Abstract
Current fire retardants are known to be toxic to humans and our environment. As environmental-friendly flame retardants (FRs), protein-based flame retardants have been studied extensively recently, even though they are not durable. In this study, we designed, synthesized and tested a durable protein-based FR through the fusion of the adhesion domain from either mussel foot protein-5 (mfp-5) or cellulose-binding domain (CBD) with flame retardant protein (SR protein and alpha casein). We first verified the expression of the recombinant proteins in Escherichia coli using Western blot. Then, we coated the fusion protein (carrying cell lysates) to cotton fabrics and wood and verified with Infrared (IR) spectroscopy. Using a vertical burning test and wood flammability test, we confirmed the flame retardancy of the materials after the protein coating. In the vertical burning test, the SR protein and alpha casein flame retardant proteins with the CBD adhesion domain showed a 50.0% and 43.3% increase in flame retardancy. The data is also consistent in the wood flame retardancy test. Confocal imaging experiments also suggested these new fire retardants can be preserved on the materials well even after washing. Overall, our results showed that flame-retardant proteins with adhesion domains are high potential candidates of green alternative flame retardants.
Keywords: Flame retardant protein, Cellulose-binding domain (CBD), Mussel foot protein-5 (mfp-5)
1. Introduction
Most textile and wooden materials can be ignited and burnt easily. Since the 1950s, flame retardants (FRs) have been widely adapted to coat flammable materials, preventing the fire from spreading and prolong the escape time during a fire accident [1]. Today, many countries, including the United States, United Kingdom, EU countries, have imposed regulations on furniture fire safety [2].
At the present stage, the halogen-containing compounds are one of the most common FRs, being routinely added to many consumer products such as plastic chairs and baby nursing pillows [[3], [4], [5]]. While these compounds demonstrated good flame retardancy, they are known to be hazardous to our health and the environment. These FRs, including the bromine-based and chlorine-based FRs (including TBBPA, PBDEs, PCBs, HBCDs, etc) are potential mutagen and their association with thyroid cancers [6,7], breast cancer [8] and prostate cancer [9] are suggested. Moreover, these widely used FRs could also influence neurodevelopment and affecting children's behaviours [10] and increasing the risk of Alzheimer's disease [11]. Because of the broad usage of these FRs, these toxic compounds widely exist in food and accumulate in animals [3,4], and hence influencing our health and the ecosystem. Nowadays, many scientists have been urging our society to develop a non-toxic and environmental-friendly alternative to replace the current ones in use.
Recently, a lot of research focused on the potential of biomacromolecules (e.g. DNA, proteins) as alternative flame retardants, because of their biodegradable and non-toxic nature [[12], [13], [14], [15]]. With the high contents of phosphorus and nitrogen in some of these molecules, they can significantly reduce flammability and eliminate the fire. When fuels burned with materials containing phosphates, the charring process will occur and isolate the fuel from burning; when ammonia is burned, it will react with oxygen to produce water and nitrogen gas, acting as an inert gas to isolate oxygen. Although these compounds have been found to be potential FRs, the durability of the coatings with these molecules is low [14,15]. Therefore, the way to enhance the retention of these compounds on surfaces is critical.
Here, we adapted the synthetic biology methods to design and tested protein-based FRs. We first selected two flame retardant proteins, SR proteins (Serine/arginine-rich splicing factor 1; SRSF1) with high nitrogen content (17.4%) and bovine alpha casein proteins with potentially high phosphorus content (7.22%) [14]. The nitrogen and phosphorus components in these proteins can form a relatively stable char layer and thereby prevent further combustion of fabrics [12,16]. We created the fusion proteins that tagged with either one of the two adhesive domains, the mussel foot protein-5 (mfp-5) or the cellulose-binding domain (CBD), and expressed them in Escherichia coli (E. coli). Previous research [[17], [18], [19]] found that these adhesive domains are adhesive to different surfaces (mfp-5 can adhere to various surfaces such as glasses and metal; and CBD can adhere to cellulose-based surfaces). We believe these modifications can increase the durability of the protein-based FRs on different materials. Further analysis also suggested these modified FR proteins have better fire retardancy and retentions than the original FR proteins. Therefore, the results from this project provided new insight to design durable and green FR that is beneficial to our daily life.
2. Materials and methodologies
2.1. Construction of flame retardant proteins and experimental overview
To test whether mfp-5 and CBD influence the fire retardancy and retention of the fire retardant proteins (SR protein and alpha casein), we cloned 9 plasmids into the pET11a vector, as shown in Table 1. These 9 plasmids can be classified into three experimental groups: (group 1) the control group with original flame-retardant protein (SR protein and alpha casein), (group 2) the flame-retardant protein fusion with adhesion domain (CBD-SR, CBD-alpha casein, mfp5-SR, mfp5-alpha casein), and (group 3) the flame-retardant proteins fused with both adhesion domain and red fluorescent protein (RFP) (Check Supplementary Table 1 for amino acid sequence and protein size).
Table 1.
