Highlights
-
•
COVID-19 forced the closure of Epilepsy monitoring units around the world limiting clinical evaluation of patients with seizures and spells.
-
•
AEEG with video increased as an out-of-hospital alternative at our center to inpatient video-EEG monitoring for diagnostic evaluation.
-
•
Technological advances in AEEG parallel minimum technical standards used for inpatient VEM but possess important and distinct strength and limitation.
-
•
AEEG can serve as a diagnostic bridge during a pandemic or when inpatient VEM is unavailable, inaccessible, or impractical.
Keywords: Ambulatory, Electroencephalography, Portable, Epilepsy, Seizure, Utility
Abstract
The COVID-19 pandemic forced temporary closure of epilepsy monitoring units across the globe due to potential hospital-based contagion. As COVID-19 exposures and deaths continues to surge in the United States and around the world, other types of long-term EEG monitoring have risen to fill the gap and minimize hospital exposure. AEEG has high yield compared to standard EEG. Prolonged audio-visual video-EEG capability can record events and epileptiform activity with quality like inpatient video-EEG monitoring. Technological advances in AEEG using miniaturized hardware and wireless secure transmission have evolved to small portable devices that are perfect for people forced to stay at home during the pandemic. Application of seizure detection algorithms and Cloud-based storage with real-time access provides connectivity to AEEG interpreters during prolonged “shut-down”. In this article we highlight the benefits of AEEG as an alternative to diagnostic inpatient VEM during the paradigm shift to mobile heath forced by the Coronavirus.
1. Introduction
Inpatient video-EEG monitoring (VEM) is the diagnostic gold standard for patients suspected of epilepsy. However, hospitalization, time-constraints, insurance approval, financial and transportation issues are practical limitations [1]. Because of the COVID-19 pandemic, hospitals across the United States restricted or cancel admissions for VEM in hospital-based epilepsy monitoring units (EMUs) [2], [3]. Telemedicine has reduced the safety risk of viral exposure to patients and their families [4]. Healthcare workers involved in patient care in an EMU risk potential exposure to infection with Coronavirus (SARS-CoV-2) or its variants [2], [5].
The number of hospital-based procedures fell during the pandemic. For example, in Italy, 206 sites saw a reduction of 76% ± 20% in EEG procedures performed [6]. Furthermore, a survey of 47 epilepsy centers across 22 countries in Europe reported that inpatient VEM was restricted due to the COVID-19 pandemic [7]. In Spain, a study of 255 epilepsy patients reported 15% had a delay in performance of epilepsy-related tests during pandemic lockdown [8].
Attempts to fill the gaps created by EMUs that closed are unable to be overcome by standard EEG due to the brevity that obviates event capture [9], [10], [11]. On the other hand, ambulatory video-EEG (AV-EEG) is an effective diagnostic alternative to inpatient VEM [12], [13] that at our center has risen in its use to offset the loss of inpatient VEM availability (personal communication, WOT 2/16/2021).
2. Methods
To address the impact of the pandemic, we performed a literature review through November 30, 2020. We compared the role of AEEG to other hospital-based techniques with the intent to highlight the role of AEEG as an alternative EEG method to evaluate patients in the post-COVID-19 era. We searched PubMed and Embase databases using broad search terms (“epilepsy AND ambulatory AND EEG) and synonyms (“epilepsy AND AEEG”). A similar search was performed for data available for pediatric population (“pediatric AND AEEG”) and (“epilepsy AND children AND AEEG”). A separate search using the same database was performed related to COVID-19 pandemic and epilepsy (“COVID-19 AND epilepsy”). Relevant articles were analyzed and information for selected topics was extracted.
3. The evolution of ambulatory EEG
The development of AEEG was inspired by Holter 50 years ago to assess dynamic changes in the electrocardiogram (ECG) of patients in an ambulatory setting [13]. Marson and McKinnon developed a four channel portable EEG device in 1972 for continuous recording using ¼ inch audiotape drawn from the success of the music industry [14]. By the mid-1970s, Ives and Wood modified AEEG recorders that could be worn over the shoulder or around the waist [15]. Three years later, Quy modeled AEEG preamplifiers so they were worn on the head, improving the signal to noise ratio [16]. By the 1980s, Bridgers and Ebersole were using AEEG recorders for clinical use with channels available for time display, event markers, and ECG channel [17]. Video was added to the audio signal and eight channel continuous EEG recorders and playback devices became commercially available prompting widespread application in the clinic [18]. Intermittent AEEG recording (i.e., 15 s every 10 min over 24 h) selected targeted samples of EEG and streamlined interpretation [19]. Today, portable lightweight AEEG devices (1 pound) can record up to 36 channels of continuous EEG with or without video. High sampling rates of 512 Hz and bit depth of at least 16-bits for analogue to digital conversion are available in most systems. Spike and seizure detection algorithms, artifact reduction, push-button activation to facilitate event recording, and quantitative EEG are available for trend analyses. Large digital storage capacities are now available to store, retrieve, and modify data from the Cloud. Current technology used for AEEG is essentially the same used for inpatient VEM, capable of reviewing, transferring, and interpreting large volumes of data [20], [21]. The minimum number of recording electrodes for standard EEG is 16, however, 25 electrodes has been recommended for long-term EEG monitoring in adults and children to provide better coverage of the anterior and inferior-basal regions of the temporal lobes [20]. Electrodes are connected to a head mounted preamplifier that digitizes and multiplexes data. Preamplifiers are connected to the recording device and are worn around the waist or over the shoulder [21]. Patients and caretakers are educated on push button activation to mark potential events on AEEG detailing the time and description of an event in a diary [21].
4. Ambulatory video-EEG
High-quality, high-definition video cameras are optional to record patient behavior during activities of daily living and increases the diagnostic yield. In a study of 59 temporal lobe epilepsy patients experiencing 262 seizures, EEG lateralized ictal onset in 64.4%, semiology lateralized ictal onset in 78%, yet combining video and EEG lateralize seizures in 94.9% [22]. Goodwin et al. offered video camcorders to 45 patients alongside AEEG and captured an ictal event in 76% (34) patients, however, only 50% (17) had an event recorded on video. There are limitations to the use of video with AEEG. Maintaining proper distance from the camera and centering it to ensure the patient remains in the field of view is crucial to good quality video recording during AVEEG. Additionally, proper use of video is reliant on familiarity with the recording equipment to ensure adequate capture of an event in case one occurs. The yield is also proportional to the amount of observation by the family or caregiver and hence is often more effective in successfully capturing events in the pediatric population [23]. AEEG without video has a lower yield than AEEG with video which aids interpretation when an event is captured [24] and facilitates diagnosis in up to 80–85% of patients undergoing AEEG [23], [25].
5. How long should we record ambulatory EEG?
AEEG is capable of recording up to 72–96 h, though 1–2 days is usually performed for most diagnostic purposes yet depends upon the individual and reasons for recording. In a retrospective study of 180 patients, Faulkner et al found an average latency of 50% of patients with the first interictal epileptiform discharge (IED) within the initial 4 h of AEEG and recovery of IEDs in 95% of patients after 48 h, concluding that 2 days of was the optimal duration for recovery of IEDs [26]. In contrast, in a smaller retrospective review of 57 AEEGs, the yield did not significantly increase after 13 h of recording [27]. In a large retrospective review of 358 AEEG in adults, Kuo et al found the yield did not increase beyond 24 h of recording IEDs but did increase for event recording [28]. Hence, 1–2 days appears optimal for interictal EEG recording, thoug longer durations may be required to capture events.
6. Clinical indications of ambulatory EEG
6.1. Diagnosis of suspected epilepsy
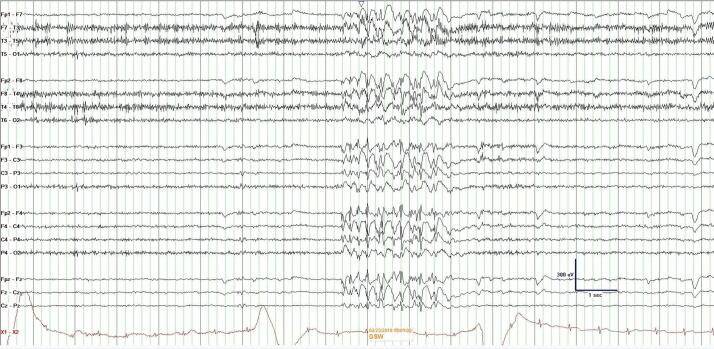
There are multiple reasons to obtain an AEEG (Table 1). Compared with other forms of EEG such as VEM, AEEG has advantages (Table 2) and disadvantages (Table 3). Keezer et al found the sensitivity of AEEG was 2.23 times greater than standard EEG in the diagnosis of patients suspected to have epilepsy (Fig. 1) [29].
