Abstract
BACKGROUND
Racial and ethnic minority groups in the United States experience a disproportionate burden of COVID-19 deaths.
OBJECTIVE
To evaluate whether outcome differences between Hispanic and non-Hispanic COVID-19 hospitalized patients exist and, if so, to identify the main malleable contributing factors.
DESIGN, SETTING, PARTICIPANTS
Retrospective, cross-sectional, observational study of 6097 adult COVID-19 patients hospitalized within a single large healthcare system from March to November 2020.
EXPOSURES
Self-reported ethnicity and primary language.
MAIN OUTCOMES AND MEASURES
Clinical outcomes included intensive care unit (ICU) utilization and in-hospital death. We used age-adjusted odds ratios (OR) and multivariable analysis to evaluate the associations between ethnicity/language groups and outcomes.
RESULTS
32.1% of patients were Hispanic, 38.6% of whom reported a non-English primary language. Hispanic patients were less likely to be insured, have a primary care provider, and have accessed the healthcare system prior to the COVID-19 admission. After adjusting for age, Hispanic inpatients experienced higher ICU utilization (non-English-speaking: OR, 1.75; 95% CI, 1.47-2.08; English-speaking: OR, 1.13; 95% CI, 0.95-1.33) and higher mortality (non-English-speaking: OR, 1.43; 95% CI, 1.10-1.86; English-speaking: OR, 1.53; 95% CI, 1.19-1.98) compared to non-Hispanic inpatients. There were no observed treatment disparities among ethnic groups. After adjusting for age, Hispanic inpatients had elevated disease severity at admission (non-English-speaking: OR, 2.27; 95% CI, 1.89-2.72; English-speaking: OR, 1.33; 95% CI, 1.10- 1.61). In multivariable analysis, the associations between ethnicity/language and clinical outcomes decreased after considering baseline disease severity (P < .001).
CONCLUSION
The associations between ethnicity and clinical outcomes can be explained by elevated disease severity at admission and limited access to healthcare for Hispanic patients, especially non-English-speaking Hispanics.
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1–7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11–15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated health-care system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
METHODS
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory- confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24–26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English- speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, to-cilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access– related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
RESULTS
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.
TABLE 1.
Cohort Characteristics and Comorbidity
| Total | Patients
|
|||||
|---|---|---|---|---|---|---|
| Non-Hispanic | Hispanic, English speaking | Hispanic, non-English speaking | P value | |||
