Abstract
Objective:
Chronic overlapping pain conditions (COPCs) represent a co-aggregation of widespread pain disorders. We characterized differences in physical and psychosocial functioning in patients with chronic migraines (CM) and those with CM and COPCs.
Background:
Patients with CM and COPCs have been identified as a distinct subgroup of patients with CM [1], and these patients may be vulnerable to greater symptom severity and burden.
Methods:
Data were extracted from CHOIR (an open-source learning healthcare system), completed at the patients’ first visit at a large tertiary care pain management center, and electronic medical records. In 1601 patients with CM, the number of non-cephalic areas of pain endorsed on a body map were used to examine differences in pain, physical and psychosocial function, adverse life experience, and health care utilization.
Results:
Patients endorsing more body map regions reported significantly worse symptoms and function across all domains. Scored on a T-score metric (mean=50, SD=10), endorsement of 1 additional body map region corresponded with a 0.69-point increase in pain interference (95% CI=0.55, 0.82; p<0.001; Cohen’s f =0.328), 1.15-point increase in fatigue (95% CI=0.97, 1.32; p<0.001; Cohen’s f =0.432), and 1.21-point decrease in physical function (95% CI=−1.39, −1.03; p<0.001; Cohen’s f = 0.560). Patients with more widespread pain reported approximately 5% more physician visits (95% CI=0.03, 0.07; p<0.001), and patients reporting adverse life events prior to age 17 endorsed 22% more body map regions (95% CI=0.11, 0.32; p<0.001).
Conclusions:
Patients with CM and other overlapping pain conditions as noted on the body map report significantly worse pain-related physical function, psychosocial functioning, increased health care utilization, and greater association with adverse life experiences, compared to those with localized CM. This study provides further evidence that patients with CM and co-occurring pain conditions are a distinct subgroup of CM and can be easily identified through patient-reported outcome measures.
Keywords: Migraine, Chronic migraine, complex widespread pain, chronic pain, outcomes registry
Introduction
Researchers have recognized a set of widespread pain disorders that co-aggregate and include temporomandibular disorder (TMD), fibromyalgia (FM), irritable bowel syndrome (IBS), vulvodynia, myalgic encephalomyelitis/chronic fatigue syndrome, interstitial cystitis/painful bladder syndrome, endometriosis, chronic tension-type headache, migraine headache, and chronic lower back pain [2]. This phenomenon of widespread pain disorders has been referred to as chronic overlapping pain conditions (COPCs).
Patients with migraine commonly experience and report pain outside the cephalic region. This overlap has been recognized by both the pain community and the headache community. The headache literature refers to pain outside the head as non-cephalic pain and many association studies have linked migraine to individual COPCs such as temporomandibular disorder, back pain, fibromyalgia, irritable bowel syndrome, and endometriosis [3–8]. The American Migraine Prevalence and Prevention study (AMPP) examined the comorbidity of pain and headache in large cross-sectional longitudinal study administered via mail and demonstrated that chronic pain was 2.4 times more likely in patients with CM vs episodic migraine (EM) [9]. The Chronic Migraine Epidemiology and Outcomes study (CAMEO) also looked at the intersection between migraine and chronic widespread pain. This cross-sectional and longitudinal Internet study characterized the course of EM and CM [10] using a self-reported web-based design to determine comorbidities in migraine. They concluded that the odds of developing CM among those with EM at baseline increased by 43% with each additional non-cephalic pain site in an unadjusted regression analysis [11]. After adjustment for demographic features and headache day frequency, this effect was attenuated but remained significant, and further adjustments for anxiety, depression, allodynia, obesity, headache frequency, and acute medication use, non-cephalic pain remained significantly associated with CM onset [11]. Subsequently, the CAMEO data were used to perform a latent class analysis demonstrating that chronic non-cephalic pain is indeed a separate and naturally occurring subgroup of CM experiencing a moderate–severe phenotype of migraine [1]. Few headache groups have utilized the standard patient-reported outcomes measures for patients with non-cephalic chronic pain, and there appears to be a disconnect between how migraine pain is assessed functionally as compared to all other forms of non-cephalic pain. Clinicians need tools that will rapidly identify this CM subgroup, which we will refer to as CM plus widespread pain. To that end, we examined the prevalence of non-cephalic pain symptoms in a sample of patients with CM. Our hypothesis was that the phenotype of increased symptom burden and disability in regard to physical, psychological and social health would increase as the number of areas of pain in the body increased. In other words, for patients with more widespread pain, we expected to see greater indications of medical need and symptom burden as compared to CM alone. To characterize this phenotype, we have used an open source, learning healthcare system, CHOIR (Collaborative Health Outcomes Information Registry), to collect multiple dimensions of physical, psychological and social functioning in patients seen at a large, tertiary referral pain management center. CHOIR uses both legacy measures as well as Patient Reported Outcome Measurement Information System (PROMIS) measures. PROMIS was developed by the NIH as a way of standardizing the multiple dimensions of a person’s health experience with comparative metrics across the United States population.
Despite their potential utility, the PROMIS measures have not been frequently applied to specific migraine populations; a literature search revealed only three prior studies utilizing these measures in migraines [12–14]. PROMIS measures, initially validated to be used as research tools, are increasingly being used in clinical environments to provide efficient and comprehensive characterization of the multidimensional impact of chronic pain on the patient population [15].
Additional goals for this study were to characterize the association between CM plus co-occurring non-cephalic pain and the frequency of adverse life experiences and health care utilization. Researchers have demonstrated higher adverse life experiences in patients with widespread pain [16, 17]. Tietjen and colleagues have demonstrated across multiple studies that adverse childhood experiences are more prevalent in a headache clinical population than in a community or general clinic population [18–20]. Further, this subpopulation is more likely to transition from EM to CM [21] and there is a greater frequency of childhood maltreatment in adult patients reporting migraine and co-occurring non-cephalic pain conditions [22]. We sought to replicate these findings in our patient population.
