Abstract
Background
Androgenetic alopecia (AGA) leads to thinning of scalp hair and affects 60%~70% of the adult population worldwide. Developing more effective treatments and studying its mechanism are of great significance. Previous clinical studies have revealed that hair growth is stimulated by 650-nm red light.
Objective
This study aimed to explore the effect and mechanism of 650-nm red light on the treatment of AGA by using ex vivo hair follicle culture.
Methods
Human hair follicles were obtained from hair transplant patients with AGA. Hair follicles were cultured in Williams E medium and treated with or without 650-nm red light. Real-time RT-PCR and immunofluorescence staining were used to detect the expression level of genes and proteins in hair follicles, respectively. RNA-sequencing analysis was carried out to reveal the distinct gene signatures upon 650 nm treatment.
Results
Low-level 650 nm red light promoted the proliferation of human hair follicles in the experimental cultured-tissue model. Consistently, 650 nm red light significantly delayed the transition of hair cycle from anagen to catagen in vitro. RNA-seq analysis and gene clustering for the differentially expressed genes suggests that leukocyte transendothelial migration, metabolism, adherens junction and other biological process maybe involved in stimulation of hair follicles by 650-nm red light treatment.
Conclusion
The effect of 650-nm red light on ex vivo hair follicles and the transcriptome set which implicates the role of red light in promoting hair growth and reversing of miniaturization process of AGA were identified.
Keywords: 650-nm red light, Androgenetic alopecia, Hair follicle, Low-level laser therapy, RNA sequencing
INTRODUCTION
Androgenetic alopecia (AGA) is a common condition that affects approximately 80% of Caucasian males and 50% of females respectively, by the age of 70 years and has also been associated with hair loss1,2. AGA is characterized by gradual transformation of terminal hairs to vellus-like hairs and results in thinning hairs3,4. The structure of a hair contains two parts: the hair shaft present above the epidermis and the hair follicle (HF) present below the epidermis. Besides, HFs are complex mini-organs undergoing cycles of anagen (growth), catagen (degeneration) and telogen (rest)5. A variety of genes and pathways are involved in the pathogenesis of AGA, including Wnt/β-catenin, immune and inflammatory responses, Janus-activated kinase, sonic hedgehog, etc5,6,7. Among them, Wnt/β catenin signalling pathway, which is specially important for the hair cycle and hair growth, has been shown to be negatively influenced by the increased androgen in AGA patients8. Some inflammatory markers were also found to be specifically expressed in AGA lesional bald scalp compared to the nonlesional scalp9. These differences in gene expression and signalling pathways ultimately led to the pathophysiology of AGA, such as aberrant follicle cycle, follicle miniaturization, and loss of hair. Therefore, controlling the hair cycle and promoting hair growth through maintaining anagen and shortening catagen or telogen is critical in the treatment of thinning hairs or hair loss in AGA.
Hair loss can have significant effects on patients’ psychological health and quality of life. Hair transplantation and growth factor injection can be used to treat hair loss, but so far, only two drugs (minoxidil and finasteride) have been approved by the Food and Drug Administration (FDA) for the treatment of hair loss. However, these drugs have demonstrated temporary effects or their use for women has been restricted. Therefore, there is an increasing demand for novel treatments that prevent the progression of hair loss, facilitate hair regrowth, and have minimal side effects.
It has been reported that specific wavelengths of laser light have biological effects, and near-infrared light has been used to stimulate stem cell proliferation and differentiation10. Recently, low-level laser (or light) therapy (LLLT) has been introduced as a therapeutic option for people who fail to, or are unwilling to use traditional medical therapy or undergo surgical treatment for hair loss11. LLLT appears to be a safe and effective treatment in AGA12,13 and LLLT devices have become commercially available to AGA patients. Strong evidence suggests that LLLT stimulates anagen re-entry of telogen HFs and prolongs the duration of the anagen phase14,15. Additionally, LLLT acts on mitochondria to increase reactive oxygen species levels, adenosine triphosphate (ATP) production, and induction of transcription factors that activate genes and produce proteins which are useful to the multiplying cell16,17,18.
