Abstract
PURPOSE
To our knowledge, NRG/RTOG 9804 is the only randomized trial to assess the impact of whole breast irradiation (radiation therapy [RT]) versus observation (OBS) in women with good-risk ductal carcinoma in situ (DCIS), following lumpectomy. Long-term results focusing on ipsilateral breast recurrence (IBR), the primary outcome, are presented here.
PATIENTS AND METHODS
Eligible patients underwent lumpectomy for DCIS that was mammogram detected, size ≤ 2.5 cm, final margins ≥ 3 mm, and low or intermediate nuclear grade. Consented patients were randomly assigned to RT or OBS. Tamoxifen use was optional. Cumulative incidence was used to estimate IBR, log-rank test and Gray's test to compare treatments, and Fine-Gray regression for hazard ratios (HRs).
RESULTS
A total of six hundred thirty-six women were randomly assigned from 1999 to 2006. Median age was 58 years and mean pathologic DCIS size was 0.60 cm. Intention to use tamoxifen was balanced between arms (69%); however, actual receipt of tamoxifen varied, 58% RT versus 66% OBS (P = .05). At 13.9 years' median follow-up, the 15-year cumulative incidence of IBR was 7.1% (95% CI, 4.0 to 11.5) with RT versus 15.1% (95% CI, 10.8 to 20.2) OBS (P = .0007; HR = 0.36; 95% CI, 0.20 to 0.66); and for invasive LR was 5.4% (95% CI, 2.7 to 9.5) RT versus 9.5% (95% CI, 6.0 to 13.9) OBS (P = .027; HR = 0.44; 95% CI, 0.21 to 0.91). On multivariable analysis, only RT (HR = 0.34; 95% CI, 0.19 to 0.64; P = .0007) and tamoxifen use (HR = 0.45; 95% CI, 0.25 to 0.78; P = .0047) were associated with reduced IBR.
CONCLUSION
RT significantly reduced all and invasive IBR for good-risk DCIS with durable results at 15 years. These results are not an absolute indication for RT but rather should inform shared patient-physician treatment decisions about ipsilateral breast risk reduction in the long term following lumpectomy.
INTRODUCTION
Ductal carcinoma in situ (DCIS) is diagnosed in more than 50,000 women per year in the United States, primarily through screening mammogram.1 Breast conservation with radiation is a common treatment for DCIS with four prior randomized trials2-5 and a meta-analysis6 demonstrating that adjuvant radiation reduces risk of ipsilateral breast recurrence (IBR) in the breast by about 50%. With widespread screening, DCIS is frequently diagnosed when it is smaller and lower grade creating some uncertainty of the benefit of breast radiation. To our knowledge, this prospective randomized trial is the first to evaluate the role of whole breast irradiation (radiation therapy [RT]) in the treatment of good-risk DCIS after breast conservation surgery (BCS), in contrast to prior trials that had broader eligibility criteria, including larger, high-grade, and symptomatic DCIS.2-5 The goal was to determine whether radiation significantly reduced IBR in a lower-risk DCIS population, to better guide patients and physicians on treatment decisions.
CONTEXT
Key Objective
To identify a group of women with good-risk ductal carcinoma in situ (DCIS) whose risk of local recurrence after breast conservation surgery was so low, to justify the omission of breast radiation.
Knowledge Generated
The local recurrence risk in good-risk patients without radiation, last reported with a median follow-up of 7 years, to be 1% per year, continued at that rate through 15 years of follow-up. The addition of whole breast radiation after surgery delayed and decreased the risk of both invasive and noninvasive local events significantly.
Relevance
Widespread use of screening mammography identifies good-risk DCIS in many thousands of women each year. This information supports the decision to treat patients with good-risk DCIS, who want to minimize their in-breast recurrence, and particularly invasive risk in the long term. The results of this trial provide critical information that can inform shared patient-physician treatment decisions.
