Abstract
Recent evidence suggests transplanted stem cells improve left ventricular function in diabetic induced cardiomyopathy (DICM). However, little is known about the mechanisms by which iPS cells or factors released from these cells inhibit adverse cardiac remodeling in DICM. The present study was designed to determine molecular mediators and pathways regulated by transplanted iPS cells and their conditioned media (CM) in DICM. Animals were divided into four experimental groups such as control, STZ, STZ+iPS-CM, and STZ+iPS cells. Experimental diabetes was induced in C57BL/6 mice by intraperitoneal STZ injections (100 mg/kg body weight for 2 consecutive days). Following STZ injections, iPS cells or CM was given intravenously for 3 consecutive days. Animals were humanely killed and hearts were harvested at D14. Animals transplanted iPS cells or CM demonstrated a significant reduced apoptosis, mediated by Akt upregulation and ERK1/2 downregulation, and inhibition of interstitial fibrosis via MMP-9 suppression compared with the STZ group. Oxidative stress was significantly hindered in iPS cell and CM groups as evidenced by diminished pro-oxidant expression and enhanced antioxidant (catalase and MnSOD) concentration. Echocardiography data suggest a significant improvement in cardiac function in cells and CM groups in comparison to STZ. In conclusion, our data strongly suggest that iPS cells and CM attenuate oxidative stress and associated apoptosis, and fibrosis. Moreover, we also suggest increased antioxidant levels, decreased adverse cardiac remodeling, and improved cardiac function is mediated by iPS CM and cells in DICM through multiple autocrine and paracrine mechanisms.
Keywords: Heart, Diabetes, Apoptosis, Cardiomyopathy, iPS cells, Fibrosis, Akt
Introduction
Cardiovascular complications, including atherosclerosis, myocardial infarction, cardiomyopathy, and heart failure comprise the leading cause of morbidity and mortality in diabetic patients1, 2. Specifically, diabetes induced cardiomyopathy (DICM), mediated by hyperglycemia and oxidative stress (OS), elicits adverse architectural remodeling and deleterious mediation of regulatory machinery that ultimately lead to end stage heart failure and death3–5. DICM is characterized by the presence of cardiac injury, hypertrophy, cardiac cell apoptosis, reactive oxygen species (ROS), fibrosis, and left ventricular dysfunction3, 6, 7. Although advances in pharmacological interventions have been significant, long- and short-term prognosis of DICM patients remain ominous.
Recent studies have empirically established the potential of cell based therapies to treat different cardiac diseases8–12. Moreover, adult, embryonic, and induced pluripotent stem (iPS) cells and their conditioned media (CM) have been investigated and conclusions from these studies suggest the ability of these cells to prevent apoptosis, fibrosis, oxidative stress, and hypertrophy and contribute to augmented cardiac function 11, 13–15. Of note, most of the above studies on cell therapy and CM include myocardial infarction or various other related cardiac disease models. Recently testified, transplanted bone marrow stem cells (BMSCs) in DICM increased angiogenesis as well as improved cardiac function16. However, evidence of these transplanted stem cells in the diabetic heart was minimal, suggesting an autocrine or paracrine factors released from the BMSCs purported their beneficial effects16.
With the aforementioned study in mind, the present investigation was designed to establish the advantageous impact of transplanted iPS cells derived from H9c2 cardiomyoblasts and iPS-CM on DICM. To date, various cell types have been used to generate iPS cells with significant differences reported regarding their potential to treat cardiac diseases10, 13, 17, 18. As per the best of our knowledge, there is absolutely no study available in current literature which has determined the potential of H9c2 cell-derived iPS cells to attenuate oxidative stress induced adverse cardiac remodeling in the streptozotocin induced diabetic cardiomyopathy (STZ-IDC). To determine the aptitude of cardiomyoblast-generated iPS cells and their CM to inhibit oxidative stress in DICM, we transplanted iPS-conditioned medium (iPS-CM) and induced pluripotent stem (iPS) cells in STZ-IDC hearts. Within the present study, we report significant mitigation of oxidative stress as evidenced by attenuated apoptosis, fibrosis, and reactive oxygen species as well as improved cardiac function following iPS CM and cell transplantation in the STZ-IDC heart. Additionally, we sought to identify molecular mechanisms by which iPS-CM and iPS cells exerted their effects in DICM including their participation in OS regulation. Our data suggest that OS suppression, following iPS cell and CM transplantation in DICM, is consequent to antioxidant upregulation and modulation of the Akt pathway. Our data presents novel implications in DICM induced OS dysregulation and provides new avenues for research in therapeutic targets.
MATERIALS AND METHODS
Generation of iPS Cells and Preparation of the CM
iPS cells were produced via transduction of H9c2 cells as we previously reported13. In brief, c-Myc, Klf4, Oct3/4 and Sox2 were cloned into a pBluescript SK(−) vector (Stratagene) and expression of these factors was verified by immunostaining, real time-PCR and SDS-Page and western blot analysis. Pluripotency was verified by staining our generated iPS cells with Oct 3/4 and alkaline phosphatase. Generated iPS cells were maintained in cell culture medium containing mouse embryonic fibroblast (50%, MEF)-CM and rest of the Dulbecco’s Modified Eagles Medium. Media were accompanied with antibiotics such as streptomycin and penicillin, fetal bovine serum, non-essential amino acids, basic fibroblast growth factors and leukemia inhibitor factor. To prepare iPS-CM, iPS cells were passaged, maintained in the cell cultured for 24 hrs, and were replaced with fresh media excluding LIF. Post-48 hrs, cell supernatant containing released factors was removed, filtered (0.2um filter, Millipore, USA) and labeled iPS-CM for future use.
