Abstract
The seven-transmembrane receptor CX3CR1 is a specific receptor for the novel CX3C chemokine fractalkine (FKN) (neurotactin). In vitro data suggest that membrane anchoring of FKN, and the existence of a shed, soluble FKN isoform allow for both adhesive and chemoattractive properties. Expression on activated endothelium and neurons defines FKN as a potential target for therapeutic intervention in inflammatory conditions, particularly central nervous system diseases. To investigate the physiological function of CX3CR1-FKN interactions, we generated a mouse strain in which the CX3CR1 gene was replaced by a green fluorescent protein (GFP) reporter gene. In addition to the creation of a mutant CX3CR1 locus, this approach enabled us to assign murine CX3CR1 expression to monocytes, subsets of NK and dendritic cells, and the brain microglia. Analysis of CX3CR1-deficient mice indicates that CX3CR1 is the only murine FKN receptor. Yet, defying anticipated FKN functions, absence of CX3CR1 interferes neither with monocyte extravasation in a peritonitis model nor with DC migration and differentiation in response to microbial antigens or contact sensitizers. Furthermore, a prominent response of CX3CR1-deficient microglia to peripheral nerve injury indicates unimpaired neuronal-glial cross talk in the absence of CX3CR1.
A multitude of leukocyte migration events is needed to accomplish immunosurveillance in vertebrate organisms. Blood monocytes derived from central hematopoietic organs continuously seed the periphery with sentinels specialized in antigen uptake. Antigen encounter results in the mobilization of antigen-presenting cells (APC) to afferent lymphatics and their recruitment to secondary lymphoid organs, where they trigger T-cell responses. Long-distance migration of leukocytes is accomplished via blood and lymph circulation and thus requires transendothelial migration through vessel walls. The interaction of leukocytes with vascular endothelial cells during extravasation at sites of inflammation is a highly regulated process. After an initial, predominantly selectin-mediated “rolling” step, engagement of G-protein-coupled chemokine receptors leads to activation of integrins and the establishment of firm arrest, followed by diapedesis (2, 3).
Recently a novel chemokine named fractalkine (FKN) (neurotactin [NTN]) was identified (1, 15) and shown to have unique properties. FKN has a CX3C chemokine domain and thus constitutes, according to the current chemokine nomenclature based on the spacing of N-terminal cysteines, its own CX3C family. Unlike any other known chemokine, the CX3C module was found to exist in two isoforms; one is membrane anchored and presented on an extended mucin-like stalk, and the other is a soluble form resulting from membrane-proximal proteolytic cleavage of FKN. In addition to its classical function as a chemoattractant, high-affinity interaction of FKN with its specific receptor CX3CR1 (8) mediates leukocyte arrest under flow conditions (4). In vitro data show that this firm adhesion is signaling independent and does not involve integrin activation, and may thus represent a novel mechanism in leukocyte trafficking (4, 7). FKN has been shown to be expressed on activated endothelial cells (1, 15), dendritic cells (DC) (9, 16), and neurons (6, 14). The FKN receptor, CX3CR1 (formerly V28 [18]), is a typical seven-transmembrane G-protein-coupled receptor. CX3CR1 is expressed on human monocytes and undefined subsets of NK and T cells (8). Expression of FKN and CX3CR1 in neurons and microglia, respectively, has fostered speculations that the receptor-ligand pair might be crucial for neuronal-glial cross talk (6, 14).
To investigate the in vivo role of FKN-CX3CR1 interactions, we generated a mouse mutant that lacks the FKN receptor. Our strategy was to replace the murine CX3CR1 gene with the gene encoding the enhanced green fluorescent protein (EGFP; Clontech). This approach allowed not only the generation of a mutant CX3CR1 locus but also the examination of the CX3CR1 expression pattern and migration of cells that normally express this receptor.
MATERIALS AND METHODS
Molecular cloning and generation of CX3CR1 mutant mice.
Genomic fragments of the murine CX3CR1 locus were isolated from a 129/Sv phage library (Stratagene, La Jolla, Calif.) by hybridization with a human CX3CR1 cDNA probe and used to construct a CX3CR1 targeting vector. The homologous regions of the final vector consisted of a PCR-amplified 1.2-kb fragment immediately upstream of the CX3CR1 start codon and an 8-kb KpnI/XbaI fragment spanning the 3′ end of the CX3CR1 coding exon and the 3′ flanking DNA. The GFP-neo (neomycin resistance gene) cassette replacing the first 390 bp of the CX3CR1 gene was constructed using a fragment spanning the EGFP gene including the simian virus 40 poly(A) signal (pEGFP-N1; GenBank accession no. U55762; bp 653 to 1666; Clontech) and a loxP signal flanked neo gene originally derived from pL1 neo. Embryonic stem (ES) cells (E14.1, 129/Ola) were transfected with the linearized targeting vector. G418-gancyclovir double selection yielded double-resistant colonies of which 1 out of 30 showed the right targeting event as verified by Southern blot analysis. Homologous recombinant ES cell clones were transiently transfected with a Cre recombinase expression vector, and G418-sensitive colonies were isolated. ES cell clones lacking the neo gene were injected into blastocysts to generate chimeric mice. Germ line transmission yielded CX3CR1+/GFP mice. Unless otherwise indicated, data were obtained from F2 and F3 progeny of 129/Ola × C57BL/6 matings. BALB/c CX3CR1GFP mice were derived from repeated backcrosses (>N6) to BALB/c mice.
