Abstract
Background
COVID-19 infection increases mortality in hematological malignancies. In a large meta-analysis, patients aged 60 years and older had a significantly higher risk of death than patients under 60 years of age [1]. Furthermore, a high risk of death and reduced survival in patients receiving B cell depletion therapy with prolonged COVID-19 infection was reported in a recent study [2]. High-grade B-cell lymphomas are classified as morphologically aggressive lymphomas with the presence of a high mitotic index and Ki-67 proliferation rates. They demonstrate aggressive behavior clinically as well as morphologically, and COVID-19 infection is an important factor that increases mortality in these patients. Herein, we present an elderly patient with a diagnosis of high-grade B-cell lymphoma, in whom a complete response was observed after prolonged COVID-19 infection.
Case summary
An 81-year-old female patient received her first cycle of R-CHOP (rituximab, cyclophosphamide, vincristine, and prednisolone) treatment after being diagnosed with high- grade B-cell lymphoma. After being discharged from the hospital, the patient was referred to the emergency department with complaints of fever and fatigue when she came for the second cycle of chemotherapy. Her COVID-19 PCR test was found positive. She was admitted to the infectious diseases service and favipiravir treatment was started. On the 24th day of hospitalization, it was decided to perform interim FDG-PET/CT (Fluorodeoxyglucose - Positron Emission Tomography/Computed Tomography) scan at a time that her PCR (Polymerase Chain Reaction) test was still positive. A complete metabolic response was detected in her imaging. On the 26th day, the PCR test became negative and the patient was transferred to the oncology service and received the second cycle of R-CHOP treatment.
Conclusion
Our case emphasizes that antitumor effect could be seen in a patient with SARS-CoV-2 infection and a hematologic malignancy. It also highlights being alert to prolonged COVID-19 infection in patients receiving B-cell depletion therapy.
Keywords: High-grade lymphoma, COVID-19, Prolonged COVID, Antitumor effect, Oncolytic virus, Lymphoma, Antitumor, Oncolytic effect, High-grade
COVID-19 infection is an important health problem worldwide, especially in patients receiving cancer treatment. Specially, patients with non-Hodgkin's lymphoma who receive treatment with B cell depletion may be more vulnerable and have a prolonged hospitalization [1]. In addition, there are cases that have recovered after COVID-19 infection [2,3]. Herein, we present a case who had COVID-19 infection after receiving the first cycle of R-CHOP treatment and who subsequently had a complete metabolic response in the interim PET (Positron Emission Tomography) scan.
Case presentation
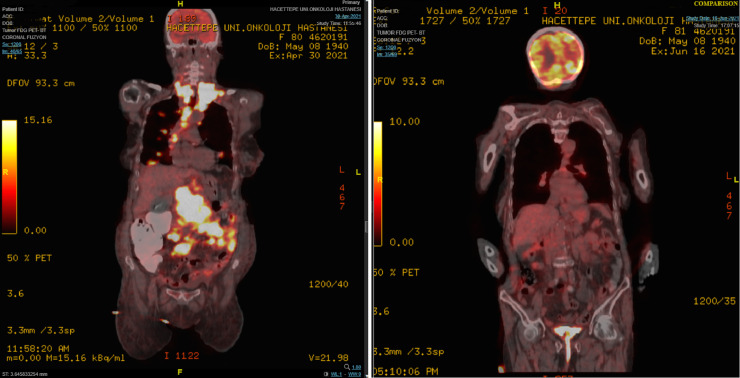
