Abstract
Background
Patients with cancer have an increased risk of complications from SARS-CoV-2 infection. Vaccination to prevent COVID-19 is recommended, but data on the immunogenicity and safety of COVID-19 vaccines for patients with solid tumours receiving systemic cancer treatment are scarce. Therefore, we aimed to assess the impact of immunotherapy, chemotherapy, and chemoimmunotherapy on the immunogenicity and safety of the mRNA-1273 (Moderna Biotech, Madrid, Spain) COVID-19 vaccine as part of the Vaccination Against COVID in Cancer (VOICE) trial.
Methods
This prospective, multicentre, non-inferiority trial was done across three centres in the Netherlands. Individuals aged 18 years or older with a life expectancy of more than 12 months were enrolled into four cohorts: individuals without cancer (cohort A [control cohort]), and patients with solid tumours, regardless of stage and histology, treated with immunotherapy (cohort B), chemotherapy (cohort C), or chemoimmunotherapy (cohort D). Participants received two mRNA-1273 vaccinations of 100 μg in 0·5 mL intramuscularly, 28 days apart. The primary endpoint, analysed per protocol (excluding patients with a positive baseline sample [>10 binding antibody units (BAU)/mL], indicating previous SARS-CoV-2 infection), was defined as the SARS-CoV-2 spike S1-specific IgG serum antibody response (ie, SARS-CoV-2-binding antibody concentration of >10 BAU/mL) 28 days after the second vaccination. For the primary endpoint analysis, a non-inferiority design with a margin of 10% was used. We also assessed adverse events in all patients who received at least one vaccination, and recorded solicited adverse events in participants who received at least one vaccination but excluding those who already had seroconversion (>10 BAU/mL) at baseline. This study is ongoing and is registered with ClinicalTrials.gov, NCT04715438.
Findings
Between Feb 17 and March 12, 2021, 791 participants were enrolled and followed up for a median of 122 days (IQR 118 to 128). A SARS-CoV-2-binding antibody response was found in 240 (100%; 95% CI 98 to 100) of 240 evaluable participants in cohort A, 130 (99%; 96 to >99) of 131 evaluable patients in cohort B, 223 (97%; 94 to 99) of 229 evaluable patients in cohort C, and 143 (100%; 97 to 100) of 143 evaluable patients in cohort D. The SARS-CoV-2-binding antibody response in each patient cohort was non-inferior compared with cohort A. No new safety signals were observed. Grade 3 or worse serious adverse events occurred in no participants in cohort A, three (2%) of 137 patients in cohort B, six (2%) of 244 patients in cohort C, and one (1%) of 163 patients in cohort D, with four events (two of fever, and one each of diarrhoea and febrile neutropenia) potentially related to the vaccination. There were no vaccine-related deaths.
Interpretation
Most patients with cancer develop, while receiving chemotherapy, immunotherapy, or both for a solid tumour, an adequate antibody response to vaccination with the mRNA-1273 COVID-19 vaccine. The vaccine is also safe in these patients. The minority of patients with an inadequate response after two vaccinations might benefit from a third vaccination.
Funding
ZonMw, The Netherlands Organisation for Health Research and Development.
Introduction
Patients with cancer affected by COVID-19 have a higher risk of admission to an intensive care unit and a higher risk of dying than patients with COVID-19 without cancer.1 Moreover, severe COVID-19 can cause a substantial delay of oncological treatment in these patients. Therefore, vaccination of patients with cancer is recommended by professional oncology societies.2, 3 Nevertheless, there is an urgent need for trials investigating the effects of COVID-19 vaccines in patients with cancer, since registration trials have largely excluded these patients, especially during active treatment with chemotherapy or immunotherapy. In a phase 3 trial with more than 30 000 volunteers, the mRNA-1273 COVID-19 vaccine (Moderna Biotech, Madrid, Spain) showed 94·1% efficacy in protecting against COVID-19.4 Local and systemic side-effects were common but mainly low grade and of short duration.
Research in context.
Evidence before this study
Patients with cancer have an increased risk of a fatal outcome of COVID-19. Vaccination to prevent COVID-19 is recommended in this patient population, but the impact of chemotherapy and immunotherapy on immunogenicity and the safety of vaccination was unknown. Therefore, we developed this study in 2020. We searched PubMed for research articles published in English between Dec 1, 2019, and July 30, 2021, using the search terms “COVID-19”, “vaccination”, “cancer”, and “solid tumours”. The findings of the search showed insufficient evidence to determine a cutoff level for an adequate antibody response to COVID-19 vaccination in these patients.
Added value of this study
SARS-CoV-2-binding antibody response at 28 days after complete vaccination with mRNA-1273 was non-inferior during immunotherapy, chemotherapy, and chemoimmunotherapy for patients with a solid tumour compared with individuals without cancer and who were not receiving cancer treatment. Moreover, vaccination was safe. Given the importance of these data for clinical decisions, in a post-hoc exploratory analysis, we defined, on the basis of neutralising capacity, a cutoff level at 300 binding antibody units per mL to discriminate between suboptimal and adequate responders. After one vaccination, only a third of the patients with cancer achieved an adequate response compared with two-thirds of the controls. However, the second vaccination resulted in an adequate response in most participants.
Implications of all the available evidence
Most patients with solid tumours develop an adequate SARS-CoV-2-binding antibody response after vaccination. However, in each patient cohort, our post-hoc analysis using a cutoff level of 300 binding antibody units per mL to define adequate responders showed that there is a small proportion of suboptimal and non-responders among patients with solid tumours who are being treated for their cancer. Since most of these patients show an increase in antibody concentration after the second vaccination, an additional booster vaccination might turn these patients into adequate responders. Notably, almost half of the suboptimal and non-responders had a spike-specific T-cell response. Further research must define whether the T-cell response might also protect these patients.
Several relatively small studies in heterogeneous populations of patients with cancer (up to 134 patients with a solid tumour) have measured the SARS-CoV-2-binding antibody response after the first or second vaccination, but without defining a cutoff level for an adequate antibody response.5, 6, 7, 8 Therefore, few data on the immunogenicity and safety of COVID-19 vaccines for patients with solid tumours receiving systemic cancer treatment are available.
The Vaccination Against COVID in Cancer (VOICE) trial was specifically designed to address this issue.9 We gained access to the mRNA-1273 COVID-19 vaccine and its registration information from the European Medicines Agency in January, 2021, and aimed to assess the immunogenicity and safety of this vaccine in patients with solid tumours being treated with chemotherapy, immunotherapy, or chemoimmunotherapy, compared with individuals without cancer.