STZ-induced Diabetes
All animal experiments were reviewed and approved by the University of Central Florida institutional animal care review board. Male and female C57BL/6 mice (Jackson laboratories) were continued on standard chow and water ad libitum. Mice were separated into 4 groups; (n=6–8 mice/group): control, STZ, STZ + iPS condition medium (iPS-CM) and STZ + iPS cells. Following fasting for 4 hours, blood from the tail vain was used to quantitate a baseline blood glucose with the One Touch Ultra glucose meter (Life Scan Inc). Thereafter, intraperitoneally (i.p.) injections of STZ (100 mg/kg STZ prepared in 50mM citrate buffer (pH 4.5)) were given for 2 consecutive days. STZ + iPS-CM group received additional intravenous (i.v.) injections of 400μl iPS-CM (supernatant of iPS cells culture) on 3 consecutive days. STZ + iPS cells group was also given i.v. injections of 400μl iPS cells (4× 105) for 3 consecutive days.
Fasting glucose levels and weight were also assessed in mice on day 3 (D3), D7, and D14 after the last STZ injection. Control mice were injected with citrate buffer (i.p.) in lieu of STZ and all parameters as aforementioned were assessed. At D14 following STZ injection, mice were sacrificed with anesthesia (pentobarbital, 80 mg/kg) followed by cervical dislocation. Hearts from these mice were removed and fixed in 4% paraformaldehyde for further analyses.
Preparation of Heart Sections For Histology and Immunostainings
Paraffin fixed heart tissue was cut into 5 μm size into serial sections, deparaffinized, and rehydrated as we published previously3. Cellular morphology and fibrosis was quantified after sections were stained with Masson’s Trichrome. Moreover interstitial fibrosis, predominantly present in the left ventricular myocardium, was quantified by computing the total blue area per mm2 with NIH Image J software. Fibrosis were constituted in 1–2 sections from n=6–8 animals/group.
Apoptosis
To detect apoptotic nuclei within the control and experimental hearts, an apoptotic cell death detection kit (Roche, USA) was used as previously published and per manufacturer’s instructions3. Total nuclei were visualized by mounting heart sections with Antifade mounting medium containing DAPI (Vectashield, USA). Sections were observed under an Olympus fluorescent and a Leica laser scanning confocal microscope. Apoptosis was quantified by counting total apoptotic nuclei (red) in 5–6 selected fields in the infarct and the peri-infarct zone area per total number of DAPI (blue) x 100% as previously described (REF).
To specifically assess cardiac myocyte apoptosis, heart sections were triple labeled with TUNEL and primary antibodies against active caspase 3 (Santa Cruz Biotechnology and Cell Signaling) and sarcomeric cardiac α-actin (1:20 dilution; Sigma). Following incubation with each primary antibody (caspase-3 and sarcomere α-actin) separately for 1 hour and intermittent washings. Secondary antibodies anti-rabbit Alexa 635 or anti-mouse Alexa 488 were applied for an additional one hour. Heart sections were mounted with mounting medium containing DAPI (Antifade Vectashield, Vector Laboratories) for nuclear visualization and viewed with a confocal microscope.
Caspase-3 Activity Assay
A caspase-3 activity assay was performed using kit from Biovision, USA. In brief, isolated heart tissue cut into small pieces and homogenized in RIPA buffer that contains a well-defined cocktail of protease inhibitors such as PMSF, sodium orthovandate, and sodium fluoride. Homogenates were prepared, centrifuged and the supernatant was collected in a 2 ml tube. Protein concentration was quantified and the caspase-3 activity was measured as per the instructions provided in the kit. The resulting reaction was examined at 405 nm in a plate reader (Bio Rad). The caspase-3 activity histogram was presented in a graph as arbitrary units (A.U.).
Phosphorylated Akt and ERK 1/2 by ELISA
Phosphorylated Akt (p-Akt) and ERK 1/2 (ERK 1/2) expression levels were examined using commercially available ELISA kits (Exalpha Biologicals, Inc., Maynard, MA). In brief, tissues were homogenized as aforementioned and concentrations of p-Akt and ERK 1/2 were quantified according to manufacturer’s instructions. The color reactions were quantitated at 450 nm using a microtiter plate reader for each ELISA. p-Akt and ERK 1/2 data were plotted on independent histograms as A.U.
Lipid Peroxides Assay
Isolated supernatant from homogenized heart tissue was used to detect the level of Lipid Peroxides (LPO). The reactions were developed as per manufacturer’s instructions provided in the LPO-CC kit (Kamiya Biomedical, Seattle, WA) and measured at 655 nm. Lipid peroxide values from each group were calculated by the following formula: (Esample - Eblank) x 50.0 / (Ecalibrator - Eblank) and plotted as A.U.
MMP-9 Concentration
MMP-9 concentration was determined for control and experimental groups using a mouse MMP-9 immunoassay kit (R&D Systems, Minneapolis, MN). Heart tissue homogenates were used to perform ELISA according to the manufacturer’s instructions. The assay reaction was developed and examined at 450 nm. The obtained results were normalized to the used protein concentration in the assay for every sample. The MMP-9 histogram was plotted as A.U.
Catalase Activity Assay
Heart tissue was homogenized and supernatant was isolated as aforementioned. Catalase activity was measured using Catalase Assay kit (Abcam, Cambridge, MA) as per manufacturer’s instructions. The coloric reaction was quantified at 570 nm and catalase activity assay results were normalized to the total amount of protein in each sample as determined by the Bradford assay. Catalase activity was plotted as A.U.
