Abstract
PURPOSE
The Groningen International Study on Sentinel nodes in Vulvar cancer (GROINSS-V)-II investigated whether inguinofemoral radiotherapy is a safe alternative to inguinofemoral lymphadenectomy (IFL) in vulvar cancer patients with a metastatic sentinel node (SN).
METHODS
GROINSS-V-II was a prospective multicenter phase-II single-arm treatment trial, including patients with early-stage vulvar cancer (diameter < 4 cm) without signs of lymph node involvement at imaging, who had primary surgical treatment (local excision with SN biopsy). Where the SN was involved (metastasis of any size), inguinofemoral radiotherapy was given (50 Gy). The primary end point was isolated groin recurrence rate at 24 months. Stopping rules were defined for the occurrence of groin recurrences.
RESULTS
From December 2005 until October 2016, 1,535 eligible patients were registered. The SN showed metastasis in 322 (21.0%) patients. In June 2010, with 91 SN-positive patients included, the stopping rule was activated because the isolated groin recurrence rate in this group went above our predefined threshold. Among 10 patients with an isolated groin recurrence, nine had SN metastases > 2 mm and/or extracapsular spread. The protocol was amended so that those with SN macrometastases (> 2 mm) underwent standard of care (IFL), whereas patients with SN micrometastases (≤ 2 mm) continued to receive inguinofemoral radiotherapy. Among 160 patients with SN micrometastases, 126 received inguinofemoral radiotherapy, with an ipsilateral isolated groin recurrence rate at 2 years of 1.6%. Among 162 patients with SN macrometastases, the isolated groin recurrence rate at 2 years was 22% in those who underwent radiotherapy, and 6.9% in those who underwent IFL (P = .011). Treatment-related morbidity after radiotherapy was less frequent compared with IFL.
CONCLUSION
Inguinofemoral radiotherapy is a safe alternative for IFL in patients with SN micrometastases, with minimal morbidity. For patients with SN macrometastasis, radiotherapy with a total dose of 50 Gy resulted in more isolated groin recurrences compared with IFL.
INTRODUCTION
Over the past 10 years, treatment for early-stage vulvar cancer has undergone major advances. Specifically, in patients with unifocal tumors < 4 cm and nonsuspicious groin nodes, sentinel node (SN) biopsy became standard of care over elective inguinofemoral lymphadenectomy (IFL). The Groningen International Study on Sentinel nodes in Vulvar cancer (GROINSS-V)-I showed that omission of IFL was safe in patients with a negative SN with an isolated groin recurrence rate after SN biopsy of 2.3% (95% CI, 0.6 to 5.0). This study also showed a major decrease in treatment-related morbidity for patients who underwent SN biopsy only.1 Simultaneously, the Gynecologic Oncology Group completed a prospective trial and found a false-negative predictive value with SN biopsy of 2.0% in patients with tumors < 4 cm.2
CONTEXT
Key Objective
Introduction of the sentinel node (SN) procedure is one of the major advances in vulvar cancer treatment. The Groningen International Study on Sentinel nodes in Vulvar cancer (GROINSS-V)-I showed that inguinofemoral lymphadenectomy could be safely omitted in SN-negative patients, resulting in significant decrease of morbidity. Patients with a metastatic SN still have to undergo lymphadenectomy and will have morbidity of this treatment. GROINSS-V-II investigated the safety of radiotherapy instead of lymphadenectomy in vulvar cancer patients with a metastatic SN.
Knowledge Generated
Radiotherapy showed to be similarly safe as compared to GROINSS-V-I data in terms of groin recurrence rate for patients with SN micrometastases. The morbidity of inguinofemoral radiotherapy was less compared with lymphadenectomy. However, for patients with SN macrometastases, the recurrence rate was higher with radiotherapy. For them, standard therapy remains lymphadenectomy, and further research is needed.
Relevance
Inguinofemoral radiotherapy could spare vulvar cancer patients with SN micrometastases the morbidity of lymphadenectomy. This should be implemented in (inter)national treatment guidelines for vulvar cancer.
Current standard of care for patients with SN metastases is further treatment with IFL. Adjuvant radiotherapy after IFL is indicated in patients with > 1 metastatic lymph node and/or extracapsular spread. Treatment-related morbidity is a major problem for these patients.3 Data analysis from all patients with a metastatic SN in GROINSS-V-I demonstrated no threshold for the size of SN metastasis below which the risk of additional metastasis was sufficiently low to safely allow omission of IFL. To prevent groin recurrences, which are often fatal, all patients with a metastatic SN therefore require additional treatment, irrespective of the size of the SN metastasis.4 GROINSS-V-II was designed to find an equally effective but less morbid treatment for patients with a metastatic SN.
The primary aim of the GROINSS-V-II study was to establish whether inguinofemoral radiotherapy is a safe alternative to IFL in vulvar cancer patients with a metastatic SN. The secondary aim was to establish the treatment-related morbidity (short- and long-term) for this management strategy. Finally, the study provided the opportunity to collect further data on the safety of omitting IFL in patients with a negative SN.
METHODS
A prospective multicenter phase II single-arm treatment trial was performed in patients with early-stage vulvar cancer planned for surgery (wide local excision and SN biopsy). Patients were recruited from 59 hospitals in 11 countries. To ensure quality of the SN biopsy, criteria were formulated for required experience (Data Supplement, online only).
Inclusion criteria were unifocal macroinvasive squamous cell carcinoma of the vulva < 4 cm; preoperative imaging of groins (computed tomography [CT], magnetic resonance imaging, or ultrasound) showing no suspicious nodes (where inguinal nodes appeared suspicious in size [> 15 mm] or morphology, metastatic disease was ruled out by fine needle aspiration cytology); and written informed consent. The primary end point of the study was the isolated groin recurrence rate at 24 months. The ethics committees of all participating centers approved the Protocol (online only). The trial was registered with the Netherlands Trial Register (NTR608/NL552).