Methods
Study design and participants
The VOICE trial is an investigator-initiated, prospective, non-inferiority trial done at three centres in the Netherlands: University Medical Centre Groningen, Erasmus Medical Centre Rotterdam, and the Netherlands Cancer Institute in Amsterdam. The trial design has been published previously.9
We enrolled participants who were aged 18 years or older with a life expectancy of more than 12 months into four cohorts: individuals without cancer (control group; cohort A), and patients with solid tumours, regardless of stage and histology, who were treated with immunotherapy (defined as single-agent monoclonal antibody against PD-1 or PD-L1; cohort B), any type or combination of cytotoxic chemotherapy (cohort C), or chemoimmunotherapy (cohort D). Cohort A consisted of partners of the patients with solid tumours. The most recent immunotherapy administration had to be within 3 months (cohorts B and D) and the most recent chemotherapy administration within 4 weeks before vaccination (cohorts C and D). Previous or current confirmed SARS-CoV-2 infection was an exclusion criterion for participation. Participants with previous or current malignancy were excluded from cohort A, and patients with an active haematological malignancy were excluded from cohorts B, C, and D. Additionally, use of immunosuppressive medication at enrolment, including chronic steroid use of more than 10 mg prednisone or equivalent, was precluded, but short-term steroid use (usually a maximum of 5 days) as co-medication with chemotherapy was allowed. Patients who started steroids after the first vaccination were not excluded from the per-protocol population. Other inclusion and exclusion criteria are listed in the protocol (appendix 1).
All patients provided written, informed consent. The trial was done in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable government regulations. The trial protocol was approved by the medical ethics committee of the University Medical Centre Groningen, which served as the central committee. There was no data and safety monitoring board.
Procedures
Participants received two doses of the mRNA-1273 vaccine (provided by the Centre for Infectious Disease Control National Institute for Public Health, Bilthoven, Netherlands), 100 μg in 0·5 mL, by intramuscular injection, 28 days apart. No guideline for scheduling of the vaccination in patients under treatment was applied. Vaccines were administered in the weeks they were made available for this study.
Blood samples were drawn by venipuncture at the participating institutes by qualified health-care workers. Blood samples for blood cell counts, routine chemistry, and SARS-CoV-2 spike S1-specific IgG serum antibody (hereafter referred to as SARS-CoV-2-binding antibody) concentrations were collected immediately before the first vaccination (baseline) and the second vaccination, and on day 28 after the second vaccination. In addition, samples to measure neutralising antibody titres against SARS-CoV-2 were obtained on day 28. Samples for peripheral blood mononuclear cells (PBMCs) were taken at baseline and on day 28 and at 6 months after the second vaccination.
SARS-CoV-2-binding antibody concentrations were measured centrally (supervised by RSvB and GdH) at the Centre for Infectious Disease Control National Institute for Public Health using a fluorescent bead-based immune assay, as previously described.10, 11 The assay's detection limit was 0·1 binding antibody units (BAU)/mL, and an antibody concentration of more than 10 BAU/mL was considered to be positive.12 In addition, neutralising antibody titres against the SARS-CoV-2 D614G variant (closely resembling the original variant against which the vaccine was developed) were measured using the plaque reduction neutralisation test (PRNT) as previously described12 in all individuals with a SARS-CoV-2-binding antibody concentration between 10 BAU/mL and up to 500 BAU/mL, and in an additional selection of 29 participants per cohort, covering the concentration range of more than 500 BAU/mL (appendix 1 p 3).12
PBMCs were isolated within 24 h after collection, and SARS-CoV-2 spike-specific IFNγ-producing T cells (hereafter referred to as spike-specific T cells) were measured. Spike-specific T cells were assessed after stimulation of PBMCs with SARS-CoV-2 spike overlapping peptide pools using an IFNγ ELISpot assay in the same individuals in whom the antibody neutralising titre was determined. The laboratory assessments are further detailed in the appendix 1 (p 2).
Starting immediately before each vaccination, a daily questionnaire was used to collect solicited adverse events. This questionnaire was collected up to day 7 after each vaccination. Since patients with cancer who receive systemic treatment encounter multiple adverse events that are related to the disease or treatment, only solicited adverse events that worsened or arose after vaccination were analysed. Serious adverse events in the first week after each vaccination, immune-related adverse events13 in cohorts B and D up to 28 days after the second vaccination, and adverse events of special interest were analysed. Adverse events of special interest are defined in the protocol (appendix 1). Information about the incidence and severity of SARS-CoV-2 breakthrough infections was collected using 3-monthly questionnaires. The last questionnaire will be sent at 12 months after the last vaccination. In this analysis, only the questionnaire 3 months after the second vaccination was analysed.
Outcomes
The primary endpoint was defined as the development of SARS-CoV-2-binding antibodies measured on day 28 after the second vaccination in participants who had not previously been infected with SARS-CoV-2. The primary endpoint was centrally reviewed at the University Medical Centre Groningen by RSNF and AB. Participants were classified as responders or non-responders based on seroconversion, defined as the presence of SARS-CoV-2-binding antibodies in individuals without measurable SARS-CoV-2-binding antibodies at baseline.
Secondary endpoints were the concentration of SARS-CoV-2-binding antibodies before the second vaccination to analyse initial response and at 6 and 12 months after the second vaccination to measure longevity, and quantification of spike-specific T cells at baseline, and at day 28 and 6 months after the second vaccination. A spike-specific T-cell response was defined as a two times or greater increase in the number of spot-forming cells (SFCs; as measured with the IFNγ ELIspot assay) from pre-vaccination to post-vaccination and 50 or more SFCs per 106 PBMCs post-vaccination.14 SARS-CoV-2-binding antibodies at 6 and 12 months and T-cell responses at 6 months after the second vaccination will be reported in a later publication.
Safety was another secondary endpoint. Adverse events were graded according to Common Terminology Criteria for Adverse Events (version 5.0).15
Prespecified exploratory endpoints were functional and phenotypical characterisation of SARS-CoV-2-specific cellular immune responses, followed by assessment of proliferative capacity, cytokine production, and phenotypical markers in a subset of patients (yet to be defined, and will be guided by the results of the primary and secondary endpoints); determination of baseline immune parameters associated with immune response to COVID-19 vaccination; and assessment of the induction of SARS-CoV-2-specific antibodies in mucosal lining fluid. These exploratory results will be reported in a later publication. Neutralising antibody titres were measured on day 28 after the second vaccination and the relationship with SARS-CoV-2-binding antibody concentrations was analysed and is reported here.
Statistical analysis
For the primary endpoint analysis, a non-inferiority design with a margin of 10% was used. For the power calculations, we initially anticipated a true response rate of 90% for cohorts A and B, and 60% for cohorts C and D. Assuming no true difference between cohorts A and B, 112 participants in cohorts A and B needed to be included to ensure 80% certainty that the upper limit of a one-sided 95% CI would exclude a difference in favour of cohort A of more than 10%. For cohorts C and D, we assumed a true difference in favour of cohort A of 30%. Adding the 10% non-inferiority margin to the anticipated true difference of 30% gave a margin of 40%. With these assumptions, 205 participants needed to be included in cohorts A, C, and D to provide 80% certainty that the upper limit of a one-sided 95% CI would exclude a difference in favour of cohort A of more than 40%. To compensate for non-evaluable patients—eg, those with SARS-CoV-2-binding antibodies at baseline—we decided to enlarge each cohort by 20%. On the basis of these power calculations (for detailed explanation see appendix 1 p 3), we aimed to enrol 246 participants in cohorts A, C, and D, and 135 participants in cohort B.
