Abstract
Background
Analysis of fluid metabolites has the potential to provide insight into the neuropathophysiology of injury in patients with traumatic brain injury (TBI).
Objective
Using a 1H nuclear magnetic resonance (NMR)-based quantitative metabolic profiling approach, this study determined (1) if urinary metabolites change during recovery in patients with mild to severe TBI; (2) whether changes in urinary metabolites correlate to injury severity; (3) whether biological pathway analysis reflects mechanisms that mediate neural damage/repair throughout TBI recovery.
Methods
Urine samples were collected within 7 days and at 6-months post-injury in male participants (n = 8) with mild-severe TBI. Samples were analyzed with NMR-based quantitative spectroscopy for metabolomic profiles and analyzed with multivariate statistical and machine learning-based analyses.
Results
Lower levels of homovanillate (R = −0.74, p ≤ 0.001), L-methionine (R = −0.78, p < 0.001), and thymine (R = −0.85, p < 0.001) negatively correlated to injury severity. Pathway analysis revealed purine metabolism to be a primary pathway (p < 0.01) impacted by TBI.
Conclusion
This study provides pilot data to support the use of urinary metabolites in clinical practice to better interpret biochemical changes underlying TBI severity and recovery. The discovery of urinary metabolites as biomarkers may assist in objective and rapid identification of TBI severity and prognosis. Thus, 1H NMR metabolomics has the potential to facilitate the adaptation of treatment programs that are personalized to the patient’s needs.
Keywords: Traumatic brain injury, Concussion, Metabolomics, Metabolic biomarkers, NMR spectroscopy, Rehabilitation, Functional recovery, Biomarkers
Highlights
-
•
NMR-based metabolomics of urine can identify metabolic fingerprints associated with functional recovery following TBI.
-
•
Metabolic profiles in urine correlate to injury severity.
-
•
Biological pathway analysis reflects mechanisms that mediate neural damage and repair processes throughout recovery.
-
•
Metabolomics provides insight into the neuropathophysiology of injury in TBI patients.
1. Introduction
Traumatic brain injury (TBI), which occurs as a result of a blow or jolt to the head, is a leading cause of death and disability worldwide (Popescu et al., 2015). In Canada, the incidence of TBI is increasing; a recent cross-sectional analysis revealed that the proportion of Canadians who reported having a TBI has more than doubled from 2005 to 2014 (Rao et al., 2017). Due to the heterogeneity of TBI presentation and underlying cause, no two TBIs are the same; therefore understanding the underlying pathophysiology of injury and recovery can be difficult. Despite advances in TBI fluid biomarker research, there is little known about the change in metabolites initially following injury and throughout recovery. The discovery of urinary metabolites that are altered following TBI would provide a valuable window into understanding the pathophysiological processes underlying TBI and subsequent recovery. Further, these biomarkers have the potential to guide future therapeutic interventions through an improved understanding of recovery processes.
Metabolomics is a powerful approach to provide quantitative assessment of endogenous small molecules within biological fluids (Beckonert et al., 2007). Nuclear magnetic resonance (NMR) spectroscopy is an amenable technique to studying metabolomics, as it permits identification of novel compounds and it requires no chemical derivatization (Emwas, 2015). NMR is capable of detecting 209 metabolites in human urine, with 108 of these being unique to NMR (Bouatra et al., 2013). Metabolomic fingerprinting has been shown to be a useful biomarker tool for a variety of neurological conditions including stroke (Naccarato et al., 2010), spinal cord injury (SCI) (Peng et al., 2014, Bykowski et al., 2021), and sport-related concussion (Wanner et al., 2021). Though recent studies hold promise, more research is needed to gain a better understanding of the pathophysiological processes involved in TBI injury severity and recovery.
The present study used a 1H NMR-based metabolomics approach to identify novel biomarkers in patients with mild-severe TBI subacutely and 6-months post-injury. It is predicted that TBI will lead to a cascade of metabolic effects detectible in urine and that alterations in the metabolomic profile will reveal biomarkers of injury severity and recovery. In line with a precision medicine approach, this study (1) determined metabolomic differences in the initial injury and 6 months post-injury urine samples in male patients with TBI; (2) based on the list of significant metabolites, revealed the underlying biochemical pathways contributing to TBI severity; (3) determined the predictive accuracy of the identified metabolites as biomarkers of TBI, and (4) indicated how these changes correlated to injury severity.
2. Materials and methods
2.1. Patient characteristics and sample collection
The present study was embedded in the UCAN Study: Understanding Neurological Recovery at the University of Calgary, Calgary, Alberta, Canada. The UCAN Study followed patients with TBI, stroke, and SCI throughout their recovery from initial injury to 6 months post-injury. Patients with TBI admitted to the Level 1 Trauma Ward at the Foothills Medical Centre, Calgary were recruited from December 2014 to September 2018 (n = 8 males, average age 45+/- 18.4 years; Table 1). Fasting morning urine samples (acquired between 6 a.m. and 9 a.m.) were collected at two different time points: within 7 days after TBI and again at 6 months post-injury. Patient’s urethral opening were wiped with an antiseptic alcohol tissue and allow the passage of urine for three seconds before filling the collection cup approximately halfway.
Table 1.
