Abstract
Background
Integration of HIV services with other health services has been proposed as an important strategy to boost the sustainability of the global HIV response. We conducted a systematic and comprehensive synthesis of the existing scientific evidence on the impact of service integration on the HIV care cascade, health outcomes, and cost-effectiveness.
Methods and findings
We reviewed the global quantitative empirical evidence on integration published between 1 January 2010 and 10 September 2021. We included experimental and observational studies that featured both an integration intervention and a comparator in our review. Of the 7,118 unique peer-reviewed English-language studies that our search algorithm identified, 114 met all of our selection criteria for data extraction. Most of the studies (90) were conducted in sub-Saharan Africa, primarily in East Africa (55) and Southern Africa (24). The most common forms of integration were (i) HIV testing and counselling added to non-HIV services and (ii) non-HIV services added to antiretroviral therapy (ART). The most commonly integrated non-HIV services were maternal and child healthcare, tuberculosis testing and treatment, primary healthcare, family planning, and sexual and reproductive health services. Values for HIV care cascade outcomes tended to be better in integrated services: uptake of HIV testing and counselling (pooled risk ratio [RR] across 37 studies: 1.67 [95% CI 1.41–1.99], p < 0.001), ART initiation coverage (pooled RR across 19 studies: 1.42 [95% CI 1.16–1.75], p = 0.002), time until ART initiation (pooled RR across 5 studies: 0.45 [95% CI 0.20–1.00], p = 0.050), retention in HIV care (pooled RR across 19 studies: 1.68 [95% CI 1.05–2.69], p = 0.031), and viral suppression (pooled RR across 9 studies: 1.19 [95% CI 1.03–1.37], p = 0.025). Also, treatment success for non-HIV-related diseases and conditions and the uptake of non-HIV services were commonly higher in integrated services. We did not find any significant differences for the following outcomes in our meta-analyses: HIV testing yield, ART adherence, HIV-free survival among infants, and HIV and non-HIV mortality. We could not conduct meta-analyses for several outcomes (HIV infections averted, costs, and cost-effectiveness), because our systematic review did not identify sufficient poolable studies. Study limitations included possible publication bias of studies with significant or favourable findings and comparatively weak evidence from some world regions and on integration of services for key populations in the HIV response.
Conclusions
Integration of HIV services and other health services tends to improve health and health systems outcomes. Despite some scientific limitations, the global evidence shows that service integration can be a valuable strategy to boost the sustainability of the HIV response and contribute to the goal of ‘ending AIDS by 2030’, while simultaneously supporting progress towards universal health coverage.
Caroline Bulstra and co-workers assess evidence on the benefits of service integration in the HIV care cascade.
Author summary
Why was this study done?
The rapid scale-up of HIV testing and antiretroviral therapy (ART) in many countries and communities over the past 2 decades has been largely achieved with stand-alone HIV programmes.
Increasing life expectancy and the side effects of ART are leading to more co-morbidities among people living with HIV, suggesting that ART programmes that also offer other treatments could improve both healthcare effectiveness and the patient experience.
Other reasons for integration of services include the hope that joint delivery of services will increase coverage and reduce costs.
The global evidence on integration of HIV services and other health services, to our knowledge, has never been synthesised, and it is thus unclear what the empirical effects of integration are.
What did the researchers do and find?
We conducted a systematic review and meta-analysis to synthesise the results of integrating HIV services and other health services for HIV care cascade outcomes (testing, linkage to care, treatment initiation, treatment adherence, retention, and viral suppression), HIV health outcomes (new infections and mortality), non-HIV health outcomes, and costs and cost-effectiveness.
In most of the 114 studies that our systematic review identified most outcomes were better in integrated compared to separate services.
What do these findings mean?
Integration of HIV services and other health services tends to improve health and health systems outcomes.
The success of integration strategies is highly context-specific, and more evidence is needed on integration in specific geographical areas and for key populations in the HIV response.
Despite such limitations, our systematic review and meta-analysis support the case for integration as a valuable and viable strategy to boost the sustainability of the HIV response and contribute to the goal of ‘ending AIDS by 2030’, while simultaneously supporting progress towards universal health coverage.
Introduction
Ambitious goals guide the global HIV response. The 2016 political declaration of the United Nations General Assembly on HIV and AIDS [1] reinforced the commitment of the international community to reach the ‘getting to zero’ targets: zero new HIV infections, zero AIDS-related deaths, and zero discrimination by 2030 [2,3]. The so-called fast-track commitments for HIV prevention aim to achieve access to combination prevention for 90% of all key populations in the HIV response (e.g., young women and adolescent girls, men who have sex with men, transgender people, sex workers and their clients, people who inject drugs, and prisoners) by the end of 2020 [2]. The UNAIDS ‘95-95-95 targets’ focus on near-universal and effective coverage with HIV testing and antiretroviral therapy (ART) for people living with HIV (PLHIV) [4]. Despite progress, these ambitious goals remain elusive in many countries [5,6]. Political and financial support for the global HIV response has stagnated or even declined in recent years [7,8]. At the same time, the number of PLHIV needing treatment is expected to further rise in the future [9,10]. The economic shock and health financing crisis triggered by the coronavirus disease 2019 (COVID-19) pandemic has further reduced political attention to HIV and will likely further widen the gap between the funding required and available to achieve the global HIV goals [11].
The rapid global scale-up of HIV testing, prevention, and treatment services over the past 2 decades has been largely achieved with stand-alone programmes operating separately from other health system functions [12]. At the same time, increasing life expectancy [13,14] and the side effects of ART have led to more co-morbidities among PLHIV [15], suggesting that more integrated ART programmes could improve the HIV patient’s experience and healthcare effectiveness. From the perspective of programmes that currently do not include HIV services, such as testing or treatment, integrating HIV services may lead to powerful benefits for patients, such as HIV status knowledge and needed ART.
In the broadest sense, integration is the joining of 2 or more health services that were previously separated in some way (for instance, delivered by different health workers or at different locations). The specific integration that was the topic of our systematic review and meta-analysis is the joining of health services for HIV and at least one other disease or condition [16,17].
Integration could improve or worsen aspects of health services. From the perspective of patients and clients, integration could reduce the time and inconvenience of utilising healthcare for several diseases or conditions, and thus improve the patient experience [17,18]. From the perspective of the providers and funders of care, integration could improve processes and resource allocation [19,20]. For example, integrating the delivery of different services that the same patient needs could increase access [19] and continuity of care [21,22], and improve the clinical coordination of treatments for different diseases and thus health outcomes [23–25]. Integration could also reduce the costs of services because of synergies in joint delivery [19,20]. However, it is also plausible that integration could increase costs, because joint delivery of services for multiple diseases reduces specialisation and the efficiency gains that it brings. Another plausible risk of integration is overburdening healthcare providers [12,26], especially in areas with high HIV prevalence.
We performed a systematic review and meta-analysis of existing empirical quantitative evidence on the integration of HIV services and other health services. We included all empirical quantitative studies that compared outcomes in an intervention and a comparator group, independent of how the intervention and comparator groups were assigned, defined and measured. The reason for the broad scope of study designs that we included in our systematic review is that in our fields of study—health systems, health services, and implementation research—experiments are overall rare, while observational study designs are common. We hope that our evidence synthesis will inform policy and implementation strategies aimed at improving the reach, quality, impact, and sustainability of HIV and other health services.
Methods
Search strategy and selection criteria
We followed the PRISMA [27] guidelines for systematic reviews and meta-analyses (S1 PRISMA Checklist). We searched Embase, Medline Ovid (the database behind PubMed), Web of Science, EconLit (ProQuest), Cochrane Central Register of Controlled Trials, and Google Scholar to identify articles published between 1 January 2010 and 28 January 2020, and manually searched the reference lists of identified studies. The search was updated on 10 September 2021 to include studies up to this date. Search strings were constructed in collaboration with a medical librarian (see S1 File for the full search strategy). We used Medical Subject Headings (MeSH) terms and ‘all fields’ terms comprising the themes healthcare integration, HIV/AIDS, utility, health, economic and healthcare quality outcome indicators, and target populations. We included all populations in our systematic review, such as the general population and PLHIV. In addition, we specifically included terms for several key populations in the HIV response in our systematic search algorithm (men who have sex with men, transgender people, sexual and gender minorities, and sex workers) to ensure that we did not miss studies of integration targeting these populations (S1 File) [5]. Database searches were restricted to studies in English.
We included full-text, peer-reviewed experimental and observational studies that provided quantitative empirical evidence on integration with both an intervention and a comparator group. We included studies on HIV care cascade outcomes (testing, linkage to care, treatment initiation, treatment adherence, retention, and viral suppression), HIV health outcomes (new infections and mortality), non-HIV health outcomes, and costs and cost-effectiveness. These outcomes align with the 5 factors of the RE-AIM Framework to evaluate the public health impact of health system interventions [28,29]: (i) reach—HIV testing and counselling; (ii) effectiveness—viral suppression and health outcomes; (iii) adoption—cost-effectiveness; (iv) implementation—linkage to care, treatment initiation, treatment retention, and adherence; and (v) maintenance—costs.
Reasons for excluding studies were (i) no integration intervention including an HIV service, (ii) integration of health system functions other than health services, (iii) no outcome of interest, (iv) no empirical evidence (e.g., editorials, perspectives, reviews, modelling studies), (v) no comparator group, (vi) non-published/non-peer-reviewed literature, (vii) study protocols, or (viii) studies whose full text was not available. All included studies reported on integration at the point of care, but integration could also have included ‘above-patient’ and ‘above-site’ levels including healthcare providers, infrastructure, resources, monitoring, evaluation, and supply chain and management [16,30]. Studies concerning all facility types were included, from hospitals to community-level services.
Three independent reviewers (CAB, MO, and AS) screened the titles and abstracts of the primary studies that our search algorithm identified, and for those records that were eligible based on our inclusion and exclusion criteria, we examined full texts to determine eligibility for data extraction. We resolved any disagreements between the independent reviewers by consensus in a discussion with another member of our author team. We did not provide the protocol online; the review was not pre-registered.
Data analysis
Two authors (MO and AS) independently extracted the following study data: (i) general information (title, authors, year of publication, journal), (ii) study characteristics (study objectives and aims, study design, study population, study size, study time and duration), (iii) geographical location and population, (v) intervention (description, rationale for integration, degree of integration, healthcare level of integration), (v) comparator, (vi) outcomes, (vii) results, and (viii) contextual factors. We performed a quality assessment of the primary studies that our systematic search identified using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool [31]. For studies that did not find that integration improved outcomes, we searched the journal articles for reasons for this finding.
We expressed results (i) as risk ratios (RRs) for both HIV and non-HIV care cascade and health outcomes, (ii) as number of infections averted for new HIV infections, and (iii) in 2018 US dollars for costs and the cost components of incremental cost-effectiveness ratios (ICERs). We did meta-analyses for those outcomes for which pooling of results was meaningful (i.e., for HIV and non-HIV care cascade and health outcomes), because the integration interventions studied were the same or functionally similar. We pooled results using inverse-variance weighting. We estimated the proportion of variation across studies that was due to heterogeneity of results rather than chance (I2 statistic) [32]. We also did meta-analyses for the subset of experimental studies only. All data analyses were done using R version 3.6.3 [33]. Ethical approval was not required for this study because we only used secondary data extracted from published studies.
Results
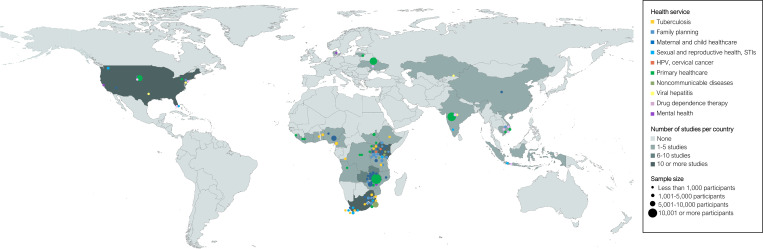
Our search identified a total of 7,118 unique publications, of which 114 met the inclusion criteria (S1 Fig) [34–147]. Of the 114 included studies, 39 were experimental and 75 were observational. Fig 1 shows the geographical location and year of publication of the included studies by type of health service. Most of the studies were conducted in East Africa (55 studies) and Southern Africa (24 studies)—primarily in Kenya (20 studies), South Africa (18 studies), Zambia (8 studies), Malawi (8 studies), and Uganda (8 studies). The most common services integrated with HIV services were maternal and child health (MCH) (28 studies), tuberculosis (TB) (16 studies), family planning (16 studies), primary healthcare (14 studies), and sexual and reproductive health (SRH) or sexually transmitted infection (STI) services (13 studies). The most common intervention was integration of HIV testing and counselling into non-HIV services (46 studies), followed by integration of additional services into ART (28 studies) and integration of ART into non-HIV programmes (21 studies). Fifty studies focused on the general population, 40 studies on women or children, and 22 studies on at least 1 of the key populations in the HIV response. Study durations differed widely (S2 Fig). While the median study duration was 2 years, 28 studies were 3 years or longer, 8 studies were 5 years or longer, and the maximum duration among all studies was 8 years. S1 Table shows the distributions of study characteristics across all studies, S2 Table shows the study characteristics for each individual study, and S3 Table shows the GRADE assessment. Table 1 shows a summary of key findings.
Fig 1. Geographical map of the included empirical studies by type of integration.
Bubble colours represent the health service integration area. Bubble sizes represent the study population size. Coordinates are dispersed up to 250 kilometres to prevent overlap of data points from similar or nearby locations. Abbreviations: HPV, human papillomavirus; STI, sexually transmitted infection. Map created using ArcGIS software by ESRI. Base map source: https://www.naturalearthdata.com/downloads/10m-cultural-vectors/10m-admin-0-countries/.
