Abstract
Purpose
Acute respiratory distress syndrome (ARDS) is characterized by uncontrollable inflammation. Cyclooxygenase-2(COX-2) and its metabolite prostaglandins are known to promote the inflammatory resolution of ARDS. Recently, a newly discovered endogenous lipid mediator, Protectin DX (PDX), was also shown to mediate the resolution of inflammation. However, the regulatory of PDX on the pro-resolving COX-2 in ARDS remains unknown.
Material and Methods
PDX (5 μg/kg) was injected into rats intravenously 12 h after the lipopolysaccharide (LPS, 3 mg/kg) challenge. Primary rat lung fibroblasts were incubated with LPS (1 μg/ml) and/or PDX (100 nM). Lung pathological changes examined using H&E staining. Protein levels of COX-2, PGDS and PGES were evaluated using western blot. Inflammatory cytokines were tested by qPCR, and the concentration of prostaglandins measured by using ELISA.
Results
Our study revealed that, COX-2 and L-PGDS has biphasic activation characteristics that LPS could induce induced by LPS both in vivo and in vitro.. The secondary peak of COX-2, L-PGDS-PGD2 promoted the inflammatory resolution in ARDS model with the DP1 receptor being activated and PDX up-regulated the inflammatory resolutionvia enhancing the secondary peak of COX-2/L-PGDS-PGD2 and activating the DP1 receptor.
Conclusion
PDX promoted the resolution of inflammation of ARDS model via enhancing the expression of secondary peak of COX-2/L-PGDS-PGD2 and activating the DP1 receptor. PDX shows promising therapeutic potential in the clinical management of ARDS.
Keywords: Acute respiratory distress syndrome, Cyclooxygenase-2, Lipocalin-type prostaglandin D synthase, Protectin DX, Inflammatory resolution
Abbreviations: ARDS, Acute respiratory distress syndrome; COX-2, Cyclooxygenases-2; H-PGDS, Hematopoietic prostaglandin D synthase; L-PGDS, Lipocalin-type prostaglandin D synthase; LPS, Lipopolysaccharide; mPGES-1, Microsomal prostaglandin E synthase-1; mPGES-2, Microsomal prostaglandin E synthase-2; PDX, Protectin DX; PGs, Prostaglandins; PGE2, Prostaglandin E2; PGD2, Prostaglandin D2; SPM, Specialized pro-resolving lipid mediators
1. 1.Introduction
Acute respiratory distress syndrome (ARDS) is a severe acute inflammatory disease, caused by various factors like pneumonia, aspiration of gastric contents and sepsis. [1], [2] Main pathological damages of ARDS were alveolar epithelial and lung endothelial barrier injury, resulting in the accumulation of protein-rich edema fluid in the alveolar space. [3] ARDS lacks of effective pharmacological treatment. [4] The resolution of inflammation serves as the self-protected character in host and it is an initiative process. Therefore, the active inflammatory resolution may become a clue to find out effective therapeutic method for ARDS.
COX-2, and prostaglandins, are associated with a variety of inflammatory diseases. In ARDS patients’ bronchoalveolar fluid, PGs levels were found to be increased. [5], However, inhibition of COX-2 has not been proven being clinically effective in ARDS [6]. In animal studies, pharmacologic inhibition or gene silencing of COX-2 would block the inflammatory resolution of Acute Lung Injury. [7] In the mouse model of carrageenin-induced pleurisy, COX-2 promotes resolution by generating pro-resolving prostaglandins. [8] Moreover, COX-2 is responsible for the producing of pro-resolving PGs, like PGD2, 15-deoxy-D12,14-PGJ2, and PGF2α. [9], [10] These works suggest that COX-2 may play both pro-inflammatory and pro-resolving roles in inflammatory diseases like ARDS.
Prostaglandins were induced by COXs, especially COX-2 when in the presence of in an inflammatory environment. Prostaglandin E2 (PGE2) was normally considered to be a pro-inflammatory PG. Previous study showed that administration of PGE2 or its receptor agonist improved lung function in mice ALI model. [11] Another prostaglandin, prostaglandin D2 (PGD2) ameliorated lung injury in ALI/ARDS model via enhancing the endothelial barrier repairment. [12], [13] Both PGE2 and PGD2 are converted from PGH2 by various synthases. PGE2 synthases consist of cPGES, microsomal prostaglandin E synthase-1(mPGES-1) and microsomal prostaglandin E synthase-2 (mPGES-2). Various pro-inflammatory stimulations can up-regulate mPGES-1, m. Meanwhile, cPGES and mPGES-2 are constitutively expressed. [14] PGD2 is induced by lipocalin-type prostaglandin D synthase (L-PGDS) and hematopoietic PGDS (H-PGDS). PGD2 acts its important role via G protein-coupled receptor DP (DP1) and the chemoattractant receptor-homologous molecule CRTH2 (DP2). PGD2 showing multiple pathophysiological characters via different receptors. [15]
Primary lung fibroblasts, which are far from being bystander cells, are important to host defense in ARDS. After the inflammatory stimulation, fibroblasts are activated following the immune response, and secreting a large number of cytokines like interleukin 6 (IL-6) and interleukin 8 (IL-8). [16] Growing evidence manifested that fibroblasts-secreted growth factors promote the alveolar barrier functions and alleviate the lung injury induced by LPS. [17], [18] Our pervious study suggested that fibroblasts regulate the inflammatory resolution by producing proresolving mediators PGD2. [19]
Formal studies have reported that endogenous lipid mediators and mechanisms can drive the resolution of inflammation the resolution of inflammation was driven by novel lipid mediators and endogenously triggered mechanisms. [20] Specialized pro-resolving lipid mediators (SPM) were identified as new genus, including Resolvins, Protectins and their aspirin-triggered forms. [21], [22] Protectin DX (10S,17S-dihydroxydocosa-4Z,7Z,11E,13Z,15E,19 Zhexaenoic acid) is a newly discovered member of this genus, which derived from natural ω-3-fatty acid docosahexaenoic acid (DHA), [23] PDX possesses anti-inflammatory and inflammation pro-resolving bioactions. [24] A study reported that PDX maintains the integrity of lung epithelium, increases the alveolar fluid clearance of ARDS in rat. [25] PDX regulates inflammatory cell infiltration via resident macrophage in LPS-induced lung injury. [26] Moreover, PDX was shown to alleviate lung injury induced by LPS via inducing primary rat type II alveolar epithelial cells proliferation and inhibiting their apoptosis in vivo and in vitro. [27]
Our studies confirmed that COX-2 has a biphasic activation pattern in LPS stimulated lung fibroblasts, showing that COX-2 and PGD2 expression levels peaked at 6 h and subsequently after 48 h. [28] Moreover, NF-κB p50/50 was responsible for regulating the secondary expression peak of COX-2 in the resolution stage of the ARDS rats model. [29] However, the downstream mechanism of secondary peak COX-2 and PGD2 in the resolution of inflammation remains unclear Furthermore, whether PDX promotes the resolution of inflammation by regulating secondary peak of the COX-2 and PGD2 has not been proved yet.
In this study, we hypothesize that the secondary peak of COX-2/L-PGDS-PGD2 a promote the resolution of inflammation in the ARDS model. Moreover, we surmise that PDX plays a pro-resolving role in ARDS by enhancing the pro-resolving COX-2/L-PGDS-PGD2 expressions and activating the DP1 receptor.
2. 2.Materials and Methods
2.1. Reagents
Protectin DX, NS-398(selective COX-2 inhibitor), AT-56 (L-PGDS inhibitor), BW245C (DP1 receptor agonist), 15(R)-15-methyl-PGD2(CRTH2/DP2 receptor agonist), MK-0524(DP1 receptor antagonist) and CAY-10471(CRTH2/DP2 receptor antagonist) were obtained from Cayman Chemical (Ann Arbor, MI, USA), Lipopolysaccharide (Escherichia coli O55: B5), BOC-2 (ALX/FPR2 receptor inhibitor) were purchased from Biomol/Enzo Life Sciences (Farmingdale, NY, USA).