Patient characteristics (n = 8 males) indicating age, initial Glasgow Coma Scale score, TBI type, comorbidities, medication use, and both the initial and 6 months post-injury Montreal Cognitive Assessment (MoCA) and Functional Independence Measure (FIM) scores.
| Patient Code | Age | Glasgow Coma Scale Score | TBI Type | Co-Morbidities | Medication Use | Initial MoCA Score | 6 Months MoCA Score | Initial FIM Score | 6 Months FIM Score |
|---|---|---|---|---|---|---|---|---|---|
| TBI_02 | 18 | 3 | Frontal | Injury to right ear, right petrous temporal bone fracture | Tylenol | 25 | 30 | 126 | 126 |
| TBI_03 | 49 | 10 | Frontal | Depression, asthma, EtOH abuse | Ducosate, sodium, fentanyl, lorazepam, phenytoin, senokot, thiomine, tobradex, multi-vits | 23 | 26 | 113 | 122 |
| TBI_07 | 18 | 6 | SDH | None | None | 26 | 27 | 124 | 125 |
| TBI_13 | 64 | 13 | DAI- Left | Multiple face lacerations, nasal fracture, liver laceration, dental injuries | Acetaminophen, ducusate sodium, heparin, quetiapine | 20 | 27 | 112 | 121 |
| TBI_19 | 46 | 8 | SDH/SAH bifrontal | None | Trazadone, testosterone, seroquel | 25 | 26 | 113 | 124 |
| TBI_24 | 68 | 15 | SDH/SAH | Chronic lower back pain, liver laceration, bilateral shoulder injuries, torn right rotator cuff | None | 21 | 23 | 126 | 123 |
| TBI_26 | 48 | 14 | SDH/SAH | None | Tylenol | 27 | 27 | 124 | 126 |
| TBI_29 | 48 | 12 | SAH- right frontal | L2, L4, L5 fracture, sciatic nerve damage, eczema, history of smoking | Tylenol, Baclofen, Pantoprazole | 23 | 23 | 115 | 122 |
Abbreviations: SDH = subdural hematoma, SAH = subarachnoid hemorrhage, DAI = diffuse axonal injury, MoCA = Montreal Cognitive Assessment, FIM = Functional Independence Measure.
Urine samples were stored at − 80 ℃ until further processing. In this study, the samples were paired using a within-subject control design to reduce the impact of confounding factors, such as diet, lifestyle factors, body mass index, and medical history. This study was reviewed and approved by the University of Calgary Conjoint Health Research Ethics Board (CHREB) and the University of Lethbridge Human Participant Research Committee in accordance to the standards set forth by the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans.
2.2. Clinical assessments
The Glasgow Coma Scale (GCS) was used to rate the initial severity of TBI for each patient. It measures three different functions: eye opening, verbal response, and motor responses, where higher scores indicate better function (Sternbach, 2000). The final GCS score was the sum of these numbers, with the following ranges: severe (GCS less than 8), moderate (GCS 8–12), and mild (GCS 13–15). The Montreal Cognitive Assessment (MoCA) was used to screen for short term memory, visuospatial abilities, executive functions, and language (Nasreddine et al., 2005). A score of 26 and higher was considered to be normal, whereas a score below 26 indicated impairment. In addition, the Functional Independence Measure (FIM) served as a global assessment of physical, social, and psychological function (Kidd et al., 1995). It included six areas of evaluation including self-care, continence, mobility, transfers, communication, and cognition. Each item was graded on a scale from 1 to 7, where 1 indicates total dependence and 7 indicates complete independence. Both the MoCA and FIM assessments were taken initially and at 6 months post-injury to measure both injury severity and recovery.
2.3. NMR sample preparation, data acquisition, and processing
Urine samples were prepared for nuclear magnetic resonance (NMR) spectroscopy as described previously (Bykowski et al., 2021). A 700 MHz Bruker Avance III HD NMR spectrometer and a room-temperature triple resonance broad band observe (TBO) probe was used to acquire the NMR data. Three-dimensional and one-dimensional shimming experiments were conducted prior to NMR data acquisition on the urine samples to correct for any inhomogeneities in the static magnetic field. The data were acquired using a one-dimensional 1H Nuclear Overhauser Effect Spectroscopy experiment with water suppression, 128k points, and 128 scans. The data was processed using zero filling to 256k points, line broadening to 0.3 Hz, and automatic phase and baseline correction. The spectra obtained from the NMR experiment were then imported into MATLAB where they underwent dynamic adaptive binning (Anderson et al., 2011), followed by manual inspection and correction of the bins, and recursive segment-wise peak alignment (Veselkov et al., 2009). In total, 354 bins were created for this analysis.
Metabolites were identified using a combination of resources, including Chenomx 8.2 NMR Suite (Chenomx Inc., Edmonton, Alberta, Canada), the Human Metabolome Database (HMDB) (Wishart et al., 2018), and Table 3 from The Human Urine Metabolome study (Bouatra et al., 2013). Once the significant metabolites were identified, they were used to carry out metabolic pathway topology and visualization tests in MetaboAnalystR version 2.0.4 package running inside R version 3.5.3 (Pang et al., 2020). Pathway topology analysis was conducted by selecting hypergeometric test for over-representation analysis and relative betweenness centrality for the topology analysis. This was done using the list of significant metabolites (Supplementary Table 1), the Kyoto Encyclopedia of Genes and Genomes (KEGG) database for Homo sapiens as the pathway library, and the HMDB (Wishart et al., 2018, Xia and Wishart, 2010), to provide metabolite pathways that have been potentially altered.