MnSOD Activity Assay
MnSOD activity in the heart homogenate supernatant was measured using a SOD-560 colorimetric assay kit (Applied Bioanalytical Labs, Sarasota, FL). MnSOD activity was performed following manufacturer’s instructions and measured at 560 nm. MnSOD activity was calculated by the following formula: 125*(100% - Ratesample/ Rateblank) x dilution factor. MnSOD activity was plotted as A.U.
Echocardiography
Noninvasive transthoracic 2D echocardiography (Sonos 5500) was performed using a 13-MHz linear probe on D14. Following 2.0% isoflurane-sedation, mice were placed in the supine position in a temperature controlled platform and chest hair was removed. Motion mode and two-dimensional images were obtained in the short axis view at the mid-papillary muscle level. The data obtained for left ventricular internal dimension-diastole (LVIDd), left ventricular internal dimension-systole (LVIDs) were calculated and converted into fractional shortening (LVIDd-LVIDs/LVIDd x 100), and ejection fraction which was plotted as a graph.
Data Analysis
Data were presented as means ± SEM. One way ANOVA followed by Tukey test was examined to determine the statistical significant difference between groups. Statistical significance was assigned when p < 0.05.
Results
iPS Cells and Their CM Attenuate STZ-induced Elevated Glucose Levels
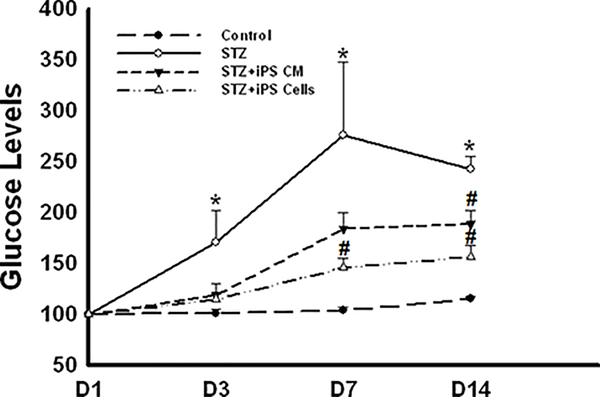
Figure 1 illustrates levels of blood glucose in control and experimental mice. A significant increase in blood glucose levels was observed in STZ mice relative to control mice (Figure 1). By D14, iPS cell or iPS-CM administration considerably improved (p<0.05) the glycemic status in these mice compared to mice receiving STZ alone (Figure 1).
Figure 1: iPS-CM and iPS cells improve glycemic control in STZ-DICM.
Line graph shows quantitative glucose levels (mg/dL) for control and experimental groups at D1, D3, D7, and D14. *p<0.05 vs. control and #p<0.05 vs. STZ.
iPS Cells and Their CM Inhibit Apoptosis in STZ-IDC
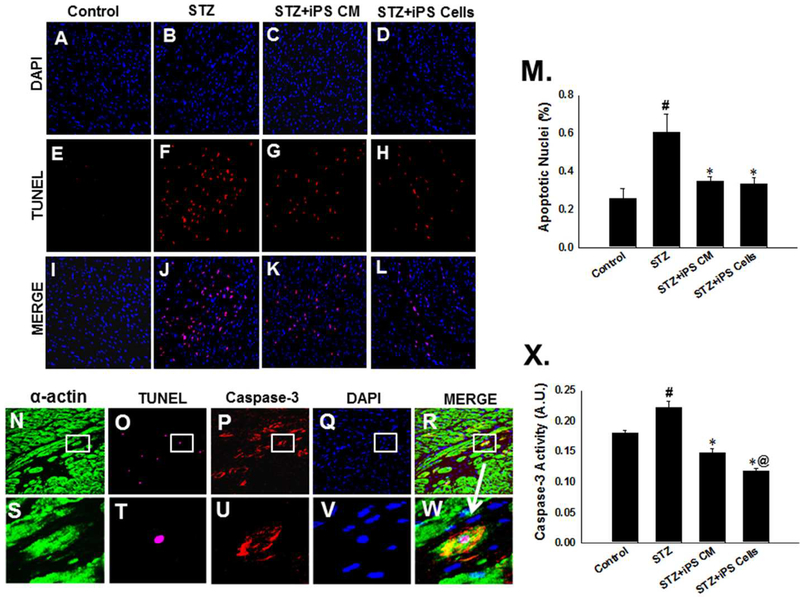
We determined the effects of iPS cells and their CM in the inhibition of apoptosis in the STZ-IDC hearts, TUNEL staining was performed at D14 (Figure 2A-L). There was a significant increase in apoptotic-positive nuclei in the STZ treated group compared with normal control (STZ: 0.60 ± 0.10 vs. Control: 0.25 ± 0.05, mean ± SEM, p<0.01, Figure 2M) were observed. Moreover, mice receiving iPS-CM or iPS cells post-STZ demonstrated significantly reduced apoptosis compared with the STZ group (iPS-CM: 0.35 ± 0.02 and iPS cells: 0.33 ± 0.03, vs. STZ: 0.60 ± 0.10, mean ± SEM, p<0.01, Figure 2M). To ascertain apoptosis occurs in cardiac myocytes following STZ treatment, heart sections from control and experimental mouse hearts were tripled labeled with TUNEL and anti-α-actin and anti-caspase-3 antibodies. Representative photomicrographs demonstrating apoptotic cardiac myocytes are shown in Figures 2N-W.