2.2. Animal procedures
Male Sprague Dawley (SD) rats (200–250 g) were purchased from SLAC Laboratory (Shanghai, China). SD rats were raised in a temperature-controlled room (22–24 °C) on a 12 h day/night cycle with free access to food and water. All animal experimental procedures were approved by the Animal Care and Use Committee Institutional of Wenzhou Medical University (Wenzhou, China).
SD rats were injected with 3 mg/kg body weight of LPS intravenously, meanwhile, control group animals were administered the same volume of sterile 0.9% saline. Rats were euthanized at the different time points: 0,6, 12, 24, 48 and 72 h after LPS stimulation.
For experimental procedures, NS-398, BOC-2, AT-56, BW-245C,15(R)-15-methyl-PGD2, MK-0524 and CAY-10471 were dissolved in DMSO and then diluted into sterile 0.9% saline for further use. At 12 h after the LPS challenge, rats were given PDX (5 μg/kg) or an equivalent volume of ethanol via tail vein injection. Rats were administered NS-398(5 mg/kg) i.v. 1 h prior than the LPS challenge or 12 h after the LPS administration. AT-56 was given intravenously (5 mg/kg) 12 h after the LPS challenge. For DP receptors studies, BW-245C,15(R)-15-methyl-PGD2, MK-0524 and CAY-10471 were injected (5 mg/kg) i.p. to rats 12 h after the LPS exposure.BOC-2(600 ng/kg) were given i.v. to rats 1 h prior to PDX injection, whereas other groups received an equal volume of DMSO/saline solution. Rats were sacrificed at 24 h humanely under anesthesia.
2.3. Western blot analysis
Rat primary lung fibroblasts and lung tissues were washed in iced PBS and harvested by using RIPA buffer supplemented with protease inhibitors. Resulting supernatant fraction was homogenized in 1 × SDS–PAGE sample buffer and boiled for 10 min at 95 °C. For the immunoblotting, protein lysates were electrophoresed via 10%,12% or 15% SDS-PAGE gels and then transferred to polyvinylidene-difluoride (PVDF) membranes. Membranes were blocked and incubated with the indicated primary antibody overnight at 4 °C. Primary antibodies against COX-2 (ab-52237), L-PGDS (ab-182141) were purchased from Abcam (Cambridge, UK), mPGES-1 antibody (DF-8592) and mPGES-2 antibody (DF-12712) were purchased from Affinity (Cincinnati, OH, USA). Bound primary antibodies were incubated with appropriate secondary antibodies for another 1 h. Protein levels were detected by using chemiluminescence reagents from Thermo Scientific (Rockford, IL, USA). Images were scanned by a UVP imaging system and analyzed by the Image Quant LAS 4000 mini system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
2.4. Cell culture
Primary lung fibroblasts were isolated from SD rats; isolation process was same as we described before. [30] For experimental procedures, fibroblasts (5 × 105 were plated in six wells plates and grown to 80% confluence. Pulmonary fibroblasts were allowed to remain in a quiescent state for 24 h by incubating them in medium containing 1% FBS (Life Technologies BRL; Grand Island, NY, USA) prior to experimental treatment. After 24 h of culture with LPS (1 μg/ml) or a control medium, fibroblasts were treated with 1,50,100 nM PDX or a vehicle solution (0.1% ethanol, as the PDX was supplied in ethanol) for an additional 24 h. BOC-2 (10 μM) was added 30 min before PDX administration. Fibroblasts were harvested at the different time points:0, 6, 12, 24, 48 and 72 h after LPS challenge for further use.
2.5. Histopathological staining
The same part of the left lung of each rat was fixed in 10% paraformaldehyde for 24 h. Lung tissues were embedded in paraffin wax, sectioned, and stained with H&E for light microscopy analysis. Acute lung injury scores were quantified by a single observer who was blinded to the treatment groups via the established histopathological scoring system. [31]
2.6. Elisa
PGE2 and PGD2 concentrations in fibroblasts cellular supernatants and rat homogenized lung tissues were measured as previously described. [32] ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA), all procedures were performed according to the manufacturers’ instructions. All analyzes were run in triplicate and repeated twice.
2.7. Quantitative real-time PCR
Total RNA samples in lungs were isolated using TRIzol reagent (Takara Bio, Kusatsu, Japan) according to the manufacturer’s protocol. The cDNA of mRNA was synthesized by the reverse transcription kit purchased from Thermo Scientific (Rockford, IL, USA). The expression of mRNA was detected by qPCR (Bio-Rad, Hercules, CA, USA) with TB Green® Premix Ex Taq™ PCR kit (Takara Bio, Kusatsu, Japan). The gene-specific primers used are listed in Table S1 and mRNA levels normalized to GAPDH. Data were calculated with using the 2-ΔΔCtmethod.
2.8. Statistical analysis
All data were presented as mean ± SEM. All data were analyzed using one-way ANOVA, followed by a Tukey test for post hoc comparisons. P < 0.05 was considered as significant. Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Biphasic activation of COX-2, L-PGDS in rat primary lung fibroblasts stimulated by LPS
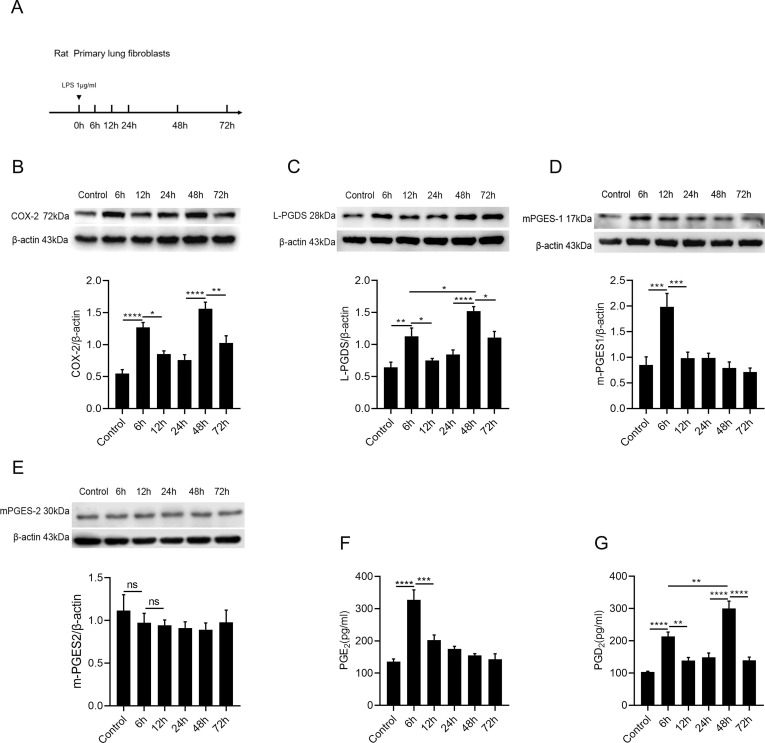
Pulmonary lung fibroblasts play an important role in inflammatory diseases and participate actively in immune response. [33] Herein, to find out the dynamic change of COX-2 expression in fibroblasts, cells were exposed to LPS for 0,6,12,24,48,72 h ( Fig. 1 A). We found that the COX-2 protein presents a biphasic expression character, COX-2 firstly peaked at 6 h and secondary one at 48 h ( Fig. 1 B).
Fig. 1.
