Abstract
Cardiac damage from chemotherapy is a known phenomenon leading to significant morbidity and mortality in the cancer surviving population, and identifying high-risk pediatric patients early is challenging. The purpose of this pilot study was to evaluate whether echo strain, cardiac MRI (CMR), and serum biomarkers are more sensitive methods for detecting cardiac toxicity than standard echo and to examine the relationship between biomarkers in patients without decreased systolic function as determined by standard echo. In this pilot study, we prospectively enrolled pediatric subjects after completion of anthracycline inclusive chemotherapy. Each subject underwent a post-treatment echocardiogram (standard with strain), serum biomarkers (N-terminal brain natriuretic peptide (NT-pro-BNP) and interleukin 1 receptor-like 1 protein (ST2)), and CMR (standard and extracellular volumes (ECVs)).We correlated the markers using Pearson correlation. We enrolled 30 subjects, 11F/19M, aged 8–21 years. Cumulative anthracycline dose (CAD) correlated with BNP (p=0.06), CMR ECV 4-chamber (p=0.05) and sagittal (p=0.01), and mitral valve E/A (p=0.02). BNP correlated with CMR ECV 4-chamber (p=0.001) and sagittal (p=0.001) and with echo average longitudinal strain (ALS) (p=0.05). This study demonstrated a significant correlation of CAD with BNP and CMR ECV. There was also a significant correlation of NT-pro-BNP with CMR ECV and ALS. Combining these parameters with standard echo has the potential to identify high-risk patients early. Further studies are needed for long-term follow-up and management in this vulnerable population.
INTRODUCTION
Improvements in treatment for childhood cancers have resulted in an increase in 5-year survival over the last 40 years to >80%, with the expectation for continued improvement. Cardiac-related mortality is 10-fold higher among childhood cancer survivors as compared with age-matched controls, representing the third leading cause of death in this population following cancer recurrence and secondary malignancy. Because of the limited regenerative capacity of cardiomyocytes, many chemotherapeutic agents, such as anthracyclines, are cardiotoxic with long-term effects on cardiac health. Cardiotoxicity can be difficult to treat and manifests at variable times during and after treatment. The effects range from decreased cardiac systolic function, diastolic dysfunction, cardiomyopathy, valvular changes, hypertension, and dysrhythmias.1 The incidence of cardiotoxicity in children ranges from 0% to 16% for clinical heart failure2 and 0%–57% for subclinical cardiotoxicity defined by decreased systolic function alone.3
Due to its non-invasive nature and availability, echocardiography is a useful screening tool to detect chemotherapy-based cardiotoxicity. The primary echocardiographic parameters monitored by oncologists are left ventricular (LV) shortening fraction (SF) and ejection fraction (EF) based on the guidelines from the 1992 Children’s Cancer Study Group Cardiology Committee of the Children’s Oncology Group, which were accepted again by the 2008 Children’s Oncology Group task force.4,5 These describe a lower limit of normal for SF of 28% and EF of 50%–55% by transthoracic echocardiography (TTE). They also define deterioration by an absolute change of 10% in either SF or EF. A major limitation of these markers is that changes in EF and SF are often late findings and irreversible damage has already occurred and often hard to treat. Current strategies for the medical treatment of heart failure yield only modest results in this patient population.6 Therefore, it is imperative to find early diagnostic markers of subclinical changes in myocardial function to prevent morbidity and mortality owing to cardiac sequelae in this vulnerable population.
Speckle tracking echocardiography (STE) is a non-invasive technique that can analyze ventricular deformation from standard echo images. Studies in adult populations showed using STE to measure longitudinal and circumferential strain revealed changes that preceded decreases in EF.7 Cardiac MRI (CMR) provides accurate measures of LV systolic function and morphology and measures myocardial fibrosis using late gadolinium enhancement (LGE) and extracellular volume (ECV). Animal studies have demonstrated the utility of CMR techniques in screening for early signs of cardiotoxicity.8 While positive LGE is infrequently seen in chemotherapy-based cardiotoxicity in adults,9 ECV values were increased compared with healthy controls.9 Serum biomarkers such as cardiac troponins and brain natriuretic peptide (BNP) were sensitive markers of cardiotoxicity in adult studies.10 Other serum biomarkers such as myeloperoxidase (MPO), a marker of oxidative stress, and interleukin 1 receptor-like 1 protein (ST2), an inflammatory mediator, correlated with cardiomyocyte damage,11 although there are no specific studies in chemotherapy-related cardiotoxicity in children.10
The purpose of this pilot study was to evaluate whether STE, CMR, and serum biomarkers are more sensitive methods for detecting cardiac toxicity than standard TTE and to examine the relationship between these markers in patients without decreased systolic function as determined by TTE. If proven effective, these methods may be incorporated into standard monitoring of post-chemotherapy cardiac toxicity to prevent morbidity and mortality from poor cardiac outcomes in these patients.
