Abstract
The hnRNP C1 and C2 proteins are among the most abundant proteins in the nucleus, and as ubiquitous components of RNP complexes, they have been implicated in many aspects of mRNA biogenesis. In this report, we have characterized a null mutation induced in embryonic stem cells by insertion of the U3His gene trap retrovirus into the first intron of the hnRNP C1/C2 gene. cDNAs encoding murine hnRNP C1 and C2 were characterized, and the predicted protein sequences were found to be highly conserved among vertebrates. A human consensus sequence, generated from over 400 expressed sequence tags, suggests two revisions to the previously published human sequence. In addition, alternatively spliced transcripts, expressed only by the murine gene, encode four novel proteins: variants of C1 and C2 with either seven additional amino acids or one fewer amino acid in a region between the oligomerization and C-terminal acidic domains. The disrupted gene was transmitted into the germ line and is tightly linked to a recessive, embryonic lethal phenotype. Homozygous mutant embryos fail to develop beyond the egg cylinder stage and are resorbed by 10.5 days of gestation, a phenotype consistent with a fundamental role in cellular metabolism. However, hnRNP C1 and C2 are not required for cell viability. Embryonic stem cell lines established from homozygous mutant blastocysts did not express detectable levels of either protein yet were able to grow and differentiate in vitro, albeit more slowly than wild-type cells. These results indicate that the C1 and C2 hnRNPs are not required for any essential step in mRNA biogenesis; however, the proteins may influence the rate and/or fidelity of one or more steps.
Primary transcripts synthesized by RNA polymerase II associate with a large number of nuclear RNA binding proteins to form hnRNP complexes (13, 30, 55). These RNA-protein complexes provide the substrates for processing and transport of nuclear pre-mRNA. Purified hnRNP complexes contain over 20 proteins, designated according to size, from A1 (32 kDa) to U (120 kDa) (41). The most abundant protein in the complex, hnRNP C1, is expressed at approximately 90 million molecules per cell, levels comparable to those for core histones (25). A related protein, hnRNP C2, is thought to arise from differential splicing of the same gene and is expressed at approximately one-third the level of hnRNP C1 (6, 35). C1 and C2 proteins form stable heterotetramers that bind cooperatively to RNA (32) and contain both an RNA recognition motif (RRM) and a unique bZIP-like RNA binding domain (31). Although the biochemical functions of hnRNP C are unknown, the protein sequence is highly conserved among vertebrates, suggesting an essential role in RNA metabolism.
Native transcripts isolated from gently disrupted nuclei sediment in sucrose gradients as 30S to 250S dispersed material that appears by electron microscopy as regular repeating arrays (5, 10) or as clusters (50) of 20- to-25-nm particles. Following mild nuclease digestion, this material is converted to morphologically homogeneous, 40S “monoparticles” (30). Purified 40S monoparticles contain heterotetrameric complexes of hnRNP C1 and C2, hnRNP A1 and B2, and hnRNP B1 and A2. These six proteins are the major constituents of the core hnRNP complex and together occupy approximately 700 nucleotides (nt) of RNA (10). Three hnRNP C tetramers together fold RNA into 19S triangular structures that nucleate the assembly of 40S hnRNP complexes in vitro (22).
Assembly and disassembly of the core hnRNP complex have recently been shown to be associated with RNA trafficking and nuclear export. hnRNP C is the only member of the core complex that does not shuttle between the nucleus and cytoplasm, as assessed by heterokaryon trafficking experiments (43). The A and B proteins contain a nuclear export signal (36), whereas the C proteins contain a nuclear retention signal (39). The retention signal appears to override export signals when both are incorporated into the same protein, suggesting that removal of hnRNP C and/or remodeling of the core complex may be a prerequisite for RNA transport from the nucleus. Since hnRNP C is the most tightly bound component of the core (5), such remodeling of riboprotein complexes is likely to be a regulated, energy-dependent process. Furthermore, the protein is preferentially phosphorylated during mitosis, suggesting that aspects of hnRNP C function are regulated during the cell cycle (29, 42).
hnRNP C has been implicated in a variety of processes including splicing, polyadenylation, and RNA turnover, but no clear consensus has emerged concerning its biochemical function. Some reports place hnRNP C within the spliceosome and suggest an active role for the protein in splicing (8, 17, 40), whereas other studies indicate that the hnRNP core proteins, including C, are displaced before the assembly of the initial spliceosome complex (4). The functional significance of interactions between hnRNP C and specific RNA sequences is also unclear. Specific sequences, including those containing runs of uridine and guanine residues, bind with relatively high affinity in a context-specific manner. However, equilibrium-binding studies have failed to uncover any preference for a limited number of sequences involved in RNA processing (18, 49).
Overshadowing any effort to study activities of hnRNP C in vitro is the fact that these highly abundant proteins may have pleiotropic effects on RNA templates that are unrelated to their function. The nuclear concentration of hnRNP C (10 μM) is sufficient to saturate all RNA in the nucleus. The bound tetramers occupy approximately 230 nt of RNA and induce changes in RNA secondary and tertiary structure (22, 44). Consequently, biochemical approaches alone are unlikely to resolve physiologic functions of hnRNP C.
Efforts to understand the function of hnRNP proteins have been hampered by the lack of practical systems for genetic analysis. In principle, the function of hnRNP C, which has been found only in vertebrates, could be addressed by gene targeting experiments followed by the derivation of null cell lines. However, this approach assumes that the protein will not be required for cell viability, an uncertain prospect in light of its abundance, sequence conservation, and presumed role in mRNA biogenesis.
Our laboratory has developed strategies for large-scale insertional mutagenesis in mice. The targeting vectors contain a selectable marker in the U3 region of the long terminal repeat (LTR) of a replication-defective Moloney murine leukemia virus (20). Infection of embryonic stem (ES) cells followed by selection for U3 gene expression generates clones in which the virus has integrated into expressed cellular genes. Genes disrupted by the provirus can be introduced into the germ line to study gene functions in vivo. This report characterizes the 4A4 mutation (53) which results from insertion of the U3His gene trap vector into the first intron of the hnRNP C1/C2 gene. Embryos homozygous for the mutation fail to develop beyond the egg cylinder stage and are resorbed between 6.5 to 10.5 days of gestation. However, cell lines derived from preimplantation mutant embryos are viable and are able to differentiate in vitro. This is the first report of a null mutation in a member of the hnRNP gene family and suggests that genetic approaches will be useful to understand the functions of even highly abundant proteins involved in mRNA biogenesis.
MATERIALS AND METHODS
Nucleic acid isolation and genotyping of embryos.
Mouse DNA was isolated from tail biopsies, cultured cells, or mouse embryos as described previously (26). Total cellular RNA was isolated from tissue culture cells or from mouse tissues by guanidinium isothiocyanate lysis followed by phenol-chloroform extraction (9). For Northern blot analysis, 20 μg of RNA was denatured in 1.1 M glyoxal, 50% dimethyl sulfoxide, and 10 mM sodium phosphate (pH 7.0) at 50°C for 1 h. Samples were separated on a 1.2% agarose gel and blotted onto Hybond N+ membrane (Amersham). Hybridization to [32P]dCTP-labeled probes (15) for both Southern and Northern analysis was carried out in 10% dextran sulfate–50% formamide–5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–1× Denhardt's solution–20 μg of salmon sperm DNA per ml.
Embryos older than E9.0 (embryonic day 9.0) or between E6.5 and E8.5 were genotyped by Southern analysis or nested PCR, respectively. PCRs (25 μl, containing 10 mM Tris-HCl [pH 8.3], 5 mM KCl, 1.5 mM MgCl2, 200 μM each deoxyribonucleoside triphosphate, each primer at 2 μM, and 2.5 U of Amplitaq [Perkin-Elmer/Cetus]) involved 30 cycles of denaturation (95°C for 45 s), primer annealing (56°C for 45 s), and primer extension (72°C for 1.5 min). The first reaction used a primer upstream of the viral integration site (Up1, 5′-AATTGTGAGGAGCGGTAGC-3′) paired with two oligonucleotides, one corresponding to genomic sequence downstream of the integration site (Dn1, 5′-CCACCCAGCGTCTATCTTCGG-3′) and the other corresponding to the viral His gene (HisB, 5′-CGCTGTATTCACGCAGGGCATCG-3′); 0.5 μl of the first PCR mixture was then used in a second PCR using a set of nested primers (Up2, 5′-ACGGTCGTGTGGTTCTTCGCTG-3′; Dn2, 5′-CGAATCCGTAAGACATCCC-3′; and HisA, 5′-AAATCGCCGGACGCGTCAGCA-3′). Wild-type embryos were identified by the presence of a single 421-nt product derived from the primer pair Up2-Dn2. Heterozygous mice were identified by the presence of both the 421-nt product and a 597-nt product from the Up2-HisB primer pair. Homozygous embryos produced only the 597-nt product, as the genomic sequence amplified by the two genomic primers (Up2-Dn2) was disrupted by the intervening viral sequence.
