Abstract
Cortisol, the end product of the hypothalamic–pituitary–adrenal axis, regulates cognitive function and emotion processing. Cushing's disease, which is characterized by a unique excess of cortisol upon clinical diagnosis, serve as an excellent in vivo “hyperexpression” model to investigate the neurobiological mechanisms of cortisol in the human brain. Previous studies have shown the association between cortisol and functional connectivity within an a priori brain network. However, the whole-brain connectivity pattern that accompanies endogenous cortisol variation is still unclear, as are its associated genetic underpinnings. Here, using resting-state functional magnetic resonance imaging in 112 subjects (60 patients with Cushing's disease and 52 healthy subjects), we performed a voxel-level brain-wide association analysis to investigate the functional connectivity pattern associated with a wide variation in cortisol levels at 8 a.m. The results showed that the regions associated with cortisol as of 8 a.m. were primarily distributed in brain functional hubs involved in self-referential processing, such as the medial prefrontal cortex, anterior and posterior cingulate cortex, and caudate. We also found that regions in the middle temporal, inferior parietal and ventrolateral prefrontal cortex, which is important for social communication tasks, and in the visual and supplementary motor cortex, which is involved in primary sensorimotor perception, were adversely affected by excessive cortisol. The connectivity between these regions was also significantly correlated with neuropsychiatric profiles, such anxiety and depression. Finally, combined neuroimaging and transcriptome analysis showed that functional cortisol-sensitive brain variations were significantly coupled to regional expression of glucocorticoid and mineralocorticoid receptors. These findings reveal cortisol-biased functional signatures in the human brain and shed light on the transcriptional regulation constraints on the cortisol-related brain network.
Keywords: Cortisol, Functional magnetic resonance imaging, Gene expression, Connectivity, Cognition
1. Introduction
Cortisol, the end product of the hypothalamus–pituitary–adrenal (HPA) axis, plays a crucial role in modulating aspects of cognitive function and emotional processing in the brain, including working memory and social stress (Harkness et al., 2011; Merz et al., 2010; Oei et al., 2006). In a nonhuman primate model, cortisol administration impaired the inhibitory control of behavior by affecting medial prefrontal cortex activity (Lyons et al., 2000). In a rat model, cortisol exposure also caused dendritic reorganization of pyramidal neurons in the prefrontal cortex (Wellman, 2001). In human brains, task-based functional magnetic resonance imaging (fMRI) studies have shown that cortisol acts on brain regions involved in reward-related circuitry, such as the basolateral amygdala, striatum and anterior cingulate cortex and medial temporal cortex (Kinner et al., 2016; Montoya et al., 2014). Stress-induced increased cortisol levels also elicit greater brain activation during working memory maintenance, which involves brain regions such as the posterior cingulate cortex, prefrontal cortex and hippocampus (Abercrombie et al., 2011; Qin et al., 2009; Weerda et al., 2010). Moreover, the severity of neuropsychological symptoms in major psychiatric disorders moderate the relationship between cortisol levels and emotional brain activation in the regions of the anterior cingulate cortex, hippocampus, insula and parietal cortex (Abercrombie et al., 2011; Quidé et al., 2020). This evidence from task-based fMRI studies has provided extensive insight into the modulating effects of cortisol on neural activity in the human brain. However, these cortisol-induced alterations in brain patterns are dependent on the particular task paradigms used, which can limit investigations to the comprehensive effects of cortisol on neural activity in the whole brain. Moreover, the effects of cortisol on the brain cannot be explained by only localized activation in a small number of regions. In contrast, this cortisol-induced alteration could also be associated with the functional coupling between brain regions, which is consistent with the prevailing “disconnection” hypothesis of psychopathology studies (Catani and ffytche, 2005; Sha et al., 2019; Xia et al., 2018).