Biphasic activation of COX-2 and L-PGDS in rat primary lung fibroblasts stimulated by LPS.(A) Rat primary lung fibroblasts were incubated with LPS (1 μg/ml) for 0, 6, 12, 24, 48, 72 h. (B–E) Relative protein levels of COX-2, L-PGDS, mPGES-1 and mPGES-2 were determined by western blot analysis. (F and G) Supernatants were collected at 0, 6, 12, 24, 48 and 72 h after LPS challenge for ELISA detection. PGE2 and PGD2 levels at each time point were determined by ELISA. Data are presented as mean ± SEM, n = 6 *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
Interestingly, we found that the L-PGDS also showed a biphasic expression, similar to that of COX-2 expression, peaking at 6 and 48 h. ( Fig. 1 C). Moreover, mPGES-1 only presented a single peak expression at 6 h ( Fig. 1 D). mPGES-2, as a constitutively expressed synthetase, [34] showed no significant changes after the LPS challenge ( Fig. 1 E). PGE2, as a pro-inflammatory prostaglandin, [35] highly expressed at 6 h only ( Fig. 1 F). In contrast, PGD2 highly peaked both at 6 h and 48 h, and the secondary peak of PGD2 was significantly higher than the first one ( Fig. 1 G). We revealed that there was a biphasic activation character of COX-2/L-PGDS-PGD2 in LPS-stimulated lung fibroblasts.
3.2. PDX enhances the secondary peak of COX-2, L-PGDS via activating the ALX receptor in vitro
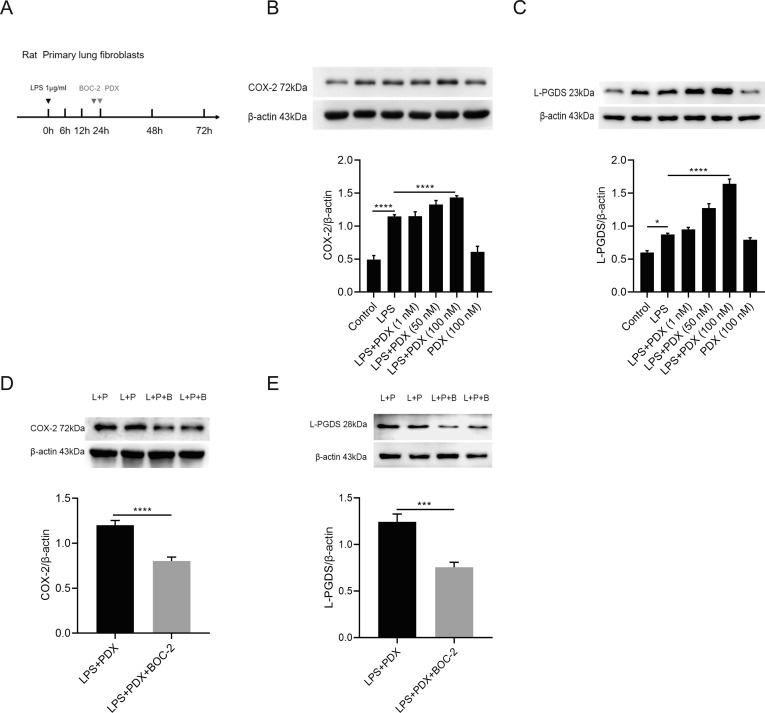
To determine the effect of Protectin DX on the secondary peak of COX-2/L-PGDS expression. COX-2 and L-PGDS protein levels were tested at 48 h with the treatment of PDX ( Fig. 2 A). Our results demonstrated that PDX enhanced COX-2 and L-PGDS protein levels in a dosage-dependent pattern ( Fig. 2 B and 2C).
Fig. 2.
PDX enhances the secondary peak of COX-2, L-PGDS via the ALX/FPR2 receptor in vitro.(A). Primary lung fibroblasts were incubated with LPS (1 μg/ml) for 48 h. Fibroblasts were treated with various concentrations of PDX (1 nM, 50 nM, 100 nM) for 24 h. BOC-2 (10 μM) was administered 30 min prior to PDX (100 nM) treatment, then cells were harvested and sonicated at 48 h. (B and C) The protein expression of COX-2 and L-PGDS after PDX treatment were tested by western blot and analyzed by densitometry compared to β-actin. (D and E) COX-2 and L-PGDS protein levels after the BOC-2 treatment were detected by western blot. Data are shown as mean ± SEM, n = 3–10. *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
We have previously demonstrated that PDX ameliorates the wound repair of the lung epithelial barrier via ALX receptor. [27] Here we would like to know whether PDX enhances the secondary peak of COX-2/L-PGDS via ALX receptor. The ALX receptor antagonist, BOC-2(10 μM) was added 30 min before the PDX treatment. As Fig. 2 D and Fig. 2 E presented, pre-treatment with BOC-2 reversed the promoting effect of PDX on the secondary peak of COX-2 and L-PGDS. suggesting the promoting effect of PDX on the expression of secondary peak COX-2, L-PGDS are via the ALX receptor.
3.3. Biphasic activation of COX-2 and L-PGDS in LPS -stimulated ARDS murine model
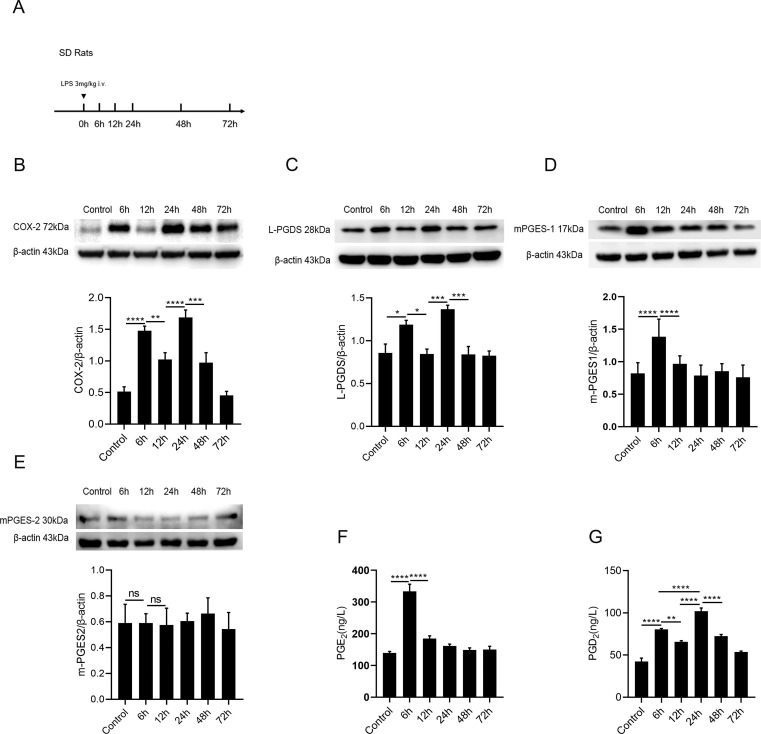
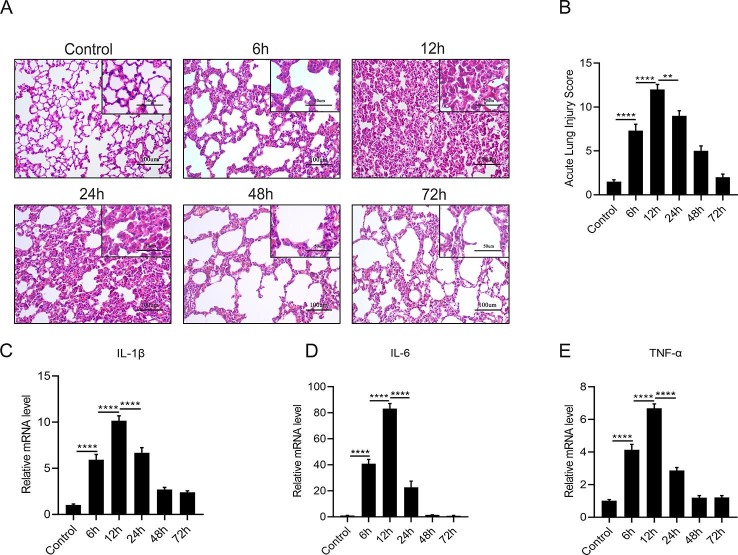
Next, to explore the activation character of COX-2 and L-PGDS in vivo, we established the self-limited ARDS model in SD rats by administrating low dosage of LPS intravenously ( Fig. 3 A). Pathomorphological changes were detected by H&E staining, compared with the control group, the lung architecture in the LPS group showed most remarkable damage at 12 h, as evidenced by the changes in lung injury score. The mRNA expression of IL-1β, IL-6 and TNF-α in the lungs, peaked at 12 h and decreased subsequently after the LPS challenge (data were shown in Figure S1). Moreover, COX-2 showed a biphasic expression pattern as presented in Fig. 3 B.COX-2 first peaked at 6 h after LPS stimulation, and the secondary peak appeared at 24 h.