A modified standard fixed-delta test was used to compare the percentage of responders in cohorts B, C, and D, with cohort A.16 When analysing our data after the database lock, it became clear that our anticipated true response rates used in the power calculations were substantially lower than the observed true response rates. Therefore, to test for non-inferiority, we decided that the anticipated true response rates in cohorts B, C, and D all had to be equal to the observed true response rate in cohort A (ie, 100%). The non-inferiority margin was kept at 10%. The primary endpoint analysis was done in the per-protocol population: participants who received two vaccinations with no major protocol deviations and who had a SARS-CoV-2-binding antibody measurement available on day 28 after the second vaccination. Participants with a positive baseline sample (>10 BAU/mL), which suggests a previous SARS-CoV-2 infection, were not included in the per-protocol population. In a post-hoc analysis, subgroups of suboptimal and adequate responders were defined based on the determined cutoff value of the SARS-CoV-2-binding antibody concentration at 28 days after the second and first vaccination. Our exploratory analysis of the association between SARS-CoV-2-binding antibody concentration and neutralising antibody titre enabled us to define a cutoff level of 300 BAU/mL to categorise responders into adequate responders (>300 BAU/mL) and suboptimal responders (>10 BAU/mL but ≤300 BAU/mL). This cutoff level was based on a PRNT titre of 40 that we considered as minimally protective (appendix 1 p 4).17 Spike-specific T-cell responses were analysed according to SARS-CoV-2-binding antibody response group in a post-hoc analysis. In addition, we did post-hoc exploratory analyses of the association between SARS-CoV-2-binding antibody concentration and lymphocyte and neutrophil counts on the first and the second vaccination days and 28 days thereafter, treatment regimen, the exact time between the most recent administration of systemic treatment before the first vaccination per patient, and the use of immunosuppressive medication.
Solicited adverse events were assessed in participants who received at least one dose of the mRNA-1273 vaccine with no major protocol deviations and who had no SARS-CoV-2-binding antibodies at baseline. Serious adverse events (all cohorts), immune-related adverse events (cohorts B and D), and adverse events of special interest (in cohorts B, C, and D) were recorded for all participants who received at least one vaccine.
Descriptive summary data (numbers and percentages) were provided for each relevant group and subgroup for all analyses. Associations between continuous variables were determined with the Spearman correlation coefficient. Two-class comparison between proportions was done using the one-sided two-proportion Z test. All analyses were done in R (version 4.1.0). p values of 0·05 or less were considered statistically significant. This trial is registered with ClinicalTrials.gov, NCT04715438.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
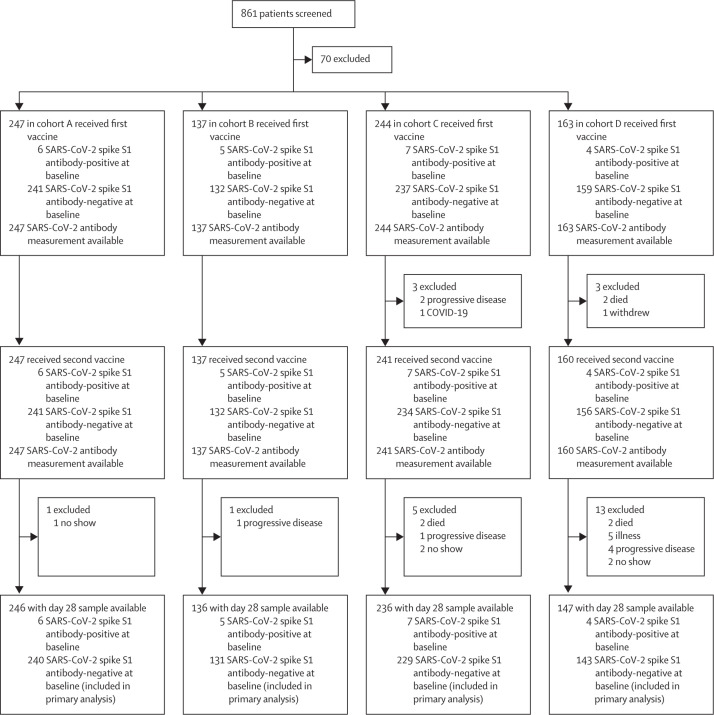
Between Feb 17 and March 12, 2021, 791 participants were enrolled. At data cutoff on June 28, 2021, the median follow-up was 122 days (IQR 118–128). For the primary endpoint, 743 participants were evaluable (per-protocol population): 240 individuals without cancer (cohort A) and 131 patients receiving immunotherapy (cohort B), 229 patients receiving chemotherapy (cohort C), and 143 patients receiving chemoimmunotherapy (cohort D; figure 1 ).
Figure 1.
Study disposition of all participants included in the study
Participants without cancer were included in cohort A, and patients with solid tumours receiving immunotherapy were included in cohort B, those receiving chemotherapy were included in cohort C, and those receiving chemoimmunotherapy were included in cohort D.
For cohort C, the target accrual of 246 patients was not reached with 244 enrolled; however, 229 patients in cohort C were evaluable where 205 evaluable patients were needed according to the power calculations. The target accrual for cohort D (the chemoimmunotherapy cohort) was also not reached due to the short timespan of early vaccine access for participants and the low number of patients eligible for cohort D during that period. Moreover, a relatively high number of patients in cohort D had rapid clinical deterioration due to progressive disease before inclusion in the trial. Reasons for patients being non-evaluable for the primary endpoint are shown in figure 1. Baseline demographics and clinical characteristics of the per-protocol population are shown in the table . Patients with respiratory tract cancers were most frequently enrolled, followed by patients with breast cancer, digestive tract cancers, and skin cancers (table). Most patients had advanced disease (table). The anti-PD-1 antibodies nivolumab and pembrolizumab were the most frequently used types of immunotherapy (appendix 1 p 14). A wide range of chemotherapy regimens were administered, and 39 (17%) of 229 patients in cohort C received chemoradiotherapy (appendix 1 p 15). Cancer treatment details are available in appendix 1 (pp 14–16). As of June 28, 2021, one patient in cohort C had been diagnosed with COVID-19, 23 days after the first vaccination. Baseline demographics and clinical characteristics of all participants enrolled in the study are provided in appendix 1 (p 17).
Table.
