Demographics and baseline characteristics of enrolled patients suggest that study results will be representative for a large proportion of the DD-CKD population.
Keywords: anaemia, chronic kidney disease, dialysis, hypoxia-inducible factor, vadadustat
KEY LEARNING POINTS
What is already known about this subject?
Erythropoiesis-stimulating agents (ESAs), including recombinant human erythropoietin and its derivatives, are a mainstay of treatment for anaemia of chronic kidney disease (CKD).
Vadadustat is an investigational, oral hypoxia-inducible factor prolyl-hydroxylase inhibitor (HIF-PHI) that stimulates endogenous erythropoietin formation and in Phase 2 trials raised and maintained haemoglobin concentrations in patients with non-dialysis-dependent (NDD) and dialysis dependent (DD)- CKD.
What this study adds?
The INNO2VATE trials are Phase 3, randomized, open-label, sponsor-blind, active-controlled trials designed to evaluate the cardiovascular safety and haematological efficacy of vadadustat versus darbepoetin alfa for the long-term treatment of anaemia in patients with DD-CKD.
In this report we describe the design and methodology of the two Phase 3 INNO2VATE trials and summarize demographic and baseline characteristics of randomized patients.
Importantly, the demographics and baseline characteristics of patients enrolled in the two studies are comparable to those typically observed in patients with DD-CKD, suggesting the results of the INNO2VATE studies will be generalizable to a large proportion of the DD-CKD population.
What impact may this have on practice or policy?
The distinct mechanism of action of vadadustat and other HIF-PHIs may yield a different and potentially improved risk–benefit profile relative to ESAs.
INTRODUCTION
Anaemia is a common complication of chronic kidney disease (CKD) that results primarily from a combination of insufficient synthesis of erythropoietin (EPO) and EPO resistance [1, 2]. Haemodialysis itself is also associated with blood loss and damage to red blood cells (RBCs) [3, 4]. Half of patients with CKD Stage G4 and the vast majority of patients on dialysis have anaemia [5]. Anaemia of CKD results in diminished health-related quality of life and is associated with an increased risk of CKD progression [6, 7], cardiovascular disease (CVD) morbidity and mortality [8–10].
Over the past three decades, recombinant human EPO and its derivatives [collectively termed erythropoiesis-stimulating agents (ESAs)], together with iron supplementation, have been the standard of care for treatment of anaemia of CKD [11]. Large randomized clinical trials testing haemoglobin (Hb) targets in the subnormal range compared with lower targets [Normal Hematocrit Trial (NHCT) [12], Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) [13]] or placebo [Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) [14]] suggested an increased risk of CVD events in patients with CKD targeted to higher Hb targets, including those dependent and those not dependent on dialysis. It is unclear whether this risk is related to higher Hb concentrations or to higher doses of ESAs used, although some evidence favours the latter hypothesis [15–17]. Of note, plasma concentrations of ESAs after intravenous (IV) injection, the most common form of administration, far exceed the physiological range of circulating endogenously produced EPO, which could potentially contribute to adverse events (AEs) in this patient population [18]. In view of the high prevalence of anaemia of CKD and risks associated with RBC transfusions and ESA therapy, there remains a need to optimize anaemia management in patients with CKD.
Recent advances in understanding the role of the hypoxia-inducible factor (HIF) pathway in regulating the body’s adaptive response to hypoxia, including stimulation of erythropoiesis [19, 20], have facilitated the development of a novel therapeutic approach in the treatment of anaemia. The transcription factor HIF-α is subject to oxygen-dependent proteolysis, mediated by a family of prolyl-hydroxylases [21]. HIF prolyl-hydroxylase inhibitors (HIF-PHIs) are a class of agents designed to stabilize HIF-α, thereby enhancing EPO transcription and resultant endogenous EPO production in the kidneys and liver (Supplementary data, Figure S1) [22–24].
Vadadustat is an orally administered HIF-PHI currently in the late stages of clinical development for the treatment of anaemia of CKD. In Phase 2 trials, vadadustat increased and maintained Hb concentrations with minimal excursions in patients with non-dialysis-dependent (NDD)- and dialysis-dependent (DD)-CKD [25–27]. The vadadustat Phase 3 programme includes four efficacy and cardiovascular safety outcome trials of vadadustat versus the ESA darbepoetin alfa. The primary objective of these trials is to investigate the safety and efficacy of vadadustat compared with darbepoetin alfa for the treatment of anaemia in patients with DD-CKD (INNO2VATE trials) or NDD-CKD (PRO2TECT trials). In this report we describe the design and methodology of the two Phase 3 INNO2VATE trials (NCT 02865850, NCT02892149) and summarize demographic and baseline characteristics of randomized patients; the PRO2TECT trials will be reported on separately.
MATERIALS AND METHODS
Study design
The INNO2VATE trials are Phase 3, randomized, open-label, sponsor-blind, active-controlled trials designed to evaluate the cardiovascular safety and haematological efficacy of vadadustat versus darbepoetin alfa for the treatment of anaemia in patients with DD-CKD. Efficacy and safety will be assessed during and after the attainment of target range Hb concentrations in patients who recently initiated dialysis and had limited exposure to ESAs (incident DD-CKD trial) or after conversion from ESA in patients on maintenance dialysis with prolonged prior exposure to ESAs (prevalent DD-CKD trial). When first registered in ClinicalTrials.gov, these studies were described as “Correction/Conversion” and “Conversion” trials, respectively. We now use the terms “incident DD-CKD” and “prevalent DD-CKD” trials, respectively, to avoid confusion and better represent the study population enrolled in these trials.