Figure 2: iPS cells and their CM inhibit apoptosis in STZ-IDC.
Representative photomicrographs of control, STZ, STZ+iPS-CM, and STZ+iPS cell hearts demonstrating total nuclei stained with DAPI in blue (A-D), apoptotic nuclei stained with TUNEL in red (E-H), and merged nuclei in pink (I-L). Magnification, 40X. M: Histogram shows quantitative % apoptotic nuclei per total nuclei from control and treated groups. #p<0.01 vs. control and *p<0.01 vs. STZ. N-R: Representative photomicrographs of apoptotic cardiac myocytes with cardiac myocytes stained with α-actin in red (N), apoptotic nuclei stained with TUNEL in pink (O), apoptotic cells stained with caspase-3 in red (P), nuclei stained with DAPI in blue (Q), merged image (R) and enhanced images in S-W. X: Histogram shows quantitative caspase-3 activity from control and experimental groups. #p<0.01 vs. control, *p<0.001 vs. STZ, and @p<0.05 vs. STZiPS-CM.
To strengthen our TUNEL apoptotic data, we additionally performed caspase-3 activity assay. As with the TUNEL data, hearts from the STZ treated mice had a significant increase in caspase-3 activity compared with controls (STZ: 0.23 ± 0.01 vs. Control: 0.18 ± 0.00, mean ± SEM, p<0.01, Figure 2X). Additionally, mice transplanted with iPS-CM or iPS cells exhibited reduced capsase-3 activity compared with STZ group (iPS-CM: 0.15 ± 0.01 and iPS cells: 0.12 ± 0.00 vs. STZ: 0.23 ± 0.01, p<0.001, Figure 2X). Of note, mice treated with iPS cells had a statistically significant difference in caspase-3 activity compared to the STZ+iPS-CM group (p<0.05, Figure 2X).
iPS Cells and Their CM Regulate Akt and ERK Pathways in the STZ-IDC Heart
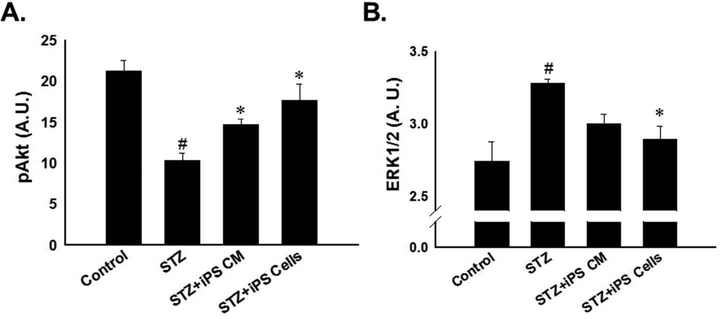
Regulation of apoptosis is mediated by various signaling molecules including Akt and ERK in ischemic cardiac diseases as well as in STZ-IDC8, 19. To this end, we examined the effects of STZ on Akt and ERK expression levels and their modulation by iPS-CM and iPS cells post-STZ administration. Phosphorylated Akt was significantly reduced in the hearts of mice treated with STZ alone compared to normal control mice, suggesting diminished cardiac cell survival (p<0.001, Figure 3A). However, upon transplantation of iPS-CM or iPS cells post-STZ, reduction of pAkt was significantly attenuated relative to the STZ group (iPS-CM: 14.71± 0.68 and iPS cells: 17.67 ± 1.99 vs. STZ: 10.37 ± 0.87, mean ± SEM, p<0.05, Figure 3A).
Figure 3: Impact of iPS cells and their CM regulate on Akt and ERK expression.
A: Histogram data demonstrate quantitative pAkt expression. #p<0.001 vs. control and *p<0.05 vs. STZ. B: Histogram shows quantitative ERK1/2 expression, determined by ELISA, was significantly decreased in hearts transplanted with iPS-cells. #p<0.01 vs. control and *p<0.05 vs. STZ.
Levels of ERK 1/2 were significantly upregulated in the hearts of mice treated with STZ alone relative to control mice hearts (p<0.01, Figure 3B). Conversely, transplanted iPS cells post-STZ significantly abrogated these effects (iPS cells: 2.89 ± 0.09 vs. STZ: 3.28 ± 0.03, mean ± SEM, p<0.05, Figure 3B). Statistical significance was not reached regarding levels of ERK 1/2 between STZ and STZ+iPS-CM groups (Figure 3B).
iPS Cells and Their CM Attenuate Oxidative Stress and Upregulate Antioxidants in the STZ-IDC Heart
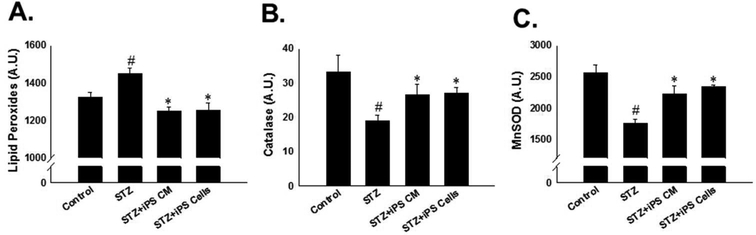
To assess the impact of iPS-CM and iPS cells on oxidative stress in the STZ-IDC heart, levels of lipid peroxide, an oxidative stress marker, catalase, and MnSOD were evaluated. Levels of lipid peroxide were significantly increased in the hearts of mice receiving STZ only compared to their respective controls (Figure 4A, p<0.001). Conversely, hearts from mice treated with iPS-CM or iPS cells demonstrated a significant reduction in levels of lipid peroxide compared to the STZ treated group (iPSCM 1251.53 ± 19.16 and iPS cells: 1253.13 ± 41.53 vs. STZ: 1450.08 ± 27.91, mean ± SEM, p<0.001, Figure 4A), suggesting downregulation of oxidative stress. Furthermore, antioxidant levels including catalase and MnSOD were assessed to determine the potential impact that iPS-CM and iPS cells may have on them. A significant downregulation in catalase and MnSOD was observed in the STZ group relative to controls (p<0.01 and p<0.001, Figure 4B-C, respectively). As hypothesized, transplantation of iPS-CM and iPS cells blunted diminished antioxidant levels compared to the STZ group (p<0.05, Figure 4B-C).