SN Biopsy
SN biopsy was performed as previously described using the combined technique: a radioactive tracer with performance of a lymphoscintigram, and blue dye.1 When routine hematoxylin-eosin staining was negative, ultrastaging was performed (one section/500 µm; one for hematoxylin-eosin, one for cytokeratin AE1/AE3-immunohistochemistry, and one spare section). In case of SN metastasis, size of the metastasis and presence of extracapsular spread were documented. When the SN could not be identified, an IFL was advised.
For centrally located tumors that crossed the (virtual) midline, the SN should be identified in both groins. Where the medial margin was < 1 cm from the midline, without crossing the midline, detection of only ipsilateral SN(s) was acceptable when the lymphoscintigram showed only unilateral drainage. Resection of the vulvar lesion was typically performed following SN biopsy.
When the SN was negative after ultrastaging, no further treatment followed. Each groin in which tumor cells were detected (regardless of size, including isolated tumor cells [ITC]) was regarded as a groin with metastatic disease.4
Radiotherapy
When definitive histopathologic examination showed metastatic disease in a SN, inguinofemoral radiotherapy was indicated. It was at the discretion of the participating center to give radiotherapy to one or both sides in case of unilateral SN involvement. The radiotherapy target volume included the inguinofemoral region and distal part of the external iliac lymph nodes (to the level of the inferior border of the sacroiliac joints). Radiotherapy had to be initiated within 6 weeks postoperation. Radiotherapy was given to a total dose of 50 Gy in 25-28 fractions of 1.8-2 Gy, five fractions/week. The target dose of 50 Gy was chosen as this was considered an effective dose for subclinical disease. Treatment planning was based on a three-dimensional volume acquired by CT with a slice thickness of 5 mm. Three-dimensional conformal treatment planning was required according to ICRU-62 guidelines, with 98% of the planning target volume required to receive at least 95% of the prescribed dose. As from the Protocol amendment in 2010, intensity-modulated radiation therapy planning or volumetric modulated arc radiotherapy was permitted if standard for the treatment center and with appropriate quality assurance in place. Treatment verification using electronic portal imaging and/or cone beam CT was required at least at the first treatment fractions and once weekly. The overall treatment time was to be kept within 110% of the prescribed time. Clinical target volume (CTV) contouring was to be done according to the atlases5,6 and with the CTV including the superficial inguinal and deep inguinofemoral nodal compartment plus a 0.5-cm margin, and the lower external iliac nodes plus a 0.5-cm margin, taking care to include the area of the metastatic SN with generous margin. The CTV-planning target volume margin was 1 cm. The use of bolus over the superficial inguinal nodes was recommended only if necessary to achieve dose homogeneity in the target volume. The vulvar area was only included in case of a specific indication (close or involved resection margins). Because of uncertainties with respect to the role of added chemotherapy with radiotherapy in vulvar cancer, it was left to the discretion of the participating institute whether or not to add chemotherapy.7
In 2015, our radiation protocol was challenged with the suggestion that the target definition ran the risk of allowing for the geographical target to be missed, based upon the circumferential margin.8 Although the original Protocol together with the figures was designed to ensure optimal radiation fields, we felt that in the intensity-modulated radiation therapy era, we should give more detail to defining the CTV to prevent any possible misunderstanding. Therefore, we included additional pictures to guide treatment planning. All participants and radiation oncologists were informed.
Follow-Up
All patients were followed by the gynecologic oncologist every 2 months during the first 2 years after completion of treatment. Follow-up included a dedicated patient history, gynecologic examination, and palpation of the groins. At each follow-up visit, presence of lymphedema (objective findings and subjective symptoms), episodes of recurrent erysipelas, and recurrences were specifically documented. Short- and long-term treatment-related toxicity was monitored in more detail for SN-positive patients who received radiotherapy at 6, 12, and 24 months using Common Terminology Criteria for adverse events (version 3, grade 0-5). Routine items scored were nausea, vomiting, mucositis, diarrhea, skin toxicity of vulva and/or groins, and edema.
Statistics
Analyses were performed with IBM Statistical Package for the Social Sciences (SPSS) version 23 and R version 3.2.1.
For analysis of recurrences, first site of recurrence was used. For analysis of disease-specific death and recurrence, competing-risk methods were used. For disease-specific death, intercurrent death was used as a competing risk. For groin recurrence, local recurrence and death were used as competing risks. Differences in recurrence and survival rates between groups were tested with log-rank and Cox regression analysis.
To evaluate the differences in categorical variables, we used Pearson's chi square. To evaluate difference in continuous variables, we used independent-sample t-testing. Follow-up time was calculated from date of primary surgery until the date of last follow-up. Patients who were alive were censored at the date of last follow-up. Time to disease-specific death was calculated from date of surgery until date of death by vulvar cancer. Overall survival (OS) was calculated from date of surgery until date of death by any cause. When patients were lost to follow-up, they were censored at the date of last visit. Per protocol patients were followed up for 24 months after end of treatment.
Stopping Rule and Protocol Amendment
To monitor safety of radiotherapy, stopping rules were formulated based on the previously reported frequency of isolated groin recurrences in patients with a metastatic lymph node who underwent IFL (in GROINSS-V-I 8.1%).4 Sequential testing was performed after every 25 patients who completed 2 years of follow-up, with a maximum of six interim analyses (150 patients). Upper and lower bounds were calculated using a Christmas tree correction9 so that—in case the 2-year probability of groin recurrence is as low as 2%—the probability of hitting the upper bound remained below a desired alpha level of 5%, while the probability of hitting the lower bound was 80%, whereas—in case the 2-year probability of groin recurrence is as high as 10%—the probability of hitting the lower bound remained below an alpha level of 5%, while the probability of hitting the upper bound was 80%. The total sample size was calculated such that (at the peak of the Christmas tree) the lower and upper bounds meet, so that at least one of the hypotheses (2-year probability of groin recurrence either 2% or 10%) is rejected. For P = .05 with a power of 0.80, 150 patients were needed (Data Supplement). It was predefined that if the isolated groin recurrence rate would exceed the upper boundary, the study would be interrupted to analyze the data in the patients with an isolated groin recurrence. In the absence of activation of the stopping rules, the study was designed to close when 150 patients with a metastatic SN completed 2 years of follow-up. Further details can be found in the Data Supplement.