Table 1.
Indications for AEEG.
|
|
|
|
|
|
|
|
|
|
ASM: Anti-seizure medication; VEM: Video Monitoring.
Table 2.
Advantages of AEEG.
|
|
|
|
|
|
|
|
|
|
|
|
EEG: Electroencephalography; VEM: Video Monitoring; rEEG: routine EEG.
Table 3.
Limitations of AEEG.
|
|
|
|
|
|
|
|
|
|
|
ASM: Anti-seizure medication; AEEG: Ambulatory EEG.
Fig. 1.
Generalized spike-and-waves at 5 Hz on AEEG in a patient with JME who previously had two non-diagnostic standard EEGs.
6.2. Differential diagnosis of seizures
The differential diagnosis of patients suspected of epilepsy is broad. Physiologic nonepileptic events are important but infrequent mimics of epilepsy, and occur in approximately 10% of individuals undergoing diagnostic VEM [30]. Syncope is the most common physiological nonepileptic event in people to mimic epileptic seizures. NEE are frequently due to psychogenic non-epileptic attacks (PNEA) in majority of patients and diagnosed in 20–30% of EMU admissions [31], [32]. One retrospective AEEG study of 324 patient recorded habitual events in 52%. The majority (54%) captured were PNEA with 31% of patients manifesting epileptic seizures, 2% with both, and 10% with syncope [32]. A larger evaluation of 502 patients retrospectively identified 47 with events; 13% were epileptic and 87% were without scalp EEG change (presumed nonepileptic) [33]. Another retrospective study of 200 patients recorded events in 110 studies (55%) with 101 events (92%) captured on video. Of those events captured with video and EEG, epileptic seizures were recorded in 17.8% and NEEs were diagnosed in 38% of the studies. Overall, AEEG with video recording was found to be a potentially useful alternative to inpatient epilepsy monitoring unit, particularly with high clinical suspicion for nonepileptic events [34]. The diagnostic impact of AEEG resulted in a reduction in testing charges by 76%, and antiseizure medication charges by 69% [35].
6.3. Detecting “silent” seizures
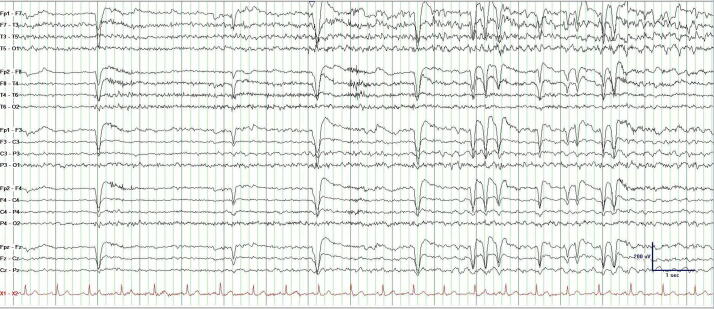
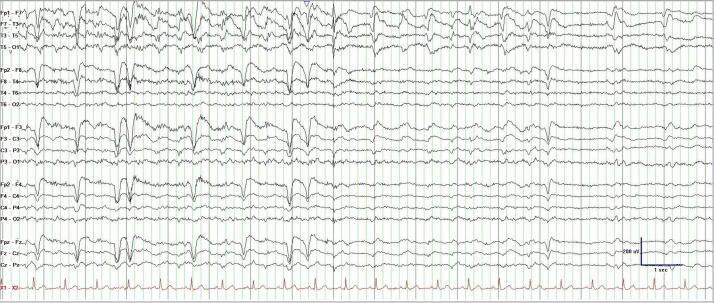
Some focal impaired awareness seizures are clinically apparent, however, when they are subtle and associated with a loss of self-awareness, they may defy detection by observers (Fig. 2, Fig. 3). Subclinical seizures are only evident on EEG where a clinical correlate is absent despite the presence of an electrographic seizure. Similarly, nocturnal seizures may defy detection due to an intervening post-ictal somnolence that supersedes recognition when patient return to sleep. In these cases, overnight AEEG monitoring has remarkably use quantifying “silent” seizures [9], [10], [11], [12], [13].
Fig. 2.
AEEG demonstrating of a 85-year-old man with recurrent left temporal focal impaired awareness seizure without self-awareness. While he denied their occurrence, his wife insisted that he be evaluated for recurrent “episodes”.
Fig. 3.
Termination of the AEEG from Fig. 2. Note the lack of post-ictal slowing that correlated with the lack of a clinical post-ictal state.
The seizure frequency reported by patients with epilepsy is often under-estimated. In one hospital study, 30% of patients admitted for inpatient VEM were always self-unaware of their seizures 1 hour after they occurred [36]. An outpatient AEEG record review of 502 reports found 38.3% of patients experiencing focal seizures were unassociated with push button event activation and absent diary entries despite electrographic confirmation of seizures in their home environment [33].
6.4. Treatment decisions
Lack of witnesses to report seizures, inadequate seizure logs, patient memory deficits, post-ictal amnesia in patients without seizure self-awareness, and those experiencing subclinical seizures compromise management [33], [36]. With respect to chronic treatment in patients with epilepsy, Oxley and Roberts retrospectively analyzed 100 AEEG recordings and found that AEEG captured events in 80% of patients and guided a change of treatment by either adjusting the dosage of ASM or starting a new ASM regime in 57% of patients [37]. Faulkner et al found in 324 AEEG studies that the results led to a change in management in 51% of his patients [32]. AEEG may be able to validate drug-resistance in patients with focal epilepsy adequately treated with ASM who report seizure freedom.
Even in patients after a first seizure, the initial diagnosis of epilepsy now includes the occurrence of a single seizure. One seizure in conjunction with an abnormal epileptiform EEG provides more than 60% likelihood that recurrence is anticipated [38]. Therefore, despite a single seizure, AEEG can yield an initial diagnosis of epilepsy when epileptiform discharges occur after a first seizure and may be especially useful to consider when an initial standard EEG is non-diagnostic [39].
Before considering ASM withdrawal, the presence of epileptiform activity is associated with a risk of seizure relapse despite prolonged seizure freedom with ASM [40], [41]. However, standard EEG in patients with epilepsy often does not detect IEDs creating a false sense of security based on limited time sampled. Koepp et al compared standard EEG with AEEG in predicting seizure recurrence following ASM reduction [43]. In this small study, 15 seizure-free patients, comparison was made before ASM reduction. AEEG was found to be superior to standard EEG in detecting epileptiform activity and better predicted seizure recurrence after withdrawal of ASM [42]. Still, the true predictive value of AEEG similar to standard EEG on recurrence remains inconsistent [43], [44].
6.5. Driving
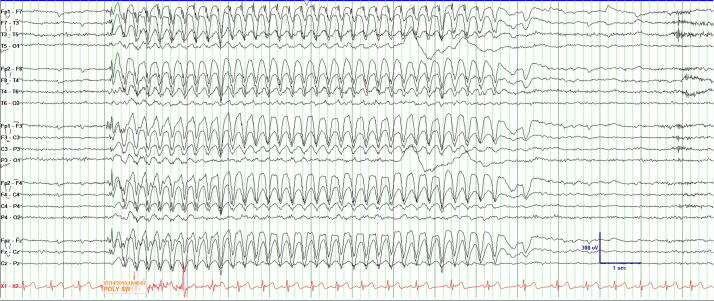
Driving privileges are a primary concern to people with epilepsy. State-specific regulations by the Department of Motor Vehicles exist requiring people with epilepsy to attain a period of prolonged seizure freedom prior to reinstatement of driving privileges. This regulation is designed to ensure personal and public safety are maintained by avoiding risk from breakthrough seizures [45]. Fattouch et al retrospectively analyzed AEEG recordings of 1100 patients and found a large number of unreported ictal events in patients who were “seizure-free”, yet still drove regularly [46]. Tatum et al carried out a survey in 236 patients with seizures and reported 14.8% of patients continued to drive despite being ineligible, with 8.9% of patients who drove undeterred by the law despite being aware of state restrictions [47]. Therefore, AEEG is ideally suited to objectively assess seizure freedom. When patients report seizure freedom, the AEEG may disclose clinical seizures or electrographic seizures to provide a clinician with greater confidence prior to safe return to driving (Fig. 4) [42].