List of construction used in this paper. Group 1 represents the control group of flame retardant proteins without adhesive domains. Group 2 represents the flame retardant proteins with mfp5 or CBD adhesive domains. Group 3 represents the flame retardant protein with the adhesive domains and RFP.
| Construct | iGEM part number | Group |
|---|---|---|
| Ptac-GST-SR-pET-11a | BBa_K1608002 | 1 |
| Alpha casein-pET-11a | BBa_K3503001 | 1 |
| Mfp-5-SR-pET-11a | BBa_K3503006 | 2 |
| Mfp-5-alpha casein-pET-11a | BBa_K3503007 | 2 |
| CBD-SR-pET-11a | BBa_K3503004 | 2 |
| CBD-alpha casein-pET-11a | BBa_K3503008 | 2 |
| Alpha casein-RFP-pET-11a | BBa_K3503009 | 3 |
| Alpha casein-mfp-5-RFP-pET-11a | BBa_K3503011 | 3 |
| Alpha casein-CBD-RFP-pET-11a | BBa_K3503010 | 3 |
The plasmid constructs were constructed either by NEBuilder HiFi DNA Assembly Master Mix (NEB) to clone DNA fragments (from Integrated DNA Technologies) into the pET11a vector, or by DNA synthesis using Genscript. The plasmids were transformed into E.coli strain BL21(DE3) following by an ampicillin selection. The colony was then picked for further analyses, after conformation of constructs by Sanger sequencing (BGI Genomics).
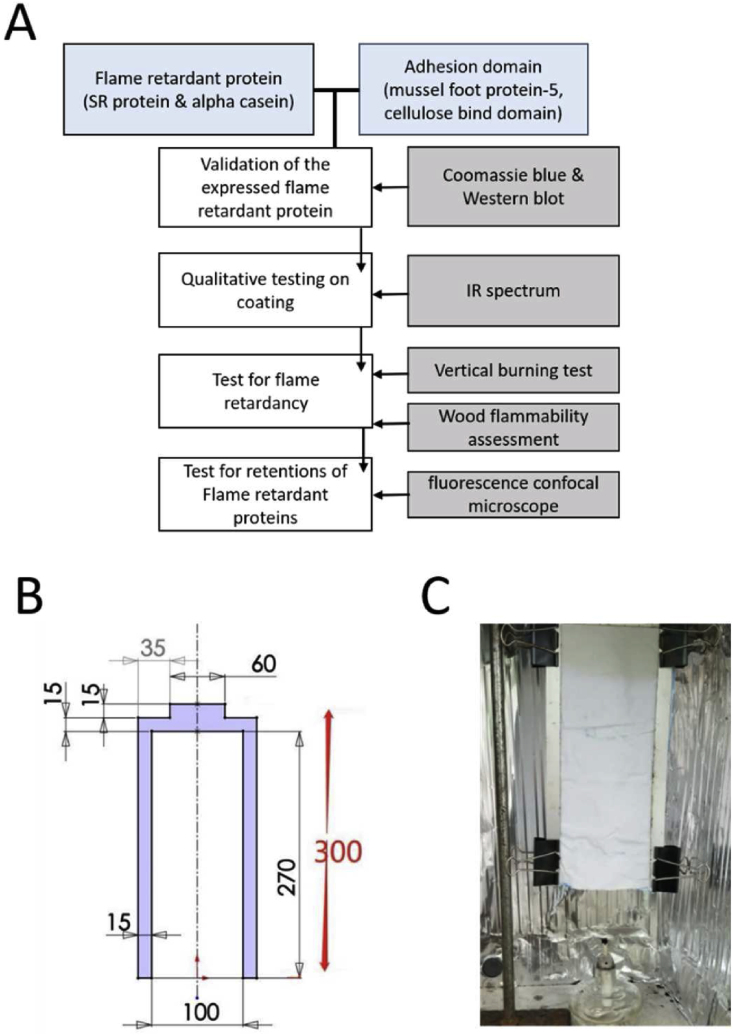
As shown in the experimental flow chart, (Fig. 1A), following transformation Coomassie blue and Western blot were used to validate the protein expression of all plasmids following IPTG induction of the T7 protomers. After that, one of the 9 proteins was used to coat cotton fabrics (100% Cotton; 300 mm*130 mm) or woodblocks (40 mm*38 mm*51 mm). In the vertical burning test and the wood flammability assessment test, (group 1) control group flame-retardant proteins will be used to compare with (group 2) flame-retardant protein fusion with adhesion domain. These tests will give us general information about the retardancy of our tested samples. In addition, (group 3) flame-retardant proteins fusion with both adhesion domain and red fluorescent were used to test the retentions of the coating following washing or soaking procedure. The detailed methods of all these experiments are further described in the following paragraphs.
Fig. 1.
Experimental setup
The figure shows (A) the overall purposes and procedures of the experiments performed in this study. The arrows represent the flow of the procedure of the experiments while the lines represent the procedures (on the right) in need to validate the purposes (on the left). (B) Design of iron plate used in the vertical burning test setup. The purple color indicates the iron plate. The number is represented in mm. (C) Vertical burning test experimental set up (modified according to the ASTM-D6413 standard). Spirit burner was put 7 cm below the cotton fabrics and removed once the cotton fabric was ignited.
2.2. Validation of the expressed flame retardant protein
To validate the protein expression in E. coli, SDS-PAGE and Western blot analysis were performed.
First, E. coli was cultured with LB media, containing 1.0 mM IPTG, at 30 °C until reaching O.D. 0.4 (wavelength: 595 nm with VICTOR multilabel plate reader). The cultured samples were then lysed by sonication with Qsonica, Q700CA sonicator. The sonication was performed by resuspending the pellet with Lysis buffer (50 mM Tris-HCl pH7.5, 20 mM NaCl), followed by cooling down on ice for 10 min. Microtip was used and set at 40% amplitude. We sonicated each sample for 45 s 3 times. After that, the sample was then centrifuged at 1200×g for 10 min and the supernatant was used for further experiments.