Isolation of genomic and cDNA clones encoding the hnRNP C1/C2 proteins.
DNA sequences (65 nt) adjacent to the 4A4 provirus were isolated by inverse PCR (iPCR) (53). This fragment was used to isolate a genomic clone containing an 11-kb insert. A SacII-HindIII fragment spanning the site of virus integration was then used to screen a random-primed ES cell cDNA library. We identified 57 positive plaques from a total of approximately 106. Two cDNA inserts were characterized and found to terminate approximately 100 bp prior to the published stop codon of the human gene. Therefore, a 970-bp AccI-PvuII cDNA fragment was isolated from one of the cDNA clones and used to screen an 8.5-day mouse embryonic cDNA λgt10 library. We identified 138 positive plaques out of a total of approximately 2.5 × 105, suggesting that hnRNP C transcripts constitute approximately 0.05% of the total mRNA of the 8.5-day embryo. Five positive plaques were purified, and all were found to contain similar 1.2-kb inserts. An insert from a single clone was isolated and found to terminate with a poly(A) tail. Together, the three overlapping cDNA clones constituted the entire coding sequence of the mouse hnRNP C1 and C2 proteins.
Isolation of ES cells.
ES cells lines were isolated essentially according to Evans and Kaufman (14), as detailed by Robertson (46). Briefly, the 4A4 mutation, which had been maintained on a C57BL/6 background, was outbred onto an 129/Sv background for three generations. Heterozygous mice were mated. Visual inspection for a vaginal plug was used to time pregnancies as day 0.5. Female mice were sacrificed on day 3.5, and the uterine horns were dissected and flushed with ES cell medium (high-glucose Dulbecco modified Eagle medium [Gibco] supplemented with 15% preselected fetal bovine serum [heat inactivated at 55°C for 30 min; HyClone], 0.1 mM 2-mercaptoethanol, and 100 mM nonessential amino acids [Gibco]). All blastocysts appeared normal, suggesting that the disruption of the hnRNP C gene has no effect on preimplantation development. Individual blastocysts were isolated and transferred to single wells of a 24-well plate containing an established feeder cell layer of gamma-irradiated primary mouse embryo fibroblasts (MEFs) with culture-conditioned ES cell medium. Blastocysts were left undisturbed for 4 days of tissue culture. During this time, the inner cell mass (ICM) begins to outgrow from the trophoblastic cells. The growth of the ICM was closely monitored between 4 and 6 days of culture and, when it reached sufficient size (46), was dissected away from the accompanying trophoblastic cells by using a blunted, drawn-out Pasteur pipette. The ICM was transferred to a culture dish containing phosphate-buffered saline (PBS) and then transferred to a 96-well plate containing 0.25% (wt/vol) trypsin and 0.04% (wt/vol) EDTA in PBS. The ICM was triturated using a 100-μl pipette tip. The resulting small clumps of cells were then seeded back into a single well of a 24-well plate containing a previously established MEF feeder layer. Subsequently, each well was trypsinized every 3 days and replated onto a fresh feeder layer. Wells in which ES cells arose were expanded to subconfluence on a 10-cm-diameter tissue culture plate. Aliquots of cells were frozen (50% Dulbecco modified Eagle medium [DMEM]–38% fetal bovine serum–12% dimethyl sulfoxide), and the remainder were expanded for further characterization.
Characterization of alternative 3′ splice sites.
Primers (5′-CTTCTATCGCCTTCTTGACG and 5′-ACACAGATAAGTTGCTGGCC) complementary to the murine hnRNP C1/C2 cDNA sequence were used to amplify intervening intron sequences by PCR (see above) involving 35 cycles of denaturation (95°C for 45 s), primer annealing (55°C for 45 s), and primer extension (72°C for 1.5 min). Two products of 200 and 320 nt were generated. The 200-nt fragment is of the size expected if there were no intervening intron sequences and was probably amplified from one or more hnRNP C pseudogenes. The 320-nt fragment, containing an intron and alternative 3′ splice sites, was cloned into a TA cloning vector (Invitrogen) and sequenced.
Cell culture and protein purification.
Cells were maintained on 0.1% gelatinized tissue culture plates in ES cell medium (see above) supplemented with leukemia inhibitory factor (1,000 U/ml; ESGRO; Gibco). Cultures were trypsinized every 2 days and replated at a 1:3 ratio with either a feeder layer of gamma-irradiated MEFs for maintenance of the cell line or for at least three generations without feeder layers for isolation of DNA, RNA, and cell extracts. For whole cell extracts, cells were harvested, washed twice with cold PBS, pelleted, and resuspended in EBC buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 0.5% NP-40 [22]; 0.5 ml/10-cm-diameter plate) containing the protease inhibitors antipain, leupeptin, pepstatin A, and chymostatin at 50 μg/ml each and 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF; Calbiochem) at 0.2% (wt/vol). Following 30 min of constant agitation at 4°C, samples were clarified by centrifugation.
Nuclear extracts (NE) were prepared as previously described (41), with slight modifications. Adherent cells were washed twice with PBS and lysed with buffer A (10 mM Tris [pH 7.4], 100 mM NaCl, 2.5 mM MgCl2; 1 ml/10-cm-diameter plate) containing 0.5% Triton X-100, aprotinin (4 μg/ml), leupeptin (1 μg/ml), soybean trypsin inhibitor (5 μg/ml), and pepstatin A (4 μg/ml). Nuclei were pelleted, resuspended in 1/10 volume of buffer A without Triton X-100 containing the same protease inhibitors as above, and sonicated on ice with three 15-s bursts, using a microtip sonicator (model XL2015; Heat Systems) at a setting of 3.5. The sonicate was centrifuged at 14,000 rpm for 15 min, and the supernatant was flash frozen in liquid N2.
A single-step chromatography purification protocol was developed based on a previous procedure (3). A 4.5-mg aliquot of either wild-type ES cell or mutant cell NE was loaded onto individual 1-ml HiTrap Q (Amersham Pharmacia Biotech) anion-exchange columns equilibrated with buffer A. The columns were washed with 5 column volumes (CV) of buffer A and then developed with a step gradient of increasing NaCl concentration (0.25, 0.5, and 1.0 M with 5 CV per step). A constant flow rate of 0.5 ml/min was maintained throughout, and protein elution was monitored at 214 nm. One-milliliter fractions were collected, aliquots were taken for protein concentration determination, and the remaining samples were precipitated with 3 volumes of 100% ethanol. Pellets were resuspended in Laemmli loading buffer and analyzed by Western blotting.
Western blotting analysis.
Protein concentrations were determined by the Bradford assay. Samples were adjusted to 1× Laemmli loading buffer (47), boiled for 10 min, and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8.75% gel. Proteins were transferred onto PolyScreen polyvinylidene difluoride membrane (NEN Life Sciences) in Tris-glycine buffer (47). Polyclonal serum from chickens (Ab-1041; Aves Laboratories) immunized against a C-terminal peptide (Val 140-Gly 290) of human hnRNP C1 was diluted 1:2,000 in BLOTTO A (5% milk and 0.05% Tween 20 in Tris-buffered saline [TBS; 10 mM Tris-HCl, 150 mM NaCl]) and bound to proteins overnight at 4°C. Membranes were washed three times for 5 min each in TBS–0.05% Tween 20, incubated with a 1:5,000 dilution of peroxidase-conjugated anti-chicken Fc fragment (Jackson ImmunoResearch) in BLOTTO A, and detected by SuperSignal ECL (enhanced chemiluminescence) (Pierce).
Nucleotide sequence accession number.
The sequences shown in Fig. 1C, 2, and 3 have been assigned GenBank accession no. AF095256, AF095257, and AF095258, respectively.