The definition of good-risk DCIS for this trial was derived from clinical and pathologic information available when the study was proposed, and included smaller, lower-grade lesions that were asymptomatic, ie, presenting with an abnormal screening mammogram. The primary end point of this trial was published at 7 years, demonstrating that radiation significantly reduced the incidence of IBR (7.2% v O.8% for observation [OBS] and RT, respectively).7 However, given the propensity for late IBR occurrence and the larger-than-expected radiation effect seen, long-term reporting was planned. This publication now reports the long-term update of IBR.
PATIENTS AND METHODS
NRG/RTOG 9804 was a randomized phase III trial conducted by NRG Oncology/RTOG (Data Supplement, online only) approved by the American College of Radiology Institutional Review Board and the institutional review boards of all participating sites. Written consent was required. Details of the study design, eligibility, ineligibility, and population have been previously reported.7 Briefly, eligible patients had DCIS detected by mammogram, underwent lumpectomy, a minimal surgical margin width of 3 mm, unifocal lesion, size on pathology or mammogram of 2.5 cm or less, and low or intermediate nuclear grade histology. Pathology details were previously published.7,8
Procedures
Initially, the trial specified RT as 50 Gy in 25 fractions or 50.4 Gy in 28 fractions until a 2001 amendment allowed 42.5 Gy in 16 fractions. No additional radiation boost to the lumpectomy cavity following breast RT was allowed.
The study opened requiring tamoxifen 20 mg/day, in both arms, based on results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial.10 In 2001, however, based on the results of the United Kingdom, Australia, and New Zealand DCIS Trial,11 the use of tamoxifen was made optional. All patients adhered to the same follow-up procedures that included annual mammography and specified clinical examination intervals.
Statistical Considerations
Details of the statistical design were published previously.7,9 Patients were randomly assigned 1:1 and stratification was by age (< 50 v ≥ 50 years), size (≤ 1 cm v > 1 cm), and pathology margins (negative re-excision v 3-9 mm v ≥ 10 mm). After tamoxifen was made optional in a 2001 amendment, tamoxifen use (yes v no) and nuclei grade (low v intermediate) were added as stratification factors.
The primary end point was IBR, defined as biopsy-proven invasive or DCIS recurrence in the ipsilateral breast. Secondary end points include invasive IBR, contralateral breast events (CBEs), overall survival (OS) and disease-free survival (DFS), distant metastasis (DM), subsequent mastectomy (M), and toxicity. DM is defined as breast cancer recurrence beyond the study breast and axillary lymph nodes; and M is defined as the removal of the study breast for any reason. DFS is defined as the first occurrence of IBR, CBE, DM, M, or death. All end points are measured from date of random assignment to date of recurrence or last follow-up for censored patients.
The focus of this analysis is long-term cumulative incidence of IBR. The study designed hypothesized that radiation would significantly reduce IBR from 6% to 3.5% (hazard ratio [HR], 0.58) at 5 years based on available data at that time and assumed that the reduction in IBR from RT would be less than in prior trials that included mostly higher grades and larger sizes of DCIS.2,12 Using Kim and Tsiatis' group sequential design approach13 with two interim and one final analyses using two-sided log-rank test statistics (significance level of 0.05), a maximum of 129 IBRs were required to detect the hypothesized reduction in the IBR cumulative incidence with a statistical power of 80%. Enrollment of 1790 patients in 6 years with a minimum follow-up period of 5 years was planned. The trial closed early because of low accrual rates. The specified primary analysis at 5 years was reported previously.7 Cumulative incidence methods14 were used to estimate the rates of IBR, CBE, and DM, with death as competing risk, and treatment arms were compared using the log-rank test.15 Gray's test comparing treatment arms was also shown.16 Kaplan-Meier was used to estimate OS and DFS and treatment arms compared with log-rank test.15 Multivariable Fine-Gray regression models17 were used to test for treatment differences, adjusting for age, pathology margins, tumor size, nuclei grade, and tamoxifen use, using backward selection at a significance level of 0.05. For the treatment variable, an HR < 1 indicates a decreased risk of recurrence for the RT arm. All patients are included in the intent-to-treat analysis, based on the treatment arm to which they were randomly assigned.