Finally, we sought to characterize the association between healthcare utilization and widespread pain as this has presented an equivocal point in the literature. Previous research has shown that widespread pain is not always associated with greater healthcare utilization [23] but this may be present in patients with widespread pain and a history of adverse life experiences [24–26]. Examination of the link between widespread pain and health care utilization in the current sample was intended to highlight the underappreciated clinical and financial burden that complex chronic pain can have for the health care system, even in a patient population often thought to have a relatively circumscribed set of pain complaints like CM.
Materials and Methods:
Procedure
The study design was a cross-sectional assessment of retrospective observational data extracted from initial clinic visits to a large, multidisciplinary tertiary care pain clinic. Data were extracted from both CHOIR, which is completed either prior or during patients’ first visit to the pain clinic, as well as their electronic medical records. All study procedures were approved by the university’s institutional review board (IRB) under a retrospective chart review protocol. As this project occurred using existing clinical data as a retrospective chart review, the requirement for written patient consent was waived. CHOIR (http://choir.stanford.edu) is an open-source learning healthcare system that incorporates patient- and clinician reported outcomes across a variety of clinical domains, including pain intensity, physical and psychosocial function, and global health. CHOIR administers both traditional long-form assessments (e.g., the Pain Catastrophizing Scale) and item response theory (IRT)-based assessments from the Patient- Reported Outcomes Measurement Information Systems (PROMIS) item banks developed by the National Institutes of Health. PROMIS assessments are based on item response theory that allow use of efficient computerized adaptive testing (CAT) data capture [27, 28]. Data from CHOIR have been used in prior empirical work [29–35]; however, no publications have presented data extracted from CHOIR specifically related to patients with migraine.
Participants
Patients were identified according to diagnosis codes entered from the International Classification of Diseases (ICD) 9th and 10th versions- data were extracted for all patients who had an ICD-9 or ICD-10 code referring to chronic migraines that was associated with their initial clinic visits. Diagnoses were given by board-certified headache and/or pain physicians.
Measures
Pain Assessment via the CHOIR body map:
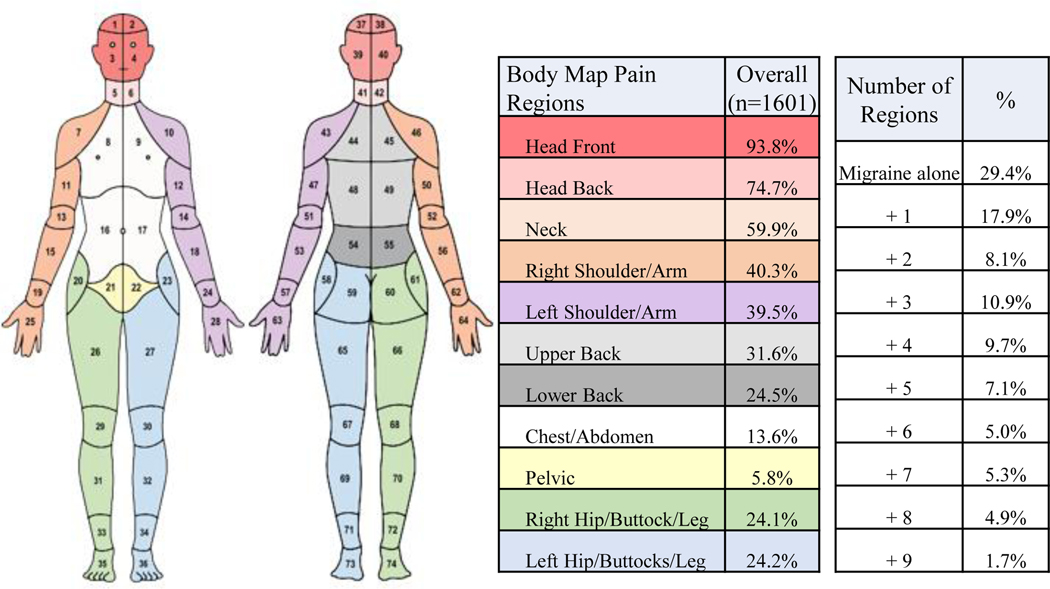
The CHOIR body map is an electronic, visual representation of the human body designed for participants to indicate the location(s) of their pain. Participants use a computer mouse or touch screen device to select each body area where they experience pain, with the instruction “select the areas where you are experiencing pain” or indicate “I have no pain.” The CHOIR body map is adapted from previously published body maps and designed to reflect areas commonly seen in chronic pain disorders. The body map was initially developed and subsequently validated using a modified Delphi approach, and assesses bodily pain across 36 anterior regions and 36 posterior regions of the body (FIGURE 1) [36, 37]. Participants reporting pain in any cranial site (regions 1, 2, 3,4, 37,38, 39, 40 in Figure 1) were considered to have “migraine only” if they received clinician diagnosis of migraine. We collapsed the 74 separate body map areas into 11 anatomically defined broader groups (Figure 1). Participants reporting pain in any of 9 condensed non-cephalic regions in addition to the cranial site(s) were considered to have some indication of non-cephalic pain. This approach is similar to that taken by both Nickel et al and Lai et al [38, 39].
Figure 1.
Body Map Regions and Pain Prevalence in Chronic Migraine Patients
PROMIS assessments.