2.4. Statistical analysis
Multivariate statistical analyses were used to determine if urinary metabolite profiles distinguished between the initial injury and 6 months post-injury samples. Prior to modeling, the data were normalized to the total metabolome (excluding the regions corresponding to water and urea), log-transformed, pareto-scaled, and mean-centered (Box and Cox, 1964, Craig et al., 2006; van den Berg et al., 2006). Bins containing significant metabolites were sorted according to the F-ranked Variable Importance Analysis based on random Variable Combination (VIAVC) analysis (Yun et al., 2015). This MATLAB based statistical programming algorithm enables identification of significant metabolites based on the Receiver Operator Characteristic (ROC) test and the subsequent Area-Under-the-Curve (AUC) analysis (Fawcett, 2005). It also employs a binary matrix resampling method, which is a more robust method for randomly sampling the data, and all multivariate supervised models were double ten-fold cross-validated (Szymanska et al., 2012). This machine learning algorithm validates the model by setting aside a randomly selected independent test set and repeating model validation multiple times until every sample has been included in the test set at least once.
An orthogonal projection to latent structures discriminant analysis (OPLS-DA) was also conducted to visualize between-group separation as a function of within-group separation (Wiklund et al., 2008). This was complemented by a Principal Components Analysis (PCA) which demonstrated the degree of separation between groups without the presence of an algorithm (unsupervised). In addition, either a paired T-tests or paired Wilcoxon-Mann-Whitney U test was used in the case of parametric or non-parametric data, respectively. A Shapiro-Wilk test was used to determine if the data for each bin was parametric or not (Goodpaster et al., 2010).
Pearson R correlations were performed between concentrations of urinary metabolites and the GCS measure (Table 2). For the correlations, the change in concentration (delta) was calculated by subtracting the initial concentration from the concentration at 6 months. The significance is assessed based on the Bonferroni corrected p-value (0.001), obtained by dividing alpha < 0.05 by the number of VIAVC F-ranked bins.
Table 2.
Pearson R values and associated p-values in n = 8 males for the change in metabolites (6 month post-injury concentration – initial concentration) correlated to the Glasgow Coma Scale score. Reported metabolites are significant based on the Bonferroni corrected threshold (alpha < 0.001).
| Metabolite | Δ Metabolites to initial injury severity (GCS) |
|---|---|
| Homovanillate L-Methionine Thymine |
R = −0.74, p = 0.001 R = −0.78, p = 0.0004 R = −0.85, p = 0.00003 |
3. Results
3.1. Overview
The current study set out to determine urinary metabolomic fingerprints of injury severity following TBI. Significantly altered metabolites were identified using univariate and multivariate statistical analyses. The clustering of the two groups as seen in the PCA and OPLS-DA revealed differences in the metabolomic profiles of TBI patients before and 6-months post-injury. The area under the curve of the ROC illustrates that the VIAVC model was able to discriminate between initial and 6-months post-injury samples with nearly perfect predictive accuracy. Furthermore, biological pathway analysis provided insights into the biochemical pathways that are altered during the recovery process. To determine the statistical relationship between metabolite levels and clinical measures, GCS scores taken at admission were correlated to changes in metabolite levels. The results obtained from the correlation revealed potential metabolites that play a role in TBI severity.
3.2. Patient characteristics
A total of 8 patients with TBI were recruited. GCS scores reveal the number of patients with severe (n = 2), moderate (n = 3), and mild (n = 3) TBI. Initial MoCA scores display two patients with normal scores and six patients with impairment. MoCA scores at 6 months revealed six patients with normal scores and two patients with impairment. These values and FIM scores are summarized in Table 1.
3.3. Metabolomic analysis
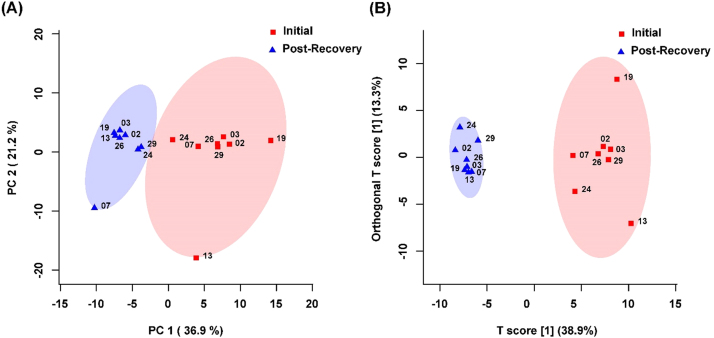
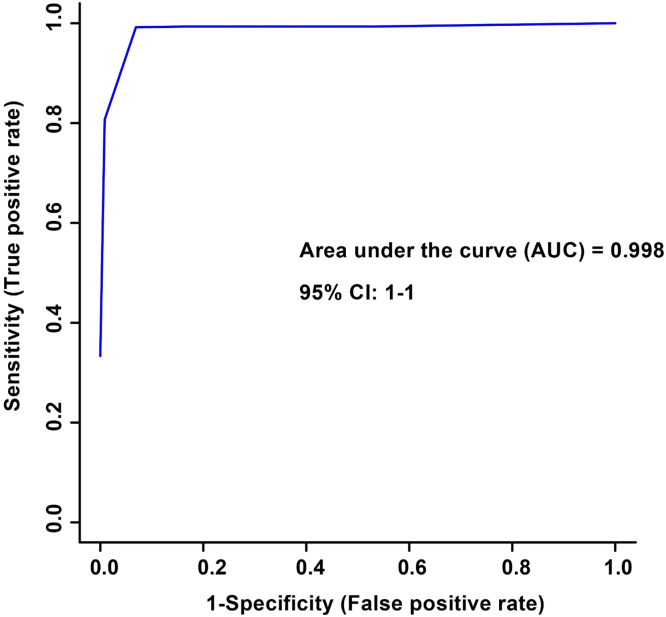
The spectral bins found to be significant by either the paired T-test/Wilcoxon Mann-Whitney test (134 bins) or the VIAVC best subset (27 bins) were used for subsequent analysis. PCA demonstrated a large degree of unsupervised group separation (Fig. 1A). The OPLS-DA plot illustrates significant group separation between the initial injury and 6 month post-injury samples (R2Y = 0.96, p < 0.001; Q2 = 0.79, p < 0.001), Fig. 1B). This supervised model indicates a change in the metabolic profiles of patients over the course of the recovery process. Metabolites that contributed the most to the group separation are provided in Supplementary Table 1. Metabolites are ranked in order of significance according to the paired T-test/Wilcoxon Mann-Whitney analysis. Receiver Operator Characteristic (ROC) curves were also generated. An area-under-the-curve equal to 0.998 was achieved, with a 95% confidence interval of 1–1 (Fig. 2).