The protein ladder and samples were first loaded to the SDS-PAGE gel (Biorad mini protean tetra system). Then we run the gel at 60 V for 1 h and then increase to 120 V for another hour. The gel was washed with Mili-Q water for 10 min on a shaker and soak it with Coomassie Brilliant Blue. It was then incubated at room temperature for an hour on a shaker and washed with Mili-Q water. The gel was then soaked overnight with Mili-Q on a shaker.
Western blot analysis was performed by transferring the SDS-PAGE gel to 1x transfer buffer and then undergoing the Cassette assembly (1.5 mm with 10 min running). Blocking buffer (1 g non-fat dry milk + 20 mL TBST) was then used to block the membrane for an hour on a shaker. The primary antibody (anti-his antibody; hrp antibody from Abcam) was then diluted in blocking buffer at 1:2000 dilution, and the membrane was then incubated in diluted primary antibody overnight at 4° Celsius.
2.3. Investigation of the flame retardancy of the engineered protein
2.3.1. Flame retardant treatment
To investigate the flame retardancy of the engineered protein, 1 mM IPTG was added to the bacterial cultures at 6 h after incubation and then further incubate overnight. The culture samples were then lysed by sonication (as described in the last section). Then the cotton fabrics (300 mm*130 mm; 100% cotton) were coated by soaking in the supernatant of the lysate (protein concentrations: 0.125 mg/ml) at room temperature overnight (soaking in Mili-Q water was used as a control for the coating). Then, the cotton fabrics were dried at room temperature until the moisture content percentage is stable, reaching a moisture content of 13.55–14.37%, as measured by testo 606-1-Moisture meter.
2.3.2. Infrared (IR) spectroscopy
IR spectroscopy was used to determine whether the fire retardant proteins had been coated on the cotton fabrics. The cotton fabrics samples, which went through the flame retardant coating treatment, were analyzed by the Bruker Alpha IR spectrometer to detect the IR spectrum. Cotton fabrics with coating treatment using Mili-Q water, as well as, non-coated cotton fabrics were used as a control to compare with other samples being treated with cell protein lysates.
2.3.3. Vertical burning test
In order to determine the flame retardancy of the proteins coated on the cotton fabrics, the vertical burning test was performed with a device built according to the ASTM-D6413 standard (ASTM D6413, n.d.). The testing cotton fabric sample (300 mm*130 mm) was placed between two iron plates and fixed with 4 Elliot folders about 7 cm above the spirit burner (Fig. 1B & 1C). The device also includes a surrounding aluminium chamber with only one-sided ventilation in order to minimize the influence of air current. We started counting the burning time after the cotton fabric is ignited and stopped when there's no visible flame. The spirit burner was displaced once the cotton fabric was ignited.
2.3.4. Wood flammability assessment
Wooden furniture is commonly used in households, offices, and the hotel industry. Thus, we here used the BS476-part4:1970 test to assess the flame retardancy of wood. This test is commonly used as a fire retardancy test for building materials and structures (non-combustibility test for materials) (BS476, n.d.). Wooden blocks, with a size of 40 mm*38 mm*51 mm and average moisture content 12.6%, were first coated with different cell lysates (with protein concentrations: 0.125 mg/ml) containing different proteins (by immersing the wooden blocks into lysates overnight) and then tested by the Institute of development and Quality in Macau SAR, China.
2.3.5. Retentions of flame retardant proteins
Mfp-5 and CBD were fused into the flame retardant protein to improve retention of the FR protein. We investigate the retentions of the proteins (also fused in RFP) using the Nikon A1MP + fluorescence confocal microscope (Nikon, Japan). More specifically, we used the microscope to detect the RFP signals on the cotton fabric samples, which underwent the flame retardant coating treatment, with/without being washed or soaked gently with Mili-Q water. By comparing the intensity of RFP signals before and after washing/soaking, we can study how much proteins stayed on the surfaces of the cotton fabrics, and thereby study the retentions of flame retardant proteins. A two-photon excitation wavelength at 1100 nm was used to activate the RFP; and the whole emission wavelength (600–680 nm) was scanned after excitation to detect the RFP signals.
3. Results
3.1. Expression of flame-retardant proteins
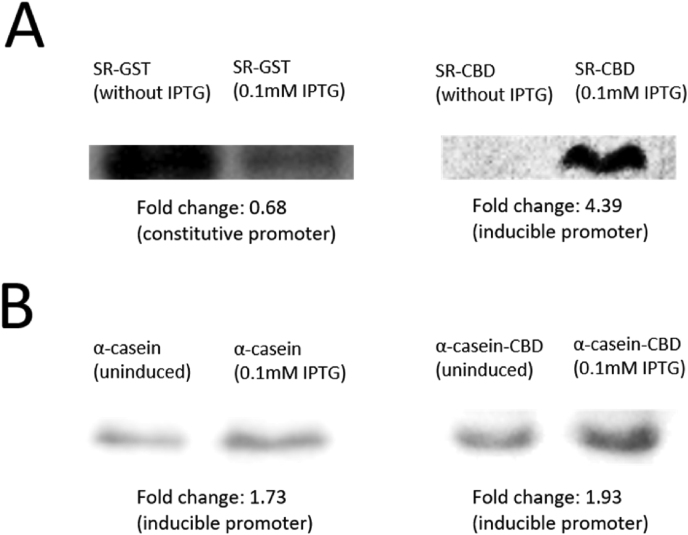
To validate our concept of applying synthetic biology to optimize flame-retardant proteins, 9 plasmids (Table 1) with different FR proteins that with or without adhesion domains, were constructed and expressed into BL21 strain. The expression of the proteins was confirmed by using Coomassie blue staining and Western blot analysis (Fig. 2). As expected, the expression of SR-CBD, alpha casein and alpha casein -CBD can be significantly induced after IPTG induction. Except for the SR-GST protein, the IPTG treatment cannot further increase the protein level as a strong endogenous expression is already found.
