Abstract
Non-invasive respiratory support (NIRS) has increasingly been used in the management of COVID-19-associated acute respiratory failure, but questions remain about the utility, safety, and outcome benefit of NIRS strategies. We identified two randomised controlled trials and 83 observational studies, compromising 13 931 patients, that examined the effects of NIRS modalities—high-flow nasal oxygen, continuous positive airway pressure, and bilevel positive airway pressure—on patients with COVID-19. Of 5120 patients who were candidates for full treatment escalation, 1880 (37%) progressed to invasive mechanical ventilation and 3658 of 4669 (78%) survived to study end. Survival was 30% among the 1050 patients for whom NIRS was the stated ceiling of treatment. The two randomised controlled trials indicate superiority of non-invasive ventilation over high-flow nasal oxygen in reducing the need for intubation. Reported complication rates were low. Overall, the studies indicate that NIRS in patients with COVID-19 is safe, improves resource utilisation, and might be associated with better outcomes. To guide clinical decision making, prospective, randomised studies are needed to address timing of intervention, optimal use of NIRS modalities—alone or in combination—and validation of tools such as oxygenation indices, response to a trial of NIRS, and inflammatory markers as predictors of treatment success.
Introduction
Of the 246 million people infected with the SARS-CoV-2 virus by Oct 29, 2021, almost 5 million had died.1 Among patients admitted to hospital with COVID-19, the predominant presenting feature is hypoxaemic respiratory failure, which often requires additional respiratory support over and above standard oxygen therapy. However, best practice remains unknown for this population and will be contingent on local availability of resources and trained staff.
Non-invasive respiratory support (NIRS) is routinely used in other conditions associated with acute hypoxaemic respiratory failure.2 Continuous positive airway pressure (CPAP) can be delivered via a hood or helmet, or a tight-fitting partial or full face mask. Bilevel positive airway pressure ventilation (BiPAP), primarily used in hypercapnic (type 2) respiratory failure, uses a similar interface to deliver additional inspiratory support over a continuous positive-pressure background. High-flow nasal oxygen (HFNO) devices, which can deliver 30–60 L/min (or higher) humidified gas flow via specially adapted nasal cannulae, reduce dead-space ventilation and deliver dynamic positive airway pressure. A recent meta-analysis showed that treatment with NIRS strategies (helmet or face mask non-invasive ventilation or HFNO) was associated with a lower risk of death in adults with acute hypoxaemic respiratory failure compared with standard oxygen therapy.2
Initial guidance from WHO cautioned against the use of NIRS, citing potential risks of health-care worker infection through aerosolisation of viral particles, and patient self-inflicted lung injury from prolonged spontaneous hyperventilation.3 However, the evidence base was so weak that 26 separate guidelines, most published before April 2020, offered a highly conflicting range of recommendations, including the following: avoidance of HFNO but use of CPAP only as a bridge to mechanical ventilation; use of HFNO but avoidance of non-invasive ventilation; or a preference for HFNO over non-invasive ventilation.4
Rising case numbers in China, Europe, and the USA in the spring of 2020, allied with shortages of mechanical ventilators and intensive care unit (ICU) beds,5 led to NIRS being increasingly adopted outside ICUs, with guidelines altered accordingly. Nonetheless, the role and benefits of CPAP and HFNO in the management of COVID-19 remain contentious, with lively debates about the timing of intubation and the risk–benefit balance between patient self-inflicted lung injury and ventilator-induced lung injury.6, 7, 8, 9 In this Personal View, we aim to provide an overview of what has been learnt so far about outcomes in patients with COVID-19 who received one or more NIRS modalities. We reviewed observational studies and randomised controlled trials (RCTs) of NIRS in COVID-19 pneumonia, examining duration of use, outcomes, predictors of success (ie, survival) or failure (ie, death or need for subsequent invasive mechanical ventilation), and changes in clinical practice over the course of the COVID-19 pandemic. We present our personal opinion as to what the findings mean for clinical decision making, and outline future directions for research and clinical practice.
Key messages.
-
•
NIRS techniques (HFNO, CPAP, and BiPAP) were increasingly used in patients with COVID-19 respiratory failure with the aim of avoiding the need for invasive mechanical ventilation before data on safety and efficacy were available from RCTs
-
•
Only two RCTs comparing two modalities of NIRS in patients with COVID-19 have been reported, but many retrospective and prospective observational studies of NIRS have been published; marked heterogeneity exists between studies in terms of patient populations, including age and existing comorbidities, baseline illness severity, ward settings, and techniques used, either alone or in combination
-
•
Ceiling of treatment (respiratory support) was variably reported; regardless of non-invasive modality, patients who were candidates for full treatment escalation had better outcomes (78% survival at study end vs 30% for patients in whom NIRS was a ceiling of treatment)
-
•
37% of patients for full treatment escalation who received NIRS progressed to invasive ventilation; the two RCTs indicate that non-invasive ventilation reduces the need for intubation compared with high-flow nasal oxygen
-
•
In multivariable analyses, age, comorbidities, baseline illness severity, degree of respiratory dysfunction before initiation of NIRS, response in respiratory variables to a trial of NIRS, and baseline concentrations of inflammatory markers were commonly identified as predictors of NIRS success or failure
-
•
Well designed RCTs are needed to provide an evidence base for optimised selection and use of NIRS devices, and appropriate use of prone positioning and adjuvant therapies, in the context of an individualised approach to management
BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. HFNO=high-flow nasal oxygen. NIRS=non-invasive respiratory support. RCT=randomised controlled trial.
Studies of NIRS in COVID-19
Search strategy and selection criteria
We searched PubMed, Embase, ScienceDirect, Scopus, Google Scholar, and medRxiv for relevant studies published in English from Jan 1, 2020, to June 1, 2021, using the search terms (“COVID” OR “COVID-19” OR “SARS nCoV”) AND (“CPAP” OR “continuous positive airway pressure” OR “NIV” OR “non-invasive ventilation” OR “NIPPV” OR “non-invasive positive pressure ventilation” OR “HFNO” OR “high-flow nasal oxygen” OR “HFNOT” OR “high-flow nasal oxygen therapy” OR “HFNC” OR “high-flow nasal cannula” OR “BiPAP” OR “bilevel positive pressure ventilation”). The following terms were also included to improve the scope and relevance of the search: “ARDS” OR “acute respiratory failure” OR “ARF” OR “hypoxaemia” OR “prone”. Articles that did not include an assessment of CPAP, non-invasive ventilation, or HFNO to treat COVID-19 pneumonia were excluded. Reference lists (including those from reviews) were searched for articles not otherwise identified. Review articles were excluded unless they contained data not reported elsewhere. Letters, position papers, guidelines, case reports, and case series were excluded if they did not present original data or if the evidence presented was of poor quality or relevance. Nomenclature was often inconsistent, with the term non-invasive ventilation being used to describe either BiPAP alone, or both BiPAP and CPAP. Both are included as non-invasive ventilation where the precise modality was not specified. Literature searches were conducted by SW, PA, JG, and MS on the basis of prespecified inclusion criteria, which are summarised in the appendix (p 1). SW, PA, MS, and HEM reviewed 98 full-text articles for relevance to patient outcomes with the use of NIRS, conducted additional searches to avoid omissions, and identified other relevant papers that did not appear under the search categories. MS read each identified paper to confirm the accuracy of data extraction. The PRISMA statement guided our review and reporting.10
Data extraction and interpretation
The following data were extracted, where stated: country in which the study was done, type of hospital (university or community), clinical setting (ward, high-dependency unit, or ICU), type of NIRS used (HFNO, CPAP, or BiPAP), number of patients receiving a particular NIRS option, rates and duration of support in patient subsets in which the outcome was deemed to be a success or a failure (ie, outcome of subsequent invasive mechanical ventilation or death), complication rates, and mortality rates (eg, in-ICU, in-hospital, 28-day, 30-day, or 60-day mortality). Where provided, device-specific outcome data were recorded for patients who were candidates for full treatment escalation (including mechanical ventilation), if considered necessary, or for those in whom NIRS represented a ceiling of treatment. Physiological and laboratory predictors of NIRS success or failure, and associated values, were also extracted, including any multivariable and univariate analyses, where stated. Summary statistics were generated using Microsoft Excel software 2010 (version 14.7.2) as mean and SE if normally distributed, as determined by the Kolmogorov-Smirnov test of normality, or median and IQR if not. Mortality rates are reported as in the original papers (ie, unadjusted).
