Abstract
Background
In 2015, the Korean Atopic Dermatitis Association (KADA) working group published consensus guidelines for treating atopic dermatitis (AD).
Objective
We aimed to provide updated consensus recommendations for systemic treatment of AD in South Korea based on recent evidence and experience.
Methods
We compiled a database of references from relevant systematic reviews and guidelines on the systemic management of AD. Evidence for each statement was graded and classified based on thestrength of the recommendation. Forty-two council members from the KADA participated in three rounds of voting to establish a consensus on expert recommendations.
Results
We do not recommend long-term treatment with systemic steroids forpatients with moderate-to-severe AD due to the risk of adverse effects. We recommend treatment with cyclosporine or dupilumab and selective treatment with methotrexate or azathioprine for patients with moderate-to-severe AD. We suggest treatment with antihistamines as an option for alleviating clinical symptoms of AD. We recommend selective treatment with narrowband ultraviolet B for patients with chronic moderate-to-severe AD. We do not recommend treatment with oral antibiotics for patients with moderate-to-severe AD but who have no signs of infection. We did not reach a consensus on recommendations for treatment with allergen-specific immunotherapy, probiotics, evening primrose oil, orvitamin D for patients with moderate-to-severe AD. We also recommend educational interventions and counselling for patients with AD and caregivers to improve the treatment success rate.
Conclusion
We look forward to implementing a new and updated consensus of systemic therapy in controlling patients with moderate-to-severe AD.
Keywords: Atopic dermatitis, Consensus, Republic of Korea, Systemic treatment, Therapeutics
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory cutaneous disorder characterized by severe itching1. It is one of the most common inflammatory dermatological diseases in South Korea, with an estimated prevalence of 10%~20% among children and 1%~3% among adults2. Treatment of AD, which is a chronic and relapsing disease, depends on the severity of the patients. However, assessments of AD severity are somewhat arbitrary. The Korean Atopic Dermatitis Association (KADA) recently defined an eczema area and severity index (EASI) score of 16~22 as moderate AD, whereas an EASI score of ≥23 was considered severe AD3. They agreed to increase the severity score if the numerical rating scale for pruritus was ≥7 or the Dermatology Life Quality Index >10.
The previous consensus guidelines for treating AD were published in 20154, and updated guidelines are needed. The updated consensus recommendations described here have been developed by KADA based on recent evidence and experience. In particular, there have been significant advances in systemic therapies, which are used mainly to treat moderate-to-severe AD. The new systemic treatment recommendations were developed taking into account the South Korean healthcare system and are designed to improve patient adherence. These updated treatment consensus, which is the latest, evidence-based consensus recommendations, propose a systematic and integrated treatment algorithm that includes basic, active, proactive, and adjunctive AD treatments4. This algorithm focuses on the systemic management of AD using systemic steroids, immunomodulators, biologics, antihistamines, phototherapy, allergen-specific immunotherapy (ASIT), antimicrobials, and adjunctive treatments.
MATERIALS AND METHODS
To update the guidelines for the systemic management of AD in South Korea, the KADA established a task force team of 12 dermatologists, representing AD experts nationwide. Task force team members performed extensive, up-to-date literature reviews of systemic therapy for moderate-to-severe adult AD. Based on the evidence reviewed, they developed a total of 14 Patient characteristics, type of Intervention, Control, and Outcome (PICO) questions regarding AD systemic therapy and requested expert opinions on each of these questions (Table 1). The study was also approved by the Institutional Review Board of the Catholic University of Korea (approved no. KC21ZASI0075).
Table 1. Expert consensus recommendations for the treatment of AD.
| PICO | Recommendation strength | Grade of evidence | % of respondents | Mean agreement score |
|---|---|---|---|---|
| Long-term systemic treatment with steroids is not recommended for patients with moderate-to-severe AD due to risk of adverse effects. | D | V | 100 | 9.5 |
| Cyclosporine is recommended for patients with moderate-to-severe AD. | A | Ia | 97.6 | 9.1 |
| Selective use of methotrexate is recommended for patients with moderate-to-severe AD. | B | Ib | 79.5 | 7.3 |
| Selective use of azathioprine is recommended for patients with moderate-to-severe AD. | B | Ib | 64.1 | 6.4 |
| Dupilumab is recommended for patients with moderate-to-severe AD. | A | Ia | 94.7 | 8.3 |
| Oral H1 antihistamines could be helpful to improve clinical symptoms in patients with moderate-to-severe AD. Optional use of antihistamines is recommended for these patients, if standard treatment with systemic or topical immunomodulators is insufficient. | B | II | 90.5 | 8.3 |
| Narrowband ultraviolet B is recommended as a selective treatment for patients with chronic moderate-to-severe AD. | B | Ia | 92.9 | 8.4 |
| Selective use of allergen-specific immunotherapy is recommended for patients with moderate-to-severe AD. | B | Ib | 52.4 | 6.8 |
| Oral antibiotics are not recommended for patients with moderate-to-severe AD without apparent signs of infection. | B | IIb | 85.7 | 8.1 |
| Selective use of oral antifungal agents is recommended for patients with head-and-neck AD. | B | IIb | 57.1 | 6.6 |
| Limited use of probiotics is proposed as adjuvant therapy for patients with moderate-to-severe AD. | C | IIb | 33.3 | 5.6 |
| Limited use of evening primrose oil is proposed as adjuvant therapy for patients with moderate-to-severe AD. | C | IIb | 50 | 6.6 |
| Limited use of vitamin D is proposed as adjuvant therapy for patients with moderate-to-severe AD. | C | IIb | 31 | 5.4 |
| Educational interventions and counselling for patients with AD and caregivers are recommended for successful treatment. | A | Ia | 100 | 9.5 |
AD: atopic dermatitis, PICO: Patient Characteristics, Type of Intervention, Control, and Outcome.
Database and literature searches
The members of the task force team individually performed a comprehensive database search. This search was conducted in the PubMed, Scopus, Cochrane library, and KoreaMed databases for articles published between 1 January 2005 and 30 June 30 2019, using combinations of the terms “atopic eczema”, “atopic dermatitis”, “antihistamine”, “antimicrobial, “antifungal”, “antiviral”, “corticosteroids”, “cyclosporine”, “azathioprine”, “methotrexate”, “mycophenolate mofetil”, “biologics”, “allergen-specific immunotherapy”, “probiotics”, “prebiotics”, “vitamin D”, “essential fatty acid”, “small molecule inhibitors”, and “education”. These searches were supplemented by manual searches of the reference lists from relevant systematic reviews and guidelines from other research groups. The members collected all relevant statements relating to AD management.
Evaluation of the literature
The members of the working group graded the evidence and then classified the strength of the recommendation for each statement. The evidence for each statement was graded as follows: level 1a, systematic review (with homogeneity) of randomized controlled trials (RCTs); level 1b, individual RCT (with narrow confidence interval); level 2a, systematic review (with homogeneity) of cohort studies; level 2b, individual cohort study (including low-quality RCTs); level 2c, “outcome” research; level 3a, systematic review (with homogeneity) of case-control studies; level 3b, individual case-control study; level 4, case series (and poor-quality cohort and case-control studies); and level 5, expert opinion. The strength of the recommendation was classified as A (level 1), B (levels 2 and 3), C (level 4), or D (level 5).
Consensus process
Fifty-five council members of the KADA were asked to provide their level of agreement with each draft statement, using a voting scale of 1~10 (where 1 denotes strong disagreement and 10 strong agreement). Forty-two South Korean experts participated in the vote. Each voting score was allocated to one of the three groups: 1~3 (disagreement), 4~6 (neutrality), and 7~10 (agreement). Consensus was defined as ≥70% of participants providing a score within the 7~10 range (agreement). Consensus recommendations were derived from three rounds of voting.