Previous studies have indicated that 650-nm red light is the most effective and practical way for stimulating hair growth via LLLT treatment. However, mechanisms by which LLLT reduces hair loss are not yet clearly understood. Therefore, we examined the effects of 650 nm light on hair growth using cultured human HFs and identified potential transcriptional mechanisms via RNA sequencing (RNA-seq) analysis.
MATERIALS AND METHODS
Human hair follicle organ dissection and culture
Occipital scalp HF units were obtained during the hair transplant of AGA patients. All participants signed informed consents and were notified about the study before participation. The study was approved by ethics committee of Huashan Hospital of Fudan University (ethical approval number 2019M-008) and was performed in accordance with the principles embodied in the declaration of Helsinki. The informed consent was obtained from all patients who provided samples. Anagen VI follicles were isolated from the human scalp and cultured in William’s E medium (Gibco BRL, Grand Island, NY, USA) containing 2 mM glutamine, 10 ng/ml hydrocortisone, 100 U/ml penicillin, and 100 mg/ml streptomycin in individual wells of 12-well plates at 37.0℃ in a 5% CO2 incubator. HFs that grew to lengths of 0.3~0.5 mm after 24 hours were selected for subsequent LLLT treatment experiments19.
Hair follicle treatment with 650 nm light
Light emitting diodes (LEDs; 650 nm) (CHARMWIN, Beijing, China) were used as red light sources. The wavelength was remeasured with a Spectral Flickering Irradiance Meter (SFIM-300_V310, Hangzhou, China) and was confirmed to show a peak wavelength of 650 nm. The energy density was 0.8 J/cm2 (exposure time: 5 minutes) and 1.6 J/cm2 (exposure time: 10 minutes). The selected HFs were divided into 3 groups, with different LLLT exposure times: 5 minutes, 10 minutes, and control (0 minute). Each group contained 6 HFs and laser treatment occurred on alternate days. Mediums were changed every other days and images of HFs were also captured every alternate day using a stereomicroscope (Olympus, Tokyo, Japan), to measure the elongation of hair shaft. Twenty-four hours after the first 650-nm LED treatment, HFs from each group were collected and fixed in 4% paraformaldehyde for immunofluorescence staining or lysed with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) for RNA extraction.
RNA isolation, reverse transcription and real-time PCR
Total RNA was extracted from HFs using TRIzol according to the manufacturers’ instruction (Invitrogen). Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA) according to the manufacturers’ protocol. SYBR Premix Ex Taq (TakaRa Biotech, Tokyo, Japan) and an ABI Prism 7900 Detector System (Applied Biosystems) were used to perform real-time RT-PCR. The expression levels of the housekeeping gene GADPH were assessed as an internal control. The data obtained from the assays were analyzed using SDS 2.3 software (Applied Biosystems).
Immunofluorescence staining
HF samples were embedded in paraffin and sectioned. The sections were blocked with bovine serum albumin and incubated with an antibody to Ki67 (Abcam, Cambridge, UK), an indicator of cell proliferation, overnight at 4℃. The sections were then incubated with Alexa Fluor 488 (Invitrogen) secondary antibody for 1 h at room temperature, and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime Biotechnology, Nanjing, China). Stained sections were viewed and photographed using fluorescence microscopy (Olympus).
RNA-sequencing data analysis
Up to 1~2 µg of total RNA was extracted from untreated or 650 nm light-treated HFs and used to prepare sequencing libraries with a TruSeq RNA Library Prep Kit (Illumina, San Diego, CA, USA). A HiSeq X Ten genome analyzer (Illumina) was used to perform the RNA sequencing. FastQC was used to assess sequence quality and FASTX-Toolkit was used to filter out the low-quality reads. The data was analyzed by Kallisto (version 0.44.0; Invitrogen) for quick transcript quantification without alignment. The index we used was downloaded from the Ensembl database (Homo_sapiens.GRCh38.cdna.all.fa.gz) and was built in the Kallisto index (a Kallisto module). We ran Kallisto quant with a bootstrap parameter-b 100 to produce an h5 file as input for sleuth analysis. The expression level was quantified by transcript per million (TPM) and counts. Sleuth (version 0.30.0) and DEseq2 (version 1.20.0) analysis were utilized to detect the differentially expressed transcripts (DETs) or genes (DEGs). A padj value <0.1 (adjusted by false discovery rate) was set to indicate DETs and the genes encoded by these transcripts were considered DEGs. The gene ontology (GO) enrichment analysis and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of DEGs were performed using the R package cluster Profiler (version 3.8.1; R Foundation for Statistical Computing, Vienna, Austria). The R package ggplot2 (version 3.0.0) was used to draw the bar plot and the volcano plot, while the pheatmap (version 1.0.12) was used to draw the heatmap.