In 2010, one major Protocol amendment was made, after activation of the stopping rule for patients with a metastatic SN who underwent radiotherapy. In June 2010, with 91 SN-positive patients included, the stopping rule was activated because the isolated groin recurrence rate in this group went above our predefined threshold. Interim analysis showed that among 10 patients with an isolated groin recurrence, nine had SN metastases > 2 mm and/or extracapsular spread. Based on this interim analysis, it was decided to amend the Protocol and to allow only patients with SN micrometastases (≤ 2 mm) to receive inguinofemoral radiotherapy, whereas those with SN macrometastases (> 2 mm) reverted back to standard of care and underwent IFL, with adjuvant radiotherapy only when there were more than one lymph node metastases and/or extracapsular spread.
RESULTS
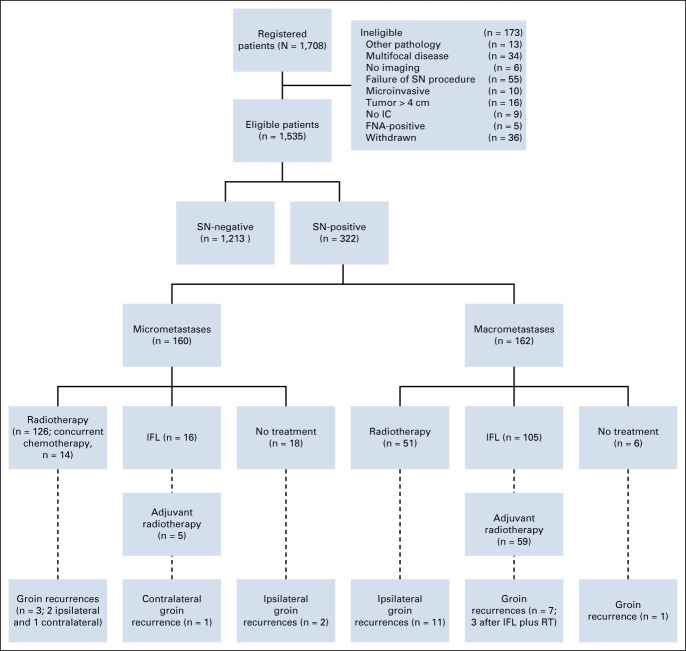
Between December 2005 and October 2016, 1708 patients were registered. A total of 173 patients were ineligible for a variety of reasons (Fig 1). Among the 1,535 eligible patients, 1,213 had a negative SN (79.0%) and 322 a metastatic SN (21.0%). Tumor and imaging characteristics of the included patients are listed in Table 1. The median follow-up for all patients was 24.3 months (IQR, 23.3-25.9 months).
FIG 1.
Flowchart of GROINSS-V II. FNA, fine needle aspiration cytology; IC, informed consent; IFL, inguinofemoral lymphadenectomy; RT, radiation therapy; SN, sentinel node.
TABLE 1.
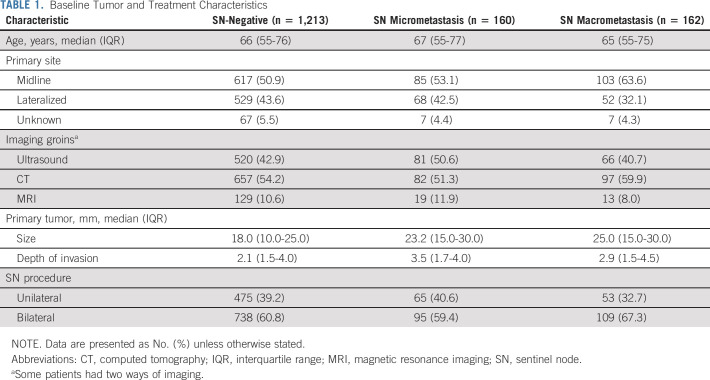
Baseline Tumor and Treatment Characteristics
Patients With SN Micrometastases ≤ 2 mm
Among 322 patients with a metastatic SN, 160 (49.7%) had micrometastases. Of these 160 patients, 126 (78.8%) received inguinofemoral radiotherapy as prescribed by protocol (81 bilateral, 38 unilateral, 7 unknown). In 14 out of 126 patients (11.1%), radiotherapy was combined with chemotherapy (nine cisplatin and five cisplatin and fluorouracil). Sixteen of 160 patients (10.0%) underwent an IFL instead of inguinofemoral radiotherapy and 18 (11.3%) underwent no further treatment for a variety of reasons (patient refused any further treatment, severe comorbidity, and/or [very] elderly; Fig 1).
Two years after primary surgery, overall six isolated groin recurrences had occurred in 160 patients with a SN micrometastasis (3.8% at 2 years, 95% CI, 0.8 to 6.8). Among 126 patients who received inguinofemoral radiotherapy, only two isolated ipsilateral groin recurrences were diagnosed and in addition one in a contralateral SN-negative groin (this patient had undergone bilateral inguinofemoral radiotherapy). Another two of six groin recurrences occurred in patients who had no further treatment (radiotherapy nor IFL). Furthermore, one groin recurrence occurred in the contralateral SN-negative groin, after unilateral IFL for a metastatic SN. For those 18 patients who did not receive any additional treatment, the ipsilateral isolated groin recurrence rate was higher than for those who were treated per protocol with inguinofemoral radiotherapy (at 2 years, 11.8% [95% CI, 0 to 27.2] ipsilateral groin recurrence after no adjuvant treatment v 1.6% [95% CI, 0 to 3.9] after radiotherapy, P = .006; Fig 2).
FIG 2.