Therefore, task-free functional connectivity (FC) in resting-state fMRI is a powerful tool that can be used to investigate the functional correlation of regional spontaneous brain activity by examining low-frequency fluctuations of blood oxygenation level-dependent fMRI signals. This approach not only has the potential to overcome limitations of task-dependent brain patterns but also has been widely used to characterize functional network architecture at the whole-brain level in healthy and diseased cohorts (Biswal et al., 2010; Fornito et al., 2015; Power et al., 2011; van den Heuvel and Sporns, 2019). Accordingly, intrinsic FC has illustrated the organization of large-scale cerebral functional networks in the human brain, such as the default-mode network (DMN, e.g., the posterior cingulate, medial prefrontal and lateral temporal cortex), visual network (VN, e.g., the medial and lateral visual cortices) and limbic network (LN, e.g., the orbitofrontal cortex and amygdala)(Power et al., 2011; Yeo et al., 2011). Regarding cortisol secretion, previous studies have shown that endogenous cortisol levels are associated with FC between the medial prefrontal cortex and amygdala in healthy subjects, defined as the DMN and LN, and involved in self-referential processing and emotional regulation (Bressler and Menon, 2010; Menon, 2011). Another study showed a negative association between cortisol variations and FC within regions of the DMN, such as the medial prefrontal cortex and posterior cingulate cortex, which serve as potential brain functional signatures of stress-related vulnerability (W. Zhang et al., 2019). However, these seed-based findings are hypothesis-driven and identify cortisol-sensitive brain FC by measuring the correlation between seed regions defined by a priori knowledge, which could lead to potential bias and a lack of global and independent views on the neural mechanisms of cortisol in response to excess cortisol. Therefore, it is necessary to extensively investigate the FC signatures associated with cortisol variations at the whole-brain level. However, the cortisol-sensitive whole-brain FC pattern is still not clear.
Molecular studies have shown that cortisol acts in the brain to support adaptation to stress by directly modulating the activity of two types of nuclear receptors, mineralocorticoid receptors and glucocorticoid receptors (de Kloet et al., 2005). Mineralocorticoid receptors mediate the onset of the stress response, whereas glucocorticoid receptors are involved in termination of the stress response. The mineralocorticoid receptor known as nuclear receptor subfamily 3, group C, member 2 (NR3C2) is a protein encoded by the NR3C2 gene in humans. Mineralocorticoid receptors are widely expressed in the cingulate cortex, amygdala and inferior frontal cortex (de Kloet, 2013; Klok et al., 2011). The glucocorticoid receptor known as nuclear receptor subfamily 3, group C, member 1 (NR3C1) is the receptor to which cortisol binds. Glucocorticoid receptors are highly dense in the occipital cortex and parietal cortex in the human brain (Joels, 2018; Perlman et al., 2007). Thus, whether cortisol-associated FC architecture is constrained by the spatial distribution of mineralocorticoid and glucocorticoid receptors remains unknown. Thus, identifying the cortisol-sensitive functional network signature and its associated gene transcription profiles could contribute to revealing the modulatory effects of cortisol on the neural network and genetic underpinnings in the human brain.
Cushing's disease (CD) is a common neuroendocrine disorder that is characterized by the unique excess of endogenous cortisol upon clinical diagnosis. Therefore, this disease naturally serves as a valuable in vivo “hyperexpression” model to elucidate the effect of cortisol on brain function (Y. Zhang et al., 2021). This study is the first to apply a novel data-driven approach, a voxel-wise brain-wide association study (BWAS)(Cheng et al., 2015; Cheng et al., 2016; Gong et al., 2018), to extensively explore the FC signatures associated with cortisol variation across a wide range at the whole-brain level and how they are linked to clinical variables. This approach is in line with genome-wide association studies, in which genotype information is pooled to identify significant genetic variations associated with specific traits (Hirschhorn and Daly, 2005). Second, Neurosynth (Yarkoni et al., 2011), a platform for the large-scale synthesis of task fMRI data, was used to explore the cognitive processes associated with cortisol-affected functional brain systems. Finally, we explored the topological relationship between cortisol-associated FC architecture and the expression profiles of a glucocorticoid receptor (NR3C1) and a mineralocorticoid receptor (NR3C2) from the Allen Human Brain Atlas (Hawrylycz et al., 2012).