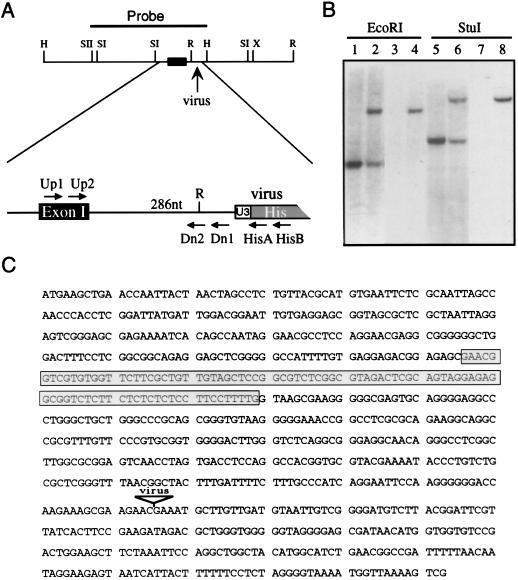
FIG. 1.
The 4A4 provirus inserts into an intron of the hnRNP C1/C2 gene. (A) Restriction map of a 3.7-kb genomic fragment encompassing the site of viral integration. A SacII-HindIII fragment (solid line) used to probe an ES cell cDNA library and the location of the first hnRNP C1/C2 exon (black box) are indicated. The virus integration site is enlarged, showing the distance (286 nt) to the first exon and the locations of primers used for genotyping embryos (Up1, Up2, Dn1, Dn2, HisA, and HisB). H, HindIII; R, EcoRI; SI, SacI; SII, SacII; X, XbaI. (B) Southern blot analysis of wild-type D3 cells (lanes 1, 3, 5, and 7) and mutant cells heterozygous for the 4A4 provirus (lanes 2, 4, 6, and 8). A flanking sequence probe (lanes 1, 2, 5, and 6) detects a single-copy sequence in wild-type DNA as well as an additional fragment corresponding to the virus-occupied allele in 4A4 cells. A virus-specific probe (lanes 3, 4, 7, and 8) detects only the occupied allele. (C) Sequence surrounding the site of viral integration in 4A4 cells showing the first exon of the mouse hnRNP C1/C2 gene (boxed) and the flanking sequence isolated by iPCR.
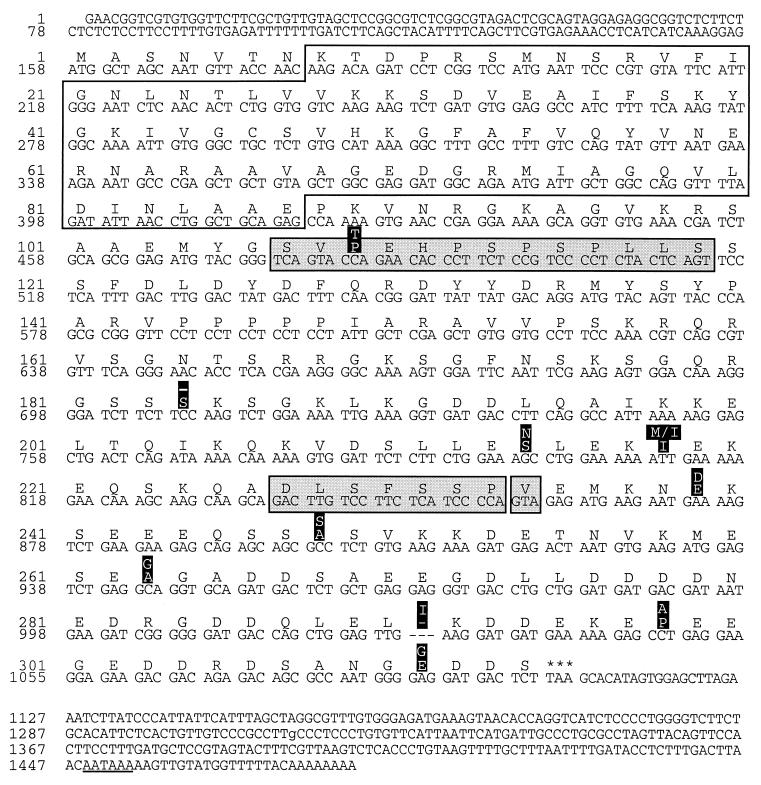
FIG. 2.
cDNA sequences of the mouse hnRNP C1/C2 gene. The consensus sequence of a transcript encoding the largest form of murine hnRNP C2 is shown. The alternatively spliced 39-nt exon missing in C1 transcripts is shown (residues 107 to 119, first shaded box). Additional murine sequences that arise by alternative splicing, encoding seven residues (227 to 233) or one residue (234), are also indicated (second and third shaded boxes, respectively). Single amino acid differences between the murine and human hnRNP C proteins are highlighted (white on black), with the human sequence shown above. The RRM is enclosed (residues 8 to 87).
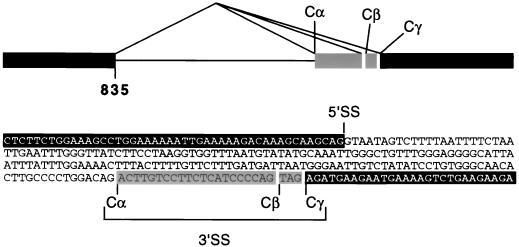
FIG. 3.
Species-specific splicing of murine hnRNP C. A region of genomic DNA containing an intron and flanking exons (white on black and shaded) was amplified by PCR and sequenced as shown. Comparisons of genomic and cDNA sequences reveal that murine hnRNP C transcripts splice from a single 5′ splice site (5′SS) located at nt 835 of the composite cDNA (Fig. 2) to one of three alternative 3′ splice sites (Cα, Cβ, and Cγ). For comparison, human hnRNP C transcripts use the Cβ 3′ splice site exclusively.
RESULTS
The 4A4 provirus integrates into the hnRNP C gene.
The 4A4 cell line was generated by infecting D3-ES cells with the U3His gene trap retrovirus and selecting for l-histidinol-resistant clones. 4A4 cells contain a single intact provirus as assessed by Southern blot hybridization, and expression of the proviral His gene occurs via transcripts that initiate in the flanking cellular DNA (53). To identify the gene disrupted in the 4A4 cell line, a 65-nt sequence, extending to an MseI site immediately upstream of the provirus, was isolated by iPCR (54). When used as a probe in Southern blot analyses, the iPCR fragment hybridized to single-copy cellular DNA and could therefore distinguish the wild-type genomic sequence from the locus occupied by the provirus (Fig. 1B). For example, a unique fragment corresponding to the wild-type allele is detected in StuI digested DNA from the parental D3 cell line (Fig. 1B, lane 5). An additional fragment is detected in DNA from the 4A4 clone (Fig. 1B, lane 6). The more slowly migrating fragment also hybridized to a virus-specific probe (Fig. 1B, lanes 7 and 8) and corresponded to the expected size of the intact 6.8-kb U3His provirus. These data confirm that the 4A4 mutant cell line contains a single provirus and suggest that no rearrangements occurred within the virus or flanking cellular DNA as a result of virus integration.
To characterize the gene disrupted in 4A4 cells, we tested the 65-nt iPCR fragment for hybridization to transcripts expressed in normal cells but were unsuccessful. Therefore, the iPCR product was used to isolate a larger region of genomic DNA from a λDASH I D3-ES cell library. A single clone was isolated, mapped, and sequenced on either side of the integration site (Fig. 1C). The genomic clone provided several fragments for use as probes in Northern blot experiments (data not shown). A 950-nt SacII-HindIII fragment (Fig. 1A), spanning the provirus integration site, hybridized to a prominent 1.4-kb transcript and was used to screen two separate ES cell cDNA libraries (see Materials and Methods). Three independent clones were isolated and sequenced.
The 5′ ends of the cDNA sequences were identical to a region in the flanking genomic DNA. The match extended for 94 nt and ended at a consensus 5′ splice site located 286 nt upstream of the virus integration site (Fig. 1C, shaded box). The cDNA sequences were also compared against the GenBank database by using the Gapped BLAST program (1). All three cDNAs were orthologous to the human hnRNP C1/C2 gene (7). Moreover, patterns of alternative splicing that generate the human C1 and C2 proteins were also observed, with the C1 and C2 transcripts represented by one and two cDNAs, respectively. Together, these results indicate that the 4A4 provirus inserted into an intron of the murine hnRNP C1/C2 gene. The original 65-nt iPCR sequence is entirely contained within the intron, accounting for the failure of this probe to hybridize to cellular transcripts.
Murine hnRNP C1 and C2 cDNA sequences.