Identified publications
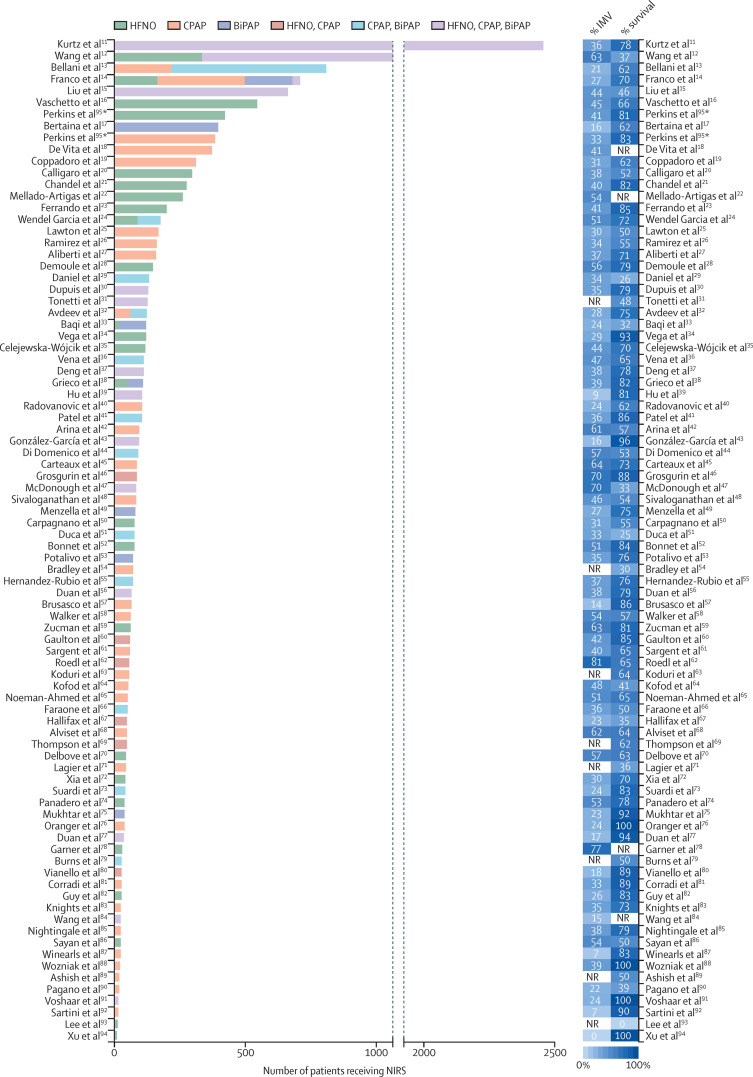
Search terms yielded 84 articles relating to a total of 13 140 patients after exclusion of duplicates, and after screening titles, abstracts, and full text for relevance specifically to outcomes with the use of NIRS in patients with COVID-19 (appendix p 1).11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 Most papers originated from western European countries (62 studies), China (nine), and the USA (seven). A report of one large RCT from the UK was published as a preprint95 after our original search and, because of its size, is included for completeness, giving a total of 13 931 patients (figure 1 ).
Figure 1.
Summary of studies identified for review
85 studies (two multicentre RCTs, 22 prospective observational studies, and 61 retrospective observational studies) were included in our review. BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. HFNO=high-flow nasal oxygen. IMV=invasive mechanical ventilation. NIRS=non-invasive respiratory support. NR=not reported. RCT=randomised controlled trial. *RCT by Perkins et al95 included CPAP versus conventional oxygen therapy and HFNO versus conventional oxygen therapy, and therefore appears twice.
Other than two multicentre RCTs from Italy and the UK,38, 95 22 prospective and 61 retrospective observational studies were identified, reporting a median of 71 patients (IQR 40–127) per study.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 Of these observational studies, 34 were multicentre and 49 single-centre studies. In total, 14 studies were based in community hospitals, 35 in university hospitals, and 16 in mixed institutions; the type of hospital was not specified in 20 studies. Patients were located in non-ICU wards (24 studies), in ICUs (42), or in both (six); the location was not specified in 13 reports.
In total, 29 papers reported outcomes with CPAP, 23 with HFNO, and 6 with BiPAP as the sole modality of treatment. Use of two modalities was reported in 17 studies, and all three in 17 studies, although the reports rarely specified whether individual patients received more than one modality. In some cases, patients escalated from HFNO to CPAP or BiPAP to offer increased respiratory support; in others, CPAP or BiPAP were switched to HFNO due to device, mask, or hood intolerance. 16 studies reported the levels of positive end-expiratory pressure used (median 10 cm H2O [IQR 7·5–11·0]). The median duration of NIRS, where reported, is shown in tables 1–3.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95 Results were variable, but most studies revealed a shorter duration of NIRS use in those for whom the intervention was deemed to be a failure (ie, outcome of death or escalation to invasive mechanical ventilation, depending on the ceiling of treatment).
Overall, survival in patients receiving NIRS across the full 85 studies was 66·3% (8702 of 13 125 patients), with survival reported from 7 days to 60 days. 59 studies reported longer-term outcomes (hospital survival or 60-day survival), in which 65% of patients (6132 of 9388) survived. The observational studies were too heterogeneous in terms of patient demographics, disease severity, and ward location to draw conclusions about superiority of one technique over another. The Italian RCT from Grieco and colleagues38 compared HFNO with BiPAP in 109 patients; although the difference in median days free of respiratory support was not statistically significant, the rate of endotracheal intubation was significantly lower in the BiPAP group (30%) compared with those randomised to HFNO (51%), with more days free of invasive mechanical ventilation. Hospital mortality was similar between the groups. The Recovery-RS study from the UK was a three-arm, open-label, adaptive RCT with 1272 patients randomly assigned to receive CPAP, HFNO, or conventional oxygen therapy.95 The primary composite outcome of need for tracheal intubation or mortality within 30 days was significantly lower for those who received CPAP (36·3%) compared with conventional oxygen therapy (44·4%). There was no difference between HFNO (44·4%) and conventional oxygen therapy (45·1%). Compared with oxygen therapy, there were relative reductions in mortality of 13·9% for CPAP and 6·5% for HFNO, and relative reductions in the need for intubation of 19·1% for CPAP versus oxygen and 1·2% for HFNO.
Very few papers reported complications specifically related to the use of NIRS. Rates were low for studies that did report complications.27, 37, 46, 80, 87, 95
Outcomes of NIRS in COVID-19
Patients with NIRS as ceiling of treatment
22 studies comprising 1050 patients (median number of patients per study 26·5 [IQR 13–67]) specifically reported outcomes in those patients with a ceiling of treatment that included a do-not-intubate order (table 1 ). All but five were from Italian or UK centres. Some studies included patients not offered high-dependency unit or ICU admission. Mean age, where reported, was 70 (SE 1·5) years. 15 studies (704 patients) used CPAP only, whereas HFNO was used in two small studies totalling 23 patients. Five studies (323 patients) used a combination of NIRS approaches.
Table 1.
Outcomes in patients with NIRS used as a ceiling of treatment
| Patients, N | Location* | Survival assessment | Survival, n (%) |
Duration of support, median (IQR) days |
||
|---|---|---|---|---|---|---|
| NIRS success† | NIRS failure† | |||||
| HFNO only | ||||||
| Delbove et al (France)70 | 11 | Ward | Hospital | 5 (45%) | .. | .. |
| Lee et al (Korea)93 | 12 | Ward | Hospital | 0 (0%) | .. | .. |
| Total‡ | 23 | .. | .. | 5 (22%); range 0–45% | .. | .. |
| CPAP only | ||||||
| Aliberti et al (Italy)27 | 65 | HDU | Hospital | 29 (45%) | 7 (4–12) | 5 (3–10) |
| Alviset et al (France)68 | 8 | HDU, ICU | Hospital | 6 (75%) | .. | .. |
| Arina et al (UK)42 | 16 | ICU | Hospital | 2 (13%) | .. | .. |
| Bradley et al (UK)54 | 70 | Ward | 30-day | 21 (30%) | 5 (2–9) | 2 (1–4) |
| Brusasco et al (Italy)57 | 14 | HDU | Hospital | 10 (71%) | .. | .. |
| Coppadoro et al (Italy)19 | 130 | Ward | Hospital | 36 (28%) | 5 (4–8) | 4 (2–5) |
| Kofod et al (Denmark)64 | 26 | Ward | Hospital | 2 (8%) | .. | .. |
| Lagier et al (France)71 | 44 | Ward | HFNO ward | 16 (36%) | 10 (4–25) | .. |
| Lawton et al (UK)25 | 89 | HDU | Hospital | 25 (28%) | .. | .. |
| Noeman-Ahmed et al (UK)65 | 11 | HDU | Hospital | 1 (9%) | .. | .. |
| Ramirez et al (Italy)26 | 38 | Ward | Hospital | 11 (29%) | .. | .. |
| Sivaloganathan et al (UK)48 | 24 | HDU | Hospital | 4 (17%) | .. | .. |
| Vaschetto et al (Italy)16 | 140 | Ward | 60-day | 38 (27%) | .. | .. |
| Walker et al (UK)58 | 19 | Ward, ICU | Hospital | 3 (16%) | .. | .. |
| Winearls et al (UK)87 | 10 | Respiratory HDU | 28-day | 6 (60%) | .. | .. |
| Total‡ | 704 | .. | .. | 210 (30%); range 8–75% | .. | .. |
| CPAP, BiPAP | ||||||
| Bellani et al (Italy)13 | 215 | HDU | 60-day | 70 (33%) | .. | .. |
| Burns et al (UK)79 | 28 | Respiratory ward | Hospital | 14 (50%) | 5 (1–14) | 3 (1–13) |
| Di Domenico et al (Italy)44 | 27 | HDU | Hospital | 3 (11%) | .. | .. |
| Faraone et al (Italy)66 | 25 | Ward | Hospital | 3 (12%) | .. | .. |
| Total‡ | 295 | .. | .. | 90 (31%); range 11–50% | .. | .. |
| HFNO, CPAP, BiPAP | ||||||
| Franco et al (Italy)14 | 28 | HDU | Hospital | 8 (29%) | .. | .. |
| Total | 28 | .. | .. | 8 (29%) | .. | .. |
BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. HDU=high-dependency unit. HFNO=high-flow nasal oxygen. ICU=intensive care unit. NIRS=non-invasive respiratory support.
Hospital location, if stated.
Success means survival of patient receiving NIRS; failure means death of patient receiving NIRS.
Survival figures totalled regardless of timing (28-day, 30-day, 60-day, or hospital survival); range represents range of values reported across studies.
Median duration of NIRS treatment was reported in only five studies; duration of treatment was shorter for those in whom NIRS was deemed to be a failure (ie, outcome of death). Overall survival rate was 29·8% (median 29% [IQR 15–45]) with a survival cutoff ranging from 28 days to 60 days or hospital discharge.
Patients for full escalation to invasive ventilation
40 studies reported data from 5120 patients for whom full escalation of treatment was specifically stated, with outcomes provided for 4669 patients (table 2 ). Median number of patients per study was 61 (IQR 36–102). 27 of the studies included patients from hospitals in Italy, France, and the UK. Mean age was 64 (SE 1–2) years. When used as the sole NIRS modality, progression to invasive ventilation occurred in a median of 41% (IQR 29–52) of patients initially managed with HFNO (11 studies; 1331 patients), 39% (IQR 31–51) of those managed with CPAP (20 studies; 2445 patients), and 27% (range 23–28) of those managed with BiPAP (three studies; 270 patients). For the ten studies comprising 1074 patients receiving CPAP, BiPAP, HFNO, or a combination, 330 (31% [IQR 36–70]) required invasive mechanical ventilation. Overall, of 5120 patients, 1880 (37%) progressed to invasive ventilation.