Fig. 1.
(A) Principal Components Analysis (PCA) and (B) Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) scores plots for n = 8 males. This analysis was carried out using a list of urinary metabolites found to be statistically significant by either paired t-test/Wilcoxon Mann-Whitney or VIAVC testing. The 95% confidence interval is indicated by the shaded ellipses. In the case of the PCA scores plots the x- and y-axis show the data variance explained by principle components 1 and 2, respectively. In the case of the OPLS-DA scores plot the x- and y-axis show the predictive (between group) and orthogonal (within group) variation, respectively. The numbers represent the patient code for each individual sample. The following are the cross-validation and permutation measures for the OPLS-DA figure: R2Y = 0.959 (p < 0.0005), Q2 = 0.785 (p < 0.0005).
Fig. 2.
Receiver Operator Characteristic (ROC) curve for n = 8 males. The corresponding area under the curve (AUC) and confidence interval are indicated. The ROC curve was constructed using the metabolites determined to be significantly altered based on the VIAVC best subset which corresponds to 27 bins. The predictive accuracy was 99.7% when all bins from the best subset were included.
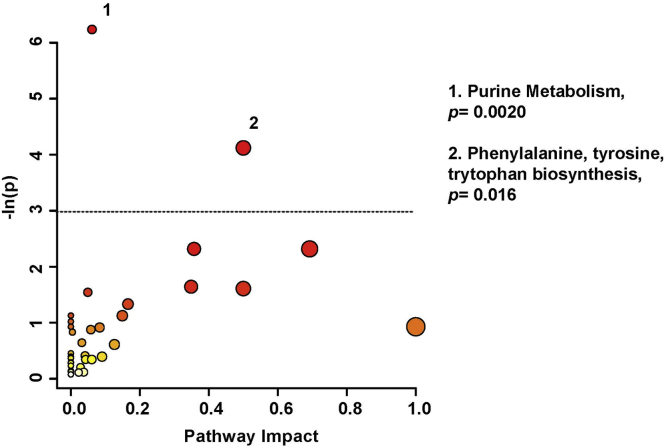
Pathway topology analysis (Fig. 3) illustrates the potential pathway impact based on changes to the patients’ urinary metabolic profiles, presented in increasing order of impact. Metabolic pathways significantly affected were purine metabolism (p < 0.01), and phenylalanine, tyrosine, & tryptophan biosynthesis (p < 0.05). Pathway analysis was based on bins significant by the VIAVC best subset, the paired T-test, and the Wilcoxon Mann-Whitney test.
Fig. 3.
Metabolic Pathway Analysis; a higher value on the y-axis indicates a lower p-value for the pathway and the x-axis provides the pathway impact, which is a measure of how affected each pathway is by the metabolites identified as significantly altered. The color of each circle is an indication if the p-value, with darker colors being more significant. The size of the circle is proportional to the pathways impact factor. Only pathways with a p-value less than 0.05, represented by the dotted line, are labeled. This analysis was carried out using the list of metabolites that were identified to be significantly altered by the paired t-test/Mann-Whitney test or the VIAVC best subset. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Metabolomic signatures correlate with injury severity
Table 2 provides the Pearson R correlation values when comparing the change in concentration of each metabolite (delta) to the GCS scores. Homovanillate (R = −0.74, p ≤ 0.001), L-methionine (R = −0.78, p < 0.001), and thymine (R = −0.85, p < 0.001) negatively correlated to injury severity and were significant based on the Bonferroni corrected threshold.
4. Discussion
The present study evaluated the feasibility of identifying metabolomic signatures in urine that correlate to initial injury severity and recovery in patients with TBI. These findings suggest that a metabolomics approach combined with machine learning analysis of urine samples is feasible, and specific metabolite profiles can be correlated to injury severity. The ROC model indicates high predictive accuracy and near-perfect classification of the initial metabolomic profiles compared to 6 months post-injury (Fig. 2). This observation demonstrates that the TBI profiles are separated with a high degree of sensitivity, and that the model provides a robust predictor of the group separation shown within the OPLS-DA analysis (Fig. 1B). Thus, there are profound differences in the types of metabolites found in urine over the course of the patients’ recovery. The following discusses the most robust metabolic pathways altered by TBI and recovery processes.