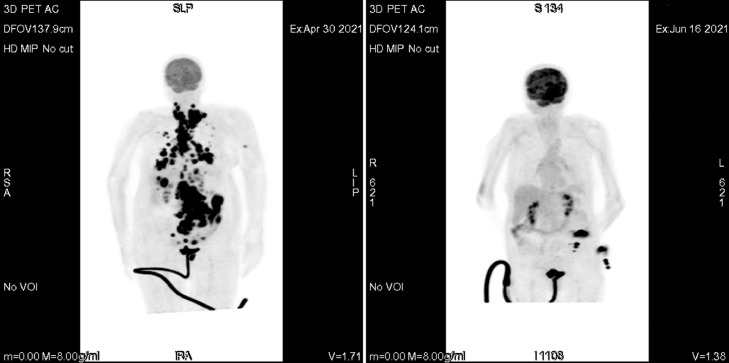
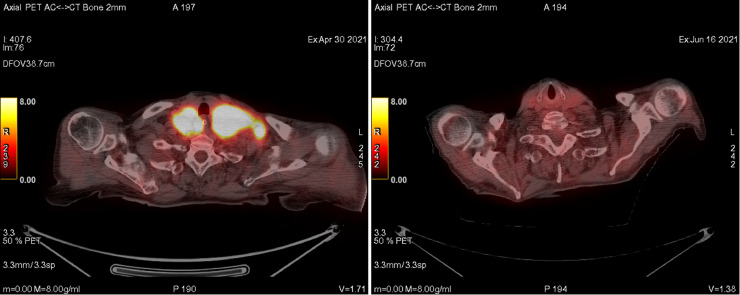
An 81-year-old female patient presented to emergency department with the complaint of abdominal distension and pain that had persisted for 4 months. Her medical history included asthma and hypertension. Her family history had no significant disease. On her examination, a palpable swelling was found in left hypochondriac and umbilical side. Computed tomography (CT) of the thorax, abdomen and pelvis was suggestive of lymphoproliferative disease with several lymph nodes, multiple lung nodules, bilateral pleural fluid, and a 43 × 37 mm mass in the left supraclavicular area. Additionally, a solid mass extending posteriorly at the level of the pancreatic head, wall thickening in the jejunum, and nodules suspicious for metastasis in the liver were demonstrated. She was admitted to the oncology floor and biopsy obtained from the largest lesion at the level of pancreatic head. Her biopsy was reported as high-grade B-cell lymphoma. In the staging PET scan, widespread FDG uptake was demonstrated consistent with metabolically active disease in the neck, thorax, abdominopelvic region, many lymph nodes, most conglomerated, and in parenchymal lung nodules, a lesion adjacent to the left humerus, and areas of thickening in the intestines (Fig. 2). Upon diagnosis, standard dose R-CHOP regimen was started. She was discharged a few days after chemotherapy. When she returned to the hospital for the second cycle, she had complaints of fever and malaise. and a PCR test for COVID-19 infection was positive. Favipiravir treatment was started, but she did not receive steroids. On the 24th day of her treatment, while the PCR test was still positive, interim FDG (fluorodeoxyglucose)-PET / CT was performed. It demonstrated that the conglomerate lymph nodes and pathological intestinal wall thickening observed in previous imaging had disappeared. Furthermore, the size of the parenchymal lung nodules had decreased and FDG uptake disappeared. There was no FDG uptake compatible with metabolically active disease (Figs. 1 -3 ). Two days after the interim PET scan, her PCR test was negative, and the second cycle of R-CHOP was administered.
Fig. 2.
Comparison of FDG-PET/CT (in coronal plane) at diagnosis and FDG-PET/CT after COVID-19 infection. (The left panel is the image at the time of diagnosis. The right panel is the post-COVID-19 image.)
Fig. 1.
Comparison of FDG-PET/CT at diagnosis and FDG-PET/CT after COVID-19 infection. (The left panel is the image at the time of diagnosis. The right panel is the post-COVID-19 image.)
Fig. 3.
Comparison of FDG-PET/CT (in axial plane) at diagnosis and FDG-PET/CT after COVID-19 infection. (The images of neck lymph nodes- The left panel is the image at the time of diagnosis. The right panel is the post-COVID-19 image.)