Fig. 4.
AEEG demonstrating a 9-second burst of 3–3.5 Hz generalized spike-and-slow waves with left frontopolar predominant in a patient with juvenile absence epilepsy reporting seizure freedom.
7. Safety
AEEG is typically not used in the presurgical evaluation of patients with drug resistant epilepsy due to safety concerns [48], [49], [50]. Diagnostic AEEG has no significant risks beyond those associated with performance of standard EEG [51]. Electrodes need to be adequately disinfected to prevent transmission of contagious disease such as viral hepatitis, Creuzfeldt-Jacob disease, and HIV [52]. Similar to continuous EEG monitoring (cEEG), AEEG equipment should be disinfected for COVID-19 with a commercially available germicidal disposable wipe or solution, which is virucidal and bactericidal with at least 70% isopropyl alcohol, prior to next use [2], [53]. Electronic equipment should be covered with washable or plastic coverings or cases to facilitate this cleaning. Where possible, disposable electrodes should be used. Difficult to clean parts should have “rest period” between uses [53], [54], [55]. Rest periods are warranted because infectious sources such as the Coronaviruses can be found on inanimate surfaces for up to 9 days without proper disinfection [54].
Technologists performing and interacting with patients during a pandemic should use personal protective equipment (PPE) during hook-ups and take-downs. These should include the use of an isolation gown, gloves, and medical face mask or shield, when near or in contact with patients (Fig. 5) [2], [54].
Fig. 5.
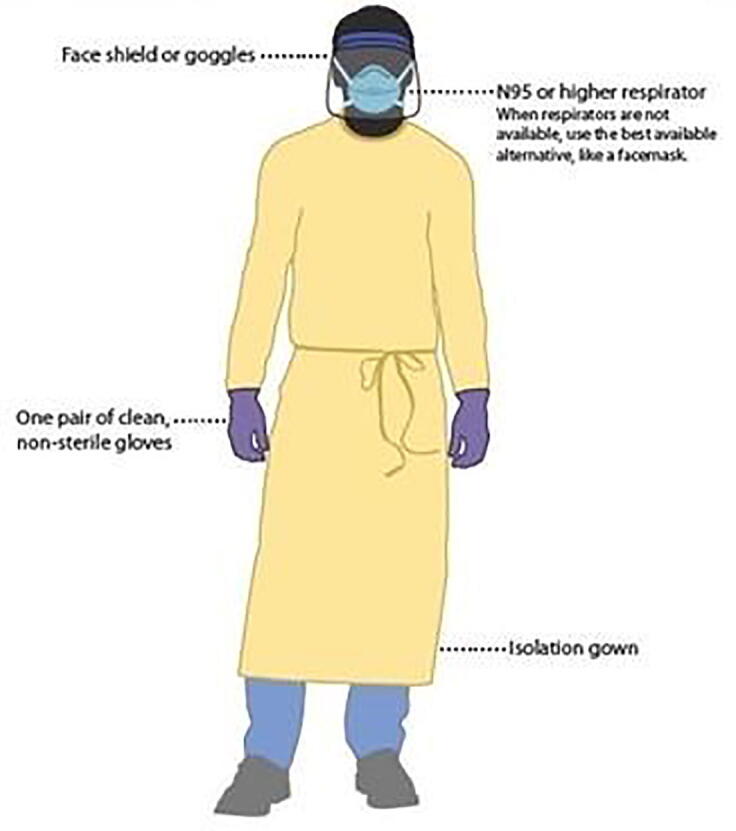
COVID-19 Personal Protective Equipment (PPE) for healthcare personnel.
Electrical safety can be established by avoiding electrical current leakage through proper use of fuses, outlets to ground the equipment, grounding electrode, and by avoiding use of extension cords [56]. Patients should be advised to avoid contact of the electrical machinery and wiring with water to maximize safety and minimize electrical current exposure that has been linked to skin burns and potentially more serious injury [56].
8. Cost
It is imperative that during a healthcare crisis such as a pandemic, judicious economic strategies are employed for the benefit of patients and organizations. In a study of 255 epilepsy patients by Fonseca et al., approximately 30% of patient reported a reduction in income directly linked to the impact of the COVID-19 pandemic [8]. In a large retrospective cohort study of 13,958 patients, outpatient AEEG cost was significantly lower compared to inpatient VEM monitoring. Interestingly, overall epilepsy-related healthcare expenditures in patients undergoing AEEG was lower in the first 12 months after the initial study compared with patients who underwent inpatient VEM [57]. These findings were similar to original economic models which indicated that AEEG was up to 66% cheaper than inpatient VEM [25]. Since January 1, 2020, in the United States, reimbursement for long-term inpatient EEG monitoring modified the fee structure for VEM and AEEG reimbursement for professional and technical components though still incurs hospital costs to patients for inpatient VEM.
9. Utility
9.1. Clinical usefulness
The utility of AEEG has been demonstrated in multiple class III and IV studies (Table 4). Obtaining sleep is the most effective method to “activate” the EEG [58] and increase the yield of AEEG for recording IEDs and seizures [37], [59], [60]. Dash et al. prospectively reviewed AEEG recordings of 101 patients and found a diagnostic yield of 72% [10]. Morris et al. evaluated patients undergoing 16-channel AEEG and found usefulness in 74.4% patients. In that study, seizures were captured in 11.9%, and IEDs captured in 26.2% of the patients [59]. Faulkner et al. found utility in 68% of 324 AEEG recordings with 52% of patients experiencing typical events, and 36% containing IEDs [32]. In patients 60 years and older, 58 AEEG studies identified diagnostic results in 37% of the patients including IEDs in 26% and NEE in 16% of patients [60]. Smaller studies (n = 26 patients) undergoing AEEG recorded nonepileptic events without EEG changes in 46% of the patients [61] emphasizing variability of study results and population biases. Nonetheless, multiple retrospective studies support a high diagnostic yield of AEEG to differentiate epileptic from events without EEG changes (presumed nonepileptic events).
Table 4.
Summary of AEEG studies.