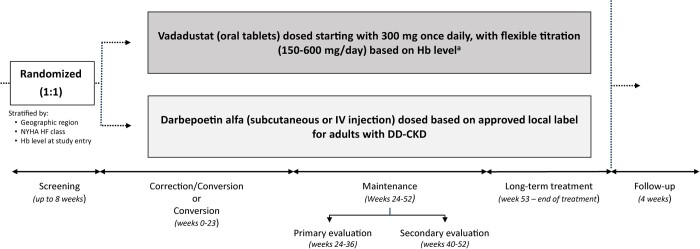
Following a screening period of up to 8 weeks, eligible patients were randomized 1:1 to vadadustat or darbepoetin alfa, stratified by geographic region (USA versus Europe versus other regions), New York Heart Association Heart Failure (NYHA HF) Class (0/I versus II/III) and Hb concentration at entry (incident DD-CKD trial: <9.5 versus ≥9.5 g/dL; prevalent DD-CKD trial: <10 versus ≥10 g/dL). Patients in both trials entered four sequential study periods for treatment and evaluation of safety and efficacy: a correction or conversion period (Weeks 0–23); (ii) a maintenance period (Weeks 24–52), comprising both primary (Weeks 24–36) and secondary (Weeks 40–52) efficacy evaluation periods; a long-term treatment period (Weeks 53 to end of treatment); and a 4-week safety follow-up period (Figure 1). The INNO2VATE trials use an event-driven design and terminate when a prespecified number of major adverse cardiovascular events (MACEs) accrue across both trials based on power calculation (vide infra).
FIGURE 1.
Study design. After a screening period of up to 8 weeks, eligible patients were randomized 1:1 to vadadustat or darbepoetin alfa. Patients in both trials entered four sequential study periods for treatment and evaluation of safety and efficacy: a correction or conversion period (Weeks 0–23), a maintenance period (Weeks 24–52) comprising both a primary (Weeks 24–36) and secondary (Weeks 40–52) efficacy evaluation period, a long-term treatment period (Weeks 53 to end of treatment) and a 4-week safety follow-up period. aStudy drug is titrated to achieve target Hb levels (USA: 10–11 g/dL; non-USA: 10–12 g/dL).
Study endpoints
The primary safety endpoint is time to first adjudicated MACE, defined as the composite endpoint of all-cause mortality, non-fatal myocardial infarction (MI) or non-fatal stroke. A key secondary endpoint is time to first expanded MACE, a composite endpoint of MACE plus hospitalization for heart failure, or thromboembolic event (excluding vascular access failure).
The primary efficacy endpoint is a change in Hb between baseline (mean pre-treatment Hb concentration) and the primary evaluation period (mean Hb concentration from Weeks 24 to 36). The key secondary efficacy endpoint is a change in Hb between baseline and the secondary evaluation period (mean Hb concentration from Weeks 40 to 52). Other efficacy endpoints include the proportion of patients with Hb concentrations within the target range (10–11 g/dL in USA, 10–12 g/dL in non-USA) during the primary evaluation period (Weeks 24–36), proportion of patients with Hb concentrations within the target range during the secondary evaluation period (Weeks 40–52) and time to achieve stable Hb concentrations within the target range. Safety and efficacy assessments involving changes in Hb concentrations are based on measurements from a central laboratory following protocol-specified blood draws. Exploratory endpoints include changes in the biomarkers hepcidin and vascular endothelial growth factor (VEGF).
A listing of safety and efficacy endpoints is provided in Table 1.
Table 1.
Primary and key secondary safety and efficacy endpoints and selected endpoints of special interest
| Primary safety endpoint | Time to first MACE (all cause mortality, non-fatal MI, non-fatal stroke) |
| Key secondary safety endpoints | Time to first expanded MACE: MACE plus hospitalization for HF or thromboembolic event, excluding vascular access failure |
| Time to cardiovascular mortality, non-fatal MI or non-fatal stroke | |
| Time to cardiovascular mortality | |
| Time to all-cause mortality | |
| Other safety endpoints |
|
| Primary efficacy endpoint | Change in average Hb between baseline and the primary evaluation period (Weeks 24–36) |
| Key secondary efficacy endpoint | Change in average Hb value between baseline and the secondary evaluation period (Weeks 40–52) |
| Other efficacy endpoints |
|
| Endpoints of special interest |
|
TEA, treatment emergent adverse event.
Eligibility
Eligibility criteria were chosen to maximize the generalizability of results to patients with anaemia of DD-CKD. Eligible patients were adults (≥18 years) with anaemia of DD-CKD, with serum ferritin ≥100 ng/mL and transferrin saturation (TSAT) ≥20%, who had not received a RBC transfusion within 8 weeks prior to randomization. For the incident DD-CKD study, patients were required to have initiated maintenance dialysis (haemodialysis or peritoneal dialysis) within 16 weeks prior to screening, with 8–11 g/dL. Initial eligibility criteria specified Hb <10 g/dL, which was later amended to the current range. Eligibility also included receipt of limited doses of ESAs. Initial eligibility criteria excluded patients who had received more than one dose of long acting ESA (darbepoetin alfa or methoxy polyethelyene glycol epoetin beta), or two or more doses of short-acting ESA within 8 weeks prior to screening, later amended to up to two doses of long-acting and up to four doses of short-acting ESA. A subsequent protocol amendment removed the restriction on ESA use in the eight weeks prior and during the initial to screening period. Patients were excluded if they met the following criteria for ESA resistance within 8 weeks prior to screening: epoetin >7700 U/dose three times per week or darbepoetin alfa >23 000 U/week, or methoxy polyethylene glycol-epoetin beta >100 μg every other week or >200 μg every month.