Figure 4: iPS cells and their CM attenuate oxidative stress and upregulate antioxidants in the STZ-IDC heart.
A: Histogram shows quantitated lipd peroxide concentration in control and experimental groups. #p<0.001 vs. control and *p<0.001 vs STZ. B: Histogram demonstrates catalase is significantly upregulated in the STZ+iPS-CM and STZ+iPS cell groups. #p<0.01 vs. control and *p<0.05 vs STZ. C: Histogram of quantitative MnSOD expression following iPS-CM or iPS cell transplantation. #p<0.01 vs. control and *p<0.05 vs STZ.
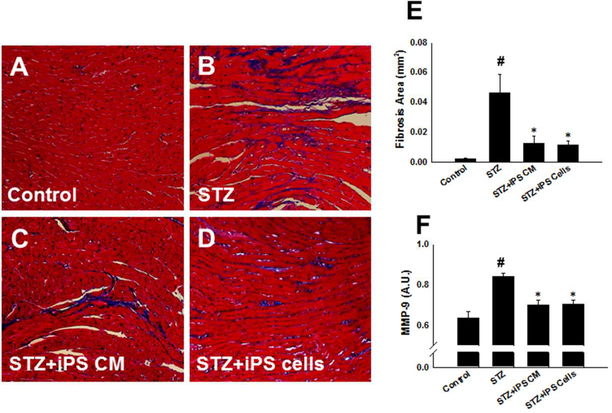
iPS Cells and Their CM Inhibit Cardiovascular Fibrosis in the STZ-IDC Heart
To determine whether transplanted iPS cells and their CM attenuate interstitial cardiac fibrosis in the STZ-IDC heart, we completed Masson’s trichrome staining and calculated interstitial fibrosis (Figure 5A-D). Blue area demonstrating fibrosis in the STZ- group was significantly greater compared to the control (STZ: 0.05 ± 0.01 vs. control: 0.00001 ± 0.0001, mean ± SEM, p<0.01, Figure 5E). Following transplantation of iPS-CM or iPS cells, our quantitative data suggests there was significantly reduced interstitial fibrosis compared with the STZ group (iPS-CM: 0.01 ± 0.00 and iPS cells: 0.01 ± 0.00 vs. STZ: 0.05 ± 0.01, mean ± SEM, p<0.05, Figure 5E).
Figure 5: iPS cells and their CM inhibit fibrosis in the STZ-IDC heart.
A-D: Representative photomicrographs from sections stained with Masson’s trichrome 14 days following cell and CM transplantation. Magnification, 40X. E: Histogram data demonstrate quantitative interstitial fibrotic area in STZ-IDC mouse hearts transplanted with iPS-CM and iPS cells. #p<0.01 vs. control and *p<0.05 vs STZ. F: Histogram data demonstrate MMP-9 expression is significantly diminished in STZ-IDC hearts transplanted with iPS-CM or iPS cells. #p<0.001 vs. control and *p<0.01 vs STZ.
To determine whether inhibition of fibrosis following iPS-CM or iPS cell transplantation was associated with changes in expression of matrix metalloproteinases, levels of MMP-9, a well-documented purport rater of fibrotic dysregulation, was determined using an enzyme-linked immunoassay. Hearts from STZ mice showed significantly heightened MMP-9 expression compared with controls (p<0.001, Figure 5F). Importantly, MMP-9 expression was meaningfully weakened in hearts from the iPS-CM and iPS cell groups relative to the STZ group (p<0.01, Figure 5F) suggesting a connection between fibrosis inhibition and modulation of MMP-9 expression by iPS-CM and iPS cells in the STZ-IDC heart.
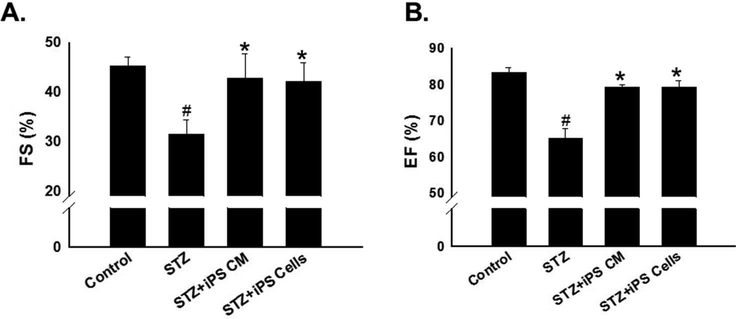
Improvement in Cardiac Function Following CM or Cell Transplantation in STZ-IDC
M-mode echocardiography was used to define the impact of CM or cell transplantation on heart function in mice at 2 weeks post STZ treatment. Fractional shortening and ejection fraction were significantly reduced in the STZ treated groups compared to control (p<0.001, Figure 6A-B). Notably, fraction shortening and ejection fraction were both improved in iPS-CM and iPS cell treatment groups compared to the STZ animals: (Figure 6A-B, p<0.01).
