Abstract
Background & Aims:
Perturbations in the early life gut microbiome are associated with increased risk for complex immune disorders like inflammatory bowel diseases. We previously showed maternal antibiotic-induced gut dysbiosis vertically transmitted to offspring increases experimental colitis risk in IL-10 gene deficient (IL-10−/−) mice, a finding that may result from the loss/lack of essential microbes needed for appropriate immunological education early in life. Here, we aimed to identify key microbes required for proper development of the early life gut microbiome that decrease colitis risk in genetically susceptible animals.
Methods:
Metagenomic sequencing followed by reconstruction of metagenome-assembled genomes (MAGs) was performed on fecal samples of IL-10−/− mice with and without antibiotic-induced dysbiosis to identify potential missing microbial members needed for immunological education. One high value target strain was then engrafted early and/or late into the gut microbiomes of IL-10−/− mice with antibiotic-induced dysbiosis.
Results:
Early, but not late life engraftment of a single dominant Bacteroides strain of non-antibiotic treated IL-10−/− mice was sufficient to restore the development of the gut microbiome, promote immune tolerance, and prevent colitis in IL-10−/− mice that had antibiotic-induced dysbiosis.
Conclusion:
Restitution of a keystone microbial strain missing in the early life antibiotic-induced gut dysbiosis results in recovery of the microbiome, proper development of immune tolerance, and reduced risk for colitis in genetically prone hosts.
Keywords: Inflammatory bowel diseases, dysbiosis, immune tolerance, keystone microbes
Introduction
Inflammatory bowel diseases (IBD) are chronic disorders of unclear etiology that arise from convergence of environmental, genetic, and microbial factors. However, the rapid increase in prevalence and incidence of IBD over the past century suggests environmental factors and shifts in the gut microbiome, rather than genetic drift, are major risk factors.1-3 In this regard, several retrospective clinical studies have suggested that antibiotic exposure in early life increases risk for IBD by creating a dysbiosis that can impair the maturation of the host immune system 4-6, but none have been able to establish causality and mechanism. Here, we test this notion by employing a well-established murine model of experimental colitis that presents with many of the epidemiological features of human IBD.7, 8 Our model involves the administration of the broad spectrum antibiotic cefoperazone (CPZ) to perturb the gut microbiota during the peripartum period of interleukin (IL)-10 deficient (IL-10 KO) dams which is then vertically transmitted to the offspring7. The acquired gut microbiota skews immune development, increasing risk and severity of both spontaneous colitis and chemical-induced colitis in offspring.
This study was designed to answer the following questions: (1) Is there a window of immune development early in life that is critically dependent on proper gut microbiome development? (2) Does this window close later in life, resulting in long-term colitis risk, especially in genetically prone hosts? (3) If immune tolerance to missing commensal microbes does not develop, is late life exposure associated with increased colitis risk in genetically prone hosts? (4) Can proper immune development be restored with a single indigenous microbial strain absent from CPZ-induced gut perturbations to reduce risk of spontaneous colitis? and (5) When does this intervention have to take place?
Our findings provide proof-of-concept that the long-term risk for colitis associated with early life antibiotic-induced gut dysbiosis can be reversed through microbial restitution. We identified and cultivated a dominant Bacteroides strain that is associated with colitis outcomes using genome-resolved metagenomics. Our analyses showed that this strain (‘Bacteroides sp. CL1-UC’) is capable of engrafting into an antibiotic-induced dysbiotic gut microbiota, altering subsequent microbial membership and significantly reducing risk for spontaneous colitis development, but only if the intervention is performed early in life. Without early immune tolerance to missing gut microbes, later life exposure is associated with increased colitis risk in a genetically prone host.
Materials and Methods
Animals
For these studies, germ-free C57Bl/6 IL-10 KO mice fed autoclaved 5K67 ((LabDiet, MO, USA) were bred in the UChicago Gnotobiotic Research Animal Facility (IACUC protocol 72101).
Shotgun metagenomics and genome-resolved metagenomics
Metagenomic sequencing for fecal DNA samples was performed via Illumina HiSeq at the Marine Biological Laboratory (Woods Hole, MA) which yielded paired-end reads of 2x150 nucleotides. Noisy sequences were denoised with ‘iu-filter-quality-minoche’9 using default parameters.10 The remaining reads were co-assembled using MEGAHIT11 v1.0.3 with a minimum scaffold length of 2.5 kbp, and scaffold header names were simplified using anvi’o12 v6.1 (available from https://merenlab.org/software/anvio). Scaffolds were subsequently binned as previously described (Supplemental Method)12, 13. Bins with <10% of redundancy, and with either >2Mbp in length or >70% of estimated completion were defined as metagenome-assembled genomes (MAGs), manually curated using the same interactive interface. The program ‘nvi-summariz’ reported final FASTA files for MAGs and CheckM14 to infer their taxonomy using 43 single-copy gene markers.
Statistical analysis
Mann-Whitney U test was performed with GraphPad Prism (GraphPad Software, CA) to compare 16s rRNA gene copy number, Shannon diversity index, T cell populations, body weights, histological scores, fecal LCN-2 levels, and cytokine levels between NT and CPZ groups. For 16S rRNA gene amplicon sequencing analysis, PERMANOVA tests were computed with QIIME215 to assess bacterial composition differences between treatment groups or for unweighted and weighted UniFrac distance data. Statistical significance was achieved at p < 0.05. MAG detection rates between CPZ-colitis group vs. CPZ-no-colitis group at 11 weeks of age were compared using student’s t-test and p-values were adjusted for multiple-test using the Benjamini-Hochberg method. 16 The criterion for significance was set at a false discovery rate (FDR) < 0.05.
Data Availability
We made raw sequencing data for all metagenomes publicly available (see Table S1 for MG-RAST IDs). In addition, we have made available as anvi’o profiles (1) individual co-assemblies and the final set of metagenome-assembled genomes (doi:10.6084/m9.figshare.11955060) which offer reproducible access to detection and coverage statistics of each genome across metagenomes, and (2) pangenomes (doi:10.6084/m9.figshare.11968674 for Figure 1B and doi:10.6084/m9.figshare.11968692 for Figure 1C) which offer reproducible access to functions and sequences of each gene cluster and their distribution across genomes. The raw sequencing data for 16S rRNA gene amplicon sequences reported in this study are also publicly available (MG-RAST mgp82768).