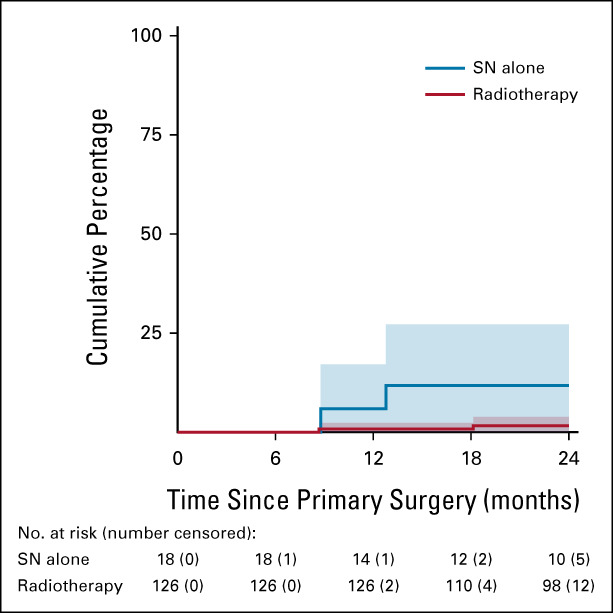
Isolated ipsilateral groin recurrences in patients with SN micrometastases ≤ 2 mm. Radiotherapy versus no further treatment (HR, 0.11; 95% CI, 0.02 to 0.76). Patients who underwent IFL instead of radiotherapy were excluded. HR, hazard ratio; IFL, inguinofemoral lymphadenectomy; SN, sentinel node.
To rule out unfavorable nodal characteristics as cause of higher groin recurrence rate in patients who received no adjuvant treatment, we compared metastasis size in both groups. The mean size of SN metastasis was larger in the patients who received radiotherapy than in those who received no adjuvant treatment (0.77 mm [standard deviation 0.66 mm] v 0.39 mm [standard deviation 0.54 mm], P = .023). When comparing SN metastases > 0.2 mm-2 mm (n = 102) with SNs containing only ITC or metastases ≤ 0.2 mm (n = 56), no ipsilateral isolated groin recurrences were observed in the patients with ITC or metastases ≤ 0.2 mm when treated according to the study Protocol. Eleven patients with ITC or metastases ≤ 0.2 mm underwent no radiotherapy or IFL, and one of them experienced an ipsilateral groin recurrence.
Local and distant recurrences for all patients are summarized in the Data Supplement.
Patients With SN Metastases > 2 mm
Among 322 patients with a metastatic SN, 162 (50.3%) had macrometastases. Fifty-one patients with a SN macrometastasis received only radiotherapy to the groins (31.5%%; 39 bilateral, eight unilateral, and four unknown), in seven patients (13.7%) combined with chemotherapy (cisplatin). A unilateral or bilateral IFL was performed in 105 patients (64.8%), of whom 59 (56.2%) also received adjuvant radiotherapy. In six patients, no further treatment was given after removal of the SN for a variety of reasons (Fig 1).
In 162 patients with SN macrometastases, 19 groin recurrences were diagnosed (12.2% at 2 years; 95% CI, 7.1 to 17.4). Groin recurrence rate at 2 years was 22.0% (95% CI, 10.5 to 33.5) in patients who underwent inguinofemoral radiotherapy versus 6.9% (95% CI, 2.0 to 11.8) in patients who underwent IFL with or without adjuvant radiotherapy (P = .011, Fig 3). Size and number of metastases, and presence of extracapsular spread were not different in both groups. One groin recurrence occurred in a patient with two SN macrometastases who received no adjuvant treatment. No groin recurrences occurred in a contralateral nonirradiated groin. For the patients who underwent IFL, the groin recurrence rate for those with adjuvant radiotherapy was not different from those who did not undergo adjuvant radiotherapy (P = .43). Intention-to-treat analyses were comparable (Data Supplement).
FIG 3.
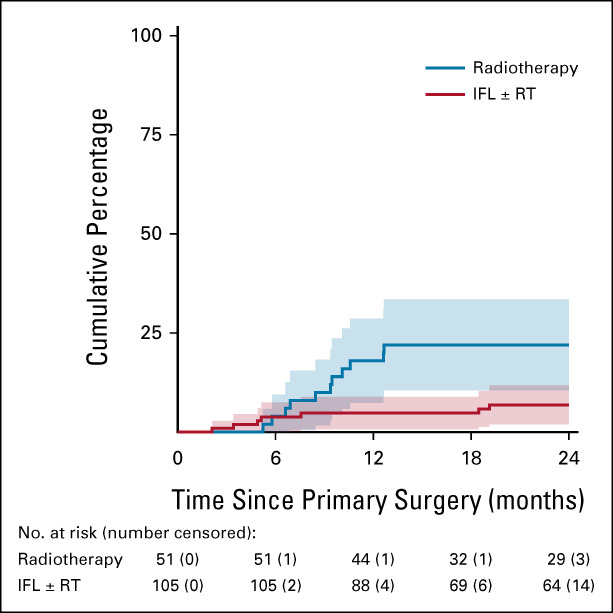
Isolated ipsilateral groin recurrences in patients with SN macrometastases > 2 mm. Per protocol analysis, radiotherapy versus IFL (HR, 3.2; 95% CI, 1.2 to 8.3). Six patients who received no treatment were excluded from this analysis. HR, hazard ratio; IFL, inguinofemoral lymphadenectomy; RT, radiation therapy; SN, sentinel node.
No groin recurrences were observed in seven patients who received chemoradiation. There was a trend toward better disease control in patients who received chemoradiation (P = .091; more details are available in the Data Supplement).
Patients With a Negative SN
Isolated groin recurrences were diagnosed in 31 out of 1213 SN-negative patients (2.7% at 2 years, 95% CI, 1.7 to 3.6). All patients with groin recurrences were analyzed to find a possible explanation for cause of failure. In two patients, revision of the pathology slides showed that metastases were present in retrospect. In four cases, not all SNs visualized on the lymphoscintigram were removed. In the other cases, no clear explanation was found.
Treatment-Associated Morbidity
The combination of a SN biopsy with subsequent radiotherapy to the groins was associated with mostly low-grade toxicity (Data Supplement). For nausea, vomiting, mucositis, and anal and urinary incontinence, only grade 1-2 toxicity was reported. Diarrhea was most frequently reported 4-6 weeks and 6 months after radiotherapy. At 4-6 weeks after treatment, grade 1, 2, or 3 diarrhea was reported in 21 out of 171 (12.3%), 4 out of 171 (2.3%), and 1 out of 171 (0.6%), respectively. At 6 months, these percentages were 7.9%, 0.0%, and 0.7%, respectively. Frequently reported toxicity was related to the skin in the irradiated groin. Most patients reported this 4-6 weeks after radiotherapy: 21.3% grade 1, 14.8% grade 2, and 1.3% grade 3 toxicity. At 6 months, skin toxicity decreased significantly (7.2% grade 1, 0.7% grade 2, and 0.7% grade 3). No grade 4-5 toxicity was reported.