Flow cytometry.
The staining reagents used in these studies included the phycoerythrin-coupled antibodies anti-CD3ɛ, anti-CD11c, anti-NK1.1, anti-CD4, and anti-Gr1; the biotinylated antibodies anti-CD11b and anti-CD11c; and the APC-coupled antibodies anti-CD11b, anti-CD8α, and anti-CD3ɛ. Unless indicated otherwise, the reagents were obtained from PharMingen, San Diego, Calif. For CX3CR1 surface staining, cells were incubated with an FKN/NTN-Fc fusion peptide (NTN-Fc; 10 μg/ml; kindly provided by Millennium Biotherapeutics) and subsequently stained with Cy5-conjugated F(ab′)2 goat anti-human immunoglobulin G1 (IgG1; Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.). Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson, Mountain View, Calif.) using CellQuest software (Becton Dickinson). For intracellular cytokine staining, cells were fixed in 1% paraformaldehyde, washed, and kept overnight in phosphate-buffered saline (PBS)–EDTA containing 1% fetal calf serum. The next day, cells were washed and stained in buffer containing 0.1% saponin with C17.15.10, a rat IgG2a against mouse interleukin-12 (IL-12) p40.
Immunohistochemistry.
For cryostat sections (10 to 16 μm), tissues had to be fixed in paraformaldehyde (4%) to preserve GFP. Sections were stained by overnight incubation with the indicated antibodies, washing, and subsequent incubation with the fluorochrome-labeled secondary reagent for 3 h at 25°C. The reagents used were biotin-coupled anti-CD3ɛ antibody (PharMingen), neuronal nucleus-specific antibody NeuN (Chemicon, Temecula, Calif.), and Cy5-conjugated F(ab′)2 sheep anti-mouse IgG (Jackson ImmunoResearch). Visual data were acquired with an Axioplan 2 fluorescent microscope (Carl Zeiss, Jena, Germany) equipped with a Cooke Corporation SensiCam charge-coupled device camera using SlideBook software (Intelligent Imaging Corporation).
Thioglycolate-induced peritonitis.
Six-week-old BALB/c wild-type (wt) and CX3CR1GFP/GFP (N6) mice were administered 1 ml of 4% thioglycolate broth intraperitoneally. Differential cell counts were obtained by flow cytometric analysis of the peritoneal lavage 12, 24, and 72 h after injection.
Contact hypersensitivity assay.
A 3% solution of oxazolone sensitizing agent (Sigma) was prepared in ethanol-acetone (3:1), and 150 μl were applied to the shaved abdomens of 6-week-old BALB/c wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice. Six days after sensitization, mice were challenged by application of 25 μl of 1% oxazolone in olive oil-acetone (3:1) on the dorsal side of the right ear. Control ears were challenged with vehicle alone. Ear thickness was measured immediately before challenge and 24 h later, using an engineer's micrometer (Mitutoyo).
STAg injections.
Toxoplasma gondii tachyzoite extract (STAg) injections were carried out as described elsewhere (19). Briefly, 6- to 8-week-old (C57BL/6 × 129)F3 mice were injected intravenously with 25 μg of STAg. Spleens were isolated, and single-cell suspensions were prepared. Cells were plated at 106 cells/ml in 24-well plates in complete RPMI 1640 medium and cultured for 18 h in the presence of GolgiPlug (1 μl/ml of culture; PharMingen). The cells were then used for intracellular staining.
Peripheral nerve injury.
Facial motor nerve transections of avertin-anesthetized 8-week-old wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice were carried out as described elsewhere (17). Briefly, 1, 3, 7, 14, and 21 days after axotomy, mice (n = 4 per time point per genotype) were overdosed with avertin and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were saturated in 15% sucrose and cryosectioned coronally (12 μm). To determine cell proliferation, some animals were injected intraperitoneally with 0.2 mCi of [3H]thymidine 2 h before being killed. Quantification of the microglial cell numbers in the facial nerve nucleus cross sections (about 0.25 mm2) was performed using the NIH Image analyzing program.
RESULTS AND DISCUSSION
Generation of CX3CR-1GFP mice.
