Abstract
PURPOSE
Venetoclax is an oral BCL-2 inhibitor with single-agent activity in patients with relapsed or refractory multiple myeloma (RRMM) with t(11;14) translocation. Venetoclax efficacy in RRMM may be potentiated through combination with agents including bortezomib, dexamethasone, and daratumumab.
METHODS
This phase I study (NCT03314181) evaluated venetoclax with daratumumab and dexamethasone (VenDd) in patients with t(11;14) RRMM and VenDd with bortezomib (VenDVd) in cytogenetically unselected patients with RRMM. Primary objectives included expansion-phase dosing, safety, and overall response rate. Secondary objectives included further safety analysis, progression-free survival, duration of response, time to progression, and minimal residual disease negativity.
RESULTS
Forty-eight patients were enrolled, 24 each in parts 1 (VenDd) and 2 (VenDVd). There was one dose-limiting toxicity in part 1 (grade 3 febrile neutropenia, 800 mg VenDd). Common adverse events with VenDd and VenDVd included diarrhea (63% and 54%) and nausea (50% and 50%); grade ≥ 3 adverse events were observed in 88% in the VenDd group and 71% in the VenDVd group. One treatment-emergent death occurred in part 2 (sepsis) in the context of progressive disease, with no other infection-related deaths on study with medians of 20.9 and 20.4 months of follow-up in parts 1 and 2, respectively. The overall response rate was 96% with VenDd (all very good partial response or better [≥ VGPR]) and 92% with VenDVd (79% ≥ VGPR). The 18-month progression-free survival rate was 90.5% (95% CI, 67.0 to 97.5) with VenDd and 66.7% (95% CI, 42.5 to 82.5) with VenDVd.
CONCLUSION
VenDd and VenDVd produced a high rate of deep and durable responses in patients with RRMM. These results support continued evaluation of venetoclax with daratumumab regimens to treat RRMM, particularly in those with t(11;14).
INTRODUCTION
Multiple myeloma (MM) is a heterogenous plasma cell dyscrasia that varies in clinical presentation, responsiveness to therapy, and underlying cytogenetic abnormalities. Despite the introduction of novel therapeutic options, most patients will relapse and become refractory to available therapies.1-4 Regimens combining drugs with synergistic or additive mechanisms of action are key to controlling MM, which has a high degree of clonal heterogeneity that contributes to disease progression, resistance to treatment, and relapse.5-8 To maximize treatment outcomes, it is critical that patients receive optimal treatment throughout their disease course; however, there is currently no guidance on an optimal choice of therapy for individual patients.
CONTEXT
Key Objective
Venetoclax has shown meaningful clinical activity in relapsed or refractory multiple myeloma (RRMM), particularly in the presence of t(11;14), which may be enhanced by combination with agents that increase BCL-2 dependency or eliminate BCL-2–independent subclones. This study evaluated venetoclax with daratumumab and dexamethasone (VenDd) in patients with t(11;14) RRMM and VenDd with bortezomib (VenDVd) in patients with cytogenetically unselected RRMM.
Knowledge Generated
VenDd and VenDVd demonstrated tolerable safety and very encouraging efficacy in patients with RRMM with approximately 20 months of follow-up. A notably high rate of deep and durable responses was observed with VenDd in heavily pretreated patients with t(11;14).
Relevance (S. Lentzsch)
VenDd and VenDVd demonstrated high efficacy in RRMM with t(11;14), highlighting the utility of a biomarker-driven treatment. Given the data of the BELLINI Trial (Lancet Oncol 21:1630-1642, 2020), randomized clinical trials are needed to evaluate whether venetoclax combined with daratumumab for RRMM carrying t(11;14) leads to a significantly better progression-free survival and overall survival but moreover has no detrimental effects on overall survival.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
Evasion of apoptosis and resistance to anticancer drugs in MM can be driven by prosurvival proteins, including BCL-2, BCL-XL, and MCL-1.9,10 MM cells have variable dependence on these prosurvival proteins, and cells harboring the t(11;14) translocation have an increased dependency on BCL-2 for survival.11-13 Venetoclax is a highly selective, oral BCL-2 inhibitor that has activity against MM, particularly in the presence of t(11;14),14 which has been identified as a predictive biomarker for venetoclax activity.15 Venetoclax activity may be enhanced by combination with agents that increase BCL-2 dependency. Bortezomib and dexamethasone increase sensitivity to venetoclax by driving BCL-2 dependency through upregulation of NOXA and shifting BIM loading to BCL-2, respectively.16-18 In phase I studies, venetoclax monotherapy and venetoclax with dexamethasone and bortezomib have yielded promising activity in patients with relapsed or refractory multiple myeloma (RRMM) with and without t(11;14).14,19
The emergence of treatment-resistant subclones with novel genomic alterations or differential oncogenic dependencies is a feature of relapse in MM.20 Elimination of resistant subclones that may arise because of selective pressure from venetoclax treatment is necessary for optimal treatment outcomes. The CD38 monoclonal antibody daratumumab promotes MM cell death through immune-mediated mechanisms, including complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis with expansion of clonal effector T cells, and reduction of regulatory T cells and, therefore, could eliminate emergent resistant subclones.21 Additionally, BCL-2 inhibition with venetoclax was demonstrated to enhance adaptive immunity by increasing the proportion of CD4+ and CD8+ effector memory cells (TEM and TEMRA) in the blood of healthy volunteers and in intratumoral xenografted mice and by augmenting the efficacy of immune checkpoint blockade.22
This study evaluated venetoclax with daratumumab and dexamethasone (VenDd) in patients with t(11;14) RRMM and VenDd with bortezomib (VenDVd) in cytogenetically unselected patients with RRMM. The current trial examined the effects of selected therapeutics against defined molecular targets with the aim of developing a precision medicine strategy for the treatment of defined RRMM subgroups.