Acute toxicities were previously reported.7 Late RT toxicities (> 90 days from treatment start) were scored for the RT arm only using the RTOG/European Organization for Research and Treatment of Cancer Late Radiation Morbidity Scheme. The data safety monitoring committee oversaw the trial until released for reporting. Statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute Inc). Data cutoff was on February 12, 2020. The trial was registered with ClinicalTrials.gov (NCT00003857).
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
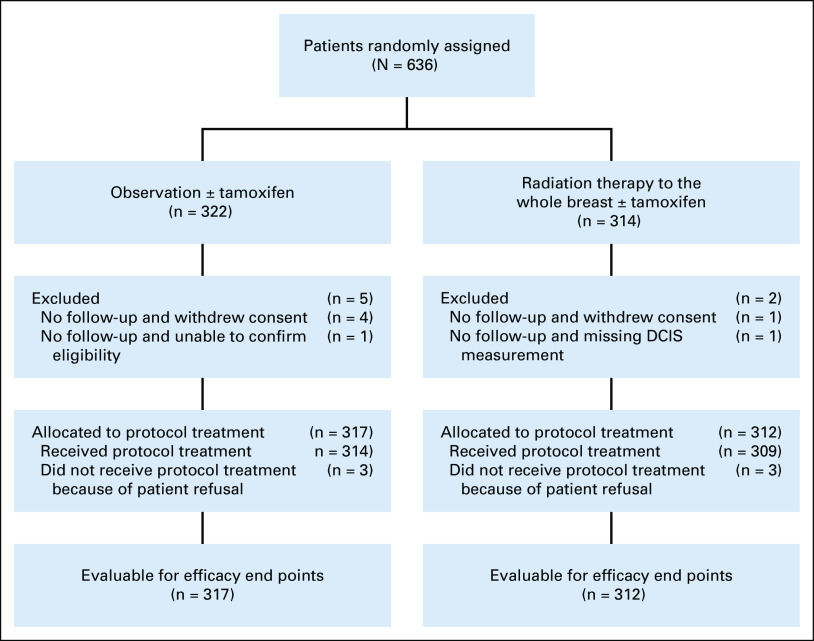
Between December 1999 and July 2006, 636 women from almost 200 institutions across the United States and Canada were consented and randomly assigned. For this long-term update, analyses were done on all patients with follow-up data (n = 629). The CONSORT diagram is shown in Figure 1.
FIG 1.
CONSORT diagram. DCIS, ductal carcinoma in situ.
Table 1 lists the patient and tumor characteristics. Median age was 58 years, and 76% were postmenopausal at study entry. Mean pathologic size was 0.60 cm and for 61% of those enrolled, the DCIS size was ≤ 0.5 cm. The highest nuclear grade was one in 44% and two in 56%. For 65%, the negative margin width was ≥ 1.0 cm and this included 49% of the population that had a completely negative re-excision specimen. Intention to use tamoxifen was balanced between treatment arms; however, actual receipt of tamoxifen was different between arms at 58% RT versus 66% OBS (P = .05).
TABLE 1.
Patient Characteristics
At the first reporting of this trial with a median follow-up of 7 years, the IBR of 3.6% and 6.7%, respectively, at 5 and 7 years in the OBS arm approximated prediction. The incidence of IBR in the RT arm reached statistical significance because the HR from radiation was at 0.11,7 much lower than was hypothesized or seen in prior trials.2-6 Long-term reporting was not prespecified in the protocol; however, given the lower-than-expected HR from radiation at 5 and 7 years, it was planned to determine the durability of these results in this good-risk DCIS population with a protracted natural history.
