Abstract
Background:
Microribose nucleic acids (miRNAs) are implicated in the progression of lung adenocarcinoma. MicroRNA-345-5p (miR-345-5p) is a recently identified anti-oncogene in some human cancers, but its functional role and possible molecular mechanism in lung adenocarcinoma remain unknown. This study aimed to identify the biological function and underlying mechanism of miR-345-5p in lung adenocarcinoma cells.
Methods:
In this study, lung adenocarcinoma tissues and adjacent tissues were collected in the First Affiliated Hospital of Anhui Medical University between April 2016 and February 2017. The expression of miR-345-5p and ras homolog family member A (RhoA) in lung adenocarcinoma tissues and human lung adenocarcinoma cell lines (A549, H1650, PC-9, and H441) was detected by reverse transcription quantitative polymerase chain reaction analysis. Functional assays including colony formation, flow cytometry analysis, wound healing, and transwell assays were performed to assess the proliferation, apoptosis, migration, and invasion of lung adenocarcinoma cells. In addition, RNA pulldown and luciferase reporter assays were conducted to evaluate the relationship between miR-345-5p and RhoA. Difference between the two groups was analyzed with Student's t test, while that among multiple groups was analyzed with one-way analysis of variance.
Results:
MiR-345-5p expression displayed lower level in lung adenocarcinoma tissues (0.241 ± 0.095 vs.1.000 ± 0.233, t = 19.247, P < 0.001) and cell lines (F = 56.992, P < 0.001) than control tissues and cells. Functional experiments demonstrated that upregulation of miR-345-5p inhibited the malignant phenotypes of lung adenocarcinoma cells via suppressing cell proliferation, migration, invasion, and facilitating cell apoptosis. Additionally, RhoA was verified to be the downstream target of miR-345-5p. Expression of RhoA was downregulated by overexpression of miR-345-5p in PC-9 (0.321 ± 0.047 vs. 1.000 ± 0.127, t = 8.536, P < 0.001) and H1650 (0.398 ± 0.054 vs. 1.000 ± 0.156, t = 4.429, P = 0.011) cells. Rescue assays revealed that overexpression of RhoA rescued the suppressive effects of miR-345-5p upregulation on proliferation, migration, and invasion of lung adenocarcinoma cells. Further, miR-345-5p was found to regulate the Rho/Rho-associated protein kinase (ROCK) signaling pathway by downregulation of RhoA in lung adenocarcinoma cells.
Conclusions:
MiR-345-5p plays a tumor suppressor role in lung adenocarcinoma cells by downregulating RhoA to inactivate the Rho/ROCK pathway.
Keywords: MicroRNA-345-5p, Lung adenocarcinoma, Ras homolog family member A (RhoA), Rho/Rho-associated protein kinase (ROCK)
Introduction
Lung cancer accounts for the highest proportion of cancer morbidity and mortality in the world, and about 90% of lung cancer-related deaths are caused by metastasis.[1,2] Lung cancer can be divided into two categories, small cell lung carcinoma and non-small cell lung carcinoma (NSCLC). Lung adenocarcinoma is the significant histological subtype of NSCLC with high metastasis rate.[3] Although multiple diagnosis and treatment options have been developed in the past few years, the 5-year survival rate of lung adenocarcinoma is 15%.[4] Fortunately, emerging evidence has suggested the association between microribose nucleic acids (miRNAs) and the initiation and development of lung adenocarcinoma. Therefore, further research regarding the molecular mechanism underlying lung adenocarcinoma is urgently needed to improve the treatment of lung adenocarcinoma.
MiRNAs are small non-coding RNAs of about 21 to 25 nucleotides that exert crucial functions in translational suppression or post-transcriptional mRNA degradation.[5] Dysregulated miRNAs are involved in a variety of biological cellular processes in cancers, such as cell stemness, differentiation, and epithelial-mesenchymal transition (EMT) process.[6–9] In addition, many studies indicated that miRNAs are implicated in lung adenocarcinoma cell self-renewal,[10] metastasis,[11] and chemoresistance.[12] It was reported that by mediating the hedgehog signaling, miR-182-5p targets gliotactin family zinc finger 2 to modulate chemosensitivity of cisplatin-resistant lung adenocarcinoma cells.[13] MiR-629-5p contributes to the invasive capacity of lung adenocarcinoma by promoting the permeability of endothelial cells.[14]
MicroRNA-345-5p (miR-345-5p) expression is at a high level in the serum of prostate cancer patients, and serves as an oncogenic gene to promote cell migration.[15] On the other hand, miR-345-5p serves as a tumor suppressor to inhibit migration and EMT process of gastric cancer cells.[16] Additionally, miR-345-5p is underexpressed in pancreatic cancer and directly targets C-C motif chemokine ligand 8 to suppress cell proliferation and invasion.[17] These results signified that miR-345-5p has different biological functions in human carcinomas. Nonetheless, the function and underlying molecular mechanism of miR-345-5p in lung adenocarcinoma has not been studied.
Herein, we endeavored to identify the biological function and underlying mechanism of miR-345-5p in lung adenocarcinoma cells.
Methods
Ethical approval
This study was approved by Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 2019-022). We conducted all the experiments in conformity with relevant guidelines. Prior to the collection of specimens, the patients or their guardians had agreed to use the lung adenocarcinoma tissues for research. Written informed consents were signed by all participants.
Clinical specimens
Thirty-five specimens of lung adenocarcinoma tissues were collected from patients (21 males and 14 females, aged from 22 to 68 years, with an average age of 44.1 ± 12.0 years) who were pathologically diagnosed with lung adenocarcinoma and underwent resection of lung adenocarcinoma tumors in the First Affiliated Hospital of Anhui Medical University between April 2016 and February 2017. Sample size was determined using G power for power analysis. The tumor adjacent tissues were located no less than three centimeters away from the lung adenocarcinoma tissues. None of the patients received any pre-operative treatments prior to surgery. The collected specimens were instantly frozen in liquid nitrogen and stored at −80°C for further utilization.