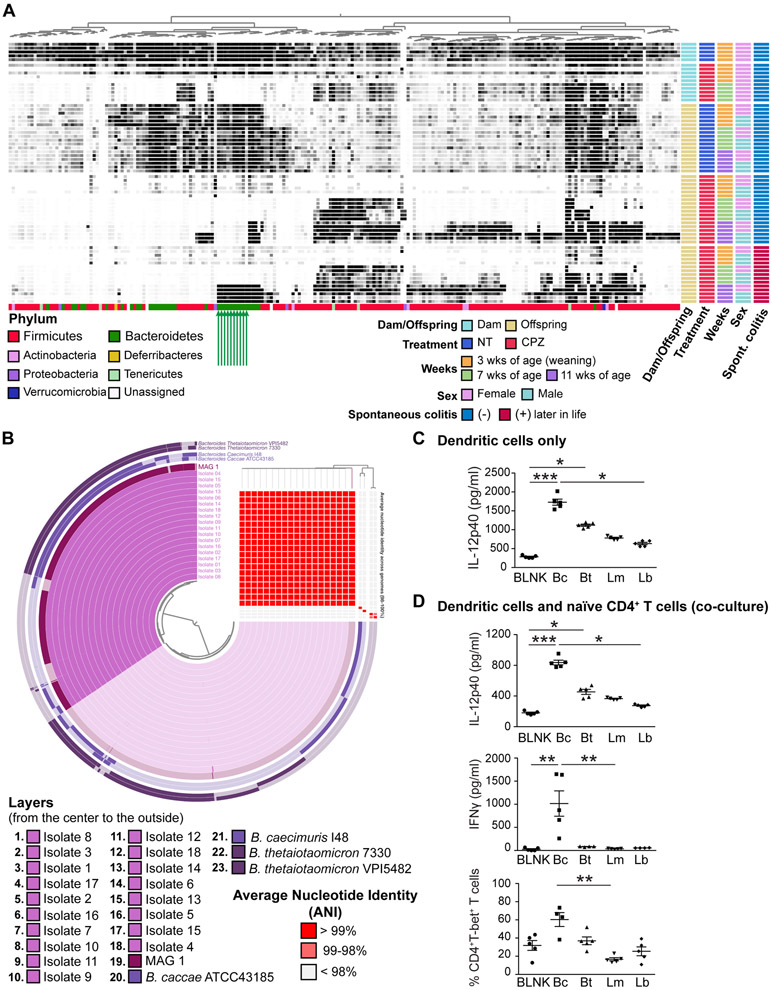
Figure 1. Identification and cultivation of a key commensal Bacteroides strain that is important for host immune development.
(A) The detection rates of bacterial metagenomic-assembled genomes (MAGs) in fecal samples obtained from dams and pups over time. Each column represents a single MAG and each row represents an individual sample. The darker the cell, the greater the relative abundance. Metadata for each sample is presented to the right of the heat map. Colored bars at the bottom of the heatmap represent the MAG annotations at the phylum level. Green arrows indicate CPZ-sensitive MAGs that are residents of IL-10 KO dams and their NT offspring that did not develop colitis, but which emerged later in life of the CPZ group offspring that develop colitis. (B) Pangenome analysis and assessment of average nucleotide identity (ANI) for 18 assembled genomes based on whole genome sequencing for 18 isolated cultivars compatible with MAG1 shown together with MAG1 and the four most similarly related complete genomes registered in the BLAST database. Each circular layer represents an individual MAG and darker areas in each layer show the gene clusters contained by each MAG contains. The ANI is presented in the right upper area corner in this figure. Each column represents an individual MAG corresponding to the circular layer. Each row presents an individual MAG in the order corresponding to the order of round layers from top to bottom. Red cell means > 99% identity. Detailed results of ANI are presented in Table S4. (C) IL-12p40 production of dendritic cells (DCs). DCs were isolated from spleen of male IL-10 KO mice at 7 weeks of age and stimulated using bacterial lysates from Bacteroides strain CL1-UC(Bc), B. thetaiotaomicron (Bt), Lactobacillus murinus (Lm), and Lachnospiraceae bacterium (ATCC BAA-2281) (Lb), or BLNK: blank control with sterile PBS (n = 5 per group). (D) IL-12p40 and IFNγ production as well as Th1 differentiation in co-culture assays of DCs and naïve CD4+ T cells from male animals. Flow cytometry data represent the percentage of live TCRβ+CD4+T-bet+ cells out of live TCRβ+CD4+ cells (n = 4-5 per group). *p < 0.05, **p < 0.01, and ***p < 0.001. Data represent mean ± SEM for (C) and (D).
Results
Identification and cultivation of a novel commensal Bacteroides strain essential for host immune development in early life
Via 16S rRNA gene amplicon sequencing analysis, we previously demonstrated that many amplicon sequencing variants (ASVs) from the phylum Bacteroidetes were eradicated with CPZ treatment of dams during the peripartum period and were also absent from vertically transferred gut microbiota of offspring at 3 and 7 weeks of age.7 However, some ASVs appeared to reemerge at 11 weeks of age, particularly in pups that developed spontaneous colitis later in life. It is notable that the reemerging ASVs were part of the commensal bacteria in the no treatment (NT) maternal and offspring gut microbiota of IL-10 KO mice, yet none developed colitis. For a genome-resolved investigation of microbial succession to identify microbial members that likely contribute to the colitis phenotype, we performed metagenomic sequence assemblies and binning of reconstruct metagenome-assembled genomes (MAGs) from the same fecal DNA previously used for 16S rRNA amplicon sequencing.7 Samples included those harvested from NT and peripartum CPZ-exposed dams at weaning as well as 4 weeks after weaning, and NT and CPZ offspring at 3, 7, and 11 weeks of age (Supplementary Table S1). No spontaneous colitis was observed in NT mice throughout the study while only some, but not all, CPZ mice developed colitis at 12 to 23 weeks of age. However, neither NT or CPZ offspring exhibited clinical symptoms of colitis at the time of fecal collections. Along with NT samples, we included samples from CPZ pups that did (CPZ-colitis group) or did not (CPZ-no-colitis group) develop overt spontaneous colitis later in life. From the total set of 66 metagenomes, we reconstructed 216 non-redundant total MAGs recruiting on average 42.5% of reads from each metagenome (Table S1). Figure 1A displays the distribution of these MAGs across samples, where each column represents an individual MAG and each row represents an individual sample. Analysis of MAGs via anvi’o12 revealed gut bacterial composition at nearly strain level.
Ten MAGs belonging to the phylum Bacteroidetes (MAGs 1-10; marked with green arrows in Figure 1A with details in Table 1) exhibited unique detection patterns in both female and male mice: while they were clearly detected in NT dams and NT offspring at all time points, they were absent in dams and their offspring following peripartum CPZ exposure. Interestingly, these MAGs appeared to reemerge in the CPZ-colitis group but not in their CPZ-no-colitis group counterparts at 11 weeks of age in both sexes. These findings suggest that MAGs 1-10 represent bacterial populations that (1) are resident microbiota of our specific pathogen free (SPF) IL-10 KO mice, (2) are extinguished by CPZ, (3) appear to be non-pathogenic when the host acquires them as commensal bacteria early in life, and (4) can be colitogenic when initial exposure of the naïve, genetically susceptible host occurs late in life.