Statistical analysis
All statistical analyses of data from our experiments were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The independent two-group t-test and one-way ANOVA test were used for the evaluation of significance between different groups, and a p-value of less than 0.05 was considered significant. All experiments were performed in triplicate.
RESULTS
Treatment with 650-nm red light promoted human hair growth in an ex vivo culture model
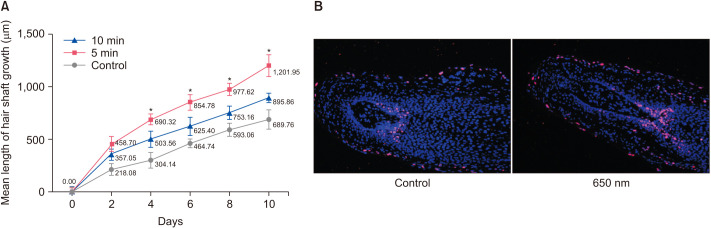
The isolated human scalp HFs were cultured in vitro and divided into three groups (control, 5 minutes, 10 minutes) and the elongation of hair shaft was measured every other day. Compared to the control group, both 5 minutes and 10 minutes treatment with 650 nm red light increased the mean length of hair shaft growth (Fig. 1A). Moreover, HFs treated for 5 minutes showed significantly increased length compared to the control group (p<0.05), while there was no significant difference between the length of HFs treated for 10 minutes and the control group (p>0.05). Ki67 is a typical marker of cell proliferation and the expression level of Ki67 in the hair matrix area was used to assess the proliferation of HFs. As shown in Fig. 1B, proliferation enhancement by treatment with 650-nm red light was confirmed via immunofluorescence staining. HFs were collected after 4 days of red-light therapy and the proportion of Ki67-positive cells (red fluorescence) relative to DAPI-stained cells (blue fluorescence) was recorded. Compared to HFs in the control group without light therapy, the proportion of Ki67-positive cells in the 650 nm light-treated group was significantly increased. These data indicate that 650 nm red light stimulates the HF and promotes hair growth in an ex vivo model.
Fig. 1. Hair shaft elongation and cell proliferation detected in cultured human hair follicles. (A) Hair shaft length was measured every other day. (B) immunofluorescence staining images showing the localization of the cell proliferation marker Ki67, and the cell nucleus was stained with DAPI.
Treatment with 650-nm red light postponed hair catagen transition in an in vitro culture model
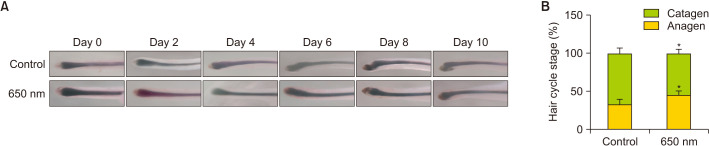
The hair cycle plays an important role in AGA, and the percentage of HFs in the anagen phase was significantly decreased in AGA patients. Therefore, we investigated the hair cycle of cultured HFs treated with or without 650 nm red light. Macroscopic images of cultured HFs were obtained every other day and the hair cycle stage was recorded. HFs show a remarkably thin hair matrix, an oval dermal papilla, and low melanin levels in the early catagen phase. These changes occurred on day 6 in HFs in the control group, while HFs in the 650-nm light-treated group appeared to be in anagen for 8 days (Fig. 2A). Moreover, the hair cycle stage was macroscopically quantifiable on day 8. As shown in Fig. 2B, a greater percentage of organ-cultured anagen scalp HFs remained in the 650 nm light-treated group compared to the control group (45.8%±4.8% vs. 33.3%±6.8%), while there was a lower percentage of catagen HFs in 650 nm light-treated group compared to the control group (54.1%±4.8% vs. 66.6%±6.8%) on day 8. All data suggested that 650-nm red light treatment postponed HF catagen transition and prolonged the anagen stage.