For the prevalent DD-CKD study, patients were required to have received maintenance dialysis for at least 12 weeks prior to screening and to be currently receiving any form of ESA therapy, with Hb 8–11 g/dL (USA) or 9–12 g/dL (non-USA).
In both studies, patients were excluded if their anaemia was thought to be due to a cause(s) other than CKD, including active or recent bleeding, or if they had uncontrolled hypertension or had recent CVD events (e.g. MI; surgical or percutaneous intervention for coronary, cerebrovascular or peripheral artery disease; surgical or percutaneous valvular replacement or repair; sustained ventricular tachycardia or stroke within 12 weeks prior to or during screening). Eligibility criteria across the two trials are presented in Table 2.
Table 2.
Final eligibility criteria
| INNO2VATE incident trial | INNO2VATE prevalent trial |
|---|---|
| NCT02865850 (n = 369) | NCT02892149 (n = 3554) |
|
|
| |
| Hb eligibility | |
| Hb 8–11 g/dL | USA: Hb 8–11 g/dL |
| Non-USA: Hb 9–12 g/dL | |
| ESA treatment | |
| Limited prior exposure to ESA therapy (see text for details) Cannot have met the following criteria for ESA resistance within 8 weeks prior to or during screening: epoetin >7700 U/dose three times per week or >23 000 U/week, darbepoetin alfa >100 μg/week or methoxy polyethylene glycol-epoetin beta >100 μg every other week or >200 μg every month | Currently maintained on ESA therapy, with a dose received within 6 weeks prior to or during screening |
| RBC transfusions | |
| No RBC transfusions within 8 weeks prior to randomization | |
| Exclusion of patients with any of the following: | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
BP, blood pressure; DVT, deep vein thrombosis; PE, pulmonary embolism.
Dosing
Vadadustat was provided as 150 mg tablets during the study and will be made available as 150, 300 and 450 mg upon regulatory approval. The starting dose for vadadustat was 300 mg once daily (two 150 mg tablets), with doses of 150, 300, 450 and 600 mg daily available for dose titration during the study after an initial 4-week fixed-dose period. Darbepoetin alfa was administered subcutaneously or intravenously; the initial dose was based on the prior dose for patients already on darbepoetin alfa and based on the product label for those not on darbepoetin alfa prior to randomization. Study drugs were titrated to achieve target Hb concentrations (USA: 10–11 g/dL; non-USA: 10–12 g/dL), which were derived from clinical practice guidelines, regulatory agency discussions and darbepoetin alfa product labelling [28]. Dose adjustments were made based on protocol-specified dose adjustment guideline algorithms, customized by regional (USA and non-USA) considerations (Supplementary data, Figure S2). Dose adjustments were based on Hb values obtained via the HemoCue (HemoCue AB, Ängelholm, Sweden) point-of-care device for expediency. Hb concentrations were measured via HemoCue every 2 weeks from Weeks 0 to 12, every 4 weeks from Weeks 12 to 52 and on a flexible monthly schedule from Week 53 to the end of the study.
Data collection
Study visits occurred at baseline and every 2 weeks through Week 12, every 4 weeks from Weeks 13 to 52 and then every 12 weeks until the end of treatment. Safety parameters [MACEs, TEAEs, vital signs, complete blood counts, RBC transfusion and ‘rescue’ assessment (vide infra)], concomitant medications and drug dispensing records were collected at every study visit. Patients who permanently discontinued study medication early or withdrew from the study prior to Week 52 continued with the schedule of activities and safety assessments through Week 52 and were followed for safety-only assessments after Week 52. Patients who stopped study medication after Week 52 were followed for safety assessments for the remainder of the study. A schedule of activities is provided in Supplementary data, Table S1.
Iron supplementation and rescue
During the trials, investigators were requested to prescribe iron supplementation (IV, oral or intradialytic) to maintain ferritin ≥100 ng/mL or TSAT ≥ 20%. RBC transfusions as rescue therapy were permitted and did not require discontinuation of study medication. Starting at Week 6, patients in both treatment arms could receive ESAs as ‘rescue’ therapy if they experienced a worsening of the symptoms of anaemia with a Hb concentration <9.5 g/dL. While receiving ESAs as rescue therapy, study drugs were temporarily discontinued. ESA rescue was stopped when Hb concentrations increased to ≥10.0 g/dL, at which time study drugs could be restarted. Restarting vadadustat followed a protocol-specified interval based on the timing of the administration of the last dose of the specific ESA used for rescue (epoetin alfa: 2 days; darbepoetin alfa: 7 days; methoxy polyethylene glycol-epoetin beta: 14 days).
Power and sample size calculation
The primary safety analysis, prespecified to be pooled from both trials, will be based upon all events that accrue over the two DD-CKD trials. The sample size has been determined based on the number of events needed to demonstrate non-inferiority of the two-sided 95% confidence interval (CI) for the hazard ratio (vadadustat versus darbepoetin alfa). We calculated that 631 MACEs will be required to have 80% power to establish non-inferiority with the upper bound of the 95% CI not exceeding 1.25 and >90% power to establish non-inferiority with a margin of 1.30. A MACE rate of 12% annually is anticipated in both treatment arms based on findings from observational studies and clinical trials [29, 30].
The primary efficacy analysis assumes that the mean change from baseline in Hb concentrations for vadadustat will be the same as for darbepoetin alfa, and the common standard deviation (SD) for the mean change from baseline is assumed to be 1.5 g/dL. Non-inferiority will be established based on a two-sided 95% CI for the difference between vadadustat and darbepoetin alfa groups, using a non-inferiority margin of −0.75 g/dL. The anticipated sample size is 150 patients per treatment group for the incident DD-CKD study and 1650 patients per treatment group for the prevalent DD-CKD study and will have >90% and >99% power, respectively, for the non-inferiority assessment.