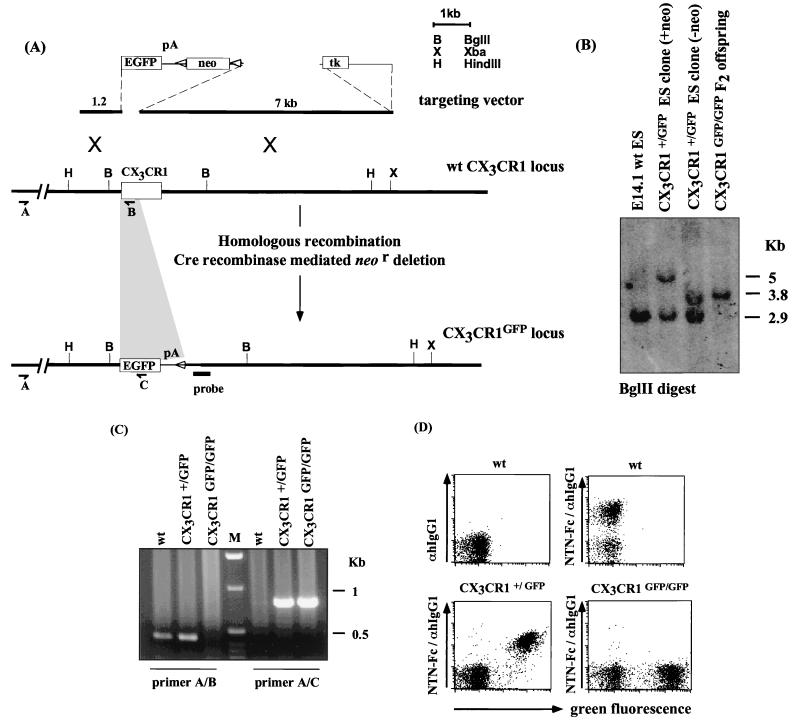
We manipulated the murine CX3CR1 locus by targeted replacement of the CX3CR1 gene with the cDNA encoding EGFP (Clontech). In the targeting construct—and the mutant locus—the EGFP gene replaces the first 390 bp of the second CX3CR1 exon encoding the N terminus of the seven-transmembrane receptor shown to be crucial for interaction with FKN (13). Genomic fragments flanking the murine CX3CR1 gene were used to construct a CX3CR1 targeting vector (Fig. 1A). Following homologous recombination and isolation of ES cell clones that harbored the expected replacement of CX3CR1 by EGFP, the loxP site-flanked neo gene was excised. The neo gene deletion was confirmed by Southern blot analysis (Fig. 1B), and the targeted ES cell clones were injected into blastocysts to generate chimeric mice. Germ line transmission of the mutant allele yielded heterozygous mutant CX3CR1+/GFP mice, which were intercrossed to generate CX3CR1GFP/GFP mice.
FIG. 1.
Targeted disruption of the murine CX3CR1 gene. (A) CX3CR1 targeting strategy. (B) Southern blot analysis of genomic DNA of E14 wt ES cells, the CX3CR1+/GFP ES clone 382, before and after Cre recombinase mediated neo gene deletion, and homozygous mutant CX3CR1GFP/GFP mice. DNA was digested with BglII and analyzed by Southern blotting using the indicated probe. (C) RT-PCR analysis of lymphoid tissues of wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice. Primer A, hybridizing to the upstream untranslated exon of CX3CR1, in combination with primer B results in amplification of a 390-bp fragment indicative of the wt CX3CR1 locus. Its combination with primer C yields a 760-bp fragment specific for the mutant CX3CR1GFP locus. (D) Flow cytometric analysis of peripheral blood of wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice. PBMNCs were enriched by Ficoll gradient. Cells were stained with NTN-Fc and Cy5-coupled goat anti-human IgG1. Cells are gated on live cells and according to scatter.
Like most chemokine receptors, CX3CR1 is encoded by a single exon. Divergence of the genomic CX3CR1 DNA sequence and the murine CX3CR1 cDNA sequence (GenBank accession no. AF074912; S. Jung, unpublished results) 15 bp upstream of the ATG indicated the presence of a 5′ untranslated exon. Replacement of the CX3CR1 coding exon by the GFP cassette in CX3CR1GFP mice should thus result in the generation of transcripts carrying the CX3CR1 untranslated exon spliced onto the GFP exon. Reverse transcription-PCR (RT-PCR) analysis shows (i) the presence of these chimeric transcripts in CX3CR1GFP mice and (ii) the absence of wt CX3CR1 transcripts in CX3CR1GFP/GFP mice (Fig. 1C). Flow cytometric analysis of peripheral blood cells of CX3CR1+/GFP mice showed the presence of a discrete green fluorescent cell population, absent in wt mice (Fig. 1D), that was also stained with NTN-Fc (Fig. 1D). All surface CX3CR1-positive cells in the blood of heterozygous mice expressed GFP, indicating appropriate GFP expression as well as biallelic expression of the FKN receptor locus. The absence of staining with the NTN-Fc protein on GFP-positive cells of homozygous mutant mice (Fig. 1D; see also Fig. 5A) further confirmed that CX3CR1GFP/GFP mice lack CX3CR1 expression and suggests that there is no alternative murine FKN receptor.