METHODS
Study population
Patients aged 8–25 years who underwent oncologic treatment in the past 10 years, received anthracycline-based chemotherapy at any dose, had completed treatment, and did not require sedation for CMR were eligible for the study. Eligible subjects were enrolled through the Cardiology-Oncology clinic at Children’s National Medical Center prospectively. Informed consent was obtained for all participants. Subjects aged 12–17 years also gave assent. Patients with significant congenital heart defects, renal injury/failure with decreased estimated glomerular filtration rate, pregnant patients, and patients with any contraindication to undergoing CMR were excluded. Patients under 8 years of age were excluded due to the likely need for sedation for CMR.
Echocardiography
Echocardiography (echo) was performed in the accredited echocardiography laboratory at Children’s National Medical Center. Echocardiographic assessments were obtained with a standard protocol to obtain two-dimensional, M mode, spectral Doppler, color Doppler, and tissue Doppler images. Echocardiograms were obtained as standard of care prior to chemotherapy as indicated in the individual chemotherapy protocols (pre-chemotherapy echo), and these data were gathered for use in this analysis. An echo was obtained contemporaneous with the CMR obtained for this study (study echo). Pre-chemotherapy echocardiograms were performed on either a Philips iE33 or GE machine. The post-chemotherapy echocardiograms performed at the time of the CMR were all performed on a Philips iE33 machine. Echocardiograms were interpreted by a pediatric cardiologist blinded to the subjects’ underlying diagnosis and treatment. Post-acquisition measurements were performed using American Society of Echocardiography guidelines.12,13 Speckle tracking analysis was completed by two cardiologists involved in the study. Philips QLab software (Philips, Best, Holland, The Netherlands) was used for speckle tracking analysis for all study echoes. Speckle tracking analysis for average longitudinal strain (ALS) was performed on a single apical four-chamber image and average circumferential strain (ACS) was performed from a single parasternal short-axis image at the level of the mid-papillary muscles.
Cardiac MRI
Subjects underwent CMR with gadolinium contrast on a Siemens Aera 1.5T MR scanner (Siemens Healthcare, Erlangen, Germany). The methods used for obtaining volumetric analysis, native T1 mapping, LGE, and ECV are described in previous work from our CMR core group.14 The CMR study included volumetric analysis to obtain left ventricular ejection fraction (LVEF). Native T1 mapping was performed in four short-axis slices (excluding apical regions to avoid partial volume effect) and a four-chamber slice using both a modified look-locker inversion recovery (MOLLI) and a saturation recovery single-shot acquisition (SASHA) sequence. LGE imaging and post-contrast T1 mapping were performed following intravenous administration of gadobutrol 0.15 mmol/kg using both the MOLLI and SASHA methods in identical slice positions. All post-contrast T1 maps were acquired between 15 and 22 min after contrast administration to assure that a dynamic equilibrium was achieved.
Following the CMR examination, maps of ECV values were created for one short-axis slice and one four-chamber slice on a per-pixel basis using the pre-contrast T1 map, the post-contrast T1 map, and the venous hematocrit at the time of intravenous placement. Six regions of interest (ROI), three in the septal wall and three in the lateral wall of the LV, were generated for the short-axis and four-chamber slices using the “middle third” technique. Abnormal ECV was defined as >28%. Care was taken to avoid including pixels containing blood pool at the endocardial border, epicardial fat, and any artifacts in the ROI.