Various domains of patient functioning (depression, anxiety, anger, physical function, pain interference, pain behavior, sleep disturbance, fatigue, social isolation, and satisfaction with social roles and activities) were assessed using computerized adaptive testing (CAT)-based instruments from Patient-Reported Outcome Measurement Information System (PROMIS) [27, 40, 41]. Descriptions of all PROMIS item banks can be found at the Assessment Center website (http://assessmentcenter.net). PROMIS item banks were initially created with a focus on reducing item overlap, such that depression items encapsulate primarily cognitive/affective features of depression (e.g., feelings of hopelessness, sadness, guilt, and decreased positive emotion), anxiety items assess affective aspects of anxiety (e.g., fear, dread), worry, and physiological aspects of anxiety (tension or dizziness), and anger items assess angry or irritable mood as well as social cognitions and efforts to regulate anger. Fatigue items capture subjective tiredness or exhaustion, and sleep disturbance items assess the depth, quality, and restorativeness of sleep. Physical function items assess patients’ perceived difficulty with engaging in discrete physical behaviors across a range of difficulty levels (e.g., standing up for short periods of time, washing or dressing oneself, or exercising for an hour). Pain interference items assess the degree of disturbance or interference due to pain symptoms across a broad range of domains (e.g., social, recreational, and occupational function), while pain behavior items were designed to assess the presence of external, expressive pain-related behaviors such as grimacing, guarding, and other behaviors that may signal a need for help from others. Social isolation items assess perceptions of being disconnected from, detached, or avoided by others, and satisfaction with social roles and activities items relate primarily to self-rated satisfaction with an individual’s ability to perform their typical life roles (e.g., with family, work, friendships, leisure). PROMIS assessments are converted to a t-score metric with a mean of t = 50 and a standard deviation of 10.
Pain intensity.
Pain intensity was computed using two 11-point numerical rating scale measures (0 reflecting “no pain” and 10 reflecting “pain as bad as it can be”), reflecting average pain over the past 7 days and worst pain over the past 7 days. NRS measures have been validated for use in chronic pain populations [42].
Pain Catastrophizing.
Pain catastrophizing, defined broadly as a cognitive and affective overreaction to actual or expected experience of pain, was assessed using the Pain Catastrophizing Scale[43]. The PCS is a 13-item measure assessing feelings of helplessness related to pain, magnification of the negative aspects of pain, and an inability to disengage from thoughts about pain. The PCS has been validated for use in chronic pain populations [44–46].
Medical utilization.
Medical utilization was assessed via 3 questions: number of physician visits (excluding hospital stays and emergency department visits) over the past 6 months, number of emergency room visits in the past 6 months, and number of overnight hospital stays in the past 6 months.
Adverse life experiences.
Adverse life experiences were assessed using 3 binary (“yes/no”) questions. Childhood adverse experiences were assessed using the question “Prior to the age of 17, did you experience any major upheaval that you think may have shaped your life or personality significantly?”, and adverse experiences as an adult were assessed similarly but with the stem “After the age of 17…” Further, patients were asked to self-report a childhood history of being neglected using the question “Do you feel you were neglected as a child?” No specific examples of adverse life experiences were included as examples to guide patient responses.
Analytic Plan
All analyses were conducted using SAS Enterprise Guide Version 7.1 (SAS Institute Inc., Cary, NC, USA). The primary predictor of interest was the number of endorsed body map regions outside of the head, using a count variable to predict differences in other examined clinical variables. Continuous variables were summarized using mean (SD) or median (25th-75th) if the distributions are skewed. Categorical variables were summarized using frequency (%). The number of non-cephalic regions endorsed in the body map were used as predictors of differences in average and worst pain intensity, PROMIS measures (pain interference, pain behavior, social isolation, satisfaction with social roles and activities, depressive symptoms, anxious symptoms, anger, sleep disturbance, fatigue), and pain catastrophizing using generalized linear models using normal distribution with identity link. The number of non-cephalic regions endorsed was also estimated as a predictor of differences in health care utilization; as these variables reflected count, rather than continuous variables, differences were estimated using negative binomial models with log link. Associations between non-cephalic regions endorsed and the binary adverse childhood experiences variables were computed using generalized linear models using gamma distribution with log link. A p value of less than 0.05 was considered as statistically significant using two-tailed tests. For all models, sex, age, education, and marital status were included as covariates to control for potential contributions of demographic factors. Cohen’s f effect size measures can be found in Table 3. Cohen’s f estimates were computed for each GLM using the following formula: f = sqrt(eta2/(1-eta2)). Benchmarks for interpreting Cohen’s f indicate the presence of a small effect size at f = .10, a medium effect size at f = .25, and a large effect size at f = .40 [47]. No a priori power analysis was conducted as we opted to use all available clinical data for this analysis, and it was deemed likely that we would be sufficiently powered given the large sample size.
Table 3.
Effect sizes of endorsed body map regions on pain and PROMIS outcomes
| Variable | Effect Size |
|---|---|
|
| |
| Pain Intensity - Average | 0.327 |
| Pain Intensity - Worst | 0.251 |
| Pain Catastrophizing Scale | 0.240 |
| PROMIS - Anger | 0.272 |
| PROMIS - Anxiety | 0.285 |
| PROMIS - Depression | 0.296 |
| PROMIS - Fatigue | 0.432 |
| PROMIS - Pain Behavior | 0.269 |
| PROMIS - Pain Interference | 0.328 |
| PROMIS - Physical Function | 0.560 |
| PROMIS - Satisfaction Roles | 0.301 |
| PROMIS - Sleep Disturbance | 0.330 |
| PROMIS - Social Isolation | 0.266 |
Abbreviations: PROMIS, Patient Reported Outcomes Measurement Information System.
The analyses were adjusted for gender, age, education and marital status.
Results
Participants.
Data were drawn between November 2013 and June 2017. The patient characteristics are summarized in Table 1. A total of 1601 patients diagnosed with chronic migraine were included in the current sample. The sample was 1498/1591 (94.2%) female and 581/1053 (55.2%) of the sample reported having a bachelor’s degree or higher. Regarding marital status, 667/1054 (63.3%) of patients reported being currently married or cohabitating with a partner, and 387/1054 (36.7%) of the sample reported being divorced, separated, widowed, or never married.