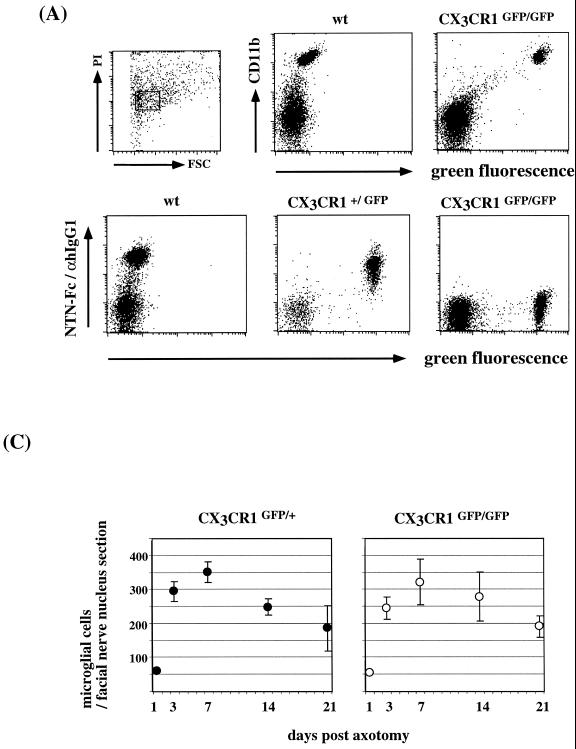
FIG. 5.
Analysis of CX3CR1 function in microglia. (A) Surface CX3CR1 staining of microglial cells isolated via Percoll density gradient from collagenase-digested brains of wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice. Cells were stained for the indicated surface markers (CD11b and CX3CR1) and gated according to scatter and viability as indicated. (B) Peripheral nerve transection experiment. Coronal section through operated and contralateral control facial nerve nuclei of axotomized CX3CR1+/GFP mouse day 7 after axotomy. Section were stained with an anti-neuronal nucleus-specific antibody (NeuN) and Cy5-coupled sheep anti-mouse IgG serum. (C) Quantitative evaluation of microglial reaction in response to facial nerve transection in operated CX3CR1+/GFP and CX3CR1GFP/GFP mice. The volume analyzed in the facial nerve nucleus cross sections represents about 0.25 mm2 by 12 μm. Results are presented as means (± standard deviations) of 16 sections obtained from four mice of each genotype per time point.
Analysis of murine CX3CR1 expression.
All CX3CR1-expressing cells in CX3CR1+/GFP mice are GFP positive. However, due to the extended half-life of the EGFP protein (>24 h), not all green fluorescent cells in CX3CR1+/GFP mice would be expected to be CX3CR1 positive. Cells which ceased to express the FKN receptor are likely to harbor residual GFP. Green fluorescence in these cells would thus indicate their derivation from a CX3CR1-positive precursor.
Peripheral blood monocytes of CX3CR1+/GFP mice, as defined by being CD11b+ and Gr1low (12), were GFP/CX3CR1 positive (Fig. 2A; see Fig. 1D for NTN-Fc staining). This finding is in accord with the reported CX3CR1 expression in human monocytes (8). Other cells of the myeloid lineage, such as neutrophils (CD11b+ and Gr1high) (12) and eosinophils, were GFP/CX3CR1 negative (Fig. 2A and data not shown). We consistently observed a slight increase of green fluorescence in neutrophils of CX3CR1+/GFP mice. Given the absence of surface CX3CR1 expression on these cells, we interpret the low GFP expression to be a relic from the granulocyte-macrophage colony-forming cell precursor stage in which both CX3CR1 and GFP are expressed in heterozygote mice (data not shown).
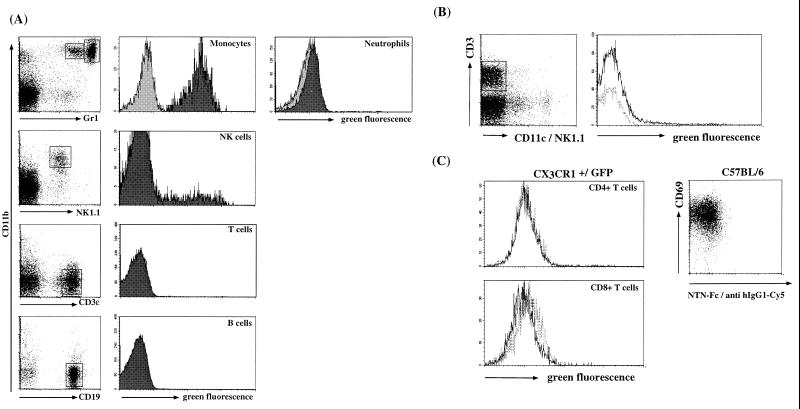
FIG. 2.