Serum biomarkers
Serum for MPO, ST2, troponin I, and N-terminal brain natriuretic peptide (NT-pro-BNP) was collected at the time of CMR via routine laboratory draw or during intravenous insertion. Complete blood count, Troponin I, and NT-pro-BNP assays were performed in the Chemistry Laboratory at Children’s National Medical Center. MPO and ST2 were performed by Quest Diagnostics.
Statistical analysis
All data were de-identified and compiled into a single study database. Paired, two-tailed t-tests were used to compare pre-echocardiographic and post-echocardiographic data for the study group. Pearson correlation coefficients were used to assess the relationship between two continuous variables. A p value ≤0.05 was used to indicate a statistically significant difference.
RESULTS
Study population
A total of 30 patients were enrolled in the study (19 male, 11 female). The most common malignancy in the study population was a primary bone malignancy (osteosarcoma and Ewing’s sarcoma) followed by lymphoma and acute lymphoblastic leukemia. Patient characteristics are summarized in table 1. None of the patients were receiving cardiac medications at the time of the study or reported any cardiac symptoms. There were eight patients who received radiation, of which four received radiation to the chest wall or lung.
Table 1.
Patient Characteristics
| Median (range) | |
|---|---|
| Gender | 19 male, 11 female |
| Age at diagnosis (years) | 12 (0.5–20) |
| Age at time of study (years) | 12.5 (8–21) |
| Cumulative anthracycline dose (mg/m2) | 330.5 (75–492) |
| Time since completion of treatment (months) | 32.6 (1.6–194) |
| Time since last anthracycline treatment (months) | 47.7 (2.5–136) |
| Time from pre-treatment echo to study echo (months) | 55.4 (8.3–167) |
| Cancer type, n (%) | |
| Osteosarcoma/Ewing’s sarcoma | 11 (37%) |
| AML | 4 (13%) |
| ALL | 5 (17%) |
| Lymphoma | 7 (23%) |
| Wilms’ tumor | 1 (3%) |
| Rhabdomyosarcoma or synovial sarcoma | 2 (7%) |
| Radiation to chest (n) | 4 (13%) |
| Previous bone marrow transplantation (n) | 4 (13%) |
ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia.
Comparison of echocardiograms
There was a statistically significant difference in measures of systolic function (EF and SF) between the pre-chemotherapy and study echoes; however, the magnitude of the change is not clinically significant. There was no statistically significant difference in the LV diastolic dimension Z-score, which was normal in both sets of echoes. With regards to diastolic function, there was no significant difference in the mitral E/A ratio, but there was a significant difference in the lateral E/E′ ratio although values remained within the normal range. The results of the echoes are summarized in table 2.
Table 2.
Comparison of pre-chemotherapy echocardiogram and study echocardiogram
| Pre-Chemotherapy echocardiogram, median (range) | Study echocardiogram, median (range) | P value | |
|---|---|---|---|
| LVIDd Z-score | 0.3 (−2.1 to 1.7) | 0.35 (−1.4 to 2.3) | 0.17 |
| SF (%) | 35.7 (30.0–44.0) | 30 (20.0–43.0) | <0.001* |
| EF (%) | 63.5 (56.0–71.0) | 60.0 (47.7–68.0) | <0.001* |
| Mitral E/A | 1.85 (1.2–4.2) | 1.9 (1.1–3.1) | 0.78 |
| Lateral E/E′ | 6 (3.2–9.0) | 4.9 (3.4–8.3) | 0.007* |
Statistically significant, p≤0.05.
EF, ejection fraction; LVIDd, left ventricular internal diameter in diastole; SF, shortening fraction.
Cardiac MRI and serum biomarker results
Relevant CMR values are listed in table 3. LVEF by MRI was normal or low normal for all subjects who underwent CMR. Fourteen subjects were found to have at least one abnormal ROI on CMR ECV as seen in figure 1. No subjects demonstrated late gadolinium enhancement. ECV measurements were similar in the four-chamber and short-axis slices.
Table 3.