Statistical analyses
The primary safety endpoint, time to the first adjudicated MACE, will be analysed as (date of first MACE − date of first dose + 1) in the safety population (randomized patients who receive one or more doses of study treatment). Patients without a MACE at the time of study closure will be censored on the date of their last study assessment. Analysis of time to the first MACE will be based on a stratified Cox regression model with study as a stratification factor. The model will also include covariates of baseline Hb, randomization strata of region (USA, Europe, non-USA/non-Europe) and NYHA HF (0/I; II/III), sex, age (>65, ≤65 years), race (white or non-white), pre-existing CVD and pre-existing diabetes mellitus. The primary MACE analysis will be based upon all events that accrue over both INNO2VATE trials. Pre-specified hierarchical testing will be conducted on other secondary safety outcomes. MACEs, thromboembolic events, hospitalizations for heart failure and for events of vascular access failure are adjudicated by an independent adjudication committee, which is blinded to the treatment allocation.
Treatment-emergent adverse events (TEAEs) will be summarized as the number and percentage of patients with TEAEs for the safety population. Descriptive summaries will also be provided for serious TEAEs (TESAEs), related TEAEs and TEAEs leading to early discontinuation of study medication. Subgroup summaries of TESAEs by age, gender and time since CKD diagnosis will be conducted. The primary efficacy endpoint, as well as all secondary endpoints, will be summarized for the randomized population using descriptive statistics by treatment group, as well as by study visit and/or analysis period. Primary and key secondary efficacy analyses will use an analysis of covariance with multiple imputation for missing data. The primary analysis model will contain treatment group, baseline Hb concentration and the two stratification factors (region and NYHA HF class) as predictor variables.
RESULTS
In total, 652 patients were screened for the incident DD-CKD trial and 369 were randomized; of those, 52.8% were in the USA and the remainder were in Europe and non-USA/non-Europe. A total of 4944 patients were screened for the prevalent DD-CKD trial and 3554 were randomized; of those 61.2% were in the USA and the remainder were in other countries in Europe, South America and Asia. The most common reason for screen failure in both trials was an out-of-range Hb concentration.
Baseline demographics and characteristics
Baseline demographics and patient characteristics for the incident DD-CKD and the prevalent DD-CKD trials are presented in Table 3.
Table 3.
Demographic and baseline characteristics
| INNO2VATE incident trial |
INNO2VATE prevalent trial |
|||||
|---|---|---|---|---|---|---|
| Characteristics | US | Non-US | Total | US | Non-US | Total |
| (n = 195) | (n = 170) | (N = 369) | (n = 2176) | (n = 1378) | (N = 3554) | |
| Age (years), mean (SD) | 57.8 (15.2) | 54.3 (13.8) | 56.0 (14.7) | 59.0 (13.4) | 56.8 (14.4) | 58.1 (13.9) |
| Male, n (%) | 124 (63.6) | 94 (55.3) | 220 (59.6) | 1181 (54.3) | 813 (59.0) | 1994 (56.1) |
| BMI (kg/m2), mean (SD) | 28.4 (6.1) | 26.4 (5.6) | 27.5 (6.0) | 30.1 (7.8) | 26.2 (5.4) | 28.6 (7.2) |
| Race, n (%) | ||||||
| White | 128 (65.6) | 139 (81.8) | 271 (73.4) | 1167 (53.6) | 1064 (77.2) | 2231 (62.8) |
| Black or African-American | 56 (28.7) | 17 (10.0) | 73 (19.8) | 779 (35.8) | 97 (7.0) | 876 (24.6) |
| Asian | 9 (4.6) | 11 (6.5) | 20 (5.4) | 63 (2.9) | 112 (8.1) | 175 (4.9) |
| American Indian or Alaska Native | 0 | 1 (0.6) | 1 (0.3) | 46 (2.1) | 3 (0.2) | 49 (1.4) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 18 (0.8) | 1 (0.1) | 19 (0.5) |
| Other | 1 (0.5) | 0 | 1 (0.3) | 25 (1.1) | 62 (4.5) | 87 (2.4) |
| Multiple | 0 | 1 (0.6) | 1 (0.3) | 7 (0.3) | 6 (0.4) | 13 (0.4) |
| Not reported | 1 (0.5) | 0 | 1 (0.3) | 71 (3.3) | 33 (2.4) | 104 (2.9) |
| Smoking, n (%) | ||||||
| Never smoked | 112 (57.4) | 129 (75.9) | 245 (66.4) | 1354 (62.2) | 877 (63.6) | 2231 (62.8) |
| Former smoker | 74 (37.9) | 30 (17.6) | 104 (28.2) | 643 (29.5) | 350 (25.4) | 993 (27.9) |
| Current smoker | 9 (4.6) | 10 (5.9) | 19 (5.1) | 179 (8.2) | 151 (11.0) | 330 (9.3) |
| Geographic region, n (%) | ||||||