Table 1. Summary of key findings.
| Outcome | Relationship to global policy goals | Total number of studies (number of experimental studies) | Total study population size | Number of studies (number of experimental studies) | I2 (meta-analysis) | Pooled RR (95% CI) or mean outcome (range) based on all studies | Pooled RR (95% CI) based on experimental studies only | ||
|---|---|---|---|---|---|---|---|---|---|
| Unfavourable outcome | No difference | Favourable outcome | |||||||
| Uptake of HIV services | First 95 of the 95-95-95 targets | 37 (11) | 637,148 | 1 (0) | 1 (0) | 34 (11) | 99.0% | 1.67 (1.41–1.99), p < 0.001 | 1.42 (1.28–1.58), p < 0.001 |
| HIV testing yield | First 95 of the 95-95-95 targets | 10 (1) | 461,486 | 3 (0) | 2 (1) | 5 (0) | 97.7% | 0.68 (0.38–1.24), p = 0.185 | 0.91 (0.61–1.36), p = 0.652 |
| ART initiation | Second 95 of the 95-95-95 targets | 19 (6) | 271,689 | 2 (1) | 1 (0) | 16 (5) | 99.8% | 1.42 (1.16–1.75), p = 0.002 | 1.50 (0.97–2.33), p = 0.064 |
| Time until ART initiation | Second 95 of the 95-95-95 targets | 5 (1) | 3,052 | 0 (0) | 0 (0) | 5 (1) | 93.1% | 0.45 (0.20–1.00), p = 0.050 | 0.13 (0.05–0.29), p < 0.001 |
| Retention in care | Third 95 of the 95-95-95 targets | 19 (7) | 66,151 | 3 (1) | 4 (2) | 11 (4) | 93.4% | 1.68 (1.05–2.69), p = 0.031 | 1.46 (0.67–3.17), p = 0.282 |
| ART adherence | Third 95 of the 95-95-95 targets | 7 (3) | 52,140 | 1 (0) | 2 (2) | 4 (1) | 98.8% | 1.13 (0.95–1.34), p = 0.146 | 1.06 (0.91–1.23), p = 0.245 |
| Viral suppression | Third 95 of the 95-95-95 targets | 9 (6) | 24,615 | 0 (0) | 2 (1) | 6 (5) | 46.1% | 1.19 (1.03–1.37), p = 0.025 | 1.23 (1.00–1.51), p = 0.054 |
| HIV-free survival among infants | ‘Zero new infections’ of the ‘getting to zero’ strategy | 5 (2) | 242,196 | 0 (0) | 3 (1) | 2 (1) | 99.5% | 1.04 (0.98–1.11), p = 0.135 | 1.11 (1.03–1.20), p = 0.033 |
| HIV infections averted | ‘Zero new infections’ of the ‘getting to zero’ strategy | 4 (3) | 2,181 | 0 (0) | 1 (1) | 3 (3) | N/A1 | 1.16 infections averted per 100-person-years (range 0.0–3.6) | N/A |
| AIDS-related mortality | ‘Zero AIDS-related deaths’ of the ‘getting to zero’ strategy | 8 (4) | 39,630 | 2 (1) | 3 (1) | 3 (2) | 97.8% | 0.72 (0.47–1.11), p = 0.118 | 0.99 (0.66–1.51), p = 0.985 |
| Uptake of other health services | Non-HIV-related outcomes | 32 (11) | 278,042 | 2 (0) | 4 (2) | 24 (8) | 98.4% | 2.42 (1.59–3.66), p < 0.001 | 2.03 (1.31–3.15), p = 0.005 |
| Treatment success for other diseases/conditions | Non-HIV-related outcomes | 21 (5) | 40,452 | 0 (0) | 8 (3) | 11 (2) | 81.1% | 1.56 (1.11–2.20), p = 0.014 | 1.64 (0.75–3.58), p = 0.156 |
| Non-AIDS-related mortality | Non-HIV-related outcomes | 6 (2) | 25,879 | 1 (1) | 1 (1) | 4 (0) | 94.7% | 0.43 (0.16–1.17), p = 0.083 | 1.00 (0.01–1.62), p = 0.997 |
| HIV-only costs | Costs and cost-effectiveness | 6 (1) | 119,830 | 2 (0) | 0 (0) | 4 (1) | N/A1 | 1.06 (range 0.59–1.92)1 | N/A |
| Non-HIV costs | Costs and cost-effectiveness | 2 (0) | 202 | 0 (0) | 0 (0) | 2 (0) | N/A1 | 0.62 (range 0.50–0.73)1 | N/A |
| HIV and non-HIV costs | Costs and cost-effectiveness | 2 (1) | 8,027 | 0 (0) | 0 (0) | 2 (1) | N/A1 | 0.83 (range 0.69–0.97)1 | N/A |
| Costs of integrated services versus HIV-only costs | Costs and cost-effectiveness | 7 (1) | 132,306 | N/A2 | N/A2 | N/A2 | N/A1 | 2.31 (range 1.20–6.12)1 | N/A |
| Cost-effectiveness | Costs and cost-effectiveness | 6 (2) | 142,881 | 0 (0) | 1 (0) | 5 (2) | N/A1 | N/A | N/A |
Colours indicate the outcome groups: UNAIDS 95-95-95 targets, purple; UNAIDS ‘getting to zero’ targets, blue; non-HIV-related outcomes, green; and costs and cost-effectiveness, orange.
1Meta-analysis not possible because of insufficient poolable data.
2Costs of both HIV and non-HIV services as compared to the costs of HIV services only; it was thus impossible to judge whether integration increased or reduced costs.
Abbreviations: ART, antiretroviral therapy; N/A, not applicable; RR, risk ratio.
HIV cascade of care
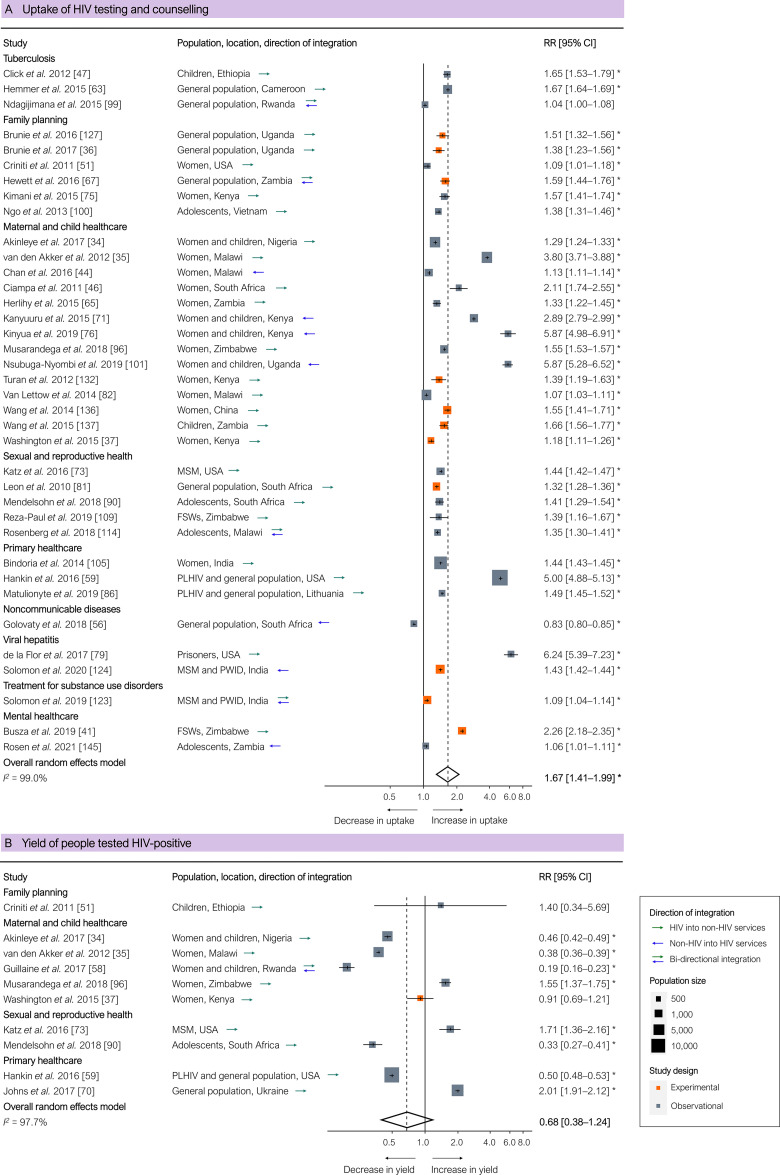
HIV testing and counselling uptake was significantly higher for integrated health services based on all 37 studies (pooled RR 1.67 [95% CI 1.41–1.99], p < 0.001; Fig 2A), including in all 11 experimental studies (pooled RR 1.42 [95% CI 1.28–1.58], p < 0.001). One study—evaluating integration of HIV, hypertension, and diabetes screening in rural South Africa—reported lower uptake of HIV testing, which the authors attributed to a lack of human resources and inadequate staff training [56]. Integration yielded a higher percentage of HIV-positive people in 4 out of 10 studies and a lower percentage in the remaining studies (Fig 2B).
Fig 2. Results of integration of HIV services: Uptake of HIV testing and counselling and yield of people testing HIV-positive.
(A) Uptake of HIV testing and counselling. (B) Yield of people testing HIV-positive. Outcomes are related to the ‘first 95’ of the 95-95-95 target for the HIV cascade of care. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. Abbreviations: CI, confidence interval; FSWs, female sex workers; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; RR, risk ratio.
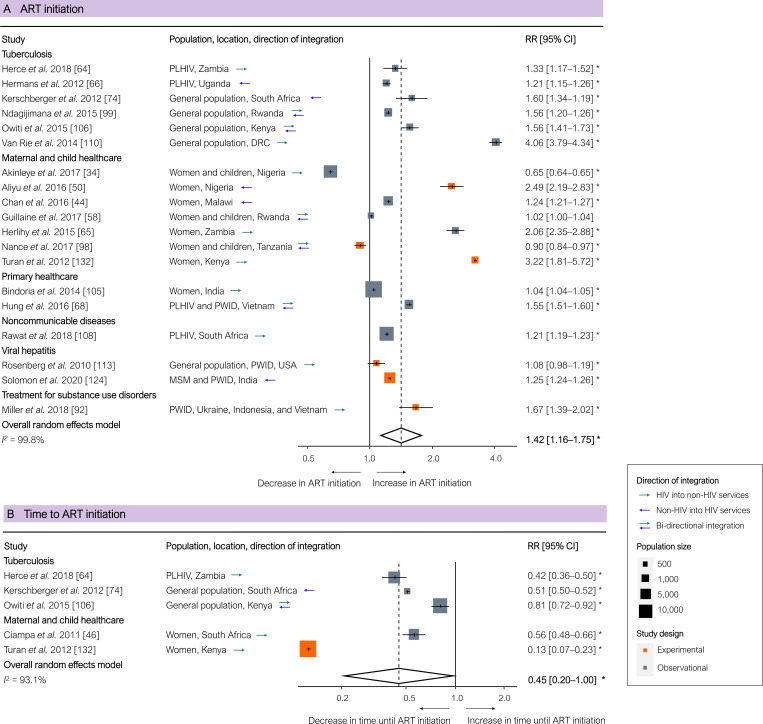
ART initiation was significantly higher in integrated programmes based on all 19 studies (pooled RR 1.42 [95% CI 1.16–1.75], p = 0.002; Fig 3A), but non-significantly higher based on the 6 experimental studies only (pooled RR 1.50 [95% CI 0.97–2.33], p = 0.064). Two studies that examined integration of HIV testing and counselling with MCH services reported lower initiation rates of ART and decreased prevention of mother-to-child transmission, which the authors attributed to poor linkage to HIV services beyond testing and counselling [34,98]. People initiated ART significantly faster in integrated programmes, measured in 5 studies, all of which reported on integration with TB or MCH services (pooled RR 0.45 [95% CI 0.20–1.00], p = 0.050; Fig 3B).
Fig 3. Results of integration of HIV services: ART initiation and time until ART initiation.
(A) ART initiation. (B) Time until ART initiation. Outcomes are related to the ‘second 95’ of the 95-95-95 HIV cascade of care. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; DRC, Democratic Republic of the Congo; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; RR, risk ratio.