Table 1.
Demographic and Clinical Characteristics of Patients
| Variable | Sample (N=1601) |
|---|---|
|
| |
| Age, Mean (SD) | |
| Age | 42.8 (14.1) |
| Gender, % | |
| Female | 1498/1591 (94.2%) |
| Male | 93/1591 (5.8%) |
| Education, % | |
| Some College or Lower | 472/1053 (44.8%) |
| Bachelor Degree or Higher | 581/1053 (55.2%) |
| Relationship Status, % | |
| Divorced/Separated/Widowed/Never Married | 387/1054 (36.7%) |
| Living Together/Married | 667/1054 (63.3%) |
| Adverse Life Experience, % | |
| Experience of upheaval prior to age 17 | 329/1002 (32.8%) |
| Experience of upheaval after age 17 | 134/ 315 (42.5%) |
| Neglected as a child | 150/1013 (14.8%) |
| Pain and Psychosocial Measure, Mean (SD) | |
| Pain Intensity - Average | 5.4 (2.1) |
| Pain Intensity - Worst | 7.5 (2.0) |
| Pain Catastrophizing Scale | 23.2 (12.2) |
| PROMIS Anger | 50.7 (10.0) |
| PROMIS Social Isolation | 48.3 (9.3) |
| PROMIS Anxiety | 56.4 (9.3) |
| PROMIS Depression | 54.6 (9.6) |
| PROMIS Fatigue | 61.0 (9.2) |
| PROMIS Pain Behavior | 59.1 (4.5) |
| PROMIS Pain Interference | 64.2 (7.0) |
| PROMIS Physical Function | 40.1 (8.7) |
| PROMIS Satisfaction Role | 43.7 (9.5) |
| PROMIS Sleep Disturbance | 56.6 (9.4) |
| Health Care Utilization, Median (25th, 75th) | |
| Physician Visit | 4.0 (2.0, 6.0) |
| Emergency Visit | 0.0 (0.0, 1.0) |
| Hospital Overnight | 0.0 (0.0, 0.0) |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; SD, Standard Deviation; CI, Confidence Interval; PCS, Pain Catastrophizing Scale.
Clinical Variables.
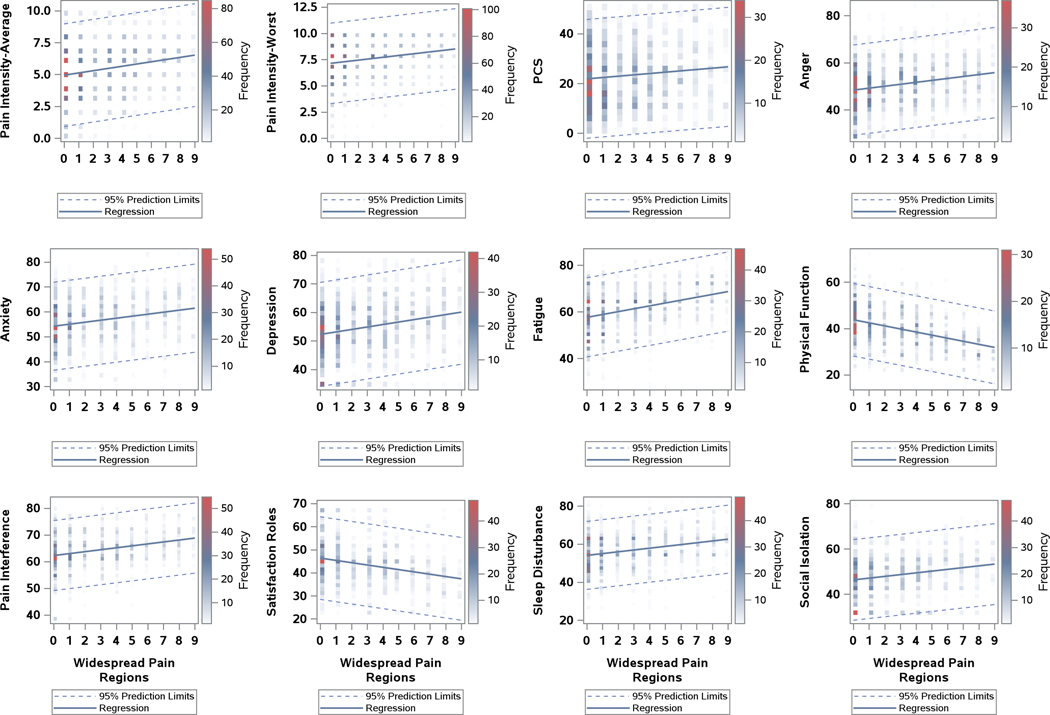
Of the 1601 patients with CM, 470 endorsed no other regions of pain on the body map, 1131 patients reported pain in 1 or more areas outside of the head, 17.9% had one additional region of pain and the other percentage breakdown is visualized in Figure 1. The body map (Figure 1) has been color coded into the condensed regions. Pain was most commonly experienced in the front of the head (93.8%). An additional 74.7% of patients had pain in the back of the head, and 59.9% had pain in the neck. The bilateral shoulder regions were the next most highly marked areas of pain with 40.3% and 39.5% for the right and left shoulder/arm respectively. Figure 2 reflects the associations between body map regions endorsed and differential levels of symptom burden and disability. Analyses indicated a linear association between more widespread pain and significantly worse symptoms and function across all domains: average and worst pain intensity, depressive and anxious symptoms, anger, fatigue, sleep disturbance, physical function, pain-related interference, social isolation, and pain catastrophizing. The number of endorsed body map regions demonstrated a strong relationship with physical function and fatigue, and moderate relationships and other symptom domains (see Table 3). A significant association was noted in terms of health care utilization (number of physician visits, emergency department visits, and overnight hospital stays) that increased as the number of additional regions endorsed on the body map increased (Table 2). Similarly, there was a significantly higher number of endorsed body map regions among patients endorsing higher frequencies of adverse life experiences before the age of 17, adverse life experiences after the age of 17, and childhood neglect (Table 2).