METHODS
Study Design and Conduct
The phase I portion of this multicenter, dose-escalation and dose-expansion study enrolled patients in 17 sites in the United States, Australia, Canada, Denmark, and France (NCT03314181). VenDd was evaluated in patients with t(11;14) RRMM in part 1, and VenDVd was evaluated in cytogenetically unselected patients with RRMM in part 2 (Data Supplement, online only). Dose escalation was based on a Bayesian optimal interval design, and dose-limiting toxicities (DLTs) were assessed to identify an optimal expansion-phase dose. The study was approved by the Institutional Review Board or Ethics Committee at each participating center; all patients provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and with the current International Conference on Harmonisation and Good Clinical Practice guidelines. The data cutoff date was October 16, 2020.
Patients
Both parts enrolled patients age ≥ 18 years with RRMM and with documented evidence of progression per International Myeloma Working Group (IMWG) criteria23,24 during or after their last treatment regimen. Patients had an Eastern Cooperative Oncology Group performance status of ≤ 2, acceptable laboratory parameters, and measurable disease confirmed by central laboratory at screening. Patients in part 1 had t(11;14) as determined by fluorescence in situ hybridization (FISH) and must have received ≥ 1 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory imide drug (IMiD). Patients in part 2 must have received one to three prior lines of therapy and could not be refractory to PIs. Full enrollment criteria are listed in the study Protocol (online only).
Treatments and Assessments
For dose escalation, patients received venetoclax once daily at 400 mg. Upon determination of acceptable safety after cycle 1, additional patients were enrolled to receive once-daily 800 mg venetoclax. VenDd was administered in 28-day cycles. Daratumumab was initially given intravenously (IV; 16 mg/kg) but the Protocol was later amended to deliver daratumumab subcutaneously (SC; 1,800 mg). Daratumumab was given weekly for the first two cycles, every 2 weeks for cycles 3-6, then every 4 weeks thereafter. Dexamethasone was given at 40 mg weekly; dose reductions to 20 mg were allowed for those who were underweight or ≥ 75 years old. Dexamethasone was administered IV for the first dose and IV or orally for subsequent doses. VenDVd was administered in 21-day cycles for cycles 1-8 and 28-day cycles thereafter. Daratumumab was given as above weekly for the first three cycles, every 3 weeks for cycles 4-8, and every 4 weeks thereafter. Dexamethasone was given at 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12, and 15 of cycles 1-3; days 1, 2, 4, 5, 8, 9, 11, and 12 of cycles 4-8; and 40 mg weekly thereafter. Bortezomib was administered at 1.3 mg/m2 SC (preferred) or IV on days 1, 4, 8, and 11 of cycles 1-8 (Data Supplement). Per Protocol amendment, antibiotic prophylaxis was mandated for patients receiving venetoclax with bortezomib. For all other patients, anti-infective prophylaxis and granulocyte colony-stimulating factor were recommended per institutional guidelines; granulocyte colony-stimulating factor could not be used during cycle 1. Patients could receive immunoglobulin replacement therapy per institutional guidelines. See the Data Supplement for details of anti-infective prophylaxis.
FISH analysis at screening was performed on CD138-enriched bone marrow aspirates to assess t(11;14) and known prognostic cytogenetic markers in MM. High-risk cytogenetics was defined as the presence of t(4;14), t(14;16), or del(17p). The threshold for determining positivity by FISH per central laboratory testing was based on the analytical cutoff determined for each probe (t(11;14), ≥ 2%; t(4;14), ≥ 3%; t(14;16), ≥ 4%; and del(17p), ≥ 9%). DLTs for dose finding were determined during cycle 1; adverse events (AEs) occurring following cycle 1 may also be considered DLTs (Protocol). Survival information (alive or deceased; if deceased, the date and cause of death) was collected every 12 weeks ± 2 weeks until death, loss of follow-up, withdrawn consent, a time period of 18 months after the study's last patient's first dose, or study termination. Disease assessments were performed per IMWG criteria23,24 within 7 days of day 1, cycle 1, and day 1 of all cycles thereafter. Minimal residual disease (MRD) was assessed in bone marrow aspirates by next-generation sequencing in patients at the time of suspected complete response (CR) or stringent CR (sCR), and at 6- and 12-month postconfirmation of CR or sCR. Patients with missing or unevaluable MRD status were considered MRD-positive. Blood samples for venetoclax pharmacokinetics in dose escalation were collected predose, and 2, 4, 8, and 24 hours postdose on day 2 of cycle 1 and on day 1 of cycle 2. Additional predose blood samples were collected on day 1 of cycles 3, 7, and 12.
Outcomes
The primary objectives were to evaluate the safety and tolerability of VenDd and VenDVd during dose escalation, determine the expansion-phase dose, and further evaluate the safety and preliminary efficacy during dose expansion. Efficacy end points included response rates (overall response rate [ORR defined as ≥ partial response], very good partial response or better [≥ VGPR], and CR or better [≥ CR]) per IMWG criteria,23,24 progression-free survival (PFS), time to progression (TTP), time to response (TTR), duration of response (DOR), and MRD-negative rate (10−5). Exploratory analyses of MRD negativity at 10−4 and 10−6 thresholds were also performed. Venetoclax pharmacokinetics was a secondary objective.
Statistical and Pharmacokinetic Analyses
This was a hypothesis-generating study, and the sample size was determined on the basis of safety assessments. If the true AE rate is 10%, then there is a 92.8% chance of observing at least 1 AE with enrollment of 25 patients in each of the two parts. The primary efficacy end points for parts 1 and 2 were ORR, ≥ VGPR rate, and ≥ CR rate. Response rates were summarized using descriptive statistics with point estimates. Patients were evaluable for disease assessment from the first dose of study treatment until disease progression, start of a new MM therapy, or death, whichever occurred first. Descriptive statistics for PFS, TTP, TTR, DOR, and overall survival (OS) were summarized with 95% CIs using Kaplan-Meier methodology. Patients were evaluable for time-to-event end points from the first dose of study treatment until the occurrence of disease progression or death, whichever occurred first. Censoring was conducted per the statistical analysis plan for the study. Treatment-emergent adverse events (TEAEs), defined as occurring within 30 days following cessation of treatment, were summarized using the Medical Dictionary for Regulatory Activities and graded by severity per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. All patients who received at least one dose of study treatment were included in safety and efficacy analyses. Pharmacokinetic parameters were determined using noncompartmental methods, including maximum observed plasma concentration (Cmax), time to Cmax (peak time, Tmax), and area under the plasma concentration-time curve over a 24-hour dose interval (AUC24).