| USA | 195 (100.0) | 0 | 195 (52.8) | 2176 (100.0) | 0 | 2176 (61.2) |
| Europe | 0 | 42 (24.7) | 42 (11.4) | 0 | 404 (29.3) | 404 (11.4) |
| Non-USA/non-Europe | 0 | 128 (75.3) | 128 (34.7) | 0 | 974 (70.7) | 974 (27.4) |
| Type of dialysis, n (%) | ||||||
| Haemodialysis | 171 (87.7) | 154 (90.6) | 325 (88.1) | 2034 (93.5) | 1245 (90.3) | 3279 (92.3) |
| Peritoneal | 17 (8.7) | 14 (8.2) | 35 (9.5) | 141 (6.5) | 131 (9.5) | 272 (7.7) |
| Years on dialysis, mean (SD) | 0.13 (0.26) | 0.14 (0.14) | 0.14 (0.21) | 3.87 (3.86) | 4.10 (4.27) | 3.96 (4.02) |
| Vascular access, n (%)a | ||||||
| Arteriovenous fistula | 67 (34.4) | 101 (59.4) | 168 (45.5) | 1525 (70.1) | 1036 (75.2) | 2561 (72.1) |
| Arteriovenous graft | 8 (4.1) | 1 (0.6) | 9 (2.4) | 283 (13.0) | 47 (3.4) | 330 (9.3) |
| Temporary (i.e. non-tunnelled dialysis) catheter | 18 (9.2) | 23 (13.5) | 41 (11.1) | 33 (1.5) | 24 (1.7) | 57 (1.6) |
| Tunnelled dialysis catheter | 81 (41.5) | 29 (17.1) | 110 (29.8) | 199 (9.1) | 155 (11.2) | 354 (10.0) |
| Not applicable (peritoneal dialysis) | 18 (9.2) | 16 (9.4) | 38 (10.3) | 145 (6.7) | 135 (9.8) | 280 (7.9) |
| Other | 1 (0.5) | 2 (1.2) | 3 (0.8) | 1 (0.0) | 2 (0.1) | 3 (0.1) |
| Comorbidities/aetiology of CKD, n (%)a | ||||||
| Autoimmune/glomerulonephritis/vasculitis | 8 (4.1) | 45 (26.5) | 53 (14.4) | 94 (4.3) | 264 (19.2) | 358 (10.1) |
| Cystic/hereditary/congenital disease | 2 (1.0) | 12 (7.1) | 14 (3.8) | 34 (1.6) | 98 (7.1) | 132 (3.7) |
| Diabetes | 114 (58.5) | 47 (27.6) | 163 (44.2) | 1227 (56.4) | 387 (28.1) | 1614 (45.4) |
| Hypertension | 117 (60.0) | 43 (25.3) | 164 (44.4) | 1311 (60.2) | 490 (35.6) | 1801 (50.7) |
| Interstitial nephritis/pyelonephritis | 1 (0.5) | 21 (12.4) | 22 (6.0) | 7 (0.3) | 149 (10.8) | 156 (4.4) |
| Neoplasms/tumours | 1 (0.5) | 0 | 1 (0.3) | 4 (0.2) | 10 (0.7) | 14 (0.4) |
| Other | 10 (5.1) | 28 (16.5) | 39 (10.6) | 167 (7.7) | 233 (16.9) | 400 (11.3) |
| Laboratory values and BP, mean (SD) | ||||||
| Pre-baseline ESA (U/kg/week) | 159.5 (123.9) | 119.1 (77.9) | 149.6 (115.2) | 119.5 (122.5) | 104.1 (83.0) | 113.6 (109.2) |
| Baseline Hb (g/dL) | 9.5 (1.0) | 9.0 (1.2) | 9.3 (1.1) | 10.0 (0.8) | 10.6 (0.8) | 10.2 (0.8) |
| Ferritin (ng/mL) | 520.4 (319.9) | 475.7 (407.5) | 499.3 (362.9) | 962.8 (499.0) | 655.3 (575.0) | 843.6 (550.5) |
| TSAT (%) | 33.5 (12.1) | 31.8 (10.1) | 32.8 (11.3) | 38.6 (13.1) | 36.7 (13.6) | 37.9 (13.3) |
| TIBC (μg/dL) | 219.9 (37.2) | 235.1 (42.5) | 226.9 (40.3) | 206.8 (34.9) | 217.3 (37.3) | 210.9 (36.2) |
| Hepcidin (ng/mL) | 126.1 (114.4) | 123.7 (106.8) | 124.7 (110.2) | 209.4 (136.6) | 164.2 (135.6) | 192.0 (138.0) |
| CRP (mg/dL) | 1.0 (1.8) | 0.9 (1.4) | 0.9 (1.6) | 1.1 (2.0) | 0.9 (1.7) | 1.0 (1.9) |
| Total cholesterol (mg/dL) | 156.6 (42.8) | 177.7 (45.6) | 167.2 (46.0) | 149.2 (40.5) | 164.0 (43.9) | 154.9 (42.5) |
| LDL cholesterol (mg/dL) | 78.6 (34.5) | 97.7 (37.1) | 87.8 (37.3) | 72.0 (32.9) | 86.7 (35.4) | 77.7 (34.6) |
| HDL cholesterol (mg/dL) | 46.3 (17.8) | 46.3 (17.3) | 46.5 (17.6) | 46.7 (16.9) | 43.4 (15.0) | 45.4 (16.3) |
| Triglycerides (mg/dL) | 162.1 (93.5) | 178.2 (122.4) | 170.1 (107.9) | 155.7 (115.4) | 171.2 (109.0) | 161.7 (113.2) |
| Systolic BP (mmHg) | 143.4 (22.4) | 142.2 (19.2) | 142.8 (20.9) | 145.5 (23.7) | 139.3 (20.4) | 143.1 (22.7) |
| Diastolic BP (mmHg) | 76.3 (12.7) | 79.6 (13.3) | 77.8 (13.0) | 75.8 (13.5) | 77.1 (12.7) | 76.3 (13.2) |
| Prior medications, n (%) | ||||||
| Diuretics | 92 (47.2) | 60 (35.3) | 152 (41.2) | 651 (29.9) | 393 (28.5) | 1044 (29.4) |
| Beta-blocking agents | 134 (68.7) | 86 (50.6) | 220 (59.6) | 1435 (65.9) | 702 (50.9) | 2137 (60.1) |
| ACE inhibitors | 33 (16.9) | 35 (20.6) | 68 (18.4) | 478 (22.0) | 241 (17.5) | 719 (20.2) |
| Angiotensin II receptor blockers | 42 (21.5) | 41 (24.1) | 83 (22.5) | 486 (22.3) | 331 (24.0) | 817 (23.0) |
| Calcium channel blockers | 131 (67.2) | 97 (57.1) | 228 (61.8) | 1132 (52.0) | 612 (44.4) | 1744 (49.1) |
| Insulin | 74 (37.9) | 35 (20.6) | 109 (29.5) | 813 (37.4) | 276 (20.0) | 1089 (30.6) |
| Blood glucose-lowering drugs, excl. insulin | 37 (19.0) | 15 (8.8) | 52 (14.1) | 325 (14.9) | 94 (6.8) | 419 (11.8) |
| Aldosterone antagonist | 3 (1.5) | 4 (2.4) | 7 (1.9) | 30 (1.4) | 13 (0.9) | 43 (1.2) |
| HMG CoA reductase inhibitors | 99 (50.8) | 42 (24.7) | 141 (38.2) | 1124 (51.7) | 365 (26.5) | 1489 (41.9) |