Table 2.
Outcomes in candidates for full escalation to intubation and mechanical ventilation
| Patients, N | Location* | Need for IMV, n (%) | Survival assessment | Survival, n (%) |
Duration of support, median (IQR) days |
||
|---|---|---|---|---|---|---|---|
| NIRS success† | NIRS failure† | ||||||
| HFNO only | |||||||
| Bonnet et al (France)52 | 76 | ICU | 39 (51%) | 60-day | 64 (84%) | .. | .. |
| Celejewska-Wójcik et al (Poland)35 | 116 | .. | 51 (44%) | 30-day | 81 (70%) | 7 (5–11) | 2 (2–6) |
| Chandel et al (USA)21 | 272 | Ward, ICU | 108 (40%) | Hospital | 223 (82%) | 4 (2–7) | 2 (1–4) |
| Delbove et al (France)70 | 35 | Ward, ICU | 20 (57%) | Hospital | 28 (80%) | 6 (4–8) | 2 (1–5) |
| Franco et al (Italy)14 | 163 | HDU | 47 (29%) | 30-day | 137 (84%) | .. | .. |
| Garner et al (USA)78 | 30 | .. | 23 (77%) | .. | .. | .. | .. |
| Grieco et al (Italy)38 | 55 | ICU | 28 (51%) | 28-day | 44 (80%) | .. | 0·9 (0·2–2·7) |
| Panadero et al (Spain)74 | 40 | Respiratory HDU | 21 (53%) | 20-day | 31 (78%) | 6 (5–8) | 2 (1–4) |
| Perkins et al (UK)95 | 414 | HDU, ICU | 170 (41%) | 30-day | 337 (81%) | .. | 1 (0–4) |
| Vega et al (Italy, Argentina)34 | 120 | Ward | 35 (29%) | .. | 111 (93%) | .. | .. |
| Xu et al (China)94 | 10 | .. | 0 (0%) | Hospital | 10 (100%) | .. | .. |
| Total‡ | 1331 | .. | 542 (41%); range 0–77% | .. | 1066 of 1301 (82%); range 70–100% | .. | .. |
| CPAP only | |||||||
| Aliberti et al (Italy)27 | 92 | HDU | 34 (37%) | Hospital | 83 (90%) | 7 (4–12) | 3 (2–5) |
| Alviset et al (France)68 | 39 | HDU, ICU | 24 (62%) | Hospital | 27 (69%) | 4 (3–7) | 2 (2–3) |
| Arina et al (UK)42 | 77 | ICU | 47 (61%) | Hospital | 51 (66%) | 2 (1–6) | 3 (1–5) |
| Brusasco et al (Italy)57 | 50 | HDU | 7 (14%) | Hospital | 45 (90%) | .. | .. |
| Carteaux et al (France)45 | 85 | ICU, HDU | 54 (64%) | 28-day | 62 (73%) | 4·0 (1·5–5·5) | 2 (1–3) |
| Coppadoro et al (Italy)19 | 176 | Ward | 54 (31%) | Hospital | 154 (88%) | 6 (4–9) | 4 (3–7) |
| Corradi et al (Italy)81 | 27 | ICU | 9 (33%) | Hospital | 24 (89%) | .. | .. |
| De Vita et al (Italy)18 | 367 | HDU | 150 (41%) | .. | .. | 8 (5–12) | 4 (2–6) |
| Franco et al (Italy)14 | 330 | HDU | 82 (25%) | 30-day | 230 (70%) | .. | .. |
| Kofod et al (Denmark)64 | 27 | Ward | 13 (48%) | Hospital | 20 (74%) | .. | .. |
| Lawton et al (UK)25 | 76 | HDU, ICU | 23 (30%) | Hospital | 58 (76%) | .. | .. |
| Nightingale et al (UK)85 | 24 | ID ward | 9 (38%) | Hospital | 19 (79%) | 4·5 (2·5–5·5) | 0·2 (0·1–0·4) |
| Noeman-Ahmed et al (UK)65 | 41 | HDU | 21 (51%) | Hospital | 33 (80%) | .. | .. |
| Perkins et al (UK)95 | 377 | HDU, ICU | 126 (33%) | 30-day | 315 (83%) | .. | .. |
| Ramirez et al (Italy)26 | 121 | Ward | 41 (34%) | Hospital | 97 (80%) | .. | .. |
| Sivaloganathan et al (UK)48 | 58 | HDU | 27 (46%) | Hospital | 8 of 11 (73%) | 3·0 (1·7–5·5) | 0·7 (0·2–1·3) |
| Vaschetto et al (Italy)16 | 397 | Ward | 180 (45%) | 60-day | 314 (79%) | 2 (1–3) | 3 (1–5) |
| Walker et al (UK)58 | 44 | HDU, ICU | 24 (54%) | Hospital | 33 (75%) | .. | .. |
| Winearls et al (UK)87 | 14 | HDU, ICU | 1 (7%) | 28-day | 14 (100%) | .. | .. |
| Wozniak et al (UK)88 | 23 | ICU | 9 (39%) | ICU | 23 (100%) | 5·9 (3·6)§ | .. |
| Total‡ | 2445 | .. | 935 (38%); range 7–64% | .. | 1610 of 2031 (79%); range 66–100% | .. | .. |
| BiPAP only | |||||||
| Franco et al (Italy)14 | 177 | HDU | 49 (28%) | 30-day | 123 (69%) | .. | .. |
| Grieco et al (Italy)38 | 54 | ICU | 16 (30%) | 28-day | 45 (83%) | .. | 1·2 (0·3–3·0) |
| Mukhtar et al (Egypt)75 | 39 | ICU | 9 (23%) | Hospital | 36 (92%) | .. | .. |
| Total‡ | 270 | .. | 74 (27%); range 23–30% | .. | 204 (76%); range 69–92% | .. | .. |
| HFNO, CPAP | |||||||
| Gaulton et al (USA)60 | 59 | ICU | 25 (42%) | End of study | 50 (85%) | .. | .. |
| Vianello et al (Italy)80 | 28 | ICU | 5 (18%) | 15-day post-ICU | 25 (89%) | .. | .. |
| Total‡ | 87 | .. | 30 (34%); range 18–42% | .. | 75 (86%); range 85–89% | .. | .. |
| CPAP, BiPAP | |||||||
| Avdeev et al (Russia)32 | 61 | HDU | 17 (28%) | Hospital | 44 (72%) | 8·0 (6·3–11·0) | 3·0 (2·5–8·0) |
| Bellani et al (Italy)13 | 583 | HDU | 123 (21%) | 60-day | 428 (73%) | .. | .. |
| Di Domenico et al (Italy)44 | 63 | HDU | 36 (57%) | Hospital | 45 (71%) | .. | .. |
| Faraone et al (Italy)66 | 25 | Ward | 9 (36%) | Hospital | 22 (88%) | 11 (10)§ | 5 (5)§ |
| Hernandez-Rubio et al (Spain)55 | 70 | HDU | 26 (37%) | 28-day | 53 (76%) | .. | .. |
| Total‡ | 802 | .. | 211 (26%); range 21–57% | .. | 592 (74%); range 71–88% | .. | .. |
| HFNO, CPAP, BiPAP | |||||||
| Duan et al (China)77 | 36 | ICU, HDU | 6 (17%) | Hospital | 34 (94%) | .. | .. |
| Duan et al (China)56 | 66 | .. | 25 (38%) | Hospital | 52 (79%) | 10·1 (6·0–12·3) | 1·6 (0·6–4·9) |
| McDonough et al (USA)47 | 83 | ICU | 58 (70%) | Hospital | 25 of 76 (33%) | .. | .. |
| Total‡ | 185 | .. | 89 (48%); range 17–70% | .. | 111 of 178 (62%); range 33–94% | .. | .. |
BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. HDU=high-dependency unit. HFNO=high-flow nasal oxygen. ICU=intensive care unit. ID=infectious diseases. IMV=invasive mechanical ventilation. NIRS=non-invasive respiratory support.
Hospital location, if stated.
Success means survival of patient receiving NIRS; failure means need for subsequent invasive mechanical ventilation or death of patient receiving NIRS.
Survival figures totalled regardless of timing (28-day, 30-day, 60-day, 15-day post-ICU, hospital, or end-of-study survival); range represents range of values reported across studies.
Mean (SD) reported.
Overall survival, using each study end value, was 78% (3658 of 4669 patients). Median survival was similar with all modalities: 79% (IQR 73–89) for CPAP only, 83% (79–89) for HFNO only, 76% (69–92) for BiPAP only, and 78% (72–88) for patients who received CPAP, BiPAP, or HFNO. The median duration of NIRS treatment was longer among patients for whom NIRS was a success in 13 of the 16 studies reporting data.
Patients with full escalation not specified
42 studies comprising 7761 patients (median number of patients per study 79 [IQR 41–127]) reported outcomes in patients for whom a do-not-intubate order was not specified, or in patients who were reported to have died while on NIRS but for whom the do-not-intubate status was not provided (table 3 ). 29 studies from European centres, five from China, and four from the USA were reported. Mean age was 64 (SE 1·1) years. When used as the sole NIRS modality, progression to invasive ventilation occurred in 42% (IQR 30–54) of those initially managed with HFNO (12 studies; 1570 patients), 29% (20–36) of those managed with CPAP (eight studies; 387 patients), and 23% (16–38) of those who received BiPAP (three studies; 569 patients). For the 21 studies comprising 5061 patients who received CPAP, BiPAP, or HFNO, 35% (IQR 23–44) required invasive ventilation.