4.1. Adenosine, inosine, and deoxyinosine
Adenosine is a purine derivative and was significantly up-regulated over the course of recovery. A previous study suggested that adenosine, derived from the breakdown of ATP, mitigates ischemia and that traumatic insult coincides with a concomitant increase in brain interstitial adenosine levels (Kochanek et al., 2013). Several studies support the presence of adenosine in cerebrospinal fluid as an endogenous neuroprotective agent. For instance, adenosine may reduce excitotoxicity and moderate the risk of microvascular thrombosis (Clark et al., 1997). Adenosine has also been experimentally proven to inhibit the generation of toxic oxygen metabolites and play a role in regulating neutrophil activity during the immune response (Cronstein, 1994). To date, there is paucity of evidence in the literature supporting the presence of adenosine in urine as an additional marker of this phenomenon. Findings from this study may address this gap.
As a major degradation product of adenosine, inosine has been shown to have neuroprotective and immunomodulatory effects. The observed up-regulations of inosine in this study supports its postulated roles in suppressing macrophage, neutrophil, and lymphocyte activity and attenuating levels of pro-inflammatory mediators (Hasko et al., 2004). Deoxyinosine is a constituent of DNA and is the equivalent of inosine found in RNA, and both are the result of the deamination of adenine to hypoxanthine (Novotny et al., 2000). Deoxyinosine was up-regulated following recovery and may have a similar neuroprotective role to inosine, although this has not been explored further in literature. In combination, metabolomic signatures of adenosine and its associated purine derivatives, inosine and deoxyinosine, support their implication in pathological processes following TBI.
4.2. Xanthine, hypoxanthine, and xanthosine
Xanthine and hypoxanthine are part of the purine nucleotide degradation pathway (Yamamoto et al., 2005) and were present at increased levels following recovery. Xanthosine is a purine nucleoside whereby xanthine is bound to ribofuranose (PubChem, 2020). Thus, the presence of these three metabolites in the urine further confirms the abundance of purine metabolites following TBI.
4.3. ADP and guanosine
As breakdown products of ATP and GMP, respectively, ADP and guanosine are also part of purine nucleotide degradation (Yamamoto et al., 2005) and were both up-regulated in this study. ATP is one of the most abundant purine molecules in the body, and dephosphorylation yields ADP. Increased glycolytic flux in the wake of TBI and ATP production may underlie this finding (Jalloh et al., 2015). Similarly, Stovell et al. (2020) found a decline in ATP during the acute phase of TBI and hypothesized that this reduction stems from neurons undergoing energy failure. However, this decrease has also been found in stroke, which may suggest that ATP is a biomarker for CNS injury or other types of tissue injury more generally. Further recent experimental findings have demonstrated that increased guanosine levels attenuate neurochemical alterations following TBI (Dobrachinski et al., 2019).
Overall, the above altered metabolites revealed purine metabolism as a major pathway significantly altered during recovery from TBI and this is supported by ample previous evidence for the neuroprotective role of purines in the nervous system (Stone, 2002, Jackson et al., 2016). For example, purine derivatives can restore tissue perfusion and down-regulate inflammation (Morelli et al., 2011) and their consistent observed up-regulation throughout recovery suggests that they are fulfilling a neuroprotective role.
4.4. Homovanillate
The change in homovanillate concentration had a significant negative correlation to patients’ initial injury severity as assessed by the GCS. Homovanillate is the major metabolite of dopamine, and therefore, its levels may be reflective of the body’s dopamine levels (Felice and Kissinger, 1976). Jenkins et al. (2018) demonstrated that patients with TBI have been shown to have reduced binding to dopamine transporters in the striatum. They explained that the reduced dopamine transporter expression could be due to damaged striatal regions resulting in dopamine cell loss or simply due to low levels of dopamine itself. The present data corroborate this claim by showing that the change in homovanillate levels is negatively correlated to initial severity scores, hence homovanillate levels are higher initially than at 6 months. This observation may indicate that dopamine abnormalities become more evident in later stages of TBI pathology, as the reduction in homovanillate in the urine may reflect a concurrent reduction in circulating dopamine levels. Homovanillate therefore has the potential to serve as an indirect biomarker of reduced circulating dopamine.
4.5. L-Methionine
L-Methionine also presented with a significant negative correlation to patients’ GCS scores. This essential amino acid is implicated in angiogenesis and vascular remodeling, which are processes stimulated by TBI (Zhang et al., 2013). In addition, a decrease in blood methionine levels amongst patients with mild to severe TBI may be related to injury severity (Dash et al., 2016). Accordingly, the opposite trend would be expected in urine, where increased levels of methionine would indicate greater severity. At 6 months post-injury, the concentration of L-methionine was lower than the initial levels, suggesting attenuation of TBI pathology, and potentially indicating recovery.