Table 1.
Metagenome-assembled genomes (MAGs) 1-10 information
| Length (Mbp) | Percent of completion (%) |
Percent of redundancy (%) |
Phylum | Class | Order | Family | Genus | |
|---|---|---|---|---|---|---|---|---|
| MAG 1 | 4.29 | 99.3 | 2.2 | Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides |
| MAG 2 | 2.13 | 96.4 | 1.4 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 3 | 2.23 | 92.8 | 0.7 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 4 | 2.43 | 92.8 | 0.7 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 5 | 2.67 | 96.4 | 0.7 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 6 | 2.43 | 92.8 | 1.4 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 7 | 2.02 | 96.4 | 0.7 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 8 | 2.51 | 71.2 | 1.4 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 9 | 2.47 | 95.7 | 1.4 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
| MAG 10 | 2.44 | 97.8 | 1.4 | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | - |
To further study the potential role of these MAGs in both early life immune tolerance development, later life immune system skewing, and onset of colitis, we isolated resident bacterial strains corresponding to these MAGs from fecal samples obtained from 11-week-old CPZ-colitis group male mice, a time point where their reemergence was observed. Pangenomics analysis with anvi’o was performed to design 4 targeted PCR primer sets to gene clusters unique for each of the MAGs 1-10 (Supplemental Method, Figure S1A, and Tables S2 and S3). Primers were validated using DNA from fecal samples containing genomes of MAGs 1-10, i.e. fecal samples from 11-week-old CPZ-colitis group males used for metagenomic shotgun sequencing (Figure S1B). Targeted PCR was then performed on isolates cultivated anaerobically from the same fecal samples corresponding to MAGs 1-10. We subcultivated an isolate that was positive for all 4 validated primer sets for each MAG and obtained 18 cultivars that appeared to be MAG 1. We were unable to establish cultivars for MAGs 2-10 which we attributed to the significantly greater relative abundance of MAG 1 relative to MAGs 2-10, giving advantage to MAG 1 in outcompeting other strains under selective growth conditions. Whole genome sequencing (WGS) was performed on these 18 isolates. Whole genomes for each isolate were assembled with PATRIC using raw sequence data. While the top 2 NCBI BLAST database hits for MAG 1 were Bacteroides caccae ATCC41385 and Bacteroides caecimuris I48, the top 2 BLAST hits for WGS assembled genomes for the 18 isolates were Bacteroides thetaiotaomicron 7330 and Bacteroides thetaiotaomicron VPI-5482. Both pangenomics and average nucleotide identity (ANI) analysis using anvi’o that included MAG 1, the 18 cultivar genomes, as well as the genomes of B. caecimuris I48, B. thetaiotaomicron 7330, B. caccae ATCC41385, and B. thetaiotaomicron VPI-5482 supported that the 18 WGS-assembled genomes originated from a single strain (ANI 99.99%) that was closely related to MAG 1 (ANI average 99.84%). These assembled genomes were distinct from the four Bacteroides species/strains based on BLAST results (ANI 81.32% to 90.17%) (Figure 1B and Table S4). Comprehensive genome analysis (CGA) via PATRIC revealed our isolate was most closely related to isolates from human feces, including Bacteroides sp. CAG:754 and reference organism Bacteroides finegoldii DSM 17565 (Figure S1C). However, our isolate was from murine feces and unique enough to be considered a novel strain, which we provisionally named Bacteroides strain CL1-UC (Bc).
We next examined whether communities with or without Bc exhibited immunogenic properties using both in-vivo and in-vitro approaches. As previously shown, vertical transmission of maternal CPZ-induced dysbiosis to their offspring skews their CD4+ T cell subpopulations at 3 weeks of age towards Th1 and Th17 development.7 This time, using the identical peripartum-CPZ treatment protocol (Figure S2A), we examined regulatory T cells (Tregs), Th1 cells, and Th17 cells at 7 weeks of age. The CPZ group exhibited significant increases in Th1 and Th17 cells in spleen (SPLN) (p < 0.05 for both), as well as Th17 cells in mesenteric lymph nodes (MLNs) (p < 0.05) compared to their NT counterparts. A similar trend was seen in colonic lamina propria (LP) (Figures S2B and S2C). Thus, the pro-inflammatory pattern is similar to that previously observed at 3 weeks of age.7 In contrast, we observed no differences in dendritic cell (DC) IL-12p40 production of NT and CPZ mice following stimulation with vehicle, NT stool, or CPZ stool. Additionally, 7-weeks-old NT and CPZ feces elicited similar IL-12p40 production from NT and CPZ DCs (Figure S2D). Thus, the persistent imbalance of CD4+ T cell subsets observed in CPZ mice appears to arise from missing, yet essential gut microbiome-derived cues for proper early life immunological development rather than from emergence of disease-promoting pathobionts. We next determined if the imbalance in CD4+ T cell subpopulations promotes immune activation in vitro and in vivo. We harvested both SPLN and MLN cells from CPZ and NT IL-10 KO offspring at 7 weeks of age followed by non-specific stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. CPZ group animals exhibited significantly increased IFNγ-producing CD4+ splenic T cells (p < 0.05) and significantly increased IL-17A-producing MLN CD4+ T cells (p < 0.05) compared to the NT group (Figures S2E and S2F). Thus, CPZ offspring exhibit skewing in CD4+ T cells at 7 weeks of age that produces pro-inflammatory Th1 and Th17 cytokines, IFNγ and IL-17A, relative to NT offspring. To assess the colitogenic potential of the skewed T cell subsets in vivo, we transferred splenic CD4+ T cells of 7-week-old CPZ and NT IL-10 KO mice into age- and sex-matched RAG1 KO mice. We prepared 2 groups; NT recipients with CD4+ T cells from NT IL-10 KO offspring and CPZ recipients with CD4+ T cells from CPZ IL-10 KO offspring. The control group only received PBS vehicle and did not exhibit body weight loss or other clinical symptoms of colitis. In contrast, CPZ RAG1 KO recipients experienced earlier and more severe weight loss compared to NT recipients (Figure S3A). The CPZ recipient survival rate was also decreased compared to NT recipients (Figure S3B). Fecal LCN-2, a biomarker of colonic inflammation, rose more rapidly over time in CPZ recipients beginning 2 weeks after CD4+ T cell transfer (p < 0.001) (Figure S3C). The histology score for colitis in animals that survived the entire 8-week post-CD4+ T cell transfer observation period was significantly higher in CPZ recipients as compared to NT recipients (Figure S3D). TNF-α and IL-6 cytokine levels were higher in CPZ recipient colonic mucosa as compared to NT counterparts (Figure S3E). Thus, CD4+ T cells of 7-week-old offspring with maternal CPZ-induced dysbiosis are more colitogenic in vivo as compared to their NT counterparts. Together, these studies show that the types of gut microbes acquired early in life play a deterministic role in the development of the host immune system. Antibiotic-induced dysbiosis during early life causes improper development of CD4+ T cells that persists and increases risk for colitis later in life in genetically susceptible hosts. This is consistent with other reports showing Th1 and DCs play important roles in the promoting colitis in IL-10 KO mice.8, 17-19
We next compared the immunogenic properties of Bc to B. thetaiotaomicron (Bt), given their genomic similarity determined via BLAST. Because of the known immune pathogenesis of spontaneous colitis in IL-10 KO mice,8, 17, 18 we investigated the immunological impact of Bt and Bc on Th1 cells and DCs. Direct stimulation of CD4+ T cells with lysates of either Bc or Bt did not induce IFNγ or IL-2 production by T cells alone, suggesting a T cell-extrinsic effect (Figure S1D). To further examine the immunogenic impact of Bc on T cells mediated by stimulation with DCs, splenic DCs from 7-week-old IL-10 KO mice were stimulated with lysates of Bt, Lactobacillus murinus (Lm), and Lachnospiraceae bacterium (Lb).17 IL-12p40 production by DCs of both male and female mice was significantly induced by exposure to Bc lysate (Figures 1C and S1E). Bc also stimulated more IL-12p40 and IFNγ production and promoted Th1 differentiation (Figures 1D and S1F) when splenic DCs and naïve CD4+ T cells of 7-week-old IL-10 KO mice were cocultured. Gating strategies for flow cytometry are shown in Figure S1G. Overall, our results show that Bc has unique immunogenic properties that play a role in host immune development early in life through a T cell extrinsic mechanism.