Table 3.
Outcomes in patients for whom escalation to intubation and mechanical ventilation was not specified
| Patients, N | Location* | Need for IMV, n (%) | Survival assessment | Survival, n (%) |
Duration of support, median (IQR) days |
||
|---|---|---|---|---|---|---|---|
| NIRS success† | NIRS failure† | ||||||
| HFNO only | |||||||
| Baqi et al (Pakistan)33 | 21 | Ward, ICU | 5 (24%) | Hospital | 11 (52%) | .. | .. |
| Calligaro et al (South Africa)20 | 293 | ICU, ward | 111 (38%) | Hospital | 139 of 269 (52%) | 6 (3–9) | 4 (2–6) to death; 2 (0·5–5) to IMV |
| Carpagnano et al (Italy)50 | 78 | HDU | 24 (31%) | Hospital | 43 (55%) | .. | .. |
| Demoule et al (France)28 | 146 | ICU | 82 (56%) | 60-day | 115 (79%) | .. | .. |
| Ferrando et al (Spain)23 | 199 | ICU | 82 (41%) | ICU | 146 of 171 (85%) | .. | .. |
| Guy et al (France)82 | 27 | Respiratory ward | 7 (26%) | Hospital | 19 of 23 (83%) | .. | .. |
| Mellado-Artigas et al (Spain)22 | 259 | ICU | 140 (54%) | .. | .. | .. | .. |
| Sayan et al (Turkey)86 | 24 | ICU | 13 (54%) | Hospital | 12 (50%) | .. | .. |
| Wang et al (USA)12 | 331 | .. | 100 (30%) | Hospital | 189 (57%) | 10·2 (6·7–15·5) | 1·4 (0·5–3·8) |
| Wendel Garcia et al (Europe)24 | 87 | ICU | 45 (52%) | ICU | 70 (80%) | .. | .. |
| Xia et al (China)72 | 43 | .. | 13 (30%) | Hospital | 30 (70%) | 5 (3–7) | 3·5 (1·5–6·5) |
| Zucman et al (France)59 | 62 | ICU | 39 (63%) | ICU | 50 (81%) | .. | .. |
| Total‡ | 1570 | .. | 661 (42%); range 24–63% | .. | 824 of 1255 (66%); range 50–85% | .. | .. |
| CPAP only | |||||||
| Ashish et al (UK)89 | 18 | Ward | .. | Hospital | 9 (50%) | .. | .. |
| Knights et al (UK)83 | 26 | .. | 9 (35%) | Hospital | 19 (73%) | .. | .. |
| Koduri et al (UK)63 | 56 | Ward | .. | Hospital | 36 (64%) | .. | .. |
| Oranger et al (France)76 | 38 | Respiratory ward | 9 (24%) | 7-day | 38 (100%) | .. | .. |
| Potalivo et al (Italy)53 | 71 | ED, ward | 25 (35%) | 60-day | 54 (76%) | 3·9 (2·0)§ | 3·6 (2·6)§ |
| Radovanovic et al (Italy)40 | 105 | Respiratory HDU | 25 (24%) | Hospital | 65 (62%) | .. | .. |
| Sargent et al (UK)61 | 58 | .. | 23 (40%) | Hospital | 38 (65%) | .. | .. |
| Sartini et al (Italy)92 | 15 | Non-ICU | 1 (7%) | 14-day | 9 of 10 (90%) | .. | .. |
| Total‡ | 387 | .. | 92 of 313 (29%); range 7–40% | .. | 268 of 382 (70%); range 50–100% | .. | .. |
| BiPAP only | |||||||
| Baqi et al (Pakistan)33 | 100 | Ward, ICU | 38 (38%) | Hospital | 28 (28%) | .. | .. |
| Bertaina et al (Spain, Italy, China, Cuba, Ecuador, Germany)17 | 390 | .. | 62 (16%) | Hospital | 243 (62%) | .. | .. |
| Menzella et al (Italy)49 | 79 | Respiratory ward | 21 (27%) | Hospital | 59 (75%) | 8·7 (3·9)§ | 6·3 (4·2) to death; 2·9 (3·2) to IMV§ |
| Total‡ | 569 | .. | 131 (23%); range 16–38% | .. | 330 (58%); range 28–75% | .. | .. |
| HFNO, CPAP | |||||||
| Grosgurin et al (Switzerland)46 | 85 | HDU | 33 (39%) | 28-day | 75 (88%) | 4·1 (2·9)§ | 1·2 (0·7)§ |
| Hallifax et al (UK)67 | 48 | Respiratory HDU | 11 (23%) | Hospital | 15 of 43 (35%) | .. | .. |
| Pagano et al (Italy)90 | 18 | HDU | 4 (22%) | Hospital | 7 (39%) | .. | .. |
| Thompson et al (UK)69 | 47 | .. | .. | 30-day post-discharge | 29 (62%) | .. | .. |
| Total‡ | 198 | .. | 48 of 151 (32%); range 22–39% | .. | 126 of 193 (65%); range 35–88% | .. | .. |
| CPAP, BiPAP | |||||||
| Daniel et al (USA)29 | 131 | .. | 44 (34%) | Hospital | 34 (26%) | .. | .. |
| Duca et al (Italy)51 | 78 | ED | 26 (33%) | Hospital | 20 (25%) | .. | .. |
| Suardi et al (Italy)73 | 41 | ICU, ward | 10 (24%) | Hospital | 34 (83%) | .. | .. |
| Vena et al (Italy)36 | 111 | Ward, ICU | 53 (47%) | Hospital | 72 (65%) | .. | .. |
| Wendel Garcia et al (Europe)24 | 87 | ICU | 43 (49%) | ICU | 55 (63%) | .. | .. |
| Total‡ | 448 | .. | 176 (39%); range 24–49% | .. | 215 (48%); range 25–83% | .. | .. |
| HFNO, CPAP, BiPAP | |||||||
| Deng et al (China)37 | 110 | Ward | 42 (38%) | Hospital | 86 (78%) | .. | .. |
| Dupuis et al (France)30 | 128¶ | ICU | 45 (35%) | 60-day | 100 (79%) | .. | .. |
| González-García et al (Spain)43 | 93 | .. | 15 (16%) | Hospital | 89 (96%) | .. | .. |
| Hu et al (China)39 | 105 | Ward | 9 (9%) | Hospital | 85 (81%) | 6 (3·5–8·5) | 3 (2–11) |
| Kurtz et al (Brazil)11 | 2423 | ICU | 865 (36%) | Hospital | 1893 (78%) | .. | .. |
| Liu et al (China)15 | 652 | ICU | 288 (44%) | 28-day post-ICU admission | 297 (46%) | HFNO 9 (5–11); NIV 6 (4–10); HFNO+NIV 11 (8–19) | HFNO 4 (2–7); NIV 4 (2–8); HFNO+NIV 9 (6–15) |
| Patel et al (USA)41 | 104 | Ward | 37 (36%) | Hospital | 89 (86%) | 3·1 (2·7)§ | 5·4 (3·3)§ |
| Roedl et al (Germany)62 | 57 | ICU | 46 (81%) | ICU | 37 (65%) | .. | .. |
| Tonetti et al (Italy)31 | 127 | ED, ward, HDU | .. | 28-day | 61 (48%) | .. | .. |
| Voshaar (Germany)91 | 17 | Ward | 4 (24%) | Hospital | 17 (100%) | .. | .. |
| Wang et al (China)84 | 26 | .. | 4 (15%) | .. | .. | .. | .. |
| Wang et al (USA)12 | 747 | .. | 580 (78%) | Hospital | 214 of 711 (30%) | 11·2 (6·8–17·6) | 2·6 (0·8–6·7) |
| Total‡ | 4589 | .. | 1935 of 4462 (43%); range 9–81% | .. | 2968 of 4527 (66%); range 30–100% | .. | .. |
BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. ED=emergency department. HDU=high-dependency unit. HFNO=high-flow nasal oxygen. ICU=intensive care unit. ID=infectious diseases. IMV=invasive mechanical ventilation. NIRS=non-invasive respiratory support. NIV=non-invasive ventilation (CPAP or BiPAP).
Hospital location, if stated.
Success means survival of patient receiving NIRS; failure means need for subsequent invasive mechanical ventilation or death of patient receiving NIRS.
Survival figures totalled regardless of timing (7-day, 14-day, 28-day, 60-day, 28-day post-ICU admission, 30-day post-discharge, or ICU or hospital survival); range represents the range of values reported across studies.
Mean (SD) reported.
16 of the 128 received oxygen only.
Overall survival (ranging from ICU survival to 60-day survival) was 64·1%, reflecting the mix of patients who were or were not candidates for full escalation. Survival was similar with all individual modalities: 70% (IQR 53–81) for HFNO only, 69% (63–87) for CPAP only, and 62% (28–75) for BiPAP only. For studies of patients receiving CPAP, BiPAP, or HFNO, median survival was 65% (IQR 41–83). In eight of ten studies reporting, median duration of NIRS treatment was longer in patients for whom the intervention was a success.
Predictors of failure of NIRS
54 papers described risk factors for failure of NIRS, in which the outcome was need for invasive ventilation or death if not for escalation (appendix pp 2–14). The larger studies performed multivariable analyses, albeit on widely differing variables. A summary of frequently reported multivariable and univariate factors is shown in panel 1 . Multivariable risk factors for failure included increasing age, number of comorbidities, illness severity, degree of hypoxaemia on hospital admission, degree of respiratory failure before institution of NIRS—often described as pulse oximetry oxygen saturation to fraction of inspired oxygen (SpO2/FiO2) ratio, partial pressure of arterial oxygen to FiO2 (PaO2/FiO2) ratio, or SpO2/FiO2 ratio divided by respiratory rate (ROX index)—poor improvement in respiratory function following a trial of NIRS over 1–6 h, and a raised concentration of C-reactive protein. Univariate risk factors included other inflammatory markers, male sex, and respiratory rate, but these were often not carried over as independent predictors.