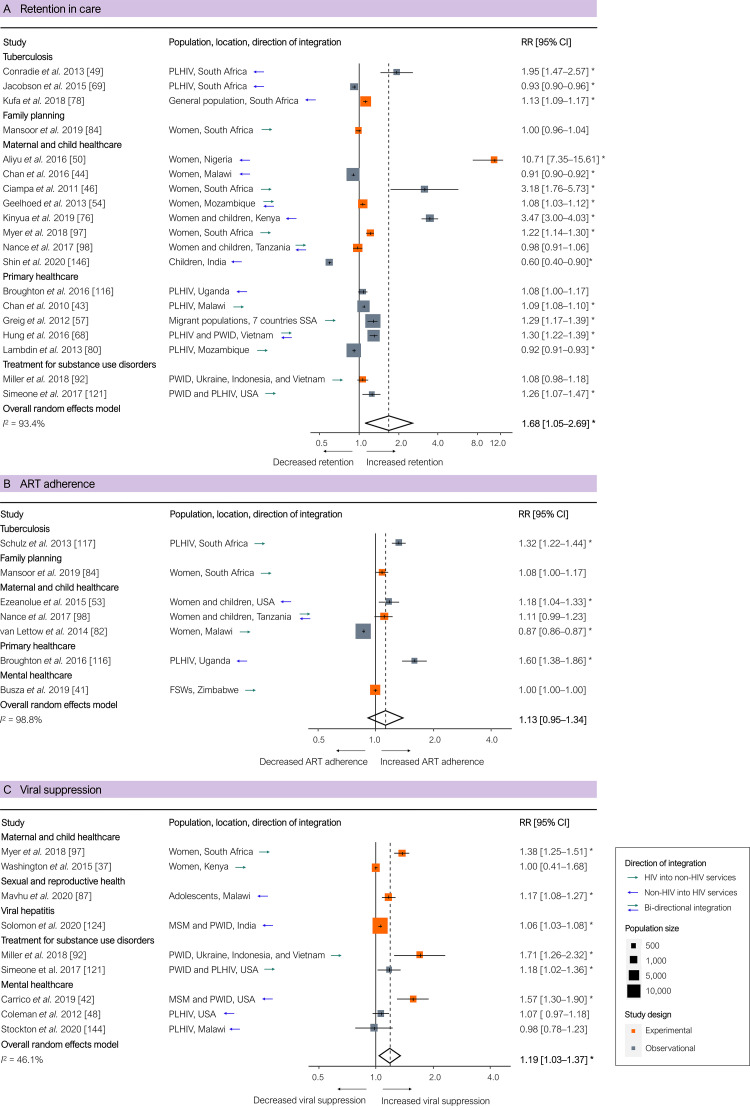
Retention in care was significantly higher in integrated programmes based on all 19 studies (pooled RR 1.68 [95% CI 1.05–2.69], p = 0.031; Fig 4A), but non-significantly higher based on the 7 experimental studies only (pooled RR 1.46 [95% CI 0.67–3.17], p = 0.282). ART adherence, was non-significantly higher in integrated programmes based on all studies (pooled RR 1.13 [95% CI 0.95–1.34], p = 0.146; Fig 4B) and based on the 3 experimental studies only (pooled RR 1.06 [95% CI 0.91–1.23], p = 0.245). One study reported lower retention and adherence in integrated antenatal care and HIV services, which the researchers attributed to non-retention when women who initiated ART in antenatal care eventually had to switch from ART in antenatal care to ART in stand-alone HIV services [82]. Viral suppression, measured in 9 studies, was significantly higher in integrated programmes based on all 9 studies (pooled RR 1.19 [95% CI 1.03–1.37], p = 0.025; Fig 4C), and borderline significantly higher based on the 6 experimental studies only (pooled RR 1.23 [95% CI 1.00–1.51], p = 0.054). One study reported no significant difference in viral suppression between integrated and separate services, which the researchers attributed to the scarcity of staff to provide HIV services and insufficient adherence support in the integrated programmes [37].
Fig 4. Results of integration of HIV services for PLHIV: Retention in care, ART adherence, and viral suppression of those on ART.
(A) Retention in care. (B) ART adherence. (C) Viral suppression of those on ART. Outcomes are related to the ‘third 95’ of the 95-95-95 targets for the HIV cascade of care. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; FSWs, female sex workers; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; RR, risk ratio; SSA, sub-Saharan Africa.
New HIV infections, HIV-related mortality, and stigma
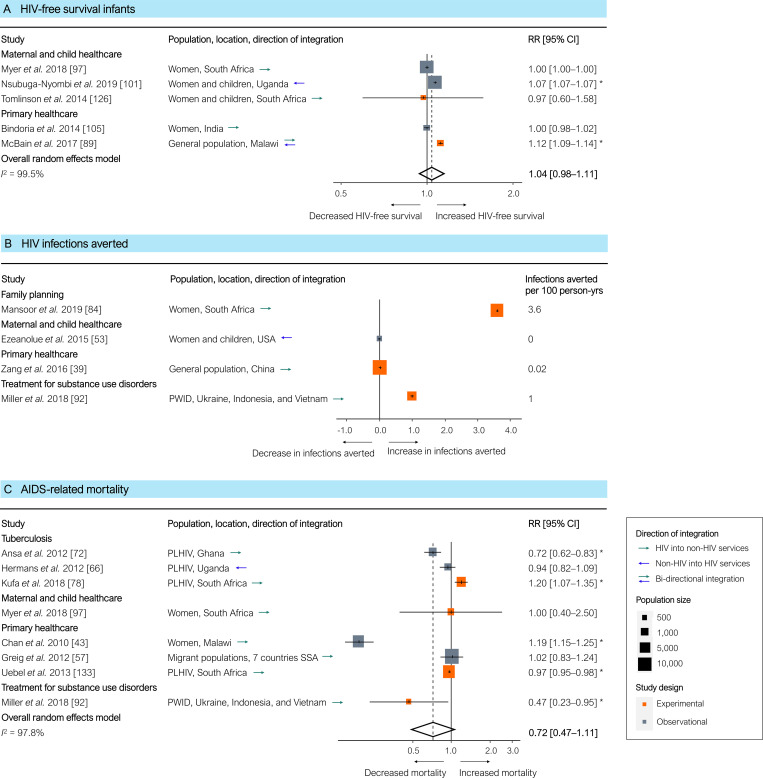
HIV-free survival among infants was only modestly and non-significantly higher in integrated programmes based on all 5 studies (pooled RR 1.04 [95% CI 0.98–1.11], p = 0.135; Fig 5A), but it was significantly higher based on the 2 experimental studies only (pooled RR 1.11 [95% CI 1.03–1.20], p = 0.033). Reported explanations for the moderate improvements in HIV-free survival outcomes were a low overall prevalence of mother-to-child transmission of HIV [97,105], national scale-up of ART for prevention of mother-to-child transmission [126], and other events promoting HIV testing and treatment initiation during the intervention period [105]. The number of HIV infections averted in integrated compared to separate services was similar in 2 studies and higher in 2 other studies (Fig 5B). AIDS-related mortality, measured in 8 studies, was non-significantly lower in integrated programmes (pooled RR 0.72 [95% CI 0.47–1.11], p = 0.118; Fig 5C). The increased mortality in integrated services in 1 study was attributed to weak implementation of integration and lack of coordination of integrated service delivery, affecting overall quality of care [78]. One study provided quantitative comparative outcomes related to the third UNAIDS ‘getting to zero’ target of ‘zero discrimination’ [3]. In this study, the integrated delivery of clinical HIV services and psychosocial interventions for adolescents in Zambia was significantly associated with reduced stigma (adjusted prevalence rate ratio 0.49 [95% CI 0.28–0.88]) and reduced negative community attitudes towards HIV (adjusted prevalence rate ratio 0.77 [95% CI 0.62–0.96]) [145].
Fig 5. Results of HIV service integration: HIV-free survival among infants, HIV infections averted, and AIDS-related mortality.
(A) HIV-free survival among infants. (B) HIV infections averted. (C) AIDS-related mortality. Outcomes are related to the ‘getting to zero’ targets for HIV/AIDS. Each estimate in (A) and (C) indicates the effect size as derived from a single study, either directly or by recalculating reported outcomes. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. Infections averted (B) are shown in 100 person-years. Abbreviations: CI, confidence interval; PLHIV, people living with HIV; PWID, people who inject drugs; RR, risk ratio; SSA, sub-Saharan Africa.
Non-HIV-related outcomes
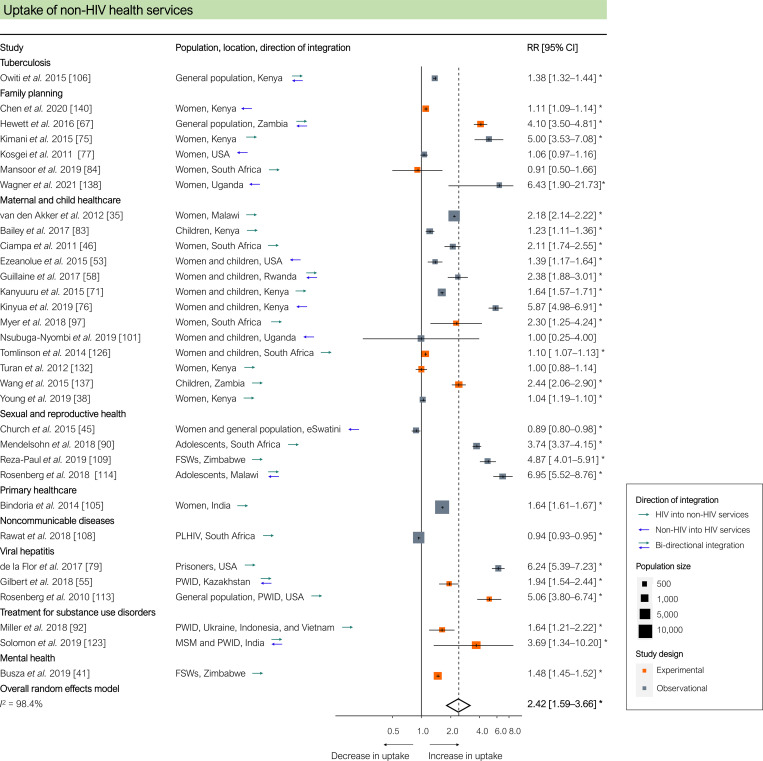
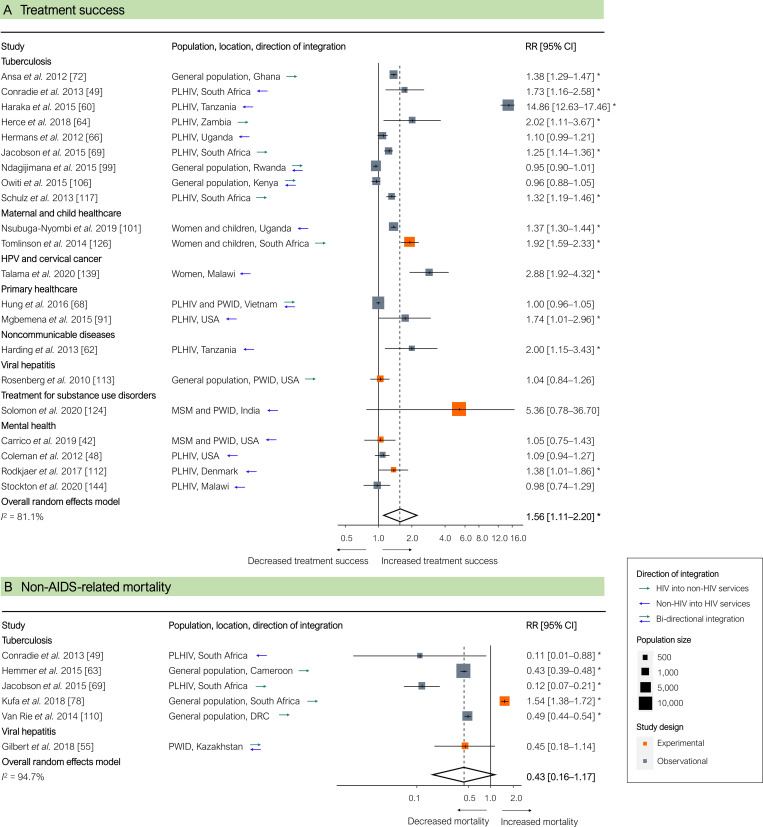
Uptake of other health services, measured in 32 studies, was significantly higher in integrated programmes (pooled RR 2.42 [95% CI 1.59–3.66], p < 0.001; Fig 6). In 2 studies, uptake of non-HIV services was significantly lower in integrated services, which the authors attributed to high HIV client loads, insufficient staff training to provide broader service packages, and insufficient human resource capacity to provide additional services [45,108]. Treatment success for diseases or conditions other than HIV, measured in 21 studies, was significantly higher in integrated programmes (pooled RR 1.56 [95% CI 1.11–2.20], p = 0.014; Fig 7A). Mortality from causes other than HIV (either TB or viral hepatitis), measured in 6 studies, was non-significantly lower in integrated programmes (pooled RR 0.43 [95% CI 0.16–1.17], p = 0.083; Fig 7B).
Fig 6. Results of HIV service integration: Uptake of non-HIV health services.
Each estimate indicates the effect size as derived from a single study, either directly or through recalculating reported outcomes. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. Abbreviations: CI, confidence interval; FSWs, female sex workers; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; RR, risk ratio.
Fig 7. Results of HIV service integration: Treatment success for non-HIV-related diseases and conditions and non-AIDS-related mortality.
(A) Treatment success for non-HIV related diseases and conditions. (B) Non-AIDS-related mortality. Each estimate indicates the size of the relationship between integration exposure and outcome. We measured these relationships as RRs; asterisks indicate statistically significant results. The diamond at the bottom of each panel shows the overall random-effects meta-analytical estimate. CI, confidence interval; DRC, Democratic Republic of the Congo; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; HPV, human papillomavirus; RR, risk ratio; SSA, sub-Saharan Africa.
Costs and cost-effectiveness
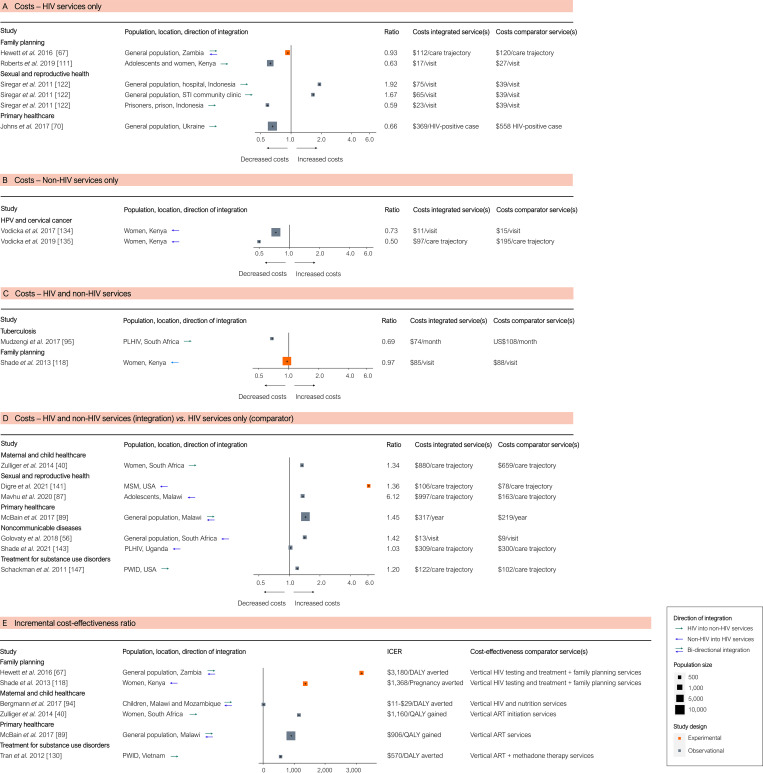
We found that costs of basic HIV and non-HIV services, measured in 10 studies, tended to be lower in integrated programmes (Fig 8). The ICERs of integrated services were universally positive, showing a wide range, from approximately US$10 to over US$3,000 per disability-adjusted life year averted or quality-adjusted life year gained (Fig 8E).