4.6. Thymine
Lastly, thymine also revealed a significant negative correlation to GCS scores. Raised urinary thymine levels may indicate dihydrothymine dehydrogenase deficiency, which catabolizes thymine to beta-aminoisobutyric acid (Bakkeren et al., 1984). This by-product has been shown to down-regulate the production of proinflammatory cytokines in adipose tissue in obesity (Tanianskii et al., 2019). Because the concentration of thymine in initial samples were on average greater than at 6 months post-injury, this pathway involving thymine could potentially provide a neuroprotective role to attenuate inflammation in the later stages of brain injury. It should be noted that the reduction in pro-inflammatory cytokines orchestrated by thymine appears to oppose a pro-inflammatory response resulting from the purines. This finding may reflect the heterogeneous inflammatory response linked to a cascade of neurochemical events after TBI. After brain injury, both pro- and anti-inflammatory cytokines are released. Some processes are beneficial and promote repair, whereas other processes may be detrimental and exacerbate injury (Ziebell and Morganti-Kossmann, 2010). Furthermore, many downstream mediators released after injury have opposing roles in the acute and chronic phases. Thus, they may have pro-inflammatory actions in one phase, and anti-inflammatory actions in another (Correale and Villa, 2004). Thus, increased levels of thymine initially appear to be neuroprotective in the acute injury phase by suppressing inflammation after injury. On the other hand, lower levels of purines initially may also be neuroprotective by promoting a beneficial acute inflammatory response and reducing inflammation during the late recovery stages.
5. Conclusion
The present study identified endogenous individual urine biomarkers that serve as indicators of initial injury severity and subsequent recovery following TBI. The rigorously tested metabolites specific to males represent a suitable starting point for uncovering injury mechanisms following TBI. Future work should explore the effects of sex-dependent injury mechanisms by comparison with an equal or greater female sample size. The within-subject design ensured that the regulation of metabolite concentrations provides a robust indicator of change in TBI symptom severity. Further validation will be needed to ascertain the prognostic potential of the identified metabolites in clinical practice. For example, to determine prognostic potential, future studies could correlate initial metabolite levels to the change in clinical scores from initial injury to 6 months post-injury. Nevertheless, the present findings indicate that analysis of urinary metabolites following TBI using 1H NMR spectroscopy is feasible and specific metabolic profiles can be associated with symptom severity. By providing potential biomarkers indicative of injury severity and recovery, 1H NMR metabolomics may lead to new and refined clinical approaches for the identification of injury severity and decision making in rehabilitation strategies. Overall, metabolomics-based biomarkers could improve personalized treatment programs for patients with TBI.
6. Limitations
Given the exploratory nature of the present study in 8 male participants, we intended to investigate potential urinary biomarkers of recovery from TBI and inform future research. This proof-of-principle study focused on males because TBI afflicts disproportionately more men than women (Colantonio et al., 2010), making it difficult to recruit female study participants. Hence, future studies should consider metabolic sexual dimorphisms in follow-up work in a mixed male and female cohort. Another limitation to this study is that the patients’ diet and mobility were not controlled for. Dietary and mobility factors are likely to change during inpatient treatment and return to home.
Other limitations include the absence of assessments of renal function and catecholamine levels. Previous studies have shown a surge of catecholamines following TBI (Woolf et al., 1987) which increases the risk of renal failure (Lim and Smith, 2007). Hence, future studies that examine urine should control for markers of renal function, including assessment of glomerular filtration rate and tubular function. Furthermore, dopamine, epinephrine, and norepinephrine cannot be directly quantified using NMR-based metabolomics. However, NMR is able to identify the breakdown products of dopamine and epinephrine, homovanillate and vanillylmandelate, respectively. Accordingly, homovanillate was identified as significantly altered in this study. Future studies should attempt to quantify catecholamine levels using LC-MS to confirm that homovanillate is a suitable proxy for dopamine levels. Moreover, further research is needed to confirm the present results are biomarkers for TBI exclusively, not markers for injury in general. For example, TBI patients should be compared to orthopedic controls to verify the role of ATP/ADP in TBI. Other confounding factors, such as body mass index, acute versus chronic drug treatment, and medical history, also require appropriate controls. Nevertheless, statistical power of the present study was improved through pairwise analysis across initial and post-injury time points, which was able to minimize the impact of confounding factors on the results.
Ethics statement
This study was approved by the University of Calgary Conjoint Health Research Ethics Board (CHREB) and the University of Lethbridge Human Participant Research Committee, and carried out in accordance with the ethical standards set forth by the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans.
Funding
This research was supported by the Hotchkiss Brain Institute at the University of Calgary (C.D., T.M., G.M.), Canadian Institutes of Health Research Project Scheme #363195 (G.M.) and Natural Sciences and Engineering Research Council of Canada Discovery Grant #05628 (G. M.). E.B. was supported by a Canadian Institutes of Health Research CGS-M studentship.
CRediT authorship contribution statement
S.D., C.H. and C.D. designed the overall study. G.M. and T.M. developed and devised the metabolomics portion of the study. C.H. and C.D. recruited the participants. S.D. collected the primary clinical data. E.B. and J.P. prepared the urine samples for NMR data acquisition and performed spectral analysis. E.B., J.P., G.M., T.M., and C.D. contributed to writing the manuscript. All authors have approved the final version and publication of this manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors would like to thank Eric Paxman, Regan King, Janis Yajure and Mark Piitz for assistance with recruitment and collections of clinical measures and urine. We thank Michael Opyr for assistance with data processing scripts in MATLAB. The authors would also like to thank the University of Lethbridge for use of the Magnetic Resonance Facility (Lethbridge, AB, Canada), which provided access to and training on the 700 MHz NMR instrument.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ibneur.2021.10.003.
Contributor Information
Chantel T. Debert, Email: chantel.debert@ahs.ca.
Tony Montina, Email: tony.montina@uleth.ca.
Gerlinde A.S. Metz, Email: gerlinde.metz@uleth.ca.
Appendix A. Supplementary material
Supplementary material
.