RESULTS AND DISCUSSION
Systemic corticosteroids
Long-term systemic treatment with steroids is not recommended for patients with moderate-to-severe AD due to the risk of adverse effects
Recommendation strength: D, grade of evidence: V
% of respondents (agreement score ≥7): 100%
Systemic steroids are known to be recommended for use at 0.5 mg/kg/day for 1 to 2 weeks during acute severe exacerbation of AD5. Two small double-blind randomized placebo-controlled clinical trials evaluated systemic steroids in patients with moderate-to-severe AD. Heddle et al.6 reported that 4 weeks of treatment with systemic steroids resulted in significant clinical improvements, compared with placebo. La Rosa et al.7 also reported that 2 weeks of treatment with systemic steroids resulted in improvements, compared with placebo. No significant adverse effects were associated with systemic steroid administration in either study. However, despite this efficacy, the majority of clinical practice guidelines and review articles recommend that patients with AD should avoid taking systemic steroids for a long period4,5,8,9,10,11. The long-term administration of systemic steroids is associated with a variety of conditions including impaired glucose tolerance, diabetes, hypertension, hyperlipidemia, gastritis, peptic ulcers, weight gain, osteoporosis, hypothalamic-pituitary-adrenal axis suppression, emotional changes, fluid retention, and opportunistic infections10,11. However, short-term administration of systemic steroids is usually considered safe11. Schmitt et al.12 performed a double-blind randomized clinical trial to compare the efficacies of prednisolone for 2 weeks (including tapering periods) and cyclosporine for 6 weeks on severe eczema. There was no significant difference in the response rate (defined as the proportion of patients whose objective SCORing Atopic Dermatitis (SCORAD) score decreased at least 50% compared with baseline) between the two groups. However, the relapse rate (75% or more of the baseline objective SCORAD score after response) among the responders within a 12-week follow-up period was significantly higher in the prednisolone than cyclosporine group. Treatment with systemic steroids may used as a transitional therapy for a short time to rapidly control acute exacerbation in severe cases of AD until other long-term therapies are stabilized10.
Systemic immunomodulators
That study investigated four commonly used systemic immunosuppressants: cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
Cyclosporine
Cyclosporine is recommended for patients with moderate-to-severe AD
Recommendation strength: A, grade of evidence: Ia
% of respondents (agreement score ≥7): 97.6%
In a systematic review of 14 RCTs, cyclosporine showed an average improvement of 53%~95% after short-term administration for 8 weeks compared with placebo, and the weekly rate of drug withdrawal due to adverse events was 0%~2%9,13,14,15,16. Although retrospective studies have shown that it can be used without serious adverse events for more than 1 year17,18, the level of evidence for long-term use (>1 year) is lower than that for short-term treatments. Therefore, cyclosporine is recommended for short-term use as a first-line drug for safe and effective treatment of moderate-to-severe AD9. A meta-analysis showed that treatment with cyclosporine for 6~8 weeks improved the clinical severity index by an average of 55%, similar to previous results19. However, after drug withdrawal, it was found to deteriorate again to the pre-treatment level. Compared with other treatments, cyclosporine proved superior to prednisolone, intravenous immunoglobulin (Ig), and ultraviolet A (UVA)/ultraviolet B (UVB), and it had similar efficacy to mycophenolate9,12,20,21,22. The overall efficacy of cyclosporine was lower than that of dupilumab, with 56% and 78% of patients showing a 75% improvement in the EASI after 12 to 16 weeks of treatment, respectively23. In a cohort study that retrospectively analyzed the incidence of serious infections in adult patients with AD who had received a systemic immunomodulator for more than 6 months, compared to methotrexate, cyclosporine had significantly reduced 6-month risk (risk ratio=0.87), whereas prednisone, azathioprine, and mycophenolate showed increased risks (risk ratios=1.78, 1.89, and 3.31, respectively)24.
Methotrexate
Selective use of methotrexate is recommended for patients with moderate-to-severe AD
Recommendation strength: B, grade of evidence: Ib
% of respondents (agreement score ≥7): 79.5%
To date, there have been no RCTs comparing methotrexate with placebo. Three small studies that compared methotrexate with azathioprine showed a 40% improvement rate after 12 weeks of treatment and a moderate improvement rate of 50%~60%, with no serious side effects, after 2 to 5 years of long-term use. There was no difference in efficacy between methotrexate and azathioprine25,26,27. When compared with cyclosporine, methotrexate showed a similar improvement rate at 16 weeks and a low frequency of adverse events28. Recent systematic reviews and guidelines from Europe and India recommended methotrexate as a second-line systemic immunotherapy treatment for adults and children with moderate-to-severe AD after cyclosporine, although the level of evidence was low. These sources also suggest that methotrexate is a relatively safe long-term treatment9,24,29,30.
Azathioprine
Selective use of azathioprine is recommended for patients with moderate-to-severe AD
Recommendation strength: B, grade of evidence: Ib
% of respondents (agreement score ≥7): 64.1%
There have been three RCTs of azathioprine, which is fewer than the number performed for cyclosporine25,31,32. In studies comparing azathioprine and placebo, azathioprine significantly reduced the AD severity score by 26%~36% after 12 weeks of treatment31,32. Azathioprine was reportedly a safe and effective maintenance treatment (from 6 months to several years) for patients with moderate-to-severe AD, similar to methotrexate25,26. Although azathioprine was effective and relatively safe in pediatric studies, the level of evidence is low because those analyses involved 1 year of medication on a small number of subjects33,34,35. Recent systematic reviews and guidelines from Europe and India recommended azathioprine as a second-line treatment for adults with moderate-to-severe AD after cyclosporine, with a warning that it may cause suppression in the bone marrow9,29,30.
Mycophenolate mofetil
A meta-analysis of mainly case series, involving 140 subjects, reported a 38.3% decrease in AD severity after mycophenolate mofetil treatment, but a prolonged duration of treatment (>1 year) was associated with herpes infections36. Another systematic review suggested that mycophenolate mofetil may be considered a maintenance therapy for severe AD, but the level of evidence was low because only a few case series were evaluated9.
In one RCT that compared enteric-coated mycophenolate with cyclosporine for 30 weeks, the two treatments showed equivalent efficacy, but mycophenolate showed a more delayed response22.
We decided not to present a recommendation for mycophenolate mofetil because the level of evidence was low (3a) due to the paucity of high-quality RCTs and systematic reviews.
Biologics
Dupilumab is recommended for patients with moderate-to-severe AD
Recommendation strength: A, grade of evidence: 1a
% of respondents (agreement score ≥7): 94.7%
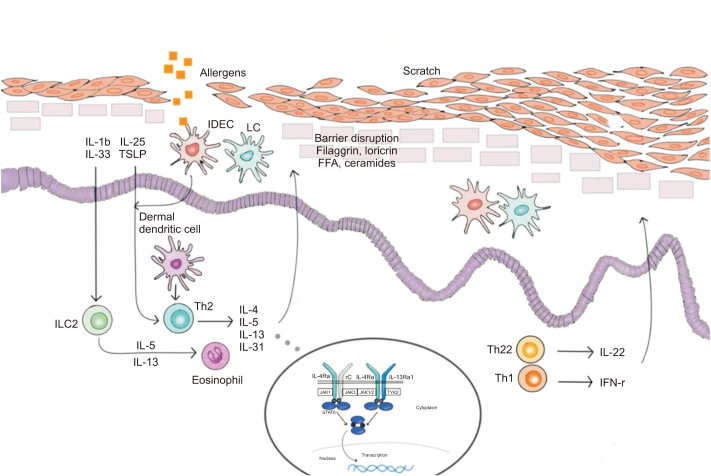
Biologics are emerging as an important treatment option for patients with moderate-to-severe AD (Fig. 1). Two cytokines, interleukin (IL)-4 and IL-13, are essential for the induction and persistence of type 2 inflammatory responses and are closely linked to the pathogenesis of AD37. These key cytokines could be a link between the skin barrier and immune deficiencies in AD.
Fig. 1. Biological therapies for atopic dermatitis. In the treatment of atopic dermatitis, each biologics acts as follows: dupilumab is an IL-4Rα monoclonal antibody, lebrikizumab and tralokinumab are IL-13 monoclonal antibodies, nemolizumab is an IL-31 receptor A monoclonal antibody, mepolizumab is an IL-5 monoclonal antibody, tezepelumab is a thymic stromal lymphopoietin monoclonal antibody, and fezakinumab is an IL-22 monoclonal antibody. IL: interleukin, IFN: interferon, IDEC: inflammatory dermal dendritic cells, LC: Langerhans cell, TSLP: thymic stromal lymphopoietin, ILC: innate lymphoid cell, FFA: free fatty acid, JAK: Janus kinase inhibitor, TYK: tyrosine kinase, STAT: signal transducers and activators of trasncription.