Fig. 2.
Confirmation of protein expression using western blotExemplary
Western blot analyses illustrating (A) Successful expression of SR-GST (53.82 kDa) and SR-CBD (53.87 kDa) in expected size. Consistent with our hypothesis, SR-CBD protein can be induced by IPTG while SR-GST protein is consistently expressed under ptac promoter. (B) Successful expression of alpha casein (25.42 kDa) and alpha casein-CBD (50.71 kDa) proteins, where both of them can be induced by IPTG. The fold change was measured with ImageJ.
3.2. Coating and infrared (IR) spectroscopy
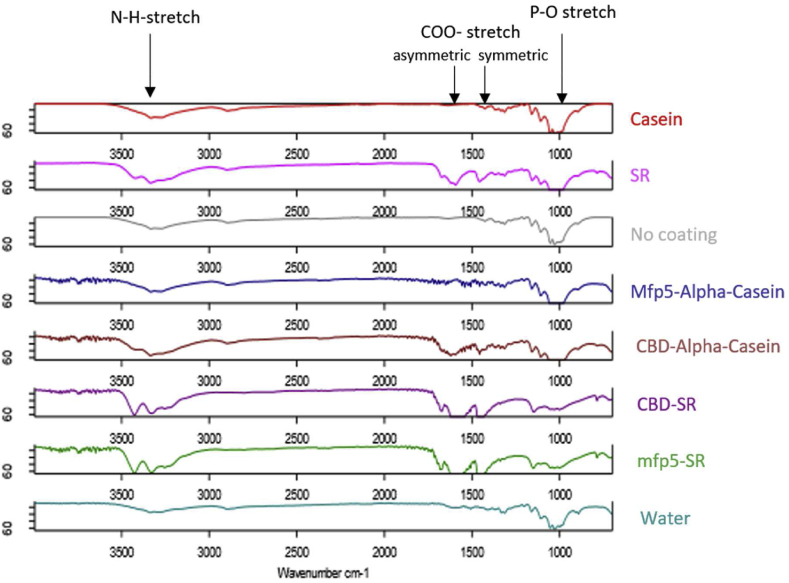
After confirming the expression of proteins, we lysed the E. coli cells with sonication and used the cell lysates for coating on cotton fabrics. The cotton fabrics were immersed into cell lysates containing different proteins (with the same protein concentrations at 0.125 mg/ml) and then dried at room temperature overnight. The coatings of the proteins were validated using Infrared (IR) spectroscopy. Consistent with previous studies [14,15], we confirmed that cotton fabrics with protein coating treatment show characteristics of the protein. As shown in the IR spectrum (Fig. 3), these cotton fabrics have some new absorption peaks at wavenumber around 3300–3500 (N-H-stretch), 1400 (COO-stretch symmetric), 1600 (COO-stretch asymmetric) and 900–1000 (P-O stretch) when compared with the Mili-Q water control and control without coating, indicating a successful coating of the proteins on the surfaces of the materials.
Fig. 3.
IR spectroscopy of cotton fabrics with different coatings
IR spectrum showing the successful coating of proteins onto the cotton fabrics. The color of the spectrum (left) represented the group (text on the right) of protein coating it belongs to.