Fig. 3.
Biphasic activation of COX-2 and L-PGDS in ARDS model.(A) LPS (3 mg/kg) or the equivalent volume of sterile 0.9% saline were injected i.v. to SD rats, lung tissues were collected at 0, 6, 12, 24, 48, 72 h for western blot analyze and ELISA detection. (B and C) Relative expression levels of COX-2 and L-PGDS protein in lung tissues were determined by western blot. (D and E) Protein expression of mPGES-1 and mPGES-2. (F and G) PGE2 and PGD2 levels were detected by ELISA. All data are presented as mean ± SEM, n = 6–10. *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Our results also showed L-PGDS had a biphasic expression characteristic in vivo as same as in fibroblasts.The expression of L-PGDS firstly increased at 6 h, and the secondary peak appeared at 24 h ( Fig. 3 C), mPGES-1 was highly expressed at 6 h only ( Fig. 3 D). mPGES-2 showed no significant change after the LPS stimulation ( Fig. 3 E). The production of PGE2 ,increased only at 6 h and then gradually decreased, in consistent with the mPGES-1 expression ( Fig. 3 F). PGD2 levels peaked at both 6 and 24 h, in consistent with the L-PGDS expression ( Fig. 3 G).
These findings uncovered that there has a biphasic expression feature of COX-2/L-PGDS-PGD2 in ARDS rat model. COX-2 might be proinflammatory at early stage (via the mPGES-1/PGE2 expression). Secondary peak of COX-2 might be pro-resolving in the resolution stage (via L-PGDS/PGD2).
3.4. The effect of COX-2 secondary peak on inflammation resolution of the rat ARDS model
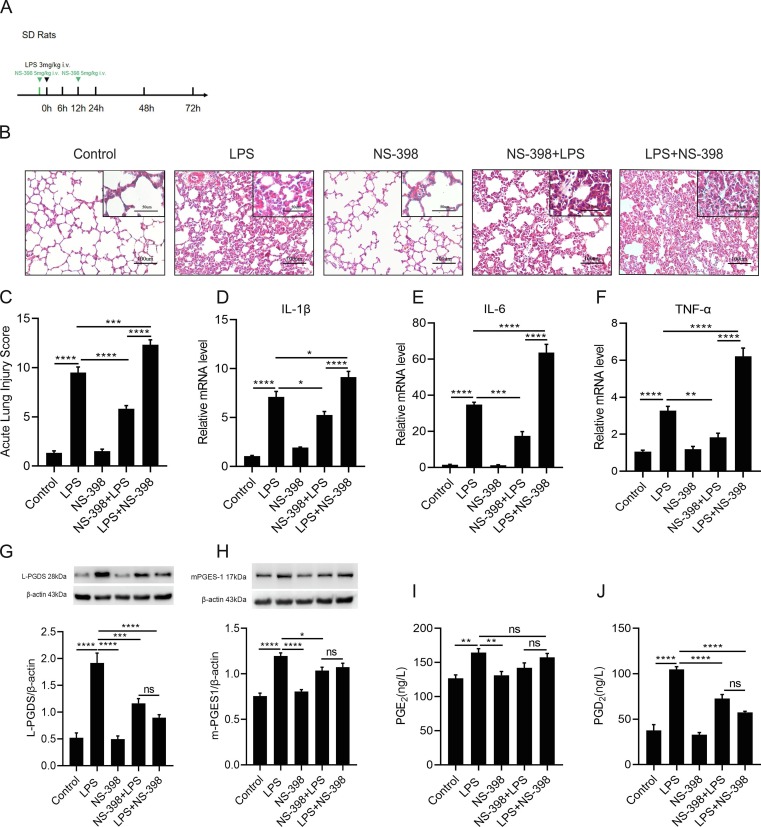
Next, to determine if secondary peak of COX-2 could play a pro-resolving role in the resolution of ARDS model. NS-398, a clinical-used selective COX-2 inhibitor, was administrated intravenously in rats 1 h before (the NS-398 + LPS group) or at 12 h (the LPS + NS-398 group) after the LPS challenge ( Fig. 4 A). Pathological features were detected at 24 h as shown in Fig. 4 B. Compared with the NS-398 + LPS group and LPS group, the LPS + NS-398 group revealed more distinct interstitial edema, hemorrhaging, thickening of alveolar walls, and inflammatory cells infiltration in the lung tissues. In contrast to the LPS group, the NS-398 + LPS group showed less pathological damage. As shown in Fig. 4 C, acute lung injury score was quantified and found to be in consistent with the pathophysiological changes. In addition, compared with the NS-398 + LPS group and the LPS group, relative mRNA levels of the proinflammatory cytokines: IL-1β, IL-6 and TNF-α were much higher in LPS + NS-398 group ( Fig. 4 D-F). These findings suggested that inhibition the latter peak of COX-2 postponed the resolution of inflammation.
Fig. 4.
The effect of COX-2 secondary peak on inflammation resolution of the rat ARDS model.(A).SD rats were administered with LPS (3 mg/kg) or the same volume of sterile intravenously, NS-398 (5 mg/kg), or the same volume of 0.9% saline was intravenously administered to SD rats 1 h prior to the LPS stimulation (NS-398 + LPS), administration at 12 h after the LPS challenge (LPS + NS-398), lung sections were collected at 24 h. (B) Representative H&E staining of the lung (original magnification, 200x; inset, 400x). (C) Acute lung injury scores of each group. (D–F) The mRNA expression level of inflammatory cytokines: IL-1β, IL-6, TNF-α. (G and H) Relative expression level of L-PGDS and mPGES-1 protein in the lung was determined by western blot. (I and J) The concentrations of PGE2 and PGD2 in lungs were detected by ELISA. Data are presented as mean ± SEM, n = 6. *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
Moreover, the COX-2 inhibition significantly decreased the protein expression of L-PGDS at 24 h. No significant difference of L-PGDS protein expression was observed between the NS-398 + LPS group and the LPS + NS-398 group ( Fig. 4 G). Pre-treatment of NS-398 decreased the protein level of mPGES-1in ARDS model ( Fig. 4 H), no significant change in the PGE2 production was observed between LPS group and the NS-398 + LPS group ( Fig. 4 I). Inhibition of the COX-2 reduced the production of PGD2 at 24 h ( Fig. 4 J).
In summary, inhibition of the secondary peak COX-2 in the resolution stage of inflammation caused more significant lung damages compared to the inhibition of the first peak of COX-2 in the early stage of inflammation. Based on these findings, we surmise that the secondary peak of COX-2 plays a key role in promoting the resolution of inflammation in ARDS.