Cell culture and treatment
Cell Bank of the Chinese Academy of Sciences (Shanghai, China) provided the human lung adenocarcinoma cell lines (A549, H1650, PC-9, and H441) and a normal human bronchial epithelial cell line (BEAS-2B). The above-mentioned cells were incubated in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1/10 fetal bovine serum (FBS) in a humidified atmosphere (5% CO2, 37°C). Cell transfection was performed using Lipofectamine 2000 transfection reagent (Invitrogen). MiR-345-5p mimics are chemically synthesized to enhance endogenous miR-345-5p functions. MiR-345-5p inhibitor is chemically modified to specifically target miR-345-5p and weaken the gene silencing effects of endogenous miR-345-5p. Commercially obtained plasmids including miR-345-5p mimics (GCUGACUCCUAGUCCAGGGCUC), negative control (NC) mimics, miR-345-5p inhibitor (GCUGACUCCUAGUCCAGGGCUC), NC inhibitor, empty pcDNA3.1, and pcDNA3.1/ras homolog family member A (RhoA) (RiboBio, Guangzhou, China) were transfected into lung adenocarcinoma cells for 48 h as per the product manuals. Some cells were treated with 1 μmol/L of CCG-1423 (formula: C18H13ClF6N2O3), the RhoA inhibitor, for 48 h.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis
TRIzol reagent (Invitrogen) was applied for the extraction of total RNA from tissues and cells. Based on the PrimeScript RT reagent Kit (Takara, Kyoto, Japan), reverse transcription was conducted. A SYBR Prime Script RT-PCR Kit (Takara) was applied for RT-qPCR on a StepOnePlus System (Applied Biosystems, Waltham, MA, USA). The expression levels of miR-345-5p and RhoA were calculated with the 2−ΔΔCt method. The primer sequences were presented as below: miR-345-5p, F: 5′-TGAGGGGCAGAGAGCGAGACTTT-3′, R: 5′-CTCAACTGGTGTCGTGGA-3′; RhoA, F: 5′-TCGAGGTGGATGGAAAGCAG-3′, R: 5′-CACAAGACAAGGCACCCAGA-3′; U6, F: 5′-CTCGCTTCGGCAGCACA-3′, R: 5′-AACGCTTCACGAATTTGCGT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), F: 5′-AACGGATTTGGTCGTATTG-3′, R: 5′-GGAAGATGGTGATGGGATT-3′. All reactions were performed as follows: 94°C, 60 s; 94°C, 30 s; 60°C, 30 s; 72°C, 60 s; 40 cycles. GAPDH and U6 were used as the endogenous references.
Colony formation assay
Pancreatic enzyme digestion was used to isolate the cells which were incubated in RPMI 1640 medium. Subsequently, cells (1 × 103 cells/well) were plated into 6-well plates and cultivated with 5% CO2 at 37°C. The cultivation was terminated after observing visible cell clones. After the cells were washed and dehydrated, they were immobilized with methanol, and dyed with violet. Finally, five fields were randomly selected and ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to calculate the number of colonies.
5-Ethynyl-2′-deoxyuridine (EdU) pulse-chase incorporation
Cell proliferation was measured by the EdU assay using the Cell-Light EdU DNA Cell Proliferation Kit (RiboBio). The transfected cells were cultured in 30-mm Petri dishes to a normal growth stage. In accordance with the product manuals for a Click-iT™ EdU flow cytometry kit (Invitrogen), cell immobilization, permeabilization, and EdU test were conducted. Finally, ten fields of each dish were randomly selected for counting EdU-labelled cells and calculating the percentage.
Transwell assay
Cell invasive capability was detected by a transwell invasion assay. As instructed by the supplier, cells were inoculated into the upper chambers which were coated with Matrigel and supplemented with 200 μL of serum-free medium. As to that of the lower chambers, RPMI 1640 containing 1/10 FBS was added. Twenty-four hours after cultivation, 4% paraformaldehyde and crystal violet were separately used to immobilize and stain the cells for 10 min. Finally, the invaded cells were observed and counted.
Wound healing assay
Cells were cultured in RPMI 1640 containing 1/10 FBS. After cultivating for 24 h, a sterile plastic 200 mL micropipette tip was used to scratch the monolayer. Then, the monolayer was washed, and incubated in serum-free RPMI 1640. At 0 and 24 h, a low-magnification fluorescence microscope (Olympus IX71, Tokyo, Japan) was applied to photograph the cells. AlphaEase FC (Version 4.0, Alpha Innotech, San Francisco, CA, USA) was adopted to measure the widths of the wound lines.
Flow cytometry analysis
Cell apoptosis was detected by an annexin V/propidium iodide (PI) kit (BD Bioscience, Hercules, NJ, USA). Before flow cytometry analysis was carried out, cells were transfected and cultured in a six-well plate. Forty-eight hours later, the digested cells were dyed using annexin V-fluorescein isothiocyanate from a cell apoptosis kit (BD Bioscience), and the apoptotic rate was analyzed using a flow cytometer.
Bioinformatics analysis
Based on the PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html) and PicTar (https://pictar.mdc-berlin.de/) online databases which predict potential targets of miR-345-5p, 23 mRNAs were identified. Among the 23 targets, family with sequence similarity 126 member B (FAM126B), RhoA, kelch domain containing ten (KLHDC10), family with sequence similarity 214 member B (FAM214B) were found to bind with miR-345-5p based on RNA pulldown assay.
Luciferase reporter assay
Luciferase reporter assays were conducted to explore whether miR-345-5p directly targets RhoA 3′-untranslated regions (3′-UTRs). Mutant (Mut) and wild-type (WT) sequences of RhoA 3′-UTR were amplified and subcloned into the pmirGLO luciferase reporter vectors (Promega, Madison, WI, USA). Cotransfection of RhoA 3′-UTR WT vector or Mut vector and miR-345-5p mimics or NC mimics into PC-9 and H1650 cells was performed using Lipofectamine 2000 transfection reagent (Invitrogen). A Dual-Luciferase Reporter Assay System (Promega) was utilized to examine the luciferase activity.
Western blot analysis
Lung adenocarcinoma cells were harvested and extracted using radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Shanghai, China). The extracted cells were boiled in loading buffer, and then 12% to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels were used to separate the equal amount of cell extracts. Then, the isolated protein samples were transferred onto the polyvinylidene fluoride membranes. According to the instructions, the membranes were cultivated with the primary antibodies at 4°C overnight, followed by secondary antibodies at room temperature for 2 h. An enhanced chemiluminescence Western blot detection kit was adopted to visualize the immunoreactive bands based on the kit instructions. The GAPDH antibody was utilized for normalization. The primary antibodies are exhibited as follows: RhoA (Abcam, Cambridge, UK, ab54835); rho associated coiled-coil containing protein kinase 1 (ROCK1, Abcam, ab45171); rho associated coiled-coil containing protein kinase 2 (ROCK2, Abcam, ab71598); GAPDH (Abcam, ab8245).