Figure 2.
Differences in clinical characteristics as a function of body map regions endorsed outside of head
Abbreviations: PCS, Pain Catastrophizing Scale;PROMIS, Patient-Reported Outcomes Measurement Information System.
Notes: 0 represents migraine alone in widespread pain regions; Data points reflect frequency of patient scores for each outcome variable; PROMIS measures include Anger, Anxiety, Depression, Fatigue, Physical Function, Pain Interference, Satisfaction Roles, Sleep Disturbance and Social Isolation.
Table 2.
Differences between chronic migraine alone and chronic migraine with additional areas of pain
| Variable | Estimate | SE | (95% CI) | P-value |
|---|---|---|---|---|
|
| ||||
| Adverse Life Experience1 | ||||
| Adverse Experiences Prior to Age 17 | 0.22 | 0.05 | (0.11, 0.32) | <.001** |
| Adverse Experiences After Age 17 | 0.29 | 0.09 | (0.11, 0.47) | 0.002* |
| Neglected Experiences as Child | 0.14 | 0.07 | (0.01, 0.28) | 0.041* |
| Pain Outcome2 | ||||
| Pain Intensity - Average | 0.16 | 0.02 | (0.12, 0.19) | <.001** |
| Pain Intensity - Worst | 0.14 | 0.02 | (0.11, 0.18) | <.001** |
| Pain Catastrophizing Scale | 0.48 | 0.14 | (0.20, 0.77) | 0.001* |
| PROMIS Outcome2 | ||||
| Anger | 0.80 | 0.10 | (0.60, 1.00) | <.001** |
| Anxiety | 0.77 | 0.09 | (0.59, 0.95) | <.001** |
| Depression | 0.82 | 0.10 | (0.64, 1.01) | <.001** |
| Fatigue | 1.15 | 0.09 | (0.97, 1.32) | <.001** |
| Pain Behavior | 0.35 | 0.05 | (0.26, 0.44) | <.001** |
| Pain Interference | 0.69 | 0.07 | (0.55, 0.82) | <.001** |
| Physical Function | −1.21 | 0.09 | (−1.39, −1.03) | <.001** |
| Satisfaction Role | −0.99 | 0.11 | (−1.20, −0.78) | <.001** |
| Sleep Disturbance | 0.88 | 0.09 | (0.70, 1.07) | <.001** |
| Social Isolation | 0.80 | 0.11 | (0.59, 1.01) | <.001** |
| Health Care Utilization Outcome2 | ||||
| Physician Visits | 0.05 | 0.01 | (0.03, 0.07) | <.001** |
| Emergency Room Visits | 0.05 | 0.02 | (0.00, 0.09) | 0.040* |
| Hospital Overnights | 0.08 | 0.03 | (0.02, 0.15) | 0.016* |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; SE, Standard Error; CI, Confidence Interval.
Adverse life experiences were modeled as predictors on the number of body map region adjusted for study covariates.
Variables were modeled as outcomes as a function of body map regions and study covariates.
p-value<0.05;
p-value<0.001
Discussion
This study supports our hypothesis that greater levels of widespread pain in CM patients are associated with significantly worse physical, psychological, and social functioning. Furthermore, that CM patients with widespread pain have higher healthcare utilization and greater association with adverse life events.
Our results indicate moderate or stronger associations between an increasing number of regions endorsed on the body map and worse levels of physical and psychosocial functioning. Notably, the number of body map regions endorsed showed the strongest relationship with two domains: physical function and fatigue (Table 3). In the case of physical function, this finding may be explained by the focus of item content in this domain on discrete physical activities. As pain symptoms become more widespread, it may be more expected that pain will interfere with a broader range of physical activities (e.g., walking, exercise, or even activities of daily living). That fatigue showed a strong relationship with body map regions endorsed may be indicative of 2 underlying processes. PROMIS fatigue items also heavily emphasize the degree of function that is impaired due to a physical symptom, so this similar emphasis on functional impairment may share variance with interference in physical function due to widespread pain. Second, as the number of body map regions endorsed increased, it is also possible that patients may have begun to exhibit more signs of centralized pain processes akin to fibromyalgia, a condition in which fatigue is a common complaint. These findings reinforce a distinct and separate subgroup of CM plus widespread pain that is characterized by a higher overall degree of symptom severity and burden, and that PROMIS measures may be a useful tool in identifying these patients in a clinical setting. They also support the potential clinical utility of the body map to rapidly aid in identifying this subgroup.
We observed that the body pain experience for CM plus widespread pain seemed to follow a top-down pattern, meaning that after head, neck was the most highly selected area of pain, followed by shoulders/arms. Prior research suggests that neck pain has been more commonly involved in CM over EM [48, 49], and demonstrated to be a common location of headache commencement in the studies leading up to the PREEMPT trials of botulinum toxin for CM [50]. However, the top-down pattern may also reflect an element of central sensitization. Both functional and structural studies have suggested that repeat migraine attacks can remodel the pain network, chiefly focusing on allodynia as a marker of central sensitization [51–54]. A recent imaging study showed increased connectivity in CM vs EM between the anterior cingulate cortex, anterior insula, thalami, dorsolateral prefrontal cortex, precuneus, supramarginal gyri, and cerebellum which would also likely impact widespread pain [55]. Other COPCs (urologic chronic pelvic pain syndrome, fibromyalgia) have also demonstrated significant changes in increased brain gray matter volume and functional connectivity involving sensorimotor and insular cortices (P< 0.05 corrected) as compared to pain-free controls [56].