| Lipid-modifying agents | 104 (53.3) | 46 (27.1) | 150 (40.7) | 1175 (54.0) | 389 (28.2) | 1564 (44.0) |
| Aspirin | 63 (32.3) | 35 (20.6) | 98 (26.6) | 904 (41.5) | 419 (30.4) | 1323 (37.2) |
| Vitamin K antagonists | 6 (3.1) | 2 (1.2) | 8 (2.2) | 126 (5.8) | 60 (4.4) | 186 (5.2) |
| Oral iron | 16 (8.2) | 9 (5.3) | 25 (6.8) | 195 (9.0) | 116 (8.4) | 311 (8.8) |
| IV iron | 26 (13.3) | 13 (7.6) | 39 (10.6) | 403 (18.5) | 174 (12.6) | 577 (16.2) |
aParticipants may contribute to multiple categories. BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; HDL, high-density lipoprotein; HMG CoA, 3-Hydroxy-3-Methylglutaryl-Coenzym-A-Reductase; LDL, low-density lipoprotein; TIBC, total iron binding capacity.
The mean age of patients in the incident DD-CKD and prevalent DD-CKD trials was 56.0 (SD 14.7) and 58.1 (SD 13.9) years, respectively. In both trials, most patients were white (73.4 and 62.8%, respectively) and male (59.6 and 56.1%, respectively). Both study populations were diverse, with representation of non-White patients in the incident and prevalent DD-CKD trials including Black/African American (19.8 and 24.6%, respectively), Asian (5.4 and 4.9%, respectively), Native Hawaiian or Pacific Islander (0 and 0.5%, respectively), American Indian or Alaska Native (0.3 and 1.4%, respectively) and others (0.3 and 2.4%, respectively).
As per design, the mean time on dialysis in the incident DD-CKD trial was 0.14 (SD 0.21) years, in contrast to 3.96 (4.02) years in the prevalent DD-CKD trial. Arteriovenous fistula use in both studies was 45.5 and 72.1%, respectively. The proportion of patients treated by peritoneal dialysis was 9.5 and 7.7%, respectively.
Baseline characteristics were similar among patients in both trials, regardless of diabetes (Supplementary data, Table S2) or CVD history (Supplementary data, Table S3), except for expected differences (e.g. insulin and lipid-modifying drug use in patients with diabetes, aspirin use in patients with CVD).
DISCUSSION
The INNO2VATE programme includes two clinical trials that are designed to evaluate the safety and efficacy of oral vadadustat compared with darbepoetin alfa in patients with anaemia of DD-CKD who either initiated dialysis within 16 weeks with limited cumulative ESA use (incident DD-CKD trial) or who were receiving maintenance dialysis with active ESA treatment (prevalent DD-CKD trial).
While ESAs have been used successfully to reduce the burden of RBC transfusion in patients with DD-CKD, several studies targeting Hb concentrations in the (sub)normal range have highlighted the potential for increased cardiovascular risk in this vulnerable population. The main goal of the INNO2VATE studies is to evaluate the cardiovascular safety during long-term treatment of anaemia with vadadustat as compared with darbepoetin alfa. To this end, the primary endpoint of the INNO2VATE programme is a MACE endpoint and the programme is designed with enough power to allow for a statistical comparison of the rates of deaths and cardiovascular events between the vadadustat and darbepoetin alfa groups. Darbepoetin alfa was chosen as an active comparator since it is marketed and available globally and has a defined safety profile. To provide the most comprehensive safety information, the trials will assess changes in all-cause mortality rather than solely cardiovascular mortality as part of the MACE primary endpoint. Given the trial population, half of the deaths are expected to be caused by CVD and CVD mortality is a key secondary safety endpoint. A criterion of non-inferiority to darbepoetin alfa was chosen because ESAs are the standard of care.
In completed Phase 1 clinical trials of vadadustat in healthy volunteers, there were similar numbers of TEAEs in the placebo and vadadustat treatment arms [31]. In Phase 2 trials, the most frequently reported AEs were gastrointestinal disorders (nausea, diarrhoea and vomiting) [25–27]. Although few cardiovascular TEAEs were reported in the Phase 2 trials, those trials were neither designed nor adequately powered to find between-group differences in MACEs [25–27]. In contrast, the INNO2VATE trials have been designed specifically to address whether the treatment of anaemia by ESAs and vadadustat are associated with similar or differential cardiovascular risk.