Baseline demographic and clinical characteristics of the per-protocol population
| Cohort A (n=240) | Cohort B (n=131) | Cohort C (n=229) | Cohort D (n=143) | ||
|---|---|---|---|---|---|
| Age, years | 62 (55–69) | 66 (59–73) | 60 (50–67) | 64 (57–70) | |
| Sex | |||||
| Female | 114 (48%) | 44 (34%) | 141 (62%) | 75 (52%) | |
| Male | 126 (53%) | 87 (66%) | 88 (38%) | 68 (48%) | |
| Smoking | |||||
| Current | 32 (13%) | 13 (11%) | 18 (8%) | 21 (15%) | |
| Former | 89 (37%) | 71 (54%) | 103 (45%) | 107 (75%) | |
| Never | 119 (50%) | 47 (35%) | 108 (47%) | 15 (10%) | |
| Body-mass index, kg/m2 | 27·0 (4·0) | 27·1 (4·5) | 26·4 (4·6) | 25·8 (5·3) | |
| WHO performance status | |||||
| 0 | 222 (93%) | 90 (69%) | 132 (58%) | 61 (43%) | |
| 1 | 16 (7%) | 41 (31%) | 91 (40%) | 70 (49%) | |
| 2 | 1 (<1%) | 0 | 6 (3%) | 12 (8%) | |
| Unknown | 1 (<1%) | 0 | 0 | 0 | |
| Primary tumour localisation | |||||
| Bone or soft tissue | NA | 1 (1%) | 9 (4%) | 0 | |
| Breast | NA | 0 | 71 (31%) | 2 (1%) | |
| CNS | NA | 0 | 10 (4%) | 0 | |
| Digestive tract | NA | 4 (3%) | 65 (28%) | 1 (1%) | |
| Endocrine glands | NA | 0 | 3 (1·3%) | 0 | |
| Female genital organs | NA | 0 | 20 (9%) | 0 | |
| Head and neck | NA | 2 (1·5%) | 5 (2%) | 1 (1%) | |
| Male genital organs | NA | 0 | 19 (8%) | 0 | |
| Respiratory tract | NA | 27 (21%) | 18 (8%) | 139 (97%) | |
| Skin | NA | 64 (49%) | 0 | 0 | |
| Urinary tract | NA | 32 (24%) | 9 (4%) | 0 | |
| Other or unspecified | NA | 1 (1%) | 0 | 0 | |
| Tumour stage | |||||
| I | NA | 2 (2%) | 16 (7%) | 0 | |
| II | NA | 2 (2%) | 36 (16%) | 0 | |
| III | NA | 31 (24%) | 46 (20%) | 9 (6%) | |
| IV | NA | 96 (73%) | 129 (56%) | 134 (94%) | |
| Unknown | NA | 0 | 1 (<1%) | 0 | |
| Treatment intent | |||||
| Curative | NA | 43 (33%) | 114 (50%) | 17 (12%) | |
| Non-curative | NA | 88 (67%) | 115 (50%) | 129 (88%) | |
Data are median (IQR), n (%), or mean (SD). NA=not applicable. Percentages might not sum to 100 because of rounding. Participants without cancer were included in cohort A, and patients with solid tumours treated with immunotherapy were included in cohort B, those treated with chemotherapy were included in cohort C, and those treated with chemoimmunotherapy were included in cohort D.
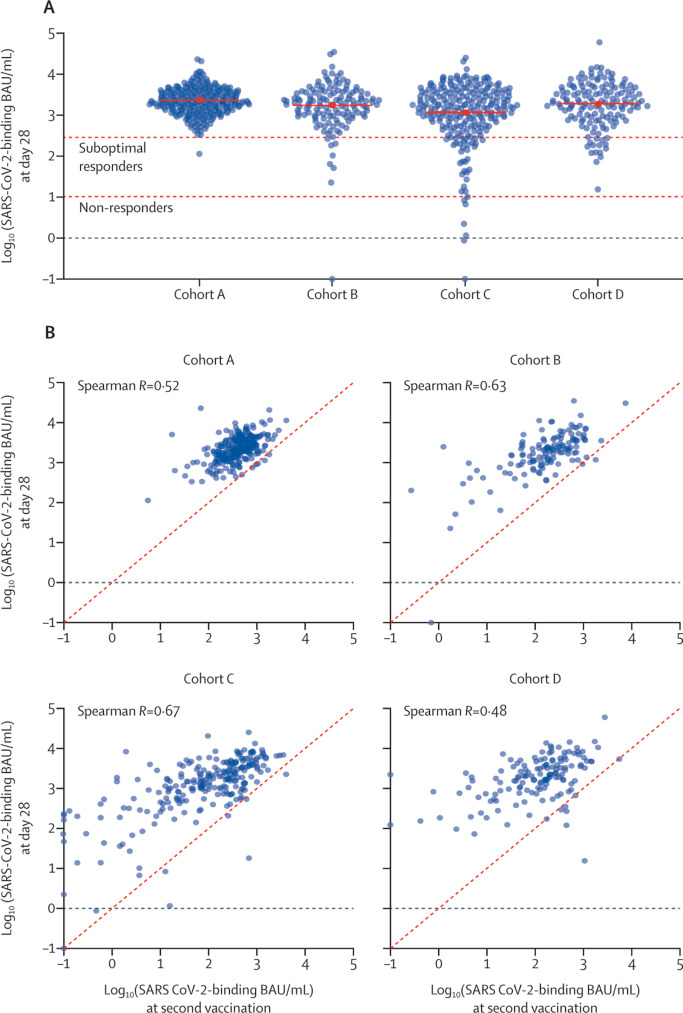
A SARS-CoV-2-binding antibody concentration of more than 10 BAU/mL on day 28 after the second vaccination was found in all 240 (100%; 95% CI 98 to 100) participants in cohort A (control cohort), in 130 (99%; 96 to >99) of 131 patients in cohort B, in 223 (97%; 94 to 99) of 229 patients in cohort C, and in all 143 (100%; 97 to 100) patients in cohort D (figure 2 A). SARS-CoV-2-binding antibodies per chemotherapy regimen for cohorts B and D are shown in appendix 1 (p 6). Appendix 1 (p 7) shows the SARS-CoV-2-binding antibody concentrations in the 22 participants excluded from the primary endpoint analysis because of a baseline antibody concentration of more than 10 BAU/mL, indicating an earlier unrecognised SARS-CoV-2 infection.
Figure 2.
SARS-CoV-2-binding antibody concentrations
Participants without cancer were included in cohort A, and patients with solid tumours receiving immunotherapy were included in cohort B, those receiving chemotherapy were included in cohort C, and those receiving chemoimmunotherapy were included in cohort D. (A) Distribution of SARS-CoV-2-binding antibody concentrations in log10-transformed BAU/mL at day 28 after second vaccination in the different cohorts. The red solid lines indicate the mean of the log10-transformed BAU/mL concentrations (ie, geometric mean). The red horizontal dashed lines indicate the following: the bottom line represents the threshold for non-responders (SARS-CoV-2-binding antibody concentration ≤10 BAU/ mL), and the top line represents the threshold between suboptimal and adequate responders (>300 BAU/mL). (B) Scatterplot of SARS-CoV-2-binding antibody concentrations in log10 transformed BAU/mL at day 28 after second vaccination versus SARS-CoV-2-binding antibody concentrations in log10 transformed BAU/mL at second vaccination day in the different cohorts. BAU=binding antibody units.