RESULTS
Patient Demographics, Baseline Characteristics, and Disposition
Forty-eight patients were enrolled between April 17, 2018, and March 14, 2019, including 24 patients with t(11;14) RRMM in part 1 (VenDd) and 24 cytogenetically unselected patients with RRMM in part 2 (VenDVd). In part 1, patients had a median age of 63 years (range, 51-76), 14 patients (58%) had International Staging System stage II or III disease, and patients had received a median of 2.5 (range, 1-8) prior lines of therapy (Table 1). In part 2, patients had a median age of 64 years (range, 41-80), 14 patients (58%) had International Staging System stage II or III disease, and patients had received a median of 1 (range, 1-3) prior line of therapy.
TABLE 1.
Patient Demographics and Baseline Characteristics
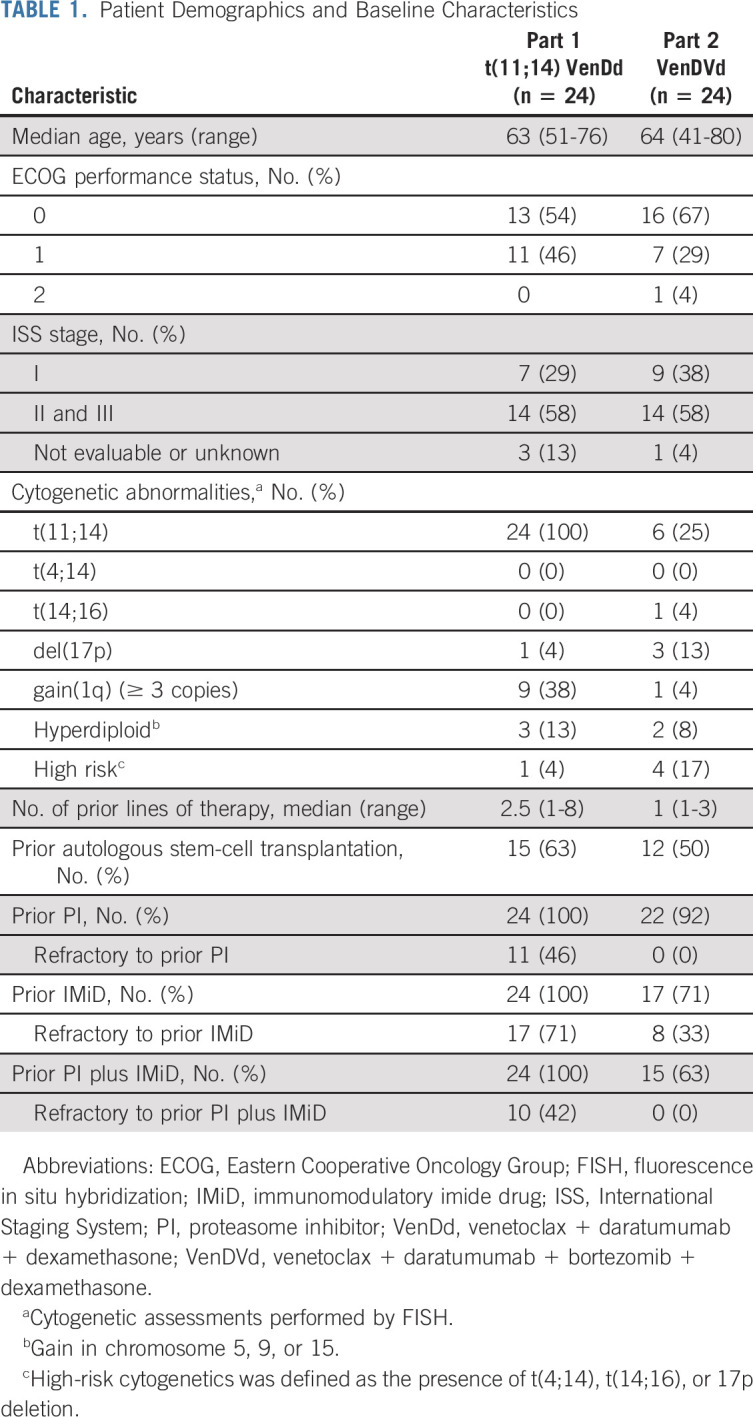
In part 1, 11 patients were enrolled in the dose-escalation cohort, and five received 400 mg venetoclax. Nine patients were enrolled in the dose-escalation cohort in part 2, and 4 received 400 mg venetoclax. The expansion-phase dose of venetoclax in both parts was 800 mg. The median time on study was 20.9 months (range, 19.2-30.0) in part 1 and 20.4 months (range, 6.3-25.7) in part 2 (Data Supplement). Eight patients in part 1 (33%) and six in part 2 (25%) had venetoclax dose reductions because of AEs (Data Supplement). Six patients (25%) in part 1 discontinued venetoclax, and the reasons included AEs (n = 1, melanoma), withdrawn consent (n = 2), progressive disease (n = 2), and opting for autologous transplantation (n = 1). In part 2, 12 patients (50%) discontinued venetoclax, and the reasons included AEs (n = 3, one instance each of knee pain, cognitive disturbance, and treatment-related nausea and abdominal pain), withdrawn consent (n = 2), and progressive disease (n = 7).