Fig. 2. Hair cycle stage detection in cultured human hair follicles. (A) Macroscopic images of cultured hair follicles were obtained every other day. (B) Macroscopic quantification of hair cycle stage on day 10 with 650-nm light treatment.
Overview of gene expression and identification of differentially expressed genes
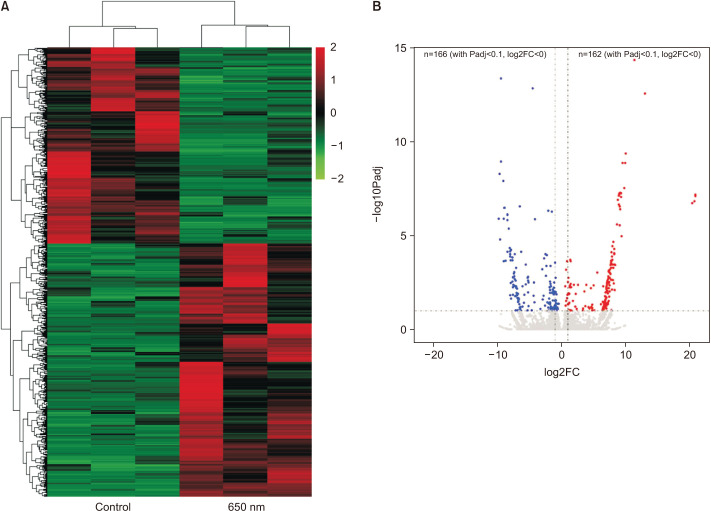
To explore the mechanisms by which 650 nm light treatment promoted HF stimulation and hair growth, we examined the genome-wide transcriptional changes in human HFs, with and without 650-nm light therapy by RNA-seq. After the stringent process of bioinformatic analysis, we found 728 DETs between the light-treated and untreated groups. Among them, 410 transcripts were upregulated, and 318 transcripts were downregulated by the treatment of 650 nm red light. The differential expression analysis and the unsupervised hierarchical clustering showed that the transcriptional changes induced by 650 nm red light treatment were consistent within the two groups and the DEGs tended to be upregulated after the treatment (Fig. 3A). The volcano plot (Fig. 3B) was generated from DEseq2 results, from which all nonzero read count transcripts were calculated and determined.
Fig. 3. Overview of gene expression and identification of differentially expressed genes. (A) The heatmap of all the differentially expressed transcripts between control and 650-nm light treatment groups. (B) The volcano plots generated from the results of DEseq2.
Transcriptome analysis of 650-nm red light-treated hair follicles
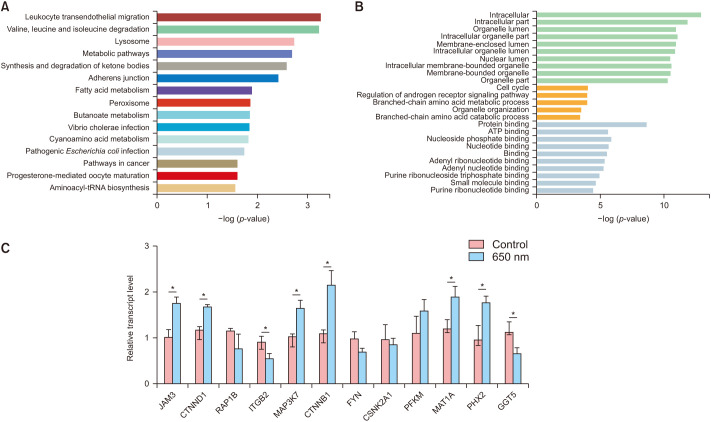
To further investigate the mechanism of 650-nm red light treatment, KEGG and GO analyses were performed using the top 500 DETs in the sleuth results. The top 15 enriched KEGG pathways are shown in Fig. 4A. Among them, the leukocyte transendothelial migration pathway, valine, leucine and isoleucine degradation pathways, the lysosome pathway, metabolic pathways, synthesis and degradation of ketone bodies pathway, and the adherens junction pathway are significant with a p-value <0.001. To further explore it, we verified the increased expression of transendothelial migration pathway related genes (JAM3, CTNND1, RAP1B, ITGB2), metabolism related genes (PFKM, MAT1A, PHX2, GGT5), and adhesion related genes (MAP3K7, CTNNB1, FYN, CSNK2A1) by RT-PCR. These results further confirmed the possible functional route of LLLT (Fig. 4C). GO analysis is composed of three parts: molecular function (MF), biological process (BP), and cellular component (CC). The results of GO analysis shown in Fig. 4B indicate that intracellular and intracellular parts are the most enriched MF pathways, while cell cycle and regulation of androgen receptor signaling are the most enriched BP pathways, and protein binding and ATP binding are the most enriched CC pathways. This work may lay a groundwork for further exploration of the mechanisms of HF stimulation via 650-nm red light treatment.