Discussion
Spontaneous remissions after bacterial or viral infection in patients with lymphoma have been reported in the literature [4, 5]. While an antitumor immune response has been thought responsible for this spontaneous remission, the exact mechanism has not been elucidated. Additionally, several cases of antitumor effect of SARS-CoV-2 infection can be found in the literature. Thus, we searched PubMed in October 2021 for full-text case reports or letters to editor using the following search strategy: “antitumor COVID-19” or “oncolytic COVID-19” or “abscopal COVID-19” or “remission COVID-19.” While most of these cases were hematological malignancies, one case was reported in a patient with melanoma. In Table 1 , we summarize the characteristics of patients presenting with remission or disease response after COVID-19 infection [6], [7], [8], [9], [10], [11].
Table 1.
Full-text case reports and letters to editor about clinical response after COVID-19 infection in the literature
| Author | Age | Sex | Diagnosis | Date of diagnosis | Prior treatment and clinical course | Date of COVID-19 infection | Response after COVID-19 infection |
|---|---|---|---|---|---|---|---|
| Herrscher et al. 2019 [6] | 84 | F | Melanoma harboring BRAFV600E mutation | February 2020 | Dabrafenib-trametinib and 20 Gy irradiation to metastatic cervical lymph node | January 2021 | Objective tumor response and reduction in size of all metastases |
| Pasin et al [7] | 20 | M | Relapsed/Refractory NK/T-cell lymphoma associated with EBV and AIHA | July 2018 (the initial record which available) | Rituximab, pembrolizumab, L-asparaginase, intravenous immunoglobulin, etoposide, SMILE, DDGP and CHOP chemotherapy | April 2020 | EBV-DNA levels decreased, and spleen enlargement was reduced, a remission of the NK lymphoma was observed |
| Challenor and Tucker [8] | 61 | M | EBV positive Hodgkin lymphoma | N/A | No prior therapy. | Immediately after diagnosis | EBV-DNA values decreased, and disease activity regressed significantly in PET/CT scan |
| Sollini et al 2021 [9] | 61 | M | Follicular lymphoma | September 2019 | Rituximab and bendamustine | April 2020 | Partial response after treatment with rituximab-bendamustine and complete response after COVID-19 |
| Rudolphi-Solero et al [10] | 55 | M | Follicular lymphoma | 2014, relaps in June 2020 | Remission after R-CHOP in 2016. Two cycles of ESHAP after relapse in June 2020 (last in September 2020) | December 16, 2020 (the date of post-COVID-19 imaging) | Interpreted as a partial response without any treatment. |
| Antwi-Amoabeng et al [11] | 76 | F | Multiple myeloma | June 2020 | One cycle of CyBorD | August 2020 | Normocellular bone marrow seen without increase in blast or plasma cells |
| Current patient | 81 | F | High grade B cell Lymphoma | April 2021 | One cycle of R-CHOP | May 2021 | Complete metabolic response on FDG PET/CT |
Abbreviations: AIHA = autoimmune hemolytic anemia; EBV = Epstein-Barr virus; F = female; M = male; CyBorD = cyclophosphamide, bortezomib and dexamethasone; DDGP = cisplatin, dexamethasone, gemcitabine and pegaspargase; ESHAP = etoposide, methylprednisolone (solumedrol), high-dose cytarabine (Ara-C), and cisplatin; R-CHOP = rituximab, cyclophosphamide, hydroxydaunorubicin (doxorubicin), vincristine (Oncovin) and prednisone; SMILE = dexamethasone, methotrexate, ifosfamide, l-asparaginase and etoposide.
COVID-19 can be fatal in patients receiving chemotherapy due to severe cytopenia and suppression of cellular immunity. Moreover, the duration of viral shedding in these patients is still unknown. In one study examining the evolution of COVID-19 infection in patients with a diagnosis of cancer, one patient treated with bendamustine and rituximab had a positive COVID-19 PCR test for 56 days [12]. Depletion of B cells in patients diagnosed with B cell lymphoma especially after treatment with an agent targeting CD-20 agent could impact the immune response and lead to persistence of SARS-CoV-2. In a multicenter study, prolonged COVID-19 was noted in patients with B-cell non-Hodgkin's lymphoma hospitalized for SARS-CoV-2 infection. In addition, it has been shown that administration of anti-CD20 therapy in the last 12 months was associated with both prolonged COVID-19 and death due to COVID-19 infection [13]. Therefore, treatment with agents that might deplete B-cells should be delayed as much as possible in patients in remission or on maintenance therapy (eg, follicular lymphoma treatment) during the COVID-19 pandemic. In this case, we had to administer the treatment as soon as possible, since our patient was diagnosed with high-grade B-cell lymphoma and the tumor burden at the time of diagnosis was high.
