Abstract
Background
Breast surgery encompasses oncologic, reconstructive, and cosmetic procedures. With the recent focus on the over‐prescribing of opioids in the literature, it is important to assess the effectiveness and safety of non‐opioid pain medication regimens including nonsteroidal anti‐inflammatory drugs (NSAIDs) or NSAID pain medications. Clinicians have differing opinions on the safety of perioperative (relating to, occurring in, or being the period around the time of a surgical operation) NSAIDs for breast surgery given the unclear risk/benefit ratio. NSAIDs have been shown to decrease inflammation, pain, and fever, while potentially increasing the risks of bleeding complications.
Objectives
To assess the effects of perioperative NSAID use versus non‐NSAID analgesics (other pain medications) in women undergoing any form of breast surgery.
Search methods
The Cochrane Breast Information Specialist searched the Cochrane Breast Cancer Group (CBCG) Specialized Register, CENTRAL (the Cochrane Library), MEDLINE, Embase, The WHO International Clinical Trials Registry Platform (ICTRP) and Clinicaltrials.gov registries to 21 September 2020. Full articles were retrieved for potentially eligible trials.
Selection criteria
We considered all randomized controlled trials (RCTs) looking at perioperative NSAID use in women undergoing breast surgery.
Data collection and analysis
Two review authors independently screened studies, extracted data and assessed risk of bias, and certainty of the evidence using the GRADE approach. The main outcomes were incidence of breast hematoma within 90 days (requiring reoperation, interventional drainage, or no treatment) of breast surgery and pain intensity 24 hours following surgery, incidence rate or severity of postoperative nausea, vomiting or both, bleeding from any location within 90 days, need for blood transfusion, other side effects of NSAID use, opioid use within 24 hours of surgery, length of hospital stay, breast cancer recurrence, and non‐prescribed NSAID use. Data were presented as risk ratios (RRs) for dichotomous outcomes and standardized mean differences (SMDs) for continuous outcomes.
Main results
We included 12 RCTs with a total of 1596 participants. Seven studies compared NSAIDs (ketorolac, diclofenac, flurbiprofen, parecoxib and celecoxib) to placebo. Four studies compared NSAIDs (ketorolac, flurbiprofen, ibuprofen, and celecoxib) to other analgesics (morphine, hydrocodone, hydromorphone, fentanyl). One study compared NSAIDs (diclofenac) to no intervention.
NSAIDs compared to placebo
Most outcomes are judged to have low‐certainty evidence unless stated otherwise. There may be little to no difference in the incidence of breast hematomas within 90 days of breast surgery (RR 0.33, 95% confidence interval (CI) 0.05 to 2.02; 2 studies, 230 participants; I2 = 0%). NSAIDs may reduce pain intensity 24 (± 12) hours following surgery compared to placebo (SMD ‐0.26, 95% CI ‐0.49 to ‐0.03; 3 studies, 310 participants; I2 = 73%). There may be little to no difference in the incidence rates or severities of postoperative nausea, vomiting, or both (RR 1.15, 95% CI 0.58 to 2.27; 4 studies, 939 participants; I2 = 81%), bleeding from any location within 90 days (RR 1.05, 95% CI 0.89 to 1.24; 2 studies, 251 participants; I2 = 8%), or need for blood transfusion compared to placebo groups, but we are very uncertain (RR 4.62, 95% CI 0.23 to 91.34; 1 study, 48 participants; very low‐certainty evidence). There may be no difference in other side effects (RR 1.12, 95% CI 0.44 to 2.86; 2 studies, 251 participants; I2 = 0%). NSAIDs may reduce opioid use within 24 hours of surgery compared to placebo (SMD ‐0.45, 95% CI ‐0.85 to ‐0.05; 4 studies, 304 participants; I2 = 63%).
NSAIDs compared to other analgesics
There is little to no difference in the incidence of breast hematomas within 90 days of breast surgery, but we are very uncertain (RR 0.33, 95% CI 0.01 to 7.99; 1 study, 100 participants; very low‐certainty evidence). NSAIDs may reduce pain intensity 24 (± 12) hours following surgery (SMD ‐0.68, 95% CI ‐0.97 to ‐0.39; 3 studies, 200 participants; I2 = 89%; low‐certainty evidence) and probably reduce the incidence rates or severities of postoperative nausea, vomiting, or both compared to other analgesics (RR 0.18, 95% CI 0.06 to 0.57; 3 studies, 128 participants; I2 = 0%; moderate‐certainty evidence). There is little to no difference in the development of bleeding from any location within 90 days of breast surgery or in other side effects, but we are very uncertain (bleeding: RR 0.33, 95% CI 0.01 to 7.99; 1 study, 100 participants; other side effects: RR 0.11, 95% CI 0.01 to 1.80; 1 study, 48 participants; very low‐certainty evidence). NSAIDs may reduce opioid use within 24 hours of surgery compared to other analgesics (SMD ‐6.87, 95% CI ‐10.93 to ‐2.81; 3 studies, 178 participants; I2 = 96%; low‐certainty evidence).
NSAIDs compared to no intervention
There is little to no difference in pain intensity 24 (± 12) hours following surgery compared to no intervention, but we are very uncertain (SMD ‐0.54, 95% CI ‐1.09 to 0.00; 1 study, 60 participants; very low‐certainty evidence).
Authors' conclusions
Low‐certainty evidence suggests that NSAIDs may reduce postoperative pain, nausea and vomiting, and postoperative opioid use. However, there was very little evidence to indicate whether NSAIDs affect the rate of breast hematoma or bleeding from any location within 90 days of breast surgery, the need for blood transfusion and incidence of other side effects compared to placebo or other analgesics. High‐quality large‐scale RCTs are required before definitive conclusions can be made.
Keywords: Female; Humans; Anti-Inflammatory Agents, Non-Steroidal; Anti-Inflammatory Agents, Non-Steroidal/adverse effects; Breast Neoplasms; Breast Neoplasms/surgery; Ketorolac; Ketorolac/therapeutic use; Pain, Postoperative; Pain, Postoperative/drug therapy; Pharmaceutical Preparations
Plain language summary
Pain medications (NSAIDs: nonsteroidal anti‐inflammatory drugs) around the time of surgery in women undergoing breast surgery
What is the aim of this review?
The aim of this review was to find out whether NSAID pain medication reduces pain around the time of any breast surgery compared to no medication (placebo) or other pain medication (e.g. opioids). Possible side effects from NSAIDS were reviewed and compared to side effects for patients either having no medication or other types of pain relief. NSAID pain medications include ketorolac, flurbiprofen, ibuprofen, and celecoxib.
Why does it matter?
NSAIDs have been shown to decrease inflammation, pain, and fever, while potentially increasing the risks of bleeding complications. There are concerns about the safety of NSAID pain medication during breast surgery, but they still may be better than other drugs (opioids) which have different side effects of concern.
What was studied in the review?
Studies included women who received either a NSAID pain medication or placebo, other pain medication, or no medication. We wanted to assess breast bleeding, pain following surgery, postoperative nausea and vomiting, bleeding from any location, need for blood transfusion, any other side effects reported from NSAID use, opioid use within 24 hours of surgery, length of hospital stay, breast cancer recurrence, and non‐prescribed NSAID use.
What did we find?
We found 12 studies with a total of 1596 participants. The evidence is current to September 2020. Seven studies compared NSAIDs (ketorolac, diclofenac, flurbiprofen, parecoxib and celecoxib) to placebo. Four studies compared NSAIDs (ketorolac, flurbiprofen, ibuprofen, and celecoxib) to other pain medications. One study compared NSAIDs (diclofenac) to no medication.
NSAIDs compared to placebo :the use of NSAIDs seems to make little difference to the incidence of breast hematoma (a collection of blood inside the armpit or breast surgical site) and bleeding from any location or increase in blood transfusions within 90 days of breast surgery. There was no increase in postoperative nausea and vomiting. NSAIDs may reduce pain within 24 hours after surgery and reduce the use of opioids within 24 hours after surgery.
NSAIDs compared to other pain medications : the use of NSAIDs may make little difference to the incidence of breast hematoma or bleeding from any location or need for blood transfusion within 90 days of breast surgery. NSAIDs may reduce pain within 24 hours and reduce the chance of nausea and vomiting after surgery. There is little to no difference in the incidence of other side effects. NSAIDS may reduce opioid use within 24 hours of surgery compared to other pain medications.
NSAIDs compared to no medication : the use of NSAIDs appears to make little to no difference in pain within 24 hours after surgery compared to no medication.
Many studies did not collect or report data on the need for blood transfusions, length of hospital stay, breast cancer recurrence, and non‐prescribed NSAID pain medication use.
What do the findings mean?
In summary, the studies were small, different to each other and did not report all the possible side effects well. Therefore we could not make firm conclusions about the benefits or harms of NSAIDs. This review provides preliminary evidence, but more studies are needed to make sure there is no harm from the use of NSAIDS after breast surgery. Good‐quality large‐scale studies are required before any definitive conclusions can be made.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 21 September 2020.
Summary of findings
Summary of findings 1. NSAID compared to placebo in women undergoing breast surgery.
| NSAID compared to placebo in women undergoing breast surgery | |||||||
| Patient or population: women undergoing breast surgery Setting: inpatient and outpatient Intervention: NSAID Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments: types of NSAIDs | ||
| Risk with placebo | Risk with NSAID | ||||||
| Incidence of breast hematoma | 35 per 1000 | 12 per 1000 (2 to 71) | RR 0.33 (0.05 to 2.02) | 230 (2 RCTs) |
⊕⊕⊝⊝ Lowa,b,c,g |
parecoxib, celecoxib | |
| Preoperative | 15 per 1000 | 15 per 1000 (1 to 230) | RR 1.00 (0.06 to 15.66) |
136 (1 RCT) | ⊕⊕⊝⊝ Lowa,g,h | parecoxib | |
| Pain intensity 24 (± 12) hours following surgery | The mean pain intensity 24 (± 12) hours following surgery ‐ perioperative was 0 | SMD 0.26 lower (0.49 lower to 0.03 lower) | ‐ | 310 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c,d,e,j | ketorolac, diclofenac | |
| Preoperative | The mean pain intensity 24 (± 12) hours following surgery ‐ preoperative was 0 | SMD 0.19 lower (0.46 lower to 0.09 higher) | ‐ | 202 (1 RCT) | ⊕⊕⊝⊝ Lowb,h | ketorolac | |
| Postoperative | The mean pain intensity 24 (± 12) hours following surgery ‐ postoperative was 0 | SMD 0.04 higher (0.50 lower to 0.57 higher) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d,e,h,i |
ketorolac | |
| Incidence rate or severity of postoperative nausea, vomiting, or both | 111 per 1000 | 128 per 1000 (64 to 252) | RR 1.15 (0.58 to 2.27) |
939 (4 RCTs) |
⊕⊕⊝⊝ Lowa,b,c,d,e,i,k | ketorolac, diclofenac, celecoxib, parecoxib | |
| Preoperative | 60 per 1000 | 177 per 1000 (25 to 1000) | RR 2.95 (0.42 to 20.64) |
831 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,e,f,i,j |
celecoxib, parecoxib | |
| Postoperative | 700 per 1000 | 399 per 1000 (252 to 644) | RR 0.57 (0.36 to 0.92) |
60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d,e,i | ketorolac | |
| Bleeding from any location within 90 days | 169 per 1000 | 178 per 1,000 (151 to 210) | RR 1.05 (0.89 to 1.24) |
251 (2 RCT) | ⊕⊕⊝⊝ LOWa,b,g | ketorolac, diclofenac | |
| Preoperative | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3.34 (0.14 to 81.03) |
203 (1 RCT) |
⊕⊝⊝⊝ Very lowb,g,h | ketorolac | |
| Need for blood transfusion | 0 per 1000 | 0 per 1000 (0 to 0) | RR 4.62 (0.23 to 91.34) |
48 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,g,i | diclofenac | |
| Preoperative | 0 per 1000 | 0 per 1000 (0 to 0) | RR 4.62 (0.23 to 91.34) |
48 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,g,i | diclofenac | |
| Other side effects of NSAID use | 62 per 1000 | 69 per 1000 (27 to 176) | RR 1.12 (0.44 to 2.86) |
251 (2 RCTs) |
⊕⊕⊝⊝ Lowa,b,g | ketorolac, diclofenac | |
| Preoperative | 65 per 1000 | 83 per 1000 (31 to 221) | RR 1.27 (0.48 to 3.38) |
203 (1 RCT) | ⊕⊕⊝⊝ Lowb,h | ketorolac | |
| Opioid use within 24 (± 12) hours of surgery | The mean opioid use within 24 (± 12) hours of surgery ‐ preoperative was 0 | SMD 0.45 lower (0.85 lower to 0.05 lower) | ‐ | 304 (4 RCTs) |
⊕⊕⊝⊝ Lowa,b,c,d,e,j | ketorolac, diclofenac, parecoxib, flurbiprofen | |
| Preoperative | The mean opioid use within 24 (± 12) hours of surgery ‐ preoperative was 0 | SMD 0.85 lower (1.20 lower to 0.50 lower) | ‐ | 136 (1 RCT) | ⊕⊕⊝⊝ Lowa,h | parecoxib | |
| Postoperative | The mean opioid use within 24 (± 12) hours of surgery ‐ postoperative was 0 | SMD 0.29 lower (0.83 lower to 0.25 higher) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d,e,g,h | ketorolac | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drug; RCT: randomized controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
We downgraded by 1 point for ≥ 1 of the following and 2 points for > 4 of the following due to risk of bias.
aUnclear or high risk selective reporting. bUnclear or high risk incomplete outcomes data. cUnclear or high risk allocation concealment. dUnclear or high risk random sequence generation. eUnclear or high risk blinding of outcome assessment. fUnclear or high risk blinding of participants and personnel.
We downgraded by 1 point for each of the following.
gImprecision. hSingle study. iSample < 100 participants. jHeterogeneity > 50%. kHeterogeneity > 75%.
Summary of findings 2. NSAID compared to other analgesic in women undergoing breast surgery.
| NSAID compared to other analgesic in women undergoing breast surgery | |||||||
| Patient or population: women undergoing breast surgery Setting: inpatient and outpatient Intervention: NSAID Comparison: other analgesic | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments: types of NSAIDs; types of other analgesics | ||
| Risk with other analgesic | Risk with NSAID | ||||||
| Incidence of breast hematoma within 90 days of breast surgery | 20 per 1000 | 7 per 1000 (0 to 160) | RR 0.33 (0.01 to 7.99) | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d,e,f | celecoxib; hydrocodone | |
| Pain intensity 24 (± 12) hours following surgery | The mean pain intensity 24 (± 12) hours following surgery ‐ perioperative was 0 | SMD 0.68 lower (0.97 lower to 0.39 lower) | ‐ | 200 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c,d,i | celecoxib, flurbiprofen, ibuprofen; fentanyl, hydrocodone, hydromorphone | |
| Postoperative | The mean pain intensity 24 (± 12) hours following surgery ‐ postoperative was 0 | SMD 0.12 lower (0.51 lower to 0.27 higher) | ‐ | 100 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | flurbiprofen, ibuprofen; fentanyl, hydromorphone | |
| Incidence rate or severity of postoperative nausea, vomiting, or both | 243 per 1000 | 44 per 1000 (15 to 138) | RR 0.18 (0.06 to 0.57) | 128 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,c | ketorolac, ibuprofen, flurbiprofen; morphine, hydromorphone, fentanyl | |
| Bleeding from any location within 90 days | 20 per 1000 | 7 per 1000 (0 to 160) | RR 0.33 (0.01 to 7.99) |
100 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c,d,e,f | celecoxib; hydrocodone | |
| Other side effects of NSAID use | 233 per 1000 | 26 per 1000 (2 to 420) | RR 0.11 (0.01 to 1.80) | 48 (1 RCT) | ⊕⊝⊝⊝ Very lowe,f,g | ibuprofen; hydromorphone | |
| Opioid use within 24 (± 12) hours of surgery | The mean opioid use within 24 (± 12) hours of surgery ‐ perioperative was 0 | SMD 6.87 lower (10.93 lower to 2.81 lower) | ‐ | 178 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c,d,i | celecoxib, ketorolac, flurbiprofen; hydrocodone, morphine, fentanyl | |
| Postoperative | The mean opioid use within 24 (± 12) hours of surgery ‐ postoperative was 0 | SMD 9.56 lower (18.48 lower to 0.64 lower) | ‐ | 78 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c,g,i | ketorolac, flurbiprofen; morphine, fentanyl | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drug; RCT: randomized controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
We downgraded by 1 point for ≥ 1 of the following and 2 points for > 4 of the following due to risk of bias.
aUnclear or high risk selective reporting. bUnclear or high risk allocation concealment. cUnclear or high risk blinding of outcome assessment. dUnclear or high risk blinding of participants and personnel.
We downgraded by 1 point for each of the following
eImprecision. fSingle study. gSample < 100 participants. hHeterogeneity > 50%. iHeterogeneity > 75%.
Summary of findings 3. NSAID compared to no intervention in women undergoing breast surgery.
| NSAID compared to no intervention in women undergoing breast surgery | |||||||
| Patient or population: women undergoing breast surgery Setting: inpatient and outpatient Intervention: NSAID Comparison: no intervention | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments: types of NSAIDs | ||
| Risk with no intervention | Risk with NSAID | ||||||
| Pain intensity 24 (±12) hours following surgery | The mean pain intensity 24 (± 12) hours following surgery ‐ perioperative was 0 | SMD 0.54 lower (1.09 lower to 0.00) | ‐ | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c,d,e | diclofenac | |
| Preoperative | The mean pain intensity 24 (± 12) hours following surgery ‐ preoperative was 0 | SMD 0.35 lower (0.97 lower to 0.28 higher) | ‐ | 40 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c,d,e | diclofenac | |
| Postoperative | The mean pain intensity 24 (± 12) hours following surgery ‐ postoperative was 0 | SMD 0.66 lower (1.30 lower to 0.02 lower) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | diclofenac | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drug; RCT: randomized controlled trial; SMD: standardised mean difference. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
We downgraded by 1 point for ≥ 1 of the following and 2 points for > 4 of the following due to risk of bias.
aUnclear or high risk selective reporting. bUnclear or high risk allocation concealment. cUnclear or high risk blinding of outcome assessment.
We downgraded by 1 point for each of the following.
dSample < 100 participants. eSingle study.
Background
Description of the condition
A breast hematoma, or a collection of blood inside the axilla or surgical breast site, is one potential complication following breast surgery. A hematoma can require additional surgery to drain and/or readmission to the hospital, and it may predispose patients to infection (Cheng 2011). Addressing these complications may limit the amount of money reimbursed to providers (Smith 2017), factoring into their decision to perform a lower yield procedure with a lower risk of complications (De Souza 2012; Smith 2017). Furthermore, hematomas may increase patients' emotional stress and physical pain (Kaoutzanis 2017). While the incidence of hematomas associated with breast surgery requiring return to the operating room is relatively low (less than 2.1%) (Collins 2012; Gobble 2014; Yan 2015), the large number of breast surgery procedures performed every year makes its negative consequences significant at a population level. Nonsteroidal anti‐inflammatory drugs (NSAIDs) are being increasingly used to treat postoperative pain, and it is unclear whether there is an association between perioperative (relating to, occurring in, or being the period around the time of a surgical operation, Merriam‐Webster 1966) NSAID use and potential increased risks of hematoma development at the surgical site.
Breast surgery encompasses oncologic, reconstructive, and cosmetic procedures. An estimated 316,120 women were diagnosed with breast cancer in 2017 in the USA, with approximately 97% of stage I and II, 93% of stage III, and 31% of stage IV patients undergoing surgical treatment (ACS 2017). Commonly performed oncologic breast procedures include lumpectomy, mastectomy, sentinel lymph node biopsy, and axillary dissection. In 2017, members of the American Society for Plastic Surgery (ASPS) performed over 600,000 reconstructive and cosmetic breast cases, including implant‐based reconstruction, autologous flap reconstruction, mastopexy, and augmentation, among others (ASPS 2017). Approximately 29% of these were reconstructive procedures, while the remaining 71% were cosmetic procedures (ASPS 2017).
Description of the intervention
The American Society of Anesthesiologists (ASA) released its most recent practice guidelines for acute pain management in the perioperative period in 2016 (Chou 2016). Medication selection for perioperative pain management is guided by patient factors, but an underlying principle is a multimodal approach, that is, where two or more drugs with differing modes of action are used to treat acute surgical pain.
Opioid drugs remain a mainstay of analgesia; however, 20 years ago Kehlet 1997 introduced the now‐common recommendation of an 'around‐the‐clock' regimen of a nonsteroidal anti‐inflammatory drug (NSAID, for example ketorolac, flurbiprofen, diclofenac, celecoxib) and/or acetaminophen (paracetamol), unless contraindicated. This concept has been more recently adapted into standardized breast surgical treatment plans (Batdorf 2015; Bonde 2015; Bonde 2016; Davidge 2013). NSAID use has demonstrated equivalent efficacy to opioids and similar postoperative bleeding when compared to controls in a wide range of surgical procedures (Gobble 2014). Perioperative NSAID use for patients undergoing endoscopic sinus surgery reduced postoperative rescue analgesics that included opioid use in many studies, with bleeding seen in 0.8% of patients (Svider 2018). Perioperative NSAID use in pediatric patients undergoing tonsillectomy concluded there was insufficient evidence to exclude an increased risk of bleeding (Lewis 2013).
How the intervention might work
NSAIDs inhibit cyclooxygenase (COX) enzymes, thereby reducing prostaglandin synthesis and an inflammatory response that causes pain. There are two types of COX enzymes: COX‐1 and COX‐2. Both types produce prostaglandins that promote inflammation, pain, and fever. Most NSAIDs are reversible inhibitors; however, aspirin binds permanently to COX enzymes, leading to a prolonged duration of effect.
The use of NSAIDs perioperatively may be associated with bleeding complications. This is because NSAID inhibition of COX‐1 reduces thromboxane A2, which mediates platelet aggregation. Most cells, including those in the stomach, express COX‐1, which provides a protective effect in gastric tissue, so NSAIDs' inhibition of COX‐1 enzymes can lead to bleeding from the stomach.
Non‐selective NSAIDs also inhibit COX‐2, and their effects can be different to those that inhibit COX‐1 enzymes. COX‐2 is the most important contributor to inflammation, hypertension, and possibly cancer. It is induced by immune cell factors, shear stress, and tumour promoters. Selective COX‐2 inhibitors target the inflammatory process while minimizing gastric and non‐gastric bleeding. They may reduce the risks of hematoma and other significant bleeding after breast surgery, while still providing adequate pain control in comparison to non‐selective COX‐1/COX‐2 inhibitors by reducing endothelial prostacyclin and consequently increasing platelet aggregation. In this regard, focusing on the NSAID ketorolac may be misleading, as this has the highest COX‐1 selectivity of all the NSAIDs (Cheng 2016; Jarupongprapa 2013; Schmidt 2016).
A retrospective analysis of perioperative ketorolac use in patients undergoing breast reduction surgery demonstrated ketorolac was associated with a three‐fold increase in developing a hematoma and the need to return to the operating room for hematoma removal (Cawthorn 2012). A randomized controlled trial (RCT) comparing a NSAID (ketorolac) to a non‐NSAID (metamizol) for postoperative pain in elective plastic surgery reported postoperative bleeding in two patients receiving a NSAID that required a return to the operating room (Marin‐Bertolin 1997). Other studies have demonstrated no difference in bleeding rates between different NSAIDs (i.e. flurbiprofen or ketorolac) and placebo (Gobble 2014; Sun 2013). In patients receiving perioperative diclofenac, the risk associated with postoperative bleeding was higher than placebo; however, none of the patients needed reoperation for bleeding or hematoma (Cheng 2016).
A large number of studies have investigated whether NSAIDs are efficacious in reducing postoperative pain. Meta‐analyses of RCTs comparing opioid medication alone versus opioids plus NSAIDs in surgical patients found that the addition of ketorolac to intravenous morphine significantly improved pain scores and reduced analgesic use compared to intravenous morphine alone; however, these benefits were not as clear when intravenous patient‐controlled opioid analgesia was compared alone to a selective COX‐2 inhibitor or a non‐selective NSAID (ASA 2012). Another meta‐analysis of RCTs found the addition of a NSAID to be superior to placebo in reducing postoperative pain (Gobble 2014). When selective COX‐2 inhibitors (rofecoxib, etoricoxib, celecoxib, and parecoxib) were administered preoperatively, they significantly reduced postoperative pain when compared to placebo (Nir 2016). However, COX‐2 inhibitors have been associated with early failure of vascular free flaps (i.e. tissue that is disconnected from its original blood supply and reconnected at another location in the body) due to thrombosis from a lack of thromboxane A2 inhibition and platelet aggregation (Al‐Sukhun 2006; Bonde 2017).
Recent evidence suggests perioperative NSAIDs (flurbiprofen axetil and ketorolac) may be associated with decreased recurrences of breast cancer by inhibiting pro‐inflammatory and pro‐tumorigenic factors in patients undergoing surgery. These inflammatory factors may impair the immune system and promote tumour recurrence and metastasis (Desmedt 2018; Forget 2013). Other non‐NSAID pain medications lack these anti‐inflammatory characteristics. Even within the different classes of NSAIDs, results vary within patient demographics. A comparison of intraoperative ketorolac versus diclofenac in patients with an increased body mass index showed that ketorolac but not diclofenac was associated with reductions in the incidence of distant metastasis (Desmedt 2018).
Why it is important to do this review
With the recent focus on the over‐prescribing of opioids in the literature, it is important to assess the effectiveness and safety of non‐opioid pain medication regimens including NSAIDs (HHS 2016). Clinicians have differing opinions on the safety of perioperative NSAIDs for breast surgery given the unclear risk/benefit ratio. A hematoma during a plastic surgery procedure can result in complete loss of the reconstruction (Mikhaylov 2018).
Previous systematic reviews, focused on evaluating NSAID analgesics in breast surgery, were limited in scope (Cheng 2016; Gobble 2014; Stephens 2015). Stephens 2015 conducted a systematic review to assess the association between perioperative use of ketorolac and the incidence of hematomas in 981 patients undergoing face and breast plastic surgery procedures. Although they found a two‐fold increase in the incidence of hematomas in patients who received ketorolac, this difference was not statistically significant (Stephens 2015). A single‐institution study by Sharma 2001 also did not find any significant association between the use of ketorolac and the incidence of hematomas in breast reconstruction procedures. The relatively small sample sizes included in these studies may potentially account for the lack of association demonstrated between perioperative NSAID use and hematoma development. Additionally, these results addressed very specific patient populations, so the conclusions may not be generalizable to patients undergoing alternate breast surgery procedures. Furthermore, some countries rarely use ketorolac as the perioperative NSAID. An evaluation of other NSAIDs more commonly used around the world needs to be completed.
As perioperative NSAID use was associated with a clinical decrease of recurrence of breast cancer, it needs to be determined if this clinical effect would outweigh contraindications to receiving NSAIDs in these patients. The American Society of Plastic Surgeons (ASPS) recommends that patients stop taking NSAIDs, including aspirin, prior to breast surgery (ASPS 2018). Research has not yet clarified the risks and benefits of all NSAIDs between cosmetic and reconstructive breast surgery patients. Assessing different proportions of COX‐1/COX‐2 inhibition and patient outcomes may provide a more uniform standard of care. There is currently no Cochrane Review on perioperative NSAID use for breast surgery. These results will help lead to more conclusive surgical practice guidelines for plastic surgeons encountering perioperative outcomes.
The impact of NSAID use is a very complex topic. The focus of this Cochrane review is on surgical site bleeding outcomes, however there are a multitude of important perioperative outcomes, including venous thromboembolism, major adverse cardiac events, acute kidney injury, gastrointestinal ulceration, and potential cancer recurrence or remission. These outcomes increase with age (Wongrakpanich 2018). In addition, other comorbidities can increase the frequency of these outcomes (Harirforoosh 2013; Wongrakpanich 2018). COX‐1 and COX‐2 inhibitor selectivity is highly relevant when assessing the risk‐benefit balance in this regard.
Objectives
To assess the effects of perioperative NSAID use versus non‐NSAID analgesics (other pain medications) in women undergoing any type of breast surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) looking at perioperative NSAID use in women undergoing breast surgery, and prioritized and presented them separately in the review.
Types of participants
We included women over the age of 18 who underwent any type of breast surgery. This included oncologic, reconstructive or cosmetic surgery. Trials included participants receiving a specified perioperative NSAID. We excluded studies that did not report results of our desired population separately.
Types of interventions
All interventions that compared perioperative systemic NSAID use. The comparisons included:
NSAID versus placebo (provides psychological effect of taking a medication with no physical effect);
NSAID versus other analgesic drug (morphine, hydrocodone, hydromorphone, fentanyl);
NSAID versus no intervention.
Terms used to refer to NSAIDs included aspirin, salicylic acid, diflunisal, propyphenazone, indomethacin, diclofenac, aceclofenac, etodolac, ketorolac, sulindac, ibuprofen, naproxen, fenoprofen, flurbiprofen, ketoprofen, mefenamic acid, meclofenamate, meloxicam, piroxicam, nabumetone, celecoxib, etoricoxib, parecoxib, and rofecoxib.
This included any non‐selective COX or selective COX‐2 inhibitors administered by the following routes (intravenous, intramuscular, rectal, or oral) during hospital admission.
Types of outcome measures
Primary outcomes
Incidence of breast hematoma within 90 days of breast surgery (requiring reoperation, interventional drainage, or no treatment). Hematomas were measured with clinical diagnosis alone or imaging.
Pain intensity 24 (± 12) hours following surgery. Pain was measured with validated pain scales including the numerical rating scale (NRS), visual analogue scale (VAS), and verbal categorical rating scale (VRS), which are ascertained from reviews on pain assessment (Hjermstad 2011; Younger 2009).
Secondary outcomes
Incidence rate or severity of postoperative nausea, vomiting, or both
Bleeding from any location within 90 days
Need for blood transfusion
Other side effects of NSAID use
Opioid use within 24 (± 12) hours of surgery
Length of hospital stay
Breast cancer recurrence
Non‐prescribed NSAID use
Severity of postoperative nausea and vomiting was reported with the following scales: Likert scales, VAS, postoperative nausea and vomiting (PONV) intensity scale (Wengritzky 2010), or PONV impact scale (Myles 2012).
We determined opioid use in studies that permitted co‐administration of opioids, evaluated mean opioid use (in mg) over various study time intervals and standardized into morphine equivalents using opioid conversion tables (Jacox 1994).
Other secondary outcomes were based on the measures used by the included studies.
Search methods for identification of studies
Electronic searches
We searched the following databases.
The Cochrane Breast Cancer Group (CBCG) Specialized Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined on the Group's website (breastcancer.cochrane.org/sites/breastcancer.cochrane.org/files/public/uploads/specialised_register_details.pdf). We considered trials with the key words "non‐steroidal anti‐inflammatory agents", "NSAID", "NSAIDS", "breast surgery", "breast augmentation", "mammoplasty" and/or "mastectomy" for inclusion in the review.
CENTRAL (the Cochrane Library, Issue 8, 2020). See Appendix 1.
MEDLINE (via OvidSP) from 1950 to 21 September 2020. See Appendix 2.
Embase (via embase.com) from 1980 to 21 September 2020. See Appendix 3.
The WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials on 21 September 2020. See Appendix 4.
ClinicalTrials.gov (clinicaltrials.gov) on 21 September 2020. See Appendix 5.
Searching other resources
We identified further studies by handsearching reference lists of identified relevant trials or reviews and obtained a copy of the full article for each reference reporting a potentially eligible trial. Where this was not possible, we attempted to contact authors to provide additional information.
Data collection and analysis
Selection of studies
Two review authors (KMK, AE) independently screened all study titles and abstracts from the results of our search strategy. In case of disagreement on study inclusion, these two review authors met to discuss eligibility and involved a third review author (GDR) if necessary. We followed a similar process during full‐text screening. The selection process was recorded in the PRISMA flow diagram and at every step, and we recorded the reasons for exclusion in the Characteristics of excluded studies tables. There were no language restrictions; if necessary, we had non‐English papers translated. References were managed with Covidence.
Data extraction and management
Two review authors (KMK, AE) independently extracted quantitative data related to the primary and secondary outcomes using standardized data extraction forms and Covidence systematic review software (Covidence). Information extracted from each included trial consisted of the following.
Characteristics of trial participants (age, type of breast surgery, breast cancer history)
Type of intervention (type, dose, duration, frequency, and perioperative administration timing of NSAID and/or non‐NSAID, placebo, or no treatment)
Type of outcome measure (occurrence and treatment of breast hematoma, level of pain, occurrence or severity of PONV, length of stay)
Study information data (e.g. study design, sample size of each study group, year, location, author list), recorded for the purposes of identification and quality appraisal
Risk of bias domains (Higgins 2011)
These study authors met and determined whether any disagreements exist in the data extracted and resolved disagreements through discussion, involving a third review author (GDR) when necessary. We identified reports pertaining to the same study, selected a single one as the primary reference for inclusion based on completeness of reporting. We did not include non‐randomized studies in our review, so we did not extract data on the following: methods used to control for confounders, adjusted and unadjusted outcome measures, and the list of variables authors have included in analyses for adjusted estimates.
Assessment of risk of bias in included studies
Two review authors (KMK, AE) independently assessed risk of bias using the Cochrane RoB 1 tool (Higgins 2011), resolved any disagreement through discussion and involved a third review author (GDR) when necessary. We evaluated the following items using the risk of bias table.
Randomized studies
Random sequence generation (to assess possible selection bias)
Allocation concealment (to assess possible selection bias)
Blinding of participants and personnel (to assess possible performance bias)
Blinding of outcome assessment (to assess possible detection bias)
Incomplete outcome data (to assess possible attrition bias)
Selective outcome reporting (to assess possible reporting bias)
Moreover, we assessed all studies to evaluate if they were at overall high risk of bias according to Higgins 2011. We considered the magnitude of bias with random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. We considered studies to be at low overall risk of bias if they used a truly random process for sequence generation, concealed allocation, had no missing outcomes data, did not selectively report prespecified outcomes, blinded participants and personnel and blinded outcome assessments. Otherwise, we classified the studies as high risk of overall bias. We did not assess the impacts of risk using a sensitivity analysis with funnel plots due to fewer than 10 studies in comparative groups.
Review question
Does perioperative (preoperative, intraoperative, postoperative) administration of NSAIDs control postoperative pain without increasing the risk of hematoma within the first 90 days of the operation?
Confounding factors
We considered the following factors to be relevant to all or most studies.
Primary diagnosis
Age (age > 75 years associated with increased risk of bleeding)
Previous severe bleeding on NSAIDS (WHO grade 3 or 4)
Prior major cardiac event (serious cardiovascular thrombotic events, myocardial infarction, and stroke)
Use of anticoagulation during the study
Performance status (Eastern Cooperative Oncology Group (ECOG))
Presence of bleeding disorder
Stage 4 or 5 chronic kidney disease
Dialysis
Co‐interventions
These potential co‐interventions could be different between treatment groups and have an impact on outcomes.
Use of over‐the‐counter NSAIDs
Use of anticoagulation
Use of antiplatelet medication
Use of over‐the‐counter or herbal medicines
Measures of treatment effect
For dichotomous outcomes (i.e. incidence of hematoma requiring reoperation within 90 days of breast surgery; incidence of hematoma requiring drainage within 90 days of breast surgery; and incidence of hematoma, regardless of treatment, within 90 days of breast surgery), we measured the effect using risk ratio (RR) and 95% confidence intervals (CIs).
For continuous outcomes collected with different scales (i.e. pain 24 (± 12) hours following surgery, postoperative nausea and vomiting at 24 (± 12) hours from surgery), we measured the effect using standardized mean differences (SMDs) with 95% CIs.
For continuous outcomes collected on the same scale (i.e. length of stay and opioid use), we measured the effect using mean differences (MDs) with corresponding standard deviation (SD) and 95% CIs.
Unit of analysis issues
We did not find any cluster‐randomized trials. Chan 1996 had five treatment arms. We only assessed treatment arms with comparable interventions. In three treatment arms bupivacaine was administered postoperatively. The first treatment arm administered diclofenac preoperatively, placebo postoperatively and bupivacaine postoperatively. The second treatment arm administered placebo preoperatively, diclofenac postoperatively and bupivacaine postoperatively. The third control arm (no intervention) only administered bupivacaine postoperatively. We compared both the first and second treatment arms to the control arm. To avoid duplicating control arm results during combined perioperative comparisons, we combined the first treatment and second treatment arms into a single group to create a single pair‐wise comparison, as outlined in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions. We made separate comparisons of the first treatment arm and control arm (preoperative) and second treatment arm and control arm (postoperative).
Dealing with missing data
We attempted to obtain missing data from study authors and perform intention‐to‐treat (ITT) analyses if possible. Otherwise, we performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals) and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We initially assessed clinical and methodological heterogeneity of included trials qualitatively with regard to patient characteristics, type of breast surgery, NSAIDs use, timing of NSAID administration, and measurement of outcomes. We extracted, recorded, and qualitatively evaluated possible sources of heterogeneity from the data.
We assessed statistical heterogeneity by calculating I2 (Higgins 2011), considered statistical heterogeneity as substantial if I2 was greater than 50% and the τ2 was greater than zero or there was a difference detected using P values in the Chi2 test for heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to use funnel plots to assess reporting bias if we had 10 or more studies for the primary outcome. However, the meta‐analysis for the primary outcomes included fewer than 10 studies (Higgins 2011).
Data synthesis
We conducted statistical analysis using Review Manager 5 software (Review Manager 2020). We used a fixed‐effect model for the meta‐analysis if it was acceptable to assume the trials were estimating the same treatment effect. We used a random‐effects model for incidence rate or severity of postoperative nausea, vomiting, or both, other side effects of NSAID use, and opioid use within 24 (± 12) hours of surgery to produce an overall summary of the average clinical treatment effect. If we detected substantial statistical heterogeneity, we did not pool results from the meta‐analysis but instead reported the data narratively.
We pooled dichotomous outcomes using Mantel‐Haenszel analysis for a fixed‐effect model or DerSimonian and Laird for a random‐effects model, and we pooled continuous outcomes using the inverse‐variance method.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for timing of drug administration (preoperative and postoperative). Information and an adequate number of studies and population samples were not available to perform a subgroup analysis for type of breast surgery, type of NSAID (non‐selective, COX‐2 inhibitor, aspirin), different drug doses, route of drug administration, timing of drug administration (intraoperative), and breast cancer status of included women (history of breast cancer, no history of breast cancer).
Sensitivity analysis
Information and an adequate number of studies and population samples were not available to perform a sensitivity analysis for studies with unclear or high risk of bias for all risk of bias domains.
Summary of findings and assessment of the certainty of the evidence
Two review authors (KMK, AE) assessed the certainty of evidence according to the GRADE approach for seven outcomes of interest, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We prepared a summary of findings table using GRADE software (GRADEproGDT), and presented findings for the following seven outcomes: incidence of breast hematoma (requiring reoperation or interventional drainage or no treatment); pain intensity 24 (± 12) hours following surgery; postoperative nausea, vomiting, or both; bleeding from any location; need for blood transfusion; other side effects of NSAID use; and opioid use within 24 (± 12) hours of surgery.
Results
Description of studies
Results of the search
We searched the main databases and trial registries on 21 September 2020. The search resulted in 5085 references imported for screening. Upon removal of duplicate records, we screened 4595 records. Following title/abstract review, 87 references were eligible for full‐text review. We excluded 74 studies after full‐text review. Figure 1 illustrates details of the process of screening and selecting studies for inclusion in the review. Following full‐text review, we included 12 studies (Bakr 2016; Bosek 1996; Chan 1996; Forget 2019; Freedman 2006; Legeby 2005; Oh 2016; Parsa 2005; Romundstad 2006a; Sun 2013; van Helmond 2016; Wen 2015). One study is ongoing (NCT03535116 2018).
1.
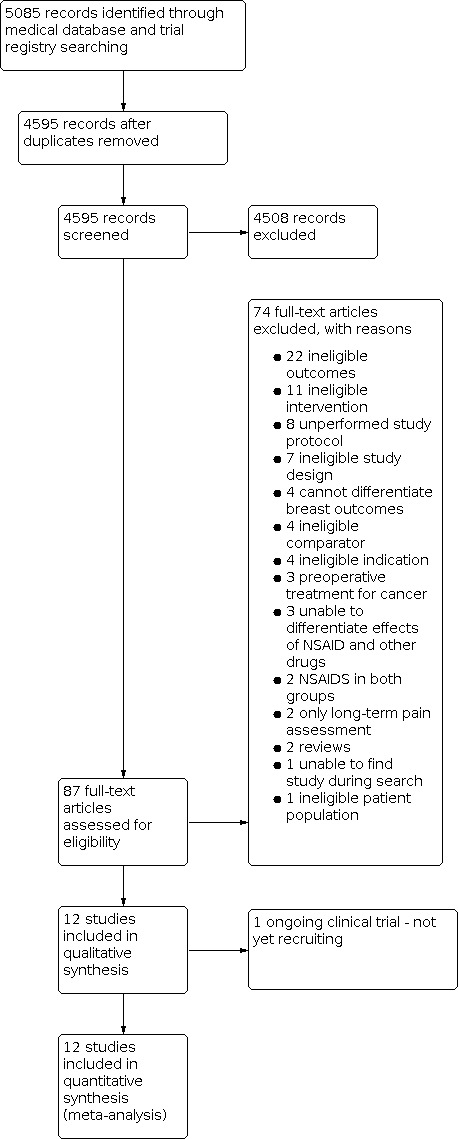
Study flow diagram.
Included studies
The 12 studies included a total of 1596 participants. Data for at least one outcome was reported in all 12 studies.
Seven studies compared 654 patients receiving NSAIDs (ketorolac (Bosek 1996; Forget 2019), diclofenac (Legeby 2005), flurbiprofen (Sun 2013), parecoxib (Romundstad 2006a), celecoxib (Parsa 2005), parecoxib and celecoxib (van Helmond 2016)) to 642 patients receiving placebo for breast surgery (prepectoral breast augmentation (Romundstad 2006a), subpectoral breast augmentation (Parsa 2005), mastectomy or lumpectomy with axillary lymph node dissection (Bosek 1996; Forget 2019), mastectomy with immediate tissue‐expander prosthesis (Legeby 2005), mastectomy with axillary lymph node dissection (Sun 2013), lumpectomy, total simple mastectomy or modified radical mastectomy (van Helmond 2016)). Of the seven studies comparing NSAIDs to placebo, six reported preoperative administration (Forget 2019; Legeby 2005; Parsa 2005; Romundstad 2006a; Sun 2013; van Helmond 2016), zero reported intraoperative administration, and four reported postoperative administration (Bosek 1996; Legeby 2005; Sun 2013; van Helmond 2016).
One study compared 40 patients receiving NSAIDs (diclofenac with bupivacaine (Chan 1996)) to 20 patients receiving no intervention (bupivacaine alone) at the beginning or end of breast surgery (lumpectomy). Chan 1996 reported preoperative and postoperative administration.
Four studies compared 120 patients receiving NSAIDs (ketorolac (Bakr 2016), flurbiprofen (Wen 2015), ibuprofen (Oh 2016), and celecoxib (Freedman 2006)) to 120 patients receiving other analgesics (morphine or tramadol (Bakr 2016), fentanyl (Wen 2015), hydromorphone (Oh 2016), hydrocodone (Freedman 2006) for breast surgery (modified radical mastectomy (Bakr 2016), modified radical mastectomy (Wen 2015), excisional biopsy or partial mastectomy or total mastectomy or modified radical mastectomy with or without lymph node dissection (Oh 2016), and subpectoral breast augmentation (Freedman 2006)). Of the four studies comparing NSAIDs to other analgesics, one reported preoperative administration (Freedman 2006), zero reported intraoperative administration, and four reported postoperative administration (Bakr 2016; Freedman 2006; Oh 2016; Wen 2015).
We present a summary of the condition, comparison, number randomized and number included in the review analyses for each study in Characteristics of included studies and summary of findings tables (Table 1; Table 2; Table 3).
Ongoing studies
Of the 80 studies eligible for full‐text review we identified one ongoing study (NCT03535116 2018). This study is not yet recruiting and will compare preoperative NSAIDs (ketorolac) to placebo and assess the development of breast hematomas. See: Characteristics of ongoing studies.
Excluded studies
We excluded 74 records with some of the reasons provided in the Characteristics of excluded studies and Figure 1. The main reasons for excluding studies were due to ineligible interventions or outcomes.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias judgments of the included studies.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
3.

