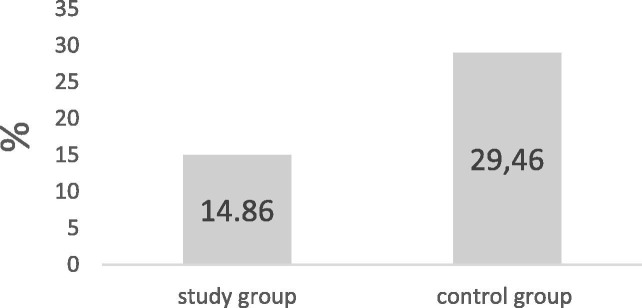Abstract
The stimulation of AT1R (Angiotensin II Receptor Type 1) by Angiotensin II has, in addition to the effects on the renin-angiotensin system, also pro-inflammatory effects through stimulation of ADAM17 and subsequent production of INF-gamma and Interleukin-6. This pro-inflammatory action stimulate the cytokine storm that characterizes the most severe forms of SARS-CoV-2 infection. We studied the effect of AT1Rab on the AT1R on 74 subjects with SARS-CoV-2 infection with respiratory symptoms requiring hospitalization. We divided the patients into 2 groups: 34 with moderate and 40 with severe symptoms that required ICU admission. Hospitalized subjects showed a 50% reduction in the frequency of AT1Rab compared to healthy reference population. Of the ICU patients, 33/40 (82.5%) were AT1Rab negative and 16/33 of them (48.5%) died. All 7 patients positive for AT1Rab survived. These preliminary data seem to indicate a protective role played by AT1R autoantibodies on inflammatory activation in SARS-CoV-2 infection pathology.
Keywords: AT1R autoantibodies, Angiotensin II, SARS-CoV-2 infection
Abbreviations: ACE-2, Angiotensin Converting Enzyme; AT1Rab, autoantibodies direct vs Angiotensin II Receptor1; AngII, Angiotensin II; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Introduction
COVID-19 can affect anyone, but the disease can create different degrees of severity, ranging from asymptomatic, to mild, to extreme symptoms. It is already known that people who have particular genetic characteristics or underlying clinical conditions, can develop a more severe infection [1], [2].
Common risk markers for COVID-19 infection, hospitalization and death have been largely studied: old age, medications, comorbidities, socio-economic status, access to health care, pregnancy, certain occupations, and race/ethnicity are proved to increase personal risk [3]. People with older age and hypertension are show to need careful observation and very early therapy in order to prevent the potential development of severe COVID-19. Patients with heart injury, hyperglycemia, diabetes or high-dose corticosteroid treatments may have a higher risk of death [4].
Kidney disease on admission proved also to be highly associated with in-hospital mortality [5]. Levels of SpO2, lymphocyte, C-reactive protein, platelet count, and high lactate dehydrogenase were confirmed to predict the prognosis of severe COVID-19 patients [6].
In last two years, we gained a deeper knowledge of the SARS-CoV-2 pathogenesis: the virus can infect host cells through the angiotensin converting enzyme (ACE2) receptor, which is part of the dual system renin- angiotensin system (RAS) on respiratory epithelium [7].
ACE2 has the main function of negative regulation of the renin-angiotensin system, a function that is altered in the binding of the Spike1 protein of Sars-CoV-2 on the same site [8].
The occupation of the ACE2 site by SARS Cov-2 favors the increase of serum AngII with consequent abnormal stimulation of the AT1R [9].
Wallukat et al. first speculated about the pathogenetic role of AT1Rab in preeclampsia [10]; several studies showed that this antibody could activate AT1R on different cell types, such as trophoblast, endothelial, mesangial and vascular smooth muscle cells, with biological responses that lead to hypertensive conditions [11].