| Total | 6097 | 4139 | 1203 | 755 | ||
|
| ||||||
| Age, y | <.001 | |||||
| 18–49 | 1989 (32.6) | 1089 (26.3) | 671 (55.8) | 229 (30.3) | ||
| 50–64 | 1633 (26.8) | 1093 (26.4) | 288 (23.9) | 252 (33.4) | ||
| 65–79 | 1623 (26.6) | 1237 (29.9) | 184 (15.3) | 202 (26.8) | ||
| ≥80 | 852 (14.0) | 720 (17.4) | 60 (5.0) | 72 (9.5) | ||
|
| ||||||
| Sex | .004 | |||||
| Male | 2916 (47.8) | 1972 (47.6) | 544 (45.2) | 400 (53.0) | ||
| Female | 3181 (52.2) | 2167 (52.4) | 659 (54.8) | 355 (47.0) | ||
|
| ||||||
| Comorbidity | ||||||
| Diabetesa | 2324 (38.1) | 1479 (35.7) | 477 (39.7) | 368 (48.7) | <.001 | |
| HbA1c > 9% | 512 (8.4) | 251 (6.1) | 142 (11.8) | 119 (15.8) | <.001 | |
| Hypertension | 3688 (60.5) | 2702 (65.3) | 563 (46.8) | 423 (56.0) | <.001 | |
| Obesity | 3109 (51.0) | 2005 (48.4) | 755 (62.8) | 349 (46.2) | <.001 | |
| COPD | 563 (9.2) | 514 (12.4) | 29 (2.4) | 20 (2.7) | <.001 | |
| Other lung disease | 301 (4.9) | 263 (6.4) | 24 (2.0) | 14 (1.9) | <.001 | |
| Asthma | 564 (9.3) | 431 (10.4) | 108 (9.0) | 25 (3.3) | <.001 | |
| Heart failure | 541 (8.9) | 449 (10.9) | 60 (5.0) | 32 (4.2) | <.001 | |
| Cancer | 566 (9.3) | 469 (11.3) | 66 (5.5) | 31 (4.1) | <.001 | |
| Kidney disease without ESRD | 1921 (31.5) | 1492 (36.1) | 236 (19.6) | 193 (25.6) | <.001 | |
| ESRD | 242 (4.0) | 141 (3.4) | 63 (5.2) | 38 (5.0) | .005 | |
| Liver disease | 367 (6.0) | 241 (5.8) | 82 (6.8) | 44 (5.8) | .4 | |
| Smoker | 1681 (27.6) | 1280 (30.9) | 267 (22.2) | 134 (17.8) | <.001 | |
|
| ||||||
| EHR creation yearb | 2020 | 1032 (16.9) | 512 (12.4) | 228 (19.0) | 292 (38.7) | <.001 |
|
| ||||||
| Has PCP | Yes | 4749 (77.9) | 3485 (84.2) | 822 (68.3) | 442 (58.5) | <.001 |
|
| ||||||
| Insurance | <.001 | |||||
| Commercial | 1981 (32.5) | 1376 (33.2) | 472 (39.2) | 133 (17.6) | ||
| Government other | 3088 (50.6) | 2372 (57.3) | 446 (37.1) | 270 (35.8) | ||
| COVID-19 HRSA | 586 (9.6) | 213 (5.1) | 153 (12.7) | 220 (29.1) | ||
| Self pay | 63 (1.0) | 29 (0.7) | 17 (1.4) | 17 (2.3) | ||
A total of 6097 patients who tested positive for COVID-19 received inpatient care at Texas Health Resources (THR) between March 3 and November 5, 2020. All values are No. (%).
Diabetes includes comorbidity code of “diabetes” or hemoglobin A1c > 9%.
Year when patients first accessed the THR health system.
Abbreviations: EHR, electronic health record; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; PCP, primary care provider.
Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
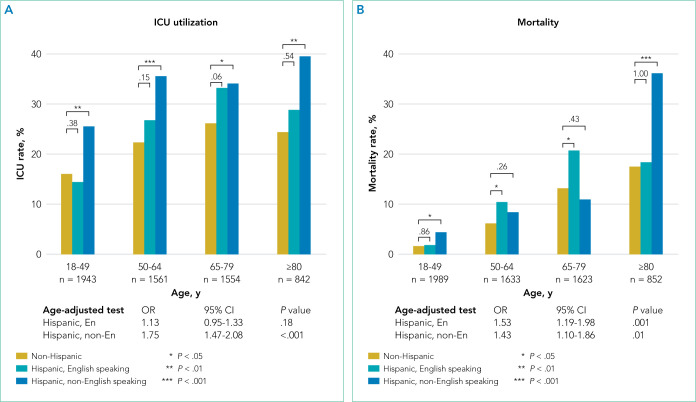
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic- language patient groups. In all age groups, non-English- speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).
FIG 1.
Intensive Care Unit Admission Rate and Mortality Rate Among Ethnic-Language Groups. P values are shown above the brackets.
Abbreviations: ICU, intensive care unit; OR, odds ratio.