We next performed proteome comparisons between Bc and Bt (B. thetaiotaomicron 7330 and B. thetaiotaomicron VPI-5482) using PATRIC which showed that of 4540 genes detected in Bc, 1426 genes (31.4%) were only in Bc, 123 genes (2.7%) were in Bc and Bt 7330, 64 genes (1.4%) were in Bc and Bt VPI-5482, and 2927 genes (64.5%) were in all strains (Table S5). Thus, most Bc genes are shared among the 3 strains. Of the 1426 genes detected only in Bc, 246 genes could be functionally annotated, of which 33 genes appeared to encode mobile element, mobilization, and membrane proteins, which could influence and/or possibly be recognized by host immune cells.
Early life engraftment by Bacteroides CL1-UC (Bc) restores development of gut microbiota in IL-10 KO offspring with antibiotic-induced dysbiosis
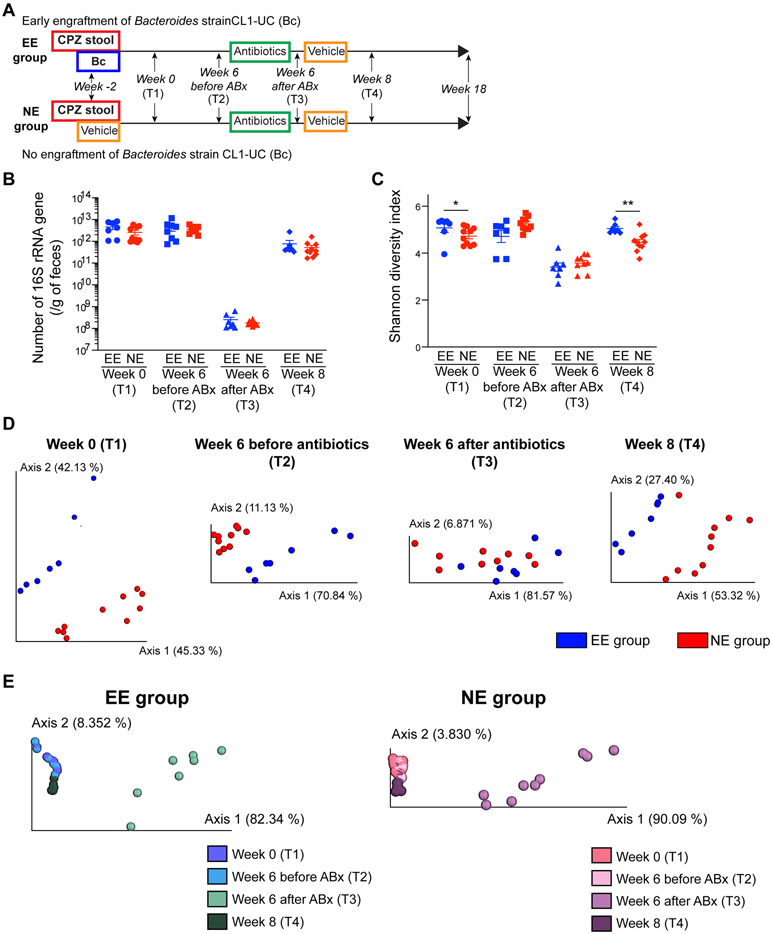
We investigated the impact of early life exposure to Bc on the gut microbiome of IL-10 KO offspring receiving CPZ-altered gut microbiota as well as on host immune development and colitis outcomes. The study was designed to answer these questions: (1) Can Bc engraftment into an early life CPZ-induced dysbiotic gut ecosystem restore immune development, even if it is eradicated later in life, reducing spontaneous colitis risk in IL-10 KO mice, i.e. immune imprinting?, and (2) Can Bc engraftment later in life (past the window of opportunity) restore commensal gut microbiota immune tolerance and reduce risk for development of spontaneous colitis in IL-10 KO mice? The first question was addressed by performing fecal microbiome transplantation (FMT) of CPZ-induced dysbiosis into male germ-free (GF) IL-10 KO mice at weaning (3 weeks of age). Donor fecal samples were collected from offspring of CPZ-treated dams in our previous study7 and the absence of Bc at 7-weeks-of-age was confirmed by MAG analysis in the current study. For early engraftment,, mice were gavaged with live Bc culture immediately following FMT (100 μl of 5 X 108 CFU/ml per day, two days) (designated early engraftment of Bc; EE group) or vehicle (no engraftment of Bc; NE group) (Figures 2A and S4A). One week after gavage, PCR using the Bc specific primer showed successful engraftment into 3-week-old mice conventionalized with CPZ-associated microbiota, whereas it remained absent in NE controls not exposed to Bc (Figure S4B). The animals were tracked for 18 weeks starting at 5 weeks of age to assess health status, including body weight and clinical colitis symptoms (Figure 2A). At week 6 (11 weeks of age), all animals were treated with a broad-spectrum antibiotic (Abx) cocktail protocol (including vancomycin, neomycin, and CPZ) applied in the drinking water for 72 hours for two purposes: (1) to test if persistent engraftment or simply early exposure to Bc was sufficient to reduce risk for spontaneous colitis, and (2) to provide additional controls for studies presented in Figure 3. After a 24-hour recovery period, all mice were moved to new cages and gavaged with sterile PBS with 20% glycerol (Figures 2A and S2A). One week after Abx exposure, Bc had been eradicated in the EE group, despite recovery of the overall microbiota community (Figure S4C).