While Phase 2 trials demonstrated sustained Hb concentrations through 16 weeks [25–27], the INNO2VATE clinical trials will evaluate the longer-term sustained effect on Hb through the course of the studies by assessing changes in Hb concentrations from baseline through the primary (Weeks 24–36) and secondary (Weeks 40–52) evaluation periods. RBC transfusion and ESA as rescue therapies were allowed for patients experiencing worsening anaemia and the number of rescues and specific reasons for administration will be evaluated.
Patients in the Phase 2 trials who received vadadustat experienced a decrease in hepcidin and ferritin and an increase in TSAT, suggesting improvement in iron utilization relative to standard therapy with ESA and IV iron [31, 32]. Potential clinical benefits of such changes include enhanced erythropoiesis. The INNO2VATE clinical trials will further elucidate these effects by assessment of iron profiles and IV iron requirements.
In addition to erythropoiesis, HIF is involved in gene transcription of numerous metabolic and enzymatic pathways [20, 21, 33] and the full extent of HIF target gene activation through HIF-PHIs used in doses that are sufficient to stimulate EPO formation is yet to be determined. Although many studies suggest potential benefits of HIF activation, other preclinical studies have reported potentially unfavourable effects [34]. Thus the clinical relevance of non-erythropoietic effects of HIF-PHI remains unclear. In addition to assessing the overall safety profile, the current studies will assess VEGF concentrations and other parameters that might indicate non-erythropoietic effects of HIF-PHIs in humans.
These trials were performed using a parallel-group, open-label design. The open-label design was chosen because blinding of the trials would have increased the complexity tremendously and introduced major practical problems, including the need for a double-dummy design, potential dosing errors, potentially inappropriate dose adjustments and delays in dosing. Because Hb concentrations are objective and are measured via a central laboratory for all efficacy endpoints, efficacy assessments are not considered to be subject to bias with an open-label design. Members of the Endpoint Adjudication Committee, which independently adjudicates major cardiovascular safety events, were blinded to the treatment allocation. However, there is an inherent bias with an open-label design that could bias TESAE and TEAE reporting.
The starting dose and the proposed dosing algorithm in this study are designed to maintain Hb concentrations within the target range in a predictable and controlled manner while minimizing excursions and sharp declines. Of note, in order to avoid Hb overshoot, a cautious approach was taken with a low vadadustat starting dose, fixed for 4 weeks, while patients randomized to darbepoetin were dosed based on their established prior dose requirement.
Patients were assigned using permuted block randomization with a 1:1 ratio to vadadustat or darbepoetin alfa. To help maintain a balance between treatment arms, randomization was stratified by geographic region, NYHA HF class and Hb concentration at study entry.
Being designed as part of the global Phase 3 programme, patients from 17 countries were enrolled in both studies; more than half of the overall study population were from the USA, ∼11% were from Europe and ∼30% from other regions. Demographic data and baseline characteristics of patients in the two trials appear similar to those of typical populations of patients with DD-CKD [10, 35]. Among patients from the USA, the proportion of those with hereditary, congenital, autoimmune or glomerular kidney disease or vasculitis was lower than in other regions and the proportion of patients with diabetes was higher. The prevalence of CKD increases dramatically with age, more than tripling from ages 40–59 to ≥60 years [35]. Patients in the incident DD-CKD and prevalent DD-CKD trials had mean ages of 56.0 and 58.1 years, respectively, with little differences across regions.
In the incident DD-CKD and prevalent DD-CKD trials, a history of CVD (coronary artery disease, MI, stroke and heart failure) was reported in 38 and 50% of patients, respectively, which is slightly lower than previously reported for patients with DD-CKD [35, 36].
Target enrolments for the incident and prevalent DD-CKD trials were 300 and 3300 patients, respectively, and the randomized populations encompass 369 and 3554 patients, respectively. Both trials were completed in January 2020.
While clinical trial programmes of several other HIF-PHIs are ongoing, so far only two comparatively small Phase 3 studies of roxadustat conducted in China in 154 patients with NDD-CKD and 305 with DD-CKD patients have been published [37, 38].
In conclusion, we enrolled >3900 patients with DD-CKD into two prospective clinical trials that will be analysed jointly to assess the safety and efficacy of vadadustat, a novel orally active HIF-PHI, relative to darbepoetin alfa. Importantly, the demographics and baseline characteristics of patients enrolled in the two studies are comparable to those typically observed in patients with DD-CKD [10, 36], suggesting the results of the INNO2VATE studies will be generalizable to a large proportion of the DD-CKD population.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
Writing and editorial assistance were provided by Prescott Medical Communications Group, Chicago, IL, USA. Editorial coordination and support were provided by Sarah Garber, PharmD, an employee of Akebia Therapeutics, who may own stock or stock options in the company.
FUNDING
This study was funded by Akebia Therapeutics.
AUTHORS’ CONTRIBUTIONS
All authors provided critical intellectual content, contributing to the interpretation of data and the drafting or revision of the manuscript, and reviewed and approved the submitted manuscript.