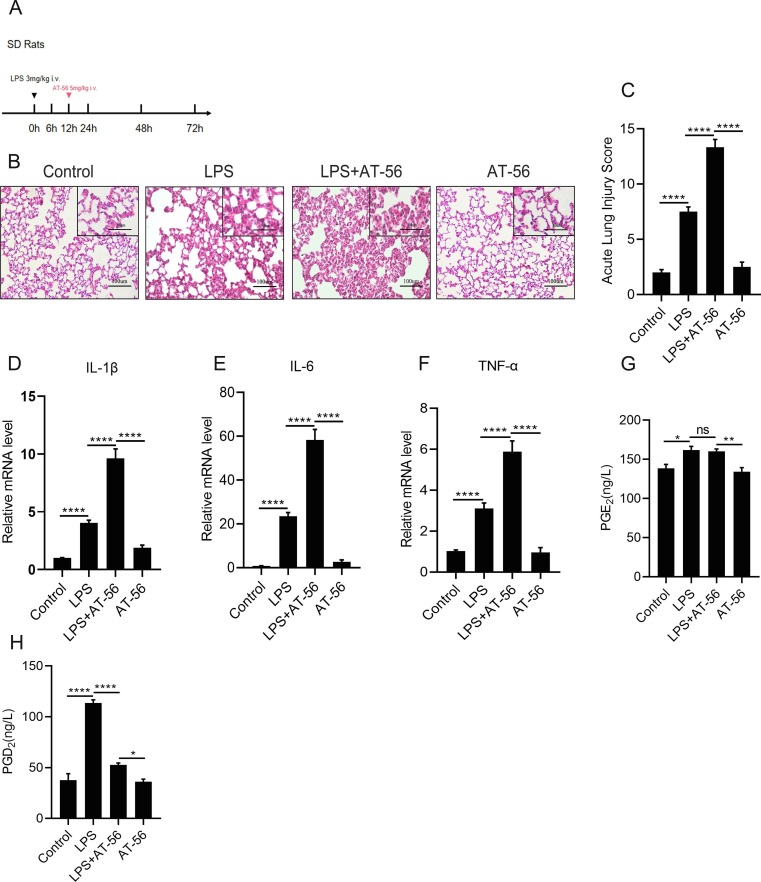
3.5. L-PGDS secondary peak promotes the inflammatory resolution in vivo
We then attempted to figure out the function of the secondary peak of L-PGDS. AT-56 was administrated to rats at 12 h after the LPS challenge ( Fig. 5 A). H&E staining ( Fig. 5 B) revealed that, compared with the LPS group, LPS + AT-56 group displayed more interstitial edema, hemorrhage, and inflammatory cells infiltration in lung tissues. In accordance with this, the lung injury scores were also elevated ( Fig. 5 C), along with the release of IL-1β, IL-6 and TNF-α ( Fig. 5 D–F). These results indicated that suppression of L-PGDS blocked ARDS resolution. ELISA results also proved that inhibiting the L-PGDS decreased the PGD2 production at 24 h ( Fig. 5 H), while no significant difference was found in PGE2 between the LPS group and the LPS + AT-56 group ( Fig. 5 G).
Fig. 5.
L-PGDS secondary peak promotes the inflammatory resolution in vivo.(A).SD rats were administered with LPS (3 mg/kg) or the same volume of sterile intravenously, AT-56 (5 mg/kg), or the same volume of 0.9% saline was injected i.v. to rats 12 h after LPS injection. (B) Pathomorphological staining of the lung tissues. (Original magnification, 200X; inset, 400X). (C) Acute lung injury scores. (D–F) The mRNA expression levels of inflammatory cytokines: IL-1β, IL-6, TNF-α were measured by qPCR. (G and H) PGE2 and PGD2 concentrations in lung tissues were measured by ELISA. Data are shown as mean ± SEM, n = 6. *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
All results above indicated that the secondary peak COX-2/L-PGDS -PGD2 was responsible for ARDS resolution in murine models.
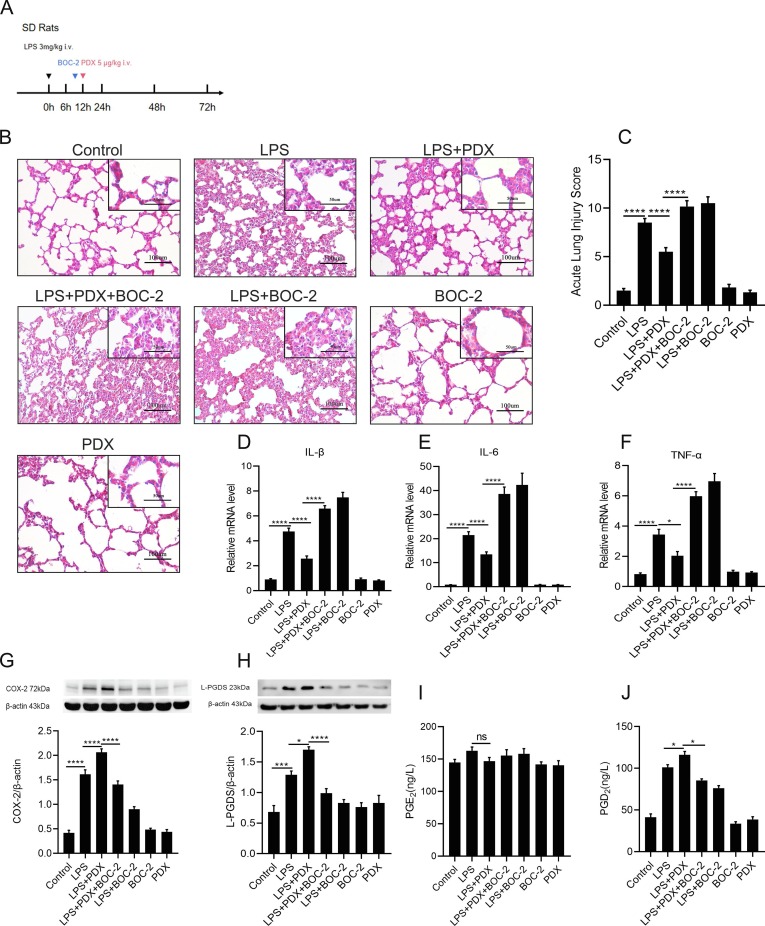
3.6. PDX promotes inflammatory resolution by enhancing the activation of ALX receptor and the COX-2/L-PGDS-PGD2 expressions in vivo
PDX have already been proved to enhance the repair of lung epithelial barrier in ALI/ARDS murine model. [27] To evaluate whether PDX promotes inflammatory resolution via activating ALX receptor and the COX-2/L-PGDS-PGD2 expressions in rat ARDS model, PDX was given at 12 h after the LPS exposure ( Fig. 6 A). H&E staining result showed that PDX markedly alleviate the morphological and histological damages induced by LPS, consistent with a decrease in acute lung injury score ( Fig. 6 B and 6C). Administration of BOC-2 (the ALX receptor inhibitor) reversed the effect of PDX on both histological damages and the release of pro-inflammatory cytokines induced by LPS ( Fig. 6 B and 6D-F), consistently with the acute lung injury score ( Fig. 6 C). These findings suggested that PDX promotes the inflammation resolution via activating the ALX receptor.
Fig. 6.
PDX promotes the inflammatory resolution by enhancing the activation of ALX/FPR2 receptor and the COX-2-L-PGDS-PGD2 expression in vivo.(A).SD rats were administered with LPS (3 mg/kg) or the same volume of sterile intravenously, PDX (5 μg/kg) were administered intravenously to rats at 12 h after LPS treatment, BOC-2 600 ng/kg was given for 1 h before PDX treatment. Lung tissues were collected at 24 h. (B) Representative pathological H&E staining sections of lung tissues. (Original magnification, 200x; inset, 400x). (C) Acute lung injury scores assessment of each group. (D–F) The mRNA expression level of Inflammatory cytokines: IL-1β, IL-6, TNF-α. (G and H) Expression levels of COX-2 and L-PGDS protein after PDX treatment was determined by western blot analysis. (I and J) Concertation of PGE2 and PGD2 were detected by ELISA. All data are presented as mean ± SEM, n = 6. *p < 0.05, **p < 0.01,***p < c0.001,****p < 0.0001.