2. Material and methods
2.1. Participants
Sixty CD patients and 54 healthy controls (HCs) were initially recruited by The First Medical Center of Chinese People's Liberation Army General Hospital between May 2017 and November 2020. The study was approved by the local Ethics Committee of the Chinese PLA General Hospital, and written informed consent was obtained from each participant. According to the latest clinical practice guidelines, CD was diagnosed by experienced endocrinologists with the combination of a low-dose dexamethasone suppression test, a high-dose dexamethasone suppression test, inferior petrosal sinus sampling, and dynamic gadolinium-enhanced MRI of the pituitary gland, which was used to increase the sensitivity of adrenocorticotropic hormone (ACTH)-secreting pituitary tumors to MRI detection (Supplementary Information). Patient diagnosis of CD was also confirmed by postsurgical pathology. In this study, we included only patients (1) who met the criteria for CD diagnosis, (2) who were 20–60 years of age and (3) for whom disease onset was more than one month prior. We excluded CD patients with large pituitary tumors, which oppress important blood vessels and optic nerves and disrupt brain morphometry. HCs matched for age, sex, and education with the patients were recruited from the local community and interviewed by experienced psychiatrists. All participants were right-handed and had normal vision and auditory sensation. Exclusion criteria for HCs and individuals with CD included a current or history of any neurological disorder (e.g., central nervous system infection, multiple sclerosis, toxic metabolic disease, Parkinson's disease, epilepsy, intracranial tumor and hypothyroidism, etc.), neurodevelopmental disorder or mental disorder; a medical condition that affects neurovascular function (e.g., hypertension); current substance abuse or dependence; MRI contraindications; pregnant or breastfeeding women and individuals who had experienced a major life event within a year (e.g., divorce, unemployment or the death of a loved one).Two HCs were excluded because no sample was collected for cortisol measurements, leaving 60 CD cases and 52 HCs.
2.2. Image acquisition
All participants were scanned the day of serum cortisol sampling. Images were acquired on a 3.0-T MR system (Discovery MR750, General Electric) with an 8-channel head coil. High-resolution structural 3D T1-weighted images were collected using a sagittal fast spoiled gradient-echo sequence with the following parameters: repetition time = 6.7 ms, echo time = 2.9 ms, flip angle = 7°, field of view = 250 × 250 mm2, number of slices = 192, and voxel size = 1 × 1 × 1 mm3 with no gap. Functional images were acquired using an echo-planar imaging sequence with the following parameters: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, thickness/gap = 3.5 mm/0.5 mm, slices = 36, field of view = 224 × 224 mm2, voxel size = 3.5 × 3.5 × 3.5 mm3, and total volume = 240. Soft earplugs were used to attenuate scanner noise, and head motion was restrained with foam padding. During functional scanning, all participants were asked to keep their eyes closed to avoid thinking anything in particular and to avoid falling asleep, as confirmed by a post-scan questionnaire.
2.3. Neuroendocrine and neuropsychological assessment
Each participant completed MRI scans and neuroendocrine and neuropsychological assessments within three days. All CD patients underwent biochemical evaluation to assess the functional status of the HPA axis. For each subject, we quantified the levels of 24-h urinary free cortisol (24-h UFC, nmol/24 h), serum cortisol (nmol/L) and plasma adrenocorticotropic hormone ACTH (pmol/L) at 8 a.m. Cortisol and ACTH levels were analyzed by chemiluminescence immunoassay. Specifically, ACTH was measured using an Immulite 2000 Analyzer (Siemens Healthcare Diagnostics Inc., LA, USA). Cortisol was detected with an ADVIA Centaur Analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA).
The majority of subjects underwent a comprehensive neuropsychological assessment using the Self-Rating Depression Scale (Zung, 1972), Self-Rating Anxiety Scale (Zung, 1971), Mini-Mental State Examination (Tombaugh, 2005) and Montreal Cognitive Assessment (Hachinski et al., 2006). Moreover, the health-related quality of life and neuropsychiatric symptoms of the CD patients were evaluated with the Cushing's Quality-of-Life questionnaire (Nelson et al., 2013) and the Chinese version of the neuropsychiatric inventory (Leung et al., 2001), respectively.