Although target accrual was not reached for cohort D, the study met its primary endpoint for each patient cohort. With the 100% response rate observed in cohort A, a non-inferiority margin of 10%, α value of 0·05, and also assuming response rates of 100% in cohorts B, C, and D, the standard modified fixed-delta test showed non-inferiority for each patient cohort (B, C, and D) compared with cohort A. Calculations for more stringent α values and non-inferiority margins are provided in appendix 2.
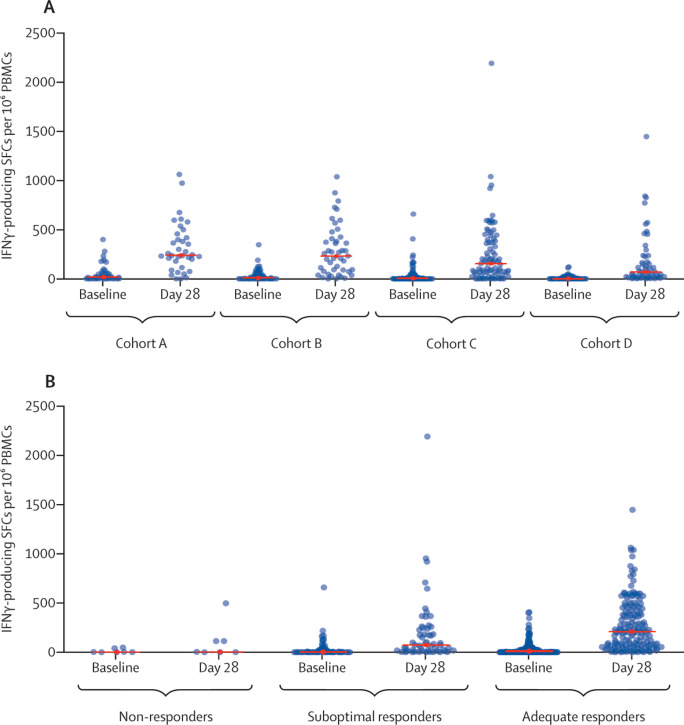
The distribution of SARS-CoV-2-binding antibody concentrations in log10 transformed BAU/mL at day 28 after second vaccination versus at day of second vaccination in the different cohorts are shown in figure 2B and spike-specific T-cell responses at day 28 after second vaccination in each cohort are shown in figure 3 A. The SARS-CoV-2-binding antibody concentration increased in most individuals after the second vaccination (figure 2A). A spike-specific T-cell response was found in 25 (69%; 95% CI 52 to 84) of 36 selected samples in cohort A, 30 (67%; 51 to 80) of 45 in cohort B, 52 (66%; 54 to 76) of 79 in cohort C, and 27 (53%; 38 to 67) of 51 in cohort D. In cohort C, some patients did not develop either an antibody response or a T-cell response (four [2%; 95% CI <1 to 4] of 229; figure 3).
Figure 3.
SARS-CoV-2 spike-specific IFNγ T-cell response
Participants without cancer were included in cohort A, and patients with solid tumours receiving immunotherapy were included in cohort B, those receiving chemotherapy were included in cohort C, and those receiving chemoimmunotherapy were included in cohort D. (A) The number of SARS-CoV-2 spike-specific IFNγ-producing SFCs per 106 PBMCs at baseline and day 28 after second vaccination in participants selected for T-cell response measurement in the four different cohorts (the red line indicates the median). (B) The number of SARS-CoV-2 spike-specific IFNγ-producing SFCs per 106 PBMCs at baseline and day 28 after second vaccination for non-responders, suboptimal responders, and selected adequate responders (the red horizontal line indicates the median). IFNγ=interferon-γ. PBMCs=peripheral blood mononuclear cells. SFCs=spot-forming cells.
In our exploratory analysis, neutralising antibody titres correlated with the SARS-CoV-2-binding antibody concentrations in each cohort (appendix 1 p 8).
At 28 days after the second vaccination, our post-hoc analysis using a cutoff value of 300 BAU/mL to categorise responders into adequate responders (>300 BAU/mL) and suboptimal responders (>10 BAU/mL but ≤300 BAU/mL) showed that there were 239 (>99%; 95% CI 98 to >99%) of 240 adequate responders in cohort A, 122 (93%; 87 to 97) of 131 in cohort B, 192 (84%; 78 to 88) of 229 in cohort C, and 127 (89%; 82 to 93) of 143 in cohort D. Thus, in the patients with cancer, nine (7%) of 131 of the patients treated with immunotherapy (cohort B), 37 (16%) of 229 of the patients treated with chemotherapy (cohort C), and 16 (11%) of 143 of the patients treated with chemoimmunotherapy (cohort D) were classified as suboptimal responders or non-responders. 28 days after the first vaccination, 159 (66%; 95% CI 60 to 72) of 241 participants in cohort A, 49 (37%; 29 to 46) of 132 patients in cohort B, 76 (32%; 27 to 39) of 234 patients in cohort C, and 52 (33%; 26 to 41) of 156 patients in cohort D had an adequate response. In further post-hoc analysis of these subgroups, a spike-specific T-cell response was found in three (43%; 95% CI 10 to 82) of seven non-responders (one in cohort B and two in cohort C), in 26 (47%; 34 to 61) of 55 suboptimal responders (five in cohort B, 18 in cohort C, and three in cohort D), and 105 (70%; 62 to 78) of 149 adequate responders (25 in cohort A, 24 in cohort B, 32 in cohort C, and 24 in cohort D; figure 3B).
Our post-hoc analyses of associations between SARS-CoV-2-binding antibody concentration and lymphocyte and neutrophil counts on the first and the second vaccination day and 28 days thereafter, the time between the most recent administration of systemic treatment before the first vaccination, and the use of immunosuppressive medication are shown in appendix 1 (pp 9–13).