In addition, PDX significantly up-regulated the protein expression of COX-2 and L-PGDS during the resolution stage. PDX significantly promoted the expression of the pro-resolving mediator PGD2 ( Fig. 6 J). No significant difference was found in the PGE2 level between the LPS group and the LPS + PDX group ( Fig. 6 I). Pre-stimulation with BOC-2 reversed the improved effect of PDX on COX-2 and L-PGDS protein expression as well as PGD2 secretion ( Fig. 6 G -H). These results indicated that PDX facilitated the inflammatory resolution via activating ALX receptor and enhancing the expression of COX-2/L-PGDS as well as the production of PGD2.
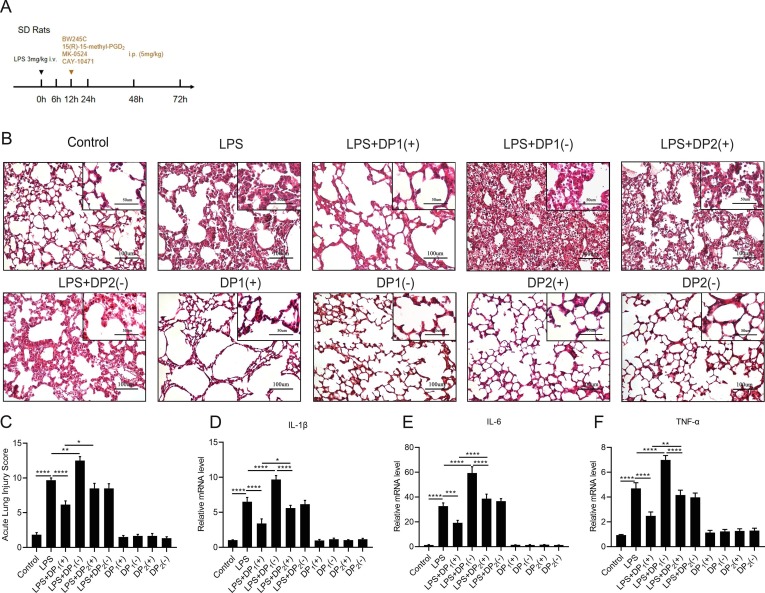
3.7. DP1 receptor is activated during the resolution phase in the murine ARDS model
Previous studies demonstrated that the PGD2-DP1 signaling pathway was responsible for the anti-inflammatory function of ALI/ARDS. [12], [13] Herein, we investigated what kind of PGD2 receptor plays the major role during the resolution phase in the murine ARDS model, BW-245C (DP1 receptor agonist), 15(R)-15-methyl-PGD2(CRTH2/DP2 receptor agonist), MK-0524(DP1 receptor antagonist) or CAY-10471(CRTH2/DP2 receptor antagonist) was intraperitoneally injected into rats at 12 h after the LPS stimulation ( Fig. 7 A). Morphological staining ( Fig. 7 B) revealed that the DP1 receptor agonist BW-245C significantly alleviated the pathological damage, while the DP1 receptor antagonist (MK-0524) aggravated the lung injury. In comparison, the agonist or antagonist of the DP2 receptor did not significantly influence the lung injury condition. As expected, the acute lung injury scores were in line with the findings of morphological staining ( Fig. 7 C). Furthermore, mRNA levels of proinflammatory cytokines: IL-1β ( Fig. 7 D), IL-6 ( Fig. 7 E), TNF-α ( Fig. 7 F) also validated the above results.
Fig. 7.
DP1 receptor is activated during the resolution phase in the rat ARDS model. (A).SD rats were administered with LPS (3 mg/kg) or the same volume of sterile intravenously, BW245C (DP1 receptor agonist 5 mg/kg), 15(R)-15-methyl-PGD2(CRTH2/DP2 receptor agonist 5 mg/kg), MK-0524(DP1 receptor antagonist 5 mg/kg) and CAY-10471(CRTH2/DP2 receptor antagonist 5 mg/kg) were injected to rats i.p. at 12 h after LPS exposure. Lung tissues were collected at 24 h. (B) Representative micrographs of pulmonary histology, as shown by H&E staining. (C) Acute lung injury score. (D–F) The mRNA levels of IL-1β, IL-6, TNF-α were measured via qPCR. Data are shown as mean ± SEM, n = 6. *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
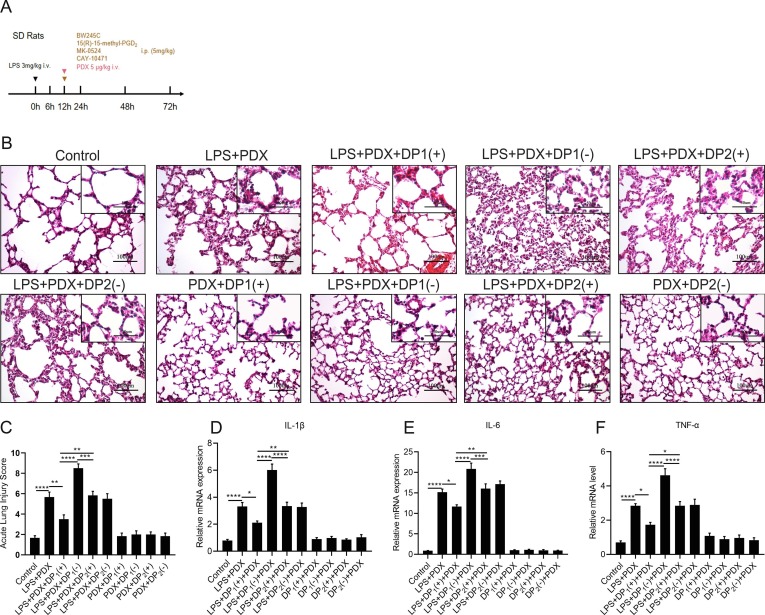
3.8. PDX promotes the inflammatory resolution through the activation of the DP1 receptor in the rat ARDS model
Then, we investigated if PDX promoted the inflammatory resolution through the activation of the PGD2 receptors,in the rat ARDS model, BW-245C,15(R)-15-methyl-PGD2, MK-0524 or CAY-10471 were given to rats with or without PDX ( Fig. 8 A). Pathological staining ( Fig. 8 B) showed that the DP1 receptor agonist enhanced the pro-resolving function of PDX, while the DP1 receptor antagonist suppressed the improved inflammatory resolution by the PDX treatment. Meanwhile, the DP2 receptor did not significantly affect the resolution of inflammation promoted by PDX ( Fig. 8 B). The acute lung injury scores were in accordance with the findings of morphological staining ( Fig. 8 C). Furthermore, inflammatory cytokines levels (IL-1β, IL-6 and TNF-α) were down-regulated after the DP1 receptor agonist treatment, while up-regulated by DP1 receptor antagonist ( Fig. 8 D-F). In contrast, the DP2 receptor did not affect the release of IL-1β, IL-6 and TNF-α ( Fig. 8 D-F). Altogether, these data suggested that PDX promotes resolution of inflammation by activating the DP1 receptor in vivo.
Fig. 8.
PDX promotes the inflammatory resolution stage through activation of DP1 receptor in the rat ARDS model.(A) SD rats were administered with LPS (3 mg/kg) or the same volume of sterile intravenously, BW245C (DP1 receptor agonist), 15(R)-15-methyl-PGD2(CRTH2/DP2 receptor agonist), MK-0524(DP1 receptor antagonist) or CAY-10471(CRTH2/DP2 receptor antagonist) were injected i.p. 5 mg/kg to rats at 12 h after LPS exposure with or without the PDX(5 μg/kg) administration. (B) Representative pathological H&E staining sections of lung tissues. (Original magnification, 200x; inset, 400x). (C) Acute lung injury scores assessment of each group. (D–F) The mRNA expression level of inflammatory cytokines: IL-1β, IL-6, TNF-α.All data are presented as mean ± SEM, n = 6. *p < 0.05, **p < 0.01,***p < 0.001,****p < 0.0001.