Fig. 4. Pathway enrichment analysis and gene expression detection. (A) The Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed transcripts (DEGs). (B) Gene ontology analysis of DEGs. (C) The mRNA levels of related genes were detected by real-time PCR in hair follicles treated with or without 650-nm light.
DISCUSSION
AGA is the most common form of hair loss in humans and is characterized by follicular miniaturization and progressive loss of hair from the scalp. HF is a regenerating, hair shaft-producing mini-organ that undergoes cyclical periods of growth (anagen), regression (catagen), and relative quiescence (telogen). In the alopecic follicle, progressive decrease in the duration of anagen and reduction of the anagen to telogen ratio increase the proportion of telogen hairs. Telogen hair are more likely to shed, thus prolonging the lag period before generation of new hair shafts and reducing the number of visible hairs20. Previous studies revealed that ex vivo HF organ culture is an ideal model for research for AGA and other types of alopecia21. Therefore, we used cultured HFs to investigate the effects of 650 nm light therapy on AGA and its associated cellular mechanisms.
Among affected males and females, AGA has caused cosmetic problems leading to psychological distress. Topical minoxidil or finasteride as well as hair transplantation are effective standard and common treatments currently available in the clinic. Many previous studies demonstrated that LLLT has been used for a variety of treatments, such as wound healing, antibiosis, anti-inflammation, and immune modulation. Recently, LLLT has been used as an alternative and adjuvant treatment for patients for whom traditional medical therapy is not adequate or appropriate. LLLT has been approved by the FDA to treat male and female pattern hair loss via home medical devices. There have been a lot of ex vivo, animal, and clinical studies evaluating the effects of LLLT on hair loss12, and it has been proposed that LLLT stimulates the growth of HFs, prolongs the anagen phase, and promotes anagen reentry22. Our present study also revealed that LLLT promotes human hair growth and prolongs the anagen phase in an in vitro HF culture model.
However, the mechanisms responsible for the efficacy of LLLT in the treatment of hair loss are not well understood. Previous studies indicated that 650-nm red light was the most effective and practical wavelength for stimulating hair growth via LLLT treatment. Therefore, we performed RNA-seq of 650-nm light-treated ex vivo HFs in this study. GO analysis of RNA-seq revealed that cell cycle and regulation of androgen receptor signaling are the most enriched BP pathways. Thus, LLLT can promote proliferation, which dependent on cell cycle. In addition, androgen receptor signaling plays an important role in both cell cycle and hair growth of HFs23. Thus, we believe that LLLT can also regulate the cell cycle and androgen receptor signaling to confront AGA. Our data revealed that 650-nm light therapy promoted HF proliferation, suggesting that 650-nm light may promote human hair growth and prolong the anagen phase by regulating the cell cycle. KEGG analysis showed that several main biological pathways, such as the leukocyte transendothelial migration, amino acid degradation, lysosome, and metabolic pathways, were involved. Prior trials of LLLT have shown a decrease in inflammatory prostaglandin E-2 and an increase in inflammatory cytokines, such as IL-6, IL-8, TNF-α24,25,26. Our transcriptional data suggested that LLLT inhibits leukocyte migration and infiltration and may play an anti-inflammatory role in protecting HFs. In particular, the CTNNB1, RAP1B, GNAI1, JAM3, CLDN5, VCAM1, CTNND1, CLDN18, ITGB2 genes are included in the leukocyte transendothelial migration pathway in KEGG. These genes are known to be expressed on the endothelium of blood vessels or leukocytes. The adherens junction is a key regulator of tissue architecture and dynamics via control of cell proliferation, motility, and survival27. During tissue inflammation, the adherens junction is disrupted27. Inflammatory processes, in turn, cannot do without endothelial cell adherens junctions28. Thus, our results may reveal that the two pathways interact with each other in the process of LLLT promoting hair growth. Besides, LLLT has been reported to increase metabolism of cell lines such as HeLa cells in the past29. Our results also found that its effect of promoting hair growth is related to the metabolic pathway and those metabolites can be produced or decomposed from acid degradation and lysosome pathways. Moreover, our real-time PCR results showed that 650 nm light treatment increased Wnt signaling pathway-related genes, such as Wnt10b and β-catenin (Supplementary Fig. 1). These data further indicated that the Wnt pathway, which is important in AGA, also played a role in the effects of LLLT treatment30.