Figure 2. Early life engraftment of a single Bacteroides strain impacts bacterial membership of the gut microbiome.
(A) Study design using male IL-10 knock-out mice. CPZ-induced dysbiosis was transplanted into germ-free pups at 3 weeks of age by fecal gavage. Following FMT, one cohort of mice was gavaged with live Bacteroides strain CL1-UC (Bc) or vehicle control representing early engraftment (EE) and no engraftment (NE) of Bc groups, respectively. (EE group: n = 7 and NE group: n = 10). Two weeks later (week 0; 5 weeks of age), animals began tracking. A cocktail of broad-spectrum antibiotics (neomycin, vancomycin, and CPZ) were administered at week 6 to perturb the gut microbiome and eradicate Bc in later stages of life, followed by observation to week 18 (T1: week 0, T2: week 6 before antibiotics treatment, T3: week 6 after antibiotics treatment [prior to the second gavage], T4: week 8 [2 weeks after the second gavage]). Gavage and treatment schedule details are presented in Figure S4A. (B) The number of 16S rRNA gene per gram of feces from EE and NE animals over time (T1-T4). (C) Shannon diversity index of EE and NE animals over time. (D) PCoA plots of 16S rRNA gene amplicon sequence weighted UniFrac distances between EE vs. NE groups at each time point (T1-T4). Bacterial compositions were different between EE and NE groups at all time points. (E) PCoA plots of weighted UniFrac distances showing over-time shifts of bacterial compositions in EE group (left panel) and NE group (right panel). The bacterial structure at T4 recovered to a state before antibiotics treatment. *p < 0.05 and **p < 0.01. Data represent mean ± SEM for (B) and (C).
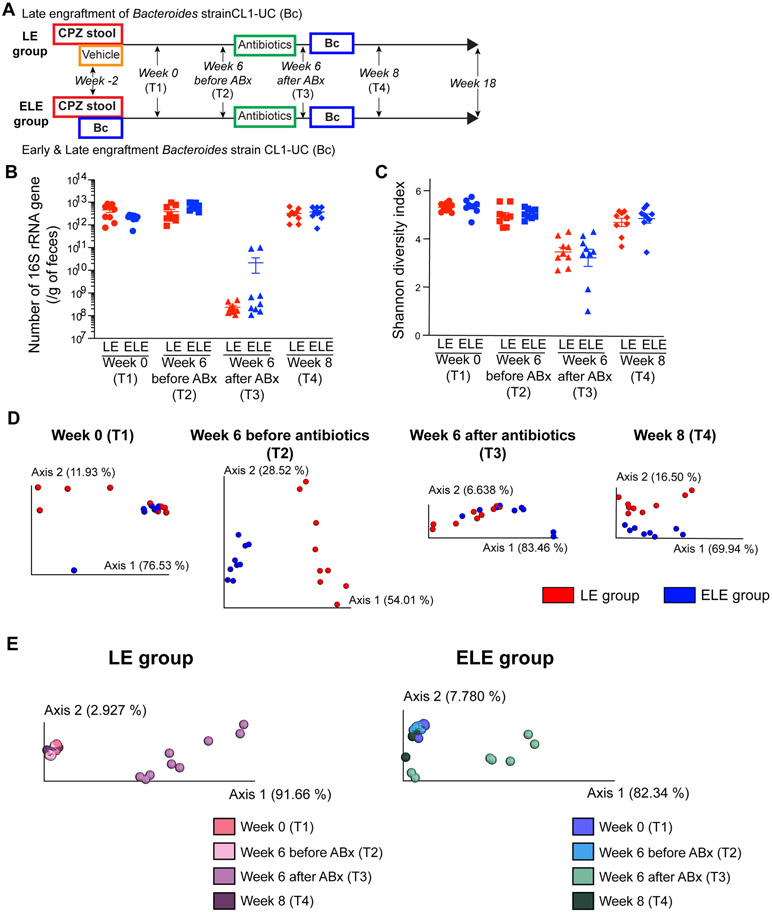
Figure 3. Later life engraftment of a Bacteroides strain does not impact gut microbiome bacterial membership.
(A) Study design to determine effects of late engraftment (LE) versus early and late engraftment (ELE) of Bc in male IL-10 KO mice. The protocol is nearly identical to NE and EE groups shown in Figure 2, except Bc is engrafted later in life in both LE and ELE groups (LE group: n = 9 and ELE group: n = 9). Late engraftment was performed at week 6 (11 weeks of age) after administration of broad-spectrum antibiotics cocktail meant to break microbiome stability and allow engraftment of Bc (T1: week 0, T2: week 6 before antibiotics treatment, T3: week 6 after antibiotics treatment [prior to the second gavage], T4: week 8 [2 weeks after the second gavage]). Gavage and treatment schedule details are presented in Figure S5A. (B) Number of 16S rRNA gene per gram of feces from LC and ELC animals over time (T1-T4). (C) Shannon diversity index of LE and ELE animals over time. (D) PCoA plots of 16S rRNA gene amplicon sequence weighted UniFrac distances between LE vs. ELE groups at each time point (T1-T4). Bacterial compositions were different between LE and ELE groups at all time points. (E) PCoA plots of weighted UniFrac distances showing shifts in gut microbiota composition over time in LE group (left panel) and ELE group (right panel). Despite antibiotics treatment and late engraftment of Bc, the bacterial structure at week 8 recovered toward that before antibiotics treatment. *p < 0.05 and **p < 0.01. Data represent mean ± SEM for (B) and (C).