Panel 1. Predictors of NIRS failure.
A variety of predictors of NIRS success (ie, survival) or failure (ie, death or need for subsequent invasive mechanical ventilation) have been identified in multivariable and univariate analyses. Frequently reported factors associated with treatment failure are listed.
Multivariable analyses
-
•
Older age
-
•
Presence, number, or type of comorbidities
-
•
Illness severity on admission to hospital or ICU (eg, higher SOFA or APACHE score)
-
•
Worse oxygenation on admission to hospital
-
•
Worse respiratory function before NIRS (measured by PaO2/FiO2, SpO2/FiO2, or ROX index)
-
•
Response in respiratory variables to NIRS (change in PaO2/FiO2, SpO2/FiO2, ROX index, or respiratory rate)
-
•
Higher circulating concentration of C-reactive protein
Univariate analyses
-
•
Older age
-
•
Male sex
-
•
Presence, number, or type of comorbidities
-
•
Illness severity on admission to hospital or ICU (eg, higher SOFA or APACHE score)
-
•
Worse oxygenation on hospital admission
-
•
Higher respiratory rate
-
•
Worse respiratory function before NIRS (measured by PaO2/FiO2, SpO2/FiO2, or ROX index)
-
•
Response in respiratory variables to NIRS (change in PaO2/FiO2, SpO2/FiO2, ROX index, respiratory rate, or minute ventilation)
-
•
Inflammatory markers (C-reactive protein, lactate dehydrogenase, D-dimer, interleukin-6, lymphopenia, or procalcitonin)
APACHE=Acute Physiology and Chronic Health Evaluation. FiO2=fraction of inspired oxygen. NIRS=non-invasive respiratory support (continuous positive airway pressure, bilevel positive airway pressure, or high-flow nasal oxygen). PaO2=partial pressure of arterial oxygen. ROX index=respiratory rate and oxygenation index (SpO2/FiO2 ratio divided by respiratory rate). SOFA=Sequential Organ Failure Assessment. SpO2=pulse oximetry oxygen saturation.
Association of time to invasive ventilation and mortality
Some studies addressed the question of whether time to invasive ventilation has an effect on mortality. Dupuis and colleagues30 reported that early invasive ventilation (within 48 h of hospital admission) was associated with a higher rate of mortality at 60 days (42·7% vs 21·9% for those intubated after 48 h), ICU-acquired pneumonia, bacteraemia, and longer ICU stay. Chandel and colleagues21 reported no significant difference in hospital mortality or any secondary endpoint between those failing non-invasive ventilation early (at a median 1 [IQR 0–1] day) or late (median 4 [3–8] days). Daniel and colleagues29 found no difference in mortality between an intubation-first group versus those intubated after a period of non-invasive ventilation; however, mortality was significantly lower in those who could be maintained on non-invasive ventilation. Likewise, Menzella and colleagues49 found a similar mortality rate in patients who had a trial with BiPAP and then required intubation compared with those who remained on BiPAP.
Mellado-Artigas and colleagues22 propensity matched patients receiving invasive ventilation on day 1 of hospital admission against patients initially managed with HFNO. Use of HFNO was associated with significant increases in ventilator-free days and a reduction in ICU length of stay with no difference in hospital mortality. However, Vaschetto and colleagues16 reported that delay in intubation after CPAP was associated with an increased mortality risk (hazard ratio 1·093, 95% CI 1·010–1·184). Wendel Garcia and colleagues24 propensity matched patients in a multinational registry who received oxygen therapy, HFNO, non-invasive ventilation, or invasive ventilation on day 1 of ICU admission. Non-invasive ventilation was associated with the highest overall ICU mortality, albeit not significantly different from mortality among those who were invasively ventilated. However, mortality in patients who did not progress to intubation was 36% in the non-invasive ventilation group, suggesting that high numbers of these patients had a ceiling of treatment. By contrast, registry data from 126 Brazilian ICUs11 showed hospital mortality rates of 4·7% (73 of 1558 patients) for those managed on NIRS (predominantly CPAP or BiPAP) alone, 53% (457 of 865) for those who required escalation from NIRS to invasive ventilation, and 59% (1042 of 1765) for those who were invasively ventilated without a prior trial of NIRS. Compared with subsets of patients treated with NIRS only, the subsets requiring invasive ventilation after NIRS or requiring direct invasive ventilation were older, included more patients with frailty or comorbidities, and had a higher median illness severity and a lower median PaO2/FiO2 ratio. Patients who received invasive ventilation after a trial of NIRS were of a similar age and had similar levels of frailty, comorbidities, and illness severity, but a lower baseline PaO2/FiO2 ratio, compared with invasively ventilated patients who did not receive prior NIRS.
Changes in use of NIRS over time
National and network database studies from ICUs in the UK,96, 97 in France, Belgium, and Switzerland,98 in Germany,99 and in Brazil,11 and a study of total hospital population from the UK,100 have reported changes in management and outcomes of patients with COVID-19 over the first wave of the pandemic,11, 96, 98, 100 and between the first and second waves.97, 99 All found temporal decreases in mortality rates and length of stay for survivors of COVID-19, and a marked shift towards NIRS with significant reductions in the use of mechanical ventilation despite, where reported, comparable degrees of illness severity. Although modelling did suggest a relationship between mortality reduction and use of NIRS,11, 100 a causal relationship between NIRS and improved outcomes has not been confirmed.
Discussion
NIRS techniques (HFNO, CPAP, and BiPAP) have been widely and increasingly used in the management of COVID-19-related hypoxaemic respiratory failure. Initial fears about transmission to health-care workers wearing personal protective equipment have abated. Notably, spontaneous manoeuvres such as breathing, speaking and, particularly, coughing have recently been shown to generate more aerosol emission than CPAP or HFNO.101 The debate about optimal timing of ventilation continues, as does uncertainty regarding the relative risks of patient self-inflicted lung injury versus ventilator-induced lung injury.6, 7, 8, 9
85 observational studies11–37,39–94,96,97 and, to date, two single RCTs38, 95 report that, overall, invasive ventilation was avoided in 7710 (61·0%) of 12633 patients with COVID-19 who received NIRS; however, the proportion does vary according to patient demographics (age and comorbidity), baseline severity of respiratory and other organ dysfunction, ceiling of treatment, degree of systemic inflammation, and response to a trial of NIRS. Overall, approximately 81% of patients with no ceiling of treatment survive to at least 30 days, whereas about 30% of patients not deemed to be suitable for mechanical ventilation survive following treatment with NIRS.
From the observational studies, there was no clear difference in outcomes between use of different modalities of NIRS. However, marked heterogeneity of the patient populations, differing locations and expertise within the hospital, and wide variation in the baseline level of respiratory failure before starting NIRS make direct comparisons problematic. The Italian RCT reported a similar hospital mortality in patients randomised to either BiPAP or HFNO, although requirement for invasive ventilation was significantly lower in the BiPAP group (30% vs 51%).38 The Recovery-RS RCT reported superiority of CPAP, but no benefit from HFNO, over conventional oxygen therapy in terms of the composite outcome of need for intubation or death within 30 days.95
The use of non-invasive oxygenation strategies in adults with acute hypoxemic respiratory failure has been well established for several decades, although there remains a relative dearth of RCT data. A recent systematic review and meta-analysis identified 25 RCTs, comprising 3804 participants, that tested helmet or face mask non-invasive ventilation or HFNO against standard oxygen therapy.2 The authors reported, with moderate certainty, that all techniques lower the risks of endotracheal intubation and all-cause in-hospital mortality, although risk of bias due to lack of blinding was deemed to be high.
A significant proportion of patients will progress from NIRS to invasive ventilation, if deemed to be appropriate, or enter an end-of-life care pathway. The observational studies, unsurprisingly, highlight increasing age, underlying comorbidities, and other organ dysfunctions as risk factors (appendix pp 2–14). The three-times higher survival rates in candidates for full escalation over those in whom NIRS represents a ceiling of respiratory support reflects the greater frailty and poorer physiological reserve of the latter group. No studies have been done to identify thresholds of frailty or physiological reserve beyond which patients will not benefit from NIRS, although resource limitations will have an impact on capacity and the ability to provide NIRS.
Care should be taken in overall interpretation of the presented data because, so far, only two prospective RCTs have been reported.38, 95 Multiple factors could influence outcome data, including changes in caseload and management practices geographically or over time. Examples of variability include the following: application of NIRS to different patient groups, such as elderly cohorts, or to cohorts deemed to be unsuitable for further therapy escalation; within-hospital location; pressure settings or flow rates used for NIRS; and duration of use. Scarcity of ICU beds, invasive ventilators, specific NIRS devices, or oxygen supply might also have dictated changes in local practice. NIRS might have been delivered at times in areas (and by staff) not normally deployed for care of the critically ill, and with a restricted ability to monitor clinical progress. Such factors will probably have changed as clinical burden, experience, and organisational structures evolved.
The use of any particular modality of NIRS is generally at the physician's discretion, and thresholds for transition to NIRS or invasive mechanical ventilation might have varied greatly between countries, centres, and clinicians, and over time. In some cases, use of NIRS might have represented a ceiling of treatment, given resource limitations or perceived futility of invasive mechanical ventilation. Again, clinical experience and resource availability might have changed such evaluations over time. Use of voluntary prone positioning and adjuvant therapies such as corticosteroids might have been applied in some cases or centres and not in others, or with differing frequencies over time. Finally, mortality rates might represent underestimates, because outcomes in some papers were provided only for ICU stay or at 7, 14, or 28 days after initiation of NIRS; in other instances, a proportion of patients were still within the particular outcome threshold at the time of publication submission. Where possible, these factors have been identified.