CMR and serum biomarker results for all patients post-chemotherapy
| Median (range) | |
|---|---|
| MRI | |
| LVEDV (mL/m2) | 93 (51.6–125) |
| LVEF (%) | 58 (49.1–71.2) |
| ECV—4 chamber (%) | 24.9 (20.4–36.0) |
| ECV—short axis (%) | 24.8 (21.2–32.3) |
| Late gadolinium enhancement | None |
| Patients with at least one abnormal ECV ROI | 14 |
| Serum biomarkers | |
| NT-pro-BNP (pg/mL) | 49.9 (0.8–302.6) |
| Troponin I (ng/mL) | <0.02 |
| MPO | 0 |
| ST2 (ng/mL) | 26 (15.0–60.0) |
ECV, extracellular volume; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; MPO, myeloperoxidase; NT-pro-BNP, N-terminal brain natriuretic peptide; ROI, region of interest; ST2, interleukin 1 receptor-like 1 protein.
Figure 1.
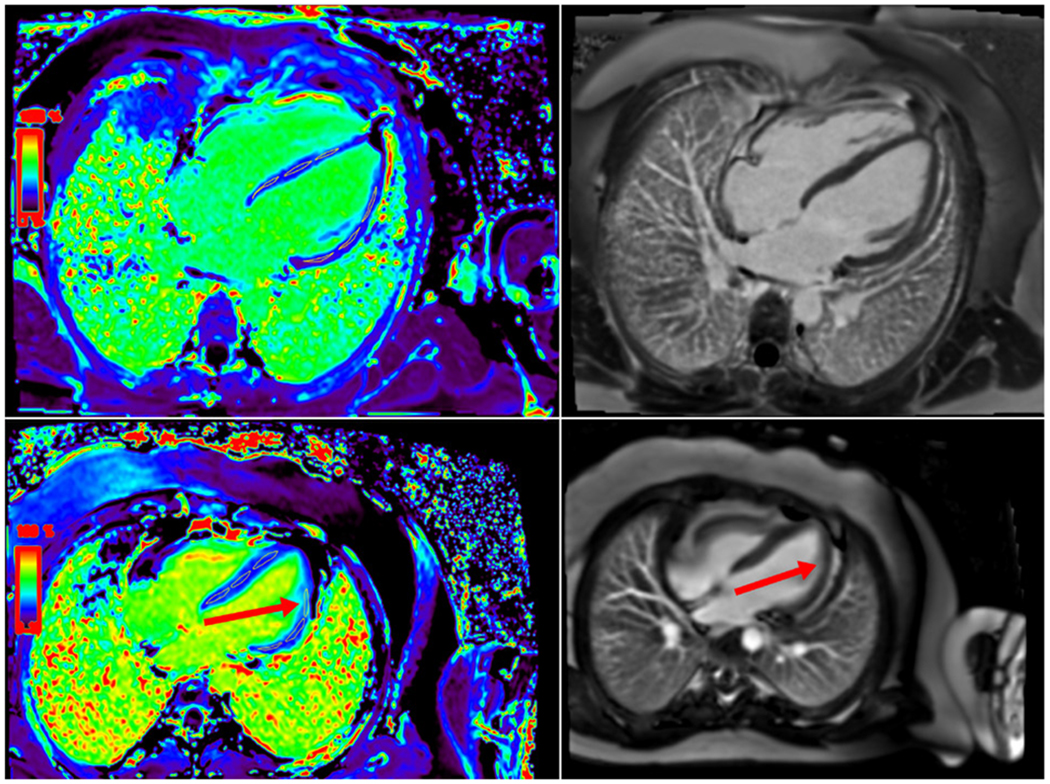
Extracellular volume (ECV) map and late gadolinium enhancement (LGE) in patients without systolic dysfunction. (Top) ECV map and LGE image showing normal ECV and no LGE. (Bottom) ECV map showing increased ECV signal in one region of interest (red arrow) without any evidence of LGE (red arrow on right).
Serum biomarker assessments were performed at the time of CMR and are also summarized in table 3. There were no subjects with positive troponin or MPO values. NT-pro-BNP values were normal for all patients (upper limit of normal=1384 pg/mL). ST2 values were abnormal in a total of six patients, whose ST2 values were greater than 35 ng/mL.