We next investigated the impact of Bc engraftment on CPZ-associated gut microbiota over time at week 0 (2 weeks after initial gavage; T1), week 6 (before Abx treatment; T2), week 6 after Abx treatment (prior to the second gavage; T3), and week 8 (2 weeks after the second gavage; T4). No differences in 16S rRNA gene copy number were observed between EE and NE groups at any of the time points. After Abx exposure, the number of 16S rRNA gene dramatically decreased (T3) but recovered 2 weeks later (T4) (Figure 2B). Shannon diversity index decreased (T3) but recovered in 2 weeks (T4), along with 16S rRNA gene copy number (Figure 2C). Shannon diversity index was significantly higher in EE compare to NE at T1 and T4. Unweighted and weighted UniFrac distances showed differences in community membership between EE and NE at all time points (T1: unweighted p = 0.05 and weighted p = 0.001, T2: unweighted p < 0.05 and weighted p = 0.001, T3: unweighted p < 0.05 and weighted p = 0.121, T4: unweighted p < 0.05 and weighted p = 0.001) (Figures S4D (unweighted) and 2D (weighted)). The shift in relative abundance of the Bacteroides genus over time arose from engraftment and eradication of Bc in EE as compared to NE (Figure S4E). NE mice did not contain Bacteroides, while the presence of Bacteroides due to Bc engraftment was detected in EE mice at T1 and T2. Bacterial composition at the phylum level in EE and NE groups at each time point is shown in Figure S4F. The Bacteroides genus corresponded to 59.7% and 53.2% of the Bacteroidetes phylum at week 0 and week 6 in the EE group at T1 and T2. The phylum Bacteroidetes as well as the genus Bacteroides was eradicated in EE mice after Abx particularly at T4, whereas mice in the NE group contained bacteria belonging to the phylum Bacteroidetes, but not to the genus Bacteroides. Importantly, at T4, community composition was indistinguishable from that observed in pre-treatment samples in both the EE and NE groups, respectively (Figures S4G (unweighted) and 2E (weighted)). Thus, early life engraftment with a single bacterial strain, such as Bc, can impact the development of the gut microbiome in the long term.
We next sought to test whether late life engraftment with Bc into CPZ-associated gut dysbiosis, i.e. outside the window of immunological development, can shift gut microbiota and lower risk of spontaneous colitis in IL-10 KO mice. To this end, a modification to the NE and EE group protocol (Figure 2A) was made where late life engraftment of Bc was performed at week 6 following Abx perturbation of the gut microbiome when mice were 11 weeks of age (Figure 3A and Figure S5A). The antibiotic cocktail was identical in the two experiments (Figures S4A and S5A). An Abx “shock” protocol is necessary to engraft a single organism, such as Bc, into a stable microbial community where pre-existing priority conditions prevent non-resident community members from joining.20, 21 In order to compare the effects of early vs. late Bc engraftment, one group of mice received Bc gavage both early and late in life (at 3 weeks of age and 11 weeks of age) (designated early and late engraftment of Bc; ELE group) and the other where only late engraftment was performed at 11 weeks of age (late engraftment of Bc; LE group). Early life engraftment of Bc was confirmed in the ELE group 1 week after the first gavage at weaning (3 weeks of age) (Figure S5B) and again 1 week after Abx and Bc gavage at 11 weeks of age (Figure S5C). Bc also successfully engrafted in the LE group (Figure S5C). Similar to our observations in the EE and NE groups, the estimated bacterial load and Shannon diversity were reduced by Abx, but recovered after 2 weeks in the LE and ELE groups (Figures 3B and 3C). Bacterial composition in the LE and ELE groups were examined via 16S rRNA gene amplicon sequencing over time at T1-T4 identically to the experiment of the EE and NE groups (Figure 3A). Unweighted and weighted UniFrac distances were significantly different between the LE and ELE groups at all time points (T1: unweighted p < 0.01 and weighted p = 0.133, T2: unweighted p = 0.001 and weighted p = 0.001, T3: unweighted p < 0.01 and weighted p < 0.01, T4: unweighted p = 0.001 and weighted p < 0.05) (Figures S5D (unweighted) and 3D (weighted)). Shifts in the relative abundances the genus Bacteroides members over time arose from engraftment and eradication of Bc in LE and ELE mice (Figure S5E). The presence of the Bacteroides genus due to Bc engraftment was detected in LE mice at T4. In ELE mice, early and late engraftment of Bc was confirmed by presence of the Bacteroides genus at T1 and T4. Bacterial composition at the phylum level in the LE and ELE groups at each time point is shown in Figure S5F. LE mice contained bacteria belonging to the Bacteroides phylum but not the Bacteroides genus before late engraftment (T1-T3), while the Bacteroides genus corresponded to 99.7% of Bacteroides phylum at T4. In ELE mice, the genus Bacteroides corresponded to 97.8%, 49.0%, 27.5%, and 58.1% of the phylum Bacteroides at T1, T2, T3, and T4, respectively. Despite Bc engraftment later life following Abx treatment, bacterial composition at T4 was similar to T1 and T2 in the LE and ELE groups, respectively (Figures S5G (unweighted) and 3E (weighted)). Thus, engraftment later in life does not impact the overall bacterial community membership. Colonization efficiency of the FMT in these studies showed that at week 0, among 98 ASVs with average relative abundance > 0.1% in the donor samples, 87 ASVs (88.8%) were detected in recipients without Bc engraftment. At the genus level, among 22 genera with average relative abundance > 0.1% in the donor samples, 20 genera (90.9%) were detected in recipients.
Colonization with a single Bacteroides strain early in life mitigates colitis development
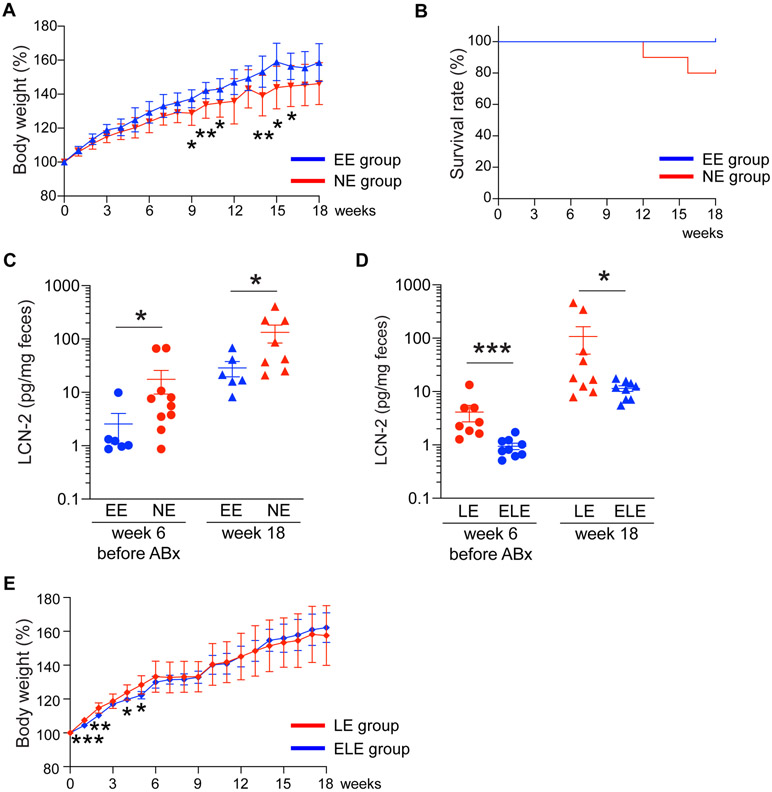
We next examined the impact of early life Bc engraftment on development of spontaneous colitis in the IL-10 KO cohorts described above. EE mice exhibited increased weight gain compared to their NE group counterparts (Figure 4A). None of the EE group animals developed clinical symptoms of colitis, while 2 out of 10 mice in the NE group reached the euthanasia criteria due to rectal prolapse and severe body weight loss during the observation period (Figure 4B). At week 6 (11 weeks of age), but before antibiotic treatment, fecal LCN-2 levels were significantly elevated in NE mice relative to the EE group (p < 0.05) (Figure 4C). Fecal LCN-2 level remained elevated in NE mice as compared to EE mice at week 18 (p < 0.05), the observation endpoint (23 weeks of age) (Figure 4C). Similarly, LE mice exhibited elevated levels of fecal LCN-2 (a commonly-used reliable marker of colonic inflammation in IL-10KO mice7) compared to ELE mice at week 6 before Abx exposure (p < 0.001) as well as at week 18 (p < 0.05) (Figure 4D), although no animals in the LE and ELE groups reached the euthanasia criteria during the observation period. The increase of body weight of the LE group tended to slow down after the late Bc engraftment (week 6) compared to ELE group (Figure 4E). Overall, early life Bc colonization prevents spontaneous colitis development despite its eradication later in life via Abx, while colonization with Bc later in life does not have this effect.