We evaluated the patients in whom lymphedema was reported more than once 6 and 12 months after treatment. Lymphedema at 6 and 12 months was less frequent following SN biopsy alone (5.1% and 4.1%, respectively), compared with SN biopsy followed by radiotherapy (16.4% and 10.7%, respectively, P < .0001). The patients who underwent IFL (with or without radiotherapy) experienced the highest incidence of lymphedema (32.0% and 22.9%, respectively; P < .001; Fig 4).
FIG 4.
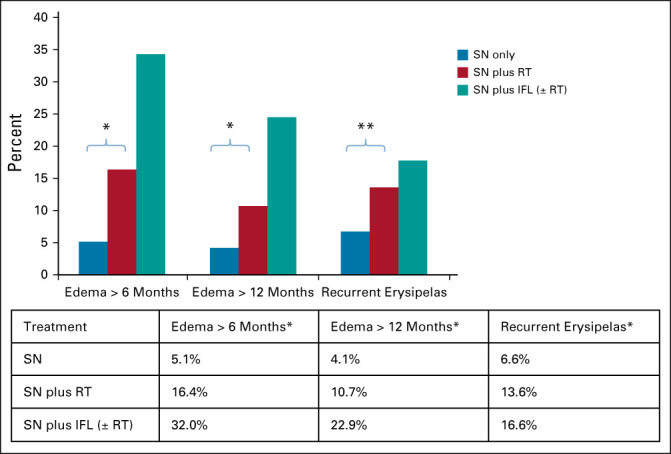
Long-term morbidity. *P < .0001; **P = .001. IFL, inguinofemoral lymphadenectomy; RT, radiation therapy; SN, sentinel node.
Survival
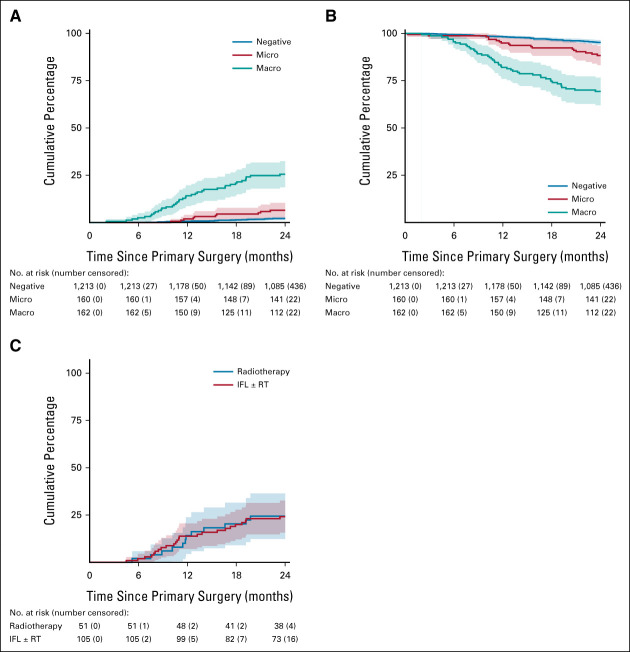
The estimated risk of disease-specific death at 2 years was 2.1% (95% CI, 1.3 to 2.9) for SN-negative patients, 6.5% (95% CI, 2.6 to 10.4) for patients with SN micrometastases, and 25.5% (95% CI, 18.6 to 32.5) for those with SN macrometastases (P < .0001; Fig 5A). OS for SN-negative patients was 95.2% (95% CI, 94.0 to 96.5) at 2 years after primary treatment, versus 88.3% (95% CI, 83.2 to 93.4) and 69.3% (95% CI, 62.0 to 76.6) for those with SN micrometastases and macrometastases, respectively (P < .0001; Fig 5B).
FIG 5.
Survival analysis of all GROINSS-V II patients. (A) Disease-specific death for all patients. (B) Overall survival for all patients. (C) Disease-specific death of patients with SN macrometastases. IFL, inguinofemoral lymphadenectomy; RT, radiation therapy; SN, sentinel node.
Of all patients who experienced a groin recurrence (n = 56), 31 died of vulvar cancer during the follow-up period of this study (OS at 2 years of 39.0% [SE 7.2]). Despite the significant difference in groin recurrence rate, the estimated risk of disease-specific death at 2 years for the patients with SN macrometastasis was similar for those who underwent IFL (± radiotherapy) compared with those who underwent radiotherapy only (24.2 [95% CI, 15.7 to 32.6] 4) v 24.4% [95% CI, 12.4 to 36.4], respectively, P = .88; Fig 5C). In the group of patients who underwent radiotherapy only, 13 out of 51 (25.5%) died of vulvar cancer, and nine following groin recurrence. Only one had a distant recurrence (pleural disease) and three recurred after first having a local recurrence. In the group that underwent IFL, 24 out of 105 (22.9%) patients died of vulvar cancer. Only four patients had a groin recurrence. However, 8 out of 105 (7.6%) had distant recurrence. Eleven patients had locally recurrent disease and progressed afterward.
DISCUSSION
GROINSS-V-II demonstrated that inguinofemoral radiotherapy in patients with a SN micrometastasis results in a very low isolated groin recurrence rate (1.6%), with acceptable treatment-related morbidity. For patients with a SN macrometastasis, inguinofemoral radiotherapy with a total dose of 50Gy is not a safe alternative for IFL in view of the higher risk of isolated groin recurrence. However, without a difference in disease-specific death. The follow-up data for SN-negative patients confirm our previous finding that omitting IFL in patients with unifocal vulvar cancer < 4 cm and a negative SN is safe.1
The major strength of our study is the large number of patients included from a large number of centers. GROINSS-V-II is, with > 1,700 recruited patients, by far the largest prospective treatment trial in patients with vulvar cancer ever performed. One of the limitations of our study was the fact that no pretreatment quality control or prospective quality assurance was performed for the radiotherapy contouring and planning. To overcome this limitation, we evaluated the radiotherapy planning of all patients who had a groin recurrence after radiotherapy for a metastatic SN and this analysis revealed no inadequacies.