To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethniclanguage groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
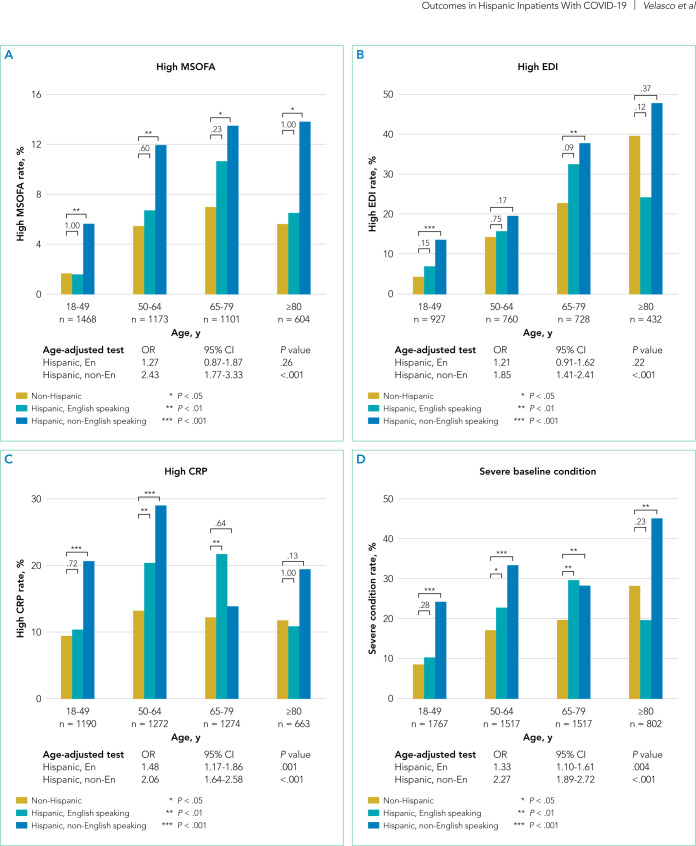
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41- 2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64- 2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).
FIG 2.
Baseline Disease Severity Among Ethnic-Language Groups. P values are shown above the brackets.
Abbreviations: CRP, C-reactive protein; EDI, Epic Deterioration Index; ICU, intensive care unit; MSOFA, Modified Sequential Organ Failure Assessment; OR, odds ratio.
We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.
TABLE 2.
Multivariable Analysis Including Demographics, Ethnicity, Comorbidity and Baseline Disease Severity (Model 2)
| Intensive care unit | Mortality | ||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| (Intercept) | 0.11 (0.08-0.15) | <.001 | (Intercept) | 0.01 (0-0.01) | <.001 |
| Age: 50–64 y | 1.18 (0.96-1.46) | .1 | Age: 50–64 y | 2.61 (1.7-4) | <.001 |
| Age: 65–79 y | 1.18 (0.96-1.46) | .1 | Age: 65–79 y | 3.99 (2.64-6.03) | <.001 |
| Age: ≥80 y | 0.82 (0.63-1.05) | .1 | Age: ≥ 80 y | 5.39 (3.48-8.37) | <.001 |
| Sex: female | 0.83 (0.72-0.96) | .01 | Sex: female | 0.83 (0.67-1.03) | .1 |
| Hispanic, English speakinga | 1.20 (0.98-1.47) | .08 | Hispanic, English-speakinga | 1.54 (1.14-2.09) | .005 |
| Hispanic, non-English speakinga | 1.47 (1.18-1.84) | <.001 | Hispanic, non-English-speakinga | 1.34 (0.97-1.87) | .08 |
| Diabetesb | 1.22 (1.05-1.42) | .01 | COPD | 1.44 (1.08-1.93) | .01 |
| COPD | 1.27 (1-1.6) | .05 | Asthma | 0.69 (0.45-1.06) | .09 |
| Heart failure | 1.34 (1.06-1.69) | .01 | Heart failure | 1.34 (1.01-1.78) | .04 |
| Kidney disease without ESRD | 2.50 (2.12-2.94) | <.001 | Kidney disease without ESRD | 3.22 (2.55-4.06) | <.001 |
| Liver disease | 1.58 (1.2-2.09) | .001 | ESRD | 1.35 (0.9-2.01) | 0.14 |
| Severe baseline condition | 4.52 (3.83-5.33) | <.001 | Liver disease | 2.62 (1.84-3.71) | <.001 |
| Smoker | 1.40 (1.11-1.76) | .004 | |||
| Severe baseline condition | 3.32 (2.67-4.13) | <.001 | |||
Variables included in the analysis were determined through a backward stepwise variable selection process (see details in Methods). Variable selection was performed independently for the two models with intensive care unit and mortality, respectively, as outcome.