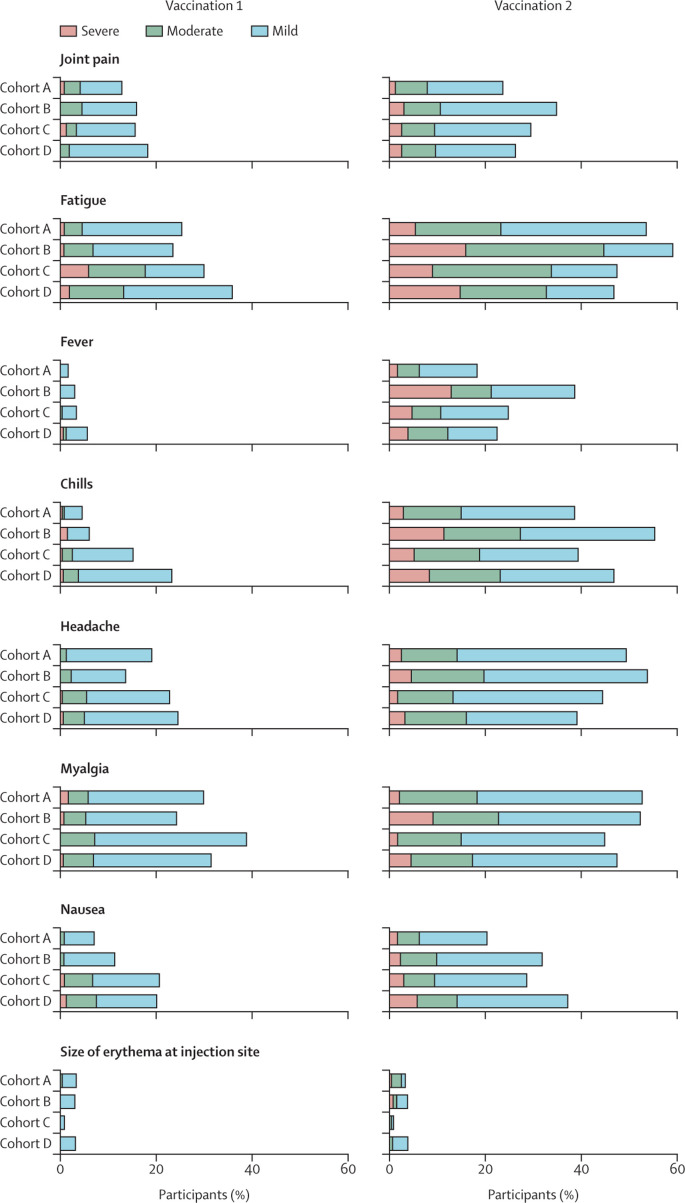
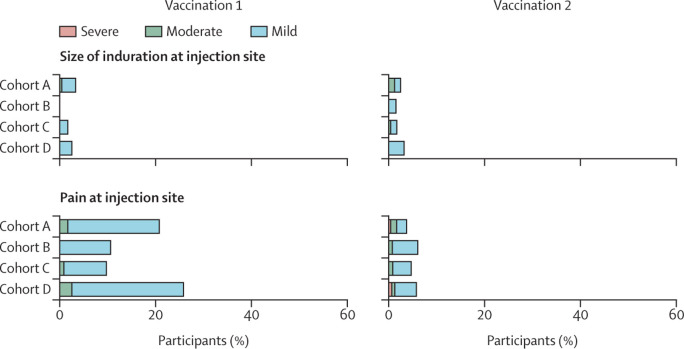
Solicited adverse events were analysed in 761 of 791 participants who received the first vaccination (239 in cohort A, 132 in cohort B, 232 in cohort C, and 158 in cohort D), and in 750 of 785 participants who received the second vaccination (238 in cohort A, 132 in cohort B, 227 in cohort C, and 153 in cohort D). Reasons for exclusion were a SARS-CoV-2-binding antibody concentration of more than 10 BAU/mL at baseline (six in cohort A, five in cohort B, seven in cohort C, and four in cohort D) and incomplete data (eight [two in cohort A, five in cohort C, and one in cohort D] after the first vaccination and 13 [three in cohort A, seven in cohort C, and three in cohort D] after the second vaccination). In all cohorts, the incidence and severity of solicited systemic adverse events (fatigue, fever, chills, headache, myalgia, joint pain, and nausea) were higher up to 7 days after the second vaccination than up to 7 days after the first one (figure 4 ). Fatigue was the most prevalent solicited systemic adverse event after the first and second vaccination in 61 (26%) of 239 and 129 (54%) of 238 participants in cohort A, 31 (23%) and 78 (59%) of 132 participants in cohort B, 71 (31%) of 232 and 111 (49%) of 227 participants in cohort C, and 57 (36%) of 158 and 73 (48%) of 153 participants in cohort D, respectively. Pain at the injection site was the most prevalent solicited local adverse event and was reported after the first and second vaccination in 50 (21%) of 239 and nine (4%) of 238 participants in cohort A, 14 (11%) and eight (6%) of 132 participants in cohort B, 23 (10%) of 232 and 11 (5%) of 227 participants in cohort C, 41 (26%) of 158 and nine (6%) of 153 participants in cohort D, respectively. Pain at the injection site was more common after the first vaccination than after the second vaccination in cohorts A, C, and D, whereas erythema and induration at the injection site were uncommon (in <5% of patients) after both vaccinations in all cohorts (figure 4).
Figure 4.
Solicited adverse events in participants who received at least one vaccination and had no seroconversion at baseline
Participants without cancer were included in cohort A, and patients with solid tumours treated with immunotherapy were included in cohort B, those treated with chemotherapy were included in cohort C, and those treated with chemoimmunotherapy were included in cohort D. Horizontally stacked bar plot showing the percentage of participants reporting solicited systemic and local adverse events up to 7 days after the first and second vaccination. The highest reported grade during the 7 days after each vaccination was included. Solicited systemic and local adverse events that were reported already at baseline were excluded. Mild indicates not interfering with daily activities, moderate indicates interfering with daily activities, and severe indicates could not perform daily activities for joint pain, fatigue, chills, headache, myalgia, nausea, and pain at injection site. For fever, mild indicates a temperature of 38·0–38·4°C, moderate indicates 38·5–38·9°C, and severe indicates 39·0°C or above. For size of erythema and induration at injection site, mild indicates 2·5–5·0 cm, moderate indicates 5·1–10·0 cm, and severe indicates larger than 10·0 cm.
Serious adverse events in the first week after each vaccination were collected for all 791 included participants. Grade 3 or worse serious adverse events occurred in no participants in cohort A, three (2%) of 137 patients in cohort B, six (2%) of 244 patients in cohort C, and one (1%) of 163 patients in cohort D (appendix 1 p 18). Of 16 serious adverse events, four were potentially related to the vaccination as judged by the local principal investigator (medical oncologist). These serious adverse events included fever (two events, one in cohort C and one in cohort D), diarrhoea (one event in cohort D), and febrile neutropenia (one event in cohort C). At data cutoff, ten patients had died (eight due to disease progression [three in cohort C and five in cohort D], one due to pneumonitis in cohort D, and one due to leukaemia as second malignancy in cohort B; appendix 1 p 19). There were no vaccine-related deaths. Adverse events of special interest were collected in all participants enrolled in cohorts B (n=137), C (n=244) and D (n=163), and included thromboembolic events (five patients), myocardial infarction (two patients), convulsion (one patient), and erythema multiforme after the first vaccination (one patient in cohort B; appendix 1 p 19). The latter progressed to Stevens-Johnson syndrome after the second vaccination and resolved after treatment with high-dose steroids (see case description in appendix 1 p 5). Two cases of thromboembolism and Stevens-Johnson syndrome were considered to be related to vaccination.
Immune-related adverse events were collected for all participants in cohorts B (n=137) and D (n=163), up to 28 days after the second vaccination, and occurred in six (4%) of 137 patients treated with immunotherapy and seven (4%) of 163 patients treated with chemoimmunotherapy (appendix 1 p 20). Most immune-related adverse events were low grade, but one patient died from pneumonitis, one patient developed grade 3 adrenal insufficiency, and one patient experienced grade 3 thrombocytopenia requiring high-dose steroids.