CONFLICT OF INTEREST STATEMENT
K.U.E. has received fees from Akebia and Bayer and grants from Amgen, Bayer, Fresenius, Genzyme, Shire and Vifor. R.A. is a consultant for and on the scientific advisory board of Akebia; has received consulting fees from Bayer, Boehringer Ingelheim, Takeda, Daiichi Sankyo, Eli Lilly, Rlypsa, Reata, Opko, ZS Pharma and Merck; is on the data safety monitoring committee for AstraZeneca, Amgen (past) and Celgene (past) and attended advisory board meetings for AbbVie, Johnson & Johnson, Boehringer Ingelheim and Relypsa. A.G.J.: none. M.J.K. is a member of the Executive Steering Committee for Akebia; a consultant for FibroGen and is on the Data and Safety Management Committee for Micelle BioPharma. K.M. is a consultant for Akebia, Kyowa Kirin, Healthy.io and Fukuda Denshi and has received grants from Kyowa Kirin, Fukuda Denshi and the National Institutes of Health. P.A.M. is a consultant for Akebia. P.P. is on the Executive Steering Committee for Akebia. M.J.S. is a consultant for Cardurian and is on the Advisory Board for Bayer and the Steering Committee for Akebia. W.C.W. is on the Executive Steering Committee for Akebia and is a consultant for AstraZeneca, Bayer, Janssen, Merck, Relypsa and Vifor Fresenius Medical Care Renal Pharma. Y.M.K.F., Z.K., W.L., G.R. and D.V. are employees of Akebia. G.M.C. has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Amgen; personal fees from Akebia during the study; personal fees and other from Ardelyx; personal fees from AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Reata, Sanifit, Vertex, Satellite Healthcare, Angion, Bayer and ReCor and other fees from CloudCath, Durect and Outset. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis 2009; 16: 94–100 [DOI] [PubMed] [Google Scholar]
- 3. Polaschegg HD. Red blood cell damage from extracorporeal circulation in hemodialysis. Semin Dial 2009; 22: 524–531 [DOI] [PubMed] [Google Scholar]
- 4. Tsukamoto T, Matsubara T, Akashi Y et al. Annual iron loss associated with hemodialysis. Am J Nephrol 2016; 43: 32–38 [DOI] [PubMed] [Google Scholar]
- 5. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014; 9: e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mix TC, Brenner RM, Cooper ME et al. Rationale—Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. Am Heart J 2005; 149: 408–413 [DOI] [PubMed] [Google Scholar]
- 7. Keane WF, Brenner BM, de Zeeuw D et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63: 1499–1507 [DOI] [PubMed] [Google Scholar]
- 8. Abramson JL, Jurkovitz CT, Vaccarino V et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int 2003; 64: 610–615 [DOI] [PubMed] [Google Scholar]
- 9. Thomas B, Matsushita K, Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 2017; 28: 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Locatelli F, Pisoni RL, Combe C et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004; 19: 121–132 [DOI] [PubMed] [Google Scholar]
- 11. Kalantar-Zadeh K. History of erythropoiesis-stimulating agents, the development of biosimilars, and the future of anemia treatment in nephrology. Am J Nephrol 2017; 45: 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besarab A, Bolton WK, Browne JK et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 13. Singh AK, Szczech L, Tang KL et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 14. Pfeffer MA, Burdmann EA, Chen CY et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 15. Perez-Garcia R, Varas J, Cives A et al. Increased mortality in haemodialysis patients administered high doses of erythropoiesis-stimulating agents: a propensity score-matched analysis. Nephrol Dial Transplant 2018; 33: 690–699. [DOI] [PubMed] [Google Scholar]
- 16. Koulouridis I, Alfayez M, Trikalinos TA et al. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis 2013; 61: 44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCullough PA, Barnhart HX, Inrig JK et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 2013; 37: 549–558 [DOI] [PubMed] [Google Scholar]
- 18. Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol 2007; 2: 1274–1282 [DOI] [PubMed] [Google Scholar]
- 19. Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013; 27: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 2012; 148: 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402 [DOI] [PubMed] [Google Scholar]
- 22. Bernhardt WM, Wiesener MS, Scigalla P et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 2010; 21: 2151–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sugahara M, Tanaka T, Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int 2017; 92: 306–312 [DOI] [PubMed] [Google Scholar]
- 24. Rainville N, Jachimowicz E, Wojchowski DM. Targeting EPO and EPO receptor pathways in anemia and dysregulated erythropoiesis. Expert Opin Ther Targets 2016; 20: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pergola PE, Spinowitz BS, Hartman CS et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016; 90: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 26. Martin ER, Smith MT, Maroni BJ et al. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol 2017; 45: 380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haase VH, Chertow GM, Block GA et al. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant 2019; 34: 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KDIGO anemia guideline: KDIGO 2012 clinical practice guideline anemia in chronic kidney disease. Summary of recommendation statements. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 29. de Zeeuw D, Akizawa T, Audhya P et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 369: 2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. U.S. Renal Data System. USRDS 2014 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014.
- 31. Hartman C, Smith MT, Flinn C et al. AKB-6548, a new hypoxia-inducible factor prolyl hydroxylase inhibitor increases hemoglobin while decreasing ferritin in a 28-day, phase 2a dose escalation study in stage 3 and 4 chronic kidney disease patients with anemia. J Am Soc Nephrol 2011; 22: 435A [Google Scholar]
- 32. Nangaku M, Khawaja Z, Luo W et al. Randomized, placebo-controlled phase 2 trials of vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), to treat anemia of chronic kidney disease (CKD). J Am Soc Nephrol 2018; 29: 171 [Google Scholar]
- 33. Chan MC, Holt-Martyn JP, Schofield CJ et al. Pharmacological targeting of the HIF hydroxylases – a new field in medicine development. Mol Aspects Med 2016; 47–48: 54–75 [DOI] [PubMed] [Google Scholar]
- 34. Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015; 5: 378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. U.S. Renal Data System. USRDS 2018 annual data report: chronic kidney disease in the United States. Vol. 1. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018
- 36. U.S. Renal Data System. USRDS 2018 annual data report: end-stage renal disease in the United States. Vol. 2. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018
- 37. Chen N, Hao C, Peng X et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 2019; 381: 1001–1010 [DOI] [PubMed] [Google Scholar]
- 38. Chen N, Hao C, Liu BC et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 2019; 381: 1011–1022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.