Figure 6: Improvement in cardiac function following CM or cell transplantation in STZ-IDC.
Heart function were performed at D14 following cell or CM transplantation. A: Histogram data show average fractional shortening for all experimental groups. #p<0.001 vs. control and *p<0.01 vs STZ. B: Histogram of average ejection fraction for control and experimental animals. #p<0.001 vs. control and *p<0.01 vs STZ.
Discussion
Diabetic induced cardiomyopathy mitigates several adverse architectural remodeling mechanisms and the functional capacity of the diseased heart. Characteristics of DICM include but are not limited to cardiac myocyte cell death consequent to apoptosis, dysregulated cell survival pathways, accumulated ROS species and downregulated antioxidants, enhanced fibrosis and pro-fibrotic factors, diminished left ventricular function, and modulated microRNA expression levels3–7. Although tremendous progress has been made toward the development of therapeutic options for the treatment of DICM, novel, effective strategies are still necessitated. Numerous studies involving stem cells and their CM have highlighted the potential of these treatment options to repair and regenerate the injured myocardium8–12. Specifically, studies have shown that transplanted stem cells and factors released from stem cells have the capacity to 1) mitigate stress induced activation of cell death mediators and subsequent apoptosis, 2) inhibit vascular and interstitial fibrosis formation, 3) prevent reactive oxygen species (ROS) accumulation through enhanced scavenging and antioxidant upregulation, 4) promote cell survival, repair, and regeneration through paracrine mechanism, and 5) enhance left ventricular output and function, to name a few3, 11, 15, 20, 21. However, little is known, to date, about the effectiveness of iPS cells in STZ-IDC and the mechanisms by which they exert their beneficial effects. To this end, the current study was undertaken to assess the impact of iPS cells and their CM on maladaptive remodeling, cardiac function, and mechanisms by which they promote cytoprotection in STZ-IDC.
DICM is well documented within currently literature and has been generated in several animal models3, 22, 23. Evidence provided within the published studies suggesting the presence of experimental DICM pathology induced by STZ include hyperglycemia, cardiac cell death, OS, hypertrophy, fibrosis, and a loss of normal contractile function3, 22, 23. Congruent with these previous studies, our DICM animals demonstrated elevated blood glucose levels, enhanced cardiac myocyte apoptosis, increased oxidative stress, activated fibrosis formation, and deleterious cardiac functional consequences, all indicative of diabetes induced cardiomyopathy.
In the current investigation, we suggest that up to two weeks following the last STZ injection, iPS cells and their CM attenuate hyperglycemia which is palpable in our generated STZ-IDC mice. Supporting evidence of our findings can be found in a multitude of investigations in which lowered blood glucose levels were reported in various diabetic models using stem cells and CM18, 24, 25. We acknowledge that mechanisms by which our iPS cells alleviate hyperglycemia remain elusive and will require further investigation in a much broader arena than just the heart as the present study resides. Additionally, whether decreased blood glucose directly contributes to the retarded DICM observed following CM and cell transplantation or the responsibility remains directly tied to the actions of the CM and iPS cells alone may be elucidated in future studies.
Cell death plays a monumental role in most cardiac pathologies with DICM no exception26. Upon iPS-CM or iPS cell transplantation in the STZ-IDC mice, apoptosis was significantly inhibited as evidenced by TUNEL and caspase-3 activity. We have previously provided evidence in accordance with the current findings suggesting iPS cells and their CM have the potential to hinder cardiac cell death3, 13. To ellucidate mechanism by which iPS cells promote cell survival, cell signaling pathways including Akt and ERK 1/2 were investigated. Mechanisms of apoptotic cell death are complex and involve the activation of various signaling molecules including MAPKs and JNKs 27. Evidence provided suggests that the Akt and ERK pathways are specifically involved in OS induced apoptosis28, 29. Additionally, previously published data have highlighted the potential of factors released from stem cells to augment the expression of key components of these pathways during cardiac stress8, 20. For the first time, we report that iPS cells moderate both pathways by upregulating pAkt and downregulating ERK 1/2 in the STZ-IDC heart. We suggest that enhanced expression of Akt, a pro-survival and cardioprotective protein, and diminished expression of ERK1/2, a maladaptive signaling molecule in stress-induced cardiac remodeling, contribute to mechanism by which iPS cells inhibit cardiac myocyte apoptosis in the STZ-IDC heart.
The etiology of DICM, in its complexity, is characterized by a multitude of metabolic dysregulations including the inability to properly maintain a balance between prooxidants and antioxidants resulting in oxidative stress30, 31. OS mediated mechanisms lead to additional maladaptions including anomalous gene expression, altered signaling cascades, and cardiac cell death promotion5, 30, 31. Because OS plays such a monumental role in the molecular and cellular pathophysiology of DICM, we investigated the impact of our iPS-CM and iPS cells on OS in the STZ-IDC model. Levels of lipid peroxide, a variant marker of OS, were significantly enhanced in the STZ mice whereas following iPS CM or cell transplantation, levels significantly reduced. To the best of our knowledge, this is the first report indicating iPS-CM and iPS cells inhibit pro-oxidant activation in STZ-IDC. Furthermore, levels of specific antioxidants were evaluated and our data suggest that iPS-CM and iPS cells ameliorate OS through upregulation of catalase and MnSOD. OS in DICM in and of itself is multifaceted. Identification of the specific molecular machinery and pathways of attenuated OS via iPS-CM and iPS cells in STZ-IDC outside the widely accepted paracrine mechanisms will require additional studies.