Figure 4. Engraftment of a single Bacteroides strain in early life prevents colitis development.
(A) Percent weight change (expressed as % of starting weight at week 0) of the tracked EE and NE animals beginning at week 0 (5 weeks of age). (B) Survival rate of animals in EE and NE groups during the 18-week observation period. Two out of ten animals in NE group reached the euthanasia criteria. (C) Fecal lipocalin-2 (LCN-2) levels before antibiotic treatment (week 6) and at the end of the observation period (week 18) in EE and NE animals. (D) Fecal lipocalin-2 (LCN-2) levels before antibiotics treatment (week 6) and at the end point of the observation period (week 18) in LE and ELE animals. (E) Percent weight change (expressed as % of starting weight at week 0) of the tracked LE and ELE animals beginning at week 0 (5 weeks of age). *p < 0.05, **p < 0.01, and ***p < 0.001. Data are represented as mean ± SEM for (A), (C)-(E).
Discussion
Our findings have broad implications to our current understanding of human IBD etiopathogenesis and in rethinking clinical best practices. Our past and current findings suggest that perturbations in the early life gut microbiome can have negative and persistent consequences to immune development that lead to increased risk for disease in genetically susceptible hosts and strongly suggest that these events arise from a “loss of function”, i.e. extinction of critical CPZ-sensitive commensal microbes in the maternal gut microbiota that are not vertically transmitted to offspring, rather than a “gain of function”, i.e. emergence of pathobionts that activate the immune system of genetically susceptible hosts to trigger the onset of disease. In support of this notion, when IL10-deficient GF dams and sires are conventionalized with CPZ-induced donor gut microbiota, reproduce, and continue to be housed in the same gnotobiotic isolator as their offspring, neither dams, sires, nor offspring develop spontaneous colitis. In contrast, offspring from CPZ-treated dams housed in SPF conditions appear to re-acquire certain CPZ-sensitive commensal gut microbiota from the environment later in life and develop spontaneous colitis.7 These microbes, in an immunologically-naïve/insufficiently-tolerized host are thought to induce an immune response that increases risk for development of spontaneous colitis. We identified and cultivated a unique indigenous Bacteroides strain (Bacteroides strain CL1-UC, Bc) using sequence-based and bioinformatics approaches from fecal samples obtained from offspring. A limitation of this approach is that only 42.5% of the metagenome sequences could be used for assembling MAGs, largely representing the more abundant bacterial genomes. Thus, less abundant bacterial genomes are not detected. Deeper sequencing to increase genomic coverage and incorporation of long-read technology could improve the percentage of sequence represented in MAGs, but whether additional informational value would be obtained is unclear. Bc successfully engrafted in recipients at 3 weeks of age that had vertically-transmitted CPZ-induced perturbed microbiota. The early engraftment of Bc significantly impacts ensuing gut bacterial assemblage, reducing risk for spontaneous colitis development, but these effects were not evident when the intervention was made later in life (Figures 2-4). This being said, we concede the possibility that there may be eventual long-term changes in the gut microbiome caused by Bc late engraftment. Engraftment of Bc into CPZ-altered gut microbiota of IL-10 KO offspring later in life required a perturbation with a brief course of antibiotics. We believe this is due to priority effects of established gut microbiota, which has already undergone a selection process to establish community stability and resilience. Under these conditions, “foreign” microbes, even if commensal under other circumstances, would be excluded from becoming part of the community.20, 21 A short course of antibiotics was therefore needed to “reset” community dynamics to allow Bc to engraft (Figures 3 and S3). Small variations of the gut microbiome can occur stochastically and are inevitable even in a strictly controlled study. This is a limitation of the technology and we cannot exclude a possible impact on our observed outcomes. Nonetheless, the finding that early, but not late engraftment, confers immune tolerance further supports the paradigm for the necessity of immunological conditioning or imprinting that only occurs in a developmental window. Future studies are planned to better define if Bc specifically elicits a generalized immune tolerance to bacterial or environmental antigens seen later in life. In addition, our MAG analysis showed some additional Bacteroides species/strains that were missing from vertically transmitted gut microbiota obtained from CPZ-treated dams returned in offspring that eventually developed colitis in a similar manner to Bc. Thus, strains related to Bc may have similar effects on host immune development. Together, by testing hypotheses that emerged from multi-omics observations through cultivation and colonization experiments, perturbations in early life immune and gut microbiome development appear to significantly affect subsequent states of health and disease and that best practices should include measures to identify host-microbiome imbalances and means to correct them at this critical life stage. These might include a focus on early life risk stratification and intervention, particularly for complex immune disorders that have been increasing in incidence and prevalence with alarming frequency over the past century. Significant perturbations of the gut microbiome are caused by factors like antibiotic exposure, diet, e.g. breast versus formula feeding, birth modality, and environment that contribute to loss of critical microbes and mediators essential to this process.22
Current modern medicine practices are rooted in identifying events temporally related to disease onset and then to intervene after-the-fact. At this point, many additionally amplifying and self-sustaining processes are set into play, particularly in genetically susceptible individuals, that result in chronic and often medically refractory disease. Our study further suggests that interventions to correct microbial imbalances must be done during an early life stage, as they may become less effective once the immune conditioning period is over. The success of any microbiome-based intervention or biotherapeutic hinges on defining the early life “healthy” gut microbiome. This remains a challenge with few solutions, as the gut microbiota of individuals varies considerably at the taxonomical level. However, by identifying microbial populations and their derived mediators that provide specific beneficial functions in a defined context such as immune development in early life could be a plausible solution. We have provided proof-of-concept that introduction of a single indigenous member of the gut microbiota (Bc) at the right time and in the right context can lower disease risk. On a practical level, it is unlikely that this particular bacteria or other murine-derived microbes will play the same role, exhibit identical biological properties, or possess the ability to engraft into human subjects because the assembly rules for acquisition and colonization of gut microbes by each host species differs.23, 24 However, similar strategies to ours could be modified to identify and develop human-specific microbes and their mediators that have biotherapeutic potential. In doing so, the knowledge gained may aid in developing new metrics and biomarkers for defining and assessing the healthy gut microbiome and in stratifying individuals who would receive the greatest benefit from early microbiome-based interventions. With this, a suite of next generation microbiome-based therapeutics is likely to emerge that are effective for both disease prevention as well as treatment of complex diseases.