It was shown the anti AT1Rab to be an allosteric agonist of the AT1R, like the AngII, and it may negatively impact graft or patient survival in kidney, heart and lung transplant [12]. Therefore, AT1Rab can directly damage endothelial and vascular smooth muscle cells, leading to transcription factors elevation associated with pro-inflammatory response [13]. These autoantibodies are elevated in patients with end stage cystic fibrosis [14]; they are more frequent in systemic sclerosis (SS) and more generally in connective tissue diseases [15], even though they can be detected also in healthy people, with a frequency that ranges between 25% and 30% [16]. AngII acts not only as a vasoconstrictor but also as a pro-inflammatory molecule through AT1R axis, which activates ADAM17 with release of pro-inflammatory citokines.
The increased protease activity of disintegrin and metalloprotease enzyme called ADAM17 induced by the fusion of viral particles with the cytoplasmic membrane is supposed to be responsible of the cleavage of TNF-α, IL6 and other pro-inflammatory molecules in SARS-CoV-2 infection. [9]
Our hypotesis is that the presence of AT1Rab, by interfering with the action of AngII, can modulate the cytokine cascade and consequently the inflammation acute phase response in people infected by SARS-CoV-2.
2. Material and methods
This was a retrospective study on seventy-four patients with a confirmed diagnosis of COVID-19 admitted to two hospitals of Abruzzo Region, Italy (L’Aquila and Teramo). Ethic Local Committee approved the study.
Serum samples were been tested for AT1Rab and data were compared with one hundred and twenty-nine healthy subjects of control group. Detection of AT1Rab was performed using quantitative ELISA, a solid phase sandwich test (CellTrend GmbH, Luckenwakde, Germany). The presence of antibodies is been detected by a colorimetric change. The OD450 measurement was conducted using a standard curve which is generated to allow the quantification of antibodies, using a control sample at varying concentrations (2.5, 5, 10, 20, 40 U/ml), assuming a positivity ≥ 10 U/mL.
We divided 74 patients into 2 groups according to severity of infection: the severe group (A), made up of forty patients, includes several and critical cases admitted to Intensive Care Unit (ICU) while the mild/moderate group (B) is composed by thirty-four patients admitted in the hospital with a mild respiratory picture.
All variables were analyzed reporting frequencies and percentages for categorical variables and mean, range, median and interquartile range (IQR) for continuous variables. Proportions between groups were assessed by the Chi-Square test or Fisher’s exact test and 95% confidence intervals was reported. Two-sample Wilcoxon rank-sum (Mann-Whitney) test was used to compare continuous variables. A p-value of < 0.05 was considered statistically significant. All analyses were performed using StataMP14, StataCorp LLC (College Station, Texas 77,845 USA).
3. Results
Seventy-four patients hospitalized because of COVID-19 and one hundred twenty-nine healthy control subjects were analyzed. The healthy control population studied was essentially composed of blood donors without significant disease. The two groups were not different for gender (p = 0.304), the age was significantly different (p < 0.001) between controls and COVID-19 patients, while there is no difference between severe and mild/moderate group, as reported in table 1 .
Table 1.
Demographics Features of COVID19 Patients and healthy control population Group.
| Covid-19 patients |
||||||
|---|---|---|---|---|---|---|
| Controls (n = 129) |
All Covid-19 Patients (n = 74) |
P* | Severe group (n = 40) |
Mild/moderate group (n = 34) |
P** | |
| Male sex – n (%) | 79 (61%) | 48 (69%) | 0.304 | 28 (78%) | 20 (59%) | 0.088 |
| Age – yr | ||||||
| (mean – range) | 54.5 (19–76) | 64.6 (29–92) | <0.001 | 64.8 (37–89) | 64.3 (29–92) | 0.331 |
| (median - IQR) | 56 (14) | 65.5 (13) | 66 (11) | 64.5 (19) | ||
*p: comparing Controls vs All Covid-19 patients; Chi Square test or Wilcoxon rank-sum (Mann-Whitney) test; p**: comparing severe group vs mild/moderate group; Chi Square test or Wilcoxon rank-sum (Mann-Whitney) test.