3.3. Vertical burning test
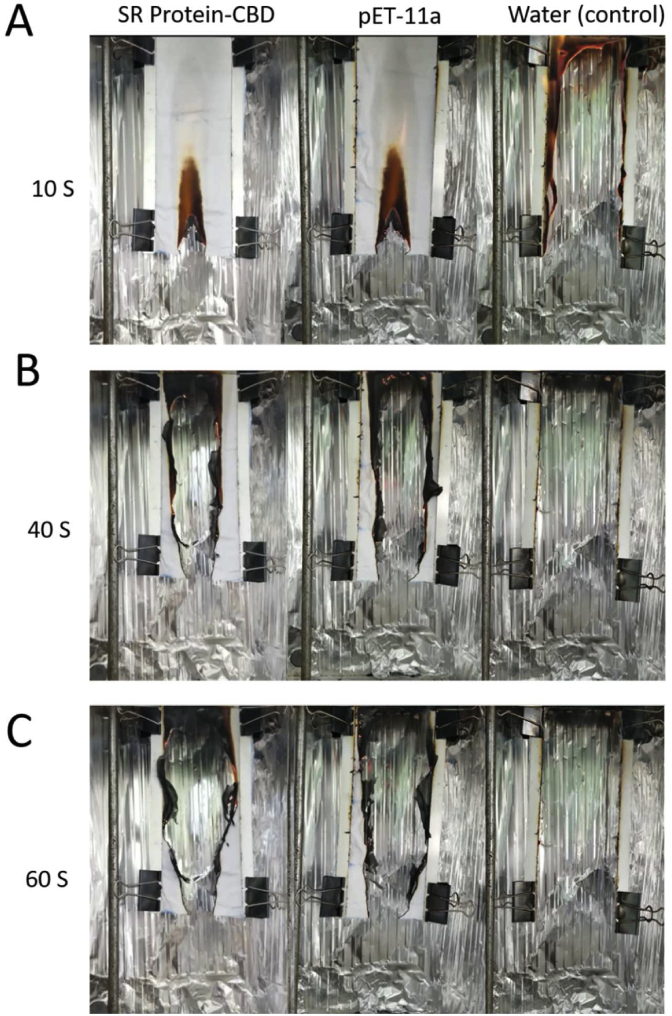
We next tested the flame retardancy of these pre-treated cotton fabrics using a vertical burning test [20], [21]. As shown in the exemplary test (Fig. 4; Supplementary Video 1), the cotton fabrics treated with proteins have higher flame retardancy than those treated with Mili-Q water. Generally, all protein lysates could have flame retardancy when compared to water control. When compared to the lysate control (pET11a only), the cotton fabrics treated with flame retardant proteins that fused with adhesion domains (including alpha casein-mfp5, alpha casein-CBD and SR-CBD) shown a higher retardancy, except the SR-mfp5. These coatings not only affect the burning speed at the beginning (Fig. 4A), but also prolong the burning time of the cotton fabrics (Table 2).
Fig. 4.
Exemplary vertical burning testsFigure
showing three exemplary vertical burning tests with three protein coating (Left: SR Protein-CBD; Middle: pET-11a; Right: Deionised Water), at (A) 10s (B) 40s (C)60s after the ignition of the cotton fabrics. See Supplementary Video 1 for details of the burning procedure across time.
Table 2.
Vertical burning test (ASTM-D6413) using the cotton fabric. Data showing mean ± SD from duplicate tests.
| Sample | Burning time (s) |
|---|---|
| SR Protein | 55.5 ± 6.5 |
| Alpha casein | 70.5 ± 17.5 |
| SR Protein-mfp-5 | 67 ± 16 |
| Alpha casein-mfp-5 | 65 ± 5 |
| SR Protein-CBD | 87 ± 15 |
| Alpha casein-CBD | 71 ± 5 |
| MiliQ water | 23.5 ± 4.5 |
| pET-11a | 54.5 ± 6.5 |
Supplementary video related to this article can be found at doi:10.1016/j.synbio.2021.10.005
The following is the supplementary data related to this article:
In addition to FR proteins, we also in fact also tested whether the monomer of proteins, the amino acid can be used as a flame retardant material. We found that (Supplementary Fig. 1) amino acids can also prolong the burning time of cotton fabrics, and that higher concentration/nitrogen composition of amino acids can further increase the burning time.
3.4. Wood flammability assessment
In order to investigate how our FR proteins perform for different real-world applications, wooden blocks (40 mm*38 mm*51 mm) were soaked in cell lysates with different proteins overnight and then further tested for flammability. Using flammability assessments, under the BS476-part4:1970 testing standard, we tested the burning time of the wooden blocks following protein coating treatments. We found that FR proteins coating can increase the burning time of the wooden blocks: all wood coated with FR proteins showed an improvement of flame retardancy in comparison with the water control and pET-11a vector (Table 3). Consistent with our vertical burning test results, the fusion proteins with of adhesion domain (CBD or mfp-5) could further increase the flame retardancy of the wooden blocks. Overall, the moisture content are similar between all of the samples and this suggested the changes in burning time are not due to the different moisture of the materials after the coating.
Table 3.
Wood flammability test under BS476 standard.
| Sample | Moisture Content percentage | Burning time (s) |
|---|---|---|
| SR Protein | 13.4% | 418 |
| Alpha casein | 12.9% | 320 |
| SR Protein-mfp-5 | 12.6% | 513 |
| Alpha casein-mfp-5 | 13.3% | 537 |
| SR Protein-CBD | 12.8% | 543 |
| Alpha casein-CBD | 12.3% | 557 |
| MiliQ water | 13% | 188 |
| pET-11a | 13.1% | 388 |
3.5. Retentions of flame retardant proteins on the cotton fabrics
To investigate whether the addition of adhesive domains can also increase the retention of FR proteins, we tagged the alpha casein, alpha casein-mfp-5, and alpha casein-CBD with red fluorescent proteins (RFPs). These engineered proteins allow us to directly observe the existence of the fusion protein by the fluorescent signals, even after washing the coated materials.