Discussion
To our knowledge, the VOICE trial is the first prospective COVID-19 vaccination trial in patients with solid tumours with a predefined primary endpoint and predefined cohorts based on treatment regimens. This trial met its primary endpoint, which was based on the SARS-CoV-2-binding antibody response 28 days after the second vaccination with mRNA-1273. Compared with individuals without cancer, the trial showed non-inferiority of two mRNA-1273 vaccinations in patients receiving immunotherapy, chemotherapy, or chemoimmunotherapy for a solid tumour. Furthermore, our post-hoc analyses showed that most of the patients developed an adequate SARS-CoV-2-binding antibody concentration of more than 300 BAU/mL. Nevertheless, nine (7%) of 131 of the patients treated with immunotherapy, 37 (16%) of 229 of the patients treated with chemotherapy, and 16 (11%) of 143 of the patients treated with chemoimmunotherapy were classified as suboptimal responders or non-responders compared with one (<1%) of 240 of the participants without cancer. Among the suboptimal and non-responders, almost half of the participants had a spike-specific T-cell response. No new safety signals were identified.
Currently, an antibody concentration threshold that represents a correlate of protection against COVID-19 is not available, but neutralising antibody concentrations are predictive of immune protection from symptomatic COVID-19.18, 19 To discriminate between suboptimal and adequate responders, we propose a cutoff level for a SARS-CoV-2-binding antibody concentration based on correlation with neutralising capacity. This cutoff level of more than 300 BAU/mL was conservatively chosen to include more than 97% of the individuals attaining a neutralising antibody titre of 40 or more. This level of neutralising antibodies was previously considered as the cutoff level for infectivity during infection with D614G SARS-CoV-2.17
In our study, 159 (66%; 95% CI 60–72) of 241 participants without cancer (the control cohort) had an adequate SARS-CoV-2-binding antibody response after the first vaccination. This percentage was lower in patients with cancer. This finding is consistent with a previous observation of a lower seroconversion rate in patients with cancer (21 of 45) than in health-care workers (31 of 32) after one dose of BNT162b2 (Pfizer-BioNTech, Mainz, Germany), another mRNA-based COVID-19 vaccine.8 However, in our study and in other recently reported studies, the majority of the patients with cancer developed seroconversion after the second vaccination.5, 6, 7 We observed that most patients with cancer had a substantial increase in the SARS-CoV-2-binding antibody concentration after the second vaccination, which suggests that a third vaccination could potentially turn suboptimal responders into adequate responders. The small proportion of patients without an adequate response after two vaccine doses supports a selective strategy of a third vaccination based on antibody concentrations, especially in light of the worldwide vaccine shortage. At a population level, this translates into many patients with cancer that would need to be tested. Hence, a validated, affordable, high-throughput test is essential. A SARS-CoV-2-binding antibody assay could suit this purpose. Meanwhile, it is important to increase the vaccination rate in the general population to protect suboptimal and non-responders.
Clinical implementation of the proposed cutoff level might be challenging because different antibody measurements are available, and there is no consensus on the definition of response and the optimal assay. Furthermore, it is not clear yet whether at later timepoints and for different vaccines, the same correlation between SARS-CoV-2-binding antibody concentration and neutralising capacity exists. Follow-up assessments at 6 months and 12 months9 in this ongoing trial will demonstrate whether longevity of the antibody concentration differs between individuals without cancer and patients with cancer who received systemic therapy. In healthy individuals who participated in a phase 1 trial of the mRNA-1273 vaccine, antibodies persisted up to 6 months after the second vaccination.20 Because immunogenicity of different vaccines against COVID-19 seems to vary, the association between SARS-CoV-2-binding antibody concentration and neutralising capacity needs to be confirmed for other vaccines before the cutoff value of 300 BAU/mL can be generalised. At present, new SARS-CoV-2 variants have become dominant globally, and partial immune escape with up to five times lower neutralising capacity has been observed, whereas T-cell responses do not seem to be substantially affected by circulating variants.21, 22, 23 So far, to what extent T-cell responses protect from severe COVID-19 and how a T-cell response should be defined remain unknown. Additional studies are needed to reveal the antibody level required for protection and the role of T cells to protect individuals from severe COVID-19.
The adverse events that we observed in our study are consistent with previous vaccination studies and the known side-effects of chemotherapy and immunotherapy. The serious adverse events, adverse events of special interest, and immune-related adverse events in this trial mainly represented disease-related and cancer treatment-related complications. Of special interest is the case of erythema multiforme after the first vaccination progressing to Stevens-Johnson syndrome after the second vaccination. Stevens-Johnson syndrome has been associated with immunotherapy,24 but a causal association with COVID-19 vaccination cannot be excluded with three cases of Stevens-Johnson syndrome after vaccination reported in the published literature so far.25, 26, 27 Although it is conceivable that treatments that boost the immune system, such as vaccination, might contribute to immune-related adverse events in patients treated with immunotherapy, the incidence of such adverse events did not seem to be higher after vaccination and were in line with the expected rate in patients receiving immunotherapy and those receiving chemoimmunotherapy. In another study, no immune-related adverse events were reported in 134 patients treated with immunotherapy who received the BNT162b2 vaccine.28 As no new safety signals have been observed in the current trial and previous studies, vaccination against COVID-19 can be supported for patients treated with chemotherapy, immunotherapy, or chemoimmunotherapy.
Because we aimed to include a broad, real-world population of patients with cancer, the study had the inherent limitation that it was not powered to draw conclusions for specific subgroups, such as patients with different tumour types or those treated with specific chemotherapy regimens. In these patients, the immune response might have been affected by the chemotherapy's timing and mechanism of action. Again, the number of patients and the variety of chemotherapy regimens precluded a meaningful analysis of the impact of specific drugs and the timing of chemotherapy. To gain insight into these factors, we have formed an international consortium to share and further analyse data of COVID-19 vaccination studies in patients with cancer. This information with additional safety and immunogenicity data of regular and additional doses of COVID-19 vaccines, is important to develop strategies to optimally protect patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours against COVID-19.
Data sharing
We have formed an International Cancer Covid Vaccination Consortium. Thus, we aim to share data of vaccination against SARS-CoV-2 studies in patients receiving systemic cancer treatment, which might allow us to define more precisely how to protect patients best in which situation. Please contact the corresponding author if you wish to participate.