RNA pulldown assay
Lung adenocarcinoma cells were lysed in radio immunoprecipitation assay lysis buffer and cultured with biotinylated miR-345-5p probes or non-biotinylated miR-345-5p probes for 1 h at 4°C. Streptavidin magnetic beads were added for harvesting the pull-down compounds, which were then subjected to RT-qPCR analysis for enrichment detection.
Statistical analysis
We used the SPSS 20.0 (St Louis, MO, USA) for the statistical analysis. Data are exhibited as the mean ± standard deviation from at least three independent assays. Levene test was used for homogeneity of variance test, and Shapiro-Wilk test was used for normal distribution test. All data are normally distributed. Comparison between two groups was conducted with Student's t test, and one-way analysis of variance followed by Dunnett post hoc test or Tukey post hoc test was adopted for comparison among multiple groups. Spearman correlation analysis was applied to analyze the expression correlation between miR-345-5p and RhoA in lung adenocarcinoma tissues. P < 0.05 was regarded to indicate a statistically significant difference.
Results
Upregulated miR-345-5p suppresses cell proliferation in lung adenocarcinoma
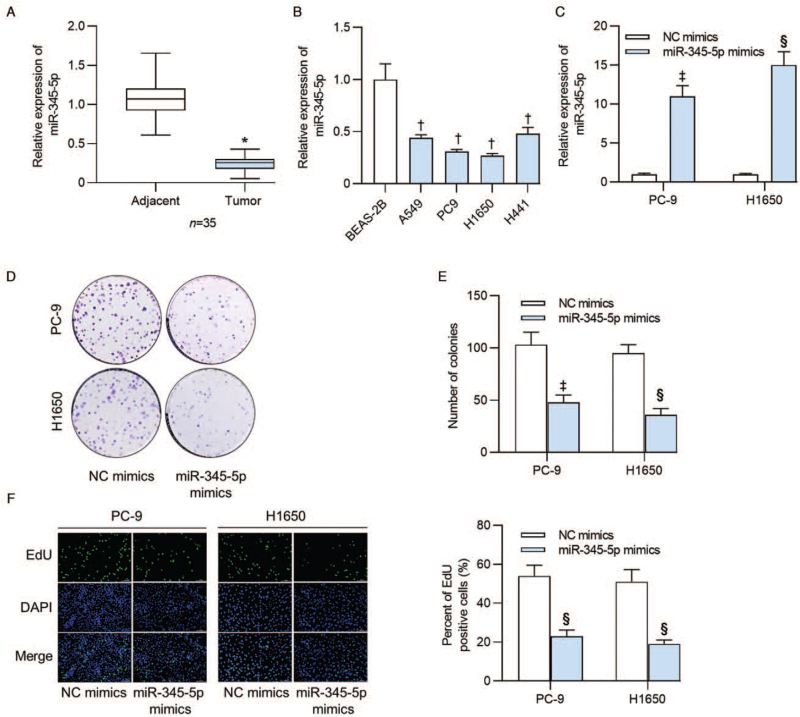
We first detected the expression status of miR-345-5p in 35 lung adenocarcinoma tissues and tumor adjacent tissues by RT-qPCR. The data manifested that miR-345-5p expression was downregulated in lung adenocarcinoma tissues relative to the tumor adjacent tissues (0.241 ± 0.095 vs. 1.000 ± 0.233, t = 19.247, P < 0.001; Figure 1A). Next, we tested the expression of miR-345-5p in normal human BEAS-2B and four human lung adenocarcinoma cell lines (A549, PC-9, H1650, and H441). Results of RT-qPCR revealed the lower miR-345-5p expression in lung adenocarcinoma cells than that in BEAS-2B cells (all P < 0.001; Figure 1B). As presented in Figure 1C, miR-345-5p expression was increased in PC-9 (11.152 ± 1.362 vs. 1.000 ± 0.122, t = 9.701, P = 0.010) and H1650 (14.958 ± 1.721 vs. 1.000 ± 0.109, t = 27.613, P < 0.001) cells after transfection of miR-345-5p mimics. Subsequently, we tested the effects of miR-345-5p overexpression on the proliferation and apoptosis of PC-9 and H1650 cells. Colony formation assay revealed that the number of colonies derived from PC-9 (47.684 ± 6.892 vs. 103.446 ± 12.132, t = 5.666, P = 0.005) and H1650 (36.251 ± 6.328 vs. 95.783 ± 8.174, t = 24.267, P < 0.001) cells was markedly decreased upon miR-345-5p upregulation [Figure 1D and 1E]. Moreover, EdU assay demonstrated that miR-345-5p mimics significantly reduced the percent of EdU positive PC-9 (23.174 ± 3.166 vs. 54.173 ± 5.479, t = 9.769, P < 0.001) and H1650 (19.230 ± 2.053 vs. 51.325 ± 6.331, t = 10.750, P < 0.001) cells [Figure 1F].
Figure 1.
MiR-345-5p expression displayed low levels in human lung adenocarcinoma tissues and cell lines and inhibited lung adenocarcinoma cell proliferation. (A) RT-qPCR was conducted to examine the expression of miR-345-5p in 35 lung adenocarcinoma tissues and tumor adjacent tissues. (B) MiR-345-5p expression levels in lung adenocarcinoma cell lines (A549, PC9, H1650 and H441) and in normal human BEAS-2B were detected by RT-qPCR. (C) Expression of miR-345-5p in cells transfected with miR-345-5p mimics was assayed by RT-qPCR analysis. (D, E) Colony formation assay measured the effects of miR-345-5p overexpression on the number of colonies formed by PC-9 and H1650 cells. (F) Percent of EdU positive cells was determined by EdU assay in PC-9 and H1650 cells transfected with NC mimics or miR-345-5p mimics. ∗P < 0.001 vs. adjacent tissues; †P < 0.001 vs. BESA-2B; ‡P < 0.05, §P < 0.001 vs. NC mimics. BEAS-2B: Bronchial epithelium transformed with Ad12-SV40 2B; DAPI: 4′,6-diamidino-2-phenylindole; EdU: 5-Ethynyl-2′-deoxyuridine; miR-345-5p: MicroRNA-345-5p; NC: Negative control; RT-qPCR: Reverse transcription quantitative polymerase chain reaction.