Median follow-up time for this analysis is 13.9 years (min-max: 0.01-20). There were a total of 52 IBR; 14 in the RT arm and 38 in the OBS arm (Table 2), of which 57% and 76%, respectively, were in the index quadrant. The cumulative incidence of IBR at 10 and 15 years, respectively, was 1.5% (95% CI, 0.5 to 3.7) and 7.1% (95% CI, 4.0 to 11.5) with RT and 9.2% (95% CI, 6.2 to 13.0) and 15.1% (95% CI, 10.8 to 20.2) with OBS (HR = 0.36 [95% CI, 0.20 to 0.66]; P = .0007, Fig 2). There were a total of 33 invasive recurrences: 10 in the RT arm and 23 in the OBS arm. The 10- and 15-year invasive IBR incidence, respectively, was 0.4% (95% CI, 0.0 to 1.9) and 5.4% (95% CI, 2.7 to 9.5) with RT and 4.3% (95% CI, 2.3 to 7.2) and 9.5% (95% CI, 6.0 to 13.9) with OBS (HR = 0.44 [95% CI, 0.21 to 0.91]; P = .024, Fig 3). The median time to any IBR was 11.5 years in the RT arm and 7.0 years in the OBS arm.
TABLE 2.
Efficacy End Points for All Randomly Assigned Patients With Follow-Up Data (n = 629)
FIG 2.
IBR for all randomly assigned patients with follow-up data. HR, hazard ratio; IBR, ipsilateral breast recurrence; OBS, observation; RL, reference level; RT, radiation therapy.
FIG 3.
Invasive IBR for all randomly assigned patients with follow-up data. HR, hazard ratio; IBR, ipsilateral breast recurrence; OBS, observation; RL, reference level; RT, radiation therapy.
The results of the IBR multivariable analysis are shown in Table 3. RT treatment remained significant for a reduction in IBR (HR = 0.34 [95% CI, 0.19 to 0.64]; P = .0007) as did tamoxifen use (HR = 0.45 [95% CI, 0.25 to 0.78]; P = .0047). Of note, age, tumor size, and grade were not associated with IBR.
TABLE 3.
Ipsilateral Breast Recurrence for All Randomly Assigned Patients With Follow-Up Data—Multivariable Analysis (n = 629)
A total of 27 underwent subsequent mastectomy, with 21 for IBR. Of the 52 IBR, 22 (42.3%) had repeat breast conservation. The treatment arms had no significant differences in subsequent mastectomy rate. There were a total of 44 CBEs (Table 2, Data Supplement), not significantly different in the two treatment arms.
The 15-year cumulative incidence of DM was 4.0% (95% CI, 1.6 to 8.2) with OBS and 2.3% (95% CI, 0.7 to 5.9) with RT (HR = 0.76 [95% CI, 0.25 to 2.38]; P = .65). There were no significant differences between arms for DM, OS, and DFS (Data Supplement).
Late RT toxicity data were only collected for patients on the RT arm. There are three (1.0%) patients with reported late grade 3 toxicities (breast pain, congestive heart failure or cardiomyopathy, and abnormal ECG) and all had right-sided treatment. There were no grade 4 or 5 late toxicities reported.
DISCUSSION
This long-term follow-up of NRG/RTOG 9804 randomized trial confirms that breast radiation following lumpectomy significantly reduces incidence of IBR for good-risk DCIS. With a median follow-up of nearly 14 years, the incidence of IBR with OBS of 9.2% and 15.1% at 10 and 15 years, respectively, was reduced to 1.5% and 7.1% from RT (HR, 0.36; 95% CI, 0.20 to 0.66). This clinical trial was conceived with the hypothesis that radiation was less beneficial for good-risk DCIS; so an HR of 0.58 was assumed from radiation, based on previously published DCIS trial results.2,12 The larger-than-expected reduction in IBR from radiation after lumpectomy in this DCIS population yielded significant results, despite this trial not meeting the original targeted accrual. The benefit of IBR prevention with adjuvant radiation continues to be seen with longer follow-up. Although the IBR in the OBS arm at 15 years may be sufficiently modest to be acceptable to some patients, all and invasive IBR continues to increase over time, particularly after 10 years of follow-up (Figs 2 and 3).