Safety
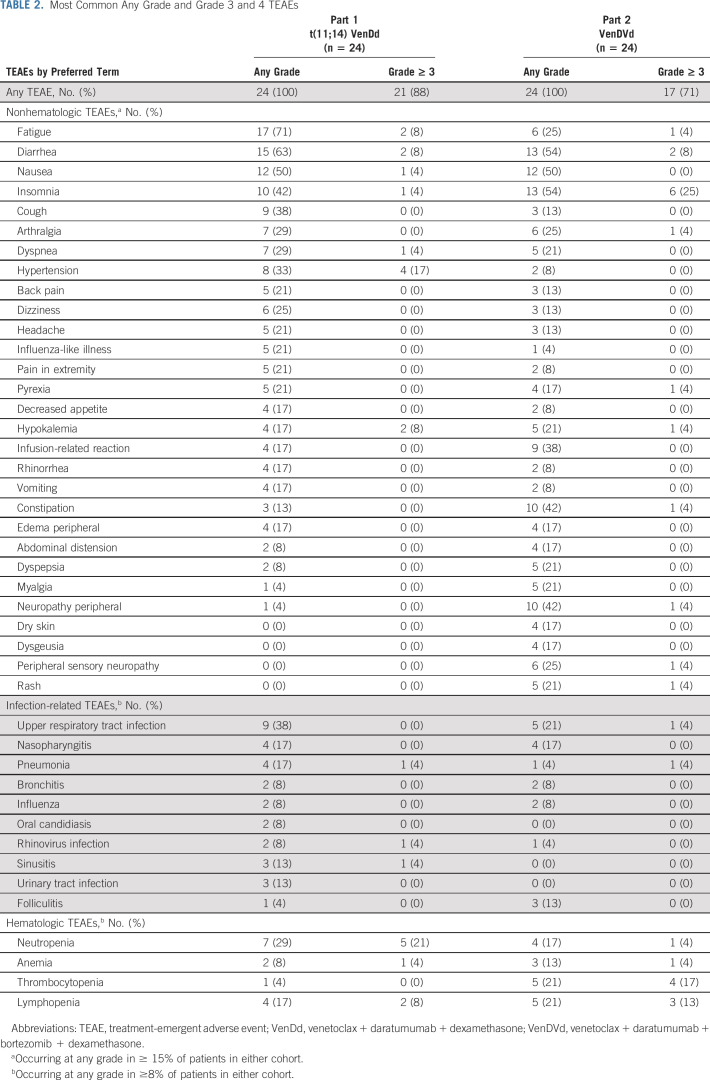
The most common TEAEs were fatigue, diarrhea, nausea, and insomnia (Table 2). Grade ≥ 3 TEAEs occurred in 21 patients (88%) treated with VenDd and 17 patients (71%) treated with VenDVd; the most common were hypertension and insomnia with VenDd and VenDVd, respectively (Table 2). There were no reports of tumor lysis syndrome. One DLT was observed, grade 3 febrile neutropenia, which resolved within 4 days of onset (800 mg VenDd). Grade ≥ 3 neutropenia was observed in five patients (21%) treated with VenDd and one patient (4%) treated with VenDVd. Peripheral neuropathy occurred in one patient (4%) treated with VenDd and 10 patients (42%) treated with VenDVd. Twenty-three patients (96%) in the VenDd arm and 15 patients (63%) in the VenDVd arm experienced an infection, with grade ≥ 3 infections in six (25%) and five patients (21%), respectively. The most common infection was upper respiratory tract infection in nine patients (38%) treated with VenDd and five patients (21%) treated with VenDVd, although all but one (VenDVd arm) were grade 1 or 2 (Data Supplement). Serious TEAEs occurred in 13 patients (54%) and eight patients (33%) treated with VenDd and VenDVd, respectively (Data Supplement). No deaths were reported with VenDd. There was one treatment-emergent death in a patient with t(11;14) treated with 400 mg VenDVd who died from sepsis while in hospice care 3 weeks after discontinuing treatment because of disease progression and therefore was not treatment-related.
TABLE 2.
Most Common Any Grade and Grade 3 and 4 TEAEs
Efficacy
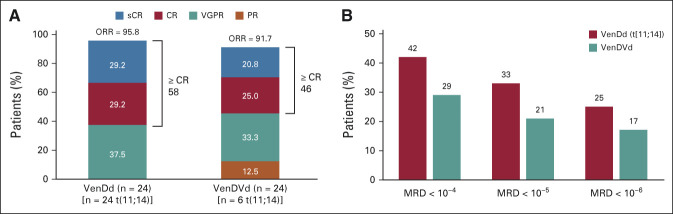
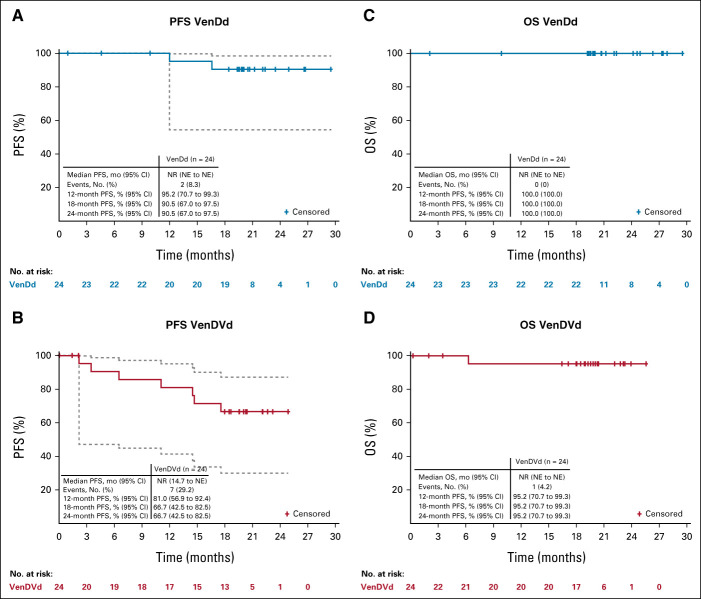
The median study follow-up time was approximately 21 months (range, 19-30) for patients treated with VenDd and approximately 21.5 months (range, 19-26) for patients treated with VenDVd. Twenty-three patients (96%; 95% CI, 78.9 to 99.9) treated with VenDd and 22 patients (92%; 95% CI, 73.0 to 99.0) treated with VenDVd achieved a confirmed response, with a ≥ CR rate of 58% (14 of 24; 95% CI, 36.6 to 77.9) and 46% (11 of 24; 95% CI, 25.6 to 67.2), respectively (Fig 1A). All patients had a ≥ 50% reduction in serum M-protein levels (Fig 2) with ≥ VGPR rates of 96% (95% CI, 78.9 to 99.9) with VenDd and 79% (95% CI, 57.8 to 92.9) with VenDVd. Two patients had unconfirmed responses but discontinued before responses could be confirmed; one in the VenDd group withdrew consent with an unconfirmed VGPR and one in the VenDVd group discontinued because of grade 2 nausea with an unconfirmed partial response. One additional patient treated with VenDVd withdrew consent before response