Specific NIRS modalities are often used for specific indications (eg, HFNO for hypoxaemia and BiPAP for hypercapnic respiratory failure). The devices are often used in tandem, in a complementary manner, in routine clinical practice, escalating to CPAP or BiPAP if HFNO proves to be inadequate, reverting to HFNO if patients become mask-intolerant, or switching between modalities for breaks, sleep, and so on. Individual patients might not tolerate a particular technique—eg, due to claustrophobia or discomfort with high air flows, or use of nasal cannulae, tight-fitting mask, or helmet. The support device needs to suit the patient rather than vice versa. Clinician expertise will be an important factor in achieving better success rates through operator confidence, optimisation of device settings such as pressures and flow rates, verbal encouragement and calming, targeted use of sedation and anxiolytics, and achievement of sleep and adequate hydration.
Various clinical scales and nomograms have been proposed to identify a potential need for intubation, but have yet to be prospectively tested. For example, Apigo and colleagues102 developed a work-of-breathing score combining respiratory rate, nasal flaring and sternocleidomastoid use during inspiration, and abdominal muscle use during expiration. Liu and colleagues15 developed a complex nomogram based on age, Glasgow coma scale, ROX (respiratory rate and oxygenation) index, use of vasopressors, and number of comorbidities to compute a probability of NIRS failure. NIRS failure is predicted in many studies by a greater degree of respiratory failure before initiation of NIRS, as well as higher concentrations of circulating inflammatory markers, indicating a more severe underlying inflammatory disease process in the lung (appendix pp 2–14). However, even studies comprising patients with moderate-to-severe respiratory failure (as judged by baseline PaO2/FiO2 ratio, SpO2/FiO2 ratio, or ROX index) found that many patients survived with NIRS alone, and this survival could often be predicted by a positive response to a trial of NIRS over several hours.14, 18, 19, 21, 27, 34, 39, 41, 44, 46, 48, 53, 54, 55, 56, 59, 65, 66, 72, 74, 75, 77, 79, 93, 94
Clearly, the population of patients who recover with the use of NIRS alone will have a shorter length of ICU stay and a high survival rate; this association has been demonstrated in UK ICU database reports both across the first wave96 and between the first and second waves.97 In the first report,96 use of invasive ventilation fell from 85·0% during February–March 2020 to 61·1% in April–July 2020, despite equivalent patient demographics and illness severity; length of ICU stay in survivors fell from a mean (SD) of 16·5 (9·9) days to 14·1 (10·4) days, but remained unchanged in non-survivors, whereas 28-day in-hospital mortality fell from 43·6% to 33·6%. The second report97 notes that severity of respiratory failure (as judged by PaO2/FiO2 ratio in the first 24 h of ICU admission) was worse over the second wave of COVID-19, but use of invasive ventilation decreased from 72·1% in the first wave to 54·1% in the second wave; ICU length of stay in survivors fell from a median of 12 (IQR 5–28) days to 7 (4–14) days, although non-survivors were spending an extra 3 days in intensive care. Similar reductions in the use of invasive ventilation and overall mortality have been found in studies from Brazil,11 Francophone countries,98 and Germany.99 Potential confounders include increasing use of adjunct therapies such as dexamethasone, and greater overall expertise, confidence, and organisation in the management of critically ill patients with COVID-19. As noted in observational studies, much of the use of NIRS has occurred outside the ICU. Prospectively collected pan-hospital data from 247 UK hospitals during the first wave (March–August 2020) indicated that, after adjustment, there was a 19% reduction in the odds of mortality per 4-week period (odds ratio 0·81, 95% CI 0·79–0·83), and the greater use of non-invasive ventilation accounted for 22·2% (0·94, 0·94–0·96) of the reduction.100
Conclusions and future directions
Studies indicate that the use of NIRS in patients with COVID-19 is safe, reduces the need for intubation, improves resource utilisation, and might be associated with better outcomes. Moreover, NIRS might improve outcomes in patients receiving face mask oxygen who are not deemed to be suitable for escalation to invasive ventilation. However, outcomes also depend on patient factors such as age and comorbidities, and baseline illness severity prior to commencement of NIRS. From the two RCTs performed so far, non-invasive ventilation appears to be superior to HFNO in reducing the need for intubation, but many uncertainties remain.
The observational studies generally indicate that the duration of NIRS support is shorter in patients for whom NIRS fails (ie, in those who do not survive NIRS or who require escalation to invasive ventilation); however, there remains no clear indication as to the optimal timing of intubation in relation to subsequent outcomes. The current literature is conflicting, with studies variously reporting worse outcomes with either early intubation or late intubation, or no difference.16, 21, 22, 24, 29, 30, 49 Studies also report prolonged use (>1 week) of NIRS in a substantial number of patients who survived and did not progress to mechanical ventilation (Table 2, Table 3).
Clearly, the ideal evidence base would comprise the findings of well-designed, clinically relevant, prospective, randomised studies with appropriate outcomes. Such outcomes include duration of mechanical ventilation, need for ICU admission, length of stay in the ICU and in hospital, and both short-term and long-term mortality and morbidity. Unfortunately, few prospective studies in critically ill patients with COVID-19 have examined outcomes beyond 28–30 days, despite the fact that many patients remain hospitalised beyond this point.97 Long-term consequences in all patients with COVID-19 receiving different forms of non-invasive or invasive respiratory support, and over differing durations, are also unknown.
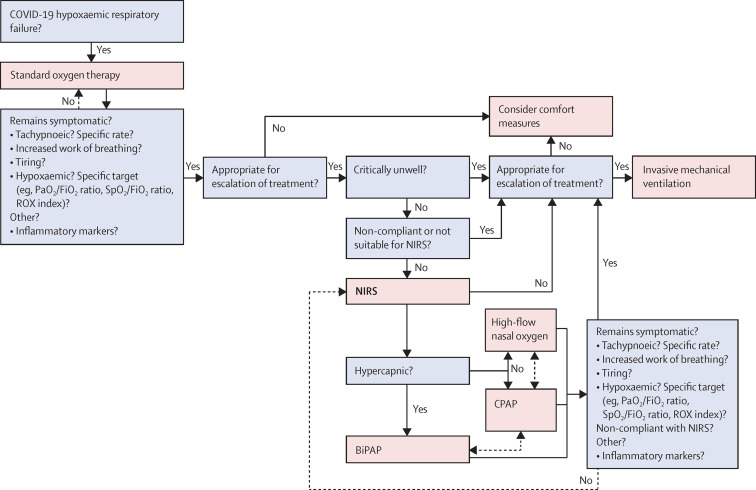
Figure 2 provides an overview of various decision points along the patient pathway, based on our distillation of the available evidence. However, many questions remain and we list in panel 2 what we consider to be priority areas for research. These include indications for the institution of NIRS (eg, identification of demographic factors, laboratory markers, and physiological and clinical characteristics that might be associated with treatment success or failure) and the question of whether early institution of NIRS can positively modify disease progression, as suggested by Lawton and colleagues.25 Studies should aim to provide an evidence base for an individualised approach to management, with optimised selection and use of appropriate NIRS device(s), and appropriate use of prone positioning. Objective criteria that assess the likelihood of patients with frailty and chronic comorbidity benefiting from either a trial of NIRS or progression to invasive ventilation need to be identified. Likewise, reliable markers predictive of NIRS failure need to be prospectively validated, especially across different hospital settings and countries, to guide optimal timing of intubation. Whether some or all patients benefit more from earlier intubation when NIRS failure is predicted rather than when it occurs is, we feel, a crucial question. Patients should not be left to struggle unduly for prolonged periods, but shortages of invasive ventilators and ICU beds might dictate otherwise at times.
Figure 2.
Decision points for respiratory support along the pathway of care
Suggestions for decision making in the provision of respiratory support for patients with COVID-19 hypoxaemic respiratory failure, based on our review of the available evidence. Choices marked by dashed lines are optional (eg, switch between high-flow nasal oxygen and CPAP, depending on availability of equipment). BiPAP=bilevel positive airway pressure non-invasive ventilation. CPAP=continuous positive airway pressure non-invasive ventilation. FiO2=fraction of inspired oxygen. NIRS=non-invasive respiratory support. PaO2=partial pressure of arterial oxygen. ROX index=respiratory rate and oxygenation index (SpO2/FiO2 ratio divided by respiratory rate). SpO2=pulse oximetry oxygen saturation.
Panel 2. Unanswered questions and priorities for research.
-
•
Which physiological and laboratory markers can be used to guide optimal timing of intubation and invasive mechanical ventilation in terms of patient outcomes (duration of mechanical ventilation, length of stay in the ICU and in hospital, and short-term and long-term mortality and morbidity)?
-
•
Does early institution of NIRS positively modify disease progression and outcomes?
-
•
Acknowledging the various indications and variability in patient compliance for the different modalities of NIRS, how can they best be used—singly or in combination—for individual patients (ie, personalised medicine)?
-
•
Does prone positioning during NIRS offer any outcome benefit?
-
•
Can thresholds of frailty or chronic comorbidity be identified beyond which patients will not benefit from either NIRS or invasive ventilation?
-
•
Should optimal strategies differ during periods of resource limitation, in different hospital settings and different geographical regions, and for patients deemed to be unlikely to benefit from escalation to invasive ventilation?
ICU=intensive care unit. NIRS=non-invasive respiratory support.
We advocate an international, collaborative, and coordinated approach to the design of future prospective, randomised studies, with prespecified methods and outcomes. Given careful selection of centres that have matched equipment in adequate supply, sufficiently powered RCTs should be possible across a broad range of centres. Whether such objective criteria for the selection of patients who are likely to benefit from a trial of NIRS can be applied during periods of resource limitation is also worth examining in future studies to gauge the impact on outcomes of rationing interventions.
This online publication has been corrected. The corrected version first appeared at thelancet.com/respiratory on November 16, 2021
Declaration of interests
MS and HEM were involved in the development of the UCL Ventura continuous positive airway pressure (CPAP) device, the manufacture of which was funded by the UK Department of Health and Social Care on a non-profit basis. MS chaired an educational webinar for Fisher & Paykel, which manufactures high-flow nasal oxygen devices. He reports grants, advisory board honoraria, and speaker's fees from Amormed, Apollo Therapeutics, Bayer, Baxter, Biotest, Critical Pressure, DSTL, General Electric, NewB, Roche Diagnostics, and Shionogi, outside of the submitted work. HEM consults for Scuba Travel International, which is developing a new closed-circuit, low-oxygen-use CPAP machine. The other authors declare no competing interests.