References
- Anderson P., Mahle D., Doom T., Reo N., DelRaso N., Raymer M. Dynamic adaptive binning: an improved quantification technique for NMR spectroscopic data. Metabolomics. 2011;7(2):179–190. [Google Scholar]
- Bakkeren J., De Abreu R., Sengers R., Gabreels F., Maas J., Renier W. Elevated urine, blood and cerebrospinal fluid levels of uracil and thymine in a child with dihydrothymine dehydrogenase deficiency. Clin. Chim. Acta. 1984;140(3):247–256. doi: 10.1016/0009-8981(84)90206-7. [DOI] [PubMed] [Google Scholar]
- Beckonert O., Keun H., Ebbels T., Bundy J., Holmes E., Lindon J.C., Nicholson J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma. serum and tissue extracts. Nat. Protoc. 2007;2(11):2692–2703. doi: 10.1038/nprot.2007.376. 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- Bouatra S., Aziat F., Mandal R., Guo A.C., Wilson M.R., Knox C., Wishart D.S. The human urine metabolome. PLoS One. 2013;8(9):1–28. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box G.E.P., Cox D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B, Methodol. 1964;26(2):211–252. doi: 10.1111/j.2517-6161.1964.tb00553.x. [DOI] [Google Scholar]
- Bykowski E.A., Petersson J.N., Dukelow S., Ho C., Debert C.T., Montina T., Metz G.A.S. Urinary biomarkers indicative of recovery from spinal cord injury: a pilot study. IBRO Neurosci. Rep. 2021;10:178–185. doi: 10.1016/j.ibneur.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Carcillo J., Kochanek P.M., Obrist W.D., Jackson E.K., Mi Z., Marion D.W. Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans. Neurosurgery. 1997;41(6):1284–1292. doi: 10.1097/0000613-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Colantonio A., Harris J.E., Ratcliff G., Chase S., Ellis K. Gender differences in self reported long term outcomes following moderate to severe traumatic brain injury. BMC Neurol. 2010;10:102. doi: 10.1186/1471-2377-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J., Villa A. The neuroprotective role of inflammation in nervous system injuries. J. Neurol. 2004;251(11):1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Craig A., Cloarec O., Holmes E., Nicholson J.K., Lindon J.C. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal. Chem. 2006;78(7):2262–2267. doi: 10.1021/ac0519312. [DOI] [PubMed] [Google Scholar]
- Cronstein B. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994;76(1):5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Dash P., Hergenroeder G., Jeter C., Choi H., Kobori N., Moore A. Traumatic brain injury alters methionine metabolism: implications for pathophysiology. Front. Syst. Neurosci. 2016;10:36. doi: 10.3389/fnsys.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrachinski F., Gerbatin R.R., Sartori G., Golombieski R.M., Antoniazzi A., Nogueira C.W., Soares F.A. Guanosine attenuates behavioral deficits after traumatic brain injury by modulation of adenosinergic receptors. Mol. Neurobiol. 2019;56(5):3145–3158. doi: 10.1007/s12035-018-1296-1. [DOI] [PubMed] [Google Scholar]
- Emwas A.H. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015;1277:161–193. doi: 10.1007/978-1-4939-2377-9_13. 10.1007/978-1-4939-2377-9_13. [DOI] [PubMed] [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Pattern Recogn. Lett. 2005;72(8):861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- Felice L., Kissinger P. Determination of homovanillic acid in urine by liquid chromatography with electrochemical detection. Anal. Chem. 1976;48(6):794–795. doi: 10.1021/ac60370a018. [DOI] [PubMed] [Google Scholar]
- Goodpaster A.M., Romick-Rosendale L.E., Kennedy M.A. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal. Biochem. 2010;401(1):134–143. doi: 10.1016/j.ab.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hasko G., Sitkovsky M., Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004;25(3):152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Jackson E., Boison D., Kochanek P. Purines: forgotten mediators in traumatic brain injury. J. Neurochem. 2016;137(2):142–153. doi: 10.1111/jnc.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalloh I., Carpenter K.L., Helmy A., Carpenter T.A., Menon D.K., Hutchinson P.J. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological change. Metab. Brain Dis. 2015;30(3):615–632. doi: 10.1007/s11011-014-9628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins P., Simoni S., Bourke N., Fleminger J., Scott G., Towey D.J., Sharp D.J. Dopaminergic abnormalities following traumatic brain inury. Brain. 2018;141(3):797–810. doi: 10.1093/brain/awx357. [DOI] [PubMed] [Google Scholar]
- Kidd D., Stewart G., Baldry J., Johnson J., Rossiter D., Petruckevitch A., Thompson A.J. The functional independence measure: a comparative validity and reliability study. Disabil. Rehabil. 1995;17(1):10–14. doi: 10.3109/09638289509166622. [DOI] [PubMed] [Google Scholar]
- Kochanek P.M., Verrier J.D., Wagner A.K., Jackson E.K. In: Adenosine. Masino S., Boison D., editors. Springer; New York, NY: 2013. The many roles of adenosine in traumatic brain injury; pp. 307–322. [DOI] [Google Scholar]
- Lim H.B., Smith M. Blackwell Publishing; 2007. Systemic Complications after Head Injury: A Clinical Review. [DOI] [PubMed] [Google Scholar]