In GROINSS-V I, in which all patients with a metastatic SN underwent IFL, we observed an isolated groin recurrence rate of 3.6% after 2 years in the patients with SN micrometastasis. With respect to isolated groin recurrence rate, inguinofemoral radiotherapy with 1.6% isolated groin recurrences after 2 years as observed in this study seems therefore at least comparable to IFL, whereas radiotherapy has a more favorable morbidity profile. Our results show that radiotherapy adds morbidity compared with SN procedure alone, but lymphedema is less frequent compared with IFL; 11% versus 23% at 12 months. The GOG244 study, a prospective study on lymphedema in patients with gynecologic malignancies who underwent lymphadenectomy, showed no association with radiotherapy. However, the number of vulvar cancer patients in this study was low.10 In specific cases, radiotherapy may be contraindicated, for example, in patients who previously underwent pelvic radiotherapy or young women in whom fertility-preservation is desired. Premenopausal women should consider that (bilateral) radiotherapy will cause premature ovarian insufficiency, with associated risks of increased mortality and morbidity. Hormonal-replacement therapy is recommended in these cases (in absence of contraindications). This fact should be weighed against the decrease in morbidity.
For patients with SN macrometastasis, radiotherapy alone (50 Gy) resulted in a higher number of isolated groin recurrences compared with IFL. At this time, IFL remains the standard treatment for these patients. However, there was no difference in disease-specific death at 2 years between the group who received radiotherapy and those who underwent an IFL with or without adjuvant radiotherapy in this study. Analysis showed that among patients who received radiotherapy, a greater proportion died after isolated groin recurrence, whereas among those who underwent IFL, death with distant metastases was more common. These groups were comparable when looking at prognostic lymph node characteristics such as number of metastases, size of metastases, and presence of extranodal spread. A more detailed analysis and longer follow-up is needed to explain these findings. A third GROINSS-V study will investigate whether the efficacy of treatment can be increased by adding concurrent chemotherapy to inguinofemoral radiotherapy, while also increasing the total dose in the involved groin area from 50 to 56 Gy by applying a (simultaneous integrated) boost dose. This large international cooperative group trial started recruitment in 2021. The trend toward better disease control in patients with SN macrometastases who received chemoradiation, as observed in this study, supports the hypothesis of GROINSS-V III.
In summary, inguinofemoral radiotherapy for vulvar cancer patients with SN micrometastasis appears to be a safe alternative for IFL. The toxicity of radiotherapy is acceptable, and treatment-related morbidity is less frequent compared with IFL. For patients with SN macrometastasis, radiotherapy with a total dose of 50 Gy showed more isolated groin recurrences than IFL. Radiotherapy dose escalation in combination with chemotherapy will be investigated for such patients in GROINSS-V-III.
Brian Slomovitz
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Genentech, Incyte, Agenus, GlaxoSmithKline, GOG Foundation, Myriad Genetics, Merck, Eisai
Ignace Vergote
Consulting or Advisory Role: Amgen, AstraZeneca, Clovis Oncology, Carrick Therapeutics, Deciphera, Elevar Therapeutics, Genmab, GlaxoSmithKline, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, OCTIMET Oncology NV, Oncoinvent, Sotio, Verastem, Zentalis, Roche, Millennium
Research Funding: Roche, Genmab, Amgen, Oncoinvent
Travel, Accommodations, Expenses: Roche, AstraZeneca, Tesaro, Amgen, MSD/Merck
Mats Brännström
Stock and Other Ownership Interests: EUGIN Sweden
Martin Widschwendter
Stock and Other Ownership Interests: Sola Diagnostics, BreOva Health
Patents, Royalties, Other Intellectual Property: Patents relevant for risk prediction or diagnosis of women's cancers
Paul A. DiSilvestro
Consulting or Advisory Role: AstraZeneca, Agenus
Research Funding: Janssen Oncology, Tesaro, AstraZeneca, Genentech, AbbVie
Robert Mannel
Consulting or Advisory Role: Tesaro
Dorry Boll
Research Funding: AstraZeneca
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline
Diane Provencher
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca, AbbVie
Ingo B. Runnebaum
Consulting or Advisory Role: AbbVie (I), Amgen, AstraZeneca, Clovis Oncology, GlaxoSmithKline, Oncgnostics, Tesaro
Preben Kjølhede
Research Funding: Leo Pharma AB
Katharina E. Kieser
Honoraria: AstraZeneca
Consulting or Advisory Role: Merck
Research Funding: AstraZeneca
Nicola M. Spirtos
Research Funding: AbbVie, AstraZeneca, Genentech/Roche, Clovis Oncology, Seattle Genetics
Patents, Royalties, Other Intellectual Property: Application No. PCT/US 2019/19465 Cannabis Based Therapeutic and Method of Use Application No, US Patent 0024098766 Compounds Cannabidiol and Flavanones 63/047550 (July 1, 2020) 63/055458 (July 23, 2020)
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seagen, BBI Healthcare, Arquer Diagnostics, Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen, AstraZeneca, Genentech/Roche, Regeneron, Immunogen, Janssen Research & Development, Clovis Oncology, EMD Serono, Ergomed, Ajinomoto, Cerulean Pharma, PharmaMar, Array BioPharma, Bristol Myers Squibb, Agenus, Tesaro, TRACON Pharma, Genmab, Seattle Genetics, Iovance Biotherapeutics, Leap Therapeutics, Merck, AbbVie/Stemcentrx, AbbVie, Mersana, Eisai, BBI Healthcare, Sumitomo Dainippon Pharma Oncology
Mario M. Leitao