Prior randomized trials have addressed the question of radiation benefit after lumpectomy for treatment of DCIS.2-6 The good-risk DCIS population in this trial contrasts sharply to that enrolled in previous randomized trials as all were mammogram detected, with small mean size, widely negative margins, no high-grade lesions, and more prevalent tamoxifen use (Data Supplement).2,3,10,11,18-21 The clinical pathologic eligibility criteria for this trial successfully selected a DCIS cohort with a low IBR incidence after lumpectomy when compared with that reported by the Early Breast Cancer Trials' Collaborative Group (EBCTCG) meta-analysis of the four prior randomized trials.6 The meta-analysis demonstrated an IBR at 10 years with OBS of 28.1%, compared with the 9.2% cumulative incidence in NRG/RTOG 9804 at 10 years.
The NRG/RTOG 9804 eligibility criteria for good-risk DCIS are identical to that used in Cohort 1 of the Eastern Cooperative Oncology Group (ECOG) E5194 single-arm prospective observation trial for DCIS after BCS.22 With a median follow-up of 12.3 years, the 566 women enrolled in E5194 cohort 1 had a 12-year IBR of 14.4% compared with 11.9% seen at 12 years in NRG/RTOG 9804. When the population characteristics are compared, a notable difference is that the 31% tamoxifen use in E5194 cohort 1 is much less than the 69% in this trial. ECOG E5194 found that DCIS size was the only factor significantly associated with IBR after lumpectomy, with the highest risk in > 1 cm lesions. By contrast, NRG/RTOG 9804 did not find an association with DCIS size on multivariable analysis (Table 3); instead, tamoxifen use was the only variable besides radiation significantly associated with a reduction in IBR. The NSABP 2410,23 and UK-ANZ5 clinical trials evaluating adjuvant tamoxifen for DCIS included more high-risk cases and demonstrated a significant effect on contralateral breast cancer events and only a modest nonsignificant effect on IBR. However, a combined analysis of NSABP B17 and B24 clinical trials reported a 52% reduction in the risk of invasive IBR from the addition of radiation after BCS (HR = 0.48 [95% CI, 0.33 to 0.69]; P < .001) and an approximately 18% added benefit from tamoxifen, for 70% total reduction with combined RT and tamoxifen (HR = 0.30 [95% CI, 0.21 to 0.42]; P < .001).24 Unfortunately, NRG/RTOG 9804 did not collect the DCIS estrogen and progesterone receptor status. Central review of DCIS pathology materials on ECOG E5194 revealed that 97% were estrogen receptor–positive and 91% progesterone receptor–positive by reverse-transcription polymerase chain reaction.25 It is expected that a similar rate of positive receptors should be present for DCIS in NRG/RTOG 9804, given the comparable eligibility criteria. This perhaps supports the fact that tamoxifen is more effective for reducing IBR in low-risk hormone-sensitive DCIS than when more high-risk DCIS is present. In the UK-ANZ trial, tamoxifen was less effective in those with high-grade DCIS.5
A larger-than-expected radiation effect for good-risk DCIS was seen in NRG/RTOG 9804 with an HR of 0.36 compared with 0.46 HR from radiation in EBCTCG meta-analysis that included a more heterogeneous DCIS population. In the EBCTCG meta-analysis, IBR was 12.9% with RT after BCS at 10 years, compared with 1.5% in NRG/RTOG 9804 at that time point.6 The frequent use of tamoxifen in NRG/RTOG-9804 compared with the EBCTCG meta-analysis possibly contributed to this, or potentially the larger radiation effect reflects that good-risk DCIS is more radiosensitive. Radiation was more effective in lower-grade DCIS in the UK-ANZ trial as well.5 Research from the Netherland Cancer Institute analyzed DCIS using immunohistochemistry and found patterns suggesting that luminal and nonluminal DCIS tumor classification is feasible comparable with invasive breast cancer.26 In the setting of invasive breast cancer, a recent analysis of two postmastectomy RT clinical trials (DBCG and British Columbia) demonstrated that the HR for locoregional recurrence associated with giving radiation was 0.34 (95% CI, 0.19 to 0.61) overall and 0.12 (95% CI, 0.03 to 0.52) for luminal A tumors.27 However, an analysis of breast radiation after lumpectomy did not demonstrate larger HR from breast radiation in luminal A breast cancer relative to other subtypes.27 Nevertheless, this offers an intriguing avenue for further investigation to determine whether radiation is relatively more effective in hormone-sensitive, low-intermediate grade DCIS.