Dupilumab, a fully human IL-4Rα monoclonal antibody, inhibits both IL-4 and IL-13 signaling and proved to have efficacy and a good safety profile in randomized double-blind placebo-controlled phase III studies in patients with moderate-to-severe AD38,39,40. First, two phase 3 trials of identical design (SOLO 1 and SOLO 2)38, involving adult patients with moderate-to-severe AD inadequately controlled by topical treatments, were used to evaluate the efficacy and safety of dupilumab monotherapy. An improvement in the EASI of at least 75% from baseline (EASI-75) was observed in 51% and 44% of patients receiving dupilumab every alternate week in SOLO 1 and SOLO 2, respectively. Those results were statistically significant compared with the placebo groups. Although the overall incidence of adverse events was similar between the dupilumab and placebo groups, injection-site reactions and conjunctivitis occurred more frequently in the dupilumab groups. Based on the results of that trial, dupilumab was approved by the US Food and Drug Administration (FDA) in March 2017 for adult patients with moderate-to-severe AD. In a clinical trial investigating the efficacy and safety of dupilumab (300 mg for 52 weeks) combined with background therapy of low-or medium-potency topical corticosteroids (LIBERTY AD CHRONOS)39, the proportion of patients who reached the 16-week EASI-75 target was 69% in the every-2-weeks group, 64% in the every-week group, and 23% in the placebo group. These treatments remained equally effective at 52 weeks. Dupilumab plus topical corticosteroids also alleviated the signs and symptoms of AD and significantly improved the quality of life of adults with AD and a history of inadequate response or intolerance to cyclosporine in another phase III clinical trial (LIBERTY AD CAFÉ)40. The efficacy of dupilumab has also been verified by a systematic review, although long-term data are lacking41.
In 2018, the South Korean FDA approved dupilumab for adults with moderate-to-severe AD that is not adequately controlled by topical therapies. In March 2019, the US FDA approved dupilumab for adolescent patients aged 12~17 years with moderate-to-severe AD. A recent phase III RCT demonstrated that the efficacy and safety profile of dupilumab in adolescent patients with AD were consistent with results obtained in adult trials42. In South Korea, the use of dupilumab in adolescents with moderate-to-severe AD was approved in April 2020. In May 2020, the US FDA also approved dupilumab as the first biologic medicine suitable for children aged 6~11 years with moderate-to-severe AD. A phase II–III study involving pediatric patients aged 6 months to 6 years with moderate-to-severe AD (NCT03346434) is currently ongoing.
However, not all patients respond to dupilumab, and AD has a complex phenotype. Therefore, wider treatment options are needed. Currently, clinical trials of various biologics that target the AD-specific immune pathway are underway. IL-13 is a significant type 2 cytokine from T lymphocytes37. Monoclonal antibodies against IL-13, lebrikizumab, and tralokinumab, are currently undergoing clinical trials in patients with moderate-to-severe AD43,44. Lebrikizumab (125 mg), administered every 4 weeks for 12 weeks in combination with topical corticosteroids, generated significant improvements in the clinical conditions of patients with moderate-to-severe AD in a phase II study, and it was generally well tolerated43. Tralokinumab treatment also showed early and sustained efficacy in alleviating AD symptoms and had favorable safety and tolerability profiles in a phase IIb study44. IL-31 is a potent pruritogenic cytokine, produced by activated T-helper type 2 cells45. Nemolizumab, an anti-IL-31 receptor-A monoclonal antibody, alleviated symptoms of pruritus, dermatitis, and sleep disturbance at 12 weeks in adults with moderate-to-severe AD that was inadequately controlled by topical treatments44. Moreover, nemolizumab was well tolerated for up to 64 weeks46.
Mepolizumab inhibits the action of IL-5, which promotes differentiation and activation of eosinophils. Mepolizumab did not improve the clinical condition of patients with AD despite significantly decreasing the number of eosinophils in blood47.
Clinical trials evaluating the efficacies and safety profiles of tezepelumab48 (an anti-thymic stromal lymphopoietin monoclonal antibody) and fezakinumab49 (an IL-22 monoclonal antibody) in adults with moderate-to-severe AD are also in progress. Other biologics, such as ustekinumab (an anti-IL12/23 IgG1 kappa human monoclonal antibody) and omalizumab (a recombinant humanized anti-IgE antibody), have so far failed to alleviate symptoms in patients with AD41,50,51.
Antihistamines
Oral H1 antihistamines could be helpful to improve clinical symptoms in patients with moderate-to-severe AD. Optional use of antihistamines is recommended for these patients, if standard treatment with systemic or topical immunomodulators is insufficient
Recommendation strength: B, grade of evidence: II
% of respondents (agreement score ≥7): 90.5%
Patients with moderate-to-severe AD usually present with severe pruritus. The reasons for this are not completely understood; however, histamine is not a major pruritogen in AD. Topical and systemic immunomodulators are known to be effective in relieving pruritus in patients with AD52.
A combination of systemic immunomodulators and H1 antihistamines has been widely used to relieve pruritus in patients with moderate-to-severe AD in a real world, but recent AD treatment guidelines have noted that there is little evidence to suggest that routine use of antihistamines relieves pruritus53,54. Few RCTs have investigated the effectiveness of antihistamines to treat patients with moderate-to-severe AD. A few low-to-moderate-quality or small-sample studies fulfilled our PICO criteria and were included in that review. In the Early Treatment of the Atopic Child trials, clinical improvements in the SCORAD score were observed following treatment with 0.5 mg/kg/day cetirizine, but the results were not statistically significant. Interestingly, statistically significant corticosteroid-sparing effects were reported for infants with a SCORAD score≥25 (n=347)55. However, concomitant topical or systemic medications were permitted in that study, and the results may be interpreted in more than one way. An RCT with a 6-day runin period and multi-crossover design also demonstrated that loratadine (10 mg/day) significantly reduced pruritus in adult patients (n=16) with moderate-to-severe AD56. A combination of fexofenadine (120 mg/day) and topical 0.1% hydrocortisone butyrate also proved effective in relieving itching, compared with placebo and fexofenadine alone57. A high-quality RCT demonstrated that olopatadine, a second-generation antihistamine, alleviated nocturnal scratching behavior without affecting the quality of sleep in patients with moderate-to-severe AD58. However, that study also had a very small sample size.
Few high-quality RCTs have assessed the therapeutic efficacy of antihistamines in treating patients with AD. However, there are also few studies that have provided evidence that antihistamines are ineffective. Well-designed future studies with precisely defined baseline characteristics and outcome measures are needed to improve the level of evidence regarding treatment of AD with antihistamines.
Phototherapy
Narrowband ultraviolet B phototherapy is recommended as a selective treatment for patients with chronic moderate-to-severe AD
Recommendation strength: B, grade of evidence: 1a
% of respondents (agreement score ≥7): 92.9%
Phototherapy is often used to treat AD and may be administered using the following modalities: narrowband (NB; e.g., 311 nm) or broadband UVB, UVA (especially UVA1=340~400 nm), and photochemotherapy where UV treatment can be combined with oral or topical administration of psoralens. Other devices, such as a 308 nm excimer laser, may also be used to treat patients with refractory AD. In general, UV light sources have immunosuppressive and anti-inflammatory effects on the skin. Their mechanisms of action include immunomodulation via apoptosis of inflammatory cells, inhibition of Langerhans cells, and altered regulation of cytokine production59.
Currently, NB-UVB and UVA1 are the most common phototherapy modalities used to treat AD. To date, no clinical studies have shown an increase in the incidence of non-melanoma skin cancer following treatment with NB-UVB or UVA1. Medium-dose UVA1 was used to control acute episodes of AD exacerbation. NB-UVB may be applied to manage chronic AD. A comparison of medium-to-long wavelength UVA1 with NB-UVB did not identify significant differences in efficacy or tolerability. A recent systematic review that included 21 RCTs (961 patients) reported that the efficacies of NB-UVB and UVA1 phototherapy were similar. No serious adverse events were identified60. Another study compared combined treatment with NB-UVB and UVA with NB-UVB only AD treatment. A total of 26 adults with chronic AD were included in that study, which involved similar cumulative UV doses and numbers of treatments among the different groups. The mean response duration was significantly greater in patients treated with NB-UVB only. No differences in the complete response or in the analogue scale for pruritus were observed. The efficacies of the two treatments (NB-UVB plus UVA vs. NB-UVB only) were similar in that study61.