Correlations
Pearson correlation coefficients were calculated for all anthracycline dose, post-chemotherapy echo, CMR, and biomarker data (table 4). There are correlations between cumulative anthracycline dose (CAD) and mitral E/A ratio, CMR ECV (four-chamber and short-axis), and NT-pro-BNP There was a significant correlation between CMR ECV (four-chamber and short-axis) and NT-pro-BNP ALS also showed a correlation with NT-pro-BNP. There were no significant correlations between CAD or BNP and LVIDd Z-score, EF, or SF. There was no significant correlation between ALS and CMR ECV.
Table 4.
Pearson correlation and p values for relevant post-chemotherapy echo, cardiac MRI, and serum biomarkers as well as cumulative anthracycline dose
| Cumulative anthracycline dose |
NT-pro-BNP |
|||
|---|---|---|---|---|
| R value | P value | R value | P value | |
| LVIDd Z-score | 0.009 | 0.964 | −0.185 | 0.346 |
|
| ||||
| LVEF (%) | −0.186 | 0.343 | 0.283 | 0.137 |
|
| ||||
| LVSF (%) | −0.286 | 0.292 | 0.093 | 0.633 |
|
| ||||
| MV E/A | −0.453 | 0.016* | −0.045 | 0.818 |
|
| ||||
| Lateral E/E′ | −0.010 | 0.960 | −0.121 | 0.866 |
|
| ||||
| ALS | −0.07 | 0.723 | −0.376 | 0.045* |
|
| ||||
| ACS | 0.032 | 0.886 | −0.077 | 0.728 |
|
| ||||
| MRI LVEDV/m2 | −0.007 | 0.973 | −0.156 | 0.419 |
|
| ||||
| MRI LVEF (%) | 0.166 | 0.4 | 0.283 | 0.254 |
|
| ||||
| MRI ECV 4 chamber | 0.403 | 0.046* | 0.608 | 0.001* |
|
| ||||
| MRI ECV short axis | 0.484 | 0.011* | 0.607 | 0.001* |
|
| ||||
| NT-pro-BNP | 0.362 | 0.05* | – | – |
|
| ||||
| ST2 | −0.015 | 0.94 | −0.09 | 0.643 |
p<0.05.
ACS, average circumferential strain; ALS, average longitudinal strain; ECV, extracellular volume; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter in diastole; LVSF, left ventricular shortening fraction; NT-pro-BNP, N-terminal brain natriuretic peptide; ST2, interleukin 1 receptor-like 1 protein.
DISCUSSION
This study investigates early imaging and serum biomarkers of anthracycline cardiotoxicity in pediatric subjects. This study demonstrated a significant correlation between rising CMR ECV with increased cumulative anthracycline dose (CAD) and increased BNP. The association of CAD and CMR ECV has been reported in other studies,15 and this study provides additional evidence regarding the use of this CMR technique in screening patients who were exposed to cardiotoxic chemotherapy. While contrast-enhanced MRI is not part of standard screening guidelines for pediatric cancer survivors, our study joins a growing body of evidence9,16 showing that increasing ECV values may be a sign of early myocardial dysfunction, despite the absence of LGE.17 While the current understanding of the pathogenesis of chemotherapy-based cardiotoxicity is that of a diffuse cardiomyopathy, it is interesting as well that nearly half of the subjects in this study had at least one abnormal ROI on CMR ECV, which may suggest that early findings are patchy in nature. Our results further emphasize the need for larger studies regarding the detection of myocardial damage in these patients using non-contrast screening CMR with techniques such as native T1 mapping, as has been done in other forms of cardiomyopathy,14,18 and diagnostic contrast-enhanced CMR to add specificity in post-chemotherapy cardiac care.
We noted a statistically significant difference in the EF and SF between the pre-chemotherapy and study echoes. However, close inspection of the changes in the study population illustrates again why standard TTE alone is inadequate in fully appreciating the development of evolving cardiomyopathy in these patients as the change EF and SF values found in our study would not typically warrant intervention. An EF drop from 65% to 60% could indicate actual myocardial injury even though still in the “normal range” and thus other biomarkers are needed to optimize the timing and effectiveness of treatment.