The three cDNAs together contained the entire coding sequence for both the C1 and C2 proteins and terminated at a consensus polyadenylation site. Figure 2 shows the composite sequence of the cDNA encoding mouse hnRNP C2. The 39-nt region missing from hnRNP C1 is enclosed in a shaded box. Differences between the murine and previously reported human hnRNP C2 cDNA sequences include 10 single-codon changes and an additional 21 nucleotides that add seven amino acids to the murine sequence (amino acids 227 to 233). Furthermore, the presence of two additional nucleotides adds three amino acids to the C terminus of the murine sequence. Overall, the colinear portions of human and murine proteins are 97% identical. Except for a single amino acid in C2, the mouse and human proteins are identical over the N-terminal portion containing the RRM, highlighting the potential functional importance of this domain.
The murine and human cDNA sequences were compared to over 140 murine and 400 human hnRNP C expressed sequence tags (ESTs) obtained from the NCBI dbEST database. The colinear sequence derived from the mouse ESTs agreed with the sequence reported in Fig. 2. Moreover, none of the murine ESTs extended the 5′-terminal sequence, suggesting that the hnRNP C1/C2 gene contains a single 5′ noncoding exon and that the 4A4 provirus inserted into the first intron of the gene. However, the human ESTs differed from the previously published HeLa cDNA sequence (52) in two respects. First, a methionine at position 218 is replaced by isoleucine in all of the relevant ESTs (Fig. 2). Second, all ESTs spanning the end of the coding region contain two additional nucleotides, creating a frame shift that adds three amino acids to the carboxyl terminus of the human protein. In both cases, the sequence of the murine gene agrees with the human EST consensus.
Species-specific splicing of hnRNP C transcripts.
The most striking difference between the murine and human cDNA sequences was the 21-nt insert present in all three murine cDNAs. To determine whether the 21-nt sequence is derived from an alternatively spliced exon, a region of the murine gene was amplified by PCR and sequenced. In designing PCR primers, we postulated that cDNA sequences on either side of the 21-nt insert could be used to amplify an intervening intron. This assumption proved correct. As shown in Fig. 3, the extra murine sequence results from the use of an alternative 3′ splice site, designated Cα, located 21 nt upstream from the human splice site (Cβ). However, a sequence resembling a 3′ splice site was also observed at the murine Cβ site. Therefore, it was of interest to determine if any murine transcripts utilized the Cβ site and if any human transcripts utilized the Cα upstream site. For this, we examined all 91 ESTs (43 human and 48 mouse) from dbESTs containing this region of hnRNP C. The murine ESTs fell into three groups, indicative of transcripts that splice from a single 5′ splice site to one of three alternative 3′ splice sites (Fig. 3). Twenty-seven (56%) were derived from transcripts splicing to the upstream site (Cα), as observed in our original murine cDNAs; 13 (27%) involved transcripts using the same 3′ splice site (Cβ) as the published human sequence; 8 (17%) spliced to an AG (Cγ) three nucleotides downstream from the human site (Fig. 3). In contrast, all of the human ESTs utilized the Cβ site. The murine and human ESTs were independently cloned from a variety of tissues; therefore, the mouse gene appears to be alternatively spliced in a species-specific manner and could encode six proteins: variants of C1 and C2 utilizing each of the three splice acceptor sites (C1α, C1β, C1γ, C2α, C2β, and C2γ).
hnRNP C is essential for postimplantation mouse development.
To characterize phenotypes associated with viral disruption of the C1/C2 gene, the 4A4 ES cell line was injected into C57BL/6 blastocysts (53). The resulting chimeras transmitted the 4A4 provirus to their offspring. Mice heterozygous for the provirus were normal in all respects. However, mice homozygous for the disrupted hnRNP C gene were not detected in over 220 offspring produced from heterozygote crosses (data not shown). This analysis included progeny obtained after nine backcrosses into a C57BL/6 background; therefore, transmission of the 4A4 provirus appears to be tightly linked to a recessive embryonic lethal mutation.
To more precisely determine the time and cause of embryonic death, intercrossed females were sacrificed at various days postcoitum (dpc). Individual embryos were dissected from the deciduum and genotyped by Southern analysis (9.5 and 10.5 dpc) or by PCR (6.5 to 8.5 dpc). As shown in Table 1, the proportion of wild-type to heterozygous mutant embryos was approximately 1:2 at each stage between E6.5 and E10.5. The yield of embryos homozygous for the mutation was less than expected, presumably because they were being resorbed and could not be accurately genotyped. Except for one necrotic embryo, none of the embryos at E10.5 was homozygous for the mutation, and approximately one-fourth of all embryos (20 of 71; 27%) had undergone resorption. Greater numbers of homozygous mutant embryos were observed at earlier stages, and the proportion of resorbed embryos was lower. However, even at E6.5, a significant number of embryos had already been resorbed.
TABLE 1.
Genotypic and phenotypic analysis of embryos from hnRNP C heterozygous intercrossesa
| Stage | Phenotype
|
Genotype
|
||||
|---|---|---|---|---|---|---|
| Normal | Abnormal/ resorbed | Total | +/+ | +/− | −/− | |
| E10.5 | 51 | 1/19 | 71 | 16 | 35 | 1 |
| E8.5 | 30 | 5/3 | 38 | 8 | 22 | 5 |
| E7.5 | 60 | 13/10 | 83 | 20 | 40 | 13 |
| E6.5 | 23 | 4/5 | 32 | 7 | 16 | 4 |
Intercrossed females were sacrificed on days 6.5, 7.5, 8.5, and 10.5 of pregnancy, and embryos were dissected from the maternal deciduum. Embryos were genotyped by Southern blot analysis (E8.5 to E10.5) or by PCR amplification (E6.5 to E7.5) using nested primers as shown in Fig. 4A to C.
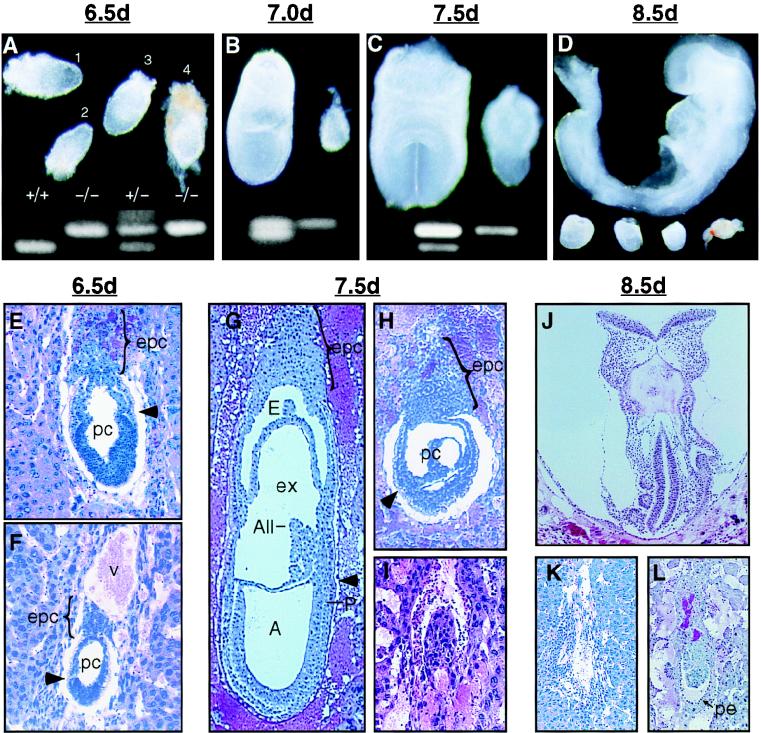
As shown in Fig. 4, embryos homozygous for the hnRNP C mutation were easily distinguished from wild-type and heterozygous siblings by E7.0. At E6.5, surviving mutant embryos appeared similar to but slightly smaller than wild-type and heterozygous littermates (Fig. 4A, embryos 2 and 4). The process of gastrulation begins at about 6.5 and transforms the bilaminar egg cylinder into a multilayered embryo as cells migrate through the primitive streak to form an intermediate mesoderm layer. However, none of over 130 mutant embryos displayed morphological features of early gastrulation (Fig. 4B to D). Histological analysis (Fig. 4E to L) confirmed that mutant embryos progress to the egg cylinder stage (Fig. 4F) but failed to display any specific morphological defect that would account for embryonic lethality. Regardless of gestational age, mutant embryos appeared to arrest at the egg cylinder stage and were resorbed at various times thereafter (Fig. 4H, I, K, and L).
FIG. 4.