RT was mostly delivered with conventional fractionation in NRG/RTOG 9804, and all IBR in the RT group occurred in those women (data not shown). No IBR was observed in the 10% receiving hypofractionated RT. As well, boost RT was not allowed and the low incidence of LR overall suggests that boost use is unlikely to be of significant benefit in this population. The RT delivered in this study was well tolerated with a low rate of late toxicity. It is anticipated that the more modern radiation treatment planning in current practice, including contouring targets and applying breast cancer–specific dose constraints to normal tissue, can reduce this even further.
The fear of recurrence, as well as the actual diagnosis of IBR, can be a stressful situation for patients with breast cancer. It is important to clearly communicate the estimated IBR rates for patients who have good-risk DCIS, with a goal of reaching a shared decision regarding the use of RT or not. This trial demonstrates that clinical pathologic criteria reliably identify good-risk DCIS resulting in reduced incidence of IBR events after lumpectomy. Practitioners can now use commercially available multigene expression assays to assist adjuvant radiation decision making for DCIS by providing individualized risk prognostication for IBR after lumpectomy alone at 10 years,25,28 and benefit from radiation.29 The IBR incidence of 9.2% at 10 years in the OBS arm in this trial is very similar to those reported by the low-risk groups from these assays making their relative clinical utility in the good-risk DCIS enrolled in NRG/RTOG 9804 unclear. Furthermore, these assays do not reflect the patient perspective and acceptance of risk. Different patients have different comfort levels with risk; for some, an IBR of 14.2% at 15 years in the OBS arm of NRG/RTOG 9804 may be acceptable, whereas others may opt for the addition of RT to achieve lower or delayed risk. This analysis from NRG/RTOG 9804 uniquely provides important long-term incidences of recurrence in this good-risk DCIS population to inform this discussion.
In summary, breast radiation significantly reduces incidence of IBR and specifically invasive recurrence for good-risk DCIS following lumpectomy with durable results through 15 years. Radiation is the most effective approach for reducing IBR following lumpectomy in this population. In discussing radiation after BCS with these patients, factors such as age, life expectancy, and willingness (if estrogen receptor–positive) to take antiestrogen therapy should be taken into consideration in this patient-doctor shared decision. Since IBR risk continues to increase through at least 15 years, with radiation conferring both a delay and decrease in this risk, the data presented support the decision to treat patients who wish to minimize their IBR and particularly the invasive cancer risk in the long term.
Beryl McCormick
Stock and Other Ownership Interests: Varian Medical Systems
Henry M. Kuerer
Leadership: American Society of Breast Surgeons, ASCO
Honoraria: Genomic Health
Consulting or Advisory Role: Targeted Medical Education Inc, Genomic Health International
Speakers' Bureau: PER
Patents, Royalties, Other Intellectual Property: NEJM Group, McGraw-Hill Publishing
Eileen Rakovitch
Honoraria: AstraZeneca
Research Funding: Genomic Health International
Barbara L. Smith
Patents, Royalties, Other Intellectual Property: Royalty as textbook editor
Judith O. Hopkins
Consulting or Advisory Role: AIM Specialty Health
Lori J. Pierce
Stock and Other Ownership Interests: PFS Genomics
Patents, Royalties, Other Intellectual Property: UpToDate, PFS Genomics
Uncompensated Relationships: Bristol Myers Squibb, Exact Sciences
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1250431/summary
Kenneth N. M. Sumida
Employment: Hawaii Oncology Inc
Danny Vesprini
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca Canada
Julia R. White
Research Funding: Intraop Medical
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 3535
PRIOR PRESENTATION
Presented at the American Society for Radiation Oncology, San Antonio, TX, October 21, 2018.