Honoraria: Intuitive Surgical
Consulting or Advisory Role: Intuitive Surgical, Ethicon/Johnson & Johnson, Medtronic, Takeda
Research Funding: KCI
Travel, Accommodations, Expenses: Intuitive Surgical
Melissa A. Geller
Research Funding: Tesaro, Genentech, FATE Therapeutics, Morphotek, Bayer
Karl Tamussino
Other Relationship: Medtronic
Daniel H. Tobias
Consulting or Advisory Role: Ethicon
Jayanthi S. Lea
Consulting or Advisory Role: Roche
Brynhildur Eyjolfsdottir
Other Relationship: Intuitive Surgical
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology
Research Funding: AbbVie, Genentech/Roche, Morphotek, Merck, Regeneron
Travel, Accommodations, Expenses: Roche/Genentech
Ranjit Manchanda
Honoraria: AstraZeneca
Linda Van Le
Consulting or Advisory Role: EyePoint Pharmaceuticals, Novartis, Advarum, Neurotech, Iveric, Gemini Therapeutics, Naegis
Research Funding: GOG Partners Trial
Bradley J. Monk
Leadership: US Oncology
Honoraria: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai, Geistlich Pharma, Genmab/Seattle Genetics, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Merck, Mersana, Myriad Pharmaceuticals, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, GOG Foundation, Starton Therapeutics, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics
Consulting or Advisory Role: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoCare, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai, Geistlich Pharma, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Merck, Mersana, Myriad Pharmaceuticals, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Novartis, Amgen, Genentech, Lilly, Janssen, Array BioPharma, Tesaro, Morphotek, Pfizer, Advaxis, AstraZeneca, Immunogen, Regeneron, Nucana
Carien L. Creutzberg
Consulting or Advisory Role: Merck
Research Funding: Elekta, Varian Medical Systems
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Gaffney at jcopodcast.libsynpro.com
PRIOR PRESENTATION
Presented at the European Society of Gynecological Oncology (ESGO) Conference, November 2-5, 2019, Athens, Greece and the Society of Gynecologic Oncology (SGO) 2020 Virtual Annual Meeting, May 12, 2020.
SUPPORT
Supported by the Dutch Cancer Society (KWF Kankerbestrijding), NRG Oncology.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Maaike H. M. Oonk, Brian Slomovitz, Peter J. W. Baldwin, Jacobus van der Velden, Joanne A. de Hullu, Ignace Vergote, Ate G. J. van der Zee
Administrative support: Maaike H. M. Oonk
Provision of study materials or patients: Maaike H. M. Oonk, Brian Slomovitz, Peter J. W. Baldwin, Helena C. van Doorn, Jacobus van der Velden, Joanne A. de Hullu, Katja N. Gaarenstroom, Brigitte F. M. Slangen, Ignace Vergote, Mats Brännström, Eleonora B. L. van Dorst, Willemien J. van Driel, Ralph H. Hermans, David Nunns, Martin Widschwendter, David Nugent, Cathrine M. Holland, Aarti Sharma, Paul A. DiSilvestro, Robert Mannel, Dorry Boll, David Cibula, Al Covens, Diane Provencher, Ingo B. Runnebaum, David Luesley, Patricia Ellis, Timothy J. Duncan, Ming Y. Tjiong, Derek J. Cruickshank, Preben Kjølhede, Charles F. Levenback, Jiri Bouda, Katharina E. Kieser, Connie Palle, Nicola M. Spirtos, David M. O'Malley, Mario M. Leitao, Melissa A. Geller, Kalyan Dhar, Viren Asher, Karl Tamussino, Daniel H. Tobias, Christer Borgfeldt, Jayanthi S. Lea, Jo Bailey, Margareta Lood, Brynhildur Eyjolfsdottir, Stephen Attard-Montalto, Krishnansu S. Tewari, Ranjit Manchanda, Pernille T. Jensen, Par Persson, Linda Van Le
Collection and assembly of data: Maaike H. M. Oonk, Peter J. W. Baldwin, Helena C. van Doorn, Jacobus van der Velden, Joanne A. de Hullu, Katja N. Gaarenstroom, Brigitte F. M. Slangen, Ignace Vergote, Mats Brännström, Eleonora B. L. van Dorst, Willemien J. van Driel, Ralph H. Hermans, David Nunns, Martin Widschwendter, David Nugent, Cathrine M. Holland, Aarti Sharma, Paul A. DiSilvestro, Robert Mannel, Dorry Boll, David Cibula, Al Covens, Diane Provencher, Ingo B. Runnebaum, David Luesley, Patricia Ellis, Timothy J. Duncan, Ming Y. Tjiong, Derek J. Cruickshank, Preben Kjølhede, Charles F. Levenback, Jiri Bouda, Katharina E. Kieser, Connie Palle,Nicola M. Spirtos, David M. O'Malley, Mario M. Leitao, Melissa A. Geller, Kalyan Dhar, Viren Asher, Karl Tamussino, Daniel H. Tobias, Christer Borgfeldt, Jayanthi S. Lea, Jo Bailey, Margareta Lood, Brynhildur Eyjolfsdottir, Stephen Attard-Montalto, Krishnansu S. Tewari, Ranjit Manchanda, Pernille T. Jensen, Par Persson, Linda Van Le, Bradley J. Monk
Data analysis and interpretation: Maaike H. M. Oonk, Hein Putter, Geertruida H. de Bock, Carien L. Creutzberg, Ate G. J. van der Zee
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of GROINSS-V II
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Brian Slomovitz
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Genentech, Incyte, Agenus, GlaxoSmithKline, GOG Foundation, Myriad Genetics, Merck, Eisai
Ignace Vergote
Consulting or Advisory Role: Amgen, AstraZeneca, Clovis Oncology, Carrick Therapeutics, Deciphera, Elevar Therapeutics, Genmab, GlaxoSmithKline, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, OCTIMET Oncology NV, Oncoinvent, Sotio, Verastem, Zentalis, Roche, Millennium
Research Funding: Roche, Genmab, Amgen, Oncoinvent
Travel, Accommodations, Expenses: Roche, AstraZeneca, Tesaro, Amgen, MSD/Merck
Mats Brännström
Stock and Other Ownership Interests: EUGIN Sweden
Martin Widschwendter
Stock and Other Ownership Interests: Sola Diagnostics, BreOva Health
Patents, Royalties, Other Intellectual Property: Patents relevant for risk prediction or diagnosis of women's cancers
Paul A. DiSilvestro
Consulting or Advisory Role: AstraZeneca, Agenus
Research Funding: Janssen Oncology, Tesaro, AstraZeneca, Genentech, AbbVie