Flow cytometric analysis of peripheral blood and resting and activated T cells of wt (light grey) and CX3CR1+/GFP (dark grey) mice. (A) Heparinized peripheral blood was subjected to erythrocyte lysis. Cells were stained for the indicated cell surface markers, (CD11b, Gr1, NK1.1, CD3ɛ, and B220). Granulocyte and monocyte analysis was performed on cells gated for viability; lymphocyte analysis was performed after gating for viability and scatter. (B) Splenocytes were stained for the indicated cell surface markers (CD11c, NK1.1, and CD3ɛ). Histogram data are gated to exclude NK cells and DC. Dashed lines, wt; solid lines, CX3CR1+/GFP cells. (C) Concanavalin A-activated wt (dashed lines) and CX3CR1+/GFP (solid lines) T-cell blasts were harvested on day 2 of culture; wt cells were stained for CD69 and surface CX3CR1 with NTN-Fc/Cy5-labeled goat anti-human IgG1. CX3CR1+/GFP T-cell blasts were stained for CD4 and CD8.
A GFP-positive subset of NK cells was also detected, which is consistent with expression of CX3CR1 in human NK cells (8). GFP+ NK cells were found in peripheral blood as well as lymphoid and nonlymphoid organs such as the liver (Fig. 2A and data not shown) and constituted 5 to 30% of all NK cells in different CX3CR1+/GFP mice.
Murine peripheral blood B and T lymphocytes were negative for CX3CR1 and GFP expression (Fig. 2A), except for minute subsets of CD11b+ cells constituting 0.5 to 1% of the respective circulating lymphocyte populations (data not shown). This is in contrast to the reported functional expression of CX3CR1 on major subsets of human T cells (4, 5). To further investigate this point, we analyzed splenic T cells of wt and CX3CR1+/GFP mice before and 2 days after activation with concanavalin A for NTN-Fc staining and green fluorescence (Fig. 2B and C). The results confirmed the absence of FKN receptor expression on conventional resting and activated murine T cells.
NTN-Fc staining and GFP expression in CX3CR1+/GFP mice indicated CX3CR1 expression in subsets of both CD8α− (so-called myeloid) and CD8α+ (lymphoid) DC (data not shown; S. Jung et al., unpublished data). Bright green fluorescent DC were surface CX3CR1 positive, while GFPlow cells were FKN receptor negative. Among cutaneous DC populations, CD11clow F4/80+ Langerhans cells expressed high levels of GFP and were surface CX3CR1 positive (data not shown).
It has previously been reported that CX3CR1 is expressed in the resident brain macrophage population, the microglia (6, 14). This was confirmed by the demonstration of GFP expression and NTN-Fc staining of microglia of CX3CR1+/GFP mice (see Fig. 5A) but not astrocytes or oligodendrocytes (data not shown). In contrast, other resting tissue macrophages, such as hepatic Kupffer cells and splenic or peritoneal macrophages, expressed neither GFP nor CX3CR1 in CX3CR1+/GFP mice (data not shown and Fig. 3A).