2.4. Resting-state fMRI data preprocessing
The resting-state fMRI data were preprocessed using SPM12 and Data Processing Assistant for Resting-State fMRI (DPABI, http://www.restfmri.net/forum/DPARSF). The first 10 vol of the functional images were discarded to avoid initial steady-state problems. Then, functional images were spatially realigned to the first image for motion correction and corrected for slice acquisition temporal delay. Any images indicating head motion at 2-mm translation or a 2° rotation in any direction were excluded. Subsequently, functional images were coregistered to each participant's segmented gray matter T1 image, spatially normalized to Montreal Neurological Institute (MNI) space and resampled to 4-mm isotropic voxels to reduce computational complexity and burden. Next, the global signal, white matter signal, cerebrospinal fluid signal and 24-motion head motion parameters (i.e., 6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items) were regressed from the data. Linear detrending and bandpass filtering (0.01–0.08 Hz) were carried out to reduce the effects of low-frequency drift and high-frequency physiological noise. Finally, to better control for head motion, we corrected head motion using a “scrubbing” procedure on the preprocessed images (Power et al., 2014). Briefly, volumes with a framewise displacement exceeding a threshold of 0.5 mm and their adjacent volumes (1 back and 2 forward) were replaced with data interpolated from the nearest neighbor within the fMRI image from each subject.
2.5. Brain-wide association study
To identify cortisol-related FC in the whole brain, we performed a voxel-wise BWAS based on serum cortisol at 8 a.m. using a published, available BWAS toolbox (Gong et al., 2018) (https://github.com/weikanggong/BWAS).
Step 1: Calculate the voxel-wise FC
In this study, each preprocessed fMRI image included 19,158 voxels, which were obtained from the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002). This was generated by extracting overlapping voxels in the automated anatomical labeling template and the thresholded prior gray matter probability map (>0.2) provided by SPM12. For each voxel, the time series was extracted to perform Pearson correlation with other voxels in the whole brain, followed by Fisher's r-to-z transformation. Next, the relationship between the FC of each pair of voxels and cortisol dosage was examined by a general linear model across subjects while controlling for age and sex.
Step 2: Chart the association map in relation to the cortisol variation
This procedure was also implemented in the BWAS toolbox. In this approach, all FCs with a p-value smaller than a certain cluster-defining threshold (z-score) of 5 (corresponding to a p-threshold of 3 × 10−7), which was considered a valid threshold for whole-brain analysis in the original study (Gong et al., 2018), were identified. A measure for the association (MA) between voxel i and the cortisol level was then defined as MA = Nα, where Nα was the number of cortisol-associated FCs (z-score>5) between voxel i and every other voxel in the brain. Accordingly, an MA map was created, in which each statistic of each voxel (MA value) represents the number of cortisol-affected links (z-score>5).
Step 3: Identification of FC clusters linked to cortisol dosage
As a parallel analysis, which is independent of analysis in step 2 at the voxel level, we also tested the association of cortisol level with FC at the cluster level, i.e. testing whether FC clusters formed by spatially connected regions were larger than those expected by chance, with a familywise error rate–corrected p-value<0.05 for each cluster as determined by random field theory. This procedure was also implemented with the BWAS toolbox. Each identified FC cluster included a number of voxelwise FC variables linked to two regions of interest. To further control the false positive rate of FC clusters that we identified, we used a relatively strict threshold to assess the cluster size of regions of interest with more than 10 voxels, following the methods of previous studies (Cheng et al., 2016; Hart et al., 2012; Konrad et al., 2006; Wittmann et al., 2005).
Additionally, we tested the effect of group on the significant associations between the FC clusters and cortisol variation. Specifically, for each identified FC cluster, time series of two regions were extracted to calculate Pearson correlation coefficient, which was used to examine the effect of group by a generalized linear regression model with the following formula across all participants:
2.6. Correlation with neuropsychological variables
To investigate the clinical outcomes of cortisol-associated FC clusters, we performed partial correlation analysis of neuropsychological scores. Specifically, for each subject, we first calculated the FC coefficients of significant FC clusters identified by the BWAS analysis described above. For each FC cluster, the time series were extracted in each region of interest by averaging the blood oxygenation level–dependent signals of all significant voxels within that region. The FC coefficient was evaluated between each pair of regions of interest by calculating the Pearson correlation coefficient and then applying Fisher's z transformation. Then, for each subject, the mean FC coefficient was obtained by averaging all these FC coefficients. Finally, we performed a separate partial correlation analysis between mean FC scores and each neuropsychological variable across available subjects; this analysis included data from the Self-Rating Depression Scale, the Self-Rating Anxiety Scale, the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Cushing's Quality-of-Life questionnaire and the Chinese version of the Neuropsychiatric Inventory. The statistical threshold for correlation analysis was set to a Bonferroni-corrected threshold of p < 0.05/6 after permuting the clinical score labels assigned as each set of variables (n = 10,000). In addition, we performed separate correlation analyses of Self-Rating Depression Scale, Self-Rating Anxiety Scale, Mini-Mental State Examination and Montreal Cognitive Assessment scores within the CD and HC groups.