Allocation
If the study adequately described the method of random sequence generation, we categorized the study to be at low risk of bias. Eleven studies were described as randomized and provided adequate information relating to the random sequence generation used (Bakr 2016; Chan 1996; Forget 2019; Freedman 2006; Legeby 2005; Oh 2016; Parsa 2005; Romundstad 2006a; Sun 2013; van Helmond 2016; Wen 2015). The randomisation process was unclear in one study due to the lack of information provided (Bosek 1996).
No information was provided or it was unclear what method of allocation concealment was used in 5 of the 12 studies (Bosek 1996; Chan 1996; Freedman 2006; Parsa 2005: van Helmond 2016). Seven studies clearly reported the allocation concealment (Bakr 2016; Forget 2019; Legeby 2005; Oh 2016; Romundstad 2006a; Sun 2013; Wen 2015).
Blinding
Ten studies clearly reported blinding of participants and personnel (Bakr 2016; Bosek 1996; Chan 1996; Forget 2019; Legeby 2005; Oh 2016; Romundstad 2006a; Sun 2013; van Helmond 2016; Wen 2015). The risk of performance bias was high in two studies (Freedman 2006; Parsa 2005). Freedman 2006 and Parsa 2005 did not blind either their participants or their personnel.
The method of blinding of outcome assessment was reported in seven studies (Forget 2019; Legeby 2005; Oh 2016; Romundstad 2006a; Sun 2013; van Helmond 2016; Wen 2015). Three studies had unclear risk of detection bias (Bakr 2016; Bosek 1996; Chan 1996). No information was provided regarding the method used to blind the assessment of outcomes in three studies (Bakr 2016; Bosek 1996; Chan 1996). Studies by Freedman 2006 and Parsa 2005 had high risk of detection bias as both studies did not blind their study personnel to outcome assessment.
Incomplete outcome data
In eight studies the completeness of outcome data was adequate and we classified them as having a low risk of bias for this domain. Legeby 2005 and van Helmond 2016 reported high rates of exclusions and we judged incomplete reporting of outcomes as high risk of bias. Parsa 2005 did not report SDs with mean opioid use within 24 (± 12) hours of surgery. We judged incomplete reporting of outcome data as a high risk of attrition bias. Forget 2019 reported missing data from one patient for pain intensity 24 (± 12) hours following surgery in a participant receiving placebo.
Selective reporting
The results for the primary and secondary outcomes were reported in four studies (Bakr 2016; Forget 2019; Oh 2016; van Helmond 2016). We judged studies with trial registrations or protocols confirming reported outcomes as low risk of reporting bias. Bosek 1996, Chan 1996, Freedman 2006, Legeby 2005, Parsa 2005, Romundstad 2006a, Sun 2013 and Wen 2015 did not have available trial registrations or protocols. We considered studies without trial or protocol registrations at high risk of reporting bias.
Other potential sources of bias
None identified.
Effects of interventions
See: Table 1; Table 2; Table 3
NSAID versus placebo
See: Table 1.
Incidence of breast hematoma within 90 days of breast surgery (requiring reoperation, interventional drainage, or no treatment)
Of the seven studies comparing perioperative NSAIDs to placebo, two reported incidence of breast hematoma within 90 days of breast surgery. Pooled analysis from two studies (Romundstad 2006a; van Helmond 2016) suggests that administering NSAIDs (parecoxib, celecoxib) may not result in a difference in the incidence of breast hematomas within 90 days of breast surgery compared to placebo groups (RR 0.33, 95% CI 0.05 to 2.02; 2 studies, 230 participants; I2 = 0%; low‐certainty evidence; Figure 4; Analysis 1.1). Romundstad 2006a reported one incidence of breast hematoma in 68 patients taking one dose of parecoxib 40 mg IV (preoperative) compared to one incidence of breast hematoma in 68 patients taking saline IV placebo, administered before the start of sedation for breast augmentation surgery. van Helmond 2016 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. van Helmond 2016 reported no incidences of breast hematoma in 48 patients taking two doses of parecoxib 40 mg IV (preoperative) administered 30 minutes before the start of sedation and oral celecoxib 200 mg daily (postoperative) day 1 to 5 compared to three incidences of breast hematoma in 46 patients taking placebo IV administered 30 minutes before the start of sedation and oral tablets daily postoperative day 1 to 5, for lumpectomy, total simple mastectomy or modified radical mastectomy.
4.

Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.1 Breast hematoma.
1.1. Analysis.

Comparison 1: NSAID versus placebo, Outcome 1: Breast hematoma
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, one reported incidence of breast hematoma within 90 days of breast surgery. Romundstad 2006a suggests there may be no difference in the development of breast hematomas within 90 days of breast surgery between preoperative NSAIDs (parecoxib) and placebo (RR 1.00, 95% CI 0.06 to 15.66; 1 study, 136 participants; low‐certainty evidence).
Pain intensity 24 (± 12) hours following surgery
Of the seven studies comparing perioperative NSAIDs to placebo, four reported pain 24 (± 12) hours following surgery (Bosek 1996; Forget 2019; Legeby 2005; Sun 2013). Pooled analysis from three studies (Bosek 1996; Forget 2019; Legeby 2005) suggest NSAIDs (ketorolac, diclofenac) may reduce pain intensity 24 (± 12) hours following surgery compared to placebo (SMD ‐0.26, 95% CI ‐0.49 to ‐0.03; 3 studies, 310 participants; I2 = 73%; low‐certainty evidence; Figure 5; Analysis 1.3). Sun 2013 reported pain scores of zero in the NSAID and placebo groups, and therefore we did not include this study in the analysis.
5.

Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.3 Pain intensity 24 (± 12) hours following surgery.
1.3. Analysis.

Comparison 1: NSAID versus placebo, Outcome 3: Pain intensity 24 (± 12) hours following surgery
Validated pain scales included the numerical rating scale (NRS), visual analog scale (VAS), and verbal categorical rating scale (VRS), which were ascertained from reviews on pain assessment (Hjermstad 2011; Younger 2009). Bosek 1996 reported no differences in pain 18 hours after surgery in 40 patients with ketorolac 30 mg IV or ketorolac 30 mg by Jackson‐Pratt drain compared to 20 patients taking saline placebo administered near the end of surgery for mastectomy or lumpectomy with axillary lymph node dissection. Forget 2019 reported no difference in pain scores 24 hours after surgery in 96 patients with ketorolac 30 mg IV compared to 106 patients with saline IV administered during the induction of anesthesia. Legeby 2005 reported lower pain scores the first 20 hours after surgery in 25 patients with diclofenac 50 mg suppositories rectally (preoperative and postoperative) compared to 23 patients with saline suppositories rectally administered every 8 hours for 3 days administered 1 hour before the start of mastectomy with immediate tissue‐expander prosthesis surgery. Sun 2013 reported no pain within 24 hours of surgery with flurbiprofen 50 mg IV (preoperative and postoperative) and intralipid placebo IV administered 15 minutes before the surgical incision and 6 hours later for mastectomy with axillary lymph node dissection. Legeby 2005 and Sun 2013 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. Pain intensity scores were reported in mean and standard deviation (SD) by Bosek 1996 and Legeby 2005. Pain intensity scores were calculated from values provided by Forget 2019.
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, one reported postoperative pain within 24 hours of surgery (Forget 2019). Forget 2019 suggests preoperative NSAIDs (diclofenac) may not reduce postoperative pain intensity within 24 (± 12) hours of surgery compared to placebo (SMD ‐0.19, 95% CI ‐0.46 to 0.09; 1 study, 202 participants; low‐certainty evidence).
Postoperative
Of the four studies comparing postoperative NSAIDs to placebo, one reported postoperative pain within 24 hours of surgery (Bosek 1996). Bosek 1996 suggests postoperative NSAIDs (ketorolac) may not reduce postoperative pain intensity within 24 (± 12) hours of surgery compared to placebo, but we are very uncertain (SMD 0.04, 95% CI ‐0.50 to 0.57; 1 study, 60 participants; very low‐certainty evidence).
Incidence rate or severity of postoperative nausea, vomiting, or both
Of the seven studies comparing perioperative NSAIDs to placebo, four reported incidence rate or severity of postoperative nausea, vomiting, or both (Bosek 1996; Legeby 2005; Parsa 2005; Romundstad 2006a). Pooled analysis from these four studies suggest NSAIDs (ketorolac, diclofenac, celecoxib, parecoxib) may not reduce the incidence rates or severities of postoperative nausea, vomiting, or both compared to placebo (RR 1.15, 95% CI 0.58 to 2.27; 4 studies, 939 participants; I2 = 81%; low‐certainty evidence; Figure 6; Analysis 1.5).
6.

Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.5 Incidence rate or severity of postoperative nausea, vomiting, or both.
1.5. Analysis.

Comparison 1: NSAID versus placebo, Outcome 5: Incidence rate or severity of postoperative nausea, vomiting, or both
Postoperative nausea, vomiting, or both were highest in the NSAID group (71/480) compared to placebo group (51/458). Bosek 1996 reported significantly less postoperative nausea, vomiting, or both measured every 6 hours, in 16/40 patients with ketorolac 30 mg IV or ketorolac 30 mg by Jackson‐Pratt drain compared to 14/20 patients taking saline placebo administered near the end of surgery for mastectomy or lumpectomy with axillary lymph node dissection. Legeby 2005 reported postoperative nausea, vomiting, or both in 14/25 patients with diclofenac 50 mg suppositories rectally compared to 12/23 patients with saline suppositories rectally administered every 8 hours for 3 days administered 1 hour before the start of mastectomy with immediate tissue‐expander prosthesis surgery. Legeby 2005 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. Parsa 2005 reported postoperative nausea, vomiting, or both in 6/347 patients with celecoxib 40 mg by mouth compared to 0/347 patients with placebo by mouth administered 30 minutes before subpectoral breast augmentation. Romundstad 2006a reported significantly more postoperative nausea, vomiting, or both measured during the first 24 hours in 41/68 patients taking 1 dose of parecoxib 40 mg IV compared to 25/68 patients taking saline IV placebo, administered before the start of sedation for breast augmentation surgery.
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, two reported incidence rate or severity of postoperative nausea, vomiting, or both (Parsa 2005; Romundstad 2006a). Pooled analysis from these two studies suggest preoperative NSAIDs (celecoxib, parecoxib) may not reduce the incidence rates or severities of postoperative nausea, vomiting, or both compared to placebo, but we are very uncertain (RR 2.95, 95% CI 0.42 to 20.64; 2 studies, 831 participants; I2 = 55%; very low‐certainty evidence).
Postoperative
Of the four studies comparing postoperative NSAIDs to placebo, one reported incidence rate or severity of postoperative nausea, vomiting, or both. Bosek 1996 suggests postoperative NSAIDs (ketorolac) may reduce the incidence rates or severities of postoperative nausea, vomiting, or both compared to placebo, but we are very uncertain (RR 0.57, 95% CI 0.36 to 0.92; 1 study, 60 participants; very low‐certainty evidence).
Bleeding from any location within 90 days
Of the seven studies comparing perioperative NSAIDs to placebo, three reported bleeding from any location within 90 days (Forget 2019; Legeby 2005, Sun 2013). Pooled analysis from two studies (Forget 2019; Legeby 2005) suggest NSAIDs (ketorolac, diclofenac) may not increase bleeding from any location within 90 days compared to placebo (RR 1.05, 95% CI 0.89 to 1.24; 2 studies, 251 participants; I2 = 8%; low‐certainty evidence; Analysis 1.7). Sun 2013 had no estimable results with zero events in the 30 patients in the NSAID and 30 patients in the placebo groups. Forget 2019 reported no difference in the incidence of bleeding from any location within 90 days in 1/96 patients with ketorolac 30 mg IV compared to 0/107 patients with saline IV administered at induction of anesthesia. Legeby 2005 reported no difference in the incidence of bleeding from any location within 90 days in 24/25 patients with diclofenac 50 mg suppositories rectally (preoperative and postoperative) compared to 22/23 patients with saline suppositories rectally administered every 8 hours for 3 days administered 1 hour before the start of mastectomy with immediate tissue‐expander prosthesis surgery. However, Legeby 2005 reported the volume of blood loss was significantly higher with diclofenac compared to placebo. Sun 2013 reported no difference in the incidence of bleeding in 0/30 patients with flurbiprofen 50 mg IV (preoperative and postoperative) compared to 0/30 patients with intralipid placebo IV administered 15 minutes before the surgical incision and 6 hours later for mastectomy with axillary lymph node dissection. Legeby 2005 and Sun 2013 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. As Sun 2013 had no estimable results, with zero events in the NSAID and placebo groups, we removed this study from the analysis.
1.7. Analysis.

Comparison 1: NSAID versus placebo, Outcome 7: Bleeding from any location within 90 days
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, one reported bleeding from any location within 90 days (Forget 2019). Forget 2019 suggests preoperative NSAIDs (ketorolac) likely does not increase bleeding from any location within 90 days compared to placebo, but we are very uncertain (RR 3.34, 95% CI 0.14 to 81.03; 1 study, 203 participants; very low‐certainty evidence).
Need for blood transfusion
Of the seven studies comparing perioperative NSAIDs to placebo, two reported need for a blood transfusion (Forget 2019; Legeby 2005). Legeby 2005 suggests that NSAIDs (diclofenac) may not increase bleeding from any location within 90 days compared to placebo, but we are very uncertain (RR 4.62, 95% CI 0.23 to 91.34; 1 study, 48 participants; very low‐certainty evidence; Analysis 1.9). Legeby 2005 reported 2/25 patients receiving a blood transfusion with diclofenac 50 mg suppositories rectally (preoperative and postoperative) compared to 0/23 patients with saline suppositories rectally administered every 8 hours for 3 days administered 1 hour before the start of mastectomy with immediate tissue‐expander prosthesis surgery. Legeby 2005 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. Forget 2019 reported no difference in the need for blood transfusion in 0/96 patients with ketorolac 30 mg IV compared to 0/107 patients with saline IV administered at induction of anesthesia. Forget 2019 had no estimable results, with zero events in the NSAID and placebo groups, and so we removed this study from the analysis.
1.9. Analysis.

Comparison 1: NSAID versus placebo, Outcome 9: Need for blood transfusion
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, one reported need for a blood transfusion (Legeby 2005). Pooled analysis from Legeby 2005 suggests NSAIDs (diclofenac) may not increase bleeding from any location within 90 days compared to placebo, but we are very uncertain (RR 4.62, 95% CI 0.23 to 91.34; 1 study, 48 participants; very low‐certainty evidence).
Other side effects of NSAID use
Of the seven studies comparing perioperative NSAIDs to placebo, three reported other side effects of NSAID use (Forget 2019; Legeby 2005, Sun 2013). Pooled analysis from two studies (Forget 2019; Legeby 2005) suggest NSAIDs (ketorolac, diclofenac) may not differ in other side effects compared to placebo (RR 1.12, 95% CI 0.44 to 2.86; 2 studies, 251 participants; I2 = 0%; low‐certainty evidence; Analysis 1.11). Forget 2019 reported other side effects in 8/96 patients with ketorolac 30 mg IV (preoperative) compared to 7/107 patients with saline IV administered at induction of anesthesia. Legeby 2005 reported other side effects in 0/25 patients with diclofenac 50 mg suppositories rectally (preoperative and postoperative) compared to 1/23 patients with saline suppositories rectally (hypoventilation with respiratory rate less than 6 breaths/min) administered every 8 hours for 3 days, administered 1 hour before the start of mastectomy with immediate tissue‐expander prosthesis surgery. Sun 2013 reported no difference in other side effects in 0/30 patients with flurbiprofen 50 mg IV (preoperative and postoperative) compared to 0/30 patients with intralipid placebo IV administered 15 minutes before the surgical incision and 6 hours later for mastectomy with axillary lymph node dissection. Legeby 2005 and Sun 2013 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. Sun 2013 had no estimable results, with zero events in the NSAID and placebo groups with 30 patients in each group.
1.11. Analysis.