Fig 8. Results of integration of HIV services and economic outcomes: Costs and cost-effectiveness.
(A–D) Costs of (A) HIV services only, (B) non-HIV services only, (C) HIV and non-HIV services combined, and (D) integrated non-HIV and HIV services compared to HIV services only. (E) Cost-effectiveness as ICERs. Each cost estimate indicates the effect size derived from a single study, either directly or through recalculating reported outcomes. The estimates represent the costs of services in integrated compared to separate services. The ICERs measure the cost-effectiveness of integration, compared to the cost-effectiveness of stand-alone HIV service delivery as reported in the studies. Abbreviations: ART, antiretroviral therapy; DALY, disability-adjusted life year; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratio; MSM, men who have sex with men; PLHIV, people living with HIV; PWID, people who inject drugs; QALY, quality-adjusted life year; STI, sexually transmitted infection.
Discussion
Our systematic review of integration of HIV services and other health services identified 114 relevant empirical peer-reviewed studies. Generally, outcomes were better in integrated compared to separate services: The meta-analytical averages for HIV testing and counselling, ART initiation, retention in HIV care, and viral suppression rates were all higher in integrated services. Similarly, uptake of non-HIV health services and non-HIV health outcomes were on average better in integrated services.
To our knowledge, this is the first systematic review and meta-analysis that quantifies the global evidence on integration of HIV services and other health services for a broad scope of health systems and health outcomes. Our systematic review was comprehensive in 4 important aspects. First, we included both directions of integration: HIV services integrated into services for other diseases or conditions, and services for diseases other than HIV—such as depression or hypertension screening and treatment—integrated into HIV services [17,26]. Second, we designed our search algorithm such that it identified all primary studies on the integration of HIV services with any other types of services. Third, we included a broad scope of outcomes in our review: HIV care cascade outcomes (testing, linkage to care, treatment initiation, treatment adherence, retention, and viral suppression), HIV health outcomes (new infections and mortality), non-HIV health outcomes, and costs and cost-effectiveness. Fourth, we included all study types as long as the study included a comparator of some sort for effect or impact estimation, i.e., we included experimental and observational quasi- and non-experimental studies in our review. Our results are broadly in line with earlier findings from systematic and scoping reviews on integration of services with narrower scopes of integration interventions or outcomes. Previous reviews on the integration of HIV and TB [148,149], MCH [22,150], family planning [151–153], SRH [154], primary healthcare [155], noncommunicable disease [23,151], and mental health [156] services generally found that integration led to improved service uptake and better health outcomes, but that high-quality evidence was limited [148,151–153,157–159]. Our synthesis also broadly concurs with the previous literature indicating that service integration evidence is particularly scarce for key populations [154], cost-effectiveness outcomes [156], and long-term impacts [19,20,151,156,160–162].
Our results should be useful for policy makers, practitioners, and researchers concerned with health system structures and processes supporting HIV services over the coming decades. Whether or not to integrate services and how to design integrated delivery models will likely depend on a wide range of factors, including the existing health system, resources, and HIV and other disease burdens. Although integration was generally successful, several specific integration interventions failed to improve outcomes. We identified several reasons why integration interventions were not successful. Integration failed to improve outcomes when it resulted in increased flows of more diverse patients in already overburdened health systems [45,108]. When integration remained limited to individual stages in the care cascade (for instance, when integration focused only on adding HIV testing or ART initiation) health outcomes did not improve in some studies, because of losses in other stages of the care cascade (such as retention and adherence) [34,82,98]. Other major reasons for integration failures were (i) imperfect fidelity of integration implementation (e.g., because of limited availability, training, or coordination of health workers) and (ii) low quality of care [45,56,78,82,108]. Overall, the studies identified in our review rarely reported on implementation fidelity, and if they did, fidelity was typically not quantified [45,78,82,98,108]. Future research should aim to capture both the fidelity of integration interventions, as well as meaningful and necessary local adaptations.
Quality of care is a critical element of studies of health service integration—it is plausible that integration either increases quality of care (because integration ensures that individual patients receive services that are well coordinated across multiple healthcare needs) or decreases quality of care (because integration decreases the specialization of service delivery structures and processes, leading to lower average quality of care for individual services). A key indicator of clinical quality of HIV care, viral suppression [163–165], consistently improved across different integration interventions covered in our systematic review, but with widely varying effect sizes. Data on other important aspects of quality of care were not available in the studies we identified in our systematic review. In particular, the studies covered did not provide estimates of the relationships between integration interventions and subjective perceptions of quality of care, the patient experience, and patient satisfaction. Such measures of peoples’ and patients’ subjective evaluations of health services are important—fundamentally, because they operationalize the health system outcomes of ‘patient satisfaction’ [166] and ‘responsiveness’ [167] and, instrumentally, because subjective evaluations influence health service uptake, retention, and adherence [168]. Future studies of integration of HIV services and other health services should include subjective measures and more detailed objective measures of quality of care. One promising approach is patient-reported outcome and experience measures (PROMs and PREMs) [169], and in particular PROM and PREM elicitation via digital devices.
Another important objective on the global HIV agenda is ‘zero discrimination’ [3]: We explicitly searched for outcomes of integration interventions related to discrimination and stigma, but found only 1 article that provided quantitative evidence [145]. However, emerging evidence suggests that interventions explicitly aimed at achieving ‘zero discrimination’ may produce greater population health benefits in jurisdictions with highly unequal access to care [170,171]. Such evidence will be important because integration could plausibly reduce discrimination by ‘normalising’ HIV services [172], but it may also cause discrimination, e.g., by reducing the privacy of vulnerable people [173,174]. Only 22 out of the 114 studies reported outcomes for key populations, demonstrating important knowledge gaps [159,175–181].
The current empirical evidence base on the impact of service integration on costs and cost-effectiveness does not provide insights into whether integration could indeed result in the hypothesized mitigation of the impact of declining resources for HIV. Our review showed that empirical evidence on the costs and cost-effectiveness of integration is limited and highly heterogeneous in terms of measured outcomes. For instance, some studies reported only HIV-related costs pre- and post-integration, while others reported only non-HIV costs. Furthermore, the complex trade-off between per-patient efficiency gains and overall programmatic costs requires careful attention when implementing integration strategies, yet these 2 components are rarely considered together. The existing empirical cost-effectiveness analyses [40,67,94] and modelling studies [182–191] show that, even if per-patient costs of service delivery go down, the increase in patient volume due to integration increases overall programme costs and resource needs. Finally, none of the studies assessed the overall healthcare quality impact of integration alongside changes in costs: Especially if resources are more stretched due to integration, per-patient costs may be lower, but so may be quality of care. Advancing our understanding of the economic impacts of integration will require more experimental or quasi-experimental studies that combine top-down macro-costing and bottom-up micro-costing approaches with quality and impact estimates of service delivery, e.g., through measuring health outcomes and conducting satisfaction surveys among patients and providers [192,193].
Our study had several limitations. First, as with any systematic review, the outcomes of our review are prone to publication bias, because studies reporting clearly positive findings may be more likely to be published than studies reporting null or negative findings. Second, the exclusion of case reports and ‘grey’ literature (e.g., project reports, conference abstracts) may have limited the range of evidence, especially for novel integration models. For example, our review excluded descriptions of HIV service integration into primary healthcare in Latin America [194], provision of hormone therapy alongside HIV services for transgender people [173], and HPV vaccination programmes integrated into HIV prevention and testing at secondary schools. Third, the experimental and observational primary studies that we synthesize in our meta-analysis may be biased. Findings from experimental studies provide the highest quality comparison, but may not be generalisable outside research settings. Evidence from quasi-experimental studies could remedy this situation by providing evidence with both high internal and high external validity [195,196]. Although some studies had relatively long study durations (4 years or more), none of them specifically reported on sustainment of the effects of service integration. Despite these limitations, for many outcomes our synthesis is encouraging, because the magnitudes of effects were large and consistent across the different study types.
In conclusion, our results support the integration of HIV services with other health services. The evidence indicates that integration of HIV testing and counselling services into non-HIV health programmes for people at risk of acquiring HIV, and integration of non-HIV services into ART programmes for PLHIV, tends to lead to improved service uptake and health benefits for HIV and other diseases or conditions. However, the effects of integrating HIV services into broader health systems and the economic impacts of integration are less clear and require further study. In addition, integration success will depend on a wide range of determinants and path dependencies, such as local epidemics and health system structures and processes. Despite the need for more studies on specific integration opportunities and key populations, it seems likely that integration of HIV and other health services can contribute to reaching the UNAIDS target of ending AIDS by 2030, while simultaneously meeting other universal healthcare targets.
Supporting information
(PDF)
(PDF)
Figure shows the duration of studies on the x-axis (by bins, each indicating minimum up to and including the maximum duration) and the number of studies per bin on the y-axis.
(PDF)
(PDF)
(PDF)
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; CHWs, community health workers; EIMC, early infant male circumcision; HCV, hepatitis C virus; MCH, maternal and child health; MSM, men who have sex with men; NCDs, noncommunicable diseases; PMTCT, prevention of mother-to-child transmission; PNC, postnatal care; PWID, people who inject drugs; PrEP, pre-exposure prophylaxis; SRH, sexual and reproductive health; STI, sexually transmitted infection; TB, tuberculosis; VMMC, voluntary medical male circumcision.
(PDF)
(PDF)
Acknowledgments
All participants of the UNAIDS Expert Group on Integration to the ‘target-setting for 2025 and resource needs and impact estimation for 2020–2030’ exercise were part of this work: George Ayala, MPact Global Action for Gay Men’s Health and Rights, US; Adele S. Benzaken, AIDS Health Care Foundation, Brazil; Georgina Caswell, Global Network of People Living with HIV, South Africa; Mandisa Dukashe, HIV Survivors and Partners Network, South Africa; Cleiton Euzebio de Lima, UNAIDS, Brazil; Christopher Fontaine, UNAIDS, Switzerland; Marcelo Freitas, Ministry of Health, Brazil; Jed Friedman, World Bank, US; Peter Ghys, UNAIDS, Switzerland; Peter Godfrey-Faussett, UNAIDS, Switzerland; Marelize Gorgens, World Bank, US; Mauro Guarinieri, INPUD Secretariat, Switzerland; Shannon Hader, UNAIDS, Switzerland; Jose A. Izazola-Licea, UNAIDS, Switzerland; Saule Kassymova, Ministry of Health, Kazakhstan; Edward Kelley, World Health Organization, Switzerland; Jules Kim, Asia Pacific Network of Sex Workers, Australia; Margaret E. Kruk, Harvard T.H. Chan School of Public Health, US; Daniel Low-Beer, World Health Organization, Switzerland; Foster Mafiala, SRHR Africa Trust, Malawi; Marvin Manzanero, Ministry of Health, Belize; Ismail Mesbah, El Kettar Hospital, Algeria; David E. Munar, Howard Brown Health, US; Asiya Ismail A. Odugleh-Kolev, World Health Organization, Switzerland; Gerson Pereira, Ministry of Health of Brazil, Brazil; Asa Radix, Callen-Lorde Community Health Center, US; Jorge Saavedra, AHF México Wellness Center, Mexico; Tim Sladden, United Nations Population Fund, US; John Stover, Avenir Health, US; Nuria Toro, World Health Organization, Switzerland; Sergio Torres-Rueda, London School of Hygiene & Tropical Medicine, UK; Jenny Vaughan, STOPAIDS, UK. The authors want to thank Wichor Bramer from the Erasmus MC Medical Library for developing and updating the search strategies. The authors would also like to thank UNAIDS for organizing and hosting a 3-day expert group meeting in Rio de Janeiro, Brazil, 3–5 March 2020, to discuss the findings of this work.
Abbreviations
- ART
antiretroviral therapy
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ICER
incremental cost-effectiveness ratio
- MCH
maternal and child health
- PLHIV
people living with HIV
- RR
risk ratio
- SRH
sexual and reproductive health
- STI
sexually transmitted infection
- TB
tuberculosis
Data Availability
All relevant data are within the manuscript and its Supporting Information files. No primary data were obtained for this study.