We also demonstrated that greater healthcare utilization in patients with CM and co-occurring widespread pain than those with isolated CM. Prior studies have demonstrated greater health care utilization and expenditures in CM versus EM [57–60]. However, there have not been any comparisons between CM and CM with widespread pain in terms of health care utilization. These findings thus extend prior research and highlight the potential value of identifying these patients as high-need early in treatment due to the likelihood that they will ultimately require and utilize more medical and other treatment resources.
Further, our results indicated that patients with more widespread pain in addition to CM were significantly more likely to report childhood neglect, and adverse experiences both in childhood and adulthood. This finding supports previous analyses by Tietjen and colleagues indicating an association between adverse life experiences and widespread pain in patients with migraine [22, 61] but also highlights the possibility of assessing this information in a relatively brief format. Due to concerns about heightened patient burden, CHOIR assessments do not typically include a full assessment of adverse life experiences. However, our results suggest that even a brief assessment of adverse life experiences and childhood neglect may provide a useful preliminary indicator that may highlight the need for more intensive evaluation in this domain to direct diagnosis and treatment. Identifying adverse life experiences early could lead to additional treatment possibilities; for example, there are emerging psychotherapy options for patients with centralized forms of chronic pain that emphasize processing of emotions related to adverse life experiences [62, 63]. Another key consideration in interpreting these findings is the possibility of co-morbid PTSD. Although PTSD symptoms were not assessed in this sample, this would be a valuable point for future research. It is likely that a subset of patients in the current sample had some degree of co-occurring PTSD symptoms stemming from these adverse life experiences. Although there is some evidence that PTSD symptoms may decrease with time without intervention in some patients, these symptoms may last for years in others and can impede chronic pain treatment unless they are properly addressed [64]. Identifying patients who endorse adverse life experiences may thus be a useful indicator that a full assessment for PTSD is indicated. Fortunately, evidence-based treatments do exist to treat patients with chronic pain and PTSD as comorbid conditions [65, 66].
It is notable that typical metrics used for assessing migraine impact such as MIDAS and HIT6 were not included in the current sample, which reflects a focus on assessments that apply to a broader range of chronic pain complaints. The use of PROMIS assessments reflects a desire to rapidly phenotype pain and functional indicators across all patients presenting for pain treatment, and to efficiently identify those patients who may need more resource-intensive treatment as quickly as possible.
At present there are changes in the recommendations of common data elements for headache studies ( https://www.commondataelements.ninds.nih.gov/headache.) These recommendations reflect an attempt to bring unity to a diverging field, and while the headache common data elements do include some PROMIS measures, the assessments are extensive and potentially burdensome to the patient. PROMIS allows for computerized adaptive testing that shortens the duration of the user experience significantly. PROMIS is also used for other pain conditions, creating standardization among disease processes, and allowing for the possibility of inter-disease comparisons and interdisciplinary research [67]. PROMIS measures are validated in comparison to the broader US population. Consequently, our results are intended to establish a basis of comparison for future studies in CM and widespread pain. These assessments (CHOIR and PROMIS) are freely available and already in use in pain clinics. Our goal in using these patient assessment tools, was to use the minimum number of tests to accurately identify and treat our patient population, however we do recognize the importance of migraine metrics in order to provide a base of comparison for the literature and future studies should incorporate both PROMIS and traditional assessments.
The limitations of our study include the cross-sectional nature of the data, which precludes inferences regarding temporal precedence or causality of the examined effects. We present these findings primarily as a characterization of a CM subpopulation that would benefit from replication and expansion in future longitudinal and clinical studies. We were also limited by our inability to reliably assess medications, interventional procedures, co-occurring substance use, and medical comorbidities, all of which may further influence the clinical status and utilization of medical resources noted in this study. We urge additional attention to these factors in future studies. We also acknowledge that these patients were selected by their ICD9/10 diagnosis codes and not by ICHD-3 criteria which would imply a greater degree of heterogeneity to our sample. Consequently, we encourage interpretation of these findings as reflecting the realities of chronic pain and migraine in a tertiary care setting rather than a general neurology or primary care setting. Further, and despite the apparent utility of the body map assessment to serve as a marker for widespread pain, it would be of value for future studies to utilize more rigorous screening and diagnosis to establish the presence of COPCs in a CM population, and longitudinally follow this cohort to determine stability in symptoms and treatment response. Similarly, our use of a non-validated measure of adverse life experiences presents a set of preliminary findings that require replication using validated and more intensive assessments in future studies.
Overall, this study confirms in a clinical setting, what has been demonstrated in large data sets; [1] that within CM there is a distinct subpopulation of CM plus widespread pain. Furthermore, detecting this subset can be easily and rapidly distinguished by utilizing the body map and PROMIS measures, in addition to traditional diagnostic strategies. Our results highlight the need for multi-dimensional assessment in both CM and CM plus widespread pain. The identification and characterization of this subpopulation has the potential to support the development of improved risk and treatment stratification paradigms for this group focusing not just on cephalic pain but also on restoration of important psychosocial domains of function commonly affected by chronic pain.
Acknowledgments
Financial Support: This work was funded by a donation for Helen and Richard Elkus, the Redlich Pain Endowment, and NIH K24DA029262 (SM).
Institutional Review Board Approval: The Stanford University Institutional Review Board approved the study
Footnotes
Conflict of Interest: Dr. Barad reports participation in clinical trials for Allergan, Teva, ATI, Lilly. She has also consulted for Lilly. Dr. Sturgeon reports a scientific advisory board membership with TribeRx, Dr. Aggarwal reports no COI, Dr. Mackey reports no COI.