Comparison 1: NSAID versus placebo, Outcome 11: Other side effects of NSAID use
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, one reported other side effects of NSAID use (Forget 2019). Forget 2019 suggests preoperative NSAIDs (ketorolac) may not result in a difference in other side effects compared to placebo (RR 1.27, 95% CI 0.48 to 3.38; 1 study, 203 participants; low‐certainty evidence).
Opioid use within 24 (± 12) hours of surgery
Of the seven studies comparing perioperative NSAIDs to placebo, five reported opioid use within 24 (± 12) hours of surgery (Bosek 1996; Legeby 2005; Parsa 2005; Romundstad 2006a; Sun 2013). Pooled analysis from four studies (Bosek 1996; Legeby 2005; Romundstad 2006a; Sun 2013) suggest NSAIDs (ketorolac, diclofenac, parecoxib, flurbiprofen) may reduce opioid use within 24 hours of surgery compared to placebo (SMD ‐0.45, 95% CI ‐0.85 to ‐0.05; 4 studies, 304 participants; I2 = 63%; low‐certainty evidence; Analysis 1.13). Parsa 2005 was not incorporated into the pooled analysis due to missing standard deviations. Parsa 2005 reported a mean hydrocodone tablet morphine equivalent use of 30.5 mg in 347 patients with celecoxib 40 mg by mouth compared to 51.5 mg in 348 patients with placebo by mouth administered 30 minutes before subpectoral breast augmentation.
1.13. Analysis.

Comparison 1: NSAID versus placebo, Outcome 13: Opioid use within 24 (± 12) hours of surgery
Bosek 1996 reported a mean morphine IV use of 1.7 ± 3.1 mg in 40 patients with ketorolac 30 mg IV or ketorolac 30 mg by Jackson‐Pratt drain (postoperative) compared to 2.7 ± 3.9 mg in 20 patients taking saline placebo 18 hours after surgery for mastectomy or lumpectomy with axillary lymph node dissection. Legeby 2005 reported a mean morphine or ketobemidone use of 26.8 ± 18.1 mg in 25 patients with diclofenac 50 mg suppositories rectally (preoperative and postoperative) compared to 35.9 ± 13.6 mg in 23 patients with saline suppositories rectally for mastectomy with immediate tissue‐expander prosthesis surgery. Romundstad 2006a reported a mean acetaminophen 500 mg and codeine 30 mg tablet morphine equivalent use of 9.9 ± 5.85 mg in 68 patients taking 1 dose of parecoxib 40 mg IV (preoperative) compared to 15.3 ± 6.75 mg in 68 patients taking saline IV placebo, administered before the start of sedation for breast augmentation surgery. Sun 2013 reported a mean fentanyl IV morphine equivalent use of 0.18 ± 0.04 mg in 30 patients with flurbiprofen 50 mg IV (preoperative and postoperative) compared to 0.18 ± 0.03 mg in 30 patients with intralipid placebo IV administered 15 minutes before the surgical incision and 6 hours later for mastectomy with axillary lymph node dissection. Legeby 2005 and Sun 2013 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these.
Timing of drug administration
Preoperative
Of the six studies comparing preoperative NSAIDs to placebo, two reported opioid use within 24 (± 12) hours of surgery (Parsa 2005; Romundstad 2006a). Romundstad 2006a suggests preoperative NSAIDs (parecoxib) may not result in a difference in opioid use within 24 hours of surgery compared to placebo (SMD ‐0.85, 95% CI ‐1.20 to ‐0.50; 1 study, 136 participants; low‐certainty evidence). As Parsa 2005 did not report SDs in the NSAID and placebo groups, we removed it from the analysis.
Postoperative
Of the four studies comparing postoperative NSAIDs to placebo, one reported opioid use within 24 (± 12) hours of surgery (Bosek 1996). Bosek 1996 suggests postoperative NSAIDs (ketorolac) may not result in a difference in opioid use within 24 hours of surgery compared to placebo, but we are very uncertain (SMD ‐0.29, 95% CI ‐0.83 to 0.25; 1 study, 60 participants; very low‐certainty evidence).
Length of hospital stay
Of the seven studies comparing perioperative NSAIDs to placebo, one reported length of hospital stay (Forget 2019). Forget 2019 suggests preoperative NSAIDs (ketorolac) may not result in a difference in length of hospital stay compared to placebo (SMD 0.18, 95% CI ‐0.09 to 0.46; 1 study, 203 participants; low‐certainty evidence). Forget 2019 reported a mean length of hospital stay of 3.9 ± 1.2 days with ketorolac 30 mg IV (preoperative) compared to 3.7 ± 1 days with saline IV administered at induction of anesthesia.
Breast cancer recurrence
None of the included studies reported breast cancer recurrence.
Non‐prescribed NSAID use
None of the included studies reported non‐prescribed NSAID use.
NSAID versus other analgesic
See: Table 2.
Incidence of breast hematoma within 90 days of breast surgery (requiring reoperation, interventional drainage, or no treatment)
Of the four studies comparing perioperative NSAIDs to other analgesics, one reported incidence of breast hematoma within 90 days of breast surgery (Freedman 2006). Freedman 2006 suggests administering NSAIDs (celecoxib) may not result in a difference in the development of breast hematomas within 90 days of breast surgery compared to other analgesics (hydrocodone), but we are very uncertain (RR 0.33, 95% CI 0.01 to 7.99; 1 study, 100 participants; very low‐certainty evidence; Figure 7; Analysis 2.1). No incidence of breast hematoma was reported in 50 patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days compared to one incidence of breast hematoma in 50 patients taking hydrocodone 5 mg by mouth as needed, for subpectoral breast augmentation surgery. Freedman 2006 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these.
7.

Forest plot of comparison: 3 NSAID vs other analgesic, outcome: 3.1 Incidence of breast hematoma within 90 days of breast surgery.
2.1. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 1: Incidence of breast hematoma within 90 days of breast surgery
Pain intensity 24 (± 12) hours following surgery
Of the four studies comparing perioperative NSAIDs to other analgesics, four reported pain 24 (± 12) hours following surgery (Bakr 2016; Freedman 2006; Oh 2016; Wen 2015). Pooled analysis from three studies (Freedman 2006; Oh 2016; Wen 2015), suggest NSAIDs (celecoxib, flurbiprofen, ibuprofen) may reduce pain intensity 24 (± 12) hours following surgery compared to other analgesics (fentanyl, hydrocodone, hydromorphone) (SMD ‐0.68, 95% CI ‐0.97 to ‐0.39; 3 studies, 200 participants; I2 = 89%; low‐certainty evidence; Analysis 2.2). Bakr 2016 reported no differences in pain up to 24 hours after surgery in 20 patients with ketorolac 60 mg IV compared to 20 patients taking morphine 5 mg IV administered at the end of modified radical mastectomy. We did not include Bakr 2016 in the pooled analysis due to missing SDs.
2.2. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 2: Pain intensity 24 (± 12) hours following surgery
Validated pain scales included the NRS, VAS, and VRS, which were ascertained from reviews on pain assessment (Hjermstad 2011; Younger 2009). Freedman 2006 reported a greater reduction in pain in 50 patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days compared to 50 patients taking hydrocodone 5 mg by mouth as needed, for subpectoral breast augmentation surgery. Oh 2016 reported ibuprofen 800 mg IV (postoperative) was not able to provide proper pain control compared to hydromorphone 2 mg IV in five consecutive patients for excisional biopsy or partial mastectomy or total mastectomy or modified radical mastectomy with or without lymph node dissection, therefore patient enrolment was stopped after 18 patients. Wen 2015 reported no differences in pain 24 hours after surgery in 20 patients with flurbiprofen 3 mg/kg IV (postoperative) compared to 20 patients taking fentanyl 15 µg/kg IV administered postoperatively for modified radical mastectomy. Freedman 2006 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these.
Timing of drug administration
Postoperative
Of the three studies comparing postoperative NSAIDs to other analgesics, three reported pain 24 (± 12) hours following surgery (Bakr 2016; Oh 2016; Wen 2015). Pooled analysis from two studies (Oh 2016; Wen 2015), suggests there is likely no difference in pain intensity 24 (± 12) hours following surgery between postoperative NSAIDs (flurbiprofen, ibuprofen) and other analgesics (fentanyl, hydromorphone) (SMD ‐0.12, 95% CI ‐0.51 to 0.27; 2 studies, 100 participants; I2 = 0%; moderate‐certainty evidence). We did not include Bakr 2016 in the pooled analysis due to missing SDs.
Incidence rate or severity of postoperative nausea, vomiting, or both
Of the four studies comparing perioperative NSAIDs to other analgesics, four reported incidence of postoperative nausea, vomiting or both (Bakr 2016; Freedman 2006; Oh 2016; Wen 2015). Pooled analysis from three studies (Bakr 2016; Oh 2016; Wen 2015) suggest NSAIDs (ketorolac, ibuprofen, flurbiprofen) likely reduce the incidence rates or severities of postoperative nausea, vomiting, or both compared to other analgesics (morphine, hydromorphone, fentanyl) (RR 0.18, 95% CI 0.06 to 0.57; 3 studies, 128 participants; I2 = 0%; moderate‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 4: Incidence rate or severity of postoperative nausea, vomiting, or both
Bakr 2016 reported postoperative nausea, vomiting, or both in 0/20 patients with ketorolac 60 mg IV (postoperative) compared to 4/20 patients taking morphine 5 mg IV administered at the end of modified radical mastectomy. Oh 2016 reported postoperative nausea, vomiting, or both in 0/18 patients taking ibuprofen IV (postoperative) compared to 1/30 patients taking hydromorphone IV for excisional biopsy or partial mastectomy or total mastectomy or modified radical mastectomy with or without lymph node dissection. Wen 2015 reported significantly less postoperative nausea, vomiting, or both within 48 hours in 2/20 patients with flurbiprofen 3 mg/kg IV (postoperative) compared to 12/20 patients taking fentanyl 15 µg/kg IV administered postoperatively for modified radical mastectomy. Bakr 2016, Oh 2016, and Wen 2015 examined the effect of administering NSAIDs during the postoperative phase. Freedman 2006 reported patients taking hydrocodone 5 mg by mouth, as needed, had a significantly higher number of days (91 days) of nausea compared to 43 days of nausea in patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days. Freedman 2006 reported results in metric units that could not be converted and compared to the other studies.
Bleeding from any location within 90 days
Of the four studies comparing perioperative NSAIDs to other analgesics, four reported bleeding from any location within 90 days (Bakr 2016; Freedman 2006; Oh 2016; Wen 2015). Bakr 2016, Freedman 2006, Oh 2016 and Wen 2015 had no estimable results, with zero events in the NSAID and other analgesics groups. Freedman 2006 suggests there may be no difference in the development of bleeding from any location within 90 days of breast surgery between NSAIDs (celecoxib) and other analgesics (hydrocodone), but we are very uncertain (RR 0.33, 95% CI 0.01 to 7.99; 1 study, 100 participants; very low‐certainty evidence). No incidence of bleeding from any location was reported in 50 patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days compared to one incidence of bleeding from any location in 50 patients taking hydrocodone 5 mg by mouth as needed, for subpectoral breast augmentation surgery. Freedman 2006 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these. Bakr 2016, Oh 2016, and Wen 2015 examined the effect of administering NSAIDs during the postoperative phase.
Need for blood transfusion
None of the included studies reported the need for a blood transfusion.
Other side effects of NSAID use
Of the four studies comparing perioperative NSAIDs to other analgesics, three reported other side effects of NSAID use (Bakr 2016; Freedman 2006; Oh 2016). Oh 2016 suggested there may be no difference in other side effects between postoperative NSAIDs (ibuprofen) and other analgesics (hydromorphone), but we are very uncertain (RR 0.11, 95% CI 0.01 to 1.80; 1 study, 48 participants; very low‐certainty evidence; Analysis 2.6). Bakr 2016 and Freedman 2006 had no estimable results, with zero events in the NSAID and other analgesics groups, and so we did not include them in the analysis. Oh 2016 reported other side effects in 0/18 patients taking ibuprofen IV (postoperative) compared to 7/30 patients taking hydromorphone IV for excisional biopsy or partial mastectomy or total mastectomy or modified radical mastectomy with or without lymph node dissection. Bakr 2016 reported other side effects in 0/20 patients with ketorolac 60 mg IV (postoperative) compared to 0/20 patients taking morphine 5 mg IV administered at the end of modified radical mastectomy. Freedman 2006 reported other side effects in 0/50 patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days compared to 0/50 patients taking hydrocodone 5 mg by mouth as needed, for subpectoral breast augmentation surgery.
2.6. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 6: Other side effects of NSAID use
Opioid use within 24 (± 12) hours of surgery
Of the four studies comparing perioperative NSAIDs to other analgesics, three reported opioid use within 24 (± 12) hours of surgery (Bakr 2016; Freedman 2006; Wen 2015). Pooled analysis from these three studies suggest NSAIDs (ketorolac, celecoxib, flurbiprofen) may reduce opioid use within 24 hours of surgery compared to other analgesics (morphine, hydrocodone, fentanyl) (SMD ‐6.87, 95% CI ‐10.93 to ‐2.81; 3 studies, 178 participants; I2 = 96%; low‐certainty evidence; Analysis 2.7). Bakr 2016 reported a mean morphine IV use of 7 ± 2.51 mg in 20 patients with ketorolac 60 mg IV (postoperative) compared to 80 ± 19.47 mg in 20 patients with morphine 5 mg IV administered at the end of modified radical mastectomy. Freedman 2006 reported a mean hydrocodone tablet morphine equivalent use of 34 mg ± 22 mg in 50 patients taking celecoxib 400 mg by mouth (preoperative and postoperative) 1 to 2 hours prior to surgery and each morning after surgery for 7 days compared to 110 mg ± 34 mg in 50 patients taking hydrocodone 5 mg by mouth, as needed, for subpectoral breast augmentation surgery. Wen 2015 reported a mean fentanyl morphine equivalent use 48 hours after surgery of 26.8 mg ± 1.8 mg in 18 patients with flurbiprofen 3 mg/kg IV (postoperative) compared to 80.7 mg ± 4.8 mg in 20 patients taking fentanyl 15 µg/kg IV administered postoperatively for modified radical mastectomy. Freedman 2006 examined the effect of administering NSAIDs during both the preoperative and postoperative phase and did not report data separately for these.
2.7. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 7: Opioid use within 24 (± 12) hours of surgery
Timing of drug administration
Postoperative
Of the three studies comparing postoperative NSAIDs to other analgesics, two reported opioid use within 24 (± 12) hours of surgery (Bakr 2016; Wen 2015). Pooled analysis from these two studies suggest postoperative NSAIDs (ketorolac, flurbiprofen) may reduce opioid use within 24 hours of surgery compared to other analgesics (morphine, fentanyl) (SMD ‐9.56, 95% CI ‐18.48 to ‐0.64; 2 studies, 78 participants; I2 = 96%; low‐certainty evidence).
Length of hospital stay
None of the included studies reported length of hospital stay.
Breast cancer recurrence
None of the included studies reported breast cancer recurrence.
Non‐prescribed NSAID use
None of the included studies reported non‐prescribed NSAID use.
NSAID versus no intervention
See: Table 3.
Incidence of breast hematoma within 90 days of breast surgery (requiring reoperation, interventional drainage, or no treatment)
None of the included studies reported incidence of breast hematoma.
Pain intensity 24 (± 12) hours following surgery
Only one study compared perioperative NSAIDs to no intervention (Chan 1996). Chan 1996 reported pain intensity 24 (± 12) hours following surgery. This study suggests NSAIDs (diclofenac) may not reduce pain intensity 24 (± 12) hours following surgery compared to no intervention, but we are very uncertain (SMD ‐0.54, 95% CI ‐1.09 to 0.00; 1 study, 60 participants; very low‐certainty evidence; Analysis 3.1). Chan 1996 reported pain intensity in 40 patients receiving NSAIDs (preoperative diclofenac with bupivacaine and postoperative diclofenac with bupivacaine) compared to 20 patients receiving no intervention (bupivacaine alone) for breast lumpectomy surgery. VAS were reported only at 30 minutes, 60 minutes, 120 minutes and 48 hours postoperative (Hjermstad 2011; Younger 2009). Pain intensity at 120 minutes following surgery was 0.25 ± 0.74 in patients receiving diclofenac 75 mg IM (preoperative and postoperative) compared to 0.75 ± 1.18 patients receiving no intervention.
3.1. Analysis.