Generally recognized, DICM is associated with significant alterations in the chemical and cellular components of the extracellular matrix (ECM) resulting in cardiac fibrosis3, 32. Fibrosis, although a reparative process, catapults the heart into further dysfunction and diminished ventricular output. Our data demonstrate the ability of iPS-CM and iPS cells to inhibit the formation of cardiac fibrosis in the STZ-IDC heart. This data is supported by previous reports indicating iPS cells inhibit fibrosis in a host of cardiac anomalies3, 13. To go a step further, we identified MMP-9, a pro-fibrotic protein, as a downstream target of IPs-CM and IPs cell signaling. Importantly, MMP-9 was tremendously reduced post iPS-CM and iPS cell administration. Our data implies inhibition of fibrosis in the STZ-IDC heart by iPS-CM and iPS cells is, in part, a result of mediation to the TIMP/MMP fibrotic pathway.
Well documented, activation of adverse cardiac remodeling mechanisms in response to cardiac cell assault contributes to left ventricular dysfunction in the setting of DICM3, 33. Previous studies have reported improved cardiac function following transplantation of various stem cells including embryonic stem (ES) and iPS cells into the injured myocardium13, 19. Moreover, transplanted CM, generated from mesenchymal stem cells (MSCs) and ES cells retaining released paracrine factors, has been reported to contribute to improved fractional shortening in the infarcted heart21, 34. Our functional data within the present study suggest that both transplanted iPS-CM and iPS cells abet augmented fractional shortening and ejection fraction in STZ-IDC which further corroborates these previous findings.
Mechanisms of CM and stem cell therapy are multifactorial and complex at best. Within the current study, we suggest the beneficial effects purported by the CM in STZ-IDC were attributable to paracrine mechanisms facilitated by factors released from iPS cells within the media which is congruent with previous CM studies14, 21. However, mechanisms of iPS cell therapy, as a whole, are much more multifaceted incorporating not only paracrine mechanisms of factors released from the cells but also autocrine mechanisms of the cells themselves. Previous studies have suggested that cells injected intraperitoneally largely accumulate in the lung, but have been shown to reach other organs as well35, 36. Although the survival and engraftment of transplanted stem cells within the heart were not examined, we suggest that both paracrine and autocrine influences were driving forces facilitating the therapeutic potential observed within the study. However, future studies are necessitated to identify which of the two mechanisms is the dominant mediator of beneficial effects.
In conclusion, we have shown for the first time that iPS cells and their CM inhibit apoptosis and fibrosis and enhance cardiac repair and function in the STZ-IDC heart. Specifically we suggest 1) iPS-CM and iPS cells promote glycemic control; 2) iPS cells inhibit apoptosis in STZ-IDC consequent to upregulation of Akt and downregulation of ERK1/2; 3) transplantation of iPS-CM and iPS cells ameliorate oxidative stress through upregulation of antioxidants; 4) STZ-IDC induced fibrosis is diminished by iPS-CM and iPS cells through MMP-9 inhibition; 5) cardiac function is significantly improved in STZ-IDC mice following iPS-CM and iPS cell transplantation. Within the present study, we have demonstrated the therapeutic benefits of iPS-CM and iPS cells in STZ-IDC as well as identified mechanisms by which they exert their effects. We have also presented data implying a novel mediator of cardiac dysfunction. Further investigations are warranted to delineate the complex molecular and cellular mechanisms by which DICM affects the heart and from which iPS-CM and iPS cells invoke their restorative protection.
ACKNOWLEDGEMENTS
The authors would like to thank Reetu Singla and Latifa Abdelli for their technical assistance and Carley Glass for assistance with the written manuscript. The performed work in this study was supported, in part, by grants from the National Institutes of Health [1R01HL090646–01, and 5R01HL094467–02 to DKS].
Footnotes
CONFLICT OF INTEREST: None declared.