| Study | Type of Study | No. of patients | Age Range | No. of channels | AEEG recording duration | AEEG with Video | Yield (Seizures) | Yield (IED) | Yield (NEE) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Ebersole and Leroy (1983) | N/A | 40 | Adults or adolescents | 3 | 16–24 h | No | 17.5% | 47.5% | NA | Concurrence between both AEEG and inpatient telemetry interpretation of normal vs EA was 77% overall (79% for focal and 100% for generalized interictal abnormalities). All seizures noted on inpatient telemetry were detected by AEEG |
| Bridgers and Ebersole (1985) | Retrospective | 500 | 2 months-82 yrs. | 3 | 6–24 h | No | 6.4% | 11% | 15.3% | EA were found in 17.4% of patients. Among patients who had both EEG and AEEG, there was a 61% increase in the yield of EA and a 21-fold increase in detecting seizures with AEEG. |
| Cull (1985) | N/A | 62 | 13–81 yrs. | 4 | 24 h | No | NA | 33.8% | 6.5% | EA were detected in 34% by AEEG vs 26% by rEEG. Patients who had clinical attacks once a week or more frequently showed an improved yield on AEEG but was not helpful when attacks were less frequent. |
| Oxley and Roberts (1985) | Retrospective | 100 | 16–50 yrs. | 4 | 48–216 h | No | 54% | NA | 32% | Clinical event was recorded in 80% of the patients and it led to a change in treatment in 57% of the patients. |
| Morris et al (1994) | Retrospective | 344 | 6 months-69 yrs. | 16 | 32 h | No | 11.9% | 26.2% | 36.3% | 16 channel AEEG system was clinically useful in 74.4% of patients and 67.5% of patients had useful findings on AEEG with previously non-diagnostic rEEG. |
| Liporace et al (1988) | Prospective | 46 | NA | 16 | 24 h | No | 15% | 33% | NA | AEEG is better than sdEEG in detecting epileptiform discharges or seizures. This study recommended the selection of a computer-assisted AEEG over a sdEEG in patients with presumed epilepsy and a non-diagnostic rEEG. |
| Tatum et al (2001) | Retrospective | 502 | 1 month- 93 yrs. | 16 | 28.5 h (mean) | No | 8.50% | NA | NA | 38.3% of the seizures went unrecognized by the patient leading to underreporting of seizures which impacts optimal diagnosis and treatment. |
| Zarkou et al (2011) [96] | Retrospective | 19 | N/A | NA | NA | No | NA | NA | NA | AEEG contributed to diagnosis in 5% of the patients. This study states that a repeat EMU admission rather than an ambulatory recording should be considered if an initial EMU admission for event classification is indeterminate. |
| Dash et al (2012) | Prospective | 101 | 13–60 yrs. | 24 | 15–96 h | No | 9.9% | NA | 30.8% | AEEG contributed to clinical diagnosis in 71% of patients and is useful in patients with frequent clinical spells to differentiate between epileptic and non-epileptic events, to quantify seizures and epileptiform activity. |
| Faulkner et al (2012) | Retrospective | 324 | 12–79 yrs. | 32 | 72–96 h | No | 15.7% | 36% | 29.6% | AEEG changed the diagnosis in 51% of patients with a high diagnostic utility in the detection of paroxysmal events. |
| McCormick et al (2014) | Retrospective | 26 | NA | NA | NA | No | NA | NA | 46% | AEEG can be a useful alternative to inpatient VEM in the diagnosis of non-epileptic events. |
| Lawley et al (2016) | Retrospective | 88 | 2–80 yrs | 32 | 24–48 | Yes | 6.8% | NA | 55.7% | The diagnostic utility of AEEG was 67.0%. Implementation of video AEEG protocols in a secondary care center appears to have high diagnostic utility, particularly for patients with psychogenic nonepileptic seizures |
| Manfredonia et al (2016) | Retrospective | 111 | 2–81 yrs | NA | NA | Yes | 55.9% | 14.5% | 85.5% | A total of 27 patients (24.3%) had changes in medical treatment following video-AEEG, most frequently antiepileptic drug introduction/increase when epileptic seizures were captured. This proportion was similar between patients with or without a previously established diagnosis of epilepsy. |
| Doshi et al (2017) | Retrospective | 105 (40 with AEEG) | NA | NA | 24 h | No | NA | 17.50% | NA | AEEG is superior to repeat rEEG and sdEEG in capturing IEDs after non-diagnostic rEEG. Higher yield of AEEG might be due to expected prolonged EEG sampling and more likelihood of capturing sleep |
| Geut et al (2017) | Retrospective | 104 (52 with sdEEG) | 48 yrs. (median age) | 21 | 16–24 h | No | NA | 40% | NA | The sensitivity of sdEEG was 45% and AEEG was 63% for detection of IEDs. AEEG has similar efficacy as sdEEG for detection of IEDs in first unprovoked seizure patients with normal rEEG. |
| Tolchin et al (2017) | Retrospective | 156 | 60–94 yrs. | 32 | 24–72 h | No | 2.6% | 26.9% | 16% | Geriatric epilepsy can easily be clinically confused with common problems of the elderly such as dementia and orthostatic hypotension. AEEG can be helpful in distinguishing geriatric epilepsy from these other disorders. |
| Syed et al (2019) | Retrospective | 9221 | 40–68 yrs. (adults) 7–15 yrs. (pediatric) | NA | 72 h (median) | Yes | 0.5% | 88.9% | NA | Ambulatory VEM outcome was equal to inpatient VEM in adults (54.4% vs 61.7%), but lower than inpatient VEM in children (86.3% vs 72.5%). |
| Primiani et al (2020) | Retrospective | 200 | 12–101 yrs | NA | 23–175 h | Yes | 55% | 17.8% | 38% | Ambulatory VEM may be a useful alternative to inpatient epilepsy monitoring unit, for clarification of diagnosis, particularly when clinical suspicion for nonepileptic events is high.. |
IED-Interictal epileptiform discharges; NEE-Non-epileptic events; NA-Not available; EA-Epileptiform abnormality; AEEG-Ambulatory EEG; rEEG-Routine EEG; sdEEG-Sleep deprived EEG; VEM-Video EEG monitoring.
*PubMed search of relevant articles until the year 2020 which talk about clinical utility of AEEG have been included. Articles discussing the role of AEEG in ASM withdrawal and pre-surgical assessment have been excluded.
9.2. Comparison with standard EEG
During technical set-up and removal of standard EEG or AEEG, the same safety risk applies to both the technologist and patient. Early work by Bridges and Ebersole compared standard EEG to AEEG in 500 patients where IEDs were present in 11% undergoing standard EEG and increased to 61% with AEEG [17]. Similar incremental findings in detection differences were reported in a study involving 62 patients who experienced frequent events where IEDs were detected in 33.8% on AEEG, but was not superior to standard EEG in seizure detection [62]. In patients undergoing a repeat EEG compared to those undergoing an AEEG after an initial non-diagnostic standard EEG, Doshi found a greater yield of IEDs increasing from 1.9% to 17.5% during AEEG monitoring in 105 patients [63].
Liporace et al. performed a class II study comparing the yield of a sleep deprived EEG (sdEEG) and 16 channel AEEG in 46 patients following a non-diagnostic EEG and found deeper stages of sleep were achieved with AEEG and the yield of detecting IEDs was 33% [9] similar to Cull’s study of 62 patients. Moreover, in this prospective study involving 2 blinded reviewers higher yield for detection of seizures was present in 15% of AEEG studies (mean 24 h of recording) compared to none in the sdEEG cohort [9]. Geut et al. noted higher sensitivity with AEEG (63%) compared to sdEEG (45%) of studies focused on detection of IEDs [64].
9.3. Comparison with inpatient VEM
The diagnostic usefulness of inpatient VEM has been reported to range between 19% and 75% [20], [65]. In comparison, the usefulness of AEEG similarly has ranged up to 72% [10], [32]. However, AEEG with video that fails to capture the clinical event on video has decreased clinical yield compared to inpatient VEM. In the largest study to date, Syed et al evaluated 9221 AVEEG reports across 28 states and found at least one patient-activated pushbutton event was captured on video in 54% of recordings. Epileptiform activity was reported in 18.0% of AVEM recordings: 88.9% were interictal, 0.5% were ictal, and 10.6% reflected both interictal and ictal records. Compared to AVEM, inpatient VEM confirmed more representative events in both adult and pediatric samples [66]. In another large single-center retrospective study, Zaroku et al. contrasted the usefulness of repeat EMU admission compared with AEEG recordings after an initial indeterminate EMU admission [67]. Of 805 hospitalizations for VEM, 80% of patients received a diagnosis after an initial admission. The investigators found 13% of first EMU admissions were indeterminate and a second admission for VEM session was performed in 13 (12%) people where 8 (62%) received a diagnosis. A third or fourth admission for VEM remained non-diagnostic in five patients. In contrast, nineteen (18%) patients had ambulatory EEG monitoring after an indeterminate admission, with only one (5%) patient receiving a diagnosis. However, in another retrospective study by Fox and colleagues, when a tandem AEEG monitoring session was performed immediately following a non-diagnostic inpatient VEM session, this resulted in event capture in 48.4% of the patients. In patients released from the EMU, 32.3% of the events that were recorded on AEEG occurred within 24 h of discharge where EDs were recorded in only 27.4% of patients [68] demonstrating utility of sequential AEEG following an unsuccessful inpatient VEM as opposed to a separate study. At our institution, AEEG increased overall by 34% while inpatient VEM decreased 21% during 2020 precipitated by the lockdown imposed by COVID-19 (personal communication, WOT 1/15/2021).
10. Pediatric AEEG
The COVID-19 pandemic also caused drastic changes in managing children with epilepsy. In a survey of 212 pediatric neurologists from 49 countries, 90.6% noted reduced access to EEG services and 93.4% reported closed or severely limited admissions to EMUs for VEM. Many respondents resorted to relying on clinical history alone and/or review of home video to diagnose first seizures and epilepsy [69].