Funding Statement
CAB, JACH, and TB received funding by the United Nations Joint Programme on HIV/AIDS (https://www.unaids.org/en). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.United Nations. Political declaration on HIV and AIDS: on the fast-track to accelerate the fight against HIV and to end the AIDS epidemic by 2030. New York: United Nations; 2016. doi: 10.1093/oxfordhb/9780199560103.003.0005 [DOI] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Fast-track—ending the AIDS epidemic by 2030. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS. UNAIDS 2011–2015 strategy: getting to zero. Geneva: Joint United Nations Programme on HIV/AIDS; 2013. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2034_UNAIDS_Strategy_en_1.pdf. [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS. Seizing the moment: tackling entrenched inequalities to end epidemics—global AIDS update 2020. Geneva: Joint United Nations Programme on HIV/AIDS; 2020. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_global-aids-report_en.pdf. [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS. UNAIDS data 2020. Geneva: Joint United Nations Programme on HIV/AIDS; 2020. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf. [Google Scholar]
- 7.Kaiser Family Foundation. U.K. considering temporary reduction in foreign aid spending, from 0.7% to 0.5% of GNI. San Francisco: Kaiser Family Foundation; 2020. [cited 2021 Jan 16]. Available from: https://www.kff.org/news-summary/u-k-considering-temporary-reduction-in-foreign-aid-spending-from-0-7-to-0-5-of-gni/. [Google Scholar]
- 8.US Department of State, Office of the US Global AIDS Coordinator and Health Diplomacy. The United States President’s emergency plan for AIDS relief: 2019 annual report to Congress. Washington (DC): US Department of State; 2019. [cited 2021 Oct 13]. Available from: https://www.state.gov/wp-content/uploads/2019/09/PEPFAR2019ARC.pdf. [Google Scholar]
- 9.Atun R, Chang AY, Ogbuoji O, Silva S, Resch S, Hontelez JAC, et al. Long-term financing needs for HIV control in sub-Saharan Africa in 2015–2050: a modelling study. BMJ Open. 2016;6:e009656. doi: 10.1136/bmjopen-2015-009656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hontelez JAC, Chang AY, Ogbuoji O, De Vlas SJ, Bärnighausen T, Atun R. Changing HIV treatment eligibility under health system constraints in sub-Saharan Africa: investment needs, population health gains, and cost-effectiveness. AIDS. 2016;30:2341–50. doi: 10.1097/QAD.0000000000001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulstra CA, Reddy CL, Atun R, Bärnighausen T, Hontelez JAC. Impact of the COVID-19-related global recession on the financing of the global HIV response. AIDS. 2021;35:1143–6. doi: 10.1097/QAD.0000000000002872 [DOI] [PubMed] [Google Scholar]
- 12.Bekker LG, Alleyne G, Baral S, Cepeda J, Daskalakis D, Dowdy D, et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society—Lancet Commission. Lancet. 2018;392:312–58. doi: 10.1016/S0140-6736(18)31070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–5. doi: 10.1126/science.1230413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hontelez JAC, Vlas SJ de, Baltussen R, Newell M-L, Bakker R, Tanser F, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS. 2012;26(Suppl 1):S19–30. doi: 10.1097/QAD.0b013e3283558526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan KMV, Miotti PG, Anand NP, Kline LM, Harmston C, Gulakowski R 3rd, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low-and middle-income country settings. J Acquir Immune Defic Syndr. 2014;67:S2–7. doi: 10.1097/QAI.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Integrated health services—what and why? Making health systems work series. Geneva: World Health Organization; 2008. [cited 2021 Oct 13]. Available from: https://www.who.int/healthsystems/technical_brief_final.pdf. [Google Scholar]
- 17.Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. Integration of targeted health interventions into health systems: a conceptual framework for analysis. Health Policy Plan. 2010;25:104–11. doi: 10.1093/heapol/czp055 [DOI] [PubMed] [Google Scholar]
- 18.Atun R, de Jongh TE, Secci FV, Ohiri K, Adeyi O, Car J. Integration of priority population, health and nutrition interventions into health systems: systematic review. BMC Public Health. 2011;11:780. doi: 10.1186/1471-2458-11-780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney S, Obure CD, Maier CB, Greener R, Dehne K, Vassall A. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect. 2012;88:85–99. doi: 10.1136/sextrans-2011-050199 [DOI] [PubMed] [Google Scholar]
- 20.Siapka M, Remme M, Obure CD, Maier CB, Dehne KL, Vassall A. Is there scope for cost savings and efficiency gains in HIV services? A systematic review of the evidence from low- and middle-income countries. Bull World Health Organ. 2014;92:499–511AD. doi: 10.2471/BLT.13.127639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope R, Kendall T, Langer A, Bärnighausen T. Health systems integration of sexual and reproductive health and HIV services in sub-Saharan Africa: a scoping study. J Acquir Immune Defic Syndr. 2014;67:S259–70. doi: 10.1097/QAI.0000000000000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health. 2018;23:136–48. doi: 10.1111/tmi.13014 [DOI] [PubMed] [Google Scholar]
- 23.Duffy M, Ojikutu B, Andrian S, Sohng E, Minior T, Hirschhorn LR. Non-communicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Health. 2017;22:926–37. doi: 10.1111/tmi.12901 [DOI] [PubMed] [Google Scholar]
- 24.Haldane V, Legido-Quigley H, Chuah FLH, Sigfrid L, Murphy G, Ong SE, et al. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care. 2018;30:103–15. doi: 10.1080/09540121.2017.1344350 [DOI] [PubMed] [Google Scholar]
- 25.Suthar AB, Rutherford GW, Horvath T, Doherty MC, Negussie EK. Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS. 2014;28:S175–85. doi: 10.1097/QAD.0000000000000259 [DOI] [PubMed] [Google Scholar]
- 26.Atun RA, Bennett S, Duran A. When do vertical (stand-alone) programmes have a place in health systems? Geneva: World Health Organization; 2008. [cited 2021 Oct 13]. Available from: https://www.who.int/management/district/services/WhenDoVerticalProgrammesPlaceHealthSystems.pdf. [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. doi: 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res. 2006;21:688–94. doi: 10.1093/her/cyl081 [DOI] [PubMed] [Google Scholar]
- 30.Atun R, de Jongh T, Secci F, Ohiri K, Adeyi O. A systematic review of the evidence on integration of targeted health interventions into health systems. Health Policy Plan. 2010;25:1–14. doi: 10.1093/heapol/czp053 [DOI] [PubMed] [Google Scholar]
- 31.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 32.Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040 [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [cited 2021 Oct 13]. Available from: https://www.r-project.org/. [Google Scholar]
- 34.Akinleye O, Dura G, de Wagt A, Davies A, Chamla D. Integration of HIV testing into maternal, newborn, and child health weeks for improved case finding and linkage to prevention of mother-to-child transmission services in Benue State, Nigeria. Front Public Health. 2017;5:71. doi: 10.3389/fpubh.2017.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Akker T, Bemelmans M, Ford N, Jemu M, Diggle E, Scheffer S, et al. HIV care need not hamper maternity care: a descriptive analysis of integration of services in rural Malawi. BJOG. 2012;119:431–8. doi: 10.1111/j.1471-0528.2011.03229.x [DOI] [PubMed] [Google Scholar]
- 36.Brunie A, Mucheri PNW, Akol A, Chen M, Mercer SJ, Petruney T. Integrating family planning and HIV services at the community level: formative assessment with village health teams in Uganda. Afr J Reprod Health. 2017;21:73–80. doi: 10.29063/ajrh2017/v21i2.9 [DOI] [PubMed] [Google Scholar]
- 37.Washington S, Owuor K, Turan JM, Steinfeld RL, Onono M, Shade SB, et al. Implementation and operational research: effect of integration of HIV care and treatment into antenatal care clinics on mother-to-child HIV transmission and maternal outcomes in Nyanza, Kenya: results from the SHAIP cluster randomized controlled trial. J Acquir Immune Defic Syndr. 2015;69:e164–71. doi: 10.1097/QAI.0000000000000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young N, Taetgmeyer M, Zulaika G, Aol G, Desai M, Ter Kuile F, et al. Integrating HIV, syphilis, malaria and anaemia point-of-care testing (POCT) for antenatal care at dispensaries in western Kenya: discrete-event simulation modelling of operational impact. BMC Public Health. 2019;19:1629. doi: 10.1186/s12889-019-7739-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zang X, Tang H, Min JE, Gu D, Montaner JSG, Wu Z, et al. Cost-effectiveness of the ‘One4All’ HIV linkage intervention in Guangxi Zhuang Autonomous Region, China. PLoS ONE. 2016;11:e0167308. doi: 10.1371/journal.pone.0167308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zulliger R, Black S, Holtgrave DR, Ciaranello AL, Bekker L-G, Myer L. Cost-effectiveness of a package of interventions for expedited antiretroviral therapy initiation during pregnancy in Cape Town, South Africa. AIDS Behav. 2014;18:697–705. doi: 10.1007/s10461-013-0641-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busza J, Chiyaka T, Musemburi S, Fearon E, Davey C, Chabata S, et al. Enhancing national prevention and treatment services for sex workers in Zimbabwe: a process evaluation of the SAPPH-IRe trial. Health Policy Plan. 2019;34:337–45. doi: 10.1093/heapol/czz037 [DOI] [PubMed] [Google Scholar]
- 42.Carrico AW, Neilands TB, Dilworth SE, Evans JL, Gόmez W, Jain JP, et al. Randomized controlled trial of a positive affect intervention to reduce HIV viral load among sexual minority men who use methamphetamine. J Int AIDS Soc. 2019;22:e25436. doi: 10.1002/jia2.25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan AK, Mateyu G, Jahn A, Schouten E, Arora P, Mlotha W, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Trop Med Int Health. 2010;15:90–7. doi: 10.1111/j.1365-3156.2010.02503.x [DOI] [PubMed] [Google Scholar]
- 44.Chan AK, Kanike E, Bedell R, Mayuni I, Manyera R, Mlotha W, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc. 2016;19:20672. doi: 10.7448/IAS.19.1.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Church K, Wringe A, Lewin S, Ploubidis GB, Fakudze P, Integra Initiative, et al. Exploring the feasibility of service integration in a low-income setting: a mixed methods investigation into different models of reproductive health and HIV care in Swaziland. PLoS ONE. 2015;10:e0126144. doi: 10.1371/journal.pone.0126144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–9. doi: 10.1097/QAI.0b013e31822149bf [DOI] [PubMed] [Google Scholar]
- 47.Click ES, Feleke B, Pevzner E, Fantu R, Gadisa T, Assefa D, et al. Evaluation of integrated registers for tuberculosis and HIV surveillance in children, Ethiopia, 2007–2009. Int J Tuberc Lung Dis. 2012;16:625–7. doi: 10.5588/ijtld.11.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coleman SM, Blashill AJ, Gandhi RT, Safren SA, Freudenreich O. Impact of integrated and measurement-based depression care: clinical experience in an HIV clinic. Psychosomatics. 2012;53:51–7. doi: 10.1016/j.psym.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 49.Conradie H, Khati P, Pharoah H, Adams S. Integrating tuberculosis/HIV treatment: an evaluation of the tuberculosis outcomes of patients co-infected with tuberculosis and HIV in the Breede Valley subdistrict. S Afr Fam Pract. 2013;55:478–9. doi: 10.1080/20786204.2013.10874399 [DOI] [Google Scholar]
- 50.Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3:e202–11. doi: 10.1016/S2352-3018(16)00018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Criniti SM, Aaron E, Hilley A, Wolf S. Integration of routine rapid HIV screening in an urban family planning clinic. J Midwifery Womens Health. 2011;56:395–9. doi: 10.1111/j.1542-2011.2011.00031.x [DOI] [PubMed] [Google Scholar]
- 52.Deo S, Topp S, Garcia A, Soldner M, Yagci Sokat K, Chipukuma J, et al. Modeling the impact of integrating HIV and outpatient health services on patient waiting times in an urban health clinic in Zambia. PLoS ONE. 2012;7:e35479. doi: 10.1371/journal.pone.0035479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezeanolue EE, Pharr JR, Hunt A, Patel D, Jackson D. Why are children still being infected with HIV? Impact of an integrated public health and clinical practice intervention on mother-to-child HIV transmission in Las Vegas, Nevada, 2007–2012. Ann Med Health Sci Res. 2015;5:253–9. doi: 10.4103/2141-9248.160189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geelhoed D, Lafort Y, Chissale E, Candrinho B, Degomme O. Integrated maternal and child health services in Mozambique: structural health system limitations overshadow its effect on follow-up of HIV-exposed infants. BMC Health Serv Res. 2013;13:207. doi: 10.1186/1472-6963-13-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert L, Hunt T, Primbetova S, Terlikbayeva A, Chang M, Wu E, et al. Reducing opioid overdose in Kazakhstan: a randomized controlled trial of a couple-based integrated HIV/HCV and overdose prevention intervention “Renaissance.” Int J Drug Policy. 2018;54:105–13. doi: 10.1016/j.drugpo.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golovaty I, Sharma M, Van Heerden A, Van Rooyen H, Baeten JM, Celum C, et al. Cost of integrating noncommunicable disease screening into home-based HIV testing and counseling in South Africa. J Acquir Immune Defic Syndr. 2018;78:522–6. doi: 10.1097/QAI.0000000000001713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greig J, O’Brien DP, Ford N, Spelman T, Sabapathy K, Shanks L. Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing antiretroviral treatment in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2012;59:e92–8. doi: 10.1097/QAI.0b013e31824206c7 [DOI] [PubMed] [Google Scholar]