SUPPORT
The trial was developed by the RTOG Breast Cancer Committee (now the NRG Breast Cancer Committee) and funded by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), and UG1CA189867 (NCORP) from the National Cancer Institute (NCI).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
No additional data are available for this article. Within approximately 1 year of publication, the data from this article will be available for data sharing proposals at the National Cancer Institute NCTN/NCORP data archive: https://nctn-data-archive.nci.nih.gov.
AUTHOR CONTRIBUTIONS
Conception and design: Beryl McCormick, Kathryn A. Winter, Henry M. Kuerer, Eileen Rakovitch, Barbara L. Smith, Eleanor M. Walker, Eric A. Strom, Julia R. White
Administrative support: Henry M. Kuerer
Provision of study materials or patients: Beryl McCormick, Henry M. Kuerer, Alan C. Hartford, Mark A. O'Rourke, Eric A. Strom, Kenneth N. M. Sumida, Danny Vesprini, Julia R. White
Collection and assembly of data: Beryl McCormick, Kathryn A. Winter, Henry M. Kuerer, Nour Sneige, Eileen Rakovitch, Barbara L. Smith, Alan C. Hartford, Mark A. O'Rourke, Eric A. Strom, Anthony T. Pu, Kenneth N. M. Sumida, Julia R. White
Data analysis and interpretation: Beryl McCormick, Kathryn A. Winter, Wendy Woodward, Henry M. Kuerer, Nour Sneige, Eileen Rakovitch, Barbara L. Smith, Isabelle Germain, Eleanor M. Walker, Eric A. Strom, Judith O. Hopkins, Lori J. Pierce, Danny Vesprini, Jennifer Moughan, Julia R. White
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Trial Evaluating Radiation Following Surgical Excision for Good-Risk Ductal Carcinoma In Situ: Long-Term Report From NRG Oncology/RTOG 9804
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Beryl McCormick
Stock and Other Ownership Interests: Varian Medical Systems
Henry M. Kuerer
Leadership: American Society of Breast Surgeons, ASCO
Honoraria: Genomic Health
Consulting or Advisory Role: Targeted Medical Education Inc, Genomic Health International
Speakers' Bureau: PER
Patents, Royalties, Other Intellectual Property: NEJM Group, McGraw-Hill Publishing
Eileen Rakovitch
Honoraria: AstraZeneca
Research Funding: Genomic Health International
Barbara L. Smith
Patents, Royalties, Other Intellectual Property: Royalty as textbook editor
Judith O. Hopkins
Consulting or Advisory Role: AIM Specialty Health
Lori J. Pierce
Stock and Other Ownership Interests: PFS Genomics
Patents, Royalties, Other Intellectual Property: UpToDate, PFS Genomics
Uncompensated Relationships: Bristol Myers Squibb, Exact Sciences
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1250431/summary
Kenneth N. M. Sumida
Employment: Hawaii Oncology Inc
Danny Vesprini
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca Canada
Julia R. White
Research Funding: Intraop Medical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Desantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics 2019 CA Cancer J Clin 69438–4512019 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from the National Surgical Adjuvant Breast and Bowel Project B-17 J Clin Oncol 16441–4521998 [DOI] [PubMed] [Google Scholar]
- 3.Julien J, Bijker N, Fentimen I, et al. Radio-therapy in breast conserving treatment for ductal carcinoma in situ: First results of the EORTC randomized phase III trial 10853-EORTC Breast Cancer Cooperatie Group and EORTC Radiotherapy Group Lancet 355528–5332000 [DOI] [PubMed] [Google Scholar]
- 4.Emdin S, Granstrand B, Ringberg A, et al. SweDCIS: Radiotherapy after sector resection for ductal carcinoma in situ of the breast—Results of a randomized trial in a population offered mammography screening Acta Oncol 45536–5432006 [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial Lancet Oncol 1221–292011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists' Collaborative Group Overview of the randomized trial of radiotherapy in ductal carcinoma in situ of the breast J Natl Cancer Inst Monogr 41162–1772010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick B, Winter K, Hudis C, et al. RTOG 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation J Clin Oncol 33709–7152015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward W, Sneige N, Winter K, et al. Web based pathology assessment in RTOG 9804 J Clin Path 67777–7802014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelen M.The randomization and stratification of patients to clinical trials J Chronic Dis 27365–3751974 [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomized controlled trial Lancet 3531993–20002000 [DOI] [PubMed] [Google Scholar]