assessments were conducted. Thirty-three percent of patients treated with VenDd and 21% treated with VenDVd achieved an MRD-negative (< 10-5) response (Fig 1B). In those who achieved ≥ CR, 8 of 14 (57%) in the VenDd arm and 5 of 11 (45%) in the VenDVd arm had MRD negativity (< 10-5), and of those, 36% and 36%, respectively, maintained MRD-negative (< 10-5) responses for more than 6 months (Data Supplement). The median TTR was 1 month with VenDd and 0.7 months with VenDVd (Fig 3). Median DOR was not reached (NR) in both arms; the 18-month estimated DOR was 90.5% (95% CI, 67.0 to 97.5) with VenDd and 70% (95% CI, 45.1 to 85.3) with VenDVd (Data Supplement). Median PFS was NR in both arms (Figs 4A and 4B). The 18-month estimated PFS was 90.5% (95% CI, 67.0 to 97.5) with VenDd and 66.7% (95% CI, 42.5 to 82.5) with VenDVd. The 18-month TTP rate was 90.5% (95% CI, 67.0 to 97.5) with VenDd and 66.7% (95% CI, 42.5 to 82.5) with VenDVd. Overall survival was NR in both parts (Figs 4C and 4D).
FIG 1.
(A) Response rates and (B) MRD negativity rates in patients treated with VenDd in part 1a and those treated with VenDVd in part 2.b aThe 95% CIs for response rates in part 1 were 78.9 to 99.9 for ORR, 36.6 to 77.9 for ≥ CR, and 78.9 to 99.9 for ≥ VGPR. bThe 95% CIs for response rates in part 2 were 73.0 to 99.0 for ORR, 25.6 to 67.2 for ≥ CR, and 57.8 to 92.9 for ≥ VGPR. CR, complete response; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response; VenDd, venetoclax, daratumumab, and dexamethasone; VenDVd, venetoclax, daratumumab, bortezomib, and dexamethasone.
FIG 2.
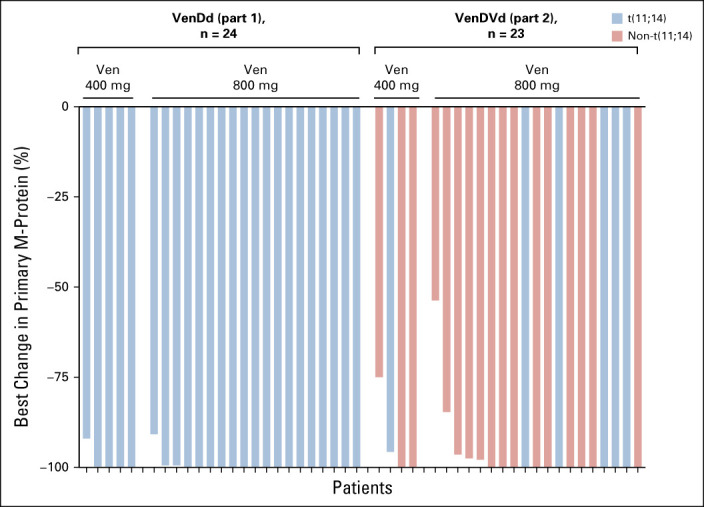
Best change in primary M-protein levels. Ven, Venetoclax; VenDd, venetoclax, daratumumab, and dexamethasone; VenDVd, venetoclax, daratumumab, bortezomib, and dexamethasone.
FIG 3.
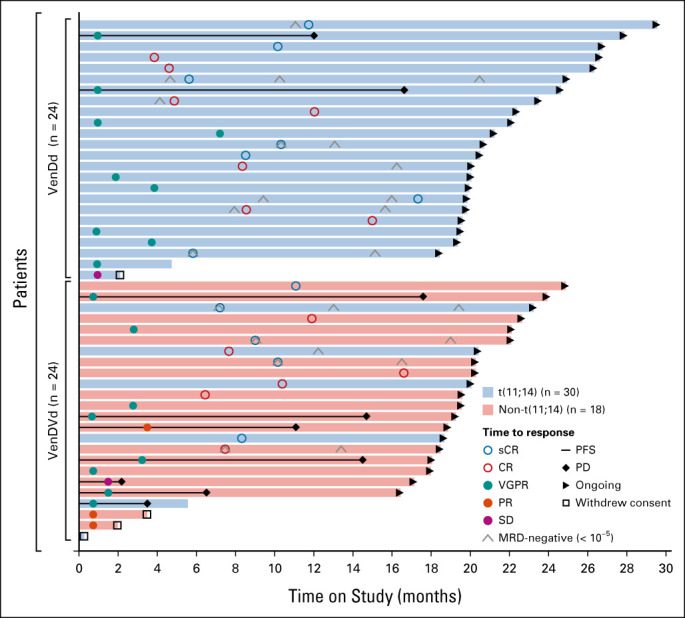
Swimlane plot showing best responses, disease progressions, and time on study. CR, complete response; MRD, minimal residual disease; PD, progressive disease; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; SD, stable disease; VenDd, venetoclax, daratumumab, and dexamethasone; VenDVd, venetoclax, daratumumab, bortezomib, and dexamethasone; VGPR, very good partial response.
FIG 4.
PFS in (A) patients treated with VenDd in part 1 and (B) those treated with VenDVd in part 2, and OS in (C) patients treated with VenDd in part 1 and (D) those treated with VenDVd in part 2. Dashed lines represent 95% CI bands. NE, not estimable; NR, not reached; OS, overall survival; PFS, progression-free survival; VenDd, venetoclax, daratumumab, and dexamethasone; VenDVd, venetoclax, daratumumab, bortezomib, and dexamethasone.