Serum biomarkers are an emerging tool in the surveillance of chemotherapy-induced cardiotoxicity, but there are few guidelines delineating which biomarkers are of use. We showed NT-pro-BNP correlated with worsening ALS, CMR ECV and CAD. This is consistent with adult studies that have shown that the rise in BNP precedes the decline in EF.10 Studies in adults have also shown that troponin I is a biomarker that becomes elevated prior to the development of LV dysfunction.10 In our study group, however, there were no patients with elevated troponin I. We suspect this is likely secondary to our younger population as well as time from completion of chemotherapy. High-sensitivity troponin assay (hs-cTn) has been shown to be more sensitive, compared with troponin I, in detecting earlier myocardial injury. However, since hs-cTn is not available at our institution, we were unable to include that in our study. A longitudinal study with long-term follow-up would be needed to determine the utility of troponin I and hs-cTn as a biomarker in the pediatric population. Lastly, the biomarker ST2, which has been shown to correlate with the development of cardiomyopathy in patients with muscular dystrophy,19 did not correlate with measures of cardiac dysfunction in our study. This is consistent with adult studies that have examined this novel biomarker in patients with cancer.20,21 However, ST2 was elevated in 20% of our subjects and may also require a longitudinal study for further evaluation. Further studies are needed to fully evaluate the clinical utility of all serum biomarkers in surveillance of chemotherapy-related cardiotoxicity.
Studies in adult patients have shown that STE measuring longitudinal and circumferential strain can reveal changes that precede changes in EF and SF.7 Our study did not find significant correlations between worsening ALS or ACS and decreasing EF and SF. This could be secondary to the smaller study size and the variability of time from completion of chemotherapy. Echocardiogram image quality may also have affected the ALS or ACS results. However, other studies in pediatric patients have shown subtle changes in LV strain measurements in asymptomatic patients,22 suggesting the need for larger studies to determine the magnitude of expected changes and their correlation with cardiotoxicity.
Echocardiography remains the primary surveillance method for chemotherapy and radiation therapy, specifically looking at the SF and EF. However, it should not be used alone. By the time a clinically significant change is noted, it is likely that the patient has deteriorated significantly. If other markers were evaluated at the same time, it may alert the clinician to monitor the patient more frequently. Further studies that follow changes in myocardial strain, screening non-contrast CMR, and post-chemotherapy contrast-enhanced CMR-derived ECV in conjunction with traditional echo surveillance in pediatric patients will need to be done to elucidate these relationships. In this study, we were able to correlate increased myocardial fibrosis by CMR-derived ECV with increased cumulative anthracycline dose, which may be useful in risk stratifying patients early on in their treatment based on the expected dose of anthracycline in their chemotherapy protocol.
Future directions
This pilot study provides insight into different potential modalities to identify cancer survivors at risk of developing significant cardiotoxicity before those changes are seen in global measures of cardiac function including EF and SF. CMR is a potential screening and diagnostic tool to identify patients with early myocardial damage in asymptomatic patients. Using a combination of the modalities will help direct physicians on the proper treatments and timing for cardiac therapies to improve outcomes in this vulnerable population.
CONCLUSIONS
Our study has demonstrated the potential benefit for a multi-modality approach in the surveillance and management of cardiac disease in cancer survivors. Changes seen in specific echo, CMR, and serum biomarkers may be more sensitive for dysfunction in these patients. These correlate with increased cumulative anthracycline dose, which can potentially guide overall risk stratification. Larger pediatric studies are needed to evaluate these modalities longitudinally. With this growing population at risk for cardiac morbidity and mortality, early detection can be the key to changing their cardiac prognosis.
Limitations
There are limitations to this pilot study that can be addressed in future studies. This study examines an overall heterogeneous group of subjects. Given the small sample size, variability of malignancies, and range of anthracycline dose, we can only speculate about the correlations noted. Furthermore, the patients in this study were a wide range of months from the completion of their chemotherapy. There were four patients who received radiation to the chest and they were not independently evaluated for the effect on echocardiographic, CMR, or biomarker changes in comparison with the radiation-free population. There was variability in the image quality of each echo, which can impact the accuracy of the ALS/ACS measurements that also prevented us from generating values for global longitudinal and circumferential strain, as has been used in adult studies. Lastly, serum biomarkers were only collected at one point in time as part of the study and not prior to chemotherapy.
Significance of this study.