Comparison 3: NSAID versus no intervention, Outcome 1: Pain intensity 24 (± 12) hours following surgery
Timing of drug administration
Preoperative
One study comparing preoperative NSAIDs to no intervention reported pain intensity 24 (± 12) hours following surgery (Chan 1996). This study suggests there may be no difference in pain intensity 24 (± 12) hours following surgery between preoperative NSAIDs (diclofenac) and no intervention, but we are very uncertain (SMD ‐0.35, 95% CI ‐0.97 to 0.28; 1 study, 40 participants; very low‐certainty evidence). Chan 1996 administered diclofenac 75 mg IM to 20 patients preoperatively. Pain intensity at 120 minutes following surgery was 0.375 ± 0.92 in patients receiving diclofenac 75 mg IM compared to 0.75 ± 1.18 patients receiving no intervention.
Postoperative
One study comparing postoperative NSAIDs to no intervention reported pain intensity 24 (± 12) hours following surgery (Chan 1996). Pooled analysis from this study suggests postoperative NSAIDs (diclofenac) may reduce pain intensity 24 (± 12) hours following surgery compared to no intervention, but we are very uncertain (SMD ‐0.66, 95% CI ‐1.30 to ‐0.02; 1 study, 40 participants; very low‐certainty evidence). Chan 1996 administered diclofenac 75 mg IM to 20 patients postoperatively. Pain intensity at 120 minutes following surgery was 0.125 ± 0.56 in patients receiving diclofenac 75 mg IM compared to 0.75 ± 1.18 in patients receiving no intervention.
Incidence rate or severity of postoperative nausea, vomiting, or both
None of the included studies reported the incidence rate or severity of postoperative nausea, vomiting, or both.
Bleeding from any location within 90 days
None of the included studies reported bleeding from any location within 90 days.
Need for blood transfusion
None of the included studies reported the need for a blood transfusion.
Other side effects of NSAID use
None of the included studies reported other side effects of NSAID use.
Opioid use within 24 (± 12) hours of surgery
None of the included studies reported opioid use within 24 (±12) hours of surgery.
Length of hospital stay
None of the included studies reported length of hospital stay.
Breast cancer recurrence
None of the included studies reported breast cancer recurrence.
Non‐prescribed NSAID use
None of the included studies reported on non‐prescribed NSAID use.
Discussion
Summary of main results
This review provides the best available evidence from 12 studies with a total of 1596 participants on the use of perioperative NSAIDs during breast surgery. We found little to no evidence of a difference between people taking perioperative NSAIDs compared to placebo, and other analgesics (hydrocodone) in the development of breast hematomas within 90 days of breast surgery. The reported incidence of breast hematoma was one in people taking analgesics, three in patients taking placebo and one in those taking NSAIDs, demonstrating similar safety profiles for developing hematomas. The one study not yet recruiting (NCT03535116 2018), will compare NSAIDs (ketorolac) to placebo and assess the development of breast hematomas.
Among such women, perioperative NSAIDs (ketorolac, diclofenac) lowered pain intensity in the first 24 hours following surgery compared to placebo. Similarly, pain intensity was lower with perioperative NSAIDs compared to other analgesics in the first 24 hours following surgery.
When comparing NSAIDs to placebo, these was little to no evidence of a difference in postoperative nausea, vomiting or both. We found evidence to suggest that the risk of postoperative nausea, vomiting or both was lower in the NSAID group compared to other analgesics. Other analgesics included the opioid medications morphine (Bakr 2016), hydromorphone (Oh 2016), and fentanyl (Wen 2015). Opioid medications directly stimulate the chemoreceptor trigger zone in the postrema of the medulla in the central nervous system, responsible for nausea and vomiting (Brunton 2017). Our findings demonstrate NSAIDs may not have these adverse effects.
There was little to no evidence of a difference between patients taking perioperative NSAIDs (diclofenac) and placebo in bleeding from any location in 90 days and need for blood transfusion. Sun 2013 reported no bleeding from any location in the preoperative and postoperative administration of NSAID or placebo groups and so we excluded this study due to no estimable results. Forget 2019 reported no need for blood transfusion in the preoperative administration of NSAID or placebo groups and we excluded this study due to no estimable results.
Overall completeness and applicability of evidence
This review provides preliminary evidence for administering perioperative NSAIDs in women undergoing breast surgery. NSAIDs were shown to reduce pain, nausea, vomiting and opioid use postoperatively in the women undergoing breast surgery. One study evaluated implant‐based reconstruction (Legeby 2005), eight studies evaluated mastectomy or lumpectomy (Bosek 1996; Chan 1996; Oh 2016; Wen 2015; Sun 2013; Bakr 2016; van Helmond 2016; Forget 2019), and three studies evaluated augmentation mammaplasty (Freedman 2006; Parsa 2005; Romundstad 2006a). None of the studies evaluated NSAIDs in women undergoing autologous breast reconstruction, breast reduction, and mastopexy surgery, limiting any conclusions with these surgeries. Women must be evaluated on an individual basis to determine appropriate patient selection for NSAID administration.
Limited evidence suggests there may be no differences in safety profiles when NSAIDs were compared to placebo, and other analgesics for the development of breast hematomas. The differences in breast cancer recurrence and non‐prescribed NSAID use remain unclear for people receiving NSAIDs; no studies evaluated these outcomes. This may be due to the fact that there are many different types of breast surgeries for different indications. The people included in eight studies received mastectomy or lumpectomy and in three studies received augmentation for cosmetic purposes. Depending on the procedure performed, patients may leave the hospital that same day or may not have breast cancer and therefore recurrence is not applicable.
We evaluated six NSAIDs. These included parecoxib (Romundstad 2006a; van Helmond 2016), celecoxib (Freedman 2006; van Helmond 2016; Parsa 2005), flubiprofen (Sun 2013; Wen 2015), ibuprofen (Oh 2016), diclofenac (Chan 1996; Legeby 2005), and ketorolac (Bosek 1996; Bakr 2016; Forget 2019). Some of these medications may not be commonly used or available internationally. Evaluating alternative NSAIDs may be beneficial for these populations. Alternative routes of administration that did not include IV, IM, or oral formulations were evaluated in one study (Legeby 2005). Evaluating different routes of administration would be beneficial for people unable to tolerate oral medications after they are discharged from the hospital. Legeby 2005 assessed the efficacy of rectal diclofenac during the perioperative period. Topical medication administration using patches, creams, ointments, lotions, and emollients may provide localized pain relief without predisposing a patient to systemic exposure. This would be beneficial for patients with multiple comorbidities or complex medication regimens susceptible to drug interactions. Performing more studies will allow for comparisons between NSAIDs. There were no studies that reported intraoperative administration of NSAIDs for any of the outcomes of interest.
Intraoperative multimodal analgesic and anesthetic regimens are likely to impact outcomes associated with NSAIDs and may be responsible for differences observed between studies (Brinck 2018). Bosek 1996, Forget 2019, Legeby 2005 and Sun 2013 evaluated pain intensity 24 hours following surgery between NSAIDs and placebo. Bosek 1996 administered thiopental and isoflurane in nitrous oxide. Forget 2019 administered propofol, ketamine, clonidine, and sufentanil. Legeby 2005 administered thiopenthone or propofol, sevoflurane, fentanyl, and lidocaine injected locally into each breast. Sun 2013 administered midazolam, etomidate, fentanyl, propofol, and ramifentanil. Absence of consideration of other components of intraoperative pain management poses potential limitations.
Further questions regarding NSAID efficacy and safety that are beyond the scope of this review and impact current practice include other adverse effects, patient comorbidities, drug interactions, and pharmacogenomics. Any predisposition to risks of bleeding, cardiovascular events, liver disease, asthma, gastrointestinal disease, and kidney disease may alter medication management.
Certainty of the evidence
This review provides evidence from 12 completed RCTs and one ongoing RCT. Of the 12 completed studies, seven compared NSAIDs to placebo, four compared NSAIDs to other analgesics, and one compared NSAIDs to no intervention. As presented in Table 1; Table 2; Table 3, the quality of the body of evidence for each outcome was moderate to very low. We were unable to perform a sensitivity analysis due to few studies in the comparative groups.
Among the studies comparing NSAIDs to placebo, no outcome had moderate‐certainty evidence. Outcomes with significant heterogeneity for comparisons between NSAIDs and placebo were: pain intensity 24 hours following surgery (preoperative and postoperative) with heterogeneity of 73%; incidence rate or severity of postoperative nausea, vomiting, or both (preoperative and postoperative) with heterogeneity of 81% and 55% in the preoperative subgroup; and opioid use within 24 hours of surgery (preoperative and postoperative) with heterogeneity of 63%. The high heterogeneity for pain intensity 24 hours following surgery (postoperative) is likely due to different times of pain assessments and routes of administration. Bosek 1996 assessed pain at 18 hours following surgery and administered ketorolac both systemically (IV) and locally (Jackson‐Pratt drain). Legeby 2005 assessed pain intensity from 0 to 20 hours following surgery and administered diclofenac rectally. The high heterogeneity for postoperative nausea, vomiting, or both (preoperative and postoperative) is likely due to the different operative anesthesia medications. Two studies (Legeby 2005; Romundstad 2006a), both reported high rates of postoperative nausea and vomiting. However, in both studies, the opioid fentanyl was administered during general anesthesia. Fentanyl is known to cause nausea and vomiting, which was apparent in both groups. Bosek 1996 administered isoflurane, another anesthetic known to commonly cause nausea and vomiting postoperatively, which resulted in nausea and vomiting in both the NSAID and placebo group. Parsa 2005 did not administer opioids or anesthesia known to cause nausea and vomiting, but demonstrated imprecise results. The high heterogeneity for opioid use within 24 hours of surgery (preoperative and postoperative) is likely due to different breast surgeries, routes of NSAID administration, types of NSAIDs, and types of postoperative opioids. Legeby 2005 administered morphine patient‐controlled analgesia following mastectomy and immediate breast reconstruction with rectal diclofenac. Romundstad 2006a administered codeine following breast augmentation with IV parecoxib. Sun 2013 administered fentanyl patient‐controlled analgesia following mastectomy with IV flurbiprofen. This may explain the inconsistency of results between these studies.
Among the studies comparing NSAIDs to other analgesics, pain intensity 24 hours following surgery (postoperative) and postoperative nausea and vomiting or both (preoperative and postoperative) had moderate‐certainty evidence. Outcomes with significant heterogeneity for comparisons between NSAIDs and other analgesics were pain intensity 24 hours following surgery (preoperative and postoperative) with heterogeneity of 89% and opioid use within 24 hours of surgery (preoperative and postoperative) with heterogeneity of 96% in the postoperative group. The high heterogeneity for pain intensity 24 hours following surgery (preoperative and postoperative) is likely due to different times of pain assessments, routes of administration and times of administration. Freedman 2006 assessed pain at 24 hours following surgery and administered celecoxib orally. Oh 2016 assessed pain 30 minutes after NSAID or other analgesic administration and administered ibuprofen IV. Wen 2015 assessed pain at 24 hours following surgery and administered flurbiprofen IV. The high heterogeneity for opioid use within 24 hours of surgery (preoperative and postoperative) is likely due to different breast surgeries, routes of NSAID administration, types of NSAIDs, and types of postoperative opioids. Bakr 2016 administered morphine IV following modified radical mastectomy with IV ketorolac. Freedman 2006 administered hydrocodone orally following breast augmentation with oral celecoxib. Wen 2015 administered fentanyl IV following modified radical mastectomy with IV flurbiprofen.
The remaining outcomes had either low‐ or very low‐certainty evidence. With low‐certainty evidence, our confidence in the effect estimate is limited or the true effect may be substantially different from the estimate of the effect. Only one study reported comparing outcomes between NSAIDs and no intervention presented in Table 3. The ability to make comparisons across studies was not possible. With all comparisons between patients taking NSAIDs and no intervention, we have very little confidence in the effect estimate or the true effect is likely to be substantially different from the estimate of effect.
The ongoing study, NCT03535116 2018 will provide a double‐blinded, placebo‐controlled randomized trial comparing preoperative NSAIDs (ketorolac) to placebo. NCT03535116 2018 will assess breast hematomas. After publication, this study will improve evidence for this outcome.
Potential biases in the review process
We strictly followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions on searching, study selection, data collection, and data analysis to reduce any potential risk of bias (Higgins 2011). This review had the potential for the following limitations: (i) no assessment of publication bias through funnel plot analysis because fewer than 10 studies were included in the meta‐analysis; (ii) subgroup analysis was planned, however the paucity of data did not allow us to perform many of these comparisons for each outcome; (iii) typical limitations of aggregate‐data meta‐analysis, where individual patient data are absent, and subgroup analysis is usually underpowered; (iv) unsuccessful attempts to obtain further outcomes information and data on the included studies from the trial authors.
Agreements and disagreements with other studies or reviews
Although thousands of women undergo oncologic, reconstructive, and cosmetic breast surgery annually, to the best of our knowledge, this is the first systematic review and meta‐analysis of randomized trials on perioperative NSAIDs in women undergoing breast surgery. Three reviews have discussed perioperative NSAIDs with similar findings (Cheng 2016; Stone 2019; Walker 2019). Cheng 2016 reviewed the efficacy of perioperative analgesia for breast cancer‐related surgery. The authors of this review used data from Legeby 2005 and concluded perioperative NSAIDs reduced pain intensity and required less opioids. However NSAIDs were associated with higher rates of postoperative bleeding. We also used data from Legeby 2005, however we considered five additional studies for comparisons (Bosek 1996; Forget 2019; Parsa 2005; Romundstad 2006a; Sun 2013). With additional studies, NSAIDs were not associated with higher rates of postoperative bleeding. As such, NSAIDs in multimodal analgesic regimens appear to be beneficial, with the possible risk of increased postoperative bleeding without conclusive recommendations until further research specific to breast surgery is performed. Walker 2019 performed a systematic review and meta‐analysis specifically evaluating the risk of hematomas in plastic surgery with NSAIDs. When examining NSAID use in breast surgery, there was no statistically significant difference in incidence of hematoma or increased bleeding after combining studies assessing ketorolac, ibuprofen, and celecoxib.
Stone 2019 reported recommendations for enhanced recovery after surgery pathways with microsurgical breast reconstruction. The authors recommended preoperative celecoxib 400 mg in the holding area, celecoxib 200 mg twice daily for 4 days postoperatively, and ibuprofen 600 mg every 6 hours as needed for 7 days after hospital discharge. These recommendations for perioperative NSAIDs were developed by pain specialists from a single institution based on experience and available literature with subjectively reported better pain control in the postoperative period.
Authors' conclusions
Implications for practice.
We found evidence to suggest that perioperative (preoperative and/or postoperative) NSAIDs may reduce pain intensity, nausea and vomiting and opioid use within 24 hours following surgery in women undergoing breast surgery. These surgeries included implant‐based reconstruction, lumpectomy, mastectomy, and augmentation mammaplasty. However, there was very little evidence to suggest whether perioperative (preoperative and/or postoperative) NSAIDs may reduce or increase the rate of breast hematoma within 90 days (requiring reoperation, interventional drainage, or no treatment) of breast surgery, bleeding from any location within 90 days of breast surgery, need for blood transfusion, and other side effects of NSAID use, in comparison to placebo, other analgesics or to no intervention. Additional good‐quality, large‐scale, RCTs are required before definite conclusions can be made.
Implications for research.
Areas of future research include evaluating the impact of perioperative NSAIDs on hospital length of stay, breast cancer recurrence and non‐prescribed NSAID use. We were unable to find these outcomes in any of the 12 included studies. By administering NSAIDs and lowering opioid doses, patients may be less likely to become over sedated and ambulate earlier following surgery. This may also reduce other associated complications postoperatively. Only a few NSAIDs have been evaluated. Some of these medications may not be available to all. Evaluating alternative NSAIDs may be beneficial for these populations. Assessing outcomes based on the pharmacologic classes of NSAIDs (COX‐1 inhibitors, selective COX‐2 inhibitors), types of NSAIDs within pharmacologic classes (diclofenac, ketorolac, ibuprofen, flurbiprofen) and times of administration (preoperative, intraoperative, postoperative) would provide information regarding the efficacy and safety of specific medications.
Pharmacogenomics (the science concerned with understanding how genetic differences among individuals cause varied responses to the same drug and with developing drug therapies to compensate for these differences, Merriam‐Webster 1997) is an evolving specialty of medicine. The impact of pharmacogenomics on the efficacy and safety of NSAIDs in response to genetic variants may reveal which patients are appropriate candidates for NSAIDs. Understanding if certain genetic factors predispose patients to different pain responses, risks of bleeding, cardiovascular events, liver disease, asthma, gastrointestinal disease, kidney disease, and pregnancy may be beneficial prior to perioperative administration of an NSAID for women undergoing breast surgery. Evaluating different routes of administration would be beneficial for patients unable to tolerate oral medications after they are discharged from the hospital (oral, rectal, IV, IM, transdermal). This would be beneficial for patients with multiple comorbidities or complex medication regimens susceptible to drug interactions. Administering perioperative NSAIDs to women undergoing a greater diversity of breast surgery procedures may optimise patient selection and allow for a greater number of recommendations for use. None of the studies evaluated NSAIDs in women undergoing autologous breast reconstruction, breast reduction, and mastopexy surgery.
Other pain medications administered intraoperatively during anesthesia are likely to impact outcomes associated with NSAIDs. Evaluating NSAIDs along with different anesthesia medications may help to further identify discrepancies between studies. With completion of the ongoing study NCT03535116 2018, we hope it will provide insight into these areas of research. RCTs are required to definitively identify the benefits or non‐benefits of NSAIDs in perioperative breast surgery.
History
Protocol first published: Issue 3, 2019
Acknowledgements
We would like to acknowledge the editorial team of the Cochrane Breast Cancer Group for their assistance preparing this full‐text review. Si Jia Zhu (Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China) for her assistance with translation of articles in Chinese. We are grateful for peer‐reviewer comments from Professor Patrice Forget, MD, PhD (University of Aberdeen; Epidemiology group; Institute of Applied Health Sciences; School of Medicine, Medical Sciences and Nutrition; NHS Grampian, Department of Anaesthesia; Aberdeen, UK); Linda Bily (Stony Brook Cancer Center, New York, USA); Bonner Cutting (Alamo Breast Cancer Foundation), Susan G Komen (Advocate‐in‐Science, Texas, USA) and Dr Amalia Karahalios (Monash University, The University of Melbourne, Melbourne, Australia).
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 breast near malignan* #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Breast] explode all trees #10 breast #11 #9 or #10 #12 MeSH descriptor: [General Surgery] explode all trees #13 MeSH descriptor: [Surgical Procedures, Operative] explode all trees #14 (Surger* or surgical*) #15 (operation* or operative*) #16 #12 or #13 or #14 or #15 #17 (#8 or #11) and #16 #18 MeSH descriptor: [Reconstructive Surgical Procedures] explode all trees #19 MeSH descriptor: [Surgery, Plastic] explode all trees #20 MeSH descriptor: [Surgical Oncology] explode all trees #21 #18 or #19 or #20 #22 #11 and #21 #23 MeSH descriptor: [Breast Neoplasms] explode all trees and with qualifier(s): [surgery ‐ SU] #24 MeSH descriptor: [Mastectomy] explode all trees #25 (breast near surger*) #26 MeSH descriptor: [Breast Implants] this term only #27 MeSH descriptor: [Breast Implantation] this term only #28 MeSH descriptor: [Mammaplasty] explode all trees #29 (breast near reconstruct*) #30 #23 or #24 or #25 or #26 or #27 or #28 or #29 #31 #17 or #22 or #30 #32 MeSH descriptor: [Analgesia] explode all trees #33 MeSH descriptor: [Analgesics] explode all trees #34 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #35 NSAID* or non steroidal anti‐inflammator* #36 MeSH descriptor: [Aspirin] explode all trees #37 aspirin #38 MeSH descriptor: [Salicylates] explode all trees #39 MeSH descriptor: [Salicylic Acid] explode all trees #40 diflusinal or salicylate* or salicylic acid #41 MeSH descriptor: [Acetaminophen] explode all trees #42 acetaminophen or paracetamol #43 MeSH descriptor: [Dipyrone] explode all trees #44 dipyrone or propyphenazone or Isopropylantipyrine #45 MeSH descriptor: [Indomethacin] explode all trees #46 indomethacin or arthrexin or indocid #47 MeSH descriptor: [Diclofenac] explode all trees #48 diclofenac or Clonac or Diclohexal or Fenac or Imflac or Viclofen or Voltaren or APO‐diclofenac #49 aceclofenac #50 MeSH descriptor: [Etodolac] explode all trees #51 MeSH descriptor: [Ketorolac] explode all trees #52 MeSH descriptor: [Sulindac] explode all trees #53 etodolac or ketorolac or ketoral or toradol or sulindac or aclin #54 MeSH descriptor: [Ibuprofen] explode all trees #55 ibuprofen or advil or Brufen or Bugesic or Dimotapp or iProfen or Nurofen or Panafen or ProVen or Rafen or Tri‐Profen #56 MeSH descriptor: [Naproxen] explode all trees #57 naproxen or Aleve or Anaprox or Crysanal or Eazydayz or Inza or Naprosyn or Naprofem or Naprogesic or Proxen #58 MeSH descriptor: [Fenoprofen] explode all trees #59 MeSH descriptor: [Flurbiprofen] explode all trees #60 fenoprofen or flurbiprofen #61 MeSH descriptor: [Ketoprofen] explode all trees #62 ketoprofen or Orudis or Oruvail #63 MeSH descriptor: [Mefenamic Acid] explode all trees #64 Mefenamic acid or ponstan #65 MeSH descriptor: [Meclofenamic Acid] explode all trees #66 Meclofenamic acid or meclofenamate or meclomen #67 Meloxicam or Meloxibell or Mobic or Movalis or Moxicam#68 MeSH descriptor: [Piroxicam] explode all trees #69 Piroxicam or Feldene or Feldene‐D or Mobilis or Mobilis D #70 nabumetone #71 MeSH descriptor: [Celecoxib] explode all trees #72 celecoxib or celebrex #73 etoricoxib #74 nabumetone or etoricoxib or arcoxia or parecoxib or dynastat or rofecoxib or vioxx #75 MeSH descriptor: [Cyclooxygenase Inhibitors] explode all trees #76 {OR #32‐#75} #77 #31 and #76
Appendix 2. MEDLINE
| # ▲ | Searches |
| 1 | exp Breast Neoplasms/ |
| 2 | (breast adj6 cancer$).tw. |
| 3 | (breast adj6 neoplasm$).tw. |
| 4 | (breast adj6 carcinoma$).tw. |
| 5 | (breast adj6 tumo?r$).tw. |
| 6 | or/1‐5 |
| 7 | exp BREAST/ |
| 8 | breast.tw. |
| 9 | or/7‐8 |
| 10 | exp General Surgery/ |
| 11 | exp SURGICAL PROCEDURES, OPERATIVE/ |
| 12 | (Surger* or surgical*).tw. |
| 13 | (operation* or operative*).tw. |
| 14 | or/10‐13 |
| 15 | (6 or 9) and 14 |
| 16 | exp Reconstructive Surgical Procedures/ |
| 17 | exp Surgery, Plastic/ |
| 18 | exp Surgical Oncology/ |
| 19 | or/16‐18 |
| 20 | 9 and 19 |
| 21 | exp Breast Neoplasms/su [Surgery] |
| 22 | exp Mastectomy/ |
| 23 | (breast adj6 surger*).tw. |
| 24 | Breast Implants/ or Breast Implantation/ |
| 25 | exp MAMMAPLASTY/ |
| 26 | (breast adj6 reconstruct*).tw. |
| 27 | or/21‐26 |
| 28 | 15 or 20 or 27 |
| 29 | exp ANALGESIA/ |
| 30 | exp ANALGESICS/ |
| 31 | exp Anti‐Inflammatory Agents, Non‐Steroidal/ |
| 32 | exp ASPIRIN/ |
| 33 | (aspirin or NSAID* or non‐steroidal anti‐inflammator*).mp. |
| 34 | exp SALICYLATES/ |
| 35 | exp Salicylic Acid/ |
| 36 | (diflusinal or salicylate* or salicylic acid).mp. |
| 37 | exp ACETAMINOPHEN/ |
| 38 | (acetaminophen or paracetamol).mp. |
| 39 | exp DIPYRONE/ |
| 40 | (dipyrone or propyphenazone or Isopropylantipyrine).mp. |
| 41 | exp INDOMETHACIN/ |
| 42 | (indomethacin or arthrexin or indocid).mp. |
| 43 | exp DICLOFENAC/ |
| 44 | (diclofenac or Clonac or Diclohexal or Fenac or Imflac or Viclofen or Voltaren or APO‐diclofenac).mp. |
| 45 | aceclofenac.mp. |
| 46 | exp ETODOLAC/ |
| 47 | exp KETOROLAC/ |
| 48 | exp SULINDAC/ |
| 49 | (etodolac or ketorolac or ketoral or toradol or sulindac or aclin).mp. |
| 50 | exp IBUPROFEN/ |
| 51 | (ibuprofen or advil or Brufen or Bugesic or Dimotapp or iProfen or Nurofen or Panafen or ProVen or Rafen or Tri‐Profen).mp. |
| 52 | exp NAPROXEN/ |
| 53 | (naproxen or Aleve or Anaprox or Crysanal or Eazydayz or Inza or Naprosyn or Naprofem or Naprogesic or Proxen).mp. |
| 54 | exp FENOPROFEN/ |
| 55 | exp FLURBIPROFEN/ |
| 56 | (fenoprofen or flurbiprofen).mp. |
| 57 | exp KETOPROFEN/ |
| 58 | (ketoprofen or Orudis or Oruvail).mp. |
| 59 | exp Mefenamic Acid/ |
| 60 | (Mefenamic acid or ponstan).mp. |
| 61 | exp Meclofenamic Acid/ |
| 62 | (Meclofenamic acid or meclofenamate or meclomen).mp. |
| 63 | (Meloxicam or Meloxibell or Mobic or Movalis or Moxicam).mp. |
| 64 | exp PIROXICAM/ |
| 65 | (Piroxicam or Feldene or Feldene‐D or Mobilis or Mobilis D).mp. |
| 66 | nabumetone.mp. |
| 67 | exp CELECOXIB/ |
| 68 | (celecoxib or celebrex).mp. |
| 69 | etoricoxib.mp. |
| 70 | (nabumetone or etoricoxib or arcoxia or parecoxib or dynastat or rofecoxib or vioxx).mp. |
| 71 | exp Cyclooxygenase Inhibitors/ |
| 72 | or/29‐71 |
| 73 | 28 and 72 |
| 74 | animals/ not humans/ |
| 75 | 73 not 74 |
| 76 | randomized controlled trial.pt. |
| 77 | controlled clinical trial.pt. |
| 78 | randomized.ab. |
| 79 | placebo.ab. |
| 80 | Clinical Trials as Topic/ |
| 81 | randomly.ab. |
| 82 | trial.ti. |
| 83 | (crossover or cross‐over).tw. |
| 84 | Pragmatic Clinical Trials as Topic/ |
| 85 | pragmatic clinical trial.pt. |
| 86 | or/76‐85 |
| 87 | Case‐Control Studies/ |
| 88 | Control Groups/ |
| 89 | Matched‐Pair Analysis/ |
| 90 | Retrospective Studies/ |
| 91 | ((case* adj5 control*) or (case adj3 comparison*) or control group*).ti,ab. |
| 92 | or/87‐91 |
| 93 | Cohort Studies/ |
| 94 | Longitudinal Studies/ |
| 95 | Follow‐Up Studies/ |
| 96 | Prospective Studies/ |
| 97 | Retrospective Studies/ |
| 98 | cohort.ti,ab. |
| 99 | longitudinal.ti,ab. |
| 100 | prospective.ti,ab. |
| 101 | retrospective.ti,ab. |
| 102 | or/93‐101 |
| 103 | 75 and 86 |
| 104 | 75 and 92 |
| 105 | 75 and 102 |
| 106 | 104 or 105 |
Appendix 3. Embase
| # | Searches |
| 1 | exp breast/ |
| 2 | exp breast disease/ |
| 3 | (1 or 2) and exp neoplasm/ |
| 4 | exp breast tumor/ |
| 5 | exp breast cancer/ |
| 6 | exp breast carcinoma/ |
| 7 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 8 | or/3‐7 |
| 9 | breast.tw. |
| 10 | 1 or 9 |
| 11 | exp surgery/ |
| 12 | exp surgical technique/ |
| 13 | (Surger* or surgical*).tw. |
| 14 | (operation* or operative*).tw. |
| 15 | or/11‐14 |
| 16 | (8 or 10) and 15 |
| 17 | exp reconstructive surgery/ |
| 18 | exp plastic surgery/ |
| 19 | exp surgical oncology/ |
| 20 | exp cancer surgery/ |
| 21 | or/17‐20 |
| 22 | 10 and 21 |
| 23 | exp breast tumor/su [Surgery] |
| 24 | exp breast cancer/su [Surgery] |
| 25 | exp mastectomy/ |
| 26 | exp breast surgery/ |
| 27 | exp breast implant/ |
| 28 | exp breast augmentation/ |
| 29 | exp breast reconstruction/ |
| 30 | (breast adj6 reconstruct*).tw. |
| 31 | (breast adj6 surger*).tw. |
| 32 | or/23‐31 |
| 33 | 16 or 22 or 32 |
| 34 | exp analgesia/ |
| 35 | exp analgesic agent/ |
| 36 | exp nonsteroid antiinflammatory agent/ |
| 37 | (NSAID* or non‐steroidal anti‐inflammator*).mp. |
| 38 | exp acetylsalicylic acid/ |
| 39 | aspirin.mp. |
| 40 | exp salicylic acid/ |
| 41 | (diflusinal or salicylate* or salicylic acid).mp. |
| 42 | exp paracetamol/ |
| 43 | (acetaminophen or paracetamol).mp. |
| 44 | exp dipyrone/ |
| 45 | exp propyphenazone/ |
| 46 | exp indometacin/ |
| 47 | (dipyrone or propyphenazone or Isopropylantipyrine or indomethacin or arthrexin or indocid).mp. |
| 48 | exp diclofenac/ |
| 49 | (diclofenac or Clonac or Diclohexal or Fenac or Imflac or Viclofen or Voltaren or APO‐diclofenac).mp. |
| 50 | exp aceclofenac/ |
| 51 | exp etodolac/ |
| 52 | exp ketorolac/ |
| 53 | exp sulindac/ |
| 54 | (etodolac or ketorolac or ketoral or toradol or sulindac or aclin).mp. |
| 55 | exp ibuprofen/ |
| 56 | (ibuprofen or advil or Brufen or Bugesic or Dimotapp or iProfen or Nurofen or Panafen or ProVen or Rafen or Tri‐Profen).mp. |
| 57 | exp naproxen/ |
| 58 | (naproxen or Aleve or Anaprox or Crysanal or Eazydayz or Inza or Naprosyn or Naprofem or Naprogesic or Proxen).mp. |
| 59 | exp fenoprofen/ |
| 60 | exp flurbiprofen/ |
| 61 | (fenoprofen or flurbiprofen).mp. |
| 62 | exp ketoprofen/ |
| 63 | (ketoprofen or Orudis or Oruvail).mp. |
| 64 | exp mefenamic acid/ |
| 65 | (Mefenamic acid or ponstan).mp. |
| 66 | exp meclofenamic acid/ |
| 67 | (Meclofenamic acid or meclofenamate or meclomen).mp. |
| 68 | exp meloxicam/ |
| 69 | (Meloxicam or Meloxibell or Mobic or Movalis or Moxicam).mp. |
| 70 | exp piroxicam/ |
| 71 | (Piroxicam or Feldene or Feldene‐D or Mobilis or Mobilis D).mp. |
| 72 | exp nabumetone/ |
| 73 | exp celecoxib/ |
| 74 | (celecoxib or celebrex).mp. |
| 75 | exp etoricoxib/ |
| 76 | (etoricoxib or arcoxia).mp. |
| 77 | exp parecoxib/ |
| 78 | (parecoxib or dynastat).mp. |
| 79 | exp rofecoxib/ |
| 80 | (rofecoxib or vioxx).mp. |
| 81 | exp prostaglandin synthase inhibitor/ |
| 82 | Cyclooxygenase Inhibitor*.mp. |
| 83 | ((COX‐1 or COX‐2) and inhibitor*).mp. |
| 84 | or/34‐83 |
| 85 | 33 and 84 |
| 86 | limit 85 to (human and (conference abstracts or embase)) |
| 87 | Randomized controlled trial/ |
| 88 | Controlled clinical study/ |
| 89 | Random$.ti,ab. |
| 90 | randomization/ |
| 91 | intermethod comparison/ |
| 92 | placebo.ti,ab. |
| 93 | (compare or compared or comparison).ti. |
| 94 | (open adj label).ti,ab. |
| 95 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 96 | double blind procedure/ |
| 97 | parallel group$1.ti,ab. |
| 98 | (crossover or cross over).ti,ab. |
| 99 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 100 | (assigned or allocated).ti,ab. |
| 101 | (controlled adj7 (study or design or trial)).ti,ab. |
| 102 | (volunteer or volunteers).ti,ab. |
| 103 | trial.ti. |
| 104 | or/87‐103 |
| 105 | exp case control study/ |
| 106 | case control study.ti,ab. |
| 107 | ((case control or case base or case matched or retrospective) adj1 (analys* or design* or evaulation* or research or stud* or survey* or trial*)).ti,ab. |
| 108 | or/105‐107 |
| 109 | exp retrospective study/ |
| 110 | exp prospective study/ |
| 111 | ((cohort or concurrent or incidence or longitudinal or followup or 'follow up' or prospective or retrospective) adj1 (analys* or design* or evaluation* or research or stud* or survey* or trial*)).ti,ab. |
| 112 | or/109‐111 |
| 113 | 86 and 104 |
| 114 | 86 and 108 |
| 115 | 86 and 112 |
| 116 | 114 or 115 |
Appendix 4. WHO ICTRP
Basic search:
1. non‐steroidal anti‐inflammatory AND breast cancer 2. non‐steroidal anti‐inflammatory AND breast surgery 3. NSAID and breast cancer 4. NSAID and breast surgery
Advanced search:
1. Condition: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention: non‐steroidal anti‐inflammatory drug
2. Condition: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention: aspirin OR salicylic acid OR diflunisal OR paracetamol OR acetaminophen
3. Condition: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention: dipyrone OR propyphenazone OR indomethacin OR diclofenac OR aceclofenac OR etodolac OR ketorolac
4. Condition: breast surgery or mastectomy or mammaplasty Intervention: Sulindac
5. Condition: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention: ibuprofen OR naproxen OR fenoprofen OR flurbiprofen OR ketoprofen OR mefenamic acid OR meclofenamate
6. Condition: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention: meloxicam OR piroxicam OR nabumetone OR celecoxib OR etoricoxib OR parecoxib OR rofecoxib
Appendix 5. ClinicalTrials.gov
Basic search:
1. Condition or disease: breast cancer or breast surgery Other terms: NSAID
2. Condition or disease: breast cancer or breast surgery Other terms: non‐steroidal anti‐inflammatory
Advanced search:
1. Condition or disease: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention/treatment: NSAID Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
2. Condition or disease: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention/treatment: aspirin OR salicylic acid OR diflunisal OR paracetamol OR acetaminophen Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
3. Condition or disease: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention/treatment: dipyrone OR propyphenazone OR indomethacin OR diclofenac OR aceclofenac OR etodolac OR ketorolac Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
4. Condition or disease: breast cancer or breast surgery or mastectomy or mammaplasty Intervention/treatment: Sulindac Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
5. Condition or disease: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention/treatment: ibuprofen OR naproxen OR fenoprofen OR flurbiprofen OR ketoprofen OR mefenamic acid OR meclofenamate Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
6. Condition or disease: breast cancer OR breast surgery or mastectomy or mammaplasty Intervention/treatment: meloxicam OR piroxicam OR nabumetone OR celecoxib OR etoricoxib OR parecoxib OR rofecoxib Recruitment: All studies Study results: All studies Study type: Interventional Study Gender: All studies
Data and analyses
Comparison 1. NSAID versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Breast hematoma | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.02] |
| 1.2 Breast hematoma by timing of drug administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Preoperative | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.66] |
| 1.3 Pain intensity 24 (± 12) hours following surgery | 3 | 310 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.49, ‐0.03] |
| 1.4 Pain intensity 24 (± 12) hours following surgery by timing of drug administration | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Preoperative | 1 | 202 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.46, 0.09] |
| 1.4.2 Postoperative | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.50, 0.57] |
| 1.5 Incidence rate or severity of postoperative nausea, vomiting, or both | 4 | 939 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.58, 2.27] |
| 1.6 Incidence rate or severity of postoperative nausea, vomiting, or both by timing of drug administration | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Preoperative | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.42, 20.64] |
| 1.6.2 Postoperative | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.36, 0.92] |
| 1.7 Bleeding from any location within 90 days | 2 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 1.8 Bleeding from any location within 90 days by timing of drug administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Preoperative | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.14, 81.03] |
| 1.9 Need for blood transfusion | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.23, 91.34] |
| 1.10 Need for blood transfusion by timing of administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Preoperative | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.23, 91.34] |
| 1.11 Other side effects of NSAID use | 2 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.44, 2.86] |
| 1.12 Other side effects of NSAID use by timing of drug administration | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.12.1 Preoperative | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.48, 3.38] |
| 1.13 Opioid use within 24 (± 12) hours of surgery | 4 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.85, ‐0.05] |
| 1.14 Opioid use within 24 (± 12) hours of surgery by timing of drug administration | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 Preoperative | 1 | 136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.20, ‐0.50] |
| 1.14.2 Postoperative | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.83, 0.25] |
| 1.15 Length of hospital stay | 1 | 203 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.09, 0.46] |
1.2. Analysis.