Upregulation of miR-345-5p promotes cell apoptosis whereas restrains cell migration and invasion in lung adenocarcinoma
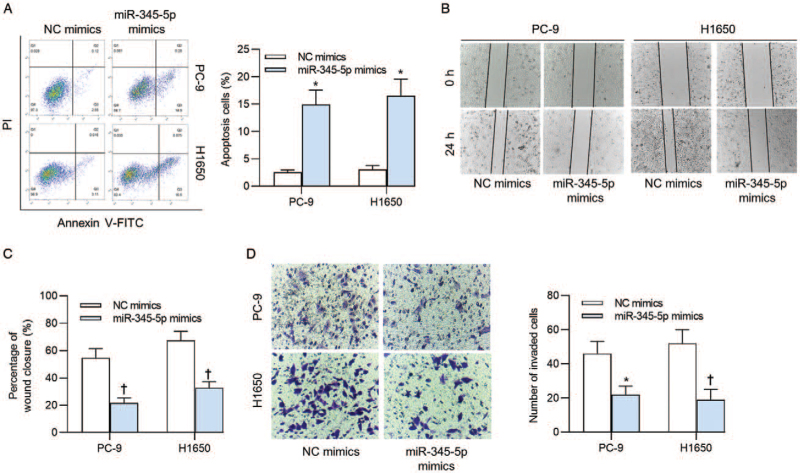
Next, we measured the effects of miR-345-5p overexpression on apoptosis, migration, and invasion of PC-9 and H1650 cells. Upregulated miR-345-5p significantly increased the percent of apoptotic PC-9 ([2.550 ± 0.421]% vs. [14.931 ± 2.653]%, t = 12.287, P < 0.001) and H1650 ([3.112 ± 0.655]% vs. [16.507 ± 3.052]%, t = 10.409, P < 0.001) cells [Figure 2A]. The results of wound healing assay illuminated that miR-345-5p mimics suppressed the migratory capacities of PC-9 ([21.272 ± 3.559]% vs. [54.511 ± 6.859]%, t = 6.823, P = 0.002) and H1650 ([32.831 ± 4.557]% vs. [67.508 ± 6.532]%, t = 6.567, P = 0.003) cells [Figure 2B and 2C]. Additionally, the decreased numbers of invaded PC-9 (22.315 ± 4.736 vs. 46.325 ± 7.026, t = 33.755, P < 0.001) and H1650 (19.774 ± 5.936 vs. 52.115 ± 7.849, t = 6.538, P = 0.003) cells were caused by upregulated expression of miR-345-5p [Figure 2D].
Figure 2.
Effects of miR-345-5p upregulation on cell apoptosis, migration, and invasion. (A) Flow cytometry analysis was conducted to detect apoptosis of PC-9 and H1650 cells after transfection of NC mimics or miR-345-5p mimics. (B, C) Cell migratory capacity upon miR-345-5p upregulation was detected via a wound healing assay. (D) The results of transwell assay revealed the cell invasive ability when miR-345-5p expression was upregulated. ∗P < 0.001, †P < 0.01 vs. NC mimics. FITC: Fluorescein isothiocyanate; miR-345-5p: MicroRNA-345-5p; NC: Negative control; PI: Prodium iodide.
RhoA serves as a direct downstream target of miR-345-5p
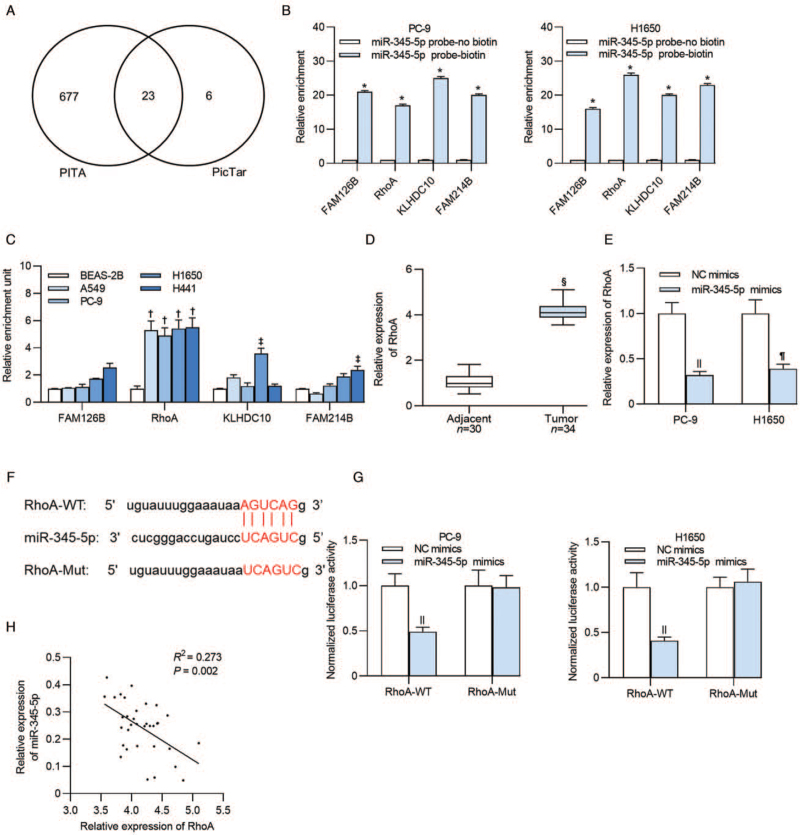
We searched the potential mRNAs for miR-345-5p using PITA and PicTar online websites to clarify the underlying molecular mechanisms of miR-345-5p in lung adenocarcinoma cells. As a result, 23 miRNAs were identified [Figure 3A]. RNA pulldown assay was performed in PC-9 and H1650 cells, and the results revealed that FAM126B (PC-9: t = 84.002, P < 0.001; H1650: t = 135.239, P < 0.001), RhoA (PC-9: t = 61.342, P < 0.001; H1650: t = 176.868, P < 0.001), KLHDC10 (PC-9: t = 76.838, P < 0.001; H1650: t = 44.259, P < 0.001), and FAM214B (PC-9: t = 229.665, P < 0.001; H1650: t = 295.480, P < 0.001) presented significant enrichment in miR-345-5p probe-biotin group [Figure 3B]. RhoA was upregulated in lung adenocarcinoma cell lines (all P < 0.001), while other mRNAs (P > 0.05) exhibited no significant expression difference in most of the cell lines [Figure 3C]. RhoA was also upregulated in lung adenocarcinoma tumor tissues (4.053 ± 0.354 vs. 1.000 ± 0.252, t = 33.133, P < 0.001; Figure 3D). Additionally, we detected the influence of miR-345-5p overexpression on RhoA expression, and found that RhoA was downregulated in PC-9 (0.321 ± 0.047 vs. 1.000 ± 0.127, t = 8.536, P < 0.001) and H1650 (0.398 ± 0.054 vs. 1.000 ± 0.156, t = 4.429, P = 0.044) cells after transfection of miR-345-5p mimics [Figure 3E]. The predicted binding sequences of miR-345-5p and RhoA were presented in Figure 3F. Based on the data from luciferase reporter assay, luciferase activity of RhoA-WT vector in PC-9 (0.492 ± 0.051 vs. 1.000 ± 0.136, t = 33.469, P < 0.001) and H1650 cells (0.411 ± 0.047 vs. 1.000 ± 0.164, t = 16.000, P < 0.001) was significantly reduced after transfection of miR-345-5p mimics, while no significant difference was found in luciferase activity of RhoA-Mut plasmids (P > 0.05) after upregulating miR-345-5p [Figure 3G]. Furthermore, we carried out Spearman correlation analysis and revealed a negative expression correlation between miR-345-5p and RhoA in lung adenocarcinoma tissues (R2 = 0.273, P = 0.002; Figure 3H).