Phenotype of hnRNP C mutant embryos. (A to D) Whole mount preparations showing failure of hnRNP C null embryos, identified by a single 597-nt PCR product (upper band), to develop beyond E6.5. (A) At E6.5 mutant embryos not yet resorbed were slightly smaller than normal littermates (the rightmost is still surrounded by Reichert's membrane). (B to D) From E7.0 to E8.5, the size difference of the mutant embryos (right and bottom) is clearly appreciated. While normal embryos undergo a rapid cellular proliferation and morphological transformation, surviving mutant embryos appear to grow only slightly, and by E8.5, many were necrotic (rightmost) and difficult to separate from maternal tissue. (E, G, and J) Sagittal sections of normal embryos at egg cylinder (E), late streak (G), and early somite (J) stages. (F) Presumed mutant embryo at the egg cylinder stage. The embryo is smaller than normal littermates, the demarcation between embryonic and extraembryonic regions (arrowhead) is not clear, and the ectoplacental cone is underdeveloped. (H) Mutant embryos at E7.5 failed to undergo gastrulation and appear similar to normal E6.5 embryos. (I) A more severely affected embryo undergoing resorption. This embryo appeared to be misoriented with regard to the mesometrial axis (all embryos are oriented with the mesometrium toward the top of the page). (K) By E8.5, the resorption process for most mutant embryos was nearly complete. (L) A surviving mutant embryo at E8.5 showing that parietal endoderm cells (arrow) had formed although no other structures were apparent. A, amniotic cavity; All, allantois; E, ectoplacental cavity; epc, ectoplacental cone; ex, exocoelom; P, primitive streak; pc, proamniotic cavity; pe, parietal endoderm; v, blood vessel.
Derivation of ES cells from mutant blastocysts.
One explanation for the phenotype of the mutant embryos is that disruption of hnRNP C leads to a generalized cell lethal defect but that mutant embryos persist until implantation due to maternally derived stores of mRNA or protein. Although the majority of maternal RNA is rapidly degraded following the onset of de novo transcription at the first cell division (2, 16, 23), it has been suggested that abundant transcripts, such as hnRNP C, could persist for several days. Therefore, to determine whether hnRNP C is required for cell viability, we attempted to derive a homozygous mutant ES cell line from preimplantation blastocysts (14, 21). Previous observations from our laboratory and others (24) suggest that derivation of ES cell lines is more consistent when mice are bred onto the 129/Sv background. Therefore, mice carrying the 4A4 mutation (C57BL/6 background) were first bred with 129/Sv mice for three generations. F3 heterozygous mice were mated, and blastocysts were collected at 3.5 dpc and cultured as described in Materials and Methods. Out of 21 blastocysts isolated from four females, eight individual cell lines were established. As shown in Fig. 5A, two cell lines were homozygous for the hnRNP C mutation. Both mutant cell lines appeared morphologically similar to other ES cell lines (see Fig. 7A and F) and grew with doubling times of approximately 24 h (data not shown).
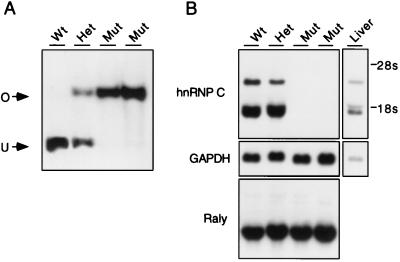
FIG. 5.
hnRNP C is not required for cell viability. (A) ES cell lines were derived from 3.5 day blastocysts and genotyped by Southern analysis. Lane 1, wild-type (wt) cells containing a single EcoRI-restricted fragment (U); lane 2, heterozygous (Het) cell line containing one copy of the unoccupied allele and one copy of the virus-occupied (O) allele; lanes 3 and 4, two mutant (Mut) homozygous cell lines containing two copies of the virus-occupied allele. (B) Cell lines shown in panel A were grown without feeder cells for three generations and analyzed by Northern blotting. The murine hnRNP C cDNA detects three transcripts (1.4, 1.9, and ∼3 kb, presumably generated by alternative 3′ polyadenylation) in wild-type and heterozygous cell lines. Expression of all three transcripts is ablated in the two mutant cell lines, indicating that hnRNP C is not essential for cell growth. When the same blot was probed with a Raly cDNA, no compensatory increase in the transcription of this related gene was observed. A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was used to ensure equal loading of RNA for each sample, and mouse liver RNA was included as a control.
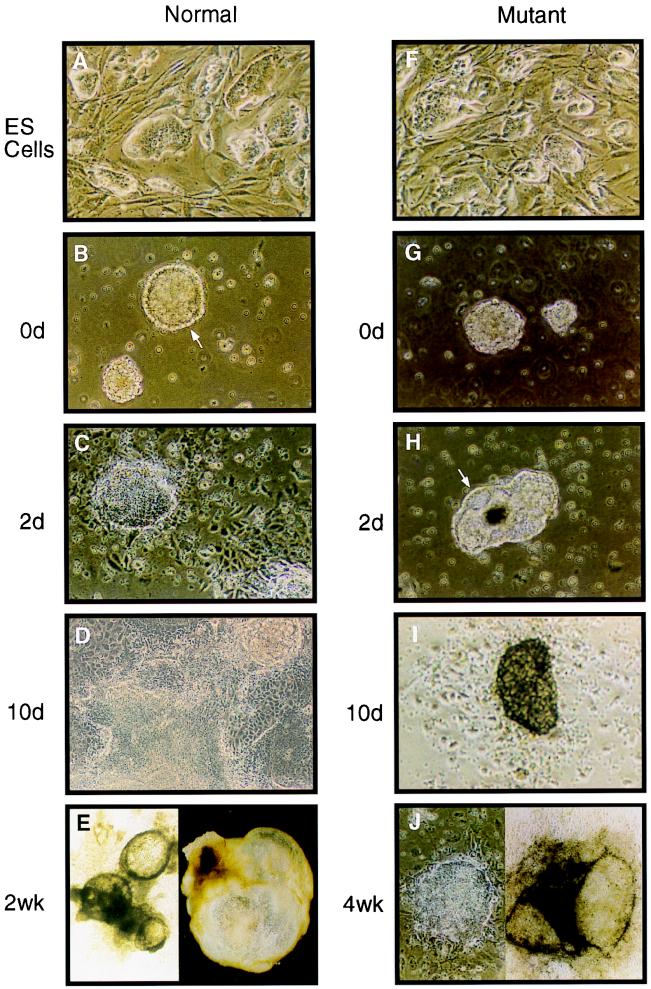
FIG. 7.
hnRNP C null ES cells differentiate in vitro. The hnRNP C null cells (F) grew in tightly packed “nests” when cultured on MEF feeder layers, indistinguishable from wild-type ES cells (A). Both mutant and wild-type ES cells were grown in suspension culture for 4 days to induce simple EB formation and then replated onto culture dishes (counted as day 0) (0d) to determine the differentiation capacity of the two cell lines. (B) Wild-type ES cells gave rise to a large number of simple EBs, many of which had already formed an outer ring of large endoderm cells with an underlying Reichert's membrane (arrow). (G) By contrast, mutant ES cells gave rise to only about 10% of the number of EBs that wild-type cells did. Of those EBs that did form, none exhibited well-differentiated endoderm cells. (C and H) Wild-type EBs quickly attached to the tissue culture plate and by 2 days had elaborated a large outgrowth of endoderm cells. By 2 days in culture, the mutant EBs were just beginning to form a ring of endoderm cells and none had attached to the tissue culture plate. (D and I) By 10 days of tissue culture, wild-type EBs had undergone extensive cellular differentiation whereas the mutant EBs that survived to this point were just beginning to attach to the culture plate. (E) In addition to the wild-type colonies that grew in flattened sheets of differentiated cells, several colonies formed large cystic EBs (right). Many of these cystic EBs coalesced to form interconnected fluid filled chambers with an underlying monolayer of differentiated cells (left). (J) Very few of the mutant EBs survived longer than 2 weeks. However, those EBs that did survive eventually (4 weeks or longer) underwent morphological differentiation similar to that of wild-type EBs, including both well-differentiated monolayers (left) and large cystic EBs. Among the cell types observed for both wild-type and mutant EBs were rhythmically beating myocardial cells.
hnRNP C is not required for cell viability.