Some evidence suggests that patients with moderate-to-severe AD may benefit from NB-UVB and UV-A1 phototherapy. Taking into account individual tolerability, NB-UVB is indicated for chronic moderate-to-severe AD and is currently preferred to broadband UVB because it is less erythemogenic. Due to difficulty obtaining access to suitable UVA1 devices compared with other phototherapy modalities, NB-UVB offers the most efficacious and cost-effective treatment for patients with chronic AD.
Allergen-specific immunotherapy
Selective use of ASIT is recommended for patients with moderate-to-severe AD
Recommendation strength: B, grade of evidence: Ib
% of respondents (agreement score ≥7): 52.4%
ASIT is a treatment approach for IgE-mediated allergic diseases. The basic principle of ASIT is to induce immunotolerance to allergens by administering them to patients in repeated, increasing doses. In total, 12 RCTs have investigated the efficacy and safety of ASIT to treat patients with AD, and those produced contradictory results62,63,64,65,66,67,68,69,70,71,72,73. Those studies involved various antigens, methods of administration, study durations, and outcome measures. Four systematic reviews have evaluated the results of ASIT studies, and those also generated different conclusions74,75,76,77. A recent Cochrane review concluded that there is limited evidence to support the view that ASIT benefits patients with AD due to the paucity of trials and participants, wide confidence intervals, extensive loss to follow-up, and lack of blinding in some studies77. In conclusion, we do not recommend ASIT for patients with moderate-to-severe AD, although this advice may change in the future after new clinical trials have been completed.
Systemic antimicrobials
Oral antibiotics are not recommended for patients with moderate-to-severe AD without apparent signs of infection
Recommendation strength: B, grade of evidence: IIb
% of respondents (agreement score ≥7): 85.7%
Selective use of oral antifungal agents is recommended for patients with head-and-neck AD
Recommendation strength: B, grade of evidence: IIb
% of respondents (agreement score ≥7): 57.1%
Some studies have suggested that Staphylococcus aureus may play a key role in AD. S. aureus can be cultured from the skin of >90% of patients with AD78. S. aureus superantigens elicit inflammation in the skin of patients with AD and exacerbate disease severity79,80. Although few researchers would dispute that systemic antibiotics can benefit patients with overtly clinically infected eczema, the clinical role of S. aureus in causing inflammatory flares in clinically uninfected eczema is less clear81. One study involving 50 children found no significant mean differences between oral flucloxacillin and placebo in children with erythema treated for 4 weeks. Another study involving 20 children and adults suggested that antibiotic treatment decreased colony counts; however, when the antibiotic was discontinued the skin was rapidly recolonized82. There is little doubt that antistaphylococcal agents can decrease the presence of S. aureus on the skin of patients with AD. However, this quantitative bacteriological change does not appear to translate into a clinically meaningful improvement in patients with AD when compared with nonantimicrobial products81. Consequently, systemic antibiotics should only be used in case of apparent and extensive bacterial superinfection.
Erythematous, scaly, and itching eczema on the face, neck, and upper thorax (i.e., head-and-neck dermatitis) is often particularly problematic in young adults with AD83. In many patients with AD, especially those with head-and-neck dermatitis, skin prick test was positive for Malassezia antigens and specific IgE antibodies84. One study demonstrated that 7 days of treatment with 200 or 400 mg itraconazole alone produced a significant clinical improvement in the head-and-neck area83. Another study showed that ketoconazole had a therapeutic effect on patients with AD who had serum IgE antibodies to yeasts85. Oral azole antifungal agents may be effective for some patients with refractory AD primarily involving the head-and-neck area or those sensitive to several species of Malassezia.
Patients with AD tend to have widespread disseminated viral infections, which are named after the causative virus: eczema molluscatum, eczema vaccinatum, or eczema herpeticum86. Damage to the skin of patients with AD may provide easier access for viruses. From a clinical perspective, eczema herpeticum is the most important disseminated cutaneous viral infection occurring in patients with AD86. The mainstay of eczema herpeticum treatment is prompt systemic antiviral therapy to limit disease duration and prevent further complications.
Adjunctive treatments
1) Probiotics/prebiotics
Limited use of probiotics are proposed as adjuvant therapy for patients with moderate-to-severe AD
Recommendation strength: C, grade of evidence: IIb
% of respondents (agreement score ≥7): 33.3%
Nineteen reports described RCTs that investigated the effects of probiotics in patients with moderate-to-severe AD87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105. Of those reports, 16 documents described significant reductions in AD severity measures such as the SCORAD score after probiotic treatment. Meanwhile, a Cochrane review published in 2018, which included results from 39 RCTs and 2,599 randomized participants with mild-to-severe eczema, failed to show that probiotics alleviate AD symptoms but recommended further studies106. Probiotics/prebiotics may be used as adjuvant therapy for patients with AD. However, the South Korean experts involved in that study were skeptical about recommendation regarding the limited use of probiotics to treat patients with moderate-to-severe AD.
2) Essential fatty acids
Limited use of evening primrose oil (EPO) is proposed as adjuvant therapy for patients with moderate-to-severe AD
Recommendation strength: C, grade of evidence: IIb
% of respondents (agreement score ≥7): 50%
EPO is a low-risk adjuvant therapy for patients with AD. It is a natural source of gamma-linolenic acid, which is an ω-6 fatty acid with anti-inflammatory properties. The effects of EPO in patients with AD have only been reported in small-scale RCTs107,108. A significant improvement in AD clinical symptoms was observed when EPO was combined with existing treatments in a study involving patients with moderate-to-severe AD107. Schalin-Karrila et al.109 reported that EPO treatment significantly decreased overall severity and inflammation grade compared with placebo in patients with moderate-to-severe AD. However, a Cochrane review published in 2012 concluded that there was insufficient evidence of any beneficial effect of EPO on patients with AD, and that further studies were needed110. Overall, our South Korean experts held a neutral position on whether to recommend essential fatty acids to treat patients with AD.
3) Vitamin D
Limited use of vitamin D is proposed as adjuvant therapy for patients with moderate-to-severe AD
Recommendation strength: C, grade of evidence: IIb
% of respondents (agreement score ≥7): 31.0%
Vitamin D has few potential side effects and may be used safely to treat patients with AD. Overall, RCTs and studies have reported inconsistent therapeutic effects of vitamin D in patients with AD111,112,113,114,115,116. A recent meta-analysis reported that the SCORAD and EASI scores decreased after vitamin D supplementation in patients with AD117,118. However, the South Korean experts involved in that study have not yet recommended using vitamin D as an adjuvant therapy to treat patients with moderate-to-severe AD.
Small molecule inhibitors
Crisaborole, a phosphodiesterase 4 inhibitor, is a novel topical agent that was approved by the FDA as a treatment for AD in 2016. Ten studies that evaluated orally administered small molecule inhibitors as potential treatments for AD were reviewed. Those included four RCTs, two open-label prospective trials, and four case series/reports. Six articles reported the efficacy of apremilast, a phosphodiesterase 4 inhibitor119,120,121,122,123,124, two articles described Janus kinase inhibitors125,126, and two articles described histamine receptor antagonists127,128.
A phase II double-blind placebo-controlled trial investigated the efficacy of 30 or 40 mg apremilast administered orally twice daily for 12 weeks to adults with moderate-to-severe AD. Patients given 40 mg apremilast exhibited a significantly greater decrease in the mean EASI than did those given placebo (31.6% vs. 11.0%)123. Adverse events associated with apremilast treatment included nausea, diarrhea, headache, and nasopharyngitis.
Two studies investigated Janus kinase inhibitors. First, a phase II RCT evaluated the efficacy and safety of baricitinib combined with topical corticosteroids in patients with moderate-to-severe AD. At 16 weeks, a significantly greater proportion of patients who received 4 mg baricitinib, compared with those given placebo, achieved EASI-50 (61% vs. 37%)125. In addition, a case series reported a marked clinical improvement in six patients with moderate-to-severe AD who were treated with tofacitinib126. Adverse events associated with Janus kinase inhibitors included headache, diarrhea, upper-respiratory-tract infections, herpes zoster reactivation, lymphopenia, neutropenia, and transaminitis, as well as elevated levels of lipids or creatinine phosphate kinase125,126.