In part 2, six patients had t(11;14) RRMM, and five (83%) responded to VenDVd. Of the 18 patients who did not have t(11;14), 17 (94%) responded to VenDVd. One patient had gain(1q) and responded to VenDVd. All four patients with high-risk cytogenetics [three with del(17p); one with t(14;16)] responded to VenDVd. In part 1, nine patients had gain(1q), and eight (89%) responded to VenDd. One patient had high-risk cytogenetics [del(17p)] and responded to VenDd.
Pharmacokinetics
Concentration versus time profiles for VenDd and VenDVd and venetoclax parameter estimates are provided in the Data Supplement. Venetoclax half-life could not be estimated because of limited sampling after Tmax. In VenDd and VenDVd dose escalation, the estimated pharmacokinetic parameters for venetoclax were consistent with those reported in literature,14,19 indicating that daratumumab with or without bortezomib did not affect venetoclax pharmacokinetics.
DISCUSSION
In this study, treatment with VenDd and VenDVd resulted in notable efficacy and acceptable safety in patients with t(11;14) RRMM and cytogenetically unselected RRMM, respectively. Although differences in the study population and treatment schedule between the VenDd and VenDVd arms prevent direct comparisons of the two arms, neither combination had new safety signals, and the addition of daratumumab to 800 mg oral daily venetoclax did not appear to alter the known safety profiles of venetoclax or daratumumab combinations.
Daratumumab with or without bortezomib did not affect the pharmacokinetics of venetoclax, and the most common AEs were mild gastrointestinal events and fatigue, consistent with the reported safety profile of venetoclax monotherapy.14 Despite the potential for increased hematologic toxicity on the basis of the safety profiles of daratumumab monotherapy and combinations,25-27 most hematologic AEs were mild with the exception of a 21% rate of grade ≥ 3 neutropenia in patients treated with VenDd. The rates of cytopenias reported in this study were consistent with those reported with other novel agents under study for MM.28 The rates of hypertension, fatigue, and grade ≥ 3 neutropenia appeared to be higher with VenDd; however, the small number of patients in each arm clouds the ability to interpret these differences. There was a higher rate of peripheral neuropathy (mostly grade 1 or 2) with VenDVd, consistent with the addition of bortezomib. Increased rates of infections have been observed with the addition of daratumumab to standard regimens in the multiple trials.26,27,29,30 The rate of grade ≥ 3 infections observed in this study (part 1, 25%; part 2, 21%) was similar to other studies with daratumumab.27,29 An increased rate of fatal infections was observed in patients treated with VenVd in the phase III BELLINI trial.31 Consequently, a Protocol amendment mandated antibiotic prophylaxis for patients receiving bortezomib in this study.
In the BELLINI trial, the rate of treatment-emergent deaths was higher in patients treated with VenVd after 19 months of follow-up; however, most of the treatment-emergent deaths occurred early in the trial, with 77% occurring within 6 months of beginning study drug treatment.31 After approximately 21 months of follow-up in this study, there was one treatment-emergent death in a patient who initially responded to VenDVd and then discontinued treatment because of disease progression and died of sepsis while in hospice care. Importantly, no other deaths were observed in the current trial.
The addition of daratumumab to PI- or IMiD-containing regimens in the CASTOR, POLLUX, and CANDOR trials resulted in improved responses (ORRs, 83%-93%) and improved PFS outcomes (12-month PFS rates, 60%-80%).26,27,29 In this study, the addition of daratumumab to venetoclax-based regimens resulted in deep and durable responses, with ORRs of more than 90% and high rates of ≥ VGPR (96% with VenDd). MRD negativity (< 10−5) rates with VenDd (33%) and VenDVd (21%) compared favorably with those observed with daratumumab combinations in the CASTOR (11%), POLLUX (30%), and CANDOR (14%) trials.26,32,33 At 18 months, 91% of patients treated with VenDd and 70% treated with VenDVd maintained their responses. These responses compare favorably with previous studies of daratumumab monotherapy (ORR 31%-36%),34,35 venetoclax monotherapy (ORR 21%),14 DVd (ORR 83%),27 and venetoclax with dexamethasone (ORR 48%-60%).36 Notable efficacy was observed with VenDd among heavily pretreated patients with t(11;14) in which 42% were refractory to both IMiDs and PIs, with an 18-month PFS rate of 91%. Comparable efficacy was observed with VenDVd, with durable responses and an 18-month PFS estimate of 67%, which compares favorably with the 12-month PFS reported for DVd.27 The rate, depth, and durability of the responses observed in this study support an additive, possibly synergistic, effect when daratumumab is added to venetoclax combinations, warranting further investigation in larger trials in BCL-2–dependent MM.
The presence of t(11;14) has been linked to increased BCL-2 dependency and improved responses to venetoclax-based therapy.11-14,18,37-39 In patients with t(11;14) RRMM in the BELLINI trial (n = 35) with 18.7 months of follow-up, VenVd prolonged PFS (median NR v 9.5 months) and improved response rates (ORR 90% v 46%) compared with Vd, without the increase in mortality observed in patients without t(11;14).31 Notably, despite a small number of patients with t(11;14) and differences in the daratumumab and dexamethasone treatment schedule in the VenDVd arm, patients with t(11;14) responded similarly to either VenDd or VenDVd. Both produced deep and durable remissions in these patients, including those with concurrent high-risk cytogenetics. Response rates in patients with t(11;14) RRMM in this trial appeared to be improved over those previously reported in similar populations treated with venetoclax monotherapy or combinations.14,31,36,40 The presence of t(11;14) has recently been identified as a predictive biomarker of venetoclax activity.15 With no apparent added benefit of bortezomib and a higher rate of neuropathy, these findings support further study of a personalized approach using VenDd to treat patients with t(11;14) RRMM.