Comparison 1: NSAID versus placebo, Outcome 2: Breast hematoma by timing of drug administration
1.4. Analysis.

Comparison 1: NSAID versus placebo, Outcome 4: Pain intensity 24 (± 12) hours following surgery by timing of drug administration
1.6. Analysis.

Comparison 1: NSAID versus placebo, Outcome 6: Incidence rate or severity of postoperative nausea, vomiting, or both by timing of drug administration
1.8. Analysis.

Comparison 1: NSAID versus placebo, Outcome 8: Bleeding from any location within 90 days by timing of drug administration
1.10. Analysis.

Comparison 1: NSAID versus placebo, Outcome 10: Need for blood transfusion by timing of administration
1.12. Analysis.

Comparison 1: NSAID versus placebo, Outcome 12: Other side effects of NSAID use by timing of drug administration
1.14. Analysis.

Comparison 1: NSAID versus placebo, Outcome 14: Opioid use within 24 (± 12) hours of surgery by timing of drug administration
1.15. Analysis.

Comparison 1: NSAID versus placebo, Outcome 15: Length of hospital stay
Comparison 2. NSAID versus other analgesic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of breast hematoma within 90 days of breast surgery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 2.2 Pain intensity 24 (± 12) hours following surgery | 3 | 200 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.97, ‐0.39] |
| 2.3 Pain intensity 24 (± 12) hours following surgery by timing of drug administration | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Postoperative | 2 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.51, 0.27] |
| 2.4 Incidence rate or severity of postoperative nausea, vomiting, or both | 3 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.57] |
| 2.5 Bleeding from any location within 90 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 2.6 Other side effects of NSAID use | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.80] |
| 2.7 Opioid use within 24 (± 12) hours of surgery | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐6.87 [‐10.93, ‐2.81] |
| 2.8 Opioid use within 24 (± 12) hours of surgery by timing of drug administration | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Postoperative | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐9.56 [‐18.48, ‐0.64] |
2.3. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 3: Pain intensity 24 (± 12) hours following surgery by timing of drug administration
2.5. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 5: Bleeding from any location within 90 days
2.8. Analysis.

Comparison 2: NSAID versus other analgesic, Outcome 8: Opioid use within 24 (± 12) hours of surgery by timing of drug administration
Comparison 3. NSAID versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain intensity 24 (± 12) hours following surgery | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.09, 0.00] |
| 3.2 Pain intensity 24 (± 12) hours following surgery by timing of drug administration | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Preoperative | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.97, 0.28] |
| 3.2.2 Postoperative | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.30, ‐0.02] |
3.2. Analysis.