2.7. Functional annotation of cortisol-affected brain regions
To refine the potential cognitive functions of cortisol-related brain regions, we used the available online platform Neurosynth (https://neurosynth.org/) to access meta-analytic brain maps from over 11,000 human neuroimaging studies. Neurosynth provides meta-analytic maps related to 50 topics encapsulating different aspects of cognitive function. Each topic illustrates a cluster of semantically related words that tend to occur together in a shared cognitive architecture. Neurosynth uses these weighted term clusters to create topic-specific association maps in which the statistical value in each voxel (z-score) represents the likelihood that the voxel is preferentially activated by the topic in question over all other topics. Then, we performed Pearson correlation analysis between our identified MA map and the Neurosynth association statistics for each of 50 topic maps across the nonzero voxels. A stringent Bonferroni-corrected threshold of p < 0.05/50 was used for multiple testing correction. We also emphasized topics with significant correlation coefficients >0.1 or < -0.1 in the figure.
2.8. Gene expression restraints on the cortisol brain network
Next, we investigated the topographical relationship between the cortisol-affected brain functional network and glucocorticoid and mineralocorticoid systems. Given that NR3C1 and NR3C2 are well-known genes that regulate the expression of glucocorticoid and mineralocorticoid receptors (Fan et al., 1989; Hollenberg et al., 1985), we used the NR3C1 and NR3C2 expression profiles to evaluate the extent of the relationship between cortisol receptor systems and functional brain architecture in the cerebral cortex. First, we separately extracted the gene expression profiles of NR3C1 and NR3C2 from the whole-brain transcriptome atlas created by Whitaker et al. (2016). For each gene, this atlas provides averaged expression values in each of 306 parcellations across cortical postmortem samples obtained from 6 adult brains. The microarray data for six donors in this atlas are available from the Allen Institute for Brain Science (http://human.brain-map.org/static/download)(Hawrylycz et al., 2012). Second, mean MA values were extracted from the nonzero voxels of the abovementioned MA map across the same 306 regions. Finally, Pearson correlation analysis was used to separately examine the relationships between the cortisol-associated MA map and NR3C1 and NR3C2 expression profiles across 306 brain regions. In addition, using a leave-one-donor-out approach and ComBat(Johnson et al., 2007), the gene expression atlas analyzed in the present study was confirmed to be robust in terms of the effects of interindividual differences and batch- and donor-induced artifacts (Romero-Garcia et al., 2019). This evidence supports the validity of extracting the spatial NR3C1 and NR3C2 expression patterns from the atlas used in the present study.
2.9. Sensitivity analysis
First, given that cortisol levels vary across different collection approaches and sources, we next performed cross-body fluid validation analysis to test the robustness of the significant regions sensitive to serum cortisol in the BWAS. Accordingly, we reperformed the BWAS using cortisol derived from urine collected for 24 h. Second, because the biological significance of global signals in relation to brain activity remains unclear and the procedure for removing global signals is still under debate (Murphy and Fox, 2017), we reprocessed the fMRI data without global signal removal. Then, we reanalyzed the MA map using fMRI data with global signals to assess the reproducibility of the cortisol-related brain functional pattern. Third, given that the level of cortisol secretion fluctuates from morning to night and because the variation in the time gap between MRI scanning and participant cortisol sampling (8 a.m.) might have implications for the BWAS results, we examined the time-dependent BWAS data and treated the time gap as the dependent variable, with age and sex as covariates.