10.1. Utility in children
The utility of AEEG has been evaluated in children with epileptic seizures and nonepileptic events (Table 5). Olson et al. retrospectively reviewed AEEG results in 167 children experiencing at least three seizures per week with 89% of children experiencing their typical spells over a mean of 1.9 days [70]. The authors felt children experiencing paroxysmal events should have frequent spells to justify obtaining AEEG. Saravanan et al. retrospectively reviewed 54 children recording IEDs in 50%, NEE in 18.5%, resulting in a change in management in 31%, and reporting AEEG was best suited for children with daily seizures [71]. Wirrell et al. prospectively recorded 16 channel AEEG in 64 children (age 0–17 years) and found a diagnostic yield of 61% to differentiate epileptic from nonepileptic events. In addition, an overall change in diagnostic category was found in 27% of children, determined seizure and IED frequency, and was able to classify seizure type or localization in 100% [72]. Overnight AEEG performed in school-aged children found recording was feasible, non-intrusive, and well tolerated [73]. A prospective study of 100 children (aged 11 days to 16 years) referred with a range of clinical questions underwent AEEG and arrived at a diagnosis in 71% of patients (nonepileptic in 45% and epileptic in 24%) [74]. The authors recommended ascertaining event frequency through telephone checks in order to improve the likelihood of recording a typical attack. Relative to classification, Adhami et al. retrospectively reviewed charts of 50 pediatric patients including AEEG and characterized the diagnosis in 70.3% and classified the seizure types in 25% concluding diagnostic AEEG is less useful for seizure classification [75]. Foley et al. compared 18 channel computer-assisted AEEG to standard EEG in 84 children and adolescents with diagnosed (n = 49) or suspected (n = 35) epilepsy. Over 1.4 days, AEEG was found to be useful in 87% of patients with events recorded in 73% of diagnosed patients (electrographic seizures in 45%), and 86% of patients suspected of epilepsy (electrographic seizures in 17%) [76]. When compared to standard EEG, AEEG offered additional accuracy in classifying seizures in pediatric patients [76] with 84% of caregivers preferring use of AEEG monitoring [76].
Table 5.
Summary of AEEG studies done in pediatric population.*
| Study | Type of Study | No. of patients | Age Range | No. of channels | AEEG recording duration | Yield (Seizures) | Yield (IED) | Yield (NEE) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Foley et al (2000) | Retrospective | 84 | 17 months-18 yrs. | 18 | 1.4 days | 17% | 69% | NA | Computer assisted AEEG is well-tolerated, reliable and useful in 87% of children. |
| Olson (2001) | Retrospective | 167 | 4 months-18 yrs. | 16 | 1–4 days | 20.38% | NA | 68% | This study demonstrates that there is a high likelihood of recording a child’s typical seizure like events on AEEG when parents report that events occur 3 days a week or more |
| Saravannan et al (2001) | Retrospective | 54 | 1–16 yrs. | 8 | 48 hrs | 5.5% | 50% | 18.50% | AEEG helped in diagnosis in 31% of the patients. Children who are experiencing at least daily (and preferably many times a day) or sleeping episodes be considered for AEEG recording. |
| Wirrell et al (2008) | Prospective | 64 | 0–17 yrs. | 16 | 32.7 days (mean) | 16% | NA | 48% | AEEG contributed to diagnosis in 73% of children leading to a change in management in 27% of the patients. The yield in differentiating epileptic from non-epileptic events was 61%. |
| Hussain et al (2013) | Prospective | 100 | 11 days-16 yrs. | 8 | NA | NA | 24% | 45% | AEEG contributed to a clinical diagnosis in 71% of children, with diagnosis of epilepsy made in 26% of the patients. |
| Iqbal et al (2014) | Retrospective | 48 | 2–21 yrs. | NA | 1–3 days | NA | NA | NA | AEEG diagnosed seizures in two-thirds of children. When AEEG is inconclusive, video telemetry provides diagnosis in a further fifth. |
| Alix et al (2015) | Retrospective | 30 | 3–16 yrs. | NA | NA | NA | NA | NA | AEEG captured an event in 65% of the studies and video telemetry captured an event in 70% of the recordings. Combining both of them will provide diagnosis in almost all the instances. |
| Adhami et al (2015) | Retrospective | 50 | 10.25 yrs. (median age) | NA | 1–3 days | NA | NA | NA | AEEG helped in event characterization in 70.3% of patients and in seizure classification in 25% of the patients. It is valuable for event characterization and less likely to be of help in seizure classification. |
| Carlson et al^ (2018) | Prospective | 33 | 1–17 yrs. | NA | 1–3 days | 42% | NA | 9% | Ambulatory VEM is similar to inpatient VEM in capturing events and diagnostic efficacy. Despite technical difficulties encountered in ambulatory settings, it didn’t affect the EEG quality and is an accessible and cost effective alternative to inpatient VEM. |
| Nagyova et al (2018) | Retrospective | 199 | 5 months-19 yrs | 16 | 1–2 days | 42.6% | NA | NA | Pediatric AEEG was clinically useful in two-thirds of patients (64.8%). The most common reason for failure of AEEG recording is inability to capture an event. |
All included studies involved AEEG without video except Carlson et al.^; IED-Interictal epileptiform discharges; NEE-Non-epileptic events; NA-Not available; EA-Epileptiform abnormality; AEEG-Ambulatory EEG; rEEG-Routine EEG; sdEEG-Sleep deprived EEG; VEM-Video EEG monitoring.
*PubMed search of relevant articles until the year 2019 which talk about clinical utility of AEEG have been included. Articles discussing the role of AEEG in ASM withdrawal and pre-surgical assessment have been excluded.
10.2. Comparison with VEM in children
Comparing AVEEG and inpatient VEM in children 1–17 years, Carlson et al. found similar yields with a typical event recorded in 64% on AEEG, and 62% of children who underwent VEM [71]. Recording quality was also similar, but technical limitations for video capture of events occurred in 52% of patients monitored by AVEEG (i.e., camera focal point and lighting) resulting in lost diagnostic information in 15% of studies. Nonetheless, 76% elected to choose AEEG as opposed to inpatient VEM [77]. Similar findings were reported by Alix et al. who found AEEG captured events in 66%, while subsequent VEM captured an event in 90% of the recordings demonstrating to conclude that both methods of long-term EEG monitoring were effective methods of recording [78].
11. AEEG innovations
Smaller wearable AEEG models continue to be developed. Dry electrodes and electrode caps may help overcome practical limitations of electrode application and also minimize artifact [79], [80]. EEG caps have been shown to be useful in situations requiring rapid diagnosis of critical conditions such as non-convulsive status epilepticus (NCSE) [81]. Surgical placement of subcutaneous or subdermal electrodes for long-term AEEG monitoring are novel approaches that have been shown to reduce the amount of artifact [79], [82]. McLaughlin et al developed a behind-the-ear EEG device for AEEG equipped with seizure detection algorithms [83]. Wireless recorders that record and transmit EEG to the computer have been tested in emergency settings during ambulance transportation with successful results [84]. These innovations make mobile EEG applicable to rapid acquisition in the ambulatory setting and make clinical application especially relevant to home telemetry during health crises. Some clinical neurophysiology laboratories may have access to real-time monitoring of outpatient AEEG as it is being acquired but this requires a high-speed internet connection, which may not be available everywhere.
11.1. Intracranial AEEG devices
The responsive neurostimulator by Neuropace (Mountain View, CA) records chronic ambulatory intracranial EEG in outpatients. Electrocorticography has a favorable spatial resolution and a signal to noise ratio when compared to scalp recording with a similar fidelity to intracranial VEM, and safety that has been established for 9 years and longer [85], [86]. King-Stephens et al retrospectively analyzed 82 patients with bitemporal seizures who were implanted with bitemporal electrodes [81]. Seizures demonstrated cyclical lateralization with notable delays beyond 30 days to the time of first bitemporal seizure [87].
11.2. Multimodal AEEG
Several ambulatory monitoring devices use physiological signal sources other than EEG including electrocardiogram [88], electromyogram (EMG) [89], body motion [90], [91], and electrodermal activity (EDA) [92], [93]. Innovative therapeutic interventions in the form of implantable devices in concert with AEEG coupled with drug and/or electrical stimulation promise the ability to predict, terminate, and provide treatment to patients with seizures in addition to activating emergency medical systems via smartphones and computer-based alerts [93]. Improving seizure detection and prediction algorithms and artificial intelligence are increasing machine accuracy in efforts to improve seizure management.