Figure 3.
MiR-345-5p targets RhoA in lung adenocarcinoma cells. (A) Targets of miR-345-5p predicted by two different databases (PITA and PicTar). (B) The significant enrichment of mRNAs (FAM126B, RhoA, KLHDC10, and FAM214B) pulled down by miR-345-5p probe was tested by an RNA pulldown assay. (C) Expression levels of FAM126B, RhoA, KLHDC10, and FAM214B in lung adenocarcinoma cells and BEAS-2B cells were examined by RT-qPCR. (D) Expression of RhoA in thirty-four tumor tissues and thirty adjacent tissues was detected. (E) RT-qPCR detected RhoA expression in PC-9 and H1650 cells after transfection of miR-345-5p mimics. (F) TargetScan predicted the binding sequences of RhoA that were complementary to the seed sequences of miR-345-5p. (G) Luciferase reporter assay presented the luciferase activity of PC-9 and H1650 cells which were transfected with NC mimics or miR-345-5p mimics. (H) Spearman's correlation analysis was applied for the analysis of the correlation between miR-345-5p and RhoA in lung adenocarcinoma tissues. ∗P < 0.001 vs. miR-345-5p probe-no biotin; †P < 0.001, ‡P < 0.01 vs. BESA-2B; §P < 0.001 vs. adjacent tissue; ||P < 0.001, ¶P < 0.05 vs. NC mimics. BEAS-2B: Bronchial Epithelium transformed with Ad12-SV40 2B; FAM126B: Family with sequence similarity 126 member B; FAM214B: Family with sequence similarity 214 member B; KLHDC10: Kelch domain containing 10; miR-345-5p: MicroRNA-345-5p; mRNA: messenger RNA; Mut: Mutant; NC: Negative control; RhoA: Ras homolog family member A; RT-qPCR: Reverse transcription quantitative polymerase chain reaction; WT: Wild type.
MiR-345-5p inhibits cell proliferation in lung adenocarcinoma via modulating RhoA
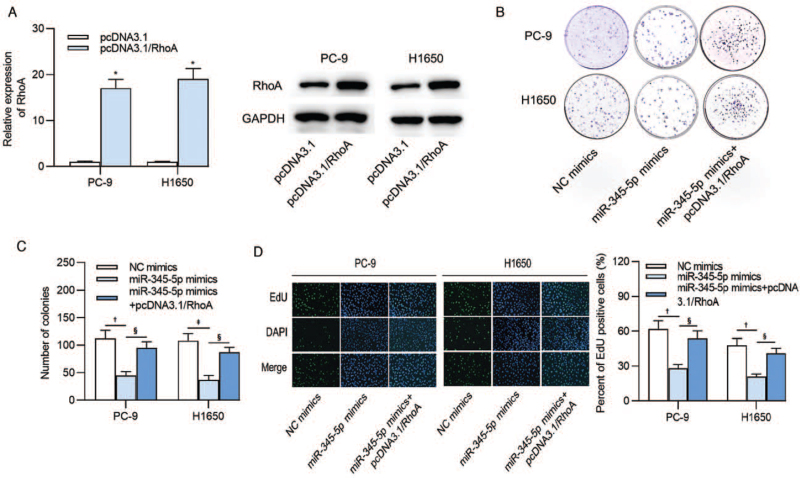
Expression of RhoA at the mRNA and protein levels was elevated after transfection of pcDNA3.1 RhoA in PC-9 (16.653 ± 1.932 vs. 1.000 ± 0.140, t = 12.226, P < 0.001) and H1650 (19.386 ± 2.374 vs. 1.000 ± 0.114, t = 57.985, P < 0.001) cells [Figure 4A]. Colony formation assay revealed that overexpressed RhoA reversed the suppressive influence of miR-345-5p mimics on the number of colonies formed by PC-9 (P = 0.004) and H1650 (P = 0.002) cells [Figure 4 B and 4C]. In addition, the results from EdU assay indicated that upregulation of RhoA rescued the reduced percent of EdU positive PC-9 (P = 0.004) and H1650 (P = 0.003) cells caused by miR-345-5p overexpression [Figure 4D].
Figure 4.