References
- 1.Lipton RB, et al. , Identifying Natural Subgroups of Migraine Based on Comorbidity and Concomitant Condition Profiles: Results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache, 2018. 58(7): p. 933–947. [DOI] [PubMed] [Google Scholar]
- 2.Maixner W, et al. , Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain, 2016. 17(9 Suppl): p. T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speciali JG and Dach F, Temporomandibular dysfunction and headache disorder. Headache, 2015. 55 Suppl 1: p. 72–83. [DOI] [PubMed] [Google Scholar]
- 4.Tietjen GE, et al. , Endometriosis is associated with prevalence of comorbid conditions in migraine. Headache, 2007. 47(7): p. 1069–78. [DOI] [PubMed] [Google Scholar]
- 5.Kucuksen S, et al. , The prevalence of fibromyalgia and its relation with headache characteristics in episodic migraine. Clin Rheumatol, 2013. 32(7): p. 983–90. [DOI] [PubMed] [Google Scholar]
- 6.Yoon MS, et al. , Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium study. Pain, 2013. 154(3): p. 484–92. [DOI] [PubMed] [Google Scholar]
- 7.Lau CI, et al. , Association between migraine and irritable bowel syndrome: a population-based retrospective cohort study. Eur J Neurol, 2014. 21(9): p. 1198–204. [DOI] [PubMed] [Google Scholar]
- 8.Plesh O, Adams SH, and Gansky SA, Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache, 2012. 52(6): p. 946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buse DC, et al. , Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry, 2010. 81(4): p. 428–32. [DOI] [PubMed] [Google Scholar]
- 10.Adams AM, et al. , The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia, 2015. 35(7): p. 563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher AI, et al. , Comorbid pain and migraine chronicity: The Chronic Migraine Epidemiology and Outcomes Study. Neurology, 2017. 89(5): p. 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton RB, et al. , Erenumab in chronic migraine: Patient-reported outcomes in a randomized double-blind study. Neurology, 2019. 92(19): p. e2250–e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearl TA, et al. , Impact of Depression and Anxiety Symptoms on Patient-Reported Outcomes in Patients With Migraine: Results From the American Registry for Migraine Research (ARMR). Headache, 2020. [DOI] [PubMed] [Google Scholar]
- 14.M TM, et al. , Smartphone-based migraine behavioral therapy: a single-arm study with assessment of mental health predictors. NPJ Digit Med, 2019. 2: p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook KF, Buckenmaier C 3rd, and Gershon RC, PASTOR/PROMIS (R) pain outcomes system: what does it mean to pain specialists? Pain Manag, 2014. 4(4): p. 277–83. [DOI] [PubMed] [Google Scholar]
- 16.Jones GT, Power C, and Macfarlane GJ, Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain, 2009. 143(1–2): p. 92–6. [DOI] [PubMed] [Google Scholar]
- 17.Generaal E, et al. , Biological stress systems, adverse life events and the onset of chronic multisite musculoskeletal pain: a 6-year cohort study. Ann Rheum Dis, 2016. 75(5): p. 847–54. [DOI] [PubMed] [Google Scholar]
- 18.Tietjen GE, et al. , Childhood maltreatment and migraine (part I). Prevalence and adult revictimization: a multicenter headache clinic survey. Headache, 2010. 50(1): p. 20–31. [DOI] [PubMed] [Google Scholar]
- 19.Gould DA, et al. , Self-reported childhood abuse in an adult population in a primary care setting. Prevalence, correlates, and associated suicide attempts. Arch Fam Med, 1994. 3(3): p. 252–6. [DOI] [PubMed] [Google Scholar]
- 20.Edwards VJ, et al. , Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry, 2003. 160(8): p. 1453–60. [DOI] [PubMed] [Google Scholar]
- 21.Tietjen GE, et al. , Childhood maltreatment and migraine (part II). Emotional abuse as a risk factor for headache chronification. Headache, 2010. 50(1): p. 32–41. [DOI] [PubMed] [Google Scholar]
- 22.Tietjen GE, et al. , Childhood maltreatment and migraine (part III). Association with comorbid pain conditions. Headache, 2010. 50(1): p. 42–51. [DOI] [PubMed] [Google Scholar]
- 23.White KP, et al. , Does the label “fibromyalgia” alter health status, function, and health service utilization? A prospective, within-group comparison in a community cohort of adults with chronic widespread pain. Arthritis Rheum, 2002. 47(3): p. 260–5. [DOI] [PubMed] [Google Scholar]
- 24.Seery MD, et al. , Lifetime exposure to adversity predicts functional impairment and healthcare utilization among individuals with chronic back pain. Pain, 2010. 150(3): p. 507–15. [DOI] [PubMed] [Google Scholar]
- 25.Finestone HM, et al. , Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse Negl, 2000. 24(4): p. 547–56. [DOI] [PubMed] [Google Scholar]
- 26.Forman-Hoffman VL, et al. , Chronic widespread pain in veterans of the first Gulf War: impact of deployment status and associated health effects. J Pain, 2007. 8(12): p. 954–61. [DOI] [PubMed] [Google Scholar]
- 27.Cella D, et al. , The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol, 2010. 63(11): p. 1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn EA, et al. , Measuring social health in the patient-reported outcomes measurement information system (PROMIS): item bank development and testing. Qual Life Res, 2010. 19(7): p. 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karayannis NV, et al. , Pain interference and physical function demonstrate poor longitudinal association in people living with pain: a PROMIS investigation. Pain, 2017. 158(6): p. 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturgeon JA, et al. , Social Disruption Mediates the Relationship Between Perceived Injustice and Anger in Chronic Pain: a Collaborative Health Outcomes Information Registry Study. Ann Behav Med, 2016. 50(6): p. 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturgeon JA, et al. , Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain, 2015. 16(3): p. 291–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturgeon JA, et al. , Contributions of physical function and satisfaction with social roles to emotional distress in chronic pain: a Collaborative Health Outcomes Information Registry (CHOIR) study. Pain, 2015. 156(12): p. 2627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturgeon JA, et al. , Clinical Profiles of Concurrent Cannabis Use in Chronic Pain: A CHOIR Study. Pain Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karayannis NV, et al. , The Impact of Social Isolation on Pain Interference: A Longitudinal Study. Ann Behav Med, 2019. 53(1): p. 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carriere JS, et al. , Pain behavior mediates the relationship between perceived injustice and opioid prescription for chronic pain: a Collaborative Health Outcomes Information Registry study. J Pain Res, 2017. 10: p. 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson K, Foote A, Kao M, & Mackey S. Somatic distributions of pain characterized with a pain registry (CHOIR) (128). The Journal of Pain, 2015. 16(4). [Google Scholar]