Robert Mannel
Consulting or Advisory Role: Tesaro
Dorry Boll
Research Funding: AstraZeneca
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline
Diane Provencher
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca, AbbVie
Ingo B. Runnebaum
Consulting or Advisory Role: AbbVie (I), Amgen, AstraZeneca, Clovis Oncology, GlaxoSmithKline, Oncgnostics, Tesaro
Preben Kjølhede
Research Funding: Leo Pharma AB
Katharina E. Kieser
Honoraria: AstraZeneca
Consulting or Advisory Role: Merck
Research Funding: AstraZeneca
Nicola M. Spirtos
Research Funding: AbbVie, AstraZeneca, Genentech/Roche, Clovis Oncology, Seattle Genetics
Patents, Royalties, Other Intellectual Property: Application No. PCT/US 2019/19465 Cannabis Based Therapeutic and Method of Use Application No, US Patent 0024098766 Compounds Cannabidiol and Flavanones 63/047550 (July 1, 2020) 63/055458 (July 23, 2020)
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seagen, BBI Healthcare, Arquer Diagnostics, Toray Medical, Takeda, InxMed, Celsion, Roche Diagnostics MSA
Research Funding: Amgen, AstraZeneca, Genentech/Roche, Regeneron, Immunogen, Janssen Research & Development, Clovis Oncology, EMD Serono, Ergomed, Ajinomoto, Cerulean Pharma, PharmaMar, Array BioPharma, Bristol Myers Squibb, Agenus, Tesaro, TRACON Pharma, Genmab, Seattle Genetics, Iovance Biotherapeutics, Leap Therapeutics, Merck, AbbVie/Stemcentrx, AbbVie, Mersana, Eisai, BBI Healthcare, Sumitomo Dainippon Pharma Oncology
Mario M. Leitao
Honoraria: Intuitive Surgical
Consulting or Advisory Role: Intuitive Surgical, Ethicon/Johnson & Johnson, Medtronic, Takeda
Research Funding: KCI
Travel, Accommodations, Expenses: Intuitive Surgical
Melissa A. Geller
Research Funding: Tesaro, Genentech, FATE Therapeutics, Morphotek, Bayer
Karl Tamussino
Other Relationship: Medtronic
Daniel H. Tobias
Consulting or Advisory Role: Ethicon
Jayanthi S. Lea
Consulting or Advisory Role: Roche
Brynhildur Eyjolfsdottir
Other Relationship: Intuitive Surgical
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology
Research Funding: AbbVie, Genentech/Roche, Morphotek, Merck, Regeneron
Travel, Accommodations, Expenses: Roche/Genentech
Ranjit Manchanda
Honoraria: AstraZeneca
Linda Van Le
Consulting or Advisory Role: EyePoint Pharmaceuticals, Novartis, Advarum, Neurotech, Iveric, Gemini Therapeutics, Naegis
Research Funding: GOG Partners Trial
Bradley J. Monk
Leadership: US Oncology
Honoraria: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai, Geistlich Pharma, Genmab/Seattle Genetics, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Merck, Mersana, Myriad Pharmaceuticals, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, GOG Foundation, Starton Therapeutics, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics
Consulting or Advisory Role: AbbVie, Advaxis, Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoCare, ChemoID, Clovis Oncology, Deciphera Pharmaceuticals, Eisai, Geistlich Pharma, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Immunomedics, Incyte, Iovance Biotherapeutics, Laekna Health Care, Merck, Mersana, Myriad Pharmaceuticals, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Senti Biosciences, Takeda, Tarveda Therapeutics, Tesaro/GSK, Vavotar Life Sciences, Vascular Biogenics, Vigeo Therapeutics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Novartis, Amgen, Genentech, Lilly, Janssen, Array BioPharma, Tesaro, Morphotek, Pfizer, Advaxis, AstraZeneca, Immunogen, Regeneron, Nucana
Carien L. Creutzberg
Consulting or Advisory Role: Merck
Research Funding: Elekta, Varian Medical Systems
No other potential conflicts of interest were reported.
REFERENCES
- 1.Van der Zee AG, Oonk MH, De Hullu JA, et al. : Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 26:884-889, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Levenback CG, Ali S, Coleman RL, et al. : Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: A Gynecologic Oncology Group study. J Clin Oncol 30:3786-3791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouwer AW, Hinten F, van der Velden J, et al. : Volume-controlled versus short drainage after inguinofemoral lymphadenectomy in vulvar cancer patients: A Dutch nationwide prospective study. Gynecol Oncol 146:580-587, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Oonk MH, van Hemel BM, Hollema H, et al. : Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early-stage vulvar cancer: Results from GROINSS-V, a multicentre observational study. Lancet Oncol 11:646-652, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Taylor A, Rockall AG, Powell ME: An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol) 19:542-550, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Gaffney DK, King B, Viswanathan AN, et al. : Consensus recommendations for radiation therapy contouring and treatment of vulvar carcinoma. Int J Radiat Oncol Biol Phys 95:1191-1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill BS, Bernard ME, Lin JF, et al. : Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynaecol Oncol 137:365-722, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Glaser S, Olawaiye A, Huang M, et al. : Inguinal nodal region radiotherapy for vulvar cancer: Are we missing the target again?. Gynecol Oncol 135:583-585, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Whitehead J: The Design and Analysis of Sequential Clinical Trials (ed 2). Chichester, United Kingdom, Wiley, 1977 [Google Scholar]
- 10.Carlson JW, Kauderer J, Hutson A, et al. : GOG 244—The lymphedema and gynecologic cancer (LEG) study : Incidence and risk factors in newly diagnosed patients. Gynecol Oncol 156:467-474, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]