Reference List
- 1.Glass CE, Singal PK, Singla DK. Stem cells in the diabetic infarcted heart. Heart failure reviews. 2010;15:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: Pathophysiology and pathogenesis. Diabetes, obesity & metabolism. 2007;9:767–780 [DOI] [PubMed] [Google Scholar]
- 3.Neel S, Singla DK. Induced pluripotent stem (ips) cells inhibit apoptosis and fibrosis in streptozotocin-induced diabetic rats. Molecular pharmaceutics. 2011;8:2350–2357 [DOI] [PubMed] [Google Scholar]
- 4.Diao X, Shen E, Wang X, Hu B. Differentially expressed micrornas and their target genes in the hearts of streptozotocin-induced diabetic mice. Molecular medicine reports. 2011;4:633–640 [DOI] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellor KM, Ritchie RH, Delbridge LM. Reactive oxygen species and insulinresistant cardiomyopathy. Clinical and experimental pharmacology & physiology. 2010;37:222–228 [DOI] [PubMed] [Google Scholar]
- 7.Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease--an overview. Toxicology mechanisms and methods. 2012;22:330–335 [DOI] [PubMed] [Google Scholar]
- 8.Singla DK, Ahmed A, Singla R, Yan B. Embryonic stem cells improve cardiac function in doxorubicin-induced cardiomyopathy mediated through multiple mechanisms. Cell transplantation. 2012;21:1919–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting AE, Sherman W. Allogeneic stem cell transplantation for ischemic myocardial dysfunction. Current opinion in organ transplantation. 2012;17:675–680 [DOI] [PubMed] [Google Scholar]
- 10.Zwi-Dantsis L, Gepstein L. Induced pluripotent stem cells for cardiac repair. Cellular and molecular life sciences : CMLS. 2012;69:3285–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascular pharmacology. 2012;57:48–55 [DOI] [PubMed] [Google Scholar]
- 12.Singla DK. Stem cells in the infarcted heart. Journal of cardiovascular translational research. 2010;3:73–78 [DOI] [PubMed] [Google Scholar]
- 13.Singla DK, Long X, Glass C, Singla RD, Yan B. Induced pluripotent stem (ips) cells repair and regenerate infarcted myocardium. Molecular pharmaceutics. 2011;8:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singla DK, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis of h9c2 cells. American journal of physiology. Heart and circulatory physiology. 2007;293:H1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singla DK, Lyons GE, Kamp TJ. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. American journal of physiology. Heart and circulatory physiology. 2007;293:H1308–1314 [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Li J, Luo R, Jiang J, Wang JA. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2008;116:104–111 [DOI] [PubMed] [Google Scholar]
- 17.Buccini S, Haider KH, Ahmed RP, Jiang S, Ashraf M. Cardiac progenitors derived from reprogrammed mesenchymal stem cells contribute to angiomyogenic repair of the infarcted heart. Basic research in cardiology. 2012;107:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon K, Lim H, Kim JH, Thuan NV, Park SH, Lim YM, Choi HY, Lee ER, Kim JH, Lee MS, Cho SG. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem cells and development. 2012;21:2642–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass C, Singla DK. Microrna-1 transfected embryonic stem cells enhance cardiac myocyte differentiation and inhibit apoptosis by modulating the pten/akt pathway in the infarcted heart. American journal of physiology. Heart and circulatory physiology. 2011;301:H2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singla DK, Singla RD, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis in h9c2 cells through pi3k/akt but not erk pathway. American journal of physiology. Heart and circulatory physiology. 2008;295:H907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DP. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem cell research. 2011;6:206–214 [DOI] [PubMed] [Google Scholar]
- 22.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948 [DOI] [PubMed] [Google Scholar]
- 23.Ma B, Xiong X, Chen C, Li H, Xu X, Li X, Li R, Chen G, Dackor RT, Zeldin DC, Wang DW. Cardiac-specific overexpression of cyp2j2 attenuates diabetic cardiomyopathy in male streptozotocin-induced diabetic mice. Endocrinology. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin P, Chen L, Yang N, Sun Y, Xu YX. Evaluation of stem cell differentiation in diabetic rats transplanted with bone marrow mesenchymal stem cells. Transplantation proceedings. 2009;41:1891–1893 [DOI] [PubMed] [Google Scholar]
- 25.Xu YX, Chen L, Hou WK, Lin P, Sun L, Sun Y, Dong QY, Liu JB, Fu YL. Mesenchymal stem cells treated with rat pancreatic extract secrete cytokines that improve the glycometabolism of diabetic rats. Transplantation proceedings. 2009;41:1878–1884 [DOI] [PubMed] [Google Scholar]
- 26.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovascular toxicology. 2003;3:219–228 [DOI] [PubMed] [Google Scholar]
- 27.Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhingra S, Sharma AK, Singla DK, Singal PK. P38 and erk1/2 mapks mediate the interplay of tnf-alpha and il-10 in regulating oxidative stress and cardiac myocyte apoptosis. American journal of physiology. Heart and circulatory physiology. 2007;293:H3524–3531 [DOI] [PubMed] [Google Scholar]
- 29.Aki T, Yamaguchi K, Fujimiya T, Mizukami Y. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line h9c2. Oncogene. 2003;22:8529–8535 [DOI] [PubMed] [Google Scholar]
- 30.Singal PK, Bello-Klein A, Farahmand F, Sandhawalia V. Oxidative stress and functional deficit in diabetic cardiomyopathy. Advances in experimental medicine and biology. 2001;498:213–220 [DOI] [PubMed] [Google Scholar]
- 31.Aydemir-Koksoy A, Bilginoglu A, Sariahmetoglu M, Schulz R, Turan B. Antioxidant treatment protects diabetic rats from cardiac dysfunction by preserving contractile protein targets of oxidative stress. The Journal of nutritional biochemistry. 2010;21:827–833 [DOI] [PubMed] [Google Scholar]
- 32.Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart failure reviews. 2013 [DOI] [PubMed] [Google Scholar]
- 33.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343 [DOI] [PubMed] [Google Scholar]
- 34.Singla DK, Singla RD, Lamm S, Glass C. Tgf-beta2 treatment enhances cytoprotective factors released from embryonic stem cells and inhibits apoptosis in infarcted myocardium. American journal of physiology. Heart and circulatory physiology. 2011;300:H1442–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson T, Stark C, Holmbom J, Rosling A, Kuusilehto A, Tirri T, Penttinen R, Ekholm E. Fate of bone marrow-derived stromal cells after intraperitoneal infusion or implantation into femoral bone defects in the host animal. Journal of tissue engineering. 2010;2010:345806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang N, Shao Y, Mei Y, Zhang L, Li Q, Li D, Shi S, Hong Q, Lin H, Chen X. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: Accumulating in the lung and secreting tumor necrosis factor alpha-stimulating gene-6. Stem cell research & therapy. 2012;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]