4. Discussion
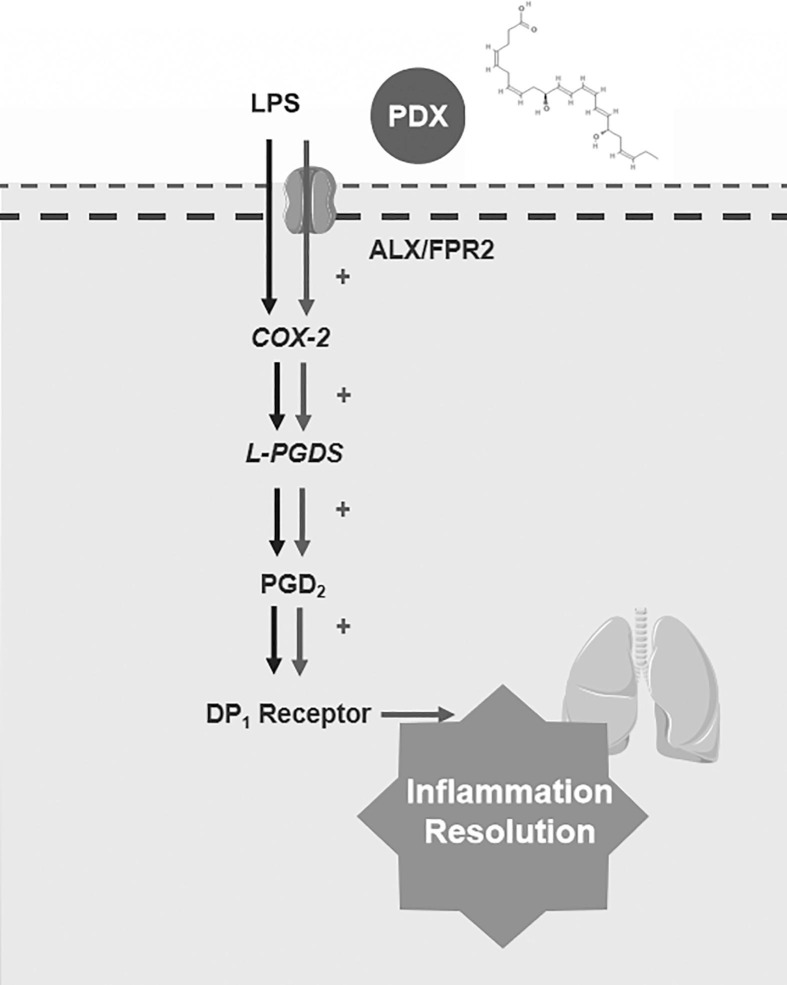
Our study uncovered that COX-2/L-PGDS-PGD2 expressions have a dual activation induced by LPS. Importantly we found that the secondary peak of COX-2/L-PGDS-induced PGD2 was responsible for the pro-resolving process in ARDS. Moreover, we showed that the DP1 receptor was activated in inflammatory resolution. This study provides evidence for a new mechanism by which PDX may promote inflammation resolution of the ARDS model through improving the expression of the secondary peak of COX-2/L-PGDS-induced PGD2. Interestingly, the ALX receptor antagonist, BOC-2, abrogated the effect of PDX on the COX-2/L-PGDS-PGD2. Altogether, these findings were summarized in Fig. 9 , showed that PDX also promotes the inflammatory resolution via activating the ALX receptor and enhances the inflammatory resolution partly via activating the DP1 receptor.
Fig. 9.
The underlying mechanism of PDX regulates the resolution of inflammation via enhancing the ALX -COX-2/L-PGDS-PGD2 pathway and activating DP1 receptor.
COX-2 is catalyzed after the inflammatory stimuli immediately. Pro-inflammatory PGs were induced by COX-2, [36] Fukunaga K. et al indicated that COX-2 plays a protective role in ALI/ARDS through COX-2-derived mediators, partly via enhancing the lipoxin signaling. [7] However, this study did not discuss the dynamic change of COX-2 expression in the ARDS model. In our LPS-stimulated ARDS model, we revealed that COX-2 was quickly and peaked at 6 h, then peaked twice at 24 h in vivo. The secondary peak of COX-2 displayed pro-resolving character which was distinguished from traditional concepts. Consistent with our study, Gilroy D.W. et al reported the biphasic expression of COX-2 in the carrageenin-induced pleurisy mice model. [8] However, the specific role of each COX-2 peak remains unclear. Herein, our findings suggested that COX-2 could be proinflammatory at early stage and be pro-resolving during the later stage of inflammation. Therefore, blindly using COXs inhibitors such as NSAIDs may postpone the resolution process of inflammatory diseases and cause unexpected damage to the ARDS patients.. This might be one of the reasons that the application of NSAIDS in patients with ARDS or sepsis in the clinical trials could not be an effective therapy. [6] Therefore, anti-inflammatory drugs should be used carefully depending on the stage of inflammation in the patients with ARDS.
Our previous findings revealed that there is a biphasic expression of COX-2 induced-PGD2 in LPS-stimulated lung fibroblasts, [19] however we did not uncover the downstream PGs synthetases. In this study, we demonstrated that mPGES-1 peaked only at 6 h assosiated with maximal PGE2 synthesis. While L-PGDS had biphasic peaks at 6 and 48 h assosiated with a biphasic PGD2 synthesis. Previous studies indicated that L-PGDS alleviated the endothelial barrier injury in ALI/ARDS by promoting the production of PGD2 in vivo. [13] We confirmed that the L-PGDS existed biphasic expression in rat ARDS model. Furthermore, we found that the secondary peak of COX-2/L-PGDS derived PGD2 promoted the inflammatory resolution of ARDS model.
PGs, such as PGE2, plays pro-inflammatory role in inflammation. [28] Previous studies indicated that PGD2 promoted the resolution of inflammation. [37] In addition, PGD2 performs its physiological function via the DP1 and CRTH2(DP2) receptors. We showed that the DP1 receptor was activated in the resolution of ARDS. Administration of the agonist of the DP1 receptor improved the resolution of inflammation, while the inhibition of the DP1 receptor aggravated lung injuries. In our self-limited ARDS model, activation or inhibition of DP2 receptors did not affect the resolution of inflammation.
As previously described, the resolution of acute inflammatory diseases is an active process. [38] There is an internal, self-protective feature among the patient with ARDS. Thus, we established a self-limited ARDS model for further study. PDX, as an endogenous “braking signal”, displays anti-inflammatory and pro-resolving characteristics. We already have determined that PDX could ameliorate the wound repair of the lung epithelial barrier. [27] In this study, we demonstrated that PDX significantly alleviated inflammatory injuries in ARDS via enhancing the expression of the secondary peak of COX-2/L-PGDS-and its metabolite PGD2. Additionally, we revealed that the pro-resolving effect of PDX was dependent on the activation of the DP1 receptor.
PDX exerts its effects via ALX receptor pathways. [25], [26], [27] Thus, our study used the ALX antagonist BOC-2. As expected, the ALX antagonist reversed the PDX induced improvement on COX-2/L-PGDS-PGD2 expression both in vivo and in vitro, suggesting that PDX up-regulates COX-2/L-PGDS-PGD2 signaling via the ALX/FPR2 receptor. Therefore, we found that the activation of the COX-2/L-PGDS-PGD2 secondary peak and the DP1 receptor may be the novel mechanism by which PDX exerts its pro-resolving effect on inflammation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 82002093), and the Natural Science Foundation of Zhejiang Province (LY19H150002, LQ20H150002).