- 11.Houghton J, George W, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia and New Zealand: Randomized controlled trial Lancet 36295–1022003 [DOI] [PubMed] [Google Scholar]
- 12.Silverstein M, Cohlan B, Gierson E, et al. Duct carcinoma in situ: 227 cases without microinvasion Eur J Cancer 28630–6341992 [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Tsiatis A.Study duration for clinical trials with survival response and early stopping rule Biometrics 4681–901990 [PubMed] [Google Scholar]
- 14.Kalbfleisch J, Prentice R.The Statistical Analysis of Failure Time Data New York, NY: John Wiley & Sons; 1980. pp 167–169 [Google Scholar]
- 15.Mantel N.Evaluation of survival data and two new rand order statistics arising in its consideration Cancer Chemother Rep 50163–1701966 [PubMed] [Google Scholar]
- 16.Gray R.A class of K-sample tests for comparing the cumulative incidence of a competing risk Ann Stat 161141–11541988 [Google Scholar]
- 17.Fine J, Gray R.A proportional hazards model for the sub-distribution of a competing risk J Am Stat Assoc 94496–5091999 [Google Scholar]
- 18.Fisher R, Costantino J, Redman C, et al. Lumpectomy comparted with lumpectomy and radiation therapy for the treatment of intraductal breast cancer N Engl J Med 3281581–15861993 [DOI] [PubMed] [Google Scholar]
- 19.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: Analysis of the European Organization for Research and Treatment of Cancer Trial 10853 J Clin Oncol 192263–22712001 [DOI] [PubMed] [Google Scholar]
- 20.Pinder SE, Duggan C, Ellis IO, et al. A new pathological system for grading DCIS with improved prediction of local recurrence: Results from the UKCCCR/ANZ DCIS trial Br J Cancer 10394–1002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast J Clin Oncol 261247–12522008 [DOI] [PubMed] [Google Scholar]
- 22.Solin L, Gray R, Hughes L, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12 year results from the ECOG-ACRIN ES 194 study J Clin Oncol 333938–39442015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor–positive ductal carcinoma in situ: A study based on NSABP protocol B-24 J Clin Oncol 301268–12732012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wapnir I, Digman J, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast turo recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS J Natl Cancer Inst 103478–4882011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solin L, Gray R, Baehner F, et al. A multigene expressions assay to predict local recurrence risk for ductal carcinoma in situ of the breasts J Natl Cancer Inst 105701–7102013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijen P, Peterse J, Antonini N, et al. Immunohistochemical categorization of ductal carcinoma in situ of the breast Br J Cancer 98137–1422008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurberg T, Trammm T, Nilesen T, et al. Intrinsic subtypes and benefit from postmastectomy radiotherapy in node-positive premenopausal breast cancer patients who received adjuvant chemotherapy-results from two independent randomized trials Acta Onc 5738–432018 [DOI] [PubMed] [Google Scholar]
- 28.Rakovitch E, Gray R, Baehner R, et al. Refined estimates of local recurrence risks by DCIS score adjusting for clinicopathological feature: A combined analysis of ECOG-ACRIN E5194 and Ontario DCIS cohort studies Breast Cancer Res Treat 169359–3692018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremer T, Whitworth PW, Patel R, et al. A biological signature for breast ductal carcinoma in situ to predict radiotherapy benefit and assess recurrence risk Clin Cancer Res 245895–59012018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available for this article. Within approximately 1 year of publication, the data from this article will be available for data sharing proposals at the National Cancer Institute NCTN/NCORP data archive: https://nctn-data-archive.nci.nih.gov.