Conclusion
Considering the high tumor burden before the first cycle of chemotherapy in our patient, it did not seem possible to achieve a complete metabolic response in a single cycle at standard doses. In addition, the patient did not receive any immunosuppressive therapy until the second cycle. These observations raise the possibility that one of the factors that contributed to the complete metabolic response was the confirmed SARS-CoV-2 infection.
Author contribution
Feride Yilmaz: Conceptualization, Writing – Original Draft, Visualization, Writing – Reviewing and Editing, Software, Serkan Yasar: Writing – Reviewing and Editing, Investigation, Meltem Caglar Tuncali: Resources, Investigation, Serkan Akin: Writing – Reviewing and Editing, Supervision, Project administration, Investigation
Conflicts of interest
The author(s) declare that they have no competing interests.
References
- 1.Dulery R., Lamure S., Delord M., et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96(8):934–944. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii H., Tsuji T., Sugitani M., et al. Prolonged persistence of SARS-CoV-2 infection during A+AVD therapy for classical Hodgkin's lymphoma: a case report. Curr Probl Cancer. 2021 doi: 10.1016/j.currproblcancer.2021.100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kos I., Balensiefer B., Roth S., et al. Prolonged course of COVID-19-associated pneumonia in a B-cell depleted patient after rituximab. Front Oncol. 2020;10:1578. doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe R., Ogawa K., Maruyama Y., Nakamura N., Abe M. Spontaneous regression of diffuse large B-cell lymphoma harbouring Epstein-Barr virus: a case report and review of the literature. J Clin Exp Hematop. 2007;47(1):23–26. doi: 10.3960/jslrt.47.23. [DOI] [PubMed] [Google Scholar]
- 5.Buckner T.W., Dunphy C., Fedoriw Y.D., et al. Complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent infections. Clin Lymphoma Myeloma Leuk. 2012;12(6):455–458. doi: 10.1016/j.clml.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Herrscher H., Sauer B., Truntzer P., Robert C. Abscopal antitumor effect in a patient with melanoma and coronavirus disease 2019. Eur J Cancer. 2021;149:91–93. doi: 10.1016/j.ejca.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasin F., Mascalchi Calveri M., Herrscher H., et al. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10141. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challenor S., Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 9.Sollini M., Gelardi F., Carlo-Stella C., Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the "flare phenomenon" to the "abscopal effect". Eur J Nucl Med Mol Imaging. 2021;48(8):2652–2654. doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolphi-Solero T., Rashki M., Fernández-Fernández J., Rivas-Navas D., Ramos-Font C., Rodríguez-Fernández A. SARS-COV-2 virus triggers immune antitumor response in a lymphoma patient. Rev Esp Med Nucl Imagen Mol. 2021 doi: 10.1016/j.remn.2021.04.005. S2253-654X(21)00102-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antwi-Amoabeng D., Ulanja M.B.., Beutler B.D., Reddy S.V. Multiple myeloma remission following COVID-19: an observation in search of a mechanism (a case report) Pan Afr Med J. 2021;39:117. doi: 10.11604/pamj.2021.39.117.30000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S., Nakamura S., Kanemasa Y., Atsuta Y., et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021;26(3):485–493. doi: 10.1007/s10147-020-01837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamure S., Duléry R., Delord M., et al. Abstract S09-02: high incidence of persistent COVID-19 among patients with lymphoma treated with B-cell depleting immunotherapy. Clin Cancer Res. 2021;27(6 suppl):S02–S09. [Google Scholar]