The histamine H4 receptor (H4R) mediates proinflammatory responses in a number of cell types involved in allergic inflammation, including T cells, mast cells, eosinophils, and dendritic cells129. In particular, stimulation of this receptor leads to upregulation of IL-31 expression in T-helper type 2 cells130. Because the H4R plays a role in inflammatory responses, it was suggested that H4R antagonists may be used as novel treatments for inflammatory skin diseases. A phase II trial was initiated to investigate the effects of the H4R antagonist JNJ-39758979 in Japanese patients with moderate AD. Unfortunately, two cases of neutropenia occurred during that study, and the drug was discontinued. However, a reduction in the mean EASI was observed in the JNJ-39758979-treated groups, suggesting that H4R antagonism may be beneficial for patients with AD127. An RCT evaluating the efficacy of the H4R antagonist ZPL-3893787 in patients with moderate-to-severe AD was performed in Europe. Treatment with ZPL-3893787 decreased the EASI by 50% compared with 27% by placebo treatment. The adverse events reported included nasopharyngitis, headache, and eczema. There were no relevant laboratory abnormalities and no cases of neutropenia in that study128.
Further studies are needed to evaluate the efficacy and safety of small molecule inhibitors because few such studies have been published. In addition, recent advances in understanding the pathogenesis of AD have led to novel therapeutic targets being identified. These include pro-inflammatory targets such as the prostaglandin D2 receptor and neurokinin 1 receptors, as well as anti-inflammatory targets such as κ-opioid receptors and Src homology 2-domain-containing inositol-5-phosphatase 1. Trials using small molecule inhibitors of these targets are now underway131.
Educational interventions
Educational interventions and counselling for patients with AD and caregivers are recommended for successful treatment
Recommendation strength: A, grade of evidence: 1a
% of respondents (agreement score ≥7): 100%
AD is a chronic pruritic inflammatory skin disease affecting children, adolescents, and adults. Due to its chronically relapsing nature, the quality of life of patients and their caregivers is significantly affected. Therefore, structured educational programs for both patients and families are important for the long-term management of AD and for treatment adherence. Although various effective therapies are available, treatment adherence has been the most important factor for the successful treatment of AD. Misconceptions among patients and caregivers regarding the disease and treatments have decreased patient adherence. The major misunderstandings that result in suboptimal management of AD include corticosteroid phobia, excessive reliance on complementary and alternative medicines (e.g., Chinese herbal medicines), and extensive dietary restrictions. In addition, associated mental health conditions such as attention-deficit hyperactivity disorder, depression, and anxiety can make treatment more challenging.
Studies show that poor adherence to AD treatment eventually results in increased direct and indirect financial costs132. Hence, structured education programs not only enhance treatment success but also decrease the financial burden of patients and caregivers. Standard educational interventions include understanding the disease and its treatment methods, learning how to cope with chronic pruritus, avoiding exacerbating factors, appropriately moisturizing and cleansing the skin daily, and eating a healthy diet.
Patient and healthcare provider education programs are important for delivering effective treatments133. Thorough and repeated interviews with patients to understand their experiences and preferences regarding disease management are crucial for strengthening treatment adherence. Nevertheless, conducting interviews and developing patient-centered educational interventions are time consuming and cannot be accomplished through routine office care visits. Accordingly, attempts have been made to develop comprehensive educational programs in various countries. These programs involve not only dermatologists but also multidisciplinary teams of pediatricians, allergists, psychologists, nurses, dieticians, and nutritionists. The education sessions have been practiced in various forms such as group sessions, workshops, written pamphlets, and videos including lectures and discussions. Jang et al.134 reported that entertaining AD education programs in South Korean schools effectively decreased the psychosocial burden of the disease.
Educational interventions empower patients and caregivers to self-manage AD and have proved effective in the long-term management of the disease. Many studies have shown that these interventions have improved the quality of life of patients and their families, increased adherence to medical treatments, decreased the severity of eczema evaluated using SCORAD or subjective severity, and relieved pruritic symptoms133,135. In Germany, a multicenter randomized trial investigating the effects of structured patient education program showed that these significantly improved patient quality of life, SCORAD scores, and psychological symptoms such as anxiety and depression in adults with moderate-to-severe AD136.
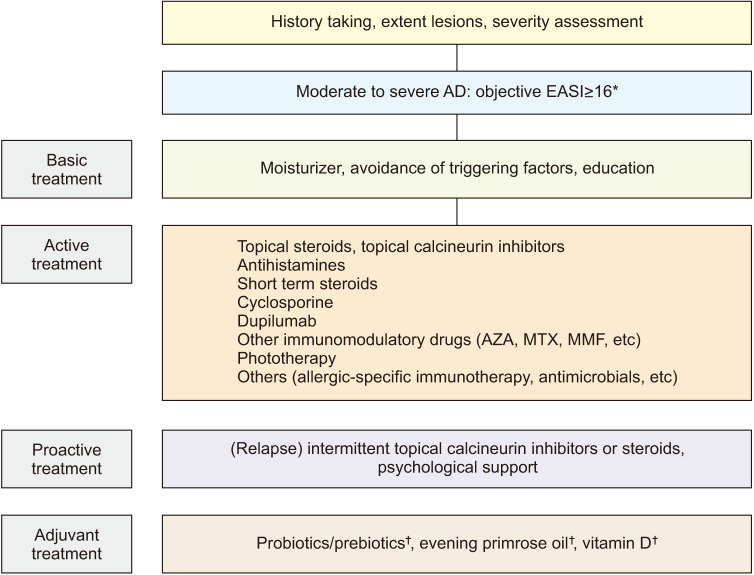
In conclusion, this updated report was produced with the assistance of a KADA panel of experts and presents a systematic review of the systemic management of AD, levels of evidence, strengths of the recommendations, and average agreement scores. It provides up-to-date evidence-based consensus recommendations and a systematic treatment algorithm for basic, active, proactive, and adjunctive AD treatments, based on previously reported concepts (Fig. 2)4.
Fig. 2. Treatment algorithm for moderate-to-severe atopic dermatitis. AD: atopic dermatitis, EASI: eczema area and severity index, AZA: azathioprine, MTX: methotrexate, MMF: mycophenolate mofetil. *Consider increasing the severity level if the patient has a diurnal or nocturnal pruritus score of ≥7 on a numerical rating scale or a Dermatology Life Quality Index of ≥10. †Adjuvant treatments may be considered but remain controversial.
For patients with moderate-to-severe AD, topical and systemic treatments are indicated, and tailored treatments are recommended, depending on the patient's condition. In addition to basic treatments such as applying moisturizer and avoiding aggravating factors, AD treatment strategies still emphasize the importance of education. To prevent exacerbation of AD, proactive treatments such as intermittent topical anti-inflammatory medication and psychosocial support are needed. ASIT or antimicrobials may be beneficial in selected cases. Adjuvant treatments are generally safe, have few side effects, and can be used at any stage of AD severity. However, we have not strongly recommended these due to discrepancy in opinion regarding their efficacy. Some of the AD drugs reviewed in this report have not yet highly recommended in Korean medical environment despite the fact that the level of evidence is not low. Therefore, further research is necessary in South Korea.
Nevertheless, the strength of this updated Consensus is that it is the algorithm reviewed and recommended by KADA experts, and they will improve the treatment of patients with AD in a real-world setting.
ACKNOWLEDGMENT
We would like to thank the 55 members of the Korean Atopic Dermatitis Association council who participated and provided their valuable advice to help us develop these recommendations.
Footnotes
CONFLICTS OF INTEREST: Joo Young Roh is a clinical investigator for Novartis, Bristol-Myers Squibb, Sanofi-Aventis, AbbVie, Boehringer Ingelheim, and Regeneron.
FUNDING SOURCE: This study was supported by Research Fund of the Korean Atopic Dermatitis Association. The funder(s) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DATA SHARING STATEMENT
The data that support the findings of this study are openly available in the Korean Atopic Dermatitis at http://www.atopy.re.kr/.