U3 gene trap vectors were designed to disrupt cellular gene expression by ablating transcription downstream from the two poly(A) sites (one in each LTR) carried by the provirus. Previous studies found high levels of a 2.2-kb His transcripts in 4A4 cells (53), approximately the size expected for hnRNP C-His fusion transcripts that terminate in the 5′ LTR. To test whether the 4A4 provirus indeed disrupts expression of the hnRNP C gene, homozygous mutant cell lines were analyzed by Northern blot analysis (Fig. 5B) using an hnRNP C cDNA probe containing sequences downstream of the virus integration site. Transcripts of 1.4, 1.9, and 3.0 kb were detected in the wild-type and heterozygous cell lines but not in the two mutant cell lines, indicating that expression is disrupted in the mutant cells. The closest known relative of hnRNP C is Raly, a gene discovered by its linkage to the lethal yellow (Ay) mutation (37). Given the similarity of these two proteins, we measured the levels of Raly transcripts to see if there was compensatory activation of this message in the mutant cells. As shown in Fig. 5B, no increase in Raly transcripts was observed in the mutant cell lines.
hnRNP C protein expression was assessed by Western blot analysis of whole cell extracts, NE, and NE partially purified by anion-exchange chromatography. hnRNP C could be detected in wild-type cell extracts containing as little as 5 μg of total protein. Heterozygous ES cells appear to contain similar levels of protein, consistent with Northern blot data (see above). No hnRNP C was detected in homozygous cells even when larger amounts (up to 300 μg) of cell protein were analyzed. However, a number of cross-reacting background bands migrating in the 40- to 45-kDa range were observed in the overloaded samples.
Since hnRNP C is specifically retained within the nucleus, preparations of NE are expected to be enriched for the protein. As seen in Fig. 6B, hnRNP C was clearly detected in samples of wild-type NE containing as little as 250 ng of total protein, and a faint signal was seen with 125 ng upon overexposure (data not shown). Again, no protein was detected in NE from mutant cells even when larger samples were analyzed. Some nonspecific background was observed when higher levels of mutant NE were examined. This background included two proteins, approximately 40 and 45 kDa in size, that migrated slightly faster and slower than hnRNP C1/C2, respectively.
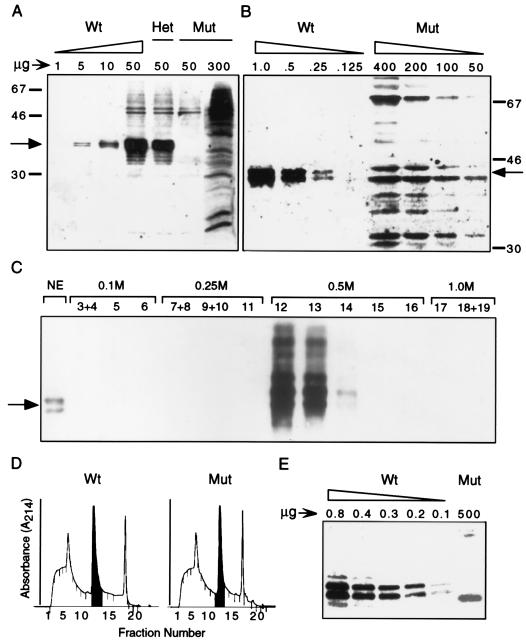
FIG. 6.
hnRNP C protein expression is not detectable in homozygous cells. Total cellular lysates (A) and NE (B) were prepared from wild-type (Wt), heterozygous (Het), and mutant (Mut) cells. The indicated amounts of protein were resolved by SDS-PAGE and analyzed by Western blotting using an anti-hnRNP C antibody. The positions of the hnRNP C doublet (C1 and C2, arrow) and protein molecular size standards (in kilodaltons) are indicated. (C) A wild-type ES cell NE was partially purified on a HiTrap Q anion-exchange column developed with a step gradient of 0.25, 0.5, and 1.0 M NaCl. Ten-microliter aliquots of the indicated 1-ml fractions, as well as 1 μg of input NE, were analyzed by Western blotting. (D) Absorbance of fractionated wild-type and mutant NE developed on Mono Q anion-exchange columns. The first two 0.5 M 1-ml fractions (shaded), containing the entire hnRNP C protein fraction, were pooled and analyzed by Western blotting (E). As much as 5,000-fold more partially purified mutant extract than wild-type extract was loaded.
To further reduce interference by cross-reacting proteins, hnRNP C was partially purified from NE by anion-exchange chromatography. Briefly, 4.5 mg of ES cell NE was loaded onto a HighTrap Q anion-exchange column and developed with a step gradient of 0.25 to 1.0 M NaCl. The relative amounts of hnRNP C in 1.0 μg of the input sample and 1.0% of each column fraction were assessed by Western blot analysis (Fig. 6C). No detectable hnRNP C was found in either the flowthrough, wash, or 0.25 M fraction, whereas nearly all of the bound hnRNP C eluted within the first 2 CV of the 0.5 M NaCl wash.
Extracts from mutant cells were fractionated on a column identical to that shown in Fig. 6D. Wild-type and mutant NE samples fractionated nearly identically, as assessed by absorbance at 214 nm. The first two 0.5 M NaCl fractions were pooled (Fig. 6D), protein concentrations were determined, and an aliquot of each sample was analyzed by Western blotting. As shown in 6E, hnRNP C1/C2 was easily detected in as little as 100 ng of the 0.5 M fraction from wild-type cells. In contrast, hnRNP C was not detected in the corresponding fraction from mutant cells, even when 5,000-fold more protein was analyzed. While chemiluminescent detection is not quantitative and cannot be used to estimate enrichment, the fractionation protocol removed all of the spuriously reacting proteins except for one 39-kDa polypeptide. As a result, hnRNP C was not detected in mutant cells under conditions that would permit detection even if the protein was expressed at 0.02% of wild-type levels. Similarly, hnRNP C transcripts were not detected in mutant cells as assessed by reverse transcriptase PCR under conditions capable of detecting transcripts expressed at levels 400 times lower than in wild-type cells (data not shown). We conclude that the 4A4 provirus induces a null mutation and that hnRNPs C1 and C2 are not required for cell viability.
Cells lacking hnRNP C differentiate in vitro.
The fact that mutant embryos arrest in development prior to gastrulation raises the possibility that hnRNP C may directly influence the differentiation potential of the developing embryo. ES cells have the ability to differentiate in vitro into a wide range of cellular phenotypes. Therefore, mutant ES cell lines lacking hnRNP C provide a model with which to test whether this protein is required for cellular differentiation. Figure 7 shows the phenotypes of both wild-type ES cells and homozygous mutant cells at various times of in vitro differentiation. After 4 days in suspension culture, both wild-type and mutant cells aggregate to form simple embryoid bodies (EBs) (Fig. 7B and G). Whereas EBs derived from wild-type ES cells had already formed a distinct outer layer of endoderm cells (Fig. 7B), formation of a similar endoderm layer in mutant EBs was delayed approximately 2 days (Fig. 7H). After replating, wild-type EBs quickly attached to the culture dish and within 2 days developed large trophoblast-like outgrowths (Fig. 7C). By 10 days in culture, wild-type cells differentiated into several morphologically distinct cell types, including blood islands and beating cardiac tissue (Fig. 7D and E). EBs derived from the mutant cells were slow to form trophoblastic outgrowths (Fig. 7I), and only about 10% survived longer than 1 week. The mutant EBs that did survive were able to differentiate in a manner morphologically similar to that of wild-type cells (Fig. 7J). However, the differentiation process took nearly 3 to 4 weeks for the mutant cells, compared to 1 to 2 weeks for wild-type cells.
DISCUSSION
Mutagenesis of murine ES cells by gene entrapment provides an effective means to analyze gene functions in mice. The mutagens permit direct selection of clones in which cellular genes have been disrupted and simplify the characterization of genes associated with recessive mutations. The present study characterized a null mutation in the murine hnRNP C gene induced by the U3His gene trap vector. The mutation was introduced into the germ line and results in early embryonic death. However, hnRNP C is not required for cell viability, as ES cells derived from homozygous mutant blastocysts are viable and undergo morphological differentiation in vitro.
cDNAs encoding murine C1 and C2 hnRNPs were characterized as part of our analysis of the 4A4 mutation, providing the second mammalian and third vertebrate hnRNP C sequenced to date. Overall, murine hnRNP C shares 97 and 78% sequence identity with the human and Xenopus proteins, respectively. C1 and C2 hnRNPs are encoded separately by both murine and human cDNAs. Neither protein is expressed in cells homozygous for the 4A4 mutation, confirming that both are expressed by a single gene. Phylogenetic conservation of the C1 and C2 proteins suggests that both have distinct functions in mRNA biogenesis.