Declaration of interests
SFO reports research grants from Novartis and Celldex Therapeutics, and consultancy fees from Bristol Myers Squibb (BMS; all paid to the institution). AAMvdV reports consultancy fees from BMS, Merck Sharpe & Dohme (MSD), Merck, Sanofi, Eisai, Pfizer, Ipsen, Roche, Pierre Fabre and Novartis, and travel support from Bayer, Roche, Novartis, and Pfizer (all paid to the institution). A-MCD reports consultancy fees from Roche, Boehringer Ingelheim, Amgen, Bayer, Pharmamar, and Sanofi (all paid to the institution); speaker fees from Eli Lilly, AstraZeneca, Jansen, Chiesi, and Takeda (all paid to the institution); and research support from BMS, AbbVie, and Amgen (all paid to the institution). EFS reports consultancy fees from Eli Lilly (all paid to the institution); speaker fees from AstraZeneca, Boehringer Ingelheim, and Daiichi Sankyo (all paid to the institution); and advisory board fees from AstraZeneca, Bayer, BMS, MSD, Merck, Novartis, Pfizer, Roche Genentech, Roche Diagnostics, and Takeda (all paid to the institution). TJNH reports advisory board fees from BMS, AstraZeneca, Merck, Pfizer, Roche, and MSD (all paid to the institution). MJ reports consultancy fees from AstraZeneca and Pierre Fabre (all paid to the institution). GFR reports funding from the Alexander von Humboldt Foundation (paid to the institution). CUB reports an advisory role at BMS, MSD, Roche, Novartis, GlaxoSmithKline, AstraZeneca, Pfizer, Lilly, GenMab, Pierre Fabre, and Third Rock Ventures; research funding from BMS, MSD, 4SC, Novartis, and NanoString (all paid to the institution); stock ownership in Uniti Cars; and being co-founder of Immagene BV. MPGK reports funding from the EU's Horizon H2020 grant (paid to the institution). JBAGH reports consultancy fees from Achilles Therapeutics, BioNTech, BMS, Immunocore, Instil Bio, Molecular Partners, MSD, Gadeta, Merck Serono, Neogene Therapeutics, Novartis, Pfizer, PokeAcel, Roche/Genentech, Sanofi, and T-Knife (paid to the institution); consultancy fees from Neogene Tx; speaker fees from Ipsen, Eisai, and Novartis (paid to the institution); research grants from Asher-Bio, BMS, BioNTech, MSD, and Novartis (paid to the institution); and stock in Neogene Tx. EGEdV reports an advisory role at Daiichi Sankyo, NSABP, and Sanofi, and research funding from Amgen, AstraZeneca, Bayer, Chugai Pharma, Crescendo, CytomX Therapeutics, G1 Therapeutics, Genentech, Nordic Nanovector, Radius Health, Regeneron, Roche, Servier, and Synthon (all paid to the institution). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study is funded by ZonMw, the Netherlands Organisation for Health Research and Development. We thank the patients and their partners, as well as the medical staff, clinical trial staff, pharmacists, nurses, and technicians at the participating sites, the referring colleagues, and the Department Clinical Trials Office at the Netherlands Comprehensive Cancer Organisation for their participation and support.
Contributors
SFO, AAMvdV, CHG, RSNF, A-MCD, EFS, TJNH, MJ, GFR, PK, CUB, MPGK, ALWH, CACMvE, NYR, DvB, JBAGH, and EGEdV contributed to conceptualisation of the study. SFO, AAMvdV, RSNF, A-MCD, EFS, TJNH, TTW, AB, JBAGH, and EGEdV were involved in data curation. SFO, AAMvdV, CHG, RSNF, RSvB, AB, DvB, JBAGH, and EGEdV did the formal analysis. SFO, AAMvdV, RSNF, DvB, JBAGH, and EGEdV were involved in funding acquisition. SFO, AAMvdV, CHG, RSvB, RSNF, A-MCD, EFS, TJNH, CUB, MvdH, GdH, CACMvE, AB, DvB, JBAGH, and EGEdV were involved in data collection and laboratory experiments. SFO, AAMvdV, CHG, RSNF, RSvB, MvdH, DvB, and EGEdV developed the methodology. SFO, AAMvdV, TTW, JBAGH, and EGEdV contributed to project administration. SFO, AAMvdV, CHG, RSNF, RSvB, A-MCD, EFS, TJNH, CUB, MPGK, CACMvE, NYR, DvB, JBAGH, and EGEdV allocated resources. RSNF and AB ran statistical software. EGEdV supervised the study. SFO, AAMvdV, CHG, RSNF, RSvB, GdH, DvB, JBAGH, and EGEdV validated the data. SFO, AAMvdV, RSNF, AB, and EGEdV made the figures. All authors contributed to writing of the original draft of the report. All authors contributed to the review and editing of the report. All authors had access to all data, and SFO, AAMvdV, CHGvK, RSNF, RSvB, GdH, TTW, AB, MvdH, DvB, and EGEdV had access to and verified raw data. The corresponding author had full access to all data and the final responsibility to submit for publication.
Supplementary Materials
References
- 1.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor. Educate. Ann Oncol. 2021;32:579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Sengupta R, Locke T, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11:233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths EA, Segal BH. Immune responses to COVID-19 vaccines in patients with cancer: promising results and a note of caution. Cancer Cell. 2021;39:1045–1047. doi: 10.1016/j.ccell.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098. doi: 10.1016/j.ccell.2021.06.009. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090. doi: 10.1016/j.ccell.2021.06.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Veldt AAM, Oosting SF, Dingemans AC, et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27:568–569. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 10.den Hartog G, Vos ERA, van den Hoogen LL, et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab172. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Hartog G, Schepp RM, Kuijer M, et al. SARS-CoV-2: specific antibody detection for seroepidemiology: a multiplex analysis approach accounting for accurate seroprevalence. J Infect Dis. 2020;222:1452–1461. doi: 10.1093/infdis/jiaa479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg CH, Ruys TA, Nanlohy NM, et al. Comprehensive longitudinal analysis of hepatitis C virus (HCV)-specific T cell responses during acute HCV infection in the presence of existing HIV-1 infection. J Viral Hepat. 2009;16:239–248. doi: 10.1111/j.1365-2893.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Studies Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Nov 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 16.Phillips KF. A new test of non-inferiority for anti-infective trials. Stat Med. 2003;22:201–212. doi: 10.1002/sim.1122. [DOI] [PubMed] [Google Scholar]
- 17.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 19.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 20.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edara VV, Pinsky BA, Suthar MS, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 23.Tarke A, Sidney J, Methot N, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: a single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. 2019;80:990–997. doi: 10.1016/j.jaad.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elboraey MO, Essa EESF. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:e139–e142. doi: 10.1016/j.oooo.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong J, Cuevas-Castillo F, Nassar M, et al. Bullous drug eruption after second dose of mRNA-1273 (Moderna) COVID-19 vaccine: case report. J Infect Public Health. 2021;14:1392–1394. doi: 10.1016/j.jiph.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dash S, Sirka CS, Mishra S, Viswan P. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021 doi: 10.1111/ced.14784. published online June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have formed an International Cancer Covid Vaccination Consortium. Thus, we aim to share data of vaccination against SARS-CoV-2 studies in patients receiving systemic cancer treatment, which might allow us to define more precisely how to protect patients best in which situation. Please contact the corresponding author if you wish to participate.