FIG. 3.
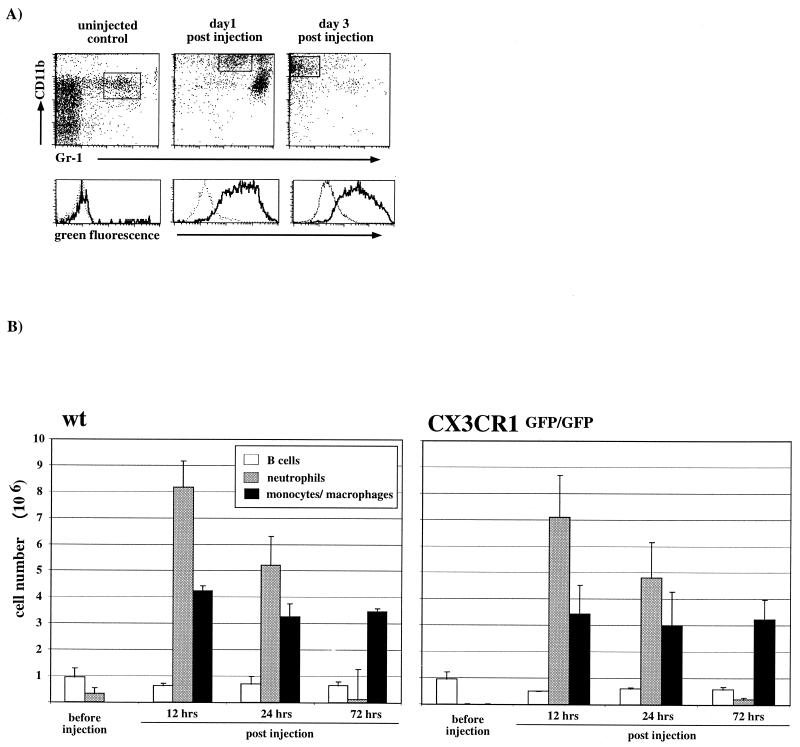
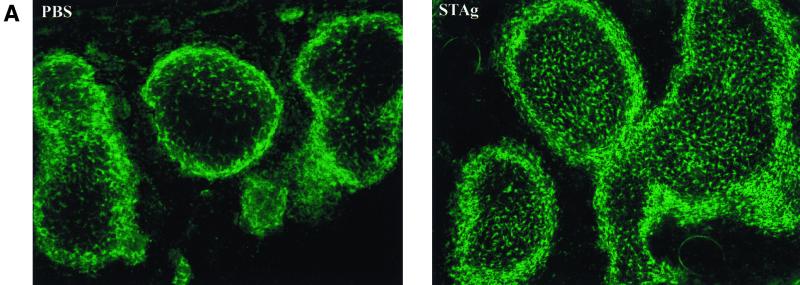
.Thioglycolate-elicited monocyte extravasation in wt and CX3CR1GFP/GFP mice. (A) Flow cytometric analysis of peritoneal lavage of CX3CR1GFP/GFP mice. The lower panel shows GFP expression profiles of gated populations, i.e., resident macrophages (control) and elicited monocytes/macrophages (day 1 and day 3). Dashed lines, wt; solid lines, CX3CR1+/GFP cells. Note the absence of GFP expression in resident macrophages (control) and the transient appearance of neutrophils (CD11b+ Gr1high) day 1 postinjection. (B) Quantitative analysis of peritoneal lavages of wt and CX3CR1-deficient mice. B cells were defined as being CD19+, neutrophils were defined as being Gr1high CD11b+, and monocytes/macrophages were defined as being CD11b+ Gr1low-negative. Data represent mean (± standard deviation) of age-matched wt BALB/c mice (n = 3 per time point) and CX3CR1GFP/GFP BALB/c mice (N6) (n = 2 per time point).
Phenotypic studies with CX3CR1-deficient mice.
Homozygous mutant CX3CR1GFP/GFP mice did not exhibit any developmental defects, were generated in normal Mendelian distribution, and were fertile. Furthermore, comparison of CX3CR1+/GFP and CX3CR1GFP/GFP mice indicated that in the absence of the FKN receptor none of the green fluorescent cell populations was absent or significantly changed in steady-state size (data not shown).
To investigate the potential role of CX3CR1 in stress situations, we subjected CX3CR1-deficient mice to a series of experiments known to elicit responses of the CX3CR1-expressing cell populations, i.e., monocytes, DC, and microglia.
To study the role of CX3CR1 in monocyte extravasation, we challenged wt and CX3CR1GFP/GFP mice by intraperitoneal injection of thioglycolate and analyzed the kinetics of the recruitment of neutrophils and monocytes from the blood. The result, summarized in Fig. 3B, indicated that in this murine model of acute peritonitis CX3CR1-FKN interactions are not essential for transendothelial migration of monocytes. Furthermore, 3 days after injection in both wt and mutant mice, the majority of recruited monocytes had lost Gr1 expression (Fig. 3A), indicating uncompromised differentiation of CX3CR1-deficient monocytes into tissue macrophages (12).
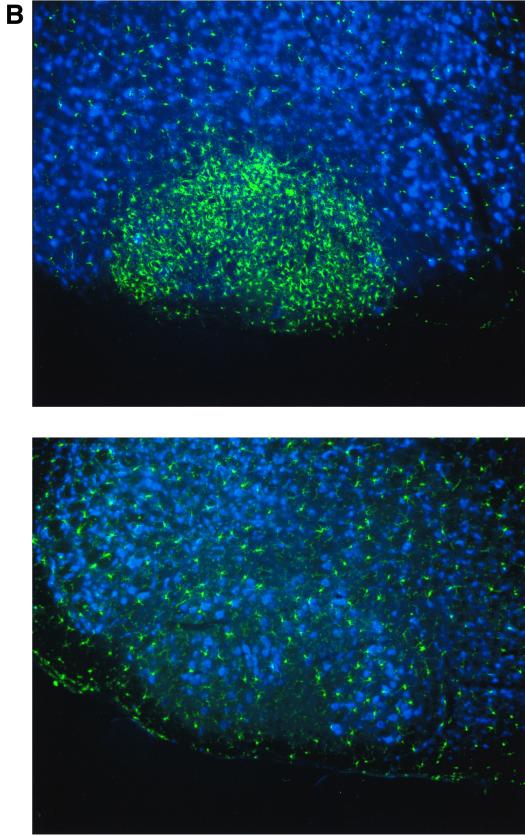
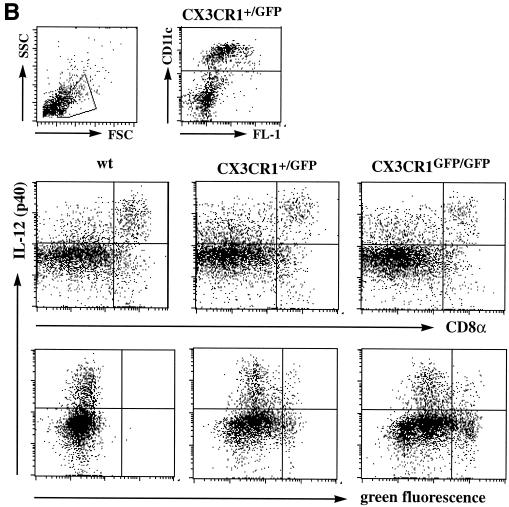
DC play a pivotal role as APC at the interphase of innate and adaptive immune defense. Engagement of pattern recognition receptors by microbial products such as lipopolysaccharide or microbial proteins leads to a rapid redistribution and differentiation of immature DC. To examine a putative role of the FKN receptor in these events, we challenged wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice with STAg. In accordance with the notion that the vast majority