Additional alternative splicing of murine hnRNP C1 and C2 transcripts generates three proteins of each type, designated C1α, C1β, and C1γ (for hnRNP C1) and C2α, C2β, and C2γ (for hnRNP C2). The murine C1β and C2β proteins are colinear with human C1 and C2, respectively. The α and γ forms, expressed only in the mouse, contain either seven additional amino acids (α) or one fewer amino acid (γ) immediately following Asp 226. Significant levels of all three types appear to be expressed, with the least abundant γ form comprising 17% of all ESTs. In addition, each type was present in cDNA libraries cloned from a variety of tissues. However, the α and γ forms are not conserved among mammals, casting doubt as to their functional significance. The variable sequence may simply serve as a spacer between a region involved in oligomerization (31) and the acidic C-terminal domain.
During the course of these studies, we found two differences between the previously published HeLa cDNA sequence (52) and all available human ESTs. Human ESTs encode isoleucine at position 218 instead of methionine and contain two additional nucleotides that extend the reading frame, resulting in the addition of three amino acids. In both cases, the murine gene sequence (Fig. 2) is the same as those of the human ESTs. The revision to Ile at position 218 is potentially significant, as it occurs in a region important for the formation of hnRNP C tetramers (31) and extends a potential amphipathic domain by seven amino acids (i.e., one α-helical turn). Similar domains in other proteins are associated with coiled-coil protein interactions (28).
This is the first null mutation described for any member of the large family of abundant hnRNP proteins. The fact that homozygous mutant embryos fail to develop beyond E6.5 (egg cylinder stage) demonstrates that hnRNP C provides nonredundant functions essential to the organism. As early embryonic death appears to be a common consequence of mutations disrupting basic cellular processes (11), the phenotype is consistent with the important role hnRNP C is thought to play in RNA metabolism. Mutations in other genes implicated in RNA processing have produced similar phenotypes. For example, a gene trap-induced mutation in Fugl—the murine homologue of yeast RNA1, a Ran GTPase-activating protein involved in nuclear transport—leads to a similar embryonic lethality at E6.5 (12). Deletion of Raly, an hnRNP C-related gene, is associated with the recessive, preimplantation lethal component of the Ay mutation (37) which also arrests development prior to gastrulation. While more severe than the hnRNP C null phenotype, Ay is genetically complex and involves more than the loss of Raly function.
Although the first 6.5 days of embryonic development are characterized by a progressive increase in cell number, very little increase is seen in the overall mass or volume of the embryo (34). However the requirement for macromolecular synthesis increases dramatically around E6.5. Prior to gastrulation, cell doubling times decrease from about 11.5 h at E5.5 to less than 5 h at E6.5 (48). An even greater increase is seen in embryonic volume. Between E5.5 and E6.5, the volume of the embryo progresses from 3.4 × 105 to 9.0 × 105 μm3, a 2.6-fold increase. By E7.5, the embryonic volume expands to approximately 1.3 × 107 μm3, a 14.4-fold increase. There is accumulating evidence that this increase in growth rate is required for mesoderm induction (45) and that mutations that depress cell proliferation cause developmental arrest at E6.5 similar to what occurs with the hnRNP C mutation (19, 27, 38, 51). Therefore, hnRNP C (and possibly Fugl and Raly) mutant embryos may arrest at the egg cylinder stage, not because of a specific developmental defect but rather due to their inability to keep pace with the large metabolic demands required at the onset of gastrulation.
An important feature of using mice as a genetic system is the potential for generating isogenic cell lines deficient in specific genes for analysis of the biochemical functions of the encoded proteins. Cell lines can be isolated, as in the present study, even when the mutation results in early embryonic death. Cell lines were established from 8 of 21 blastocysts, of which 2 were homozygous for the 4A4 mutation. Cells from homozygous mutant embryos expanded more slowly than cells from heterozygous and wild-type embryos, requiring nearly twice as long to reach each successive passaging interval. Once established, the mutant cells grew somewhat slower than normal cells.
With cell lines it was possible to assess the consequences of provirus insertion on cellular gene expression. Such experiments are difficult, it not impossible, to perform in embryos prior to E7.0. Since the 4A4 provirus is inserted into an intron, we were concerned that some transcripts might splice around the provirus, allowing residual gene expression. However, we were unable to detect hnRNP C protein or message in cells homozygous for the 4A4 provirus. Within the limits of detection, mutant cells express at least 5,000-fold less hnRNP C than wild-type cells, reflecting an essentially null mutation.
hnRNPs C1/C2, A1/B2, and B1/A2 are among the most abundant proteins in the nucleus, with levels comparable to those of the core histones. These levels are sufficient to package nuclear RNA into repeating arrays of 20- to 25-nm RNP particles (30). Riboprotein complexes, and not RNA, constitute the substrates for all transport and processing steps involved in mRNA biogenesis. Consequently, we did not expect that cells lacking hnRNP C would be viable. We considered the possibility that hnRNP C might function specifically during cell differentiation, with relatively little effect on undifferentiated ES cells. However, mutant cells retain, at least on a qualitative level, the capacity for in vitro differentiation. Moreover, ES cells are not unique in the ability to tolerate mutations in core RNPs. A murine erythroleukemia cell line that expresses only nominal levels of hnRNP A1/B2 has been described (56).
In principle, other RNA binding proteins may substitute for the loss of hnRNP C. For example, ES cells express high levels of Raly transcripts (Fig. 5B), which is 41% identical to hnRNP C at the amino acid level. However, Raly has not been found among the RNA binding proteins present in hnRNP complexes. Whether another protein can substitute for hnRNP C or not, cells appear to tolerate some plasticity in the structure of hnRNP complexes. Preliminary analysis of gene expression and RNA processing in hnRNP C-deficient cells has thus far failed to reveal differences between wild-type and mutant cells. For example, we observed no difference in the expression of a luciferase reporter gene either over a range of DNA concentrations or at various times after gene transfer (S. Banik-Maiti and H. E. Ruley, unpublished data). Similarly, no striking differences were observed (Banik-Maiti and Ruley, unpublished data) in the levels of expression or splicing patterns of a human growth hormone gene (33). In short, the loss of hnRNP C has far less effect than might be expected considering the abundance of the protein and previous notions about its function. While the available data suggest that the C1 and C2 hnRNPs are not required for any essential step in mRNA biogenesis, the proteins presumably influence the rate and/or fidelity of one or more steps, given the phenotypes observed in mutant cells and mice. While additional experiments are required for a more detailed understanding of hnRNP C functions, the hnRNP C-deficient cell lines described in this report should prove useful in such endeavors.
ACKNOWLEDGMENTS
We thank Wallace LeStourgeon for critical review of the paper, for help with hnRNP C protein purification, and for the anti-hnRNP C antibody. We also thank Edward J. Michaud for the Raly (EcDNA 1) cDNA clone; Brigid Hogan and Robert Rosenberg for providing the 8.5-day mouse embryo and ES cell cDNA libraries, respectively; and Richard Bucco, Erica White, Jin Chen, and James McAfee for helpful discussions.