Supplementary Material
Acknowledgments
We thank the Human Tissue Resource Center for histological processing (the University Chicago), the Gnotobiotic Research Animal Facility staff for GF animal husbandry (the University of Chicago) as well as the Flow Cytometry Core Facility for analysis software (Kyorin University Graduate School of Medicine).
Grant support
The present research was supported by NIDDK grants NIH P30 DK42086, RC2DK122394 (EBC), R37 DK47722 (EBC) and K01 DK111785 (VL); the Chicago GI Research Foundation and the David and Ellen Horing Research Fund.
Abbreviations
- ANI
average nucleotide identity
- ASV
amplicon sequencing variant
- CD
Crohn’s disease
- CPZ
cefoperazone
- DC
dendritic cells
- FMT
fecal microbiome transplantation
- GF
germ-free
- IBD
Inflammatory bowel diseases
- LCN-2
lipocalin-2
- MAG
metagenome-assembled genomes
- RAG1
recombination activating gene 1
- SNP
single nucleotide polymorphisms
- SPF
specific-pathogen-free
- UC
ulcerative colitis
- WGS
whole genome sequencing
Footnotes
Disclosures
The authors declare no competing interests.
References
- 1.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JD, Chen EZ, Baldassano RN, et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn's Disease. Cell Host Microbe 2015;18:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014;63:1275–83. [DOI] [PubMed] [Google Scholar]
- 4.Ortqvist AK, Lundholm C, Halfvarson J, et al. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut 2019;68:218–225. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen LH, Ortqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol 2020;5:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An D, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014;156:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi J, Bobe AM, Miyoshi S, et al. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep 2017;20:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018;3:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eren AM, Vineis JH, Morrison HG, et al. A filtering method to generate high quality short reads using illumina paired-end technology. PLoS One 2013;8:e66643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minoche AE, Dohm JC, Himmelbauer H. Evaluation of genomic high-throughput sequencing data generated on Illumina HiSeq and genome analyzer systems. Genome Biol 2011;12:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Liu CM, Luo R, et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015;31:1674–6. [DOI] [PubMed] [Google Scholar]
- 12.Eren AM, Esen OC, Quince C, et al. Anvi'o: an advanced analysis and visualization platform for 'omics data. PeerJ 2015;3:e1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmont TO, Quince C, Shaiber A, et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol 2018;3:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parks DH, Imelfort M, Skennerton CT, et al. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 2015;25:1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 1995;57:289–300. [Google Scholar]
- 17.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012;487:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74. [DOI] [PubMed] [Google Scholar]
- 19.Keubler LM, Buettner M, Hager C, et al. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm Bowel Dis 2015;21:1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 2015;125:2841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018;174:1388–1405 e21. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenburg ED, Smits SA, Tikhonov M, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016;529:212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawls JF, Mahowald MA, Ley RE, et al. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 2006;127:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We made raw sequencing data for all metagenomes publicly available (see Table S1 for MG-RAST IDs). In addition, we have made available as anvi’o profiles (1) individual co-assemblies and the final set of metagenome-assembled genomes (doi:10.6084/m9.figshare.11955060) which offer reproducible access to detection and coverage statistics of each genome across metagenomes, and (2) pangenomes (doi:10.6084/m9.figshare.11968674 for Figure 1B and doi:10.6084/m9.figshare.11968692 for Figure 1C) which offer reproducible access to functions and sequences of each gene cluster and their distribution across genomes. The raw sequencing data for 16S rRNA gene amplicon sequences reported in this study are also publicly available (MG-RAST mgp82768).
Figure 1. Identification and cultivation of a key commensal Bacteroides strain that is important for host immune development.
(A) The detection rates of bacterial metagenomic-assembled genomes (MAGs) in fecal samples obtained from dams and pups over time. Each column represents a single MAG and each row represents an individual sample. The darker the cell, the greater the relative abundance. Metadata for each sample is presented to the right of the heat map. Colored bars at the bottom of the heatmap represent the MAG annotations at the phylum level. Green arrows indicate CPZ-sensitive MAGs that are residents of IL-10 KO dams and their NT offspring that did not develop colitis, but which emerged later in life of the CPZ group offspring that develop colitis. (B) Pangenome analysis and assessment of average nucleotide identity (ANI) for 18 assembled genomes based on whole genome sequencing for 18 isolated cultivars compatible with MAG1 shown together with MAG1 and the four most similarly related complete genomes registered in the BLAST database. Each circular layer represents an individual MAG and darker areas in each layer show the gene clusters contained by each MAG contains. The ANI is presented in the right upper area corner in this figure. Each column represents an individual MAG corresponding to the circular layer. Each row presents an individual MAG in the order corresponding to the order of round layers from top to bottom. Red cell means > 99% identity. Detailed results of ANI are presented in Table S4. (C) IL-12p40 production of dendritic cells (DCs). DCs were isolated from spleen of male IL-10 KO mice at 7 weeks of age and stimulated using bacterial lysates from Bacteroides strain CL1-UC(Bc), B. thetaiotaomicron (Bt), Lactobacillus murinus (Lm), and Lachnospiraceae bacterium (ATCC BAA-2281) (Lb), or BLNK: blank control with sterile PBS (n = 5 per group). (D) IL-12p40 and IFNγ production as well as Th1 differentiation in co-culture assays of DCs and naïve CD4+ T cells from male animals. Flow cytometry data represent the percentage of live TCRβ+CD4+T-bet+ cells out of live TCRβ+CD4+ cells (n = 4-5 per group). *p < 0.05, **p < 0.01, and ***p < 0.001. Data represent mean ± SEM for (C) and (D).