In summary, VenDd and VenDVd produced high, durable responses and encouraging PFS in patients with RRMM, with no new safety signals. No treatment-related deaths were observed in this study; however, safety will be further evaluated in the ongoing portion of this study. Given the high response rate and MRD negativity associated with VenDd in patients with t(11;14) RRMM, the phase II portion of this study will enroll patients with t(11;14) RRMM to a randomized, open-label expansion cohort that will evaluate VenDd with a DVd control arm to contextualize safety results, which could inform the design of a future phase III trial. The results of this study support further exploration of targeting BCL-2 with venetoclax in combination with anti-CD38 immune therapy to treat t(11;14) RRMM.
ACKNOWLEDGMENT
AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing assistance was provided by Allison Cherry, PhD of Bio Connections, LLC, and funded by AbbVie Inc.
Nizar J. Bahlis
Honoraria: Celgene, Janssen, AbbVie, Amgen, Sanofi, Takeda, Karyopharm Therapeutics, GlaxoSmithKline, Genentech/Roche
Consulting or Advisory Role: Janssen, Celgene, Amgen, Sanofi, Takeda, Pfizer, Karyopharm Therapeutics
Research Funding: Janssen, Celgene
Rachid Baz
Consulting or Advisory Role: Karyopharm Therapeutics, BMS, AbbVie, Oncopeptides, Shattuck Labs, Sanofi
Research Funding: Karyopharm Therapeutics, Celgene, Merck Sharp & Dohme, Takeda, Signal Genetics, AbbVie, Sanofi, Janssen, BMS
Simon J. Harrison
Honoraria: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Haemalogix, Sanofi
Consulting or Advisory Role: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Haemalogix, Sanofi
Speakers' Bureau: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Terumo
Research Funding: Haemalogix, Janssen Cilag
Expert Testimony: EUSA Pharma, Terumo
Hang Quach
Consulting or Advisory Role: GlaxoSmithKline, Celgene, Karyopharm Therapeutics, Janssen-Cilag, CSL Behring, Amgen, Sanofi
Research Funding: Celgene, Amgen, Karopharm, GlaxoSmithKline, Sanofi
Torben Plesner
Consulting or Advisory Role: Janssen, Celgene, Takeda, AbbVie, Genmab, Oncopeptides, Genentech
Speakers' Bureau: Janssen, Takeda
Research Funding: Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, Roche
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie
Simon D. Gibbs
Honoraria: Janssen Oncology, Pfizer, AbbVie, Celgene/Bristol Myers Squibb, Amgen
Consulting or Advisory Role: AbbVie
Abdullah Al Masud
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jeremy A. Ross
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Orlando Bueno
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Research Funding: AbbVie
Travel, Accommodations, Expenses: AbbVie
Jonathan L. Kaufman
Honoraria: Tecnofarma
Consulting or Advisory Role: Janssen, Celgene, TG Therapeutics, Sanofi, Tecnofarma, Genentech
Research Funding: Merck, Celgene, Janssen, Sutro Biopharma, Fortis, Amgen, AbbVie/Genentech, Bristol Myers Squibb
Travel, Accommodations, Expenses: Janssen, Celgene, Bristol Myers Squibb, Sanofi, Amgen, Takeda
No other potential conflicts of interest were reported.
DISCLAIMER
AbbVie sponsored the study and participated in the design; study conduct; analysis, collection, and interpretation of the data; and the writing, review, and approval of the publication. All authors had access to the full study data and approved of the decision to submit the manuscript. The corresponding author had final responsibility for the decision to submit.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets) and other information (eg, Protocols and Clinical Study Reports) as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
AUTHOR CONTRIBUTIONS
Conception and design: Nizar J. Bahlis, Rachid Baz, Abdullah Al Masud, Jeremy A. Ross, Orlando Bueno, Jonathan L. Kaufman
Provision of study materials or patients: Nizar J. Bahlis, Rachid Baz, Hang Quach, Shir-Jing Ho, Philippe Moreau, Simon D. Gibbs, Jonathan L. Kaufman
Collection and assembly of data: Nizar J. Bahlis, Rachid Baz, Simon J. Harrison, Hang Quach, Annette Juul Vangsted, Torben Plesner, Simon D. Gibbs, Jeremy A. Ross, Orlando Bueno, Jonathan L. Kaufman
Data analysis and interpretation: Nizar J. Bahlis, Rachid Baz, Simon J. Harrison, Hang Quach, Shir-Jing Ho, Torben Plesner, Philippe Moreau, Sheryl Coppola, Xiaoqing Yang, Abdullah Al Masud, Jeremy A. Ross, Orlando Bueno, Jonathan L. Kaufman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study of Venetoclax Plus Daratumumab and Dexamethasone, With or Without Bortezomib, in Patients With Relapsed or Refractory Multiple Myeloma With and Without t(11;14)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nizar J. Bahlis
Honoraria: Celgene, Janssen, AbbVie, Amgen, Sanofi, Takeda, Karyopharm Therapeutics, GlaxoSmithKline, Genentech/Roche
Consulting or Advisory Role: Janssen, Celgene, Amgen, Sanofi, Takeda, Pfizer, Karyopharm Therapeutics
Research Funding: Janssen, Celgene
Rachid Baz
Consulting or Advisory Role: Karyopharm Therapeutics, BMS, AbbVie, Oncopeptides, Shattuck Labs, Sanofi
Research Funding: Karyopharm Therapeutics, Celgene, Merck Sharp & Dohme, Takeda, Signal Genetics, AbbVie, Sanofi, Janssen, BMS
Simon J. Harrison
Honoraria: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Haemalogix, Sanofi
Consulting or Advisory Role: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Haemalogix, Sanofi
Speakers' Bureau: AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen Cilag, Novartis, Roche/Genentech, Takeda, Terumo
Research Funding: Haemalogix, Janssen Cilag
Expert Testimony: EUSA Pharma, Terumo
Hang Quach
Consulting or Advisory Role: GlaxoSmithKline, Celgene, Karyopharm Therapeutics, Janssen-Cilag, CSL Behring, Amgen, Sanofi
Research Funding: Celgene, Amgen, Karopharm, GlaxoSmithKline, Sanofi
Torben Plesner
Consulting or Advisory Role: Janssen, Celgene, Takeda, AbbVie, Genmab, Oncopeptides, Genentech
Speakers' Bureau: Janssen, Takeda
Research Funding: Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, Roche
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie
Simon D. Gibbs
Honoraria: Janssen Oncology, Pfizer, AbbVie, Celgene/Bristol Myers Squibb, Amgen
Consulting or Advisory Role: AbbVie
Abdullah Al Masud
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jeremy A. Ross
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Orlando Bueno
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Research Funding: AbbVie
Travel, Accommodations, Expenses: AbbVie
Jonathan L. Kaufman
Honoraria: Tecnofarma
Consulting or Advisory Role: Janssen, Celgene, TG Therapeutics, Sanofi, Tecnofarma, Genentech
Research Funding: Merck, Celgene, Janssen, Sutro Biopharma, Fortis, Amgen, AbbVie/Genentech, Bristol Myers Squibb