of DC in the murine spleen is of immature phenotype and located in the marginal zone (20), most green fluorescent DC in CX3CR1+/GFP mice were located at the intersection of red and white pulp, with few GFP-positive cells in the periarteriolar lymphoid sheaths (Fig. 4A and data not shown). Intravenous STAg injection has been shown to rapidly stimulate splenic marginal zone DC to move to the central T-cell zones and produce IL-12 (19). As shown in Fig. 4A and B, neither migration nor IL-12 production in response to STAg injection seem to be impaired in the absence of CX3CR1.
FIG. 4.
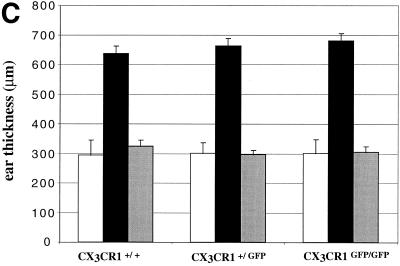
Analysis of CX3CR1 function in DC. (A) Cryosection of paraformaldehyde-fixed spleens of CX3CR1GFP/GFP mice 6 h after PBS or STAg injection indicating STAg-induced recruitment of DC to central periarteriolar lymphoid sheaths. Note that there is no depletion of GFP-positive cells from the marginal zone in the STAg-injected spleen due to recruitment of CD11b+ CD11c− blood monocytes. (B) Flow cytometric analysis of overnight-cultured DC isolated from STAg-injected spleens of wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice. Cells are gated according to scatter and CD11c expression as indicated. The fractions of IL-12-producing CD8+ DC were 50%, 50% (± 2.4%), and 49% (± 12%) for wt, heterozygous, and mutant mice, respectively. Note the absence of IL-12 (p40)-positive cells among the GFPbright DC of CX3CR1+/GFP and CX3CR1GFP/GFP mice, indicating that the FKN-positive CD8+ DC do not participate in IL-12 production. (C) Contact hypersensitivity assay. Data represent mean (± standard deviation) of results obtained from age-matched wt BALB/c mice and CX3CR1+/GFP and CX3CR1GFP/GFP BALB/c mice (N6) (n = 5 per time point). Open bars, ear thickness before challenge (day 6); black bars, ear thickness 24 h after oxazolone challenge; grey bars, control ear thickness 24 h after challenge with vehicle only.
Epidermal Langerhans cells are surface CX3CR1 positive and readily detectable in situ in CX3CR1+/GFP mice (data not shown). To investigate a potential role of CX3CR1 for cutaneous DC function, we analyzed the response of wt, CX3CR1+/GFP, and CX3CR1GFP/GFP mice to the contact sensitizer oxazolone. Langerhans cells are thought to play a dual role in the contact hypersensitivity response. In the sensitizing phase, they transport the hapten-modified proteins to regional lymph nodes and activate T cells (11), while in the eliciting phase they recruit antigen-specific T cells to the challenged skin, initiating the inflammatory response. As summarized in Fig. 4C, we did not observe any significant difference in the ear-swelling response of wt, heterozygous mutant, or CX3CR1-deficient mice. This indicates that neither migratory and nor APC functions of Langerhans cells seem to compromised in the absence of CX3CR1.