Newer forms of “rapid EEG” are able to be rapidly applied “ambulatory” EEG systems that are capable of moving with the patient. These systems inherently use a limited number of electrodes (i.e., 10 to 16 electrodes) and may be arranged in a “hairline” montage. Some algorithms also contain alarms that sound when EEG seizures are detected [94]. The utility resides in permitting on- or off-site staff to apply and record EEG quickly with subsequent web access that allows rapid interpretation of the results. During COVID-19, rapid EEG devices may demonstrate utility in critically ill patients when access to EEG is personnel is limited and on-site ability to provide rapid feedback exists. Single-channel wireless EEG is emerging as a screen tool for ambulatory patients focused on seizure detection with alarms that signal a patient’s location, smartphone real-time access, and Cloud storage [95]
12. Conclusions
AEEG is a good out-of-hospital alternative in selected patients when inpatient VEM is not feasible or available. Instead, AEEG should be utilized as a supplement as opposed to a replacement in concert with other forms of EEG including standard EEG and VEM. With limited access to inpatient VEM due to limited community resources, financial burden, or in the case of EMU closures due to the COVID-19 pandemic, outpatient AEEG is an important tool that should not be overlooked and can provide impactful evaluations to help diagnose epilepsy and nonepileptic events in adults and children. Technology continues to improve AEEG recording devices with newer sensor designs, wireless signal transmission, seizure detection algorithms, and miniaturization of AEEG hardware. The focus on ambulatory EEG alternative to healthcare forced by the COVID-19 pandemic is expected to shift more patients from inpatient diagnostic VEM to AEEG. Future studies that are needed to determine utility of newer ambulatory EEG devices.
Disclosures
None of the authors have any financial interests to disclose.
Conflicts of interest
None of the authors have potential conflicts of interest to be disclosed.
Acknowledgment
The authors thank Alison Dowdell for editorial assistance.
References
- 1.Benbadis S.R. What type of EEG (or EEG-video) does your patient need? Expert Rev Neurother. 2015;15(5):461–464. doi: 10.1586/14737175.2015.1029918. [DOI] [PubMed] [Google Scholar]
- 2.Sethi N.K. EEG during the COVID-19 pandemic: What remains the same and what is different. Clin Neurophysiol. 2020;131(7):1462. doi: 10.1016/j.clinph.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert D.V.F., Das R.R., Acharya J.N., Lee J.W., Pollard J.R., Punia V., et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society Membership. Epilepsy Curr. 2020;20(5):316–324. doi: 10.1177/1535759720956994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French J.A., Brodie M.J., Caraballo R., Devinsky O., Ding D., Jehi L., et al. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology. 2020;94(23):1032–1037. doi: 10.1212/WNL.0000000000009632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord A.S., Lombardi N., Evans K., Deveaux D., Douglas E., Mansfield L., et al. Keeping the team together: transformation of an inpatient neurology service at an urban, multi-ethnic, safety net hospital in New York City during COVID-19. Clin Neurol Neurosurg. 2020;197:106156. doi: 10.1016/j.clineuro.2020.106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assenza G., Lanzone J., Ricci L., Boscarino M., Tombini M., Galimberti C.A., et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999–2004. doi: 10.1007/s10072-020-04546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krysl D., Beniczky S., Franceschetti S., Arzimanoglou A. The COVID-19 outbreak and approaches to performing EEG in Europe. Epileptic Disorders. 2020;22(5):548–554. doi: 10.1684/epd.2020.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: a survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liporace J., Tatum W., Morris G.L., French J. Clinical utility of sleep-deprived versus computer-assisted ambulatory 16-channel EEG in epilepsy patients: a multi-center study. Epilepsy Res. 1998;32(3):357–362. doi: 10.1016/s0920-1211(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 10.Dash D., Hernandez-Ronquillo L., Moien-Afshari F., Tellez-Zenteno J.F. Ambulatory EEG: a cost-effective alternative to inpatient video-EEG in adult patients. Epileptic Disord. 2012;14(3):290–297. doi: 10.1684/epd.2012.0529. [DOI] [PubMed] [Google Scholar]
- 11.Seneviratne U., Mohamed A., Cook M., D’Souza W. The utility of ambulatory electroencephalography in routine clinical practice: a critical review. Epilepsy Res. 2013;105(1–2):1–12. doi: 10.1016/j.eplepsyres.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 12.DiGiovine MP, Massey SL, LaFalce D, et al. Video Ambulatory EEG in Children: A Quality Improvement Study. J Clin Neurophysiol. Published online September 17, 2020. doi:10.1097/WNP.0000000000000781. [DOI] [PubMed]
- 13.Holter N.J. New method for heart studies. Science. 1961;134(3486):1214–1220. doi: 10.1126/science.134.3486.1214. [DOI] [PubMed] [Google Scholar]
- 14.Marson G, Gb M, Jb M. A miniature tape recorder for many applications. Published online 1972.
- 15.Ives J.R., Woods J.F. 4-channel 24 hour cassette recorder for long-term EEG monitoring of ambulatory patients. Electroencephalogr Clin Neurophysiol. 1975;39(1):88–92. doi: 10.1016/0013-4694(75)90131-5. [DOI] [PubMed] [Google Scholar]
- 16.Rj Q. A miniature preamplifier for ambulatory monitoring of the electroencephalogram [proceedings] J Physiol. 1978;284:23P–24P. [PubMed] [Google Scholar]
- 17.Bridgers S.L., Ebersole J.S. Ambulatory cassette EEG in clinical practice. Neurology. 1985;35(12):1767–1768. doi: 10.1212/wnl.35.12.1767. [DOI] [PubMed] [Google Scholar]
- 18.Kandler R., Ponnusamy A., Wragg C. Video ambulatory EEG: A good alternative to inpatient video telemetry? Seizure. 2017;47:66–70. doi: 10.1016/j.seizure.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Ives J.R. EEG Monitoring. Gustav Fischer New York; 1982. A completely ambulatory 16-channel cassette recording system. [Google Scholar]
- 20.Tatum WO, Ruboli G, Kaplan PW, Radhakrishnan K, Koutroumanidis M, Caboclo L, et al. EEG in Diagnosis and Monitoring Epilepsy. Clinical Neurophysiology. 2018;129(5):1056-1082.21. [DOI] [PubMed]
- 21.Schomer D.L., Ambulatory E.E.G. Telemetry: How Good Is It? J Clin Neurophysiol. 2006;23(4):294–305. doi: 10.1097/01.wnp.0000228495.29014.82. [DOI] [PubMed] [Google Scholar]