What is already known about this subject?
Cancer survivors are at risk for developing cardiotoxicity.
Cardiac toxicity is the third leading cause of death in cancer survivors.
The presentation of cardiac symptoms or standard ejection fraction and shortening fraction are late findings in cancer survivors.
Echocardiographic longitudinal strain is one earlier finding of abnormality in cancer survivors.
What are the new findings?
Cumulative anthracycline dose is correlated with N-terminal brain natriuretic peptide and extracellular volumes on cardiac MRI.
N-terminal brain natriuretic peptide correlates with extracellular volumes on cardiac MRI and longitudinal strain on echocardiograms.
How might these results change the focus of research or clinical practice?
It should encourage multi-modality diagnostics to find cardiac toxicity early.
It should encourage more long-term prospective studies in cancer survivors.
Acknowledgements
We would like to acknowledge the Children’s National Medical Center Cardiac MRI Team and Cardio-Oncology clinic for their technical and logistical support in the completion of this study.
Funding
This study was funded by a Pilot Award Grant from the Clinical and Translational Science Institute at Children’s National Medical Center and in part by the National Institute of Health (NHLBI, Prime Award No. R01HL125918-01).
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This prospective observational study was approved by the Institutional Review Board at Children’s National Medical Center.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. The deidentified data that support the findings on this study are available on request from the corresponding author, ND (ndham@childrensnational.org). The data are not publicly available due to privacy or ethical reasons.
REFERENCES
- 1.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013;128:1927–95. [DOI] [PubMed] [Google Scholar]
- 2.Kremer LCM, van Dalen EC, Offringa M, et al. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol 2002;13:503–12. [DOI] [PubMed] [Google Scholar]
- 3.Kremer LCM, van der Pal HJH, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol 2002;13:819–29. [DOI] [PubMed] [Google Scholar]
- 4.Steinherz LJ, Graham T, Hurwitz R, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Children’s Cancer Study Group. Pediatrics 1992;89:942–9. [PubMed] [Google Scholar]
- 5.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 2008;121:e387–96. [DOI] [PubMed] [Google Scholar]
- 6.Wouters KA, Kremer LCM, Miller TL, et al. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol 2005;131:561–78. [DOI] [PubMed] [Google Scholar]
- 7.Thavendiranathan P, Poulin F, Lim K-D, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014;63:2751–68. [DOI] [PubMed] [Google Scholar]
- 8.Galán-Arriola C, Lobo M, Vilchez-Tschischke JP, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol 2019;73:779–91. [DOI] [PubMed] [Google Scholar]
- 9.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol 2013;111:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan L-L, Lyon AR. Role of biomarkers in prediction of cardiotoxicity during cancer treatment. Curr Treat Options Cardiovasc Med 2018;20:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colan SD, Borow KM, Neumann A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load-independent index of myocardial contractility. J Am Coll Cardiol 1984;4:715–24. [DOI] [PubMed] [Google Scholar]
- 13.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006;19:1413–30. [DOI] [PubMed] [Google Scholar]
- 14.Olivieri LJ, Kellman P, McCarter RJ, et al. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2016;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 2013;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toro-Salazar OH, Gillan E, O’Loughlin MT, et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging 2013;6:873–80. [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53. [DOI] [PubMed] [Google Scholar]
- 18.Nakamori S, Dohi K, Ishida M, et al. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 2018;11:48–59. [DOI] [PubMed] [Google Scholar]
- 19.Anderson J, Seol H, Gordish-Dressman H, et al. Interleukin 1 receptor-like 1 protein (ST2) is a potential biomarker for cardiomyopathy in Duchenne muscular dystrophy. Pediatr Cardiol 2017;38:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armenian SH, Gelehrter SK, Vase T, et al. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res 2014;20:6314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Çetin S, Babaoğlu K, Başar EZ, et al. Subclinical anthracycline-induced cardiotoxicity in long-term follow-up of asymptomatic childhood cancer survivors: assessment by speckle tracking echocardiography. Echocardiography 2018;35:234–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. The deidentified data that support the findings on this study are available on request from the corresponding author, ND (ndham@childrensnational.org). The data are not publicly available due to privacy or ethical reasons.