Comparison 3: NSAID versus no intervention, Outcome 2: Pain intensity 24 (± 12) hours following surgery by timing of drug administration
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakr 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors received no funding and had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated according to computer‐generated randomisation tables into one of 3 groups of 20 patients |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed |
| Selective reporting (reporting bias) | Low risk | Trial registration confirmed all reported outcomes were assessed |
Bosek 1996.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Chan 1996.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Forget 2019.
| Study characteristics | ||
| Methods | Belgian, multicenter, prospective, double‐blind, placebo‐controlled, parallel assignment, randomized phase III trial in high‐risk breast cancer patients | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funded by the Anticancer Fund, the Belgian Society of Anaesthesia and Resuscitation, the Foundation Saint‐Luc; the Commission du Patrimoine of the Université catholique de Louvan, St‐Luc Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial. Randomization of eligible patients was done the day before surgery and used randomisation blocks of 4. There was no stratification factor. |
| Allocation concealment (selection bias) | Low risk | In each center, a randomisation list was kept accessible exclusively to the pharmacist in charge of the preparation of the study product (ketorolac or placebo). For each patient, a sealed opaque envelope was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were given one dose of intervention or a matching placebo. Each patient was randomly assigned on a 1:1 ratio to receive either 30 mg of ketorolac or a placebo during the induction of anesthesia (pre‐incision). The placebo consisted of NaCl 0.9% (3 mL) and was identically presented to ensure double‐blinding. No dose modification was allowed because only one single dose was administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A randomisation list was kept accessible exclusively to the pharmacist in charge of the preparation of the study product. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient with missing data in placebo group for postoperative pain intensity within 24 (± 12) hours of surgery |
| Selective reporting (reporting bias) | Low risk | Trial registration confirmed all reported outcomes were assessed |
Freedman 2006.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial |
| Allocation concealment (selection bias) | Unclear risk | No information was provided regarding method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not a blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not a blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Legeby 2005.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Supported by grants from Karolinska Institute foundations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial |
| Allocation concealment (selection bias) | Low risk | A randomisation table was used to allocate patients to each group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A table of randomisation (each number corresponding to a package of identically shaped suppositories), prepared by the Hospital pharmacy, each patient received suppositories of either diclofenac (group D) or placebo (group P). The randomisation table was kept by the Hospital Pharmacy beyond the evaluation of all effect variables. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of investigators and assessing nurses to group assignment of the patients and outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 patients were excluded after randomisation from the intervention group, with reason. 6 patients were lost to follow‐up in the intervention group and placebo groups |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Oh 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors received no funding and had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done prospectively to one of the three groups using a computer‐generated table and concealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated table and concealed envelopes were used to allocate participants to each group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participants, observers, and assessors of outcomes) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind (participants, observers, and assessors of outcomes) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed except for two patients in Group IH who were withdrawn from data analysis due to missing data. |
| Selective reporting (reporting bias) | Low risk | Trial registration confirmed all reported outcomes were assessed |
Parsa 2005.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors received no funding and had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial |
| Allocation concealment (selection bias) | Unclear risk | No information was provided regarding method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not a blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not a blinded study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All randomized participants were analyzed, did not report standard deviations with mean opioid use within 24 (± 12) hours of surgery |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Romundstad 2006a.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, double‐blind, single‐dose, parallel‐group |
| Allocation concealment (selection bias) | Low risk | Patients randomized the patients in blocks of nine to 1 of 3 groups of equal size using a list of random numbers, according to the Moses‐Oakford algorithm |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded to all measurements |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analyzed |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
Sun 2013.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized control trial |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random number utilized to allocate participants to each group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomization and anesthesia procedure was done by an anesthesiologist, and the follow‐up and evaluation after the surgery was done by another anesthesiologist blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
van Helmond 2016.
| Study characteristics | ||
| Methods | Randomized prospective controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funded by Pfizer. Pfizer had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pseudo‐randomisation employed using computer‐generated blocks of six |
| Allocation concealment (selection bias) | Unclear risk | A pseudo‐random code was computer‐generated for the randomisation blocks that had a size of six. Stratified random sampling ensured equal distribution of axillary lymph node dissections over groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Observers and personnel involved in patient management were blinded to medication assignment as well as study participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers and personnel involved in patient management were blinded to medication assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was relatively high exclusion rate due to treatment failure (unsuccessful paravertebral block) in 5 patients (2 intervention group and 3 placebo group) and failure of the hospital pharmacy to deliver study drugs to the operating room on time in 30 patients. Consent was withdrawn in 2 patients (1 placebo and 1 intervention). Three patients from each group were excluded due to not meeting inclusion criteria. Study used a modified intention‐to‐treat analysis for 94 patients included in analysis. |
| Selective reporting (reporting bias) | Low risk | Trial registration confirmed all reported outcomes were assessed |
Wen 2015.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No declaration of funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial |
| Allocation concealment (selection bias) | Low risk | Secure web‐based computer‐generated randomisation process that automatically records numbers and assignments. A researcher who did not participate in the clinical trial performed the process of randomising patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | No information available for trial registrations or protocols to determine if all reported outcomes were assessed |
IM: intramuscular IV: intravenous NaCl: sodium chloride NRS: numerical rating scale NSAID: nonsteroidal anti‐inflammatory drug PONV: postoperative nausea and vomiting VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12614000507684 | Ineligible comparator. The control arm is aromatase inhibitor alone and study arm is aromatase inhibitor plus aspirin |
| Aristarco 2016 | Ineligible intervention. This a placebo‐controlled trial to evaluate the activity of exemestane and celecoxib before surgery. |
| Barker 2018 | Ineligible comparator. This is a retrospective review comparing acetaminophen to other analgesics. |
| Betambeau 2004 | Ineligible intervention. This RCT looked at the use of NSAIDs to reduce the incidence of breast cancer. |
| Blomqvist 1996 | Non‐RCT |
| Bosek 1992 | Ineligible patient population |
| Bosek 1994 | Ineligible outcomes |
| Brandao 2013 | Preoperative treatment |
| Bundred 2010 | Ineligible outcomes. This study looked only at a decrease in Ki67 between diagnosis and surgical excision. |
| Cawthorn 2012 | Non‐RCT |
| ChiCTR‐IPR‐14005271 | No data available on this study |
| CTRI/2016/10/007397 | Ineligible comparator. This RCT compared pregabalin to placebo |
| DeOliveira 2018 | Ineligible comparators. This RCT compared acetaminophen to placebo |
| Desmedt 2017 | Ineligible outcomes. The full text was not available |
| EUCTR2012‐003774‐76‐BE | Study protocol. No data available |
| Filippini 2014 | Only long‐term pain data available |
| Forget 2010 | A retrospective analysis of patients receiving intraoperative analgesics. It was not possible to differentiate effects from NSAID and other drugs |
| Forget 2013 | Study protocol with no data available |
| Forget 2014 | Ineligible comparators and non‐RCT study. This retrospective study looked at the use of ketorolac or diclofenac intraoperatively during breast surgery |
| Francis 1988 | Ineligible indication. Exmined the effect of diclofenac verus placebo on platelet aggregation |
| Hidar 2007 | Ineligible outcomes. This study only looked at postoperative drainage volume as their primary outcome |
| Himendra 1985 | Ineligible indication. A double‐blind, randomized, placebo‐controlled study was carried out to assess the efficacy of ketoprofen suppositories as a postoperative analgesic in outpatients undergoing extirpation surgery |
| Hu 2009 | Ineligible outcomes. Full text not available |
| ISRCTN06628870 | Protocol only. No data provided for study |
| ISRCTN86894592 | Ineligible intervention. Study only looks at pharmacological therapy and not breast surgery |
| Kampe 2006 | Ineligible comparators. This study compared IV paracetamol to IV dipyrone |
| Kodaka 2012 | Ineligible outcomes. Full text not available |
| Laisalmi 2001a | Non‐RCT |
| Laisalmi 2001b | Ineligible outcomes. The study only looked at renal effects of the combination of ketorolac and sevoflurane anesthesia versus placebo and sevoflurane anesthesia |
| Lakdja 1997 | Non‐RCT |
| Mahabir 2008 | Ineligible comparator. This trial compared placebo to ketorolac and bupivacaine in controlling postoperative pain in breast augmentation patients |
| Martin 2010 | Preoperative treatment |
| McCrea 2009 | Unable to differentiate data of NSAID from that of other drugs |
| Mitchell 2012 | Ineligiblecomparator. This study compared acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery |
| Molon 2009 | Non‐RCT |
| Morley‐Forster 1993 | Unable to differentiate breast surgery data from gynecological data |
| NCT00070057 | Ineligible comparator. The study compared celecoxib to surgical intervention |
| NCT00435747 | Duplicate study |
| NCT00502684 | Incorrect comparator. This study compared beta‐blocker to COX‐2 inhibitor or placebo |
| NCT01695226 | Preoperative treatment |
| NCT01806259 | The study recruitment was active. The current recruitment is unknown |
| NCT01852955 | Ineligible comparators, comparing acetomorphine to placebo |
| NCT02102555 | Ineligible comparators. This study compared acetomorphine to placebo. This study was terminated. The study closed due to difficulty enrolling subjects. No results are available |
| NCT02141139 | Ineligible comparators and outcomes. This study compared NSAID to NSAID |
| NCT02461056 | Duplicate study |
| NCT03007381 | The study was withdrawn due to a lack of funding |
| NCT03185871 | The study was withdrawn due to slow accrual and as a result was excluded from this study |
| Nguyen 2018a | Non‐RCT |
| Nguyen 2018b | Non‐RCT |
| Ohnesorge 2009 | Ineligible comparators. This study compared acetaminophen to metamizol, both of which are not NSAIDs |
| Parsa 2009 | Ineligible comparator. The study looked at NSAID versus an anti‐epileptic drug for patients undergoing all types of surgery |
| Pavlin 2002 | It was not possible to differentiate breast data from the study analysis |
| Priya 2002 | This study only compared NSAIDS in both groups |
| Retsky 2012 | Review article |
| Retsky 2013 | Review article |
| Riest 2006 | This study looked at patients scheduled for spine, breast or orthopaedic surgery. It was not possible to assess the data for just breast surgery |
| Romundstad 2006b | The data presented in this study only looked at long‐term pain assessment |
| Shaashua 2017 | Unable to extrapolate the data from NSAID and other drugs |
| Sharma 2001 | Non‐RCT, RCTs were available for assessment in this study |
| Sun 2008 | This study looked at consenting patients undergoing major plastic surgery (e.g. breast augmentation, abdominoplasty procedures). It was not possible to differentiate the data for only breast surgery. |
| Tallian 2014 | Ineligible intervention, the study compared patients undergoing intra‐abdominal and breast reconstruction surgery receiving no iIV acetaminophen with patients receiving concomitant IV acetaminophen. Furthermore the full text was not available. As a result, sufficient amount of data could not be extrapolated |
| VanHelmond 2014 | The full text was not available as a result sufficiat amount data could not be extrapolated. |
IV: intravenous NSAIDs: nonsteroidal anti‐inflammatory drugs RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT03535116 2018.
| Study name | The effect of intra‐operative ketorolac on hematoma rates in breast reduction surgery |
| Methods | Double‐blind, randomized, controlled, parallel assignment trial of breast reduction patients and their postoperative hematoma rates with the use of ketorolac |
| Participants | 100 female > 18 years of age undergoing breast reduction surgery |
| Interventions |
|
| Outcomes |
|
| Starting date | 24 May 2018 |
| Contact information | Geethan Chandran, Assistant Professor, Dr Chandran Medical Prof Corp |
| Notes | Current recruitment status: not yet recruiting; no declaration of funding or conflicts of interest |
IV: intravenous NSAID: nonsteroidal anti‐inflammatory drug
Differences between protocol and review
Paracetamol, acetaminophen, and dipyrone were not classified as a NSAID.
The seven outcomes selected for the summary of findings table in the protocol changed; that is, 'breast hematoma' outcomes were covered by 'incidence of breast hematoma within 90 days of breast surgery (requiring reoperation, interventional drainage, or no treatment)', 'postoperative pain' was updated to 'pain intensity following surgery', and 'other side effects of NSAIDs' and 'opioid use' were added.
Non‐randomized studies were not included in our review due to available RCTs, so we did not extract data on the following: methods used to control for confounders, adjusted and unadjusted outcome measures, list of variables authors have included in analyses for adjusted estimates. Use of the Risk of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) tool was not necessary to assess bias in non‐randomized studies (Sterne 2016).
Preoperative, intraoperative and postoperative NSAID use was assessed for all outcomes of interest.
Contributions of authors
Drafting the full text: KMK, AE, RP
Selecting studies: KMK, AE
Extracting data from studies: KMK, AE
Entering data into RevMan 5: KMK
Carrying out the analysis: KMK, AE
Interpreting the analysis: KMK, AE
Drafting the final review: KMK, AE
Resolving disagreements: GDR
Updating the review: KMK, AE, RP, CMC, MAM, GDR
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
KMK: none known. AE: none known. AE has received funding from Health Research Board (Ireland) under the HRB Cochrane Ireland Fellowship Scheme to undertake a Cochrane Review (grant number CTF‐2016‐1863). RP: none known. CMC: none known. CC is a co‐inventor of MileMarkerTM (a web‐based assessment software that helps drive efficiencies in medical training), and the Operative Entrustability Assessment (OEA). She is the vice‐president, an advisor, and an equity holder for EduMD LLC (a start‐up company that designs and develops application software to improve medical training programs). MAM: none known. GDR: none related to this review topic. GDR has licensed IP to Aegeria Soft Tissue, LLC (Baltimore, MD). GDR's institution has received educational grants and/or grants for investigator‐initiated studies pertaining to peripheral nerve surgery, microsurgical outcomes and/or breast reconstruction outcomes from TEI Bioschiences, LifeCell, Mentor, Sienta and AxoGen, and consulted on peripheral nerve surgery for AxoGen.
The co‐authors acknowledge the following institutional support: the Johns Hopkins Department of Plastic Surgery receives/received research support from LifeCell Corp (Branchburg, NJ) and AxoGen Inc (Alachua, FL). The Johns Hopkins Department of Plastic Surgery receives/received educational support from LifeCell Corp (Branchburg, NJ), Mentor Corp (Santa Barbara, CA), Integra LifeSciences (Plainsboro, NJ) and Sientra (Santa Barbara, CA). Products sold by these companies may be discussed in generic or brand name format, as appropriate.
New
References
References to studies included in this review
Bakr 2016 {published data only}
- Bakr MA, Amr SA, Mohamed SA, Hamed HB, Abd ER, Ahmad M, et al. Comparison between the effects of intravenous morphine, tramadol, and ketorolac on stress and immune responses in patients undergoing modified radical mastectomy. Clinical Journal of Pain 2016;32(10):889-97. [DOI] [PubMed] [Google Scholar]
Bosek 1996 {published data only}
- Bosek V, Cox CE. Comparison of analgesic effect of locally and systemically administered ketorolac in mastectomy patients. Annals of Surgical Oncology 1996;3(1):62-6. [DOI] [PubMed] [Google Scholar]
Chan 1996 {published data only}
- Chan A, Dore CJ, Ramachandra V. Analgesia for day surgery: Evaluation of the effect of diclofenac given before or after surgery with or without bupivacaine infiltration. Anaesthesia 1996;51(6):592-5. [DOI] [PubMed] [Google Scholar]
Forget 2019 {published data only}
- Forget P, Bouche G, Duhoux FP, Coulie PG, Decloedt J, Dekleermaker A, et al. Intraoperative ketorolac in high-risk breast cancer patients. A prospective, randomized, placebo-controlled clinical trial. PLoS One 2019;14(12):e0225748. [DOI: 10.1371/journal.pone.0225748] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 2006 {published data only}
- Freedman BM, Balakrishnan TP, O'Hara EL. Celecoxib reduces narcotic use and pain following augmentation mammaplasty. Aesthetic Surgery Journal 2006;26(1):24-8. [DOI] [PubMed] [Google Scholar]
Legeby 2005 {published data only}
- Legeby M, Sandelin K, Wickman M, Olofsson C. Analgesic efficacy of diclofenac in combination with morphine and paracetamol after mastectomy and immediate breast reconstruction. Acta Anaesthesiologica Scandinavica 2005;49(9):1360-6. [DOI] [PubMed] [Google Scholar]
Oh 2016 {published data only}
- Oh E, Ahn HJ, Sim WS, Lee JY. Synergistic effect of intravenous ibuprofen and hydromorphone for postoperative pain: prospective randomized controlled trial. Pain Physician 2016;19(6):341-8. [PubMed] [Google Scholar]
Parsa 2005 {published data only}
- Parsa AA, Soon CW, Parsa FD. The use of celecoxib for reduction of pain after subpectoral breast augmentation. Aesthetic Plastic Surgery 2005;29(6):441-4. [DOI] [PubMed] [Google Scholar]
Romundstad 2006a {published data only}
- Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, et al. Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesthesia and Analgesia 2006;102(2):418-25. [DOI] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun M, Liao Q, Wen L, Yan X, Zhang F, Ouyang W. Effect of perioperative intravenous flurbiprofen axetil on chronic postmastectomy pain. Journal of Central South University. Medical Sciences 2013;38(7):653-60. [DOI] [PubMed] [Google Scholar]
van Helmond 2016 {published data only}
- Helmond N, Steegers MA, Filippini-de Moor GP, Vissers KC, Wilder-Smith OH. Hyperalgesia and persistent pain after breast cancer surgery: a prospective randomized controlled trial with perioperative COX-2 inhibition. PloS One 2016;11(12):e0166601. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2015 {published data only}
- Wen Y, Wang M, Yang J, Wang Y, Sun H, Zhao J, et al. A comparison of fentanyl and flurbiprofen axetil on serum VEGF‐C, TNF‐α, and IL‐1ß concentrations in women undergoing surgery for breast cancer. Pain Practice 2015;15(6):530-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12614000507684 {published and unpublished data}
- ACTRN12614000507684. Pre-surgical aromatase inhibitor combined with non-steroidal anti inflammatory drug treatment in breast cancer. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366263 (first received 5 May 2014). [https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366263]
Aristarco 2016 {published and unpublished data}
- Aristarco V, Serrano D, Gandini S, Johansson H, Macis D, Guerrieri-Gonzaga A, et al. A randomized, placebo-controlled, phase II, presurgical biomarker trial of celecoxib versus exemestane in postmenopausal breast cancer patients. Cancer Prevention Research 2016;9(5):349‐56. [DOI] [PubMed] [Google Scholar]
Barker 2018 {published and unpublished data}
- Barker JC, DiBartola K, Wee C, Andonian N, Abdel-Rasoul M, Lowery D, et al. Preoperative multimodal analgesia decreases postanesthesia care unit narcotic use and pain scores in outpatient breast surgery. Plastic and Reconstructive Surgery 2018;142(4):443e-50e. [DOI] [PubMed] [Google Scholar]
Betambeau 2004 {published and unpublished data}
- Betambeau N, Davies G, Sacks N, Gui G, MacNeill F, Ebbs S, et al. Molecular effects of cyclooxygenase-2 (Cox-2) inhibition in primary breast cancer in vivo. Breast Cancer Research and Treatment 2004;88:S240.
Blomqvist 1996 {published and unpublished data}
- Blomqvist L, Sellman G, Strombeck JON. NSAID as pre- and postoperative medication - A potential risk for bleeding complications in reduction mammaplasty. European Journal of Plastic Surgery 1996;19(1):26-8. [Google Scholar]
Bosek 1992 {published and unpublished data}
- Bosek V, Smith DB, Cox C. Ketorolac or fentanyl to supplement local anesthesia? Journal of Clinical Anesthesia 1992;4(6):480‐3. [DOI] [PubMed] [Google Scholar]
Bosek 1994 {published and unpublished data}
- Bosek V, Cox CE. Locally or systemically administered ketorolac improves quality of pain control after mastectomy. Breast Cancer Research and Treatment 1994;32:69. [Google Scholar]
Brandao 2013 {published and unpublished data}
- Brandao RD, Veeck J, Van de Vijver KK, Lindsey P, Vries B, Elssen CH, et al. A randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancer. Breast Cancer Research 2013;15(2):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bundred 2010 {published and unpublished data}
- Bundred NJ, Cramer A, Morris J, Renshaw L, Cheung KL, Flint P, et al. Cyclooxygenase-2 inhibition does not improve the reduction in ductal carcinoma in situ proliferation with aromatase inhibitor therapy: results of the ERISAC randomized placebo-controlled trial. Clinical Cancer Research 2010;16(5):1605‐12. [DOI] [PubMed] [Google Scholar]
Cawthorn 2012 {published and unpublished data}
- Cawthorn TR, Phelan R, Davidson JS, Turner KE. Retrospective analysis of perioperative ketorolac and postoperative bleeding in reduction mammoplasty. Canadian Journal of Anaesthesia 2012;59(5):466-72. [DOI] [PubMed] [Google Scholar]
ChiCTR‐IPR‐14005271 {published and unpublished data}
- ChiCTR-IPR-14005271. Research on approaches to improving cancer patients perioperative immunity and reducing postsurgical cancer recurrence. chictr.org.cn/showprojen.aspx?proj=9564 (first received 19 September 2014).