References
- 1.Lee JH, Son SW, Cho SH. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. 2016;8:181–190. doi: 10.4168/aair.2016.8.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Han KD, Kim KM, Park YG, Lee JY, Park YM. Prevalence of atopic dermatitis in Korean children based on data from the 2008-2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol Res. 2016;8:79–83. doi: 10.4168/aair.2016.8.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JE, Shin MK, Park GH, Lee UH, Lee JH, Han TY, et al. 2019 Consensus Korean diagnostic guidelines to define severity classification and treatment refractoriness for atopic dermatitis: objective and subjective assessment of severity. Ann Dermatol. 2019;31:654–661. doi: 10.5021/ad.2019.31.6.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (Part II): systemic treatment. Ann Dermatol. 2015;27:578–592. doi: 10.5021/ad.2015.27.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker AM, Eyerich K, de Bruin-Weller MS, Thyssen JP, Spuls PI, Irvine AD, et al. Use of systemic corticosteroids for atopic dermatitis: international eczema council consensus statement. Br J Dermatol. 2018;178:768–775. doi: 10.1111/bjd.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heddle RJ, Soothill JF, Bulpitt CJ, Atherton DJ. Combined oral and nasal beclomethasone diproprionate in children with atopic eczema: a randomised controlled trial. Br Med J (Clin Res Ed) 1984;289:651–654. doi: 10.1136/bmj.289.6446.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Rosa M, Musarra I, Ranno C, Maiello N, Negri L, del Giudice MM, et al. A randomized, double-blind, placebo-controlled, crossover trial of systemic flunisolide in the treatment of children with severe atopic dermatitis. Curr Ther Res. 1995;56:720–726. [Google Scholar]
- 8.Calzavara Pinton P, Cristaudo A, Foti C, Canonica GW, Balato N, Costanzo A, et al. Diagnosis and management of moderate to severe adult atopic dermatitis: a Consensus by the Italian Society of Dermatology and Venereology (SIDeMaST), the Italian Association of Hospital Dermatologists (ADOI), the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC), and the Italian Society of Allergological, Environmental and Occupational Dermatology (SIDAPA) G Ital Dermatol Venereol. 2018;153:133–145. doi: 10.23736/S0392-0488.17.05892-8. [DOI] [PubMed] [Google Scholar]
- 9.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429–438. doi: 10.1016/j.jaci.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu SH, Drucker AM, Lebwohl M, Silverberg JI. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J Am Acad Dermatol. 2018;78:733–740.e11. doi: 10.1016/j.jaad.2017.09.074. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt J, Schakel K, Folster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. 2010;162:661–668. doi: 10.1111/j.1365-2133.2009.09561.x. [DOI] [PubMed] [Google Scholar]
- 13.Munro CS, Levell NJ, Shuster S, Friedmann PS. Maintenance treatment with cyclosporin in atopic eczema. Br J Dermatol. 1994;130:376–380. doi: 10.1111/j.1365-2133.1994.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 14.Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338:137–140. doi: 10.1016/0140-6736(91)90134-b. [DOI] [PubMed] [Google Scholar]
- 15.van Joost T, Heule F, Korstanje M, van den Broek MJ, Stenveld HJ, van Vloten WA. Cyclosporin in atopic dermatitis: a multicentre placebo-controlled study. Br J Dermatol. 1994;130:634–640. doi: 10.1111/j.1365-2133.1994.tb13111.x. [DOI] [PubMed] [Google Scholar]
- 16.Zonneveld IM, De Rie MA, Beljaards RC, Van Der Rhee HJ, Wuite J, Zeegelaar J, et al. The long-term safety and efficacy of cyclosporin in severe refractory atopic dermatitis: a comparison of two dosage regimens. Br J Dermatol. 1996;135 Suppl 48:15–20. doi: 10.1111/j.1365-2133.1996.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 17.Haw S, Shin MK, Haw CR. The efficacy and safety of long-term oral cyclosporine treatment for patients with atopic dermatitis. Ann Dermatol. 2010;22:9–15. doi: 10.5021/ad.2010.22.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol. 2007;21:85–89. doi: 10.1111/j.1468-3083.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema - a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2007;21:606–619. doi: 10.1111/j.1468-3083.2006.02023.x. [DOI] [PubMed] [Google Scholar]
- 20.Bemanian MH, Movahedi M, Farhoudi A, Gharagozlou M, Seraj MH, Pourpak Z, et al. High doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitis. Iran J Allergy Asthma Immunol. 2005;4:139–143. [PubMed] [Google Scholar]
- 21.Granlund H, Erkko P, Remitz A, Langeland T, Helsing P, Nuutinen M, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol. 2001;81:22–27. doi: 10.1080/00015550120235. [DOI] [PubMed] [Google Scholar]
- 22.Haeck IM, Knol MJ, Ten Berge O, van Velsen SG, de Bruin-Weller MS, Bruijnzeel-Koomen CA. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:1074–1084. doi: 10.1016/j.jaad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Ariëns LFM, Gadkari A, van Os-Medendorp H, Ayyagari R, Terasawa E, Kuznik A, et al. Dupilumab versus cyclosporine for the treatment of moderate-to-severe atopic dermatitis in adults: indirect comparison using the eczema area and severity index. Acta Derm Venereol. 2019;99:851–857. doi: 10.2340/00015555-3219. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss MC, Perez-Chada L, Merola JF. Comparative safety of systemic immunomodulatory medications in adults with atopic dermatitis. J Am Acad Dermatol. 2021;85:321–329. doi: 10.1016/j.jaad.2019.05.073. [DOI] [PubMed] [Google Scholar]
- 25.Schram ME, Roekevisch E, Leeflang MM, Bos JD, Schmitt J, Spuls PI. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol. 2011;128:353–359. doi: 10.1016/j.jaci.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Gerbens LAA, Hamann SAS, Brouwer MWD, Roekevisch E, Leeflang MMG, Spuls PI. Methotrexate and azathioprine for severe atopic dermatitis: a 5-year follow-up study of a randomized controlled trial. Br J Dermatol. 2018;178:1288–1296. doi: 10.1111/bjd.16240. [DOI] [PubMed] [Google Scholar]
- 27.Roekevisch E, Schram ME, Leeflang MMG, Brouwer MWD, Gerbens LAA, Bos JD, et al. Methotrexate versus azathioprine in patients with atopic dermatitis: 2-year follow-up data. J Allergy Clin Immunol. 2018;141:825–827.e10. doi: 10.1016/j.jaci.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Goujon C, Viguier M, Staumont-Sallé D, Bernier C, Guillet G, Lahfa M, et al. Methotrexate versus cyclosporine in adults with moderate-to-severe atopic dermatitis: a phase III randomized noninferiority trial. J Allergy Clin Immunol Pract. 2018;6:562–569.e3. doi: 10.1016/j.jaip.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan M, De A, Godse K, Krupa Shankar DS, Zawar V, Sharma N, et al. Guidelines on management of atopic dermatitis in India: an evidence-based review and an expert consensus. Indian J Dermatol. 2019;64:166–181. doi: 10.4103/ijd.IJD_683_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147:324–330. doi: 10.1046/j.1365-2133.2002.04989.x. [DOI] [PubMed] [Google Scholar]
- 32.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839–846. doi: 10.1016/S0140-6736(06)68340-2. [DOI] [PubMed] [Google Scholar]
- 33.Caufield M, Tom WL. Oral azathioprine for recalcitrant pediatric atopic dermatitis: clinical response and thiopurine monitoring. J Am Acad Dermatol. 2013;68:29–35. doi: 10.1016/j.jaad.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hon KL, Ching GK, Leung TF, Chow CM, Lee KK, Ng PC. Efficacy and tolerability at 3 and 6 months following use of azathioprine for recalcitrant atopic dermatitis in children and young adults. J Dermatolog Treat. 2009;20:141–145. doi: 10.1080/09546630802512646. [DOI] [PubMed] [Google Scholar]
- 35.Noguera-Morel L, Knöpfel N, Torrelo A, Hernández-Martín A. A retrospective study of systemic treatment of severe atopic dermatitis with azathioprine: effectiveness and tolerance in 11 pediatric patients. Actas Dermosifiliogr (Engl Ed) 2019;110:227–231. doi: 10.1016/j.ad.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Phan K, Smith SD. Mycophenolate mofetil and atopic dermatitis: systematic review and meta-analysis. J Dermatolog Treat. 2020;31:810–814. doi: 10.1080/09546634.2019.1642996. [DOI] [PubMed] [Google Scholar]
- 37.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4S):S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 39.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 40.de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE) Br J Dermatol. 2018;178:1083–1101. doi: 10.1111/bjd.16156. [DOI] [PubMed] [Google Scholar]
- 41.Snast I, Reiter O, Hodak E, Friedland R, Mimouni D, Leshem YA. Are biologics efficacious in atopic dermatitis? A systematic review and meta-analysis. Am J Clin Dermatol. 2018;19:145–165. doi: 10.1007/s40257-017-0324-7. [DOI] [PubMed] [Google Scholar]