- Morelli M., Carta A.R., Kachroo A., Schwarzschild M.A. Pathophysiological roles for purines: adenosine, caffeine and urate. Prog. Brain Res. 2011;183:183–208. doi: 10.1016/S0079-6123(10)83010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccarato M., Pizzuti D., Petrosino S., Simonetto M., Ferigo L., Grandi F.C., Pizzolato G., Di Marzo V. Possible anandamide and palmitoylethanolamide involvement in human stroke. Lipids Health Dis. 2010;9:47. doi: 10.1186/1476-511X-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z., Phillips N., Bedirian V., Charbonneau S., Whitehead V., Collin I., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Novotny L., Abdel-Hamid M., Hamza H. Inosine and 2′-deoxyinosine and their synthetic analogues: lipophilicity in the relation to their retention in reversed-phase liquid chromatography and the stability characteristics. J. Pharm. Biomed. Anal. 2000;24(1):125–132. doi: 10.1016/s0731-7085(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Pang Z., Chong J., Li S., Xia J. MetaboAnalystR 3.0: toward an optimized workflow for global metabolomics. Metabolites. 2020;10(5):186. doi: 10.3390/metabo10050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Zeng J., Cai B., Yang H., Cohen M.J., Chen W., Sun M.W., Lu C.D., Jiang H. Establishment of quantitative severity evaluation model for spinal cord injury by metabolomic fingerprinting. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu C., Anghelescu A., Onose G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J. Med. Life. 2015;8(3):272–277. 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4556905/#__ffn_sectitle〉 [PMC free article] [PubMed] [Google Scholar]
- Rao D., McFaull S., Thompson W., Jayaraman G. Trends in self-reported traumatic brain injury among Canadians, 2005-2014: a repeated cross-sectional analysis. CMAJ Open. 2017;5(2):E301–E307. doi: 10.9778/cmajo.20160115. [DOI] [Google Scholar]
- Sternbach G. The Glasgow Coma Scale. J. Emerg. Med. 2000;19(1):67–71. doi: 10.1016/s0736-4679(00)00182-7. [DOI] [PubMed] [Google Scholar]
- Stone T.W. Purines and neuroprotection. Adv. Exp. Med. Biol. 2002;513:249–280. doi: 10.1007/978-1-4615-0123-7.9. [DOI] [PubMed] [Google Scholar]
- Stovell M.G., Mada M.O., Carpenter T.A., Yan J., Guilfoyle M.R., Jalloh I., Carpenter K.L. Phosphorus spectroscopy in acute TBI demonstrates metabolic changes that relate to outcome in the presence of normal structural MRI. J. Cereb. Blood Flow Metab. 2020;40(1):67–84. doi: 10.1177/0271678×18799176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska E., Saccenti E., Smilde A.K., Westerhuis J.A. Double-check: validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics. 2012;8:S3–S16. doi: 10.1007/s11306-011-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanianskii D., Jarzebska N., Birkenfeld A., O’Sullivan J., Rodionov R. Beta-aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients. 2019;11(3):524. doi: 10.3390/nu11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R.A., Hoefsloot H.C.J., Westerhuis J.A., Smilde A.K., van der Werf M.J. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genom. 2006;7(1):142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselkov K., Lindon J., Ebbels T.M., Crockford D., Volynkin V.V., Holmes E., Nicholson J.K. Recursive segment-wise peak alignment of biological 1H NMR spectra for improved metabolic biomarker recovery. Anal. Chem. 2009;81(1):56–66. doi: 10.1021/ac8011544. [DOI] [PubMed] [Google Scholar]
- Wanner Z.R., Southam C.G., Boora N.S., Paxman E.J., Benson B., Montina T., Metz G.A.S., Debert C. Diagnosing sports-related concussion using urine metabolomics: a 1H NMR-based analysis. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.645829. 10.3389/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund S., Johannsson E., Sjostrom L., Mellerowicz E.J., Edlund U., Shockcor J.P., Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008;80(1):115–122. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R., Sajed T., Johnson D., Li C., Karu N., Sayeeda Z., Lo E., Assempour N., Berjanskii M., Singhal S., Arndt D., Liang Y., Badran H., Grant J., Serra-Cayuela A., Liu Y., Mandal R., Neveu V., Pon A., Knox C., Wilson M., Manach C., Scalbert A. HMBD 4.0- The human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D609–D617. doi: 10.1093/nar/gkx.1080. 29140435-D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf P.D., Hamill R.W., Lee L.A., Cox C., McDonald J.V. The predictive value of catecholamines in assessing outcome in traumatic brain injury. J. Neurosurg. 1987;66(6):875–882. doi: 10.3171/jns.1987.66.6.0875. [DOI] [PubMed] [Google Scholar]
- Xanthosine, 2020. Retrieved from PubChem, National Library of Medicine. 〈https://pubchem.ncbi.nlm.nih.gov/compound/xanthosine〉.
- Xia J., Wishart D.S. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26:2342–2344. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Moriwaki Y., Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid) Clin. Chim. Acta. 2005;356(1–2):35–57. doi: 10.1016/j.cccn.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Yun Y., Liang F., Deng B., Lai G., Goncalves C., Lu H., Liang Y. Informative metabolites identification by variable importance analysis based on random variable combination. Metabolomics. 2015;11(6):1539–1551. [Google Scholar]
- Zhang Y., Xiong Y., Mahmood A., Zhang Z., Chopp M. In: Lo E., Lok J., Ning M., Whalen M., editors. Vol. 5. Springer Series in Translational Stroke Research; 2013. Angiogenesis and functional recovery after traumatic brain injury; pp. 141–156. (Vascular Mechanisms in CNS Trauma). [Google Scholar]
- Ziebell J.M., Morganti-Kossmann M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7(1):22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material