Reference: Non-Hispanic patients.
Diabetes includes comorbidity code of “diabetes” or hemoglobin A1c > 9%.
Abbreviations: COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease.
Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
DISCUSSION
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non- Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the health-care system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
CONCLUSION
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system— including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
Footnotes
Find additional supporting information in the online version of this article.
Disclosures: The authors reported no conflicts of interest.
Funding: Portions of this study were supported by the Texas Health Resources Clinical Scholars Program and NIH grant 1R35GM136375. Dr Sheffield received grant support for this work from the National Institutes of Health (Ruth L. Kirschstein Institutional National Research Service Award, T32 CA124334).
References
- 1.Lopez L, III, Hart LH, III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719–720. doi: 10.1001/jama.2020.26443. doi: 10.1001/jama.2020.26443. [DOI] [PubMed] [Google Scholar]
- 2.Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491–1492. doi: 10.1001/jama.2020.19567. doi: 10.1001/jama.2020.19567. [DOI] [PubMed] [Google Scholar]
- 3.Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. doi: 10.3390/ijerph18020683. doi: 10.3390/ijerph18020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659–1663. doi: 10.1007/s00431-021-03955-x. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. 2020 Dec 9; https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html.
- 7.Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. doi: 10.1001/jamanetworkopen.2020.34578. doi: 10.1001/jamanetworkopen.2020.34578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. 2021 Jan 21; https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf.
- 9.Centers for Disease Control and Prevention Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.
- 10.Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517–1521. doi: 10.15585/mmwr.mm6942e1. doi: 10.15585/mmwr.mm6942e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. doi: 10.1093/ofid/ofaa638. doi: 10.1093/ofid/ofaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161–1167. doi: 10.1007/s40615-020-00872-x. doi: 10.1007/s40615-020-00872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2; doi: 10.1093/cid/ciab290. doi: 10.1093/cid/ciab290. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 14.Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20; doi: 10.1093/cid/ciab154. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 15.Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. doi: 10.1186/s12889-021-11431-2. doi: 10.1186/s12889-021-11431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326–1334. doi: 10.1513/AnnalsATS.202011-1448OC. doi: 10.1513/AnnalsATS.202011-1448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. doi: 10.1016/j.eclinm.2020.100630. doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Census Bureau US. U.S Census Bureau American Community Survey. 2019 https://www.census.gov/programs-surveys/acs.
- 20.North Texas Mass Critical Care Task Force North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. 2014 Jan; https://www.dallas-cms.org/tmaimis/dcms/assets/files/communi-tyhealth/MCC/GuidelinesAdult_JAN2014.pdf.
- 21.Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129–1137. doi: 10.1513/Annal-sATS.202006-698OC. doi: 10.1513/Annal-sATS.202006-698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489–493. doi: 10.12788/jhm.3497. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html. [PubMed]
- 24.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi: 10.1001/jamaint-ernmed.2020.6252. doi: 10.1001/jamaint-ernmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405–2415. doi: 10.1016/j.ajem.2020.08.091. doi: 10.1016/j.ajem.2020.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185–1195. doi: 10.1001/jama.2021.2747. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439–448. doi: 10.1001/jamainternmed.2020.7968. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092–1104. doi: 10.1007/s10903-021-01162-2. doi: 10.1007/s10903-021-01162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358–1360. doi: 10.1097/CCM.0000000000005126. doi: 10.1097/CCM.0000000000005126. [DOI] [PubMed] [Google Scholar]
- 30.Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209–e1217. doi: 10.1097/CCM.0000000000002699. doi: 10.1097/CCM.0000000000002699. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1–E7. doi: 10.12788/jhm.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohn D. Census considers new approach to asking about race – by not using the term at all. 2015 Jun 18; https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/