- 42.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56. doi: 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE) J Am Acad Dermatol. 2018;78:863–871.e11. doi: 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143:135–141. doi: 10.1016/j.jaci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 46.Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018;142:1121–1130.e7. doi: 10.1016/j.jaci.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 48.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013–1021. doi: 10.1016/j.jaad.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 49.Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double-blind, phase 2a trial. J Am Acad Dermatol. 2018;78:872–881.e6. doi: 10.1016/j.jaad.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khattri S, Brunner PM, Garcet S, Finney R, Cohen SR, Oliva M, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26:28–35. doi: 10.1111/exd.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HH, Li YC, Huang YC. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2016;138:1719–1722.e1. doi: 10.1016/j.jaci.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 52.Sher LG, Chang J, Patel IB, Balkrishnan R, Fleischer AB., Jr Relieving the pruritus of atopic dermatitis: a meta-analysis. Acta Derm Venereol. 2012;92:455–461. doi: 10.2340/00015555-1360. [DOI] [PubMed] [Google Scholar]
- 53.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 54.Matterne U, Böhmer MM, Weisshaar E, Jupiter A, Carter B, Apfelbacher CJ. Oral H1 antihistamines as ‘add-on’ therapy to topical treatment for eczema. Cochrane Database Syst Rev. 2019;1:CD012167. doi: 10.1002/14651858.CD012167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diepgen TL. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol. 2002;13:278–286. doi: 10.1034/j.1399-3038.2002.01047.x. [DOI] [PubMed] [Google Scholar]
- 56.Langeland T, Fagertun HE, Larsen S. Therapeutic effect of loratadine on pruritus in patients with atopic dermatitis. A multi-crossover-designed study. Allergy. 1994;49:22–26. doi: 10.1111/j.1398-9995.1994.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 57.Kawashima M, Tango T, Noguchi T, Inagi M, Nakagawa H, Harada S. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol. 2003;148:1212–1221. doi: 10.1046/j.1365-2133.2003.05293.x. [DOI] [PubMed] [Google Scholar]
- 58.Yamanaka K, Motomura E, Noro Y, Umeda K, Morikawa T, Umeda-Togami K, et al. Olopatadine, a non-sedating H1 antihistamine, decreases the nocturnal scratching without affecting sleep quality in atopic dermatitis. Exp Dermatol. 2015;24:227–229. doi: 10.1111/exd.12630. [DOI] [PubMed] [Google Scholar]
- 59.Gambichler T, Kreuter A, Tomi NS, Othlinghaus N, Altmeyer P, Skrygan M. Gene expression of cytokines in atopic eczema before and after ultraviolet A1 phototherapy. Br J Dermatol. 2008;158:1117–1120. doi: 10.1111/j.1365-2133.2008.08498.x. [DOI] [PubMed] [Google Scholar]
- 60.Pérez-Ferriols A, Aranegui B, Pujol-Montcusí JA, Martín-Gorgojo A, Campos-Domínguez M, Feltes RA, et al. Phototherapy in atopic dermatitis: a systematic review of the literature. Actas Dermosifiliogr. 2015;106:387–401. doi: 10.1016/j.ad.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, Velazquez D, Arsuaga C, Lázaro P. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19–23. doi: 10.1111/dth.12273. [DOI] [PubMed] [Google Scholar]
- 62.Di Rienzo V, Cadario G, Grieco T, Galluccio AG, Caffarelli C, Liotta G, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, open, parallel-group study. Ann Allergy Asthma Immunol. 2014;113:671–673.e1. doi: 10.1016/j.anai.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Galli E, Chini L, Nardi S, Benincori N, Panei P, Fraioli G, et al. Use of a specific oral hyposensitization therapy to Dermatophagoides pteronyssinus in children with atopic dermatitis. Allergol Immunopathol (Madr) 1994;22:18–22. [PubMed] [Google Scholar]
- 64.Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to Dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy. 1992;22:440–446. doi: 10.1111/j.1365-2222.1992.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman HS, Roth HL. Hyposensitization with alum precipitated extracts in atopic dermatitis: a placebo-controlled study. Ann Allergy. 1974;32:321–330. [PubMed] [Google Scholar]
- 66.Leroy BP, Boden G, Lachapelle JM, Jacquemin MG, Saint-Remy JM. A novel therapy for atopic dermatitis with allergen-antibody complexes: a double-blind, placebo-controlled study. J Am Acad Dermatol. 1993;28(2 Pt 1):232–239. doi: 10.1016/0190-9622(93)70033-p. [DOI] [PubMed] [Google Scholar]
- 67.Luna-Pech JA, Newton-Sanchez OA, Torres-Mendoza BM, Garcia-Cobas CY. Efficacy of sublingual immunotherapy in the severity of atopic dermatitis in children with allergic sensitization to dermatophagoides pteronyssinus. Ann Allergy Asthma Immunol. 2013;111:A8. [Google Scholar]
- 68.Novak N, Bieber T, Hoffmann M, Fölster-Holst R, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012;130:925–931.e4. doi: 10.1016/j.jaci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007;120:164–170. doi: 10.1016/j.jaci.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Qin YE, Mao JR, Sang YC, Li WX. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. 2014;53:650–655. doi: 10.1111/ijd.12302. [DOI] [PubMed] [Google Scholar]
- 71.Sánchez Caraballo JM, Cardona Villa R. Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial. ISRN Allergy. 2012;2012:183983. doi: 10.5402/2012/183983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silny W, Czarnecka-Operacz M. [Specific immunotherapy in the treatment of patients with atopic dermatitis--results of double blind placebo controlled study] Pol Merkur Lekarski. 2006;21:558–565. Polish. [PubMed] [Google Scholar]
- 73.Warner JO, Price JF, Soothill JF, Hey EN. Controlled trial of hyposensitisation to Dermatophagoides pteronyssinus in children with asthma. Lancet. 1978;2:912–915. doi: 10.1016/s0140-6736(78)91630-6. [DOI] [PubMed] [Google Scholar]
- 74.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–117. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 75.Gendelman SR, Lang DM. Specific immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Ann Allergy Asthma Immunol. 2013;111:555–561. doi: 10.1016/j.anai.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 76.Gendelman SR, Lang DM. Sublingual immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Curr Allergy Asthma Rep. 2015;15:498. doi: 10.1007/s11882-014-0498-5. [DOI] [PubMed] [Google Scholar]
- 77.Tam H, Calderon MA, Manikam L, Nankervis H, García Núñez I, Williams HC, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774. doi: 10.1002/14651858.CD008774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 79.Nomura I, Tanaka K, Tomita H, Katsunuma T, Ohya Y, Ikeda N, et al. Evaluation of the staphylococcal exotoxins and their specific IgE in childhood atopic dermatitis. J Allergy Clin Immunol. 1999;104(2 Pt 1):441–446. doi: 10.1016/s0091-6749(99)70390-8. [DOI] [PubMed] [Google Scholar]
- 80.Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658–663. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 81.Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol. 2011;164:228. doi: 10.1111/j.1365-2133.2010.10078.x. [DOI] [PubMed] [Google Scholar]
- 82.Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. J Allergy Clin Immunol. 2001;108:651–652. doi: 10.1067/mai.2001.118598. [DOI] [PubMed] [Google Scholar]
- 83.Svejgaard EL, Larsen PØ, Deleuran M, Ternowitz T, Roed-Petersen J, Nilsson J. Treatment of head and neck dermatitis comparing itraconazole 200 mg and 400 mg daily for 1 week with placebo. J Eur Acad Dermatol Venereol. 2004;18:445–449. doi: 10.1111/j.1468-3083.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 84.Wessels MW, Doekes G, Van Ieperen-Van Kijk AG, Koers WJ, Young E. IgE antibodies to Pityrosporum ovale in atopic dermatitis. Br J Dermatol. 1991;125:227–232. doi: 10.1111/j.1365-2133.1991.tb14745.x. [DOI] [PubMed] [Google Scholar]
- 85.Lintu P, Savolainen J, Kortekangas-Savolainen O, Kalimo K. Systemic ketoconazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy. 2001;56:512–517. doi: 10.1034/j.1398-9995.2001.056006512.x. [DOI] [PubMed] [Google Scholar]
- 86.Wollenberg A, Wetzel S, Burgdorf WH, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667–674. doi: 10.1016/j.jaci.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389–395. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- 88.Passeron T, Lacour JP, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy. 2006;61:431–437. doi: 10.1111/j.1398-9995.2005.00956.x. [DOI] [PubMed] [Google Scholar]
- 89.Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin Exp Allergy. 2006;36:629–633. doi: 10.1111/j.1365-2222.2006.02485.x. [DOI] [PubMed] [Google Scholar]