- 22.Serles W., Caramanos Z., Lindinger G., Pataraia E., Baumgartner C. Combining ictal surface-electroencephalography and seizure semiology improves patient lateralization in temporal lobe epilepsy. Epilepsia. 2000;41(12):1567–1573. doi: 10.1111/j.1499-1654.2000.001567.x. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin E., Kandler R.H., Alix J.J.P. The value of home video with ambulatory EEG: a prospective service review. Seizure. 2014;23(6):480–482. doi: 10.1016/j.seizure.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Tatum W.O., Hirsch L.J., Gelfand M.A., Acton E.K., LaFrance W.C., Duckrow R.B., et al. Assessment of the predictive value of outpatient smartphone videos for diagnosis of epileptic seizures. JAMA Neurol. 2020;77(5):593. doi: 10.1001/jamaneurol.2019.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunnhuber F., Amin D., Nguyen Y., Goyal S., Richardson M.P. Development, evaluation and implementation of video-EEG telemetry at home. Seizure. 2014;23(5):338–343. doi: 10.1016/j.seizure.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner H.J., Arima H., Mohamed A. Latency to first interictal epileptiform discharge in epilepsy with outpatient ambulatory EEG. Clin Neurophysiol. 2012;123(9):1732–1735. doi: 10.1016/j.clinph.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi M., Ahmed S.N. No further yield of ambulatory EEG for epileptiform discharges beyond 13 hours. The Neurodiagnostic Journal. 2017;57(3):211–223. doi: 10.1080/21646821.2017.1353799. [DOI] [PubMed] [Google Scholar]
- 28.Kuo J., Lee-Messer C., Le S. Optimal recording duration of ambulatory EEG (aEEG) Epilepsy Res. 2019;149:9–12. doi: 10.1016/j.eplepsyres.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Keezer M.R., Simard-Tremblay E., Veilleux M. The diagnostic accuracy of prolonged ambulatory versus routine EEG. Clin EEG Neurosci. 2016;47(2):157–161. doi: 10.1177/1550059415607108. [DOI] [PubMed] [Google Scholar]
- 30.Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav. 2009;15(1):15–21. doi: 10.1016/j.yebeh.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Lawley A., Evans S., Manfredonia F., Cavanna A. The role of outpatient ambulatory electroencephalography in the diagnosis and management of adults with epilepsy or nonepileptic attack disorder: a systematic literature review. Epilepsy Behav. 2015;53:26–30. doi: 10.1016/j.yebeh.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Faulkner H.J., Arima H., Mohamed A. The utility of prolonged outpatient ambulatory EEG. Seizure. 2012;21(7):491–495. doi: 10.1016/j.seizure.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Tatum W.O., Winters L., Gieron M., Passaro E.A., Benbadis S., Ferreira J., et al. Outpatient seizure identification: results of 502 patients using computer-assisted ambulatory EEG. J Clin Neurophysiol. 2001;18(1):14–19. doi: 10.1097/00004691-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Primiani CT, Rivera-Cruz A, Trudeau P, Sullivan L, MacIver S, Benbadis SR. The yield of ambulatory EEG-video monitoring. Clin EEG Neurosci. Published online August 18, 2020. doi:10.1177/1550059420949768. [DOI] [PubMed]
- 35.Martin R.C., Gilliam F.G., Kilgore M., Faught E., Kuzniecky R. Improved health care resource utilization following video-EEG-confirmed diagnosis of nonepileptic psychogenic seizures. Seizure. 1998;7(5):385–390. doi: 10.1016/s1059-1311(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 36.Blum D.E., Eskola J., Bortz J.J., Fisher R.S. Patient awareness of seizures. Neurology. 1996;47(1):260–264. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- 37.Oxley J., Roberts M. Clinical evaluation of 4-channel ambulatory EEG monitoring in the management of patients with epilepsy. J Neurol Neurosurg Psychiatry. 1985;48(9):930–932. doi: 10.1136/jnnp.48.9.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 39.Hernández-Ronquillo L, Thorpe L, Dash D, et al. Diagnostic accuracy of the ambulatory EEG vs. routine EEG for first single unprovoked seizures and seizure recurrence: the DX-seizure study. Front Neurol. 2020;11. [DOI] [PMC free article] [PubMed]
- 40.Berg A.T., Shinnar S. Relapse following discontinuation of antiepileptic drugs: a meta-analysis. Neurology. 1994;44(4):601. doi: 10.1212/wnl.44.4.601. [DOI] [PubMed] [Google Scholar]
- 41.Callaghan N., Garrett A., Goggin T. Withdrawal of anticonvulsant drugs in patients free of seizures for two years. N Engl J Med. 1988;318(15):942–946. doi: 10.1056/NEJM198804143181502. [DOI] [PubMed] [Google Scholar]
- 42.Koepp M.J., Farrell F., Collins J., Smith S. The prognostic value of long-term ambulatory electroencephalography in antiepileptic drug reduction in adults with learning disability and epilepsy in long-term remission. Epilepsy Behav. 2008;13(3):474–477. doi: 10.1016/j.yebeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Yang L., Jiang L., Lu R., Zhong J., Liu S., Tao E., et al. Correlation between the changes in ambulatory electroencephalography findings and epilepsy recurrence after medication withdrawal among the population in Southern China. Neurol Med Chir. 2013;53(1):12–16. doi: 10.2176/nmc.53.12. [DOI] [PubMed] [Google Scholar]
- 44.Verrotti A., Morresi S., Cutarella R., Morgese G., Chiarelli F. Predictive value of EEG monitoring during drug withdrawal in children with cryptogenic partial epilepsy. Neurophysiologie Clinique/Clin Neurophysiol. 2000;30(4):240–245. doi: 10.1016/s0987-7053(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 45.Drazkowski J. An overview of epilepsy and driving. Epilepsia. 2007;48:10–12. doi: 10.1111/j.1528-1167.2007.01392.x. [DOI] [PubMed] [Google Scholar]
- 46.Fattouch J., Di Bonaventura C., Lapenta L., Casciato S., Fanella M., Morano A., et al. Epilepsy, unawareness of seizures and driving license: the potential role of 24-hour ambulatory EEG in defining seizure freedom. Epilepsy Behav. 2012;25(1):32–35. doi: 10.1016/j.yebeh.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Tatum W.O., Worley A.V., Selenica M.L.B. Disobedience and driving in patients with epilepsy. Epilepsy Behav. 2012;23(1):30–35. doi: 10.1016/j.yebeh.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Schomer D.L., Ives J.R., Schachter S.C. The role of ambulatory EEG in the evaluation of patients for epilepsy surgery. J Clin Neurophysiol. 1999;16(2):116–129. doi: 10.1097/00004691-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Rizvi S.A., Téllez Zenteno J.F., Crawford S.L., Wu A. Outpatient ambulatory EEG as an option for epilepsy surgery evaluation instead of inpatient EEG telemetry. Epilepsy Behav Case Rep. 2013;1:39–41. doi: 10.1016/j.ebcr.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang B.S., Ives J.R., Schomer D.L., Drislane F.W. Outpatient EEG monitoring in the presurgical evaluation of patients with refractory temporal lobe epilepsy. J Clin Neurophysiol. 2002;19(2):152–156. doi: 10.1097/00004691-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Sinha S.R., Sullivan L.R., Sabau D., Orta D.S.J., Dombrowski K.E., Halford J.J., et al. American clinical neurophysiology society guideline 1: minimum technical requirements for performing clinical electroencephalography. Neurodiagnostic J. 2016;56(4):235–244. doi: 10.1080/21646821.2016.1245527. [DOI] [PubMed] [Google Scholar]
- 52.Scott N.K. Infection prevention: 2013 review and update for neurodiagnostic technologists. Neurodiagnostic J. 2013;53(4):271–288. [PubMed] [Google Scholar]
- 53.Simmons AM, Luck SJ. Protocol for reducing COVID-19 transmission risk in EEG research. Res Sq. Published online July 22, 2020. doi:10.21203/rs.3.pex-974/v2.