MiR-345-5p modulates lung adenocarcinoma cell proliferation by RhoA. (A) Overexpression efficiency of RhoA in PC-9 and H1650 cells. (B, C) Number of colonies following co-transfection with miR-345-5p mimics and pcDNA3.1/RhoA into PC-9 and H1650 cells. (D) The percent of EdU positive cells after the transfection of NC mimics, miR-345-5p mimics or miR-345-5p mimics and pcDNA3.1/RhoA into lung adenocarcinoma cells was examined by EdU assay. ∗P < 0.001 vs. pcDNA3.1; †P < 0.01, ‡P < 0.001 vs. NC mimics; §P < 0.01 vs. miR-345-5p mimics. EdU: 5-Ethynyl-2′-deoxyuridine; miR-345-5p: MicroRNA-345-5p; NC: Negative control; RhoA: Ras homolog family member A.
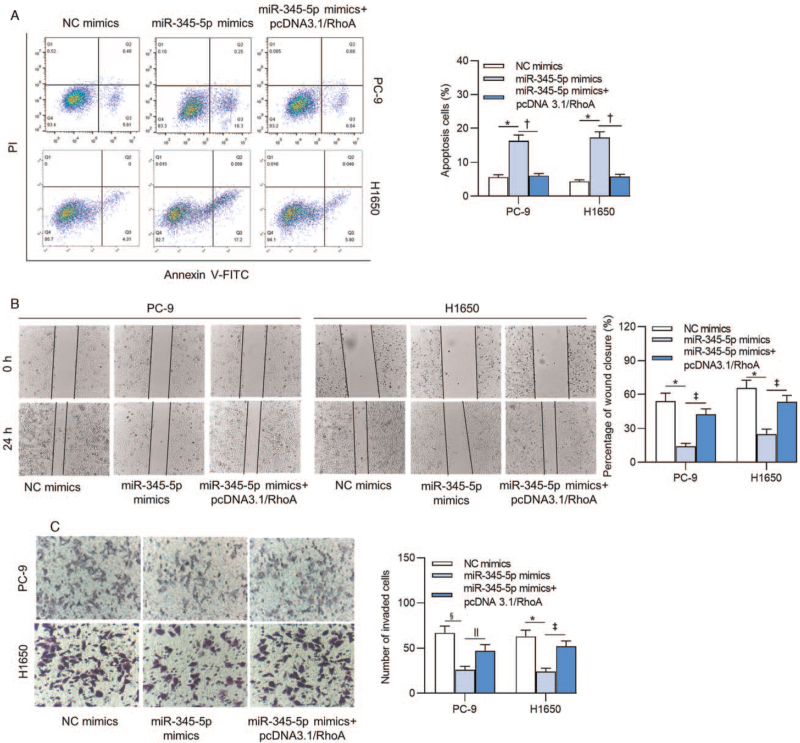
MiR-345-5p induces apoptosis and represses cell migration and invasion in lung adenocarcinoma by regulating RhoA
MiR-345-5p-induced suppression on apoptosis of PC-9 (P < 0.001) and H1650 (P < 0.001) cells was reversed by pcDNA3.1/RhoA [Figure 5A]. Moreover, repressed capacities of cell migration (PC-9: P = 0.001; H1650: P = 0.002) and invasion (PC-9: P = 0.015; H1650: P = 0.002) caused by overexpressing miR-345-5p were reversed by upregulation of RhoA [Figure 5B and 5C].
Figure 5.
MiR-345-5p regulates lung adenocarcinoma cell apoptosis, migration, and invasion via RhoA. (A) Apoptosis of PC-9 and H1650 cells transfected with the indicated plasmids was explored by flow cytometry analysis. (B, C) The migration and invasion of cells transfected with the indicated plasmids were detected by wound healing and transwell assays. ∗P < 0.001 vs. NC mimics; †P < 0.001 vs. miR-345-5p mimics; ‡P < 0.01 vs. miR-345-5p mimics; §P < 0.01 vs. NC mimics; ||P < 0.05 vs. miR-345-5p mimics. FITC: Fluorescein isothiocyanate; miR-345-5p: MicroRNA-345-5p; NC: Negative control; PI: Prodium iodide; RhoA: Ras homolog family member A.
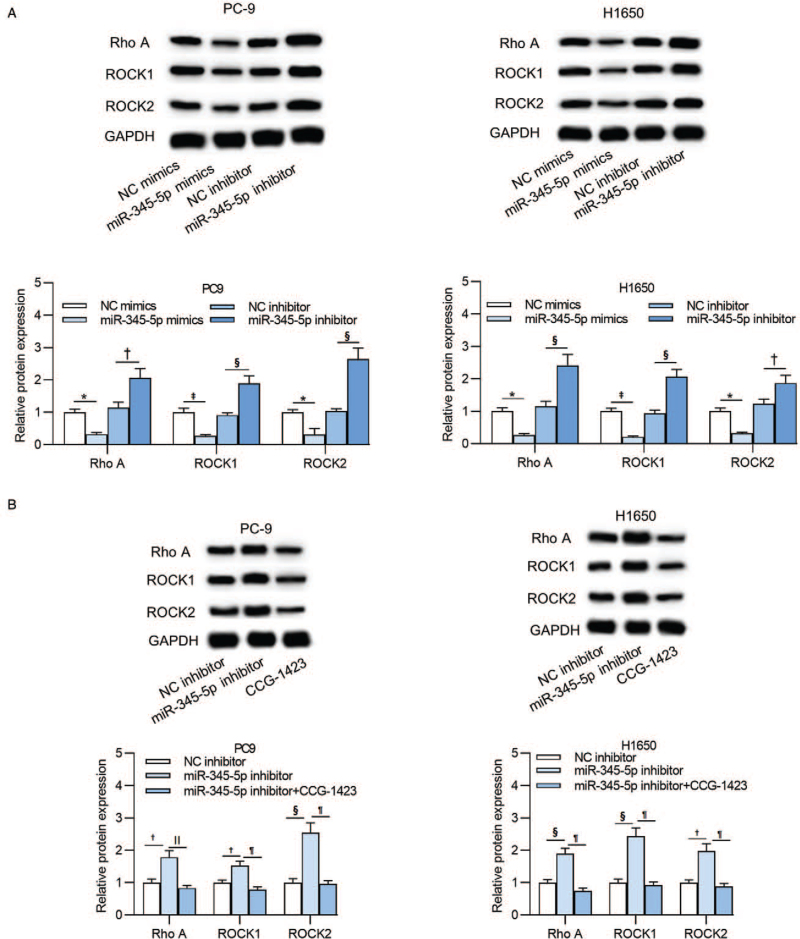
MiR-345-5p regulates the ras homolog (Rho)/Rho-associated protein kinase (ROCK) signaling in lung adenocarcinoma cells
As one of the most classical signaling pathways, the Rho/ROCK pathway participates in diverse cellular processes including cell movement, proliferation, adhesion, and EMT process. RhoA is one of the important key factors of the Rho/ROCK signaling pathway. Additionally, RhoA was validated to be the direct downstream target of miR-345-5p in this study. We speculated that miR-345-5p regulates the Rho/ROCK signaling pathway to influence the cellular development in lung adenocarcinoma. In the data presented, the protein levels of RhoA (PC-9: P = 0.006; H1650: P = 0.007), ROCK1 (PC-9: P < 0.001; H1650: P < 0.001), and ROCK2 (PC-9: P = 0.012; H1650: P = 0.002) were significantly suppressed upon miR-345-5p overexpression, and were increased after transfection of miR-345-5p inhibitor in PC-9 (RhoA: P = 0.001; ROCK1: P < 0.001; ROCK2: P < 0.001) and H1650 cells (RhoA: P < 0.001; ROCK1: P < 0.001; ROCK2: P = 0.004) [Figure 6A]. Western blot analysis showed that the increased protein levels of RhoA (PC-9: P = 0.001; H1650: P < 0.001), ROCK1 (PC-9: P < 0.001; H1650: P < 0.001), and ROCK2 (PC-9: P < 0.001; H1650: P < 0.001) induced by miR-345-5p inhibitor were subsequently decreased by CCG-1423 treatment [Figure 6B].