3. Results
3.1. Demographic and endocrinological results
We ultimately included 60 CD patients and 52 HCs in this study. No significant between-group difference (p > 0.05) was found in the demographic characteristics of the subjects (Table 1). Compared with HCs, CD patients had significantly lower Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment scores and higher Self-rating Depression Scale and Self-Rating Anxiety Scale scores (Table 1). As expected, CD patients had significantly higher levels of serum cortisol, 24-h UFC and plasma ACTH (p < 0.001, Table 1) than HCs.
Table 1.
Demographic and clinical characteristics of the participants.
| CD patients (N = 60) | HCs (N = 52) | Statistic value | P-value | |
|---|---|---|---|---|
| Age (years) | 37.77 ± 10.55 | 34.87 ± 10.75 | 1.44 | 0.15 |
| Sex (female/male) | 54/6 | 49/3 | 0.67 | 0.41 |
| Education (years) | 11.25 ± 4.15 | 11.83 ± 3.15 | −0.82 | 0.41 |
| Illness duration (months) | 43.84 ± 52.01 | – | – | – |
| Neuropsychological Tests | ||||
| MMSE | 28.02 ± 2.39 (N = 53) | 29.25 ± 0.93 (N = 51) | −3.45 | 8.22E-4 |
| MoCA | 22.57 ± 4.19 (N = 53) | 27.67 ± 2.03 (N = 51) | −7.86 | 4.10E-12 |
| SDS | 39.93 ± 9.66 (N = 54) | 27.16 ± 4.47 (N = 51) | 8.61 | 8.97E-14 |
| SAS | 37.81 ± 8.08 (N = 54) | 26.98 ± 4.54 (N = 51) | 8.40 | 2.55E-13 |
| CNPI | 12.78 ± 10.09 (N = 54) | – | – | – |
| Cushing QOL | 36.81 ± 8.46 (N = 54) | – | – | – |
| Endocrinological Tests | ||||
| Serum cortisol at 8am (nmol/L) | 725.90 ± 276.36 | 359.82 ± 106.79 | 8.98 | 8.26E-15 |
| ACTH at 8am (pmol/L) | 20.89 ± 16.05 | 5.00 ± 3.09 | 7.02 | 1.88E-10 |
| 24-h UFC (nmol/24 h) | 2349.70 ± 1534.17 (N = 51) | 258.33 ± 118.50 (N = 45) | 9.11 | 1.40E-14 |
Note: All values are expressed as the mean ± standard deviation. Abbreviations: CD: Cushing's disease patients; HC: healthy controls; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; SDS: Self-Rating Depression Scale; SAS: Self-Rating Anxiety Scale; CNPI: Chinese version of the Neuropsychiatric Inventory; Cushing QOL: Cushing Quality-of-Life Scale; ACTH: adrenocorticotropic hormone; 24-h UFC: 24-h urinary free cortisol.
3.2. BWAS of cortisol
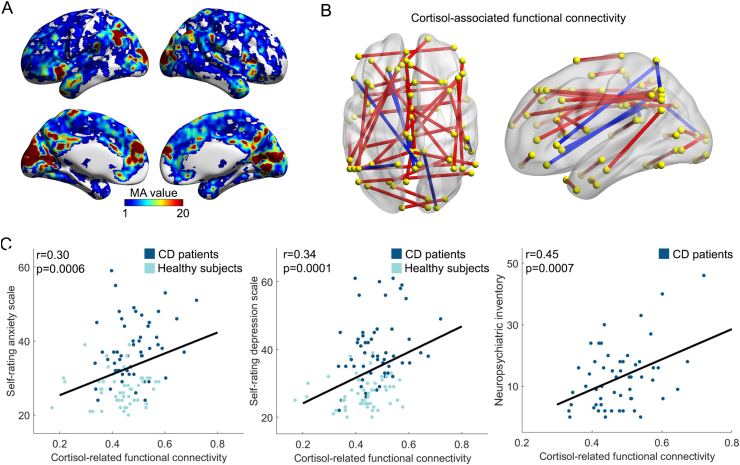
Frequency histograms of the cortisol levels for CD