- 37.Hymel Scherrer K, Development and Validation of the Collaborative Health Outcomes Information Registry (CHOIR) Body Map. Pain Reports 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickel JC, Tripp DA, and International G. Interstitial Cystitis Study, Clinical and psychological parameters associated with pain pattern phenotypes in women with interstitial cystitis/bladder pain syndrome. J Urol, 2015. 193(1): p. 138–44. [DOI] [PubMed] [Google Scholar]
- 39.Lai HH, et al. , Characterization of Whole Body Pain in Urological Chronic Pelvic Pain Syndrome at Baseline: A MAPP Research Network Study. J Urol, 2017. 198(3): p. 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilkonis PA, et al. , Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment, 2011. 18(3): p. 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riley WT, Pilkonis P, and Cella D, Application of the National Institutes of Health Patient-reported Outcome Measurement Information System (PROMIS) to mental health research. J Ment Health Policy Econ, 2011. 14(4): p. 201–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Farrar JT, et al. , Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain, 2001. 94(2): p. 149–58. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan MJL, Bishop SR, Pivik J, The Pain Catastrophizing Scale: Development and Validation. Psychological Assessement, 1995. 7(4): p. 524–532. [Google Scholar]
- 44.Van Damme S, et al. , A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain, 2002. 96(3): p. 319–24. [DOI] [PubMed] [Google Scholar]
- 45.Osman A, et al. , The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med, 2000. 23(4): p. 351–65. [DOI] [PubMed] [Google Scholar]
- 46.Osman A, et al. , Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med, 1997. 20(6): p. 589–605. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J, Statistical Power Analysis for the Behavioral Sciences. 2cd Edition. 1988, Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- 48.Carvalho GF, et al. , Comparison between neck pain disability and cervical range of motion in patients with episodic and chronic migraine: a cross-sectional study. J Manipulative Physiol Ther, 2014. 37(9): p. 641–6. [DOI] [PubMed] [Google Scholar]
- 49.Florencio LL, et al. , Neck pain disability is related to the frequency of migraine attacks: a cross-sectional study. Headache, 2014. 54(7): p. 1203–10. [DOI] [PubMed] [Google Scholar]
- 50.Blumenfeld A, et al. , Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache, 2010. 50(9): p. 1406–18. [DOI] [PubMed] [Google Scholar]
- 51.Filippi M. and Messina R, The Chronic Migraine Brain: What Have We Learned From Neuroimaging? Frontiers in Neurology, 2020. 10(1356). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Androulakis XM, et al. , Modulation of intrinsic resting-state fMRI networks in women with chronic migraine. Neurology, 2017. 89(2): p. 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwedt TJ, et al. , Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache, 2013. 53(5): p. 737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppola G, et al. , Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain, 2017. 18(1): p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MJ, et al. , Increased connectivity of pain matrix in chronic migraine: a resting-state functional MRI study. J Headache Pain, 2019. 20(1): p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kutch JJ, et al. , Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain, 2017. 158(10): p. 1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munakata J, et al. , Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache, 2009. 49(4): p. 498–508. [DOI] [PubMed] [Google Scholar]
- 58.Bloudek LM, et al. , Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain, 2012. 13(5): p. 361–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messali A, et al. , Direct and Indirect Costs of Chronic and Episodic Migraine in the United States: A Web-Based Survey. Headache, 2016. 56(2): p. 306–22. [DOI] [PubMed] [Google Scholar]
- 60.Stokes M, et al. , Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache, 2011. 51(7): p. 1058–77. [DOI] [PubMed] [Google Scholar]
- 61.Anda R, et al. , Adverse childhood experiences and frequent headaches in adults. Headache, 2010. 50(9): p. 1473–81. [DOI] [PubMed] [Google Scholar]
- 62.Lumley MA and Schubiner H, Psychological Therapy for Centralized Pain: An Integrative Assessment and Treatment Model. Psychosom Med, 2019. 81(2): p. 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yarns BC, et al. , Emotional Awareness and Expression Therapy Achieves Greater Pain Reduction than Cognitive Behavioral Therapy in Older Adults with Chronic Musculoskeletal Pain: A Preliminary Randomized Comparison Trial. Pain Med, 2020. [DOI] [PubMed] [Google Scholar]
- 64.Peres JF, Goncalves AL, and Peres MF, Psychological trauma in chronic pain: implications of PTSD for fibromyalgia and headache disorders. Curr Pain Headache Rep, 2009. 13(5): p. 350–7. [DOI] [PubMed] [Google Scholar]
- 65.Bosco MA, Murphy JL, and Clark ME, Chronic pain and traumatic brain injury in OEF/OIF service members and Veterans. Headache, 2013. 53(9): p. 1518–22. [DOI] [PubMed] [Google Scholar]
- 66.Otis JD, et al. , The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain Med, 2009. 10(7): p. 1300–11. [DOI] [PubMed] [Google Scholar]
- 67.Cook KF, et al. , PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol, 2016. 73: p. 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]