Disclosure
The authors declare that they have no conflicts of interest for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.108348.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Huppert L.A., Matthay M.A., Ware L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019;40(1):31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5(1) doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun-Buisson C., Minelli C., Bertolini G., Brazzi L., Pimentel J., Lewandowski K., Bion J., Romand J.-A., Villar J., Thorsteinsson A., Damas P., Armaganidis A., Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 5.Bernard G.R., Wheeler A.P., Russell J.A., Schein R., Summer W.R., Steinberg K.P., Fulkerson W.J., Wright P.E., Christman B.W., Dupont W.D., Higgins S.B., Swindell B.B. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N. Engl. J. Med. 1997;336(13):912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 6.Aronoff D.M. Cyclooxygenase inhibition in sepsis: is there life after death? Mediators Inflamm. 2012;2012:1–7. doi: 10.1155/2012/696897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukunaga K., Kohli P., Bonnans C., Fredenburgh L.E., Levy B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 8.Gilroy D.W., Colville-Nash P.R., Willis D., Chivers J., Paul-Clark M.J., Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 9.Ulivi V., Cancedda R., Cancedda F.D. 15-deoxy-delta 12,14-prostaglandin J(2) inhibits the synthesis of the acute phase protein SIP24 in cartilage: Involvement of COX-2 in resolution of inflammation. J. Cell Physiol. 2008;217(2):433–441. doi: 10.1002/jcp.v217:210.1002/jcp.21516. [DOI] [PubMed] [Google Scholar]
- 10.Colville-Nash P.R., Gilroy D.W., Willis D., Paul-Clark M.J., Moore A.R., Willoughby D.A. Prostaglandin F2alpha produced by inducible cyclooxygenase may contribute to the resolution of inflammation. Inflammopharmacology. 2005;12(5–6):473–480. doi: 10.1163/156856005774382616. [DOI] [PubMed] [Google Scholar]
- 11.Konya V., Maric J., Jandl K., Luschnig P., Aringer I., Lanz I., Platzer W., Theiler A., Bärnthaler T., Frei R., Marsche G., Marsh L.M., Olschewski A., Lippe I.T., Heinemann A., Schuligoi R. Activation of EP4 receptors prevents endotoxin-induced neutrophil infiltration into the airways and enhances microvascular barrier function. Br. J. Pharmacol. 2015;172(18):4454–4468. doi: 10.1111/bph.2015.172.issue-1810.1111/bph.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murata T., Aritake K., Tsubosaka Y., Maruyama T., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., Ozaki H. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc Natl Acad Sci U S A. 2013;110(13):5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horikami D., Toya N., Kobayashi K., Omori K., Nagata N., Murata T. L-PGDS-derived PGD2 attenuates acute lung injury by enhancing endothelial barrier formation. J. Pathol. 2019;248(3):280–290. doi: 10.1002/path.2019.248.issue-310.1002/path.5253. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson B., Morgenstern R., Jakobsson P.J. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59(3):207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 15.Kostenis E., Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12(4):148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Smith R.S., Smith T.J., Blieden T.M., Phipps R.P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 1997;151(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- 17.Tong L., Bi J., Zhu X., Wang G., Liu J., Rong L., Wang Q., Xu N., Zhong M., Zhu D., Song Y., Bai C. Keratinocyte growth factor-2 is protective in lipopolysaccharide-induced acute lung injury in rats. Respir. Physiol. Neurobiol. 2014;201:7–14. doi: 10.1016/j.resp.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Hu S., Li J., Xu X., Liu A., He H., Xu J., Chen Q., Liu S., Liu L., Qiu H., Yang Y.i. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther. 2016;7(1) doi: 10.1186/s13287-016-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S., Wang Q., He Q., Song X., Ye D., Gao F., Jin S., Lian Q. Novel biphasic role of Lipoxin A (4) on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts. Mediators Inflamm. 2011;2011:1–9. doi: 10.1155/2011/745340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–1288. doi: 10.1096/fsb2.v31.410.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan C.N., Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. Suppl 1(Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25(1):101–137. doi: 10.1146/immunol.2007.25.issue-110.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 23.Sancéau J.-Y., Maltais R., Poirier D., Marette A. Total Synthesis of the Antidiabetic (Type 2) Lipid Mediator Protectin DX/PDX. J Org Chem. 2019;84(2):495–505. doi: 10.1021/acs.joc.8b0197310.1021/acs.joc.8b01973.s001. [DOI] [PubMed] [Google Scholar]
- 24.Hansen T.V., Vik A., Serhan C.N. The Protectin Family of Specialized Pro-resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front Pharmacol. 2019;9:1582. doi: 10.3389/fphar.2018.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo X.-J., Hao Y.u., Cao F., Yan S.-F., Li H., Wang Q., Cheng B.-H., Ying B.-Y., Smith F.G., Jin S.-W. Protectin DX increases alveolar fluid clearance in rats with lipopolysaccharide-induced acute lung injury. Exp Mol Med. 2018;50(4):1–13. doi: 10.1038/s12276-018-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y., Zhang H.-W., Mei H.-X., Xu H.-R., Xiang S.-Y., Yang Q., Zheng S.-X., Gao Smith F., Jin S.-W., Wang Q. PDX regulates inflammatory cell infiltration via resident macrophage in LPS-induced lung injury. J Cell Mol Med. 2020;24(18):10604–10614. doi: 10.1111/jcmm.v24.1810.1111/jcmm.15679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J.-X., Li M., Hu X., Lu J.-C., Wang Q., Lu S.-Y., Gao F., Jin S.-W., Zheng S.-X. Protectin DX promotes epithelial injury repair and inhibits fibroproliferation partly via ALX/PI3K signalling pathway. J Cell Mol Med. 2020;24(23):14001–14012. doi: 10.1111/jcmm.v24.2310.1111/jcmm.16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D., Zheng S., Li W., Yang L.i., Liu Y., Zheng X., Yang Y.i., Yang L., Wang Q., Smith F.G., Jin S. Novel biphasic role of resolvin D1 on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts is partly through PI3K/AKT and ERK2 pathways. Mediators Inflamm. 2013;2013:1–11. doi: 10.1155/2013/964012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y.e., Zhang H., Luo L., Lin J., Li D., Zheng S., Huang H., Yan S., Yang J., Hao Y.u., Li H., Gao Smith F., Jin S. Resolvin D1 Improves the Resolution of Inflammation via Activating NF-κB p50/p50-Mediated Cyclooxygenase-2 Expression in Acute Respiratory Distress Syndrome [published online ahead of print, 2017 Aug 9] J. Immunol. 2017;199(6):2043–2054. doi: 10.4049/jimmunol.1700315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamm M., Roth M., Malouf M., et al. Primary fibroblast cell cultures from transbronchial biopsies of lung transplant recipients. Transplantation. 2001;71(2):337–339. doi: 10.1097/00007890-200101270-00030. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Zheng X., Cheng Y., Zhang Y.-L., Wen H.-X., Tao Z., Li H., Hao Y.u., Gao Y.e., Yang L.-M., Smith F.G., Huang C.-J., Jin S.-W. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na, K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J. Immunol. 2014;192(8):3765–3777. doi: 10.4049/jimmunol.1302421. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Lian Q.-Q., Li R.u., Ying B.-Y., He Q., Chen F., Zheng X., Yang Y.i., Wu D.-R., Zheng S.-X., Huang C.-J., Smith F.G., Jin S.-W. Lipoxin A (4) activates alveolar epithelial sodium channel, Na, K-ATPase, and increases alveolar fluid clearance. Am. J. Respir Cell Mol. Biol. 2013;48(5):610–618. doi: 10.1165/rcmb.2012-0274OC. [DOI] [PubMed] [Google Scholar]
- 33.Flavell S.J., Hou T.Z., Lax S., Filer A.D., Salmon M., Buckley C.D. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. 2008;153:S241–S246. doi: 10.1038/sj.bjp.0707487. Suppl 1(Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang H.H., Meuillet E.J. Identification and development of mPGES-1 inhibitors: where we are at? Future. Med. Chem. 2011;3(15):1909–1934. doi: 10.4155/fmc.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38(10):1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Rajakariar R., Hilliard M., Lawrence T., et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc. Natl. Acad. Sci. U S A. 2007;104(52):20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3(5):401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.