Travel, Accommodations, Expenses: Janssen, Celgene, Bristol Myers Squibb, Sanofi, Amgen, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kumar SK, Lee JH, Lahuerta JJ, et al. : Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 26:149-157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majithia N, Rajkumar SV, Lacy MQ, et al. : Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia 30:2208-2213, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi P, Kumar SK, Cerhan JR, et al. : Defining cure in multiple myeloma: A comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J 8:26, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.San-Miguel JF, Mateos MV: Can multiple myeloma become a curable disease? Haematologica 96:1246-1248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolli N, Avet-Loiseau H, Wedge DC, et al. : Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun 5:2997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli N, Maura F, Minvielle S, et al. : Genomic patterns of progression in smoldering multiple myeloma. Nat Commun 9:3363, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta AK, Fink JL, Grady JP, et al. : Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia 33:457-468, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merz M, Hielscher T, Schult D, et al. : Cytogenetic subclone formation and evolution in progressive smoldering multiple myeloma. Leukemia 34:1192-1196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slomp A, Peperzak V: Role and regulation of pro-survival BCL-2 proteins in multiple myeloma. Front Oncol 8:533, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed JC: Bcl-2-family proteins and hematologic malignancies: History and future prospects. Blood 111:3322-3330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touzeau C, Ryan J, Guerriero J, et al. : BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 30:761-764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Ross J, Peale FV, Jr, et al. : A favorable BCL-2 family expression profile may explain the increased susceptibility of the t(11;14) multiple myeloma subgroup to single agent venetoclax. Blood 128, 2016. (abstr 5613) [Google Scholar]
- 13.Gomez-Bougie P, Maiga S, Tessoulin B, et al. : BH3-mimetic toolkit guides the respective use of BCL2 and MCL1 BH3-mimetics in myeloma treatment. Blood 132:2656-2669, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Kaufman JL, Gasparetto C, et al. : Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130:2401-2409, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Silva AS, Renatino-Canevarolo R, Meads MB, et al. : Ex vivo drug sensitivity and functional genomics platform identifies novel combinations targeting intrinsic and extrinsic apoptotic signaling pathways in multiple myeloma. Blood 136:49-50, 2020 [Google Scholar]
- 16.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, et al. : Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res 67:5418-5424, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Matulis SM, Gupta VA, Nooka AK, et al. : Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia 30:1086-1093, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punnoose EA, Leverson JD, Peale F, et al. : Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther 15:1132-1144, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Moreau P, Chanan-Khan A, Roberts AW, et al. : Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 130:2392-2400, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Corre J, Cleynen A, Robiou du Pont S, et al. : Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia 32:2636-2647, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Donk N, Usmani SZ: CD38 antibodies in multiple myeloma: Mechanisms of action and modes of resistance. Front Immunol 9:2134, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohlhapp FJ, Haribhai D, Mathew R, et al. : Venetoclax increases intra-tumoral effector T cells and anti-tumor efficacy in combination with immune checkpoint blockade. Cancer Discov 11:68-79, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 20:1467-1473, 2006. [Erratum: Leukemia 21:1134, 2007] [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Harousseau JL, Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonial S, Weiss BM, Usmani SZ, et al. : Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387:1551-1560, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos M, Quach H, Mateos MV, et al. : Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 396:186-197, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Chanan-Khan A, Weisel K, et al. : Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754-766, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Ludwig H, Delforge M, Facon T, et al. : Prevention and management of adverse events of novel agents in multiple myeloma: A consensus of the European Myeloma Network. Leukemia 32:1542-1560, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Oriol A, Nahi H, et al. : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Facon T, Kumar S, Plesner T, et al. : Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104-2115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar SK, Harrison SJ, Cavo M, et al. : Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 21:1630-1642, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Bahlis NJ, Dimopoulos MA, White DJ, et al. : Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 34:1875-1884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisel K, Spencer A, Lentzsch S, et al. : Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: Subgroup analysis of CASTOR based on cytogenetic risk. J Hematol Oncol 13:115, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lokhorst HM, Plesner T, Laubach JP, et al. : Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373:1207-1219, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Usmani SZ, Weiss BM, Plesner T, et al. : Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128:37-44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman JL, Gasparetto C, Schjesvold FH, et al. : Targeting BCL-2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am J Hematol 96:418-427, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matulis SM, Gupta VA, Neri P, et al. : Functional profiling of venetoclax sensitivity can predict clinical response in multiple myeloma. Leukemia 33:1291-1296, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touzeau C, Dousset C, Le Gouill S, et al. : The Bcl-2 specific BH3 mimetic ABT-199: A promising targeted therapy for t(11;14) multiple myeloma. Leukemia 28:210-212, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touzeau C, Maciag P, Amiot M, et al. : Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia 32:1899-1907, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Harrison S, Cavo M, De La Rubia J, et al. : T(11;14) and high BCL2 expression are predictive biomarkers of response to venetoclax in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma: Biomarker analyses from the phase 3 BELLINI study. Blood 134, 2019. (abstr 142) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets) and other information (eg, Protocols and Clinical Study Reports) as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.