- 58.Guillaine N, Mwizerwa W, Odhiambo J, Hedt-Gauthier BL, Hirschhorn LR, Mugwaneza P, et al. A novel combined mother-infant clinic to optimize post-partum maternal retention, service utilization, and linkage to services in HIV care in rural Rwanda. Int J MCH AIDS. 2017;6:36. doi: 10.21106/ijma.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hankin A, Freiman H, Copeland B, Travis N, Shah B. A comparison of parallel and integrated models for implementation of routine HIV screening in a large, urban emergency department. Public Health Rep. 2016;131:90–5. doi: 10.1177/00333549161310S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haraka F, Glass TR, Sikalengo G, Gamell A, Ntamatungiro A, Hatz C, et al. A bundle of services increased ascertainment of tuberculosis among HIV-infected individuals enrolled in a HIV cohort in rural sub-Saharan Africa. PLoS ONE. 2015;10:e0123275. doi: 10.1371/journal.pone.0123275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ameh S, Klipstein-Grobusch K, Musenge E, Kahn K, Tollman S, Gómez-olivé FX. Effectiveness of an integrated approach to HIV and hypertension care in rural South Africa: controlled interrupted time-series analysis. AIDS. 2017;75:472–9. doi: 10.1097/QAI.0000000000001437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harding R, Simms V, Alexander C, Collins K, Combo E, Memiah P, et al. Can palliative care integrated within HIV outpatient settings improve pain and symptom control in a low-income country? A prospective, longitudinal, controlled intervention evaluation. AIDS Care. 2013;25:795–804. doi: 10.1080/09540121.2012.736608 [DOI] [PubMed] [Google Scholar]
- 63.Hemmer CJ, Pohl JC, Noeske J, Kuaban C, Reisinger EC. Integration of HIV services into the National Tuberculosis Program of Cameroon: the experience of the Littoral Province. Asian Pac J Trop Dis. 2015;5:525–8. doi: 10.1016/s2222-1808(15)60829-5 [DOI] [Google Scholar]
- 64.Herce ME, Morse J, Luhanga D, Harris J, Smith HJ, Besa S, et al. Integrating HIV care and treatment into tuberculosis clinics in Lusaka, Zambia: results from a before-after quasi-experimental study. BMC Infect Dis. 2018;18:536. doi: 10.1186/s12879-018-3392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herlihy JM, Hamomba L, Bonawitz R, Goggin CE, Sambambi K, Mwale J, et al. Implementation and operational research: integration of PMTCT and antenatal services improves combination antiretroviral therapy uptake for HIV-positive pregnant women in southern Zambia: a prototype for Option B+? J Acquir Immune Defic Syndr. 2015;70:e123–29. doi: 10.1097/QAI.0000000000000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermans SM, Castelnuovo B, Katabira C, Mbidde P, Lange JM, Hoepelman AI, et al. Integration of HIV and TB services results in improved TB treatment outcomes and earlier prioritized ART initiation in a large urban HIV clinic in Uganda. J Acquir Immune Defic Syndr. 2012;60:e29–35. doi: 10.1097/QAI.0b013e318251aeb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hewett PC, Nalubamba M, Bozzani F, Digitale J, Vu L, Yam E, et al. Randomized evaluation and cost-effectiveness of HIV and sexual and reproductive health service referral and linkage models in Zambia. BMC Public Health. 2016;16:785. doi: 10.1186/s12889-016-3450-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hung V, Nguyen ST, Tieu VTT, Nguyen TTT, Duong TH, Lyss S, et al. Evaluation of the integrated clinic model for HIV/AIDS services in Ho Chi Minh City, Viet Nam, 2013–2014. Public Health Action. 2016;6:255–60. doi: 10.5588/pha.16.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobson KB, Moll AP, Friedland GH, Shenoi SV. Successful tuberculosis treatment outcomes among HIV/TB coinfected patients down-referred from a district hospital to primary health clinics in rural South Africa. PLoS ONE. 2015;10:e0127024. doi: 10.1371/journal.pone.0127024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johns B, Doroshenko O, Tarantino L, Cowley P. The cost-effectiveness of integrating HIV counseling and testing into primary health care in the Ukraine. AIDS Behav. 2017;21:655–64. doi: 10.1007/s10461-016-1554-z [DOI] [PubMed] [Google Scholar]
- 71.Kanyuuru L, Kabue M, Ashengo TA, Ruparelia C, Mokaya E, Malonza I. RED for PMTCT: an adaptation of immunization’s Reaching Every District approach increases coverage, access, and utilization of PMTCT care in Bondo District, Kenya. Int J Gynecol Obstet. 2015;130:S68–73. doi: 10.1016/j.ijgo.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 72.Ansa GA, Walley JD, Siddiqi K, Wei X. Assessing the impact of TB/HIV services integration on TB treatment outcomes and their relevance in TB/HIV monitoring in Ghana. Infect Dis Poverty. 2012;1:13. doi: 10.1186/2049-9957-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katz DA, Dombrowski JC, Kerani RP, Aubin MR, Kern DA, Health DD, et al. Integrating HIV testing as an outcome of STD partner services for men who have sex with men. AIDS Patient Care STDs. 2016;30:208–14. doi: 10.1089/apc.2016.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerschberger B, Hilderbrand K, Boulle AM, Coetzee D, Goemaere E, de Azevedo V, et al. The effect of complete integration of HIV and TB services on time to initiation of antiretroviral therapy: a before-after study. PLoS ONE. 2012;7:e46988. doi: 10.1371/journal.pone.0046988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kimani J, Warren CE, Abuya T, Ndwiga C, Mayhew S, Vassall A, et al. Use of HIV counseling and testing and family planning services among postpartum women in Kenya: a multicentre, non-randomised trial. BMC Womens Health. 2015;15:104. doi: 10.1186/s12905-015-0262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinyua K, Muange P, Makenzi B, Kimani C, Amoah AO. Applying quality improvement strategies to health services for HIV-affected mother-baby pairs in rural Kenya. J Int Assoc Provid AIDS Care. 2019;18:2325958219857977. doi: 10.1177/2325958219857977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kosgei RJ, Lubano KM, Shen C, Wools-Kaloustian KK, Musick BS, Siika AM, et al. Impact of integrated family planning and HIV care services on contraceptive use and pregnancy outcomes: a retrospective cohort study. J Acquir Immune Defic Syndr. 2011;58:e121. doi: 10.1097/QAI.0b013e318237ca80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kufa T, Fielding KL, Hippner P, Kielmann K, Vassall A, Churchyard GJ, et al. An intervention to optimise the delivery of integrated tuberculosis and HIV services at primary care clinics: results of the MERGE cluster randomised trial. Contemp Clin Trials. 2018;72:43–52. doi: 10.1016/j.cct.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 79.De La Flor C, Porsa E, Nijhawan AE. Opt-out HIV and hepatitis C testing at the Dallas county jail: uptake, prevalence, and demographic characteristics of testers. Public Health Action. 2017;132:617–21. doi: 10.1177/0033354917732755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lambdin BH, Micek MA, Sherr K, Gimbel S, Karagianis M, Lara J, et al. Integration of HIV care and treatment in primary health care centers and patient retention in central Mozambique: a retrospective cohort study. J Acquir Immune Defic Syndr. 2013;62:e146. doi: 10.1097/QAI.0b013e3182840d4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leon N, Naidoo P, Mathews C, Lewin S, Lombard C. The impact of provider-initiated (opt-out) HIV testing and counseling of patients with sexually transmitted infection in Cape Town, South Africa: a controlled trial. Implement Sci. 2010;5:8. doi: 10.1186/1748-5908-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17:18994. doi: 10.7448/IAS.17.1.18994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bailey RC, Adera F, Mackesy-Amiti ME, Adipo T, Nordstrom SK, Mehta SD, et al. Prospective comparison of two models of integrating early infant male circumcision with maternal child health services in Kenya: the Mtoto Msafi Mbili Study. PLoS ONE. 2017;12:e0184170. doi: 10.1371/journal.pone.0184170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansoor LE, Yende-Zuma N, Baxter C, Mngadi KT, Dawood H, Gengiah TN, et al. Integrated provision of topical pre-exposure prophylaxis in routine family planning services in South Africa: a non-inferiority randomized controlled trial. J Int AIDS Soc. 2019;22:e25381. doi: 10.1002/jia2.25381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantell JE, Cooper D, Exner TM, Moodley J, Hoffman S, Myer L, et al. Emtonjeni—a structural intervention to integrate sexual and reproductive health into public sector HIV Care in Cape Town, South Africa: results of a Phase II study. AIDS Behav. 2017;21:905–22. doi: 10.1007/s10461-016-1562-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matulionyte R, Žagminas K, Balčiunaite E, Matulyte E, Paulauskiene R, Bajoriūniene A, et al. Routine HIV testing program in the University Infectious Diseases Centre in Lithuania: a four-year analysis. BMC Infect Dis. 2019;19:21. doi: 10.1186/s12879-018-3661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mavhu W, Willis N, Mufuka J, Bernays S, Tshuma M, Mangenah C, et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. Lancet Glob Health. 2020;8:e264–75. doi: 10.1016/S2214-109X(19)30526-1 [DOI] [PubMed] [Google Scholar]
- 88.Mayhew SH, Sweeney S, Warren CE, Collumbien M, Ndwiga C, Mutemwa R, et al. Numbers, systems, people: how interactions influence integration. Insights from case studies of HIV and reproductive health services delivery in Kenya. Health Policy Plan. 2017;32:iv67–81. doi: 10.1093/heapol/czx097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McBain RK, Petersen E, Tophof N, Dunbar EL, Kalanga N, Nazimera L, et al. Impact and economic evaluation of a novel HIV service delivery model in rural Malawi. AIDS. 2017;31:99–106. doi: 10.1097/QAD.0000000000001578 [DOI] [PubMed] [Google Scholar]
- 90.Mendelsohn AS, Gill K, Marcus R, Robbertze D, Van de Venter C, Mendel E, et al. Sexual reproductive healthcare utilisation and HIV testing in an integrated adolescent youth centre clinic in Cape Town, South Africa. South Afr J HIV Med. 2018;19:826. doi: 10.4102/sajhivmed.v19i1.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mgbemena O, Westfall AO, Ritchie CS, Hicks J, Raper JL, Overton ET, et al. Preliminary outcomes of a pilot physical therapy program for HIV-infected patients with chronic pain. AIDS Care. 2015;27:244–7. doi: 10.1080/09540121.2014.940272 [DOI] [PubMed] [Google Scholar]
- 92.Miller WC, Hoffman IF, Hanscom BS, Ha TV, Dumchev K, Djoerban Z, et al. A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment (HPTN 074): a randomised, controlled phase 3 feasibility and efficacy study. Lancet. 2018;392:747–59. doi: 10.1016/S0140-6736(18)31487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Momplaisir F, Mounzer K, Long JA. Preventive cancer screening practices in HIV-positive patients. AIDS Care. 2014;26:87–94. doi: 10.1080/09540121.2013.802276 [DOI] [PubMed] [Google Scholar]
- 94.Bergmann JN, Legins K, Sint TT, Snidal S, Amor YB, McCord GC. Outcomes and cost-effectiveness of integrating HIV and nutrition service delivery: pilots in Malawi and Mozambique. AIDS Behav. 2017;21:703–11. doi: 10.1007/s10461-016-1400-3 [DOI] [PubMed] [Google Scholar]
- 95.Mudzengi D, Sweeney S, Hippner P, Kufa T, Fielding K, Grant AD, et al. The patient costs of care for those with TB and HIV: a cross-sectional study from South Africa. Health Policy Plan. 2017;32:48–56. doi: 10.1093/heapol/czw183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musarandega R, Mutede B, Mahomva A, Nyamayaro W, Mushavi A, Lindan C, et al. Scaling up pediatric HIV testing by incorporating provider-initiated HIV testing into all child health services in Hurungwe District, Zimbabwe. J Acquir Immune Defic Syndr. 2018;77:78–85. doi: 10.1097/QAI.0000000000001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N-Y, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15:e1002547. doi: 10.1371/journal.pmed.1002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nance N, Pendo P, Masanja J, Ngilangwa DP, Webb K, Noronha R, et al. Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: a cluster-randomized trial. PLoS ONE. 2017;12:e0181919. doi: 10.1371/journal.pone.0181919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ndagijimana A, Rugigana E, Uwizeye CB, Ntaganira J. One-stop TB-HIV services evaluation in Rwanda: comparison of the 2001–2005 and 2006–2010 cohorts. Public Health Action. 2015;5:209–13. doi: 10.5588/pha.15.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngo AD, Ha TH, Rule J, Dang CV. Peer-based education and the integration of HIV and sexual and reproductive health services for young people in Vietnam: evidence from a project evaluation. PLoS ONE. 2013;8:e80951. doi: 10.1371/journal.pone.0080951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nsubuga-Nyombi T, Karamagi E, Nabitaka L, Namukose S, Calnan J, Nyakwezi S, et al. Increasing HIV-free survival of infants: reorganizing care using quality improvement for the optimal health and nutrition of HIV-positive women and their exposed infants in Uganda. J Int Assoc Provid AIDS Care. 2019;18:2325958219857724. doi: 10.1177/2325958219857724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obure CD, Sweeney S, Darsamo V, Michaels-Igbokwe C, Guinness L, Terris-Prestholt F, et al. The costs of delivering integrated HIV and sexual reproductive health services in limited resource settings. PLoS ONE. 2015;10:e0124476. doi: 10.1371/journal.pone.0124476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Obure CD, Jacobs R, Guinness L, Mayhew S, Vassall A. Does integration of HIV and sexual and reproductive health services improve technical efficiency in Kenya and Swaziland? An application of a two-stage semi parametric approach incorporating quality measures. Soc Sci Med. 2016;151:147–56. doi: 10.1016/j.socscimed.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Obure CD, Guinness L, Sweeney S, Integra Initiative, Vassall A. Does integration of HIV and SRH services achieve economies of scale and scope in practice? A cost function analysis of the Integra Initiative. Sex Transm Infect. 2016;92:130–4. doi: 10.1136/sextrans-2015-052039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bindoria SV, Devkar R, Gupta I, Ranebennur V, Saggurti N, Ramesh S, et al. Development and pilot testing of HIV screening program integration within public/primary health centers providing antenatal care services in Maharashtra, India. BMC Res Notes. 2014;7:177. doi: 10.1186/1756-0500-7-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Owiti P, Zachariah R, Bissell K, Kumar AM, Diero L, Carter EJ, et al. Integrating tuberculosis and HIV services in rural Kenya: uptake and outcomes. Public Health Action. 2015;5:36–44. doi: 10.5588/pha.14.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palma AM, Rabkin M, Simelane S, Gachuhi AB, McNairy ML, Nuwagaba-Biribonwoha H, et al. A time-motion study of cardiovascular disease risk factor screening integrated into HIV clinic visits in Swaziland. J Int AIDS Soc. 2018;21:e25099. doi: 10.1002/jia2.25099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rawat A, Uebel K, Moore D, Yassi A. Integrated HIV-care into primary health care clinics and the influence on diabetes and hypertension care: an interrupted time series analysis in Free State, South Africa over 4 years. J Acquir Immune Defic Syndr. 2018;77:476–83. doi: 10.1097/QAI.0000000000001633 [DOI] [PubMed] [Google Scholar]