- 54.San-Juan D., Jiménez C.R., Camilli C.X., de la Cruz Reyes L.A., Galindo E.G.A., Burbano G.E.R., et al. Guidance for clinical neurophysiology examination throughout the COVID-19 pandemic. Latin American chapter of the IFCN task force – COVID-19. Clin Neurophysiol. 2020;131(7):1589–1598. doi: 10.1016/j.clinph.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neurophysiologists (CSCN) CS of C, Technologists (CAET) CA of E, Canada (AETC) A of ET of, et al. Practice Guidelines for Canadian Neurophysiology Laboratories During the COVID-19 Pandemic. Canadian Journal of Neurological Sciences. Published online undefined/ed:1-6. doi:10.1017/cjn.2020.184 [DOI] [PMC free article] [PubMed]
- 56.Burgess R. Handbook of clinical neurology. Elsevier; 2019. Electrical safety; pp. 67–81. [Google Scholar]
- 57.Slater J.D., Eaddy M., Butts C.M., Meltser I., Murty S. The real-world economic impact of home-based video electroencephalography: the payer perspective. J Med Econ. 2019;22(10):1030–1040. doi: 10.1080/13696998.2019.1636382. [DOI] [PubMed] [Google Scholar]
- 58.Baldin E., Hauser W.A., Buchhalter J.R., Hesdorffer D.C., Ottman R. Utility of EEG activation procedures in epilepsy: a population-based study. J Clin Neurophysiol. 2017;34(6):512–519. doi: 10.1097/WNP.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris G.L., Galezowska J., Leroy R., North R. The results of computer-assisted ambulatory 16-channel EEG. Electroencephalogr Clin Neurophysiol. 1994;91(3):229–231. doi: 10.1016/0013-4694(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 60.Tolchin B., Lee J.W., Pavlova M., Dworetzky B.A., Sarkis R.A. Diagnostic yield of ambulatory EEGs in the elderly. Clin Neurophysiol. 2017;128(7):1350–1353. doi: 10.1016/j.clinph.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 61.McCormick J. The diagnostic utility of ambulatory EEG. Epilepsy Curr. 2014:341. [Google Scholar]
- 62.Cull R.E. An assessment of 24-hour ambulatory EEG/ECG monitoring in a neurology clinic. J Neurol Neurosurg Psychiatry. 1985;48(2):107–110. doi: 10.1136/jnnp.48.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doshi H. Comparison of utility of repeat routine EEG (rEEG), sleep-depriveD EEG (SdEEG) and ambulatory EEG (aEEG) after a normal routine EEG. J Clin Neurophysiol. 2017;34(3) [Google Scholar]
- 64.Geut I., Weenink S., Knottnerus I.L.H., van Putten M.J.A.M. Detecting interictal discharges in first seizure patients: ambulatory EEG or EEG after sleep deprivation? Seizure. 2017;51:52–54. doi: 10.1016/j.seizure.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 65.Alving J., Beniczky S. Diagnostic usefulness and duration of the inpatient long-term video-EEG monitoring: findings in patients extensively investigated before the monitoring. Seizure - Eur J Epilepsy. 2009;18(7):470–473. doi: 10.1016/j.seizure.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Syed T.U., LaFrance W.C., Loddenkemper T., Benbadis S., Slater J.D., El-Atrache R., et al. Outcome of ambulatory video-EEG monitoring in a 10,000 patient nationwide cohort. Seizure. 2019;66:104–111. doi: 10.1016/j.seizure.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Zarkou S., Grade M., Hoerth M.T., Noe K.H., Sirven J.I., Drazkowski J.F. Indeterminate EMU admissions: does repeating the admission help? Epilepsy & Behavior. 2011;20(4):706–708. doi: 10.1016/j.yebeh.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 68.Fox J., Ajinkya S., Chopade P., Schmitt S. The diagnostic utility of ambulatory EEG following nondiagnostic epilepsy monitoring unit admissions. J Clin Neurophysiol. 2019;36(2):146–149. doi: 10.1097/WNP.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 69.Wirrell E.C., Grinspan Z.M., Knupp K.G., Jiang Y., Hammeed B., Mytinger J.R., et al. Care delivery for children with epilepsy during the COVID-19 pandemic: an international survey of clinicians. J Child Neurol. 2020;35(13):924–933. doi: 10.1177/0883073820940189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olson D.M. Success of ambulatory EEG in children. J Clin Neurophysiol. 2001;18(2):158–161. doi: 10.1097/00004691-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Saravanan K., Acomb B., Beirne M., Appleton R. An audit of ambulatory cassette EEG monitoring in children. Seizure-Eur J Epilepsy. 2001;10(8):579–582. doi: 10.1053/seiz.2001.0566. [DOI] [PubMed] [Google Scholar]
- 72.Wirrell E., Kozlik S., Tellez J., Wiebe S., Hamiwka L. Ambulatory electroencephalography (EEG) in children: diagnostic yield and tolerability. J Child Neurol. 2008;23(6):655–662. doi: 10.1177/0883073808314158. [DOI] [PubMed] [Google Scholar]
- 73.Marcus C.L., Traylor J., Biggs S.N., Roberts R.S., Nixon G.M., Narang I., et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10(08):913–918. doi: 10.5664/jcsm.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussain N., Gayatri N., Downey L., Seri S., Whitehouse W., Blake A. Ambulatory electroencephalogram in children: a prospective clinical audit of 100 cases. J Pediatric Neurosci. 2013;8(3):188. doi: 10.4103/1817-1745.123660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adhami S. Ambulatory EEG in children: When is it most helpful? Epilepsy Curr. 2015;1:274. [Google Scholar]
- 76.Foley C.M., Legido A., Miles D.K., Chandler D.A., Grover W.D. Long-term computer-assisted outpatient electroencephalogram monitoring in children and adolescents. J Child Neurol. 2000;15(1):49–55. doi: 10.1177/088307380001500111. [DOI] [PubMed] [Google Scholar]
- 77.Carlson S., Kandler R.H., Moorhouse D., Ponnusamy A., Mordekar S.R., Alix J.J.P. Home video telemetry in children: A comparison to inpatient video telemetry. Seizure. 2018;61:209–213. doi: 10.1016/j.seizure.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 78.Alix J.J.P., Kandler R.H., Mordekar S.R. The value of long term EEG monitoring in children: a comparison of ambulatory EEG and video telemetry. Seizure. 2014;23(8):662–665. doi: 10.1016/j.seizure.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Casson A., Yates D., Smith S., Duncan J., Rodriguez-Villegas E. Wearable electroencephalography. IEEE Eng Med Biol Mag. 2010;29(3):44–56. doi: 10.1109/MEMB.2010.936545. [DOI] [PubMed] [Google Scholar]
- 80.Fonseca C., Cunha J.P.S., Martins R.E., Ferreira V.M., de Sa J.P.M., Barbosa M.A., et al. A novel dry active electrode for EEG recording. IEEE Trans Biomed Eng. 2007;54(1):162–165. doi: 10.1109/TBME.2006.884649. [DOI] [PubMed] [Google Scholar]
- 81.McKay J.H., Feyissa A.M., Sener U., D'Souza C., Smelick C., Spaulding A., et al. Time is brain: The use of EEG electrode caps to rapidly diagnose nonconvulsive status epilepticus. J Clin Neurophysiol. 2019;36(6):460–466. doi: 10.1097/WNP.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 82.Young G.B., Ives J.R., Chapman M.G., Mirsattari S.M. A comparison of subdermal wire electrodes with collodion-applied disk electrodes in long-term EEG recordings in ICU. Clin Neurophysiol. 2006;117(6):1376–1379. doi: 10.1016/j.clinph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 83.McLaughlin B.L., Mariano L.J., Prakash S.R., et al. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2012. An electroencephalographic recording platform for real-time seizure detection; pp. 875–878. [DOI] [PubMed] [Google Scholar]
- 84.Jakab A., Kulkas A., Salpavaara T., Kauppinen P., Verho J., Heikkilä H., et al. Novel wireless electroencephalography system with a minimal preparation time for use in emergencies and prehospital care. Biomed Eng Online. 2014;13(1):60. doi: 10.1186/1475-925X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stacey W.C., Litt B. Technology insight: neuroengineering and epilepsy—designing devices for seizure control. Nat Clin Practice Neurol. 2008;4(4):190–201. doi: 10.1038/ncpneuro0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair D, Morrell M. Nine-year prospective safety and effectiveness outcomes from the long-term treatment trial of the RNS® System (S36. 005). AAN Enterprises; 2019.
- 87.King‐Stephens D., Mirro E., Weber P.B., Laxer K.D., Van Ness P.C., Salanova V., et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56(6):959–967. doi: 10.1111/epi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H., Chen W., Bao S., Lu C., Wang L., Ma J., et al. Design of an integrated wearable multi-sensor platform based on flexible materials for neonatal monitoring. IEEE Access. 2020;8:23732–23747. [Google Scholar]
- 89.Gheryani M., Salem O., Mehaoua A. 2017 IEEE 19th International Conference on E-Health Networking, Applications and Services (Healthcom) IEEE; 2017. An effective approach for epileptic seizures detection from multi-sensors integrated in an Armband; pp. 1–6. [Google Scholar]
- 90.Marquez A., Dunn M., Ciriaco J., Farahmand F. 2017 IEEE Global Humanitarian Technology Conference (GHTC) IEEE; 2017. iSeiz: A low-cost real-time seizure detection system utilizing cloud computing; pp. 1–7. [Google Scholar]
- 91.Regalia G, Caborni C, Migliorini M, Onorati F, Picard R. Real-time seizure detection performance with Embrace alert system: One year real-life setting case study. In: ; 2017. doi:10.13140/RG.2.2.28448.48648.
- 92.Poh M.-Z., Loddenkemper T., Reinsberger C., et al. Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia. 2012;53(5):e93–e97. doi: 10.1111/j.1528-1167.2012.03444.x. [DOI] [PubMed] [Google Scholar]
- 93.Ramgopal S., Thome-Souza S., Jackson M., Kadish N.E., Sánchez Fernández I., Klehm J., et al. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav. 2014;37:291–307. doi: 10.1016/j.yebeh.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Parvizi J., Gururangan K., Razavi B., Chafe C. Detecting silent seizures by their sound. Epilepsia. 2018;59(4):877–884. doi: 10.1111/epi.14043. [DOI] [PubMed] [Google Scholar]
- 95.Lin S.-K., Wang L.-C., Lin C.-Y., Chiueh H. An ultra-low power smart headband for real-time epileptic seizure detection. IEEE J Transl Eng Health Med. 2018;6:1–10. doi: 10.1109/JTEHM.2018.2861882. [DOI] [PMC free article] [PubMed] [Google Scholar]