- 90.Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010;11:351–361. doi: 10.2165/11531420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 91.Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol. 2010;104:343–348. doi: 10.1016/j.anai.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 92.Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol. 2012;166:129–136. doi: 10.1111/j.1365-2133.2011.10596.x. [DOI] [PubMed] [Google Scholar]
- 93.Yeşilova Y, Çalka Ö, Akdeniz N, Berktaş M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24:189–193. doi: 10.5021/ad.2012.24.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2012;23:667–673. doi: 10.1111/pai.12010. [DOI] [PubMed] [Google Scholar]
- 95.Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 96.van der Aa LB, Heymans HS, van Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy. 2010;40:795–804. doi: 10.1111/j.1365-2222.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- 97.Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2012;42:112–122. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 98.Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46 Suppl:S33–S40. doi: 10.1097/MCG.0b013e31826a8468. [DOI] [PubMed] [Google Scholar]
- 99.Drago L, Iemoli E, Rodighiero V, Nicola L, De Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol. 2011;24:1037–1048. doi: 10.1177/039463201102400421. [DOI] [PubMed] [Google Scholar]
- 100.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179–185. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 101.Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892–897. doi: 10.1136/adc.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fölster-Holst R, Müller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol. 2006;155:1256–1261. doi: 10.1111/j.1365-2133.2006.07558.x. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, et al. Clinical effects of probiotic bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Med. 2010;53:37–45. [Google Scholar]
- 104.Chernyshov P. Randomized, placebo-controlled trial on clinical and immunologic effects of probiotic containing Lactobacillus rhamnosus R0011 and L. helveticus R0052 in infants with atopic dermatitis. Microb Ecol Health Disease. 2009;21:228–232. [Google Scholar]
- 105.Cukrowska B, Ceregra A, Klewicka E, Slizewska K, Motyl I, Libudzisz Z. Probiotic lactobacillus casei and lactobacillus paracasei strains in treatment of food allergy in children. Prz Pediatr. 2010;40:21–25. [Google Scholar]
- 106.Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, Murrell DF, Tang ML, Roberts A, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135. doi: 10.1002/14651858.CD006135.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2008;74:447–452. doi: 10.4103/0378-6323.42645. [DOI] [PubMed] [Google Scholar]
- 108.Chung BY, Park SY, Jung MJ, Kim HO, Park CW. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409–416. doi: 10.5021/ad.2018.30.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schalin-Karrila M, Mattila L, Jansen CT, Uotila P. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11–19. doi: 10.1111/j.1365-2133.1987.tb04085.x. [DOI] [PubMed] [Google Scholar]
- 110.Bamford JT, Ray S, Musekiwa A, van Gool C, Humphreys R, Ernst E. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;2013:CD004416. doi: 10.1002/14651858.CD004416.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, et al. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. 2012;11:327–330. [PubMed] [Google Scholar]
- 112.Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22:144–150. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 113.Camargo CA, Jr, Ganmaa D, Sidbury R, Erdenedelger K, Radnaakhand N, Khandsuren B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J Allergy Clin Immunol. 2014;134:831–835.e1. doi: 10.1016/j.jaci.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–247. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 115.Udompataikul M, Huajai S, Chalermchai T, Taweechotipatr M, Kamanamool N. The effects of oral vitamin D supplement on atopic dermatitis: a clinical trial with staphylococcus aureus colonization determination. J Med Assoc Thai. 2015;98 Suppl 9:S23–S30. [PubMed] [Google Scholar]
- 116.Hata TR, Audish D, Kotol P, Coda A, Kabigting F, Miller J, et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014;28:781–789. doi: 10.1111/jdv.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ. Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: a systematic review and meta-analysis. Nutrients. 2016;8:789. doi: 10.3390/nu8120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim G, Bae JH. Vitamin D and atopic dermatitis: a systematic review and meta-analysis. Nutrition. 2016;32:913–920. doi: 10.1016/j.nut.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 119.Abrouk M, Farahnik B, Zhu TH, Nakamura M, Singh R, Lee K, et al. Apremilast treatment of atopic dermatitis and other chronic eczematous dermatoses. J Am Acad Dermatol. 2017;77:177–180. doi: 10.1016/j.jaad.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 120.Farahnik B, Beroukhim K, Nakamura M, Abrouk M, Zhu TH, Singh R, et al. Use of an oral phosphodiesterase-4 inhibitor (apremilast) for the treatment of chronic, severe atopic dermatitis: a case report. Dermatol Online J. 2017;23:13030/qt3588p0hn. [PubMed] [Google Scholar]
- 121.Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890–897. doi: 10.1001/archdermatol.2012.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saporito RC, Cohen DJ. Apremilast use for moderate-to-severe atopic dermatitis in pediatric patients. Case Rep Dermatol. 2016;8:179–184. doi: 10.1159/000446836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simpson EL, Imafuku S, Poulin Y, Ungar B, Zhou L, Malik K, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063–1072. doi: 10.1016/j.jid.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 124.Volf EM, Au SC, Dumont N, Scheinman P, Gottlieb AB. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341–346. [PubMed] [Google Scholar]
- 125.Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80:913–921.e9. doi: 10.1016/j.jaad.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 126.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395–399. doi: 10.1016/j.jaad.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 127.Murata Y, Song M, Kikuchi H, Hisamichi K, Xu XL, Greenspan A, et al. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4 R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J Dermatol. 2015;42:129–139. doi: 10.1111/1346-8138.12726. [DOI] [PubMed] [Google Scholar]
- 128.Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:1830–1837.e4. doi: 10.1016/j.jaci.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 129.Walter M, Kottke T, Stark H. The histamine H4 receptor: targeting inflammatory disorders. Eur J Pharmacol. 2011;668:1–5. doi: 10.1016/j.ejphar.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 130.Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J Allergy Clin Immunol. 2009;123:619–625. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 131.Mobasher P, Heydari Seradj M, Raffi J, Juhasz M, Atanaskova Mesinkovska N. Oral small molecules for the treatment of atopic dermatitis: a systematic review. J Dermatolog Treat. 2019;30:550–557. doi: 10.1080/09546634.2018.1544412. [DOI] [PubMed] [Google Scholar]
- 132.Narla S, Hsu DY, Thyssen JP, Silverberg JI. Inpatient financial burden of atopic dermatitis in the United States. J Invest Dermatol. 2017;137:1461–1467. doi: 10.1016/j.jid.2017.02.975. [DOI] [PubMed] [Google Scholar]
- 133.Weber MB, Fontes Neto Pde T, Prati C, Soirefman M, Mazzotti NG, Barzenski B, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. J Eur Acad Dermatol Venereol. 2008;22:992–997. doi: 10.1111/j.1468-3083.2008.02697.x. [DOI] [PubMed] [Google Scholar]
- 134.Jang YH, Lee JS, Kim SL, Song CH, Jung HD, Shin DH, et al. A family-engaged educational program for atopic dermatitis: a seven-year, multicenter experience in Daegu-Gyeongbuk, South Korea. Ann Dermatol. 2015;27:383–388. doi: 10.5021/ad.2015.27.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lambert J, Bostoen J, Geusens B, Bourgois J, Boone J, De Smedt D, et al. A novel multidisciplinary educational programme for patients with chronic skin diseases: Ghent pilot project and first results. Arch Dermatol Res. 2011;303:57–63. doi: 10.1007/s00403-010-1082-z. [DOI] [PubMed] [Google Scholar]
- 136.Heratizadeh A, Werfel T, Wollenberg A, Abraham S, Plank-Habibi S, Schnopp C, et al. Effects of structured patient education in adults with atopic dermatitis: Multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845–853.e3. doi: 10.1016/j.jaci.2017.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the Korean Atopic Dermatitis at http://www.atopy.re.kr/.