- 109.Reza-Paul S, Lazarus L, Maiya R, Venukumar KT, Lakshmi B, Roy A, et al. Delivering community-led integrated HIV and sexual and reproductive health services for sex workers: a mixed methods evaluation of the DIFFER study in Mysore, South India. PLoS ONE. 2019;14:e0218654. doi: 10.1371/journal.pone.0218654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Rie A, Patel MR, Nana M, Vanden Driessche K, Tabala M, Yotebieng M, et al. Integration and task shifting for TB/HIV care and treatment in highly resource-scarce settings: one size may not fit all. J Acquir Immune Defic Syndr. 2014;65:e110–7. doi: 10.1097/01.qai.0000434954.65620.f3 [DOI] [PubMed] [Google Scholar]
- 111.Roberts DA, Barnabas RV, Abuna F, Lagat H, Kinuthia J, Pintye J, et al. The role of costing in the introduction and scale-up of HIV pre-exposure prophylaxis: evidence from integrating PrEP into routine maternal and child health and family planning clinics in western Kenya. J Int AIDS Soc. 2019;22(Suppl 4):e25296. doi: 10.1002/jia2.25296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodkjaer LO, Laursen T, Seeberg K, Drouin M, Johansen H, Dyrehave C, et al. The effect of a mind-body intervention on mental health and coping self-efficacy in HIV-infected individuals: a feasibility study. J Altern Complement Med. 2017;23:326–30. doi: 10.1089/acm.2016.0251 [DOI] [PubMed] [Google Scholar]
- 113.Rosenberg SD, Goldberg RW, Dixon LB, Wolford GL, Slade EP, Himelhoch S, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61:885–91. doi: 10.1176/ps.2010.61.9.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenberg NE, Bhushan NL, Vansia D, Phanga T, Maseko B, Nthani T, et al. Comparing youth-friendly health services to the standard of care through “girl Power-Malawi”: a quasi-experimental cohort study. J Acquir Immune Defic Syndr. 2018;79:458–66. doi: 10.1097/QAI.0000000000001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rutaremwa G, Kabagenyi A. Utilization of integrated HIV and sexual and reproductive health services among women in Uganda. BMC Health Serv Res. 2016;16:494. doi: 10.1186/s12913-016-1761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Broughton EI, Muhire M, Karamagi E, Kisamba H. Cost-effectiveness of implementing the chronic care model for HIV care in Uganda. Int J Qual Health Care. 2016;28:802–7. doi: 10.1093/intqhc/mzw116 [DOI] [PubMed] [Google Scholar]
- 117.Schulz SA, Draper HR, Naidoo P. A comparative study of tuberculosis patients initiated on ART and receiving different models of TB-HIV care. Int J Tuberc Lung Dis. 2013;17:1558–63. doi: 10.5588/ijtld.13.0247 [DOI] [PubMed] [Google Scholar]
- 118.Shade SB, Kevany S, Onono M, Ochieng G, Steinfeld RL, Grossman D, et al. Cost, cost-efficiency and cost-effectiveness of integrated family planning and HIV services. AIDS. 2013;27:S87–92. doi: 10.1097/QAD.0000000000000038 [DOI] [PubMed] [Google Scholar]
- 119.Shenoi SV, Moll AP, Brooks RP, Kyriakides T, Andrews L, Kompala T, et al. Integrated tuberculosis/human immunodeficiency virus community-based case finding in rural South Africa: implications for tuberculosis control efforts. Open Forum Infect Dis. 2017;4: ofx092. doi: 10.1093/ofid/ofx092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siapka M, Obure CD, Mayhew SH, Sweeney S, Fenty J, Vassall A. Impact of integration of sexual and reproductive health services on consultation duration times: results from the Integra Initiative. Health Policy Plan. 2017;32:82–90. doi: 10.1093/heapol/czx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simeone C, Shapiro B, Lum PJ. Integrated HIV care is associated with improved engagement in treatment in an urban methadone clinic. Addict Sci Clin Pract. 2017;12:19. doi: 10.1186/s13722-017-0084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siregar AYM, Komarudin D, Wisaksana R, Van Crevel R, Baltussen R. Costs and outcomes of VCT delivery models in the context of scaling up services in Indonesia. Trop Med Int Health. 2011;16:193–9. doi: 10.1111/j.1365-3156.2010.02675.x [DOI] [PubMed] [Google Scholar]
- 123.Solomon SS, Solomon S, McFall AM, Srikrishnan AK, Anand S, Verma V, et al. Integrated HIV testing, prevention, and treatment intervention for key populations in India: a cluster-randomised trial. Lancet HIV. 2019;6:283–96. doi: 10.1016/S2352-3018(19)30034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solomon SS, Quinn TC, Solomon S, McFall AM, Srikrishnan AK, Verma V, et al. Integrating HCV testing with HIV programs improves hepatitis C outcomes in people who inject drugs: a cluster-randomized trial. J Hepatol. 2020;72:67–74. doi: 10.1016/j.jhep.2019.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sweeney S, Obure CD, Terris-Prestholt F, Darsamo V, Michaels-Igbokwe C, Muketo E, et al. The impact of HIV/SRH service integration on workload: analysis from the Integra Initiative in two African settings. Hum Resour Health. 2014;12:42. doi: 10.1186/1478-4491-12-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tomlinson M, Doherty T, Ijumba P, Jackson D, Lawn J, Persson LA, et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health. 2014;19:256–66. doi: 10.1111/tmi.12257 [DOI] [PubMed] [Google Scholar]
- 127.Brunie A, Wamala-Mucheri P, Akol A, Mercer S, Chen M. Expanding HIV testing and counselling into communities: feasibility, acceptability, and effects of an integrated family planning/HTC service delivery model by village health teams in Uganda. Health Policy Plan. 2016;31:1050–7. doi: 10.1093/heapol/czw035 [DOI] [PubMed] [Google Scholar]
- 128.Topp SM, Chipukuma JM, Giganti M, Mwango LK, Chiko LM, Tambatamba-Chapula B, et al. Strengthening health systems at facility-level: feasibility of integrating antiretroviral therapy into primary health care services in Lusaka, Zambia. PLoS ONE. 2010;5:e11522. doi: 10.1371/journal.pone.0011522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Topp SM, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, Reid SE. Integrating HIV treatment with primary care outpatient services: opportunities and challenges from a scaled-up model in Zambia. Health Policy Plan. 2013;28:347–57. doi: 10.1093/heapol/czs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tran BX, Ohinmaa A, Duong AT, Nguyen LT, Vu PX, Mills S, et al. Cost-effectiveness of integrating methadone maintenance and antiretroviral treatment for HIV-positive drug users in Vietnam’s injection-driven HIV epidemics. Drug Alcohol Depend. 2012;125:260–6. doi: 10.1016/j.drugalcdep.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 131.Tran BX, Ohinmaa A, Duong AT, Do NT, Nguyen LT, Nguyen QC, et al. Changes in drug use are associated with health-related quality of life improvements among methadone maintenance patients with HIV/AIDS. Qual Life Res. 2012;21:613–23. doi: 10.1007/s11136-011-9963-y [DOI] [PubMed] [Google Scholar]
- 132.Turan JM, Steinfeld RL, Onono M, Bukusi EA, Woods M, Shade SB, et al. The study of HIV and antenatal care integration in pregnancy in Kenya: design, methods, and baseline results of a cluster-randomized controlled trial. PLoS ONE. 2012;7:e44181. doi: 10.1371/journal.pone.0044181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uebel KE, Lombard C, Joubert G, Fairall LR, Bachmann MO, Mollentze WF, et al. Integration of HIV care into primary care in South Africa: effect on survival of patients needing antiretroviral treatment. J Acquir Immune Defic Syndr. 2013;63:94–100. doi: 10.1097/QAI.0b013e318291cd08 [DOI] [PubMed] [Google Scholar]
- 134.Vodicka EL, Babigumira JB, Mann MR, Kosgei RJ, Lee F, Mugo NR, et al. Costs of integrating cervical cancer screening at an HIV clinic in Kenya. Int J Gynaecol Obs. 2017;136:220–8. doi: 10.1002/ijgo.12025 [DOI] [PubMed] [Google Scholar]
- 135.Vodicka EL, Chung MH, Zimmermann MR, Kosgei RJ, Lee F, Mugo NR, et al. Estimating the costs of HIV clinic integrated versus non-integrated treatment of pre-cancerous cervical lesions and costs of cervical cancer treatment in Kenya. PLoS ONE. 2019;14:e0217331. doi: 10.1371/journal.pone.0217331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang AL, Qiao YP, Wang LH, Fang LW, Wang F, Jin X, et al. Integrated prevention of mother-to-child transmission for human immunodeficiency virus, syphilis and hepatitis B virus in China. Bull World Health Organ. 2014;93:52–56. doi: 10.2471/BLT.14.139626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang PC, Mwango A, Moberley S, Brockman BJ, Connor AL, Kalesha-Masumbu P, et al. A cluster randomised trial on the impact of integrating early infant HIV diagnosis with the expanded programme on immunization on immunization and HIV testing rates in rural health facilities in southern Zambia. PLoS ONE. 2015;10:e0141455. doi: 10.1371/journal.pone.0141455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wagner GJ, Wanyenze RK, Beyeza-Kashesya J, Gwokyalya V, Hurley E, Mindry D, et al. “Our Choice” improves use of safer conception methods among HIV serodiscordant couples in Uganda: a cluster randomized controlled trial evaluating two implementation approaches. Implement Sci. 2021;16:41. doi: 10.1186/s13012-021-01109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Talama GC, Shaw M, Maloya J, Chihana T, Nazimera L, Wroe EB, et al. Improving uptake of cervical cancer screening services for women living with HIV and attending chronic care services in rural Malawi. BMJ Open Qual. 2020;9:e000892. doi: 10.1136/bmjoq-2019-000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Y, Begnel E, Muthigani W, Achwoka D, Mcgrath CJ, Singa B, et al. Higher contraceptive uptake in HIV treatment centers offering integrated family planning services: a national survey in Kenya. Contraception. 2020;102:39–45. doi: 10.1016/j.contraception.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Digre P, Avoundjian T, Johnson K, Peyton D, Lewis C, Barnabas RV, et al. Barriers, facilitators, and cost of integrating HIV-related activities into sexually transmitted disease partner services in Jackson, Mississippi. Sex Transm Dis. 2021;48:145–51. doi: 10.1097/OLQ.0000000000001296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dovel K, Shaba F, Offorjebe OA, Balakasi K, Nyirenda M, Phiri K, et al. Effect of facility-based HIV self-testing on uptake of testing among outpatients in Malawi: a cluster-randomised trial. Lancet Glob Health. 2020;8:e276–87. doi: 10.1016/S2214-109X(19)30534-0 [DOI] [PubMed] [Google Scholar]
- 143.Shade SB, Osmand T, Kwarisiima D, Brown LB, Luo A, Mwebaza B, et al. Costs of integrating hypertension care into HIV care in rural East African clinics. AIDS. 2021;35:911–9. doi: 10.1097/QAD.0000000000002834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stockton MA, Udedi M, Kulisewa K, Hosseinipour MC, Gaynes BN, Mphonda SM, et al. The impact of an integrated depression and HIV treatment program on mental health and HIV care outcomes among people newly initiating antiretroviral therapy in Malawi. PLoS ONE. 2020;15:e0231872. doi: 10.1371/journal.pone.0231872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosen JG, Phiri L, Chibuye M, Namukonda ES, Mbizvo MT, Kayeyi N. Integrated psychosocial, economic strengthening, and clinical service-delivery to improve health and resilience of adolescents living with HIV and their caregivers: findings from a prospective cohort study in Zambia. PLoS ONE. 2021;16:e0243822. doi: 10.1371/journal.pone.0243822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shin SS, Satyanarayana VA, Ekstrand ML, Carpenter CL, Wang Q, Yadav K, et al. The effect of community-based nutritional interventions on children of women living with human immunodeficiency virus in rural India: a 2 × 2 factorial intervention trial. Clin Infect Dis. 2020;71:1539–46. doi: 10.1093/cid/ciz1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schackman BR, Leff JA, Botsko M, Fiellin DA, Altice FL, Korthuis PT, et al. The cost of integrated HIV care and buprenorphine/naloxone treatment: results of a cross-site evaluation. J Acquir Immune Defic Syndr (1999). 2011; 56(Suppl 1):S76–82. doi: 10.1097/QAI.0b013e31820a9a66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIV and tuberculosis services in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:855–67. doi: 10.1016/S1473-3099(11)70145-1 [DOI] [PubMed] [Google Scholar]