In summary, this study demonstrates that red light of 650 nm promoted human hair growth and inhibited spontaneous catagen transition in ex vivo HFs. Furthermore, transcriptome analysis suggested that 650 nm red light promoted the activation of HFs via regulating multiple signaling pathways, such as the leukocyte transendothelial migration pathway and amino acid degradation, which lays the groundwork for further exploration of red light and associated benefits via LLLT. Ultimately, our results strongly support the benefit of LLLT in the treatment of AGA.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This study was supported by the grants from Shanghai Engineering Technology Research Center of Hair Medicine (19DZ2250500), Science and Technology Committee of Shanghai Municipality Guiding Fund (19411962400), Clinical Research Plan of SHDC (SHDC2020CR2033B) and Innovative research team of high-level local universities in Shanghai.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-33-553-s001.pdf.
The mRNA levels of related genes were detected by Real-time PCR in hair follicles treated with or without 650-nm light.
References
- 1.Severi G, Sinclair R, Hopper JL, English DR, McCredie MR, Boyle P, et al. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol. 2003;149:1207–1213. doi: 10.1111/j.1365-2133.2003.05565.x. [DOI] [PubMed] [Google Scholar]
- 2.Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10:184–189. doi: 10.1111/j.1087-0024.2005.10102.x. [DOI] [PubMed] [Google Scholar]
- 3.Fields JR, Vonu PM, Monir RL, Schoch JJ. Topical ketoconazole for the treatment of androgenetic alopecia: a systematic review. Dermatol Ther. 2020;33:e13202. doi: 10.1111/dth.13202. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Jacobo LA, Ancer-Arellano CI, Villarreal-Villarreal CD, Ortiz-Lopez R, Montufar-Martinez M, Trevino V, et al. Global expression profile and global genome methylation signatures in male patients with androgenetic alopecia. J Eur Acad Dermatol Venereol. 2020;34:e216–e218. doi: 10.1111/jdv.16169. [DOI] [PubMed] [Google Scholar]
- 5.Vasserot AP, Geyfman M, Poloso NJ. Androgenetic alopecia: combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin Ther Targets. 2019;23:755–771. doi: 10.1080/14728222.2019.1659779. [DOI] [PubMed] [Google Scholar]
- 6.Choi BY. Hair-growth potential of ginseng and its major metabolites: a review on its molecular mechanisms. Int J Mol Sci. 2018;19:2703. doi: 10.3390/ijms19092703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonthalia S, Daulatabad D, Tosti A. Hair restoration in androgenetic alopecia: looking beyond minoxidil, finasteride and hair transplantation. J Cosmo Trichol. 2016;2:1000105 [Google Scholar]
- 8.Seetharam KA. Alopecia areata: an update. Indian J Dermatol Venereol Leprol. 2013;79:563–575. doi: 10.4103/0378-6323.116725. [DOI] [PubMed] [Google Scholar]
- 9.Vogt A, Pfannes EKB, Fimmel S, Hadam S, Andruck A, Kottner J, et al. Infundibular protein and RNA microarray analyses from affected and clinically non-affected scalp in male androgenetic alopecia patients. Exp Dermatol. 2017;26:518–521. doi: 10.1111/exd.13326. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Subj. 2017;1861:441–449. doi: 10.1016/j.bbagen.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu KH, Liu D, Chen YT, Chin SY. Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2019;34:1063–1069. doi: 10.1007/s10103-019-02723-6. [DOI] [PubMed] [Google Scholar]
- 12.Panchaprateep R, Pisitkun T, Kalpongnukul N. Quantitative proteomic analysis of dermal papilla from male androgenetic alopecia comparing before and after treatment with low-level laser therapy. Lasers Surg Med. 2019;51:600–608. doi: 10.1002/lsm.23074. [DOI] [PubMed] [Google Scholar]