This work was supported by Public Health Service grants (R01HG00684 and R01RR13266 to H.E.R.) and by a grant from the Kleberg Foundation. Additional support was provided by a Cancer Center (Core) grant. D.J.W. was supported by Medical Scientist Training Grant 5T32-GM07347.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachvarova R, De Leon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev Biol. 1980;74:1–8. doi: 10.1016/0012-1606(80)90048-2. [DOI] [PubMed] [Google Scholar]
- 3.Barnett S F, LeStourgeon W M, Friedman D L. Rapid purification of native C protein from nuclear ribonucleoprotein particles. J Biochem Biophys Methods. 1988;16:87–97. doi: 10.1016/0165-022x(88)90106-6. [DOI] [PubMed] [Google Scholar]
- 4.Bennett M, Michaud S, Kingston J, Reed R. Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 1992;6:1986–2000. doi: 10.1101/gad.6.10.1986. [DOI] [PubMed] [Google Scholar]
- 5.Beyer A L, Christensen M E, Walker B W, LeStourgeon W M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- 6.Burd C G, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 7.Burd C G, Swanson M S, Gorlach M, Dreyfuss G. Primary structures of the heterogeneous nuclear ribonucleoprotein A2, B1, and C2 proteins: a diversity of RNA binding proteins is generated by small peptide inserts. Proc Natl Acad Sci USA. 1989;86:9788–9792. doi: 10.1073/pnas.86.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y D, Grabowski P J, Sharp P A, Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986;231:1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Conway G, Wooley J, Bibring T, LeStourgeon W M. Ribonucleoproteins package 700 nucleotides of pre-mRNA into a repeating array of regular particles. Mol Cell Biol. 1988;8:2884–2895. doi: 10.1128/mcb.8.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copp A J. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- 12.DeGregori J, Russ A, von Melchner H, Rayburn H, Priyaranjan P, Jenkins N A, Copeland N G, Ruley H E. A murine homolog of the yeast RNA1 gene is required for postimplantation development. Genes Dev. 1994;8:265–276. doi: 10.1101/gad.8.3.265. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfuss G, Matunis M J, Pinol-Roma S, Burd C G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 14.Evans M J, Kaufman M H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 16.Flach G, Johnson M H, Braude P R, Taylor R A, Bolton V N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1:681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forne T, Rossi F, Labourier E, Antoine E, Cathala G, Brunel C, Tazi J. Disruption of base-paired U4.U6 small nuclear RNAs induced by mammalian heterogeneous nuclear ribonucleoprotein C protein. J Biol Chem. 1995;270:16476–16481. doi: 10.1074/jbc.270.27.16476. [DOI] [PubMed] [Google Scholar]
- 18.Gorlach M, Burd C G, Dreyfuss G. The determinants of RNA-binding specificity of the heterogeneous nuclear ribonucleoprotein C proteins. J Biol Chem. 1994;269:23074–23078. [PubMed] [Google Scholar]
- 19.Hakem R, de la Pompa J L, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, Firpo E, Hui C C, Roberts J, Rossant J, Mak T W. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 20.Hicks G G, Shi E G, Chen J, Roshon M, Williamson D, Scherer C, Ruley H E. Retrovirus gene traps. Methods Enzymol. 1995;254:263–275. doi: 10.1016/0076-6879(95)54019-9. [DOI] [PubMed] [Google Scholar]
- 21.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 22.Huang M, Rech J E, Northington S J, Flicker P F, Mayeda A, Krainer A R, LeStourgeon W M. The C-protein tetramer binds 230 to 240 nucleotides of pre-mRNA and nucleates the assembly of 40S heterogeneous nuclear ribonucleoprotein particles. Mol Cell Biol. 1994;14:518–533. doi: 10.1128/mcb.14.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson M H. The molecular and cellular basis of preimplantation mouse development. Biol Rev. 1981;56:463–498. doi: 10.1111/j.1469-185x.1981.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawase E, Suemori H, Takahashi N, Okazaki K, Hashimoto K, Nakatsuji N. Strain difference in establishment of mouse embryonic stem (ES) cell lines. Int J Dev Biol. 1994;38:385–390. [PubMed] [Google Scholar]
- 25.Kiledjian M, Burd C G, Gorlach M, Portman D S, Dreyfuss G. Structure and function of hnRNP proteins. In: Mattaj I, Nagai K, editors. RNA protein interactions. Oxford, England: Oxford University Press; 1994. pp. 127–149. [Google Scholar]
- 26.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig T, Chapman D L, Papaioannou V E, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 28.Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 29.Mayrand S H, Dwen P, Pederson T. Serine/threonine phosphorylation regulates binding of C hnRNP proteins to pre-mRNA. Proc Natl Acad Sci USA. 1993;90:7764–7768. doi: 10.1073/pnas.90.16.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAfee J, Huang M, Soltaninassab S, Rech J E, Iyengar S, Lestourgeon W M. The packaging of pre-mRNA. In: Krainer A R, editor. Eukaryotic mRNA processing. Vol. 17. New York, N.Y: IRL Press at Oxford University Press; 1997. pp. 68–102. [Google Scholar]
- 31.McAfee J G, Shahied-Milam L, Soltaninassab S R, LeStourgeon W M. A major determinant of hnRNP C protein binding to RNA is a novel bZIP-like RNA binding domain. RNA. 1996;2:1139–1152. [PMC free article] [PubMed] [Google Scholar]
- 32.McAfee J G, Soltaninassab S R, Lindsay M E, LeStourgeon W M. Proteins C1 and C2 of heterogeneous nuclear ribonucleoprotein complexes bind RNA in a highly cooperative fashion: support for their contiguous deposition on pre-mRNA during transcription. Biochemistry. 1996;35:1212–1222. doi: 10.1021/bi951974k. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy E, Phillips J. Characterization of an intron splice enhancer that regulates alternative splicing of human GH pre-mRNA. Hum Mol Genet. 1998;7:1491–1496. doi: 10.1093/hmg/7.9.1491. [DOI] [PubMed] [Google Scholar]
- 34.McLaren A. Growth from fertilization to birth in the mouse. In: Elliott K, O'Connor M, editors. Embryogenesis in mammals. Vol. 40. New York, N.Y: Elsevier; 1976. pp. 47–51. [Google Scholar]
- 35.Merrill B M, Barnett S F, LeStourgeon W M, Williams K R. Primary structure differences between proteins C1 and C2 of HeLa 40S nuclear ribonucleoprotein particles. Nucleic Acids Res. 1989;17:8441–8449. doi: 10.1093/nar/17.21.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 37.Michaud E J, Bultman S J, Stubbs L J, Woychik R P. The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes Dev. 1993;7:1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- 38.Mishina Y, Suzuki A, Ueno N, Behringer R R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 39.Nakielny S, Dreyfuss G. The hnRNP C proteins contain a nuclear retention sequence that can override nuclear export signals. J Cell Biol. 1996;134:1365–1373. doi: 10.1083/jcb.134.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet. 1998;20:46–50. doi: 10.1038/1700. [DOI] [PubMed] [Google Scholar]
- 41.Pinol-Roma S, Choi Y D, Matunis M J, Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988;2:215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- 42.Pinol-Roma S, Dreyfuss G. Cell cycle-regulated phosphorylation of the pre-mRNA-binding (heterogeneous nuclear ribonucleoprotein) C proteins. Mol Cell Biol. 1993;13:5762–5770. doi: 10.1128/mcb.13.9.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 44.Portman D S, Dreyfuss G. RNA annealing activities in HeLa nuclei. EMBO J. 1994;13:213–221. doi: 10.1002/j.1460-2075.1994.tb06251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power M A, Tam P P. Onset of gastrulation, morphogenesis and somitogenesis in mouse embryos displaying compensatory growth. Anat Embryol. 1993;187:493–504. doi: 10.1007/BF00174425. [DOI] [PubMed] [Google Scholar]
- 46.Robertson E J. Teratocarcinomas and embryonic stem cells: a practical approach. 1st ed. Washington, D.C.: IRL Press; 1987. [Google Scholar]
- 47.Sambrook J, Fritsch F, Maniatis T E. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Snow M H L. Embryo growth during the immediate postimplantation period. In: Elliott K, O'Connor M, editors. Embryogenesis in mammals. Vol. 40. New York, N.Y: Elsevier; 1976. pp. 53–66. [Google Scholar]
- 49.Soltaninassab S R, McAfee J G, Shahied-Milam L, LeStourgeon W M. Oligonucleotide binding specificities of the hnRNP C protein tetramer. Nucleic Acids Res. 1998;26:3410–3417. doi: 10.1093/nar/26.14.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperling R, Sperling J. Large nuclear ribonucleoprotein particles of specific RNA polymerase II transcripts. In: Strauss P R, Wilson S H, editors. The eukaryotic nucleus: molecular biochemistry and macromolecular assemblies. Vol. 2. N.J: The Telford Press, Inc., Caldwell, Inc.; 1990. pp. 453–476. [Google Scholar]
- 51.Suzuki A, de la Pompa J L, Hakem R, Elia A, Yoshida R, Mo R, Nishina H, Chuang T, Wakeham A, Itie A, Koo W, Billia P, Ho A, Fukumoto M, Hui C C, Mak T W. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 1997;11:1242–1252. doi: 10.1101/gad.11.10.1242. [DOI] [PubMed] [Google Scholar]
- 52.Swanson M S, Nakagawa T Y, LeVan K, Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987;7:1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Melchner H, DeGregori J V, Rayburn H, Reddy S, Friedel C, Ruley H E. Selective disruption of genes expressed in totipotent embryonal stem cells. Genes Dev. 1992;6:919–927. doi: 10.1101/gad.6.6.919. [DOI] [PubMed] [Google Scholar]
- 54.von Melchner H, Reddy S, Ruley H E. Isolation of cellular promoters by using a retrovirus promoter trap. Proc Natl Acad Sci USA. 1990;87:3733–3737. doi: 10.1073/pnas.87.10.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weighardt F, Biamonti G, Riva S. The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism. Bioessays. 1996;18:747–756. doi: 10.1002/bies.950180910. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Bani M R, Lu S J, Rowan S, Ben-David Y, Chabot B. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc Natl Acad Sci USA. 1994;91:6924–6928. doi: 10.1073/pnas.91.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]