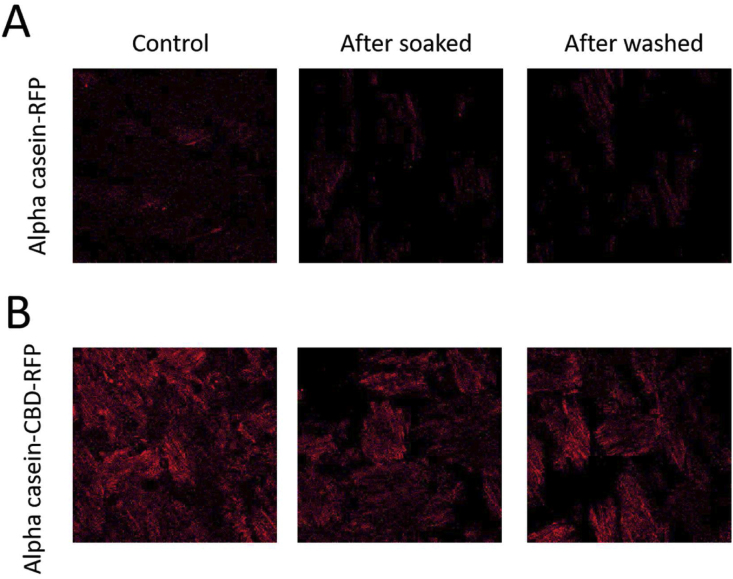
By soaking or washing out the coating of cotton fabrics, the RFP signals were captured under a confocal microscope. We found that adhesive domains (mfp-5 and CBD) can increase the retentions of protein coating (Fig. 5 and Table 4). For mfp5-alpha casein-RFP, 92.9% and 56.7% of RFP signals were retained on the coating after soaking or washing respectively; for CBD-alpha casein-RFP, 80.9%, and 59.9% of RFP signals were retained on the coating after soaking and washing respectively. In comparison, in the control group without any adhesive domains, only 12.7% post-soaking and 11.7% post-washing RFP signals were found. Overall, our results showed that the adhesion domain can improve the retentions of alpha casein on the surfaces of the cotton fabrics.
Fig. 5.
Exemplary protein retention test using confocal microscopy Figure
illustrating retentions of RFP signals (coated on the cotton fabrics) for (A) alpha casein-RFP and (B) alpha casein-CBD-RFP. Left column represented the signals without any washing/soaking procedure (control); middle column represented the signals with the soaking procedure; right column represented the signals with the washing procedure. See Table 4 for detailed analyses of RFP signals.
Table 4.
The RFP signals reflecting the percentage of proteins remained on the cotton fabrics after the washing/soaking procedure.
| Type of protein | Wavelength of control RFP peak (nm) | Intensity of control RFP peak | Wavelength of RFP peak after soaking (nm) | Intensity of RFP peak after soaking | Wavelength of RFP peak after washing (nm) | Intensity of RFP peak after washing |
|---|---|---|---|---|---|---|
| MiliQ Water | 610.7947 | 282 | NA | NA | 607.4545 | 454.9741 |
| Lysis buffer | 612.0756 | 504.2245 | 610.8234 | 177 | 611.2408 | 571.0326 |
| Alpha casein-RFP | 611.6296 | 6354.823 | 607.8721 | 808 | 609.1248 | 743.709 |
| Alpha casein-mfp-5-RFP | 610.7947 | 1407.7 | 610.7947 | 1515.54 | 607.4545 | 860 |
| Alpha casein-CBD-RFP | 609.1248 | 5582.37 | 610.7947 | 4515.046 | 611.2122 | 3442 |
4. Discussion
Consistent with previous studies, the alpha casein and SR protein had both showed flame retardancy characteristics, improving the flame retardancy for both cotton fabrics and wood in the vertical burning test and wood flammability test respectively. In the present work, we engineered these flame retardant proteins, fusing them with the mussel foot protein-5 (mfp-5) or cellulose-binding domain (CBD), and expressed them in E. coli. We proved the protein expression and sample coatings with Western blot analysis and IR spectroscopy. Then we tested and confirmed the flame retardancy of these engineered recombinant proteins. Moreover, we also tested the retentions of flame retardant proteins fused with the adhesion domains (mfp-5 or CBD). Our results indicated the fusion of adhesion domains and fire retardant proteins can improve the durability and flame retardancy of flame retardant protein coatings.
In the previous studies investigating fire retardant coatings, methods including IR spectroscopy, vertical burning test (ASTM D6413) and flammability test (BS476) are all commonly used [14], [15], [21], [22], [23], [24],. Here, by testing our genetically engineered proteins using these tests, we confirmed that our proteins can be used as a coating to increase flame retardancy in cotton fabrics and wood. Consistent with other biomolecule-based materials, while comparing our protein coatings with halogen-based fire retardants [12,[24], [25], [26]], the flam retardancy (eg. burning time or rate in vertical burning test) of proteins coatings is lower. However, these biomolecule-based FRs are still very valuable to develop because they are not toxic to humans and our environment.
Our approach using fluorescent proteins and fluorescent proteins to test the retentions of flame retardant proteins can also be helpful for further researchers to study the durability of flame retardant proteins. In comparison, previous research [14,15] investigated the durability with limiting oxygen index (LOI), which is not a direct measurement of the quantity of the coatings remaining on the surfaces. Our approach here provides an alternative way to study the durability of the protein coatings, via quantifying the amount of flame retardant proteins that remained on the surfaces of the materials.
Despite many studies [[12], [13], [14], [15]] investigated using biomolecules (proteins, nucleic acids, etc) as flame retardants, none of these studies have used genetic engineering to enhance flame retardancy. Here, we used the advanced synthetic biology approach to redesign the flame retardant proteins, and successfully increase retentions and flame retardancy. Moreover, our approach of fusing fluorescent proteins to study the durability of the coating is also novel in the field. As demonstrated by our results here, synthetic biology technologies are powerful tools to investigate flame and fire retardant materials. This possibly could open up an area of material science research, where researchers hopefully create more environmentally friendly and non-toxic flame retardants on the ground of our results. Furthermore, we here also found for the first time that the basic components of proteins, amino acids, also show some flame retardancy in the vertical burning test. As the flame retardancy of amino acids, also reflects a positive correlation with the nitrogen composition of the amino acid, we also proved the previous hypothesis [12] that nitrogen take a role in the flame retardancy of proteins.