The percent of antiAT1R positivity in the study group was 14.86% (11/74) while in the healthy control group was 29.46% (38/129) Chi Square test = 5.468 (p = 0.019) (Fig. 1 ).
Fig. 1.
AT1RAbs in study and control groups: comparison.
The severe group (A) reported AT1Rab positivity in 7/40 patients (17.5%) (Range: 10.13–52.0 UI) while the mild/moderate group (B) showed a positivity 4/34 patients (11.8%) (Range 10.01–24.92 UI) with no significant difference (Chi Square test (1) = 0.478; p = 0.489). The unpaired test results about the range of positivity on two group show no significant difference (t = 0.3224, p = 0.75).
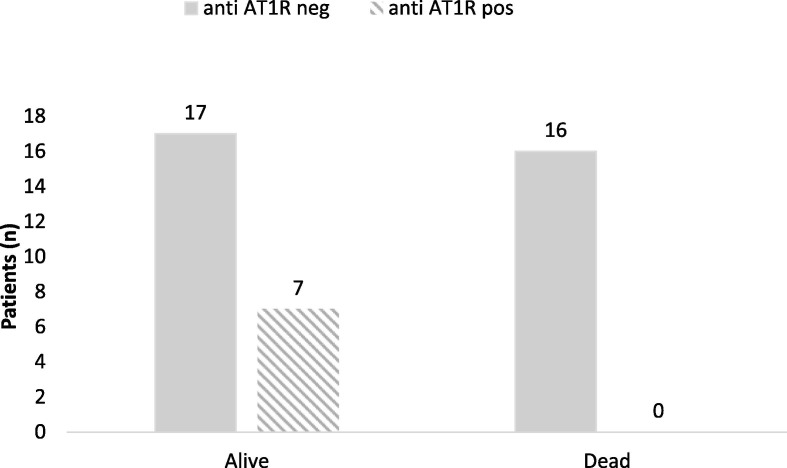
Focusing only on people admitted in ICU it was detect that 16/40 patients died (40%) and they all had not AT1R antibodies, while 7/40 (18%) who survived, in spite of their several respiratory conditions, had AT1RAbs. The remaining 17/40 (42%) had no anti-AT1Rab, so totally 33/40 (82%) of the patient afferents in ICU, had no anti-AT1Rab. Data was shown to be significant Chi Square test = 5.656, p = 0.017. (Table 2 , Fig. 2 ).
Table 2.
AT1RAbs and death/life event.
| AT1RAb |
|||
|---|---|---|---|
| EVENT | Negative n (%) |
Positive n (%) |
Total |
| Death | 16 | 0 | 16 |
| % row | 100 | 0 | 100 |
| % column | 48.48 | 0 | 40 |
| Life | 17 | 7 | 24 |
| % row | 70.83 | 29.17 | 100 |
| % column | 51.52 | 100 | 60 |
| Total | 33 | 7 | 40 |
Fig. 2.
Patients (n) by event and anti AT1R negative/positive.
4. Discussion
We divided the whole population of seventy-four people in two groups (A) and (B). The first group (A) is made of forty patients admitted in Intensive Care Unit because of their several respiratory conditions, while the second group (B) includes thirty-four patients with a mild or moderate infection, which required hospitalization as well. First, we compared the percent of AT1Rab in the hospitalized population with a healthy control group. We analyzed the dosage of AT1Rab to test a possible correlation with the clinical situation of patients affected by SARS-CoV-2: the frequency of AT1Rab (>10 U/ml) in healthy population was 29.46% (38/129), which is higher than the frequency observed in people affected by SARS-CoV-2 who required hospitalization was 14.86% (4/34) (p = 0.019). These preliminary results highlight the association between the lack of AT1Rab and severity of infection that cause the hospitalization.
We then focused on only 40 patients with COVID-19 admitted in Intensive Care Unit for respiratory monitoring, and it was clear that all the ones who died had not the AT1Rab and the only 18% of survivors endowed with them, despite their severe conditions at hospitalization.