- 149.Hudson M, Rutherford GW, Weiser S, Fair E. Linking private, for-profit providers to public sector services for HIV and tuberculosis co-infected patients: a systematic review. PLoS ONE. 2018;13:e0194960. doi: 10.1371/journal.pone.0194960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Car LT, Brusamento S, Elmoniry H, van Velthoven MHMMT, Pape UJ, Welch V, et al. The uptake of integrated perinatal prevention of mother-to-child hiv transmission programs in low- and middle-income countries: a systematic review. PLoS ONE. 2013;8:e56550. doi: 10.1371/journal.pone.0056550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hyle EP, Naidoo K, Su AE, El-Sadr WM, Freedberg KA. HIV, tuberculosis, and noncommunicable diseases: What is known about the costs, effects, and cost-effectiveness of integrated care? J Acquir Immune Defic Syndr. 2014;67:S87–95. doi: 10.1097/QAI.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Narasimhan M, Yeh PT, Haberlen S, Warren CE, Kennedy CE. Integration of HIV testing services into family planning services: a systematic review. Reprod Health. 2019;16:61. doi: 10.1186/s12978-019-0714-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilcher R, Hoke T, Adamchak SE, Cates W Jr. Integration of family planning into HIV services: a synthesis of recent evidence. AIDS. 2013;27:65–75. doi: 10.1097/QAD.0000000000000051 [DOI] [PubMed] [Google Scholar]
- 154.Rinaldi G, Kiadaliri AA, Haghparast-Bidgoli H. Cost effectiveness of HIV and sexual reproductive health interventions targeting sex workers: a systematic review. Cost Eff Resour Alloc. 2018;16:63. doi: 10.1186/s12962-018-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dudley L, Garner P. Strategies for integrating primary health services in low- and middle-income countries at the point of delivery. Cochrane Database Syst Rev. 2011;6:CD003318. doi: 10.1002/14651858.CD003318.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chuah FLH, Haldane VE, Cervero-Liceras F, Ong SE, Sigfrid LA, Murphy G, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan. 2017;32:27–47. doi: 10.1093/heapol/czw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haberlen SA, Narasimhan M, Beres LK, Kennedy CE. Integration of family planning services into HIV care and treatment services: a systematic review. Stud Fam Plann. 2017;48:153–77. doi: 10.1111/sifp.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Jongh T, Gurol-Urganci I, Allen E, Zhu NJ, Atun R. Integration of antenatal care services with health programmes: systematic review. Int J Gynaecol Obstet. 2015;6:363–4. doi: 10.7189/jogh.06.010403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Viviano M, De Beaudrap P, Tebeu PM, Fouogue JT, Vassilakos P, Petignat P. A review of screening strategies for cervical cancer in human immunodeficiency virus-positive women in sub-Saharan Africa. Int J Womens Health. 2017;9:69–79. doi: 10.2147/IJWH.S103868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haldane V, Cervero‐Liceras F. Integrating HIV and substance use services: a systematic review. J Int AIDS Soc. 2017;20:21585. doi: 10.7448/IAS.20.1.21585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Juma K, Reid M, Roy M, Vorkoper S, Temu TM, Levitt NS, et al. From HIV prevention to non-communicable disease health promotion efforts in sub-Saharan Africa: a narrative review. AIDS. 2018;32:63–73. doi: 10.1097/QAD.0000000000001879 [DOI] [PubMed] [Google Scholar]
- 162.Joseph Davey D, Myer L, Bukusi E, Ramogola-Masire D, Kilembe W, Klausner JD. Integrating human immunodeficiency virus and reproductive, maternal and child, and tuberculosis health services within national health systems. Curr HIV/AIDS Rep. 2016;13:170–6. doi: 10.1007/s11904-016-0316-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Catumbela E, Certal V, Freitas A, Costa C, Sarmento A, da Costa Pereira A. Definition of a core set of quality indicators for the assessment of HIV/AIDS clinical care: a systematic review. BMC Health Serv Res. 2013;13:236. doi: 10.1186/1472-6963-13-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yapa HM, De Neve J-W, Chetty T, Herbst C, Post FA, Jiamsakul A, et al. The impact of continuous quality improvement on coverage of antenatal HIV care tests in rural South Africa: results of a stepped-wedge cluster-randomised controlled implementation trial. PLoS Med. 2020;17:e1003150. doi: 10.1371/journal.pmed.1003150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bor J, Gage A, Onoya D, Maskew M, Tripodis Y, Fox MP, et al. Variation in HIV care and treatment outcomes by facility in South Africa, 2011–2015: a cohort study. PLoS Med. 2021;18:e1003479. doi: 10.1371/journal.pmed.1003479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roberts M, Hsiao W, Berman P, Reich M. Getting health reform right: a guide to improving performance and equity. New York: Oxford University Press; 2003. [Google Scholar]
- 167.World Health Organization. Everybody business: strengthening health systems to improve health outcomes. WHO’s framework for action. Geneva: World Health Organization; 2007. [cited 2021 Oct 13]. Available from: https://www.who.int/healthsystems/strategy/everybodys_business.pdf. [Google Scholar]
- 168.Chimbindi N, Bärnighausen T, Newell M-L. Patient satisfaction with HIV and TB treatment in a public programme in rural KwaZulu-Natal: evidence from patient-exit interviews. BMC Health Serv Res. 2014;14:32. doi: 10.1186/1472-6963-14-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 2017;17:137–44. doi: 10.1093/bjaed/mkw060 [DOI] [Google Scholar]
- 170.Joint United Nations Programme on HIV/AIDS. Evidence for elimination HIV-related stigma and discrimination. Geneva: Joint United Nations Programme on HIV/AIDS; 2020. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/eliminating-discrimination-guidance_en.pdf. [Google Scholar]
- 171.Nosyk B, Krebs E, Zang X, Piske M, Enns B, Min JE, et al. “Ending the epidemic” will not happen without addressing racial/ethnic disparities in the United States human immunodeficiency virus epidemic. Clin Infect Dis. 2020;71:2968–71. doi: 10.1093/cid/ciaa566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Viljoen L, Bond VA, Reynolds LJ, Mubekapi-Musadaidzwa C, Baloyi D, Ndubani R, et al. Universal HIV testing and treatment and HIV stigma reduction: a comparative thematic analysis of qualitative data from the HPTN 071 (PopART) trial in South Africa and Zambia. Sociol Health Illn. 2020;43:167–85. doi: 10.1111/1467-9566.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr. 2016;72:S235–42. doi: 10.1097/QAI.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Decker MR, Crago AL, Chu SKH, Sherman SG, Seshu MS, Buthelezi K, et al. Human rights violations against sex workers: Burden and effect on HIV. Lancet. 2015;385:186–99. doi: 10.1016/S0140-6736(14)60800-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jere E, Mafiala F, Jim G, Banda H, Nyasulu HM, Dambula I, et al. growing together: multisectoral investments in Malawi’s youth. Washington (DC): United States Agency for International Development; 2019. [cited 2021 Oct 13]. Available from: https://www.prb.org/wp-content/uploads/2019/08/malawi-engage-presentation-guide.pdf. [Google Scholar]
- 176.Grimsrud AT, Pike C, Bekker LG. The power of peers and community in the continuum of HIV care. Lancet Glob Health. 2020;8:e167–8. doi: 10.1016/S2214-109X(19)30544-3 [DOI] [PubMed] [Google Scholar]
- 177.Saul J, Bachman G, Allen S, Toiv NF, Cooney C, Beamon T. The DREAMS core package of interventions: a comprehensive approach to preventing HIV among adolescent girls and young women. PLoS ONE. 2018;13:e0208167. doi: 10.1371/journal.pone.0208167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mayer KH, Bekker LG, Stall R, Grulich AE, Colfax G, Lama JR. Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet. 2012;380:378–87. doi: 10.1016/S0140-6736(12)60835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.United Nations Office on Drugs and Crime. Implementing comprehensive HIV and HCV programmes with people who inject drugs. Vienna: United Nations Office on Drugs and Crime; 2012. [cited 2021 Oct 13]. Available from: https://www.unaids.org/sites/default/files/media_asset/2017_HIV-HCV-programmes-people-who-inject-drugs_en.pdf. [Google Scholar]
- 180.Burr CK, Storm DS, Hoyt MJ, Dutton L, Berezny L, Allread V, et al. Integrating health and prevention services in syringe access programs: a strategy to address unmet needs in a high-risk population. Public Health Rep. 2014;129:26–32. doi: 10.1177/00333549141291S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Roberts A, Mathers B, Degenhardt L, Reference Group to the United Nations on HIV and Injecting Drug Use. Women who inject drugs: a review of their risks, experiences and needs. Vienna: United Nations Office on Drugs and Crime; 2010. [cited 2021 Oct 13]. Available from: https://www.unodc.org/documents/hiv-aids/Women_who_inject_drugs.pdf. [Google Scholar]
- 182.Zhang L, Tao Y, Woodring J, Rattana K, Sovannarith S, Rathavy T, et al. Integrated approach for triple elimination of mother-to-child transmission of HIV, hepatitis B and syphilis is highly effective and cost-effective: an economic evaluation. Int J Epidemiol. 2019;48:1327–39. doi: 10.1093/ije/dyz037 [DOI] [PubMed] [Google Scholar]
- 183.Irungu EM, Sharma M, Maronga C, Mugo N, Ngure K, Celum C, et al. the incremental cost of delivering PrEP as a bridge to ART for HIV serodiscordant couples in public HIV care clinics in Kenya. AIDS Res Treat. 2019;2019:4170615. doi: 10.1155/2019/4170615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barocas JA, Morgan JR, Fiellin DA, Schackman BR, Eftekhari Yazdi G, Stein MD, et al. Cost-effectiveness of integrating buprenorphine-naloxone treatment for opioid use disorder into clinical care for persons with HIV/hepatitis C co-infection who inject opioids. Int J Drug Policy. 2019;72:160–8. doi: 10.1016/j.drugpo.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Owusu-Edusei K, Tao G, Gift TL, Wang A, Wang L, Tun Y, et al. Cost-effectiveness of integrated routine offering of prenatal HIV and syphilis screening in China. Sex Transm Dis. 2014;41:103–10. doi: 10.1097/OLQ.0000000000000085 [DOI] [PubMed] [Google Scholar]
- 186.Mbah MLN, Kjetland EF, Atkins KE, Poolman EM, Orenstein EW, Meyers LA, et al. Cost-effectiveness of a community-based intervention for reducing the transmission of Schistosoma haematobium and HIV in Africa. Proc Natl Acad Sci U S A. 2013;110:7952–7. doi: 10.1073/pnas.1221396110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kahn JG, Harris B, Mermin JH, Clasen T, Lugada E, Grabowksy M, et al. Cost of community integrated prevention campaign for malaria, HIV, and diarrhea in rural Kenya. BMC Health Serv Res. 2011;11:346. doi: 10.1186/1472-6963-11-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dugdale CM, Phillips TK, Myer L, Hyle EP, Brittain K, Freedberg KA, et al. Cost-effectiveness of integrating postpartum antiretroviral therapy and infant care into maternal & child health services in South Africa. PLoS ONE. 2019;14:e0225104. doi: 10.1371/journal.pone.0225104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu QH, Meyers K, Xu JJ, Chu ZX, Zhang J, Ding HB, et al. Efficacy and cost-effectiveness of early antiretroviral therapy and partners’ pre-exposure prophylaxis among men who have sex with men in Shenyang, China: a prospective cohort and costing study. BMC Infect Dis. 2019;19:663. doi: 10.1186/s12879-019-4275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ying R, Sharma M, Heffron R, Celum CL, Baeten JM, Katabira E, et al. Cost-effectiveness of pre-exposure prophylaxis targeted to high-risk serodiscordant couples as a bridge to sustained ART use in Kampala, Uganda. J Int AIDS Soc. 2015;18(4 Suppl 3):20013. doi: 10.7448/IAS.18.4.20013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vickerman P, Devine A, Foss AM, Delany-Moretlwe S, Mayaud P, Meyer-Rath G. The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sex Transm Dis. 2011;38:401–9. doi: 10.1097/OLQ.0b013e31820b8bc8 [DOI] [PubMed] [Google Scholar]
- 192.Bilinski A, Neumann P, Cohen J, Thorat T, McDaniel K, Salomon JA. When cost-effective interventions are unaffordable: Integrating cost-effectiveness and budget impact in priority setting for global health programs. PLoS Med. 2017;14:e1002397. doi: 10.1371/journal.pmed.1002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bärnighausen T, Hontelez JAC, Hauck K, Bijlmakers L, Jansen MPM, Tromp N, et al. Evidence for scaling up HIV treatment in sub-Saharan Africa: a call for incorporating health system constraints. PLOS Med. 2017;14:e1002240. doi: 10.1371/journal.pmed.1002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Luz PM, Veloso VG, Grinsztejn B. The HIV epidemic in Latin America: accomplishments and challenges on treatment and prevention. Curr Opin HIV AIDS. 2019;14:366–73. doi: 10.1097/COH.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bärnighausen T, Tugwell P, Røttingen J-A, Shemilt I, Rockers P, Geldsetzer P, et al. Quasi-experimental study designs series—paper 4: uses and value. J Clin Epidemiol. 2017;89:21–9. doi: 10.1016/j.jclinepi.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 196.Rockers PC, Tugwell P, Grimshaw J, Oliver S, Atun R, Røttingen J-A, et al. Quasi-experimental study designs series—paper 12: strengthening global capacity for evidence synthesis of quasi-experimental health systems research. J Clin Epidemiol. 2017;89:98–105. doi: 10.1016/j.jclinepi.2016.03.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Figure shows the duration of studies on the x-axis (by bins, each indicating minimum up to and including the maximum duration) and the number of studies per bin on the y-axis.
(PDF)
(PDF)
(PDF)
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; CHWs, community health workers; EIMC, early infant male circumcision; HCV, hepatitis C virus; MCH, maternal and child health; MSM, men who have sex with men; NCDs, noncommunicable diseases; PMTCT, prevention of mother-to-child transmission; PNC, postnatal care; PWID, people who inject drugs; PrEP, pre-exposure prophylaxis; SRH, sexual and reproductive health; STI, sexually transmitted infection; TB, tuberculosis; VMMC, voluntary medical male circumcision.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. No primary data were obtained for this study.