Acknowledgments
Acknowledgments
MS, HEM, the Institute of Sport, Exercise and Health, and the Bloomsbury Institute of Intensive Care Medicine are supported by the University College London Hospitals NHS Foundation Trust and University College London (UCLH/UCL) National Institute for Health Research Biomedical Research Centre. No funding was received for the writing of this Personal View.
Contributors
SW, MS, and HEM were responsible for conceptualisation of the article. SW contributed to the literature review, data analysis, writing of the first draft, and editing of all subsequent drafts. PA contributed to the literature search and review, and editing and writing of the bibliography. JG contributed to the literature search and data analysis. SC and GB-G designed and prepared figure 1. MS and HEM contributed to the literature review, data analysis, and writing and editing of all drafts, and provided higher-level intellectual input.
Supplementary Material
References
- 1.Google Coronavirus disease. Statistics: cases overview. https://www.google.com/search?client=firefox-b-d&q=deaths+from+covid+worldwide
- 2.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324:57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Jan 12, 2020. https://www.who.int/publications/i/item/10665-332299
- 4.Gorman E, Connolly B, Couper K, Perkins GD, McAuley DF. Non-invasive respiratory support strategies in COVID-19. Lancet Respir Med. 2021;9:553–556. doi: 10.1016/S2213-2600(21)00168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershengorn HB, Hu Y, Chen J-T, et al. The impact of high-flow nasal cannula use on patient mortality and the availability of mechanical ventilators in COVID-19. Ann Am Thorac Soc. 2021;18:623–631. doi: 10.1513/AnnalsATS.202007-803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Marini JJ, Camporota L. The respiratory drive: an overlooked tile of COVID-19 pathophysiology. Am J Respir Crit Care Med. 2020;202:1079–1080. doi: 10.1164/rccm.202008-3142ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobin MJ, Laghi F, Jubran A. P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care. 2020;10:105. doi: 10.1186/s13613-020-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JB, Weled B, Räsänen J, et al. Proposal for Coronavirus disease 2019 management. Crit Care Explor. 2020;2:e0127. doi: 10.1097/CCE.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruces P, Retamal J, Hurtado DE, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24:494. doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz P, Bastos LSL, Dantas LF, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47:538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JG, Liu B, Percha B, et al. Cardiovascular disease and severe hypoxemia associated with higher rates of non-invasive respiratory support failure in COVID-19. Crit Care Explor. 2021;3:e0355. doi: 10.1097/CCE.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellani G, Grasselli G, Cecconi M, et al. Noninvasive ventilatory support of COVID-19 patients outside the intensive care units (WARd-COVID) Ann Am Thorac Soc. 2021;18:1020–1026. doi: 10.1513/AnnalsATS.202008-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56 doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Health. 2021;3:e166–e174. doi: 10.1016/S2589-7500(20)30316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaschetto R, Barone-Adesi F, Racca F, et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res. 2021;7:00541–02020. doi: 10.1183/23120541.00541-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertaina M, Nuñez-Gil IJ, Franchin L, et al. Non-invasive ventilation for SARS-CoV-2 acute respiratory failure: a subanalysis from the HOPE COVID-19 registry. Emerg Med J. 2021;38:359–365. doi: 10.1136/emermed-2020-210411. [DOI] [PubMed] [Google Scholar]
- 18.De Vita N, Scotti L, Cammarota G, et al. Predictors of intubation in COVID-19 patients treated with out-of-ICU continuous positive airway pressure. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2020.12.010. published online Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppadoro A, Benini A, Fruscio R, et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: an observational study during the COVID-19 outbreak. Crit Care. 2021;25:80. doi: 10.1186/s13054-021-03502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandel A, Patolia S, Brown AW, et al. High-Flow Nasal Cannula therapy in COVID-19: using the ROX Index to predict success. Respir Care. 2021;66:909–919. doi: 10.4187/respcare.08631. [DOI] [PubMed] [Google Scholar]
- 22.Mellado-Artigas R, Ferreyro BL, Angriman F, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021;25:58. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando C, Mellado-Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24:597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care. 2021;25:175. doi: 10.1186/s13054-021-03580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawton T, Wilkinson KM, Corp A, et al. Reduced critical care demand with early CPAP and proning in COVID-19 at Bradford: a single-centre cohort. J Intensive Care Soc. 2021 doi: 10.1177/17511437211018615. published online May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez GA, Bozzolo EP, Gobbi A, et al. Outcomes of non-invasive ventilation as the ceiling of treatment in patients with COVID-19. Panminerva Med. 2021 doi: 10.23736/S0031-0808.21.04280-4. published online April 16. [DOI] [PubMed] [Google Scholar]
- 27.Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020;56 doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel P, Mecklenburg M, Massiah C, et al. Non-invasive positive pressure ventilation versus endotracheal intubation in treatment of COVID-19 patients requiring ventilatory support. Am J Emerg Med. 2021;43:103–108. doi: 10.1016/j.ajem.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupuis C, Bouadma L, de Montmollin E, et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease-2019 pneumonia. Crit Care Explor. 2021;3:e0329. doi: 10.1097/CCE.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonetti T, Grasselli G, Zanella A, et al. Use of critical care resources during the first 2 weeks (February 24–March 8, 2020) of the Covid-19 outbreak in Italy. Ann Intensive Care. 2020;10:133. doi: 10.1186/s13613-020-00750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avdeev SN, Yaroshetskiy AI, Tsareva NA, et al. Noninvasive ventilation for acute hypoxemic respiratory failure in patients with COVID-19. Am J Emerg Med. 2021;39:154–157. doi: 10.1016/j.ajem.2020.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baqi S, Naz A, Sayeed MA, et al. Clinical characteristics and outcome of patients with severe COVID-19 pneumonia at a public sector hospital in Karachi, Pakistan. Cureus. 2021;13 doi: 10.7759/cureus.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vega ML, Dongilli R, Olaizola G, et al. COVID-19 pneumonia and ROX index: time to set a new threshold for patients admitted outside the ICU. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2021.04.003. published online May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celejewska-Wójcik N, Polok K, Górka K, et al. High-flow nasal oxygen therapy in the treatment of acute respiratory failure in severe COVID-19 pneumonia: a prospective observational study. Pol Arch Intern Med. 2021;131:658–665. doi: 10.20452/pamw.16015. [DOI] [PubMed] [Google Scholar]
- 36.Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng L, Lei S, Jiang F, et al. The outcome impact of early vs late HFNC oxygen therapy in elderly patients with COVID-19 and ARDS. medRxiv. 2020 doi: 10.1101/2020.05.23.20111450. published online May 26. (preprint) [DOI] [Google Scholar]
- 38.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu M, Zhou Q, Zheng R, et al. Application of high-flow nasal cannula in hypoxemic patients with COVID-19: a retrospective cohort study. BMC Pulm Med. 2020;20:324. doi: 10.1186/s12890-020-01354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radovanovic D, Pini S, Franceschi E, et al. Characteristics and outcomes in hospitalized COVID-19 patients during the first 28 days of the spring and autumn pandemic waves in Milan: an observational prospective study. Respir Med. 2021;178 doi: 10.1016/j.rmed.2021.106323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel M, Gangemi A, Marron R, et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arina P, Baso B, Moro V, Patel H, Ambler G. Discriminating between CPAP success and failure in COVID-19 patients with severe respiratory failure. Intensive Care Med. 2021;47:237–239. doi: 10.1007/s00134-020-06304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-García JG, Pascual-Guardia S, Aguilar Colindres RJ, et al. Incidence of pulmonary embolism in patients with non-invasive respiratory support during COVID-19 outbreak. Respir Med. 2021;178 doi: 10.1016/j.rmed.2021.106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Domenico SL, Coen D, Bergamaschi M, et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: a single-center retrospective study. Intern Emerg Med. 2021;16:1051–1060. doi: 10.1007/s11739-020-02548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carteaux G, Pons M, Morin F, et al. Continuous positive airway pressure for respiratory support during COVID-19 pandemic: a frugal approach from bench to bedside. Ann Intensive Care. 2021;11:38. doi: 10.1186/s13613-021-00828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosgurin O, Leidi A, Farhoumand PD, et al. Role of intermediate care unit admission and non-invasive respiratory support during the COVID-19 pandemic: a retrospective cohort study. Respiration. 2021;100:786–793. doi: 10.1159/000516329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonough G, Khaing P, Treacy T, McGrath C, Yoo EJ. The use of high-flow nasal oxygen in the ICU as a first-line therapy for acute hypoxemic respiratory failure secondary to Coronavirus Disease 2019. Crit Care Explor. 2020;2:e0257. doi: 10.1097/CCE.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivaloganathan AA, Nasim-Mohi M, Brown MM, et al. Noninvasive ventilation for COVID-19-associated acute hypoxaemic respiratory failure: experience from a single centre. Br J Anaesth. 2020;125:e368–e371. doi: 10.1016/j.bja.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menzella F, Barbieri C, Fontana M, et al. Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome. Clin Respir J. 2021;15:779–787. doi: 10.1111/crj.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpagnano GE, Buonamico E, Migliore G, et al. Bilevel and continuous positive airway pressure and factors linked to all-cause mortality in COVID-19 patients in an intermediate respiratory intensive care unit in Italy. Expert Rev Respir Med. 2021;15:853–857. doi: 10.1080/17476348.2021.1866546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the emergency department during Italian novel coronavirus SARS-CoV2 outbreak: preliminary data on the role of helmet CPAP and non-invasive positive pressure ventilation. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnet N, Martin O, Boubaya M, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11:37. doi: 10.1186/s13613-021-00825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potalivo A, Montomoli J, Facondini F, et al. Sixty-day mortality among 520 Italian hospitalized COVID-19 patients according to the adopted ventilatory strategy in the context of an integrated multidisciplinary clinical organization: a population-based cohort study. Clin Epidemiol. 