CTRI/2016/10/007397 {published and unpublished data}
- CTRI/2016/10/007397. A study to note the effect of giving pregabalin before surgery on pain on patients going to have breast surgery. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=11107 (first received 21 October 2016). [http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=11107&EncHid=&modid=&compid=%27,%2711107det%27]
DeOliveira 2018 {published and unpublished data}
- De Oliveira Jr GS, Rodes ME, Bialek J, Kendall MC, McCarthy RJ. Single dose systemic acetaminophen to improve patient reported quality of recovery after ambulatory segmental mastectomy: A prospective, randomized, double-blinded, placebo controlled, clinical trial. Breast Journal 2018;24(3):240-4. [DOI] [PubMed] [Google Scholar]
Desmedt 2017 {published and unpublished data}
- Desmedt C, Demicheli R, Fornili M, Bachir I, Duca M, Viglietti G, et al. Differential benefit of intra-operative administration of ketorolac on breast cancer disease recurrence according to baseline body mass index. Cancer Research 2017;78(Suppl 1):4. [Google Scholar]
EUCTR2012‐003774‐76‐BE {published and unpublished data}
- EUCTR2012-003774-76-BE. Ketorolac during breast cancer surgery. clinicaltrialsregister.eu/ctr-search/search?query=2012-003774-76 (first received 9 October 2012). [https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2012-003774-76]
Filippini 2014 {published and unpublished data}
- Filippini-de Moor G, Steegers M, Helmond N, Paquay Y, Jagmont T, Vissers K, et al. Impact of extended perioperative cyclooxygenase (cox) - 2 inhibition on chronic pain after surgery for breast cancer. Pain Practice 2014;14:91-2. [Google Scholar]
Forget 2010 {published and unpublished data}
- Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesthesia and Analgesia 2010;110(6):1630-5. [DOI] [PubMed] [Google Scholar]
Forget 2013 {published and unpublished data}
- Forget P, Berliere M, Maanen A, Duhoux FP, Machiels JP, Coulie PG, et al. Perioperative ketorolac in high risk breast cancer patients. Rationale, feasibility and methodology of a prospective randomized placebo-controlled trial. Medical Hypotheses 2013;81(4):707-12. [DOI] [PubMed] [Google Scholar]
Forget 2014 {published and unpublished data}
- Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. British Journal of Anaesthesia 2014;113(Suppl 1):82-7. [DOI] [PubMed] [Google Scholar]
Francis 1988 {published data only}
- Francis JL, Dawwes RF, Roath OS. The effect of diclofenac on platelet aggregation and post-operative bleeding. Hematology Reviews 1988;2:283-91. [Google Scholar]
Hidar 2007 {published data only}
- Hidar S, Soussi K, Bibi M, Khairi HJ. Influence of ketoprofen on drainage volume after radical breast cancer surgery: a prospective randomized clinical trial. Turkish German Gynecological Association 2007;8(1):71-5. [Google Scholar]
Himendra 1985 {published data only}
- Himendra A, Rasman M, Sutisna A, Adipradja K, Santoso N. Administration of ketoprofen suppositories for out-patient post-operative analgesia. Current Medical Research and Opinion 1985;9(7):436‐41. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published and unpublished data}
- Hu SF, Zhang YL, Jiang W, Wu XY, Dai L, Chen L, et al. Effect of preemptive analgesia with flurbiprofen axetil on postoperative blood glucose and interleukin-6 levels in patients with breast cancer. Chinese Medical Journal 2009;89(39):2751-3. [PubMed] [Google Scholar]
ISRCTN06628870 {published and unpublished data}
- ISRCTN06628870. Non-steroidal anti-inflammatory drug (NSAID) use in breast surgery: prospective randomised trial. isrctn.com/search?q=ISRCTN06628870 (first received 12 September 2005). [http://www.isrctn.com/ISRCTN06628870?q=cancer%20-radiotherapy&filters=recruitmentCountry:Tunisia&sort=&offset=3&totalResults=3&page=1&pageSize=10&searchType=basic-search]
ISRCTN86894592 {published and unpublished data}
- ISRCTN86894592. Pre-surgical effects of serms, anti-COX-2 and aromatase inhibitors in breast cancer patients. isrctn.com/search?q=ISRCTN86894592 (first received 11 September 2012).
Kampe 2006 {published data only}
- Kampe S, Warm M, Landwehr S, Dagtekin O, Haussmann S, Paul M, et al. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Current Medical Research and Opinion 2006;22(10):1949‐54. [DOI] [PubMed] [Google Scholar]
Kodaka 2012 {published data only}
- Kodaka M, Hoshikawa Y, Yasuhira A, Ichikawa J, Nishiyama K, Komori M. Can preoperative flurbiprofen axetil reduce the total dose of fentanyl and sevoflurane for breast-conserving surgery? European Journal of Anaesthesiology 2012;29:149‐50. [Google Scholar]
Laisalmi 2001a {published and unpublished data}
- Laisalmi M, Eriksson H, Koivusalo AM, Pere P, Rosenberg P, Lindgren L. Ketorolac is not nephrotoxic in connection with sevoflurane anesthesia in patients undergoing breast surgery. Anesthesia and Analgesia 2001;92(4):1058-63. [DOI] [PubMed] [Google Scholar]
Laisalmi 2001b {published data only}
- Laisalmi M, Teppo AM, Koivusalo AM, Honkanen E, Valta P, Lindgren L. The effect of ketorolac and sevoflurane anesthesia on renal glomerular and tubular function. Anesthesia and Analgesia 2001;93(5):1210-3. [DOI] [PubMed] [Google Scholar]
Lakdja 1997 {published and unpublished data}
- Lakdja F, Dixmerias F, Bussieres E, Fonrouge JM, Lobera A. Preventive analgesic effect of intraoperative administration of ibuprofen-arginine on postmastectomy pain syndrome [Effet analgésique préventif d’une administration périopératoire d’ibuprofène- arginine sur le syndrome douloureux postmastectomie]. Bulletin du Cancer 1997;84(3):259-63. [PubMed] [Google Scholar]
Mahabir 2008 {published data only}
- Mahabir RC, Peterson BD, Williamson JS, Valnicek SM, Williamson DG, East WE. Locally administered ketorolac and bupivacaine for control of postoperative pain in breast augmentation patients: part II. 10-day follow-up. Plastic and Reconstructive Surgery 2008;121(2):638-43. [DOI] [PubMed] [Google Scholar]
Martin 2010 {published data only}
- Martin LA, Davies GL, Weigel MT, Betambeau N, Hills MJ, Salter J, et al. Pre-surgical study of the biological effects of the selective cyclo-oxygenase-2 inhibitor celecoxib in patients with primary breast cancer. Breast Cancer Research & Treatment 2010;123(3):829-36. [DOI] [PubMed] [Google Scholar]
McCrea 2009 {published data only}
- McCrea PH, Mitchell A, Porter G. Pain management following breast cancer surgery: a double-blind randomized controlled study comparing acetaminophen plus ibuprofen to acetaminophen plus codeine plus caffeine. Canadian Journal of Surgery 2009;52(Suppl 4):4. [Google Scholar]
Mitchell 2012 {published data only}
- Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Annals of Surgical Oncology 2012;19(12):3792-800. [DOI] [PubMed] [Google Scholar]
Molon 2009 {published and unpublished data}
- Molon V, Kruel CD, Maioli DT, Caran JZ, Lovison RC. Evaluation of the analgesic effects of the lumiracoxib compared with placebo in the 24 first postoperative hours. Revista do colegio brasileiro de cirurgioes 2009;36(1):3-8. [DOI] [PubMed] [Google Scholar]
Morley‐Forster 1993 {published data only}
- Morley-Forster P, Newton PT, Cook MJ. Ketorolac and indomethacin are equally efficacious for the relief of minor postoperative pain. Canadian Journal of Anaesthesia 1993;40(12):126-30. [DOI] [PubMed] [Google Scholar]
NCT00070057 {published and unpublished data}
- NCT00070057. Celecoxib in treating postmenopausal women who are undergoing surgery for invasive breast cancer. clinicaltrials.gov/ct2/show/NCT00070057 (first received 7 October 2003). [https://ClinicalTrials.gov/show/NCT00070057]
NCT00435747 {published and unpublished data}
- NCT00435747. Efficacy and safety of parecoxib IV/IM 40 mg vs placebo following sub muscular breast augmentation. clinicaltrials.gov/ct2/show/NCT00435747 (first received 15 February 2007). [Https://clinicaltrials.gov/show/nct00435747]
NCT00502684 {published and unpublished data}
- NCT00502684. Perioperative administration of COX 2 inhibitors and beta blockers to women undergoing breast cancer surgery. clinicaltrials.gov/ct2/show/NCT00502684 (first received 18 July 2007). [https://ClinicalTrials.gov/show/NCT00502684]
NCT01695226 {published and unpublished data}
- NCT01695226. Randomized controlled phase II trial of pre-operative celecoxib treatment in breast cancer. clinicaltrials.gov/ct2/show/NCT01695226 (first received 27 September 2012). [https://ClinicalTrials.gov/show/NCT01695226]
NCT01806259 {published and unpublished data}
- NCT01806259. Ketorolac in breast cancer surgery (KBCt). clinicaltrials.gov/ct2/show/NCT01806259 (first received 7 March 2013). [https://ClinicalTrials.gov/show/NCT01806259]
NCT01852955 {published and unpublished data}
- NCT01852955. Perioperative systemic acetaminophen to improve postoperative quality of recovery after ambulatory breast surgery. clinicaltrials.gov/ct2/show/NCT01852955 (first received 14 May 2013). [Https://clinicaltrials.gov/show/nct01852955]
NCT02102555 {published and unpublished data}
- NCT02102555. A single dose of intravenous (IV) scetaminophen versus placebo in the pre-operative setting and outcomes on post-operative pain, nausea and vomiting. clinicaltrials.gov/ct2/show/NCT02102555 (first received 3 April 2014). [Https://clinicaltrials.gov/show/nct02102555]
NCT02141139 {published and unpublished data}
- NCT02141139. Perioperative inflammation and breast cancer outcome. clinicaltrials.gov/ct2/show/NCT02141139 (first received 19 May 2014). [Https://clinicaltrials.gov/show/nct02141139]
NCT02461056 {published and unpublished data}
- NCT02461056. Synergistic effect of ibuprofen and hydromorphone for postoperative pain. clinicaltrials.gov/ct2/show/NCT02461056 (first received 3 June 2015). [Https://clinicaltrials.gov/show/nct02461056]
NCT03007381 {unpublished data only}
- NCT03007381. Ketorolac for Analgesia followiNG Autologous Breast RecOnstructiOn (KANGAROO). clinicaltrials.gov/ct2/show/NCT03007381 (first received 2 January 2017). [Https://clinicaltrials.gov/show/nct03007381]
NCT03185871 {published and unpublished data}
- NCT03185871. Celecoxib window of opportunity trial to assess tumor and stroma responses. clinicaltrials.gov/ct2/show/NCT03185871 (first received 14 June 2017). [https://ClinicalTrials.gov/show/NCT03185871]
Nguyen 2018a {published data only}
- Nguyen B, Barta R, Heinrich C. Toradol use in breast reconstruction: Risk of hematoma and benefit of post-operative pain control. Annals of Surgical Oncology 2018;25(2):185-6. [Google Scholar]
Nguyen 2018b {published and unpublished data}
- Nguyen BN, Barta RJ, Stewart CE, Heinrich CA. Toradol following breast surgery: is there an increased risk of hematoma? Plastic and Reconstructive Surgery 2018;141(6):814e-7e. [DOI] [PubMed] [Google Scholar]
Ohnesorge 2009 {published data only}
- Ohnesorge H, Bein B, Hanss R, Francksen H, Mayer L, Scholz J, et al. Paracetamol versus metamizol in the treatment of postoperative pain after breast surgery: a randomized, controlled trial. European Journal of Anaesthesiology 2009;26(8):648‐53. [DOI] [PubMed] [Google Scholar]
Parsa 2009 {published data only}
- Parsa AA, Sprouse-Blum AS, Jackowe DJ, Lee M, Oyama J, Parsa FD. Combined preoperative use of celecoxib and gabapentin in the management of postoperative pain. Aesthetic Plastic Surgery 2009;33(1):98-103. [DOI] [PubMed] [Google Scholar]
Pavlin 2002 {published data only}
- Pavlin DJ, Chen C, Penaloza DA, Polissar NL, Buckley FP. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesthesia and Analgesia 2002;95(3):627-34. [DOI] [PubMed] [Google Scholar]
Priya 2002 {published data only}
- Priya V, Divatia JV, Sareen R, Upadhye S. Efficacy of intravenous ketoprofen for pre-emptive analgesia. Journal of Postgraduate Medicine 2002;48(2):109‐12. [PubMed] [Google Scholar]
Retsky 2012 {published data only}
- Retsky M, Rogers R, Demicheli R, Hrushesky WJ, Gukas I, Vaidya JS, et al. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Research & Treatment 2012;134(2):881-8. [DOI] [PubMed] [Google Scholar]
Retsky 2013 {published data only}
- Retsky M, Demicheli R, Hrushesky WJ, Forget P, De Kock M, Gukas I, et al. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: New findings and a review. Current Medicinal Chemistry 2013;20(33):4163-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riest 2006 {published data only}
- Riest G, Peters J, Weiss M, Pospiech J, Hoffmann O, Neuhauser M, et al. Does perioperative administration of rofecoxib improve analgesia after spine, breast and orthopaedic surgery? European Journal of Anaesthesiology 2006;23(3):219-26. [DOI] [PubMed] [Google Scholar]
Romundstad 2006b {published data only}
- Romundstad L, Breivik H, Roald H, Skolleborg K, Romundstad PR, Stubhaug A. Chronic pain and sensory changes after augmentation mammoplasty: long term effects of preincisional administration of methylprednisolone. Pain 2006;124(1-2):92-9. [DOI] [PubMed] [Google Scholar]
Shaashua 2017 {published data only}
- Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clinical Cancer Research 2017;23(16):4651-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2001 {published and unpublished data}
- Sharma S, Chang DW, Koutz C, Evans GR, Robb GL, Langstein HN, et al. Incidence of hematoma associated with ketorolac after TRAM flap breast reconstruction. Plastic and Reconstructive Surgery 2001;107(2):352-5. [DOI] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun T, Sacan O, White PF, Coleman J, Rohrich RJ, Kenkel JM. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesthesia and Analgesia 2008;106(3):950-8. [DOI] [PubMed] [Google Scholar]
Tallian 2014 {published data only}
- Tallian K, Schmidt J. Impact of the perioperative use of intravenous acetaminophen in patients undergoing intraabdominal and breast reconstruction surgery. Pharmacotherapy 2014;34(10):e251. [Google Scholar]
VanHelmond 2014 {published data only}
- Helmond N, Filippini-de Moor G, Steegers M, Vissers K, Wilder-Smith O. Impact of extended perioperative cyclooxygenase (COX)-2 inhibition on quality of life after surgery for breast cancer. Pain Practice 2014;14:96. [Google Scholar]
References to ongoing studies
NCT03535116 2018 {published and unpublished data}
- NCT03535116. The effect of intra-operative ketorolac on hematoma rates in breast reduction surgery. clinicaltrials.gov/ct2/show/NCT03535116 (first received 24 May 2018).
Additional references
ACS 2017
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. Atlanta: American Cancer Society, Inc, 2017. [Google Scholar]
Al‐Sukhun 2006
- Al-Sukhun J, Koivusalo A, Törnwall J, Lindqvist C. COX-2 inhibitors and early failure of free vascular flaps. New England Journal of Medicine 2006;355(5):528-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
ASA 2012
- American Society of Anesthesiologists. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116(2):248-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
ASPS 2017
- American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf 2017. [www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf]
ASPS 2018
- American Society of Plastic Surgeons. How should I prepare for breast reduction surgery? www.plasticsurgery.org/reconstructive-procedures/breast-reduction/preparation 2018. [www.plasticsurgery.org/reconstructive-procedures/breast-reduction/preparation]
Batdorf 2015
- Batdorf NJ, Lemaine V, Lovely JK, Ballman KV, Goede WJ, Martinez-Jorge J, et al. Enhanced recovery after surgery in microvascular breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery 2015;68(3):395-402. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bonde 2015
- Bonde C, Khorasani H, Eriksen K, Wolthers M, Kehlet H, Elberg J. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: A case control study. Journal of Plastic Surgery and Hand Surgery 2015;49(6):367-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bonde 2016
- Bonde CT, Khorasani H, Elberg J, Kehlet H. Perioperative optimization of autologous breast reconstruction. Plastic and Reconstructive Surgery 2016;137(2):411-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bonde 2017
- Bonde C, Khorasani H, Hoejvig J, Kehlet H. Cyclooxygenase-2 inhibitors and free flap complications after autologous breast reconstruction: a retrospective cohort study. Journal of Plastic, Reconstructive & Aesthetic Surgery 2017;70(11):1543-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brinck 2018
- Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD012033. [DOI: 10.1002/14651858.CD012033.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunton 2017
- Brunton L, Knollmann B, Hilal-Dandan R. Goodman & Gilman's The pharmacological Basis of Therapeutics. 13th edition. New York: McGraw-Hill Education/Medical, 2017. [Google Scholar]
Cheng 2011
Cheng 2016
- Cheng GS, Ilfeld BM. An evidence-based review of the efficacy of perioperative analgesic techniques for breast cancer-related surgery. Pain Medicine 2017;18:1344-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chou 2016
- Chou R, Gordon DB, Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. Journal of Pain 2016;17(2):131-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Collins 2012
- Collins JB, Verheyden CN. Incidence of breast hematoma after placement of breast prostheses. Plastic and Reconstructive Surgery 2012;129(3):413e-20e. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed prior to 9 September 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Davidge 2013
- Davidge KM, Brown M, Morgan P, Semple JL. Processes of care in autogenous breast reconstruction with pedicled TRAM flaps: expediting postoperative discharge in an ambulatory setting. Plastic and Reconstructive Surgery 2013;132(3):339e-44e. [PMID: ] [DOI] [PubMed] [Google Scholar]
Desmedt 2018
- Desmedt C, Demicheli R, Fornili M, Bachir I, Duca M, Viglietti G, et al. Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the patient's body mass index. Journal of the National Cancer Institute 2018;110(10):1115-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Souza 2012
- De Souza JA, Ratain MJ, Fendrick AM. Value-based insurance design: aligning incentives, benefits, and evidence in oncology. Journal of the National Comprehensive Cancer Network 2012;10(1):18-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gobble 2014
- Gobble RM, Hoang HL, Kachniarz B, Orgill DP. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plastic and Reconstructive Surgery 2014;133(3):741-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- GRADEpro GDT. Version accessed prior to 9 September 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harirforoosh 2013
- Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Journal of Pharmacy & Pharmaceutical Sciences 2013;16(5):821-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
HHS 2016
- US Department of Health and Human Services (HHS), Office of the Surgeon General. Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health. Washington (DC): US Department of Health and Human Services, 2016. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hjermstad 2011
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. Journal of Pain and Symptom Management 2011;41(6):1073-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacox 1994
- Jacox A, Carr DB, Payne R. New clinical-practice guidelines for the management of pain in patients with cancer. New England Journal of Medicine 1994;330(9):651-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jarupongprapa 2013
- Jarupongprapa S, Ussavasodhi P, Katchamart W. Comparison of gastrointestinal adverse effects between cyclooxygenase-2 inhibitors and non-selective, non-steroidal anti-inflammatory drugs plus proton pump inhibitors: a systematic review and meta-analysis. Journal of Gastroenterology 2013;48(7):830-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaoutzanis 2017
- Kaoutzanis C, Winocour J, Gupta V, Ganesh Kumar N, Sarosiek K, Wormer B, et al. Incidence and risk factors for major hematomas in aesthetic surgery: analysis of 129,007 patients. Aesthetic Surgery Journal 2017;37(10):1175-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kehlet 1997
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia 1997;78(5):606-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewis 2013
- Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD003591. [DOI: 10.1002/14651858.CD003591.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin‐Bertolin 1997
- Marín-Bertolín S, De Andrés J, González-Martínez R, Valia Vera JC, Amorrortu-Velayos J. A controlled, randomized, double-blind study of ketorolac for postoperative analgesia after plastic surgery. Annals of Plastic Surgery 1997;38(5):478-84. [PMID: ] [PubMed] [Google Scholar]
Merriam‐Webster 1966
- Merriam-Webster. Perioperative - Merriam-Webster.com Dictionary. www.merriam-webster.com/dictionary/perioperative (accessed 30 April 2020). [https://www.merriam-webster.com/dictionary/perioperative]
Merriam‐Webster 1997
- Merriam-Webster. Pharmacogenomics - Merriam-Webster.com Dictionary. www.merriam-webster.com/dictionary/pharmacogenomics (accessed 30 April 2020). [Retrieved April 30, 2020, from https://www.merriam-webster.com/dictionary/pharmacogenomics]
Mikhaylov 2018
- Mikhaylov Y, Weinstein B, Schrank TP, Swartz JD, Ulm JP, Armstrong MB, et al. Ketorolac and hematoma incidence in postmastectomy implant-based breast reconstruction. Annals of Plastic Surgery 2018;80(5):472-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Myles 2012
- Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. British Journal of Anaesthesia 2012;108(3):423-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nir 2016
- Nir RR, Nahman-Averbuch H, Moont R, Sprecher E, Yarnitsky D. Preoperative preemptive drug administration for acute postoperative pain: a systematic review and meta-analysis. European Journal of Pain 2016;20(7):1025-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schmidt 2016
- Schmidt M, Lamberts M, Olsen AM, Fosbøll E, Niessner A, Tamargo J, et al. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. European Heart Journal 2016;37(13):1015–23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Smith 2017
- Smith BD, Jiang J, Shih Y, Giordano SH, Huo J, Jagsi R, et al. Cost and complications of local therapies for early-stage breast cancer. Journal of the National Cancer Institute 2017;109(1):djw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephens 2015
- Stephens DM, Richards BG, Schleicher WF, Zins JE, Langstein HN. Is ketorolac safe to use in plastic surgery? A critical review. Aesthetic Surgery Journal 2015;35(4):462-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2019
- Stone JP, Siotos C, Sarmiento S, Temple-Oberle C, Aliu O, Cooney DS, et al. Implementing our microsurgical breast reconstruction enhanced recovery after surgery pathway: vonsensus obstacles and recommendations. Plastic and Reconstructive Surgery. Global Open 2019;7(1):e1855. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svider 2018
- Svider PF, Nguyen B, Yuhan B, Zuliani G, Eloy JA, Folbe AJ. Perioperative analgesia for patients undergoing endoscopic sinus surgery: an evidence-based review. International Forum of Allergy & Rhinology 2018;8(7):837-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2019
- Walker NJ, Jones VM, Kratky L, Chen H, Runyan CM. Hematoma risks of nonsteroidal anti-inflammatory drugs used in plastic surgery procedures: a systematic review and meta-analysis. Annals of Plastic Surgery 2019;82(6S Suppl 5):S437-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wengritzky 2010
- Wengritzky R, Mettho T, Myles PS, Burke J, Kakos A. Development and validation of a postoperative nausea and vomiting intensity scale. British Journal of Anaesthesia 2010;104(2):158-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wongrakpanich 2018
- Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-Inflammatory drug use in the elderly. Aging and Disease 2018;9(1):143-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2015
- Yan C, Fischer JP, Wes AM, Basta MN, Rohrbach JI, Kovach SJ, et al. The cost of major complications associated with immediate two-stage expander/implant-based breast reconstruction. Journal of Plastic Surgery and Hand Surgery 2015;49(3):166-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Younger 2009
- Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Current Pain and Headache Reports 2009;13(1):39-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Klifto 2019
- Klifto KM, Major MR, Leto Barone AA, Payne RM, Elhelali A, Seal SM, et al. Perioperative systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in women undergoing breast surgery. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013290. [DOI: 10.1002/14651858.CD013290] [DOI] [PMC free article] [PubMed] [Google Scholar]