Figure 6.
MiR-345-5p regulates the Rho/ROCK signaling pathway in lung adenocarcinoma cells. (A) Western blot analysis detected the expression of RhoA, ROCK1, and ROCK2 proteins in PC-9 and H1650 cells transfected with NC mimics, miR-345-5p mimics, NC inhibitor or miR-345-5p inhibitor. (B) Protein expression levels of RhoA, ROCK1, and ROCK2 of PC-9 and H1650 cells after treatment of NC inhibitor, miR-345-5p inhibitor, or miR-345-5p inhibitor+CCG-1423 were measured by western blot analysis. ∗P < 0.01 vs. NC mimics; †P < 0.01 vs. NC inhibitor; ‡P < 0.001 vs. NC mimics; §P < 0.001 vs. NC inhibitor; ||P < 0.01, ¶P < 0.001 vs. miR-345-5p inhibitor. CCG-1423: RhoA inhibitor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; miR-345-5p: microRNA-345-5p; NC: negative control; Rho: Ras homolog; RhoA: Ras homolog family member A; ROCK: Rho-associated protein kinase; ROCK1: Rho associated coiled-coil containing protein kinase 1; ROCK2: Rho associated coiled-coil containing protein kinase 2.
Discussion
Previous studies have demonstrated that miR-345-5p exerts critical functions in some cancers such as prostate cancer,[15] hepatocellular carcinoma,[18] and pancreatic cancer.[17] In our research, miR-345-5p expression pattern was downregulated in lung adenocarcinoma tissues and cells. Functionally, upregulation of miR-345-5p inhibited the malignant behaviors of lung adenocarcinoma cells by suppressing cell proliferation, migration, and invasion as well as promoting cell apoptosis.
As a member of Rho GTPase family, RhoA has been recognized as a carcinogenic gene in the public eye that facilitates the growth of various types of neoplasms.[19,20] In addition, RhoA elicits significant functions in regulating cellular processes including cell invasion, proliferation, and adhesion.[21] RhoA promotes cell proliferation and migration via its downstream mediator ROCK in plenty of carcinomas including oral squamous cell carcinoma,[22] esophageal squamous cell carcinoma,[23] liver cancer,[24] and bladder cancer.[25] Additionally, RhoA can influence the phenotypes of cancer stem cells by regulation of the wingless/integrated (Wnt)/β-catenin signaling pathway,[26] and to inhibit RhoA might be a novel strategy for glioblastoma treatment.[27] The present study revealed that RhoA expression was significantly elevated in lung adenocarcinoma tissues and cell lines. In addition, RhoA was verified to be the direct downstream target of miR-345-5p in lung adenocarcinoma cells. A negative expression correlation between miR-345-5p and RhoA was identified subsequently. Furthermore, overexpression of RhoA rescued the effects of miR-345-5p on proliferation, apoptosis, migration, and invasion of lung adenocarcinoma cells.
The Rho/ROCK signaling pathway is a key modulator of actomyosin contractility, which regulates cell shape and cytoskeletal arrangement, thus regulating cellular functions including cell proliferation, differentiation, motility, and adhesion.[28] MiRNAs exert essential functions in cellular processes by regulating the Rho/ROCK signaling pathway. For example, by mediating the Rho/ROCK signaling pathway, miR-133 facilitates apoptosis of trophoblasts in human placenta tissues.[29] MiR-145 mediates the differentiation from mesenchymal stem cell to smooth muscle cells via the Rho/ROCK signaling pathway.[30] RhoA functions as one of the important key factors of the Rho/ROCK signaling pathway. In our current study, we discovered that miR-345-5p upregulation inactivated the Rho/ROCK signaling by downregulation of RhoA.
In conclusion, our study innovatively revealed that miR-345-5p expression displayed low levels in lung adenocarcinoma tissues and cells. MiR-345-5p upregulation suppressed the malignant phenotypes of lung adenocarcinoma cells by inactivation of the Rho/ROCK signaling pathway in a RhoA dependent way, which might provide a further understanding of mechanisms underlying lung adenocarcinoma.
Conflicts of interest
None.