Although we expected the retentions (or resistance to washing) of flame retardant proteins to be higher when adhesion domains are present, we didn't expect an increase of flame retardancy in these proteins. To our surprise, flame retardancy was increased when adhesion domains were fused to the proteins. One of the explanations would be the adhesion domains not only increase the retentions of proteins on the surfaces of the materials, but also increase the density (or successful rate) of coating of proteins on cotton fabrics and wood. It would be interesting for future researchers to study how to increase the density of proteins coating onto different materials, thereby, further increase the flame retardancy of protein coating materials.
Previous research shows various proteins can prolong the burning time, however, the retention of flame retardant protein on material after washing procedure is known to be very low [14,15], precluding these proteins from wide usage in the fire safety industry. In this study, we not only showed the retention can be increased with adhesion domains, but also compared the retentions of proteins fused with the mussel foot protein-5 (mfp-5) and cellulose binding domain (CBD), providing some information on the selection of adhesions domains. On average, we didn't notice differences in the performance of these two adhesion domains. However, based on previous studies [[17], [18], [19]], we know that mfp-5 can attach to surfaces of different materials while CBD would attach more specifically materials containing cellulose. Future researchers can, therefore, select the domain according to the purpose of coating and the materials they use, as our data showed that coating with either adhesion domains seem to work well.
There are a few limitations of this study that can be further investigated. Firstly, in terms of the safety aspects, toxicity tests and allergy tests can be conducted to further study whether there is any effect of flame retardant protein-coated materials on humans. Secondly, is the degradability of the protein-based FRs. Although we showed some evidence (Fig. 5 and Table 4) that adhesive proteins can increase durability of this kind of FRs, we couldn't change the fact that most of the proteins can be degraded in a relatively short time. Future research may further investigate the structures (eg. alpha helix and beta-sheet) of flame retardant proteins and improve the durability by creating more stable flame retardant proteins. On the other hand, it would be interesting to study whether it is possible to grow microorganisms on materials to serve as flame retardant coatings. One potential alternative to current FRs would be to grow engineered microorganisms that can continuously secret bio-based FRs and at the same time survive on the surfaces of the materials. Another limitation of this study is that, the fire retardancy was tested only on two types of materials – cotton (cotton fabrics) and wood, limiting the applications to fabrics (such as curtains and beddings) and wood furniture (like doors, tables and cupboards). Further fire retardancy tests on various kinds of materials would be desirable in order to widen the application of the flame retardant proteins. Finally, some more quantitative experiments such as cone calorimeter test to measure the heat release rate and smoke production rate [27]; thermogravimetric analysis (TGA) to evaluate the thermal stability and combustibility [25] can also be performed in further research to investigate the characteristics of our engineered flame retardant protein coatings, so as to give more insights to material scientists and physicists.
To the best of our knowledge, our study here is the first study that applies synthetic biology and genetic engineering to create flame retardant materials. We believe that our study provides insights and ground for future researchers to investigate using recombinant or engineered proteins in the fire safety industry, and hopefully lead to the invention of other durable and green flame retardants in the near future.
CRediT authorship contribution statement
Weng I. Leong: Data curation, Writing – original draft, Visualization, Investigation. Owen Lok In Lo: Methodology, Data curation, Writing – review & editing, Funding acquisition. Fong Tin Cheng: Methodology, Data curation, Validation, Investigation. Wai Man Cheong: Methodology, Data curation, Investigation, Funding acquisition. Leo Chi U. Seak: Supervision, Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
Declaration of competing interest
We have no conflict of interest to declare.
This statement is to certify that all Authors have seen and approved the manuscript being submitted. We warrant that the article is the Authors' original work. We warrant that the article has not received prior publication and is not under consideration for publication elsewhere. On behalf of all Co-Authors, the corresponding Author shall bear full responsibility for the submission. This research has not been submitted for publication nor has it been published in whole or in part elsewhere. We attest to the fact that all Authors listed on the title page have contributed significantly to the work, have read the manuscript, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the Synthetic and Systems Biotechnology.
All authors agree that author list is correct in its content and order and that no modification to the author list can be made without the formal approval of the Editor-in-Chief, and all authors accept that the Editor-in-Chief's decisions over acceptance or rejection or in the event of any breach of the Principles of Ethical Publishing in the Synthetic and Systems Biotechnology being discovered of retraction are final.
No additional authors will be added post submission, unless editors receive agreement from all authors and detailed information is supplied as to why the author list should be amended.