The results suggest that the presence of AT1R autoantibodies can possibly play a role in Sars-CoV-2 infection by decreasing the effect of AngII accumulation following the virus occupation of the ACE2 receptor. Since AT1Rab was present in the healthy control in absence of pathological manifestations, it could be explained as a consequence of a probable state of tolerance to the effect of the chronic stimulus of AT1Rab on its receptor. The increase of AngII due to the occupation of ACE2 by Sars-CoV-2 therefore finds, in subjects positive for anti.AT1R autoantibodies, an immune system “refractory” to acute activation, while in subjects without anti-AT1R autoantibodies can induce a violent cytokine storm.
Another hypothesis is that the presence of anti-AT1R autoantibodies may determines an increase in plasmatic AngII which makes itself available to bind to a second AT2R receptor.
AngII is the most relevant molecule of the RAS pathway and performs its function by activating the following G-protein-coupled receptors: angiotensin II receptor type 1 (AT1R) and angiotensin II receptor type 2 (AT2R).The effects exerted by these two membrane receptors are opposite, in particular, AT1R induces detrimental effects, such as inflammation, fibrosis, and altered redox balance in addition to vasoconstrictive properties, whereas AT2R is involved in protective and regenerating actions (anti-inflammatory, anti-fibrotic, neurodegenerative, metabolic) and in the release of vasodilatory molecules [21].
It is known that anti AT1R antibodies induce the increase of AT2R, by increasing the expression of AT2R at the transcriptional level of Kif-5 / IRF and post-transcriptional level of circErBb4 / miR-29a-5p; therefore the presence of antiAT1R antibodies, unbalancing the expression of AT1R / AT2R in favor of AT2R, limit detrimental effects, such as inflammation, fibrosis, vasoconstrictive properties induced from AngII through activation of AT1R. [22]
The same mechanism underlies the pharmacological efficacy of AT1R receptor blocking drugs such as sartans [17]. In fact, they block the binding of AngII to the AT1R receptor and make it available for the AT2R receptor by turning off the vasoconstrictive, proliferative and inflammatory effects. [18], [19]
Data collected is statistically significant: AT1Rab, when expressed, could be able to protect people from the virus itself or unburdening the ongoing infection. Once infected, the lack of this autoantibody could reveal a specific aptitude to hospitalization. Moreover, next to classic severity markers as old age and comorbidities, AT1Rab could be used as severity and death prognostic marker as well. In a worldwide pandemic situation, next to the categorical imperative to vaccinate, the challenge is also to highlight those factors, which could really protect people from infection, and this study shows that AT1Rab could be one of them. Our data are not in line with recent literature data [20] but it should be noted that the our evaluation of AT1Rab was carried out on the basis of the an actual positivity cut-off and not on the basis of an average values evaluation. The small data sample we examined cannot be generalized and other studies are needed to support our hypotheses about a protective role of AT1R autoantibodies in the inflammatory activation in COVID-19 pathology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Di Maria E., Latini A., Borgiani P., Novelli G. Genetic variants of the human host influencing the coronavirus-associated phenotypes (SARS, MERS and COVID-19): rapid systematic review and field synopsis. Hum Genomics. 2020;14(1):30. doi: 10.1186/s40246-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anastassopoulou C., Gkizarioti Z., Patrinos G.P., Tsakris A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum Genomics. 2020;14(1):40. doi: 10.1186/s40246-020-00290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Xu S., Yu M., Wang K.e., Tao Y.u., Zhou Y., Shi J., Zhou M., Wu B.o., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. J, Risk factors for severity and mortality in adult COVID-19 in patients in Wuhan. Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Feng, Yang Lian, Li Yuncheng, Liang Bo, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int. J. Med. Sci. 2020;17(9):1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalan R., Bornstein S.R., El-Armouche A., Rodionov R.N., Markov A., Wielockx B., Beuschlein F., Boehm B.O. The ACE-2 in COVID-19: Foe or Friend? Horm Metab. Res. 2020;52(05):257–263. doi: 10.1055/a-1155-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Li S., Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med. J. 2020;96(1137):403–407. doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toshio Hirano and Masaaki Murakami, COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome Immunity 52, 2020 https://doi.org/10.1016/j.immuni.2020.04.003 [DOI] [PMC free article] [PubMed]
- 10.Wallukat G., Homuth V., Fischer T., Lindschau C., Horstkamp B., Jüpner A., Baur E., Nissen E., Vetter K., Neichel D., Dudenhausen J.W., Haller H., Luft F.C. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clinic Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams T.A., Jaquin D., Burrello J., Philippe A., Yang Y., Rank P., Nirschl N., Sturm L., Hübener C., Dragun D., Bidlingmaier M., Beuschlein F., Reincke M. Diverse responses of autoantibodies to the angiotensin II type 1 receptor in primary aldosteronism. Hypertension. 2019;74(4):784–792. doi: 10.1161/HYPERTENSIONAHA.119.13156. [DOI] [PubMed] [Google Scholar]
- 12.E. Cozzi, F. Calabrese, M. Schiavon, P. Feltracco, M. Seveso, C. Carollo, M. Loy, M. Cardillo and F.Rea Immediate and Catastrophic Antibody-Mediated Rejection in a Lung Transplant Recipient With Anti–Angiotensin II Receptor Type 1 and Anti–Endothelin-1 Receptor Type A Antibodies. American Journal of Transplantation 2017; 17: 557–564. https://doi.org/0.1111/ajt.14053. [DOI] [PubMed]
- 13.Ariela Benigni, Paola Cassis, Giuseppe Remuzzi. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2, 247–257. https://doi.org/10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed]
- 14.Budding K et al., Anti-ETAR and anti-AT1R autoantibodies are elevated in patients with endstage cyistic fibrosis, J Cyst Fibros, 2015; 14(1):42-5 https://doi.org/ 10.1016/j.jcf.2014.07.007 . [DOI] [PubMed]
- 15.Moroncini G., et al. Agonistic antibodies in systemic sclerosis. Immunol Lett. 2018;195:83–87. doi: 10.1016/j.imlet.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Fichtner A. et al., Association of angiotensin II Type 1 receptor antibodies with graft histology, function and survival in paediatric renal transplant recipients, Nephrol Dial Transplant. 2018; 33(6): 1065-1072 https://doi.org/10.1093/ndt/gfy008. [DOI] [PubMed]
- 17.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81(5):537–540. doi: 10.1002/ddr.v81.510.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., et al. Angiotensin Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel Sanket N., Fatima Naureen, Ali Riyasat, Hussain Tahir. Emerging Role of Angiotensin AT2 Receptor in Anti-Inflammation: An Update. Curr Pharm Des. 2020;26(4):492–500. doi: 10.2174/1381612826666200115092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jelle Miedema, Marco Schreurs, Simone van der Sar – van der Brugge et al. Antibodies Against Angiotensin II Receptor Type 1 and Endothelin A Receptor Are Associated With an Unfavorable COVID19 Disease Course. Front. Immunol. 12:684142 https://doi.org/10.3389/fimmu.2021.684142. [DOI] [PMC free article] [PubMed]
- 21.D’Ardes Damiano, Boccatonda Andrea, Rossi Ilaria, Guagnano Maria Teresa, Santilli Francesca, Cipollone Francesco, Bucci Marco. Maria Teresa Guagnano, Francesca Santilli, Francesco Cipollone and Marco Bucci COVID-19 and RAS: Unravelling an Unclear Relationship. Int. J. Mol. Sci. 2020;21(8):3003. doi: 10.3390/ijms21083003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, et al. Increased AT2R expression is induced by AT1R autoantibody. Cell Death and Disease. 2020;11:432. doi: 10.1038/s41419-020-2643-5). [DOI] [PMC free article] [PubMed] [Google Scholar]