Footnotes
How to cite this article: Zhou QY, Gui SY, Zhang P, Wang M. Upregulation of miR-345-5p suppresses cell growth of lung adenocarcinoma by regulating ras homolog family member A (RhoA) and Rho/Rho associated protein kinase (Rho/ROCK) pathway. Chin Med J 2021;134:2619–2628. doi: 10.1097/CM9.0000000000001804
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Romaszko AM, Doboszyńska A. Multiple primary lung cancer: a literature review. Adv Clin Exp Med 2018; 27:725–730. doi: 10.17219/acem/68631. [DOI] [PubMed] [Google Scholar]
- 3.Shi J, Hua X, Zhu B, Ravichandran S, Wang M, Nguyen C, et al. Somatic genomics and clinical features of lung adenocarcinoma: a Retrospective Study. PLoS Med 2016; 13:e1002162.doi: 10.1371/journal.pmed.1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol 2008; 3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 5.de Sousa MC, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci 2019; 20:6249.doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SS, Peng CY, Liao YW, Chou MY, Hsieh PL, Yu CC. miR-1246 targets CCNG2 to enhance cancer stemness and chemoresistance in oral carcinomas. Cancers (Basel) 2018; 10:272.doi: 10.3390/cancers10080272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Shi L, Liang T, Wang B, Wu W, Su G, et al. MiR-696 regulates C2C12 cell proliferation and differentiation by targeting CNTFRα. Int J Biol Sci 2017; 13:413–425. doi: 10.7150/ijbs.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Hu Q, Hu LX, Lin XR, Liu JQ, Lin X, et al. miR-200b regulates epithelial-mesenchymal transition of chemo-resistant breast cancer cells by targeting FN1. Discov Med 2017; 24:75–85. [PubMed] [Google Scholar]
- 9.Chen X, Yang F, Zhang T, Wang W, Xi W, Li Y, et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J Exp Clin Cancer Res 2019; 38:99.doi: 10.1186/s13046-019-1078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, Yang Z, Long F, Luo L, Yang B, Zhu R, et al. Long noncoding RNA MALAT1 mediates stem cell-like properties in human colorectal cancer cells by regulating miR-20b-5p/Oct4 axis. J Cell Physiol 2019; 234:20816–20828. doi: 10.1002/jcp.28687. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Zhang S, Chen Z, He Z, Xu Y, Li Z. CircRNA-ENO1 promoted glycolysis and tumor progression in lung adenocarcinoma through upregulating its host gene ENO1. Cell Death Dis 2019; 10:885.doi: 10.1038/s41419-019-2127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Huang P, Li Q, Wang D, Xu CX. miR-134-5p promotes stage I lung adenocarcinoma metastasis and chemoresistance by targeting DAB2. Mol Ther Nucleic Acids 2019; 18:627–637. doi: 10.1016/j.omtn.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidl C, Panzitt K, Bertsch A, Brcic L, Schein S, Mack M, et al. MicroRNA-182-5p regulates hedgehog signaling pathway and chemosensitivity of cisplatin-resistant lung adenocarcinoma cells via targeting GLI2. Cancer Lett 2020; 469:266–276. doi: 10.1016/j.canlet.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhang H, Fan L, Mou J, Yin Y, Peng C, et al. MiR-629-5p promotes the invasion of lung adenocarcinoma via increasing both tumor cell invasion and endothelial cell permeability. Oncogene 2020; 39:3473–3488. doi: 10.1038/s41388-020-1228-1. [DOI] [PubMed] [Google Scholar]
- 15.Tinay I, Tan M, Gui B, Werner L, Kibel AS, Jia L. Functional roles and potential clinical application of miRNA-345-5p in prostate cancer. Prostate 2018; 78:927–937. doi: 10.1002/pros.23650. [DOI] [PubMed] [Google Scholar]
- 16.Feng A, Yuan X, Li X. MicroRNA-345 inhibits metastasis and epithelial-mesenchymal transition of gastric cancer by targeting FOXQ1. Oncol Rep 2017; 38:2752–2760. doi: 10.3892/or.2017.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mou T, Xie F, Zhong P, Hua H, Lai L, Yang Q, et al. MiR-345-5p functions as a tumor suppressor in pancreatic cancer by directly targeting CCL8. Biomed Pharmacother 2019; 111:891–900. doi: 10.1016/j.biopha.2018.12.121. [DOI] [PubMed] [Google Scholar]
- 18.Lin S, Zhuang J, Zhu L, Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int 2020; 20:246.doi: 10.1186/s12935-020-01293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res 2011; 71:3246–3256. doi: 10.1158/0008-5472.Can-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, et al. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res 2004; 10:4799–4805. doi: 10.1158/1078-0432.Ccr-0436-03. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Zheng Y. Cell type-specific signaling function of RhoA GTPase: lessons from mouse gene targeting. J Biol Chem 2013; 288:36179–36188. doi: 10.1074/jbc.R113.515486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeshita A, Iwai S, Morita Y, Niki-Yonekawa A, Hamada M, Yura Y. Wnt5b promotes the cell motility essential for metastasis of oral squamous cell carcinoma through active Cdc42 and RhoA. Int J Oncol 2014; 44:59–68. doi: 10.3892/ijo.2013.2172. [DOI] [PubMed] [Google Scholar]
- 23.Faried A, Faried LS, Kimura H, Nakajima M, Sohda M, Miyazaki T, et al. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer 2006; 42:1455–1465. doi: 10.1016/j.ejca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res 1999; 59:2004–2010. [PubMed] [Google Scholar]
- 25.Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res 2003; 9:2632–2641. [PubMed] [Google Scholar]
- 26.Rossol-Allison J, Stemmle LN, Swenson-Fields KI, Kelly P, Fields PE, McCall SJ, et al. Rho GTPase activity modulates Wnt3a/beta-catenin signaling. Cell Signal 2009; 21:1559–1568. doi: 10.1016/j.cellsig.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan B, Chour HH, Peh BK, Lim C, Salto-Tellez M. RhoA protein expression correlates positively with degree of malignancy in astrocytomas. Neurosci Lett 2006; 407:124–126. doi: 10.1016/j.neulet.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Johan MZ, Samuel MS. Rho-ROCK signaling regulates tumor-microenvironment interactions. Biochem Soc Trans 2019; 47:101–108. doi: 10.1042/bst20180334. [DOI] [PubMed] [Google Scholar]
- 29.Zhang WM, Cao P, Xin L, Zhang Y, Liu Z, Yao N, et al. Effect of miR-133 on apoptosis of trophoblasts in human placenta tissues via Rho/ROCK signaling pathway. Eur Rev Med Pharmacol Sci 2019; 23:10600–10608. doi: 10.26355/eurrev_201912_19755. [DOI] [PubMed] [Google Scholar]
- 30.Yeh YT, Wei J, Thorossian S, Nguyen K, Hoffman C, Del Álamo JC, et al. MiR-145 mediates cell morphology-regulated mesenchymal stem cell differentiation to smooth muscle cells. Biomaterials 2019; 204:59–69. doi: 10.1016/j.biomaterials.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]