Acknowledge
We thank Prof. Leo Tsz On LEE Prof. Ruiyu XIE and Prof. Tzu-Ming LIU for discussions and conceptual support, Stephanie Pei Wen NG and Weng I LEI for technical support, and all other members/contributors in the PuiChing 2020 team (Ieng Chon LI, Yi Fan XIANG, Sin Mei CHEONG, Cho Cheng SHE, Weng Seong LEI, Pak Chong CHEONG, Chan In NG, Nga Chi LEONG, Teng Wai HOI, Weng Si CHIO, Lok Hang CHIU, Hou IONG, Weng In LAI, Jeremy HU, Franklin YEUNG, Hao Nian MIN, Hau Yin LEUNG and Yating MO) for helping this project. We also thank the Wynn Care, Science and Technology Development Fund (FDCT) (Grand code: 0016/2020/PS), Institute for the Development and Quality (IDQ) and Faculty of Health Sciences, University of Macau for supporting this work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2021.10.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Camino G., Costa L., Martinasso G. Intumescent fire-retardant systems. Polym Degrad Stabil. 1989;23:359–376. 4. [Google Scholar]
- 2.Guillaume Eric, de Feijter Rene, van Gelderen Laurens. An overview and experimental analysis of furniture fire safety regulations in Europe. Fire Mater. 2020;44:624–639. 5. [Google Scholar]
- 3.Birnbaum Linda S., Staskal Daniele F. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuiderveen Emma AR., Chris Slootweg J., de Boer Jacob. Novel brominated flame retardants-A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere. 2020;255 doi: 10.1016/j.chemosphere.2020.126816. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton Heather M., et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–5331. doi: 10.1021/es2007462. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman Kate, et al. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: a case-control study. Environ Int. 2017;107:235–242. doi: 10.1016/j.envint.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Mughal Bilal B., Demeneix Barbara A. Flame retardants and increased risk of thyroid cancer. Nat Rev Endocrinol. 2017;13:627–628. doi: 10.1038/nrendo.2017.123. 11. [DOI] [PubMed] [Google Scholar]
- 8.Terrell Metrecia L., et al. Breast cancer among women in Michigan following exposure to brominated flame retardants. Occup Environ Med. 2016;73:564–567. doi: 10.1136/oemed-2015-103458. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pi Na, et al. Associations of serum organohalogen levels and prostate cancer risk: results from a case–control study in Singapore. Chemosphere. 2016;144:1505–1512. doi: 10.1016/j.chemosphere.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Grandjean Philippe, Philip J. Landrigan. "Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal Puja, et al. Brain bromine levels associated with Alzheimer's disease neuropathology. J Alzheim Dis. 2020;73:327–332. doi: 10.3233/JAD-190646. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malucelli Giulio, et al. Biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv. 2014;4:46024–46039. 86. [Google Scholar]
- 13.Velencoso Maria M., et al. Molecular firefighting—how modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew Chem Int Ed. 2018;57:10450–10467. doi: 10.1002/anie.201711735. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Fang, et al. Novel high-efficiency casein-based P–N-containing flame retardants with multiple reactive groups for cotton fabrics. ACS Sustainable Chem Eng. 2019;7:13999–14008. 16. [Google Scholar]
- 15.Zhang Ai-Ning, et al. Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem Eng J. 2021;410 [Google Scholar]
- 16.Levchik G.F., Grigoriev Y.V., Balabanovich A.I., Levchik S.V., Klatt M. Phosphorus–nitrogen containing fire retardants for poly (butylene terephthalate) Polym Int. 2000;49(10):1095–1100. [Google Scholar]
- 17.Carrard Géraldine, et al. Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc Natl Acad Sci Unit States Am. 2000;97:10342–10347. doi: 10.1073/pnas.160216697. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Qingye, et al. Adhesion of mussel foot proteins to different substrate surfaces. J R Soc Interface. 2013;10(79) doi: 10.1098/rsif.2012.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Chao, et al. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat Nanotechnol. 2014;9(10):858. doi: 10.1038/nnano.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan Alexander B., Galaska Mary L. Flammability testing of wool/cellulosic and wool/synthetic fiber blends: vertical flame spread and heat release results. J Fire Sci. 2020;38:522–551. 6. [Google Scholar]
- 21.Holder Kevin M., Smith Ryan J., Grunlan Jaime C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J Mater Sci. 2017;52:12923–12959. 22. [Google Scholar]
- 22.Ng Yan Hao, et al. Correlating the performance of a fire-retardant coating across different scales of testing. Polymers. 2020;12(10):2271. doi: 10.3390/polym12102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yew Ming Chian, et al. Influences of nano bio-filler on the fire-resistive and mechanical properties of water-based intumescent coatings. Prog Org Coating. 2018;124:33–40. [Google Scholar]
- 24.Cheema H.A., El-Shafei A., Hauser P.J. Conferring flame retardancy on cotton using novel halogen-free flame retardant bifunctional monomers: synthesis, characterizations and applications. Carbohydr Polym. 2013;92(1):885–893. doi: 10.1016/j.carbpol.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 25.Zope I.S., Foo S., Seah D.G., Akunuri A.T., Dasari A. Development and evaluation of a water-based flame retardant spray coating for cotton fabrics. ACS Appl Mater Interfaces. 2017;9(46):40782–40791. doi: 10.1021/acsami.7b09863. [DOI] [PubMed] [Google Scholar]
- 26.Ran Shiya, et al. Char barrier effect of graphene nanoplatelets on the flame retardancy and thermal stability of high‐density polyethylene flame‐retarded by brominated polystyrene. J Appl Polym Sci. 2014;131:15. [Google Scholar]
- 27.Kim N.K., Bruna F.G., Das O., Hedenqvist M.S., Bhattacharyya D. Fire-retardancy and mechanical performance of protein-based natural fibre-biopolymer composites. Composites Part C: Open Access. 2020;1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.