2020;12:1421–1431. doi: 10.2147/CLEP.S278709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley P, Nixon J, Wilson J, et al. Continuous positive airway pressure (CPAP) as a ceiling of care treatment for hypoxemic respiratory failure due to COVID-19. J Intensive Care Soc. 2021 doi: 10.1177/1751143721996538. published online Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrillo Hernandez-Rubio J, Sanchez-Carpintero Abad M, Yordi Leon A, et al. Outcomes of an intermediate respiratory care unit in the COVID-19 pandemic. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan J, Zeng J, Deng P, et al. High-flow nasal cannula for COVID-19 patients: a multicenter retrospective study in China. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brusasco C, Corradi F, Di Domenico A, et al. Continuous positive airway pressure in COVID-19 patients with moderate-to-severe respiratory failure. Eur Respir J. 2021;57 doi: 10.1183/13993003.02524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker J, Dolly S, Ng L, Prior-Ong M, Sabapathy K. The role of CPAP as a potential bridge to invasive ventilation and as a ceiling-of-care for patients hospitalized with Covid-19-an observational study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zucman N, Mullaert J, Roux D, et al. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46:1924–1926. doi: 10.1007/s00134-020-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaulton TG, Bellani G, Foti G, Frazer MJ, Fuchs BD, Cereda M. Early clinical experience in using helmet continuous positive airway pressure and high-flow nasal cannula in overweight and obese patients with acute hypoxemic respiratory failure from Coronavirus Disease 2019. Crit Care Explor. 2020;2:e0216. doi: 10.1097/CCE.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sargent W, Ali S, Kukran S, Harvie M, Soin S. The prognostic value of chest X-ray in patients with COVID-19 on admission and when starting CPAP. Clin Med (Lond) 2021;21:e14–e19. doi: 10.7861/clinmed.2020-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roedl K, Jarczak D, Thasler L, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care. 2021;34:167–175. doi: 10.1016/j.aucc.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koduri G, Gokaraju S, Darda M, et al. Clinical characteristics and outcomes of 500 patients with COVID pneumonia: results from a single center (Southend University Hospital) medRxiv. 2020 doi: 10.1101/2020.08.13.20163030. published online Aug 14. (preprint) [DOI] [Google Scholar]
- 64.Kofod LM, Nielsen Jeschke K, Kristensen MT, Krogh-Madsen R, Monefeldt Albek C, Hansen EF. COVID-19 and acute respiratory failure treated with CPAP. Eur Clin Respir J. 2021;8 doi: 10.1080/20018525.2021.1910191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noeman-Ahmed Y, Gokaraju S, Powrie DJ, Amran DA, El Sayed I, Roshdy A. Predictors of CPAP outcome in hospitalized COVID-19 patients. Respirology. 2020;25:1316–1319. doi: 10.1111/resp.13964. [DOI] [PubMed] [Google Scholar]
- 66.Faraone A, Beltrame C, Crociani A, et al. Effectiveness and safety of noninvasive positive pressure ventilation in the treatment of COVID-19-associated acute hypoxemic respiratory failure: a single center, non-ICU setting experience. Intern Emerg Med. 2021;16:1183–1190. doi: 10.1007/s11739-020-02562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallifax RJ, Porter BM, Elder PJ, et al. Successful awake proning is associated with improved clinical outcomes in patients with COVID-19: single-centre high-dependency unit experience. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alviset S, Riller Q, Aboab J, et al. Continuous positive airway pressure (CPAP) face-mask ventilation is an easy and cheap option to manage a massive influx of patients presenting acute respiratory failure during the SARS-CoV-2 outbreak: a retrospective cohort study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson JV, Meghani NJ, Powell BM, et al. Patient characteristics and predictors of mortality in 470 adults admitted to a district general hospital in England with Covid-19. Epidemiol Infect. 2020;148:e285. doi: 10.1017/S0950268820002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delbove A, Foubert A, Mateos F, Guy T, Gousseff M. High flow nasal cannula oxygenation in COVID-19 related acute respiratory distress syndrome: a safe way to avoid endotracheal intubation? Ther Adv Respir Dis. 2021;15 doi: 10.1177/17534666211019555. 17534666211019555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lagier JC, Amrane S, Mailhe M, et al. High-flow oxygen therapy in elderly patients infected with SARS-CoV2 with a contraindication for transfer to an intensive care unit: a preliminary report. Int J Infect Dis. 2021;108:1–3. doi: 10.1016/j.ijid.2021.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia J, Zhang Y, Ni L, et al. High-flow nasal oxygen in coronavirus disease 2019 patients with acute hypoxemic respiratory failure: a multicenter, retrospective cohort study. Crit Care Med. 2020;48:e1079–e1086. doi: 10.1097/CCM.0000000000004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suardi LR, Pallotto C, Esperti S, et al. Risk factors for non-invasive/invasive ventilatory support in patients with COVID-19 pneumonia: a retrospective study within a multidisciplinary approach. Int J Infect Dis. 2020;100:258–263. doi: 10.1016/j.ijid.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panadero C, Abad-Fernández A, Rio-Ramirez MT, et al. High-flow nasal cannula for Acute Respiratory Distress Syndrome (ARDS) due to COVID-19. Multidiscip Respir Med. 2020;15:693. doi: 10.4081/mrm.2020.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukhtar A, Lotfy A, Hasanin A, El-Hefnawy I, El Adawy A. Outcome of non-invasive ventilation in COVID-19 critically ill patients: a retrospective observational study. Anaesth Crit Care Pain Med. 2020;39:579–580. doi: 10.1016/j.accpm.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020;56 doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan J, Chen B, Liu X, et al. Use of high-flow nasal cannula and noninvasive ventilation in patients with COVID-19: a multicenter observational study. Am J Emerg Med. 2021;46:276–281. doi: 10.1016/j.ajem.2020.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garner O, Dongarwar D, Salihu HM, et al. Predictors of failure of high flow nasal cannula failure in acute hypoxemic respiratory failure due to COVID-19. Respir Med. 2021;185 doi: 10.1016/j.rmed.2021.106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burns GP, Lane ND, Tedd HM, et al. Improved survival following ward-based non-invasive pressure support for severe hypoxia in a cohort of frail patients with COVID-19: retrospective analysis from a UK teaching hospital. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax. 2020;75:998–1000. doi: 10.1136/thoraxjnl-2020-214993. [DOI] [PubMed] [Google Scholar]
- 81.Corradi F, Vetrugno L, Orso D, et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284 doi: 10.1016/j.resp.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guy T, Créac'hcadec A, Ricordel C, et al. High-flow nasal oxygen: a safe, efficient treatment for COVID-19 patients not in an ICU. Eur Respir J. 2020;56 doi: 10.1183/13993003.01154-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knights H, Mayor N, Millar K, et al. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clin Med (Lond) 2020;20:e148–e153. doi: 10.7861/clinmed.2020-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10:37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nightingale R, Nwosu N, Kutubudin F, et al. Is continuous positive airway pressure (CPAP) a new standard of care for type 1 respiratory failure in COVID-19 patients? A retrospective observational study of a dedicated COVID-19 CPAP service. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sayan İ, Altınay M, Çınar AS, et al. Impact of HFNC application on mortality and intensive care length of stay in acute respiratory failure secondary to COVID-19 pneumonia. Heart Lung. 2021;50:425–429. doi: 10.1016/j.hrtlng.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winearls S, Swingwood EL, Hardaker CL, et al. Early conscious prone positioning in patients with COVID-19 receiving continuous positive airway pressure: a retrospective analysis. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wozniak DR, Rubino A, Tan AL, et al. Positive role of continuous positive airway pressure for intensive care unit patients with severe hypoxaemic respiratory failure due to COVID-19 pneumonia: a single centre experience. J Intensive Care Soc. 2020 doi: 10.1177/1751143720971543. published online Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ashish A, Unsworth A, Martindale J, et al. CPAP management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pagano A, Porta G, Bosso G, et al. Non-invasive CPAP in mild and moderate ARDS secondary to SARS-CoV-2. Respir Physiol Neurobiol. 2020;280 doi: 10.1016/j.resp.2020.103489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voshaar T, Stais P, Köhler D, Dellweg D. Conservative management of COVID-19 associated hypoxaemia. ERJ Open Res. 2021;7:00026–02021. doi: 10.1183/23120541.00134-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JY, Kim HA, Huh K, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020;35:e223. doi: 10.3346/jkms.2020.35.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Q, Wang T, Qin X, Jie Y, Zha L, Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24:250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perkins GD, Ji C, Connolly BA, et al. An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19. medRxiv. 2021 doi: 10.1101/2021.08.02.21261379. published online Aug 4. (preprint) [DOI] [Google Scholar]
- 96.Doidge JC, Gould DW, Ferrando-Vivas P, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021;203:565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Intensive Care National Audit and Research Centre (ICNARC) ICNARC report on COVID-19 in critical care. England, Wales and Northern Ireland. June 3, 2021. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- 98.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karagiannidis C, Windisch W, McAuley DF, Welte T, Busse R. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med. 2021;9:e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Docherty AB, Mulholland RH, Lone NI, et al. Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:773–785. doi: 10.1016/S2213-2600(21)00175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamilton F, Gregson F, Arnold D, et al. Aerosol emission from the respiratory tract: an analysis of relative risks from oxygen delivery systems. medRxiv. 2021 doi: 10.1101/2021.01.29.21250552. published online Feb 1. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Apigo M, Schechtman J, Dhliwayo N, Al Tameemi M, Gazmuri RJ. Development of a work of breathing scale and monitoring need of intubation in COVID-19 pneumonia. Crit Care. 2020;24:477. doi: 10.1186/s13054-020-03176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.