Neuronal FKN expression and the expression of CX3CR1 on microglia suggest that FKN acts to mediate signals from neurons to microglia. Absence of staining of CX3CR1GFP/GFP microglial cells with NTN-Fc ruled out the existence of another FKN binding surface receptor on microglial cells (Fig. 5A). Seeding and distribution of CX3CR1-deficient microglial cells were normal in CX3CR1GFP/GFP mice. To determine whether FKN receptor function is required after trauma, we studied an animal model of peripheral nerve injury. Axotomy of the facial motor neuron induces a microglial reaction in the brain stem involving microglial migration, proliferation, and differentiation (10). Perineuronal microglial cells are thought to assist motor neuron survival and eventual axon regeneration by detachment of afferent synaptic terminals, the so-called synaptic stripping. A functional role of FKN in this process was also suggested by the finding that motor neuron axotomy in the rat results in an increase of the soluble, low-molecular-weight FKN isoform (6). GFP expression of microglial cells in CX3CR1+/GFP and CX3CR1GFP/GFP mice allowed for a high-resolution analysis of the response to the injury. Facial nerve transection of CX3CR1GFP/GFP mice resulted in a prominent microglial reaction in the brain stem that appeared normal in all aspects analyzed, including kinetics, number of recruited cells, proliferation, and differentiation, compared to wt and CX3CR1+/GFP littermate controls (Fig. 5B and C and data not shown). Furthermore, intimate association of CX3CR1GFP/GFP microglia and injured neurons indicated unimpaired neuronal-glial communication in the absence of the FKN receptor in this model.
Taken together, FKN receptor staining, flow cytometric, and immunohistological analysis of CX3CR1+/GFP mice allowed us to assign murine CX3CR1 expression to peripheral blood monocytes, subsets of NK and dendritic cells, and the brain microglia. Absence of CX3CR1 staining of these cell populations in CX3CR1GFP/GFP mice indicates that CX3CR1 is the only murine FKN receptor. Yet, as we have been unable to identify an overt phenotype of CX3CR1GFP/GFP mice, the physiological roles of FKN and its receptor CX3CR1 remain to be determined. The results of our preliminary experiments with CX3CR1-deficient mice rule out essential roles of FKN-CX3CR1 interactions in the described systems, i.e., monocyte recruitment in peritonitis, DC differentiation and migration in response to microbial antigens or contact sensitizers, and the microglial response to nerve injury. They do, however, leave room for more subtle cooperative functions of the receptor-ligand pair with other adhesion or chemoattractant receptor systems. Alternatively, FKN could have a unique function that we failed to address in the experiments described in this study. Experiments involving viral and bacterial challenge of FKN receptor-deficient mice might further elucidate the physiological role of FKN and CX3CR1. CX3CR1GFP mice, serving as a source of unmanipulated, in vivo-labeled cell populations, could be instrumental for future high-resolution studies on the developmental and dynamic properties of murine monocytes, DC, and microglial cells.
ACKNOWLEDGMENTS
We thank W. Ellmeier, R. Palframan, and Y. Pewzner-Jung for helpful discussions and critical reading of the manuscript, C. Marcondes for the microglia isolation protocol, and Millennium Biotherapeutics for providing the NTN-Fc fusion protein.
S. Jung was an associate of the Howard Hughes Medical Institute and is supported by a Special Fellowship of the Leukemia & Lymphoma Society. D. R. Littman is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 2.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Campbell J J, Hedrick J, Zlotnik A, Siani M A, Thompson D A, Butcher E C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 4.Fong A M, Robinson L A, Steeber D A, Tedder T F, Yoshie O, Imai T, Patel D D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foussat A, Coulomb-L'Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30:87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Harrison J K, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara R K, Streit W J, Salafranca M N, Adhikari S, Thompson D A, Botti P, Bacon K B, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haskell C A, Cleary M D, Charo I F. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 8.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanazawa N, Nakamura T, Tashiro K, Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S, Honjo T. Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur J Immunol. 1999;29:1925–1932. doi: 10.1002/(SICI)1521-4141(199906)29:06<1925::AID-IMMU1925>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Kreutzberg G W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 11.Kripke M L, Munn C G, Jeevan A, Tang J M, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 12.Lagasse E, Weissman I L. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 13.Mizoue L S, Bazan J F, Johnson E C, Handel T M. Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry. 1999;38:1402–1414. doi: 10.1021/bi9820614. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J A, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. . (Erratum, 389:100.) [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos E J, Sassetti C, Saeki H, Yamada N, Kawamura T, Fitzhugh D J, Saraf M A, Schall T, Blauvelt A, Rosen S D, Hwang S T. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur J Immunol. 1999;29:2551–2559. doi: 10.1002/(SICI)1521-4141(199908)29:08<2551::AID-IMMU2551>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Raivich G, Haas S, Werner A, Klein M A, Kloss C, Kreutzberg G W. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998;395:342–358. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Raport C J, Schweickart V L, Eddy R J, Shows T B, Gray P W. The orphan G-protein-coupled receptor-encoding gene V28 is closely related to genes for chemokine receptors and is expressed in lymphoid and neural tissues. Gene. 1995;163:295–299. doi: 10.1016/0378-1119(95)00336-5. [DOI] [PubMed] [Google Scholar]
- 19.Sousa C R, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinman R M, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]