- 13.Suchonwanit P, Chalermroj N, Khunkhet S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34:1107–1114. doi: 10.1007/s10103-018-02699-9. [DOI] [PubMed] [Google Scholar]
- 14.Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012;27:431–436. doi: 10.1007/s10103-011-0953-7. [DOI] [PubMed] [Google Scholar]
- 15.Sheen YS, Fan SM, Chan CC, Wu YF, Jee SH, Lin SJ. Visible red light enhances physiological anagen entry in vivo and has direct and indirect stimulative effects in vitro. Lasers Surg Med. 2015;47:50–59. doi: 10.1002/lsm.22316. [DOI] [PubMed] [Google Scholar]
- 16.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta AK, Foley KA. A critical assessment of the evidence for low-level laser therapy in the treatment of hair loss. Dermatol Surg. 2017;43:188–197. doi: 10.1097/DSS.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 18.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon OS, Oh JK, Kim MH, Park SH, Pyo HK, Kim KH, et al. Human hair growth ex vivo is correlated with in vivo hair growth: selective categorization of hair follicles for more reliable hair follicle organ culture. Arch Dermatol Res. 2006;297:367–371. doi: 10.1007/s00403-005-0619-z. [DOI] [PubMed] [Google Scholar]
- 20.Hawkshaw NJ, Hardman JA, Alam M, Jimenez F, Paus R. Deciphering the molecular morphology of the human hair cycle: Wnt signalling during the telogen-anagen transformation. Br J Dermatol. 2020;182:1184–1193. doi: 10.1111/bjd.18356. [DOI] [PubMed] [Google Scholar]
- 21.Ohn J, Kim KH, Kwon O. Evaluating hair growth promoting effects of candidate substance: a review of research methods. J Dermatol Sci. 2019;93:144–149. doi: 10.1016/j.jdermsci.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Buscone S, Mardaryev AN, Raafs B, Bikker JW, Sticht C, Gretz N, et al. A new path in defining light parameters for hair growth: discovery and modulation of photoreceptors in human hair follicle. Lasers Surg Med. 2017;49:705–718. doi: 10.1002/lsm.22673. [DOI] [PubMed] [Google Scholar]
- 23.Ceruti JM, Leiros GJ, Balana ME. Androgens and androgen receptor action in skin and hair follicles. Mol Cell Endocrinol. 2018;465:122–133. doi: 10.1016/j.mce.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai Y, Yamaguchi M, Abiko Y. Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci. 2000;108:29–34. doi: 10.1034/j.1600-0722.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- 25.Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen. 2007;15:866–874. doi: 10.1111/j.1524-475X.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim JE, Woo YJ, Sohn KM, Jeong KH, Kang H. Wnt/β-catenin and ERK pathway activation: a possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg Med. 2017;49:940–947. doi: 10.1002/lsm.22736. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol. 2013;303:27–99. doi: 10.1016/B978-0-12-407697-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 28.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymann PGB, Henkenius KSE, Ziebart T, Braun A, Hirthammer K, Halling F, et al. Modulation of tumor cell metabolism by laser photochemotherapy with cisplatin or zoledronic acid in vitro. Anticancer Res. 2018;38:1291–1301. doi: 10.21873/anticanres.12351. [DOI] [PubMed] [Google Scholar]
- 30.Han L, Liu B, Chen X, Chen H, Deng W, Yang C, et al. Activation of Wnt/β-catenin signaling is involved in hair growth-promoting effect of 655-nm red light and LED in in vitro culture model. Lasers Med Sci. 2018;33:637–645. doi: 10.1007/s10103-018-2455-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The mRNA levels of related genes were detected by Real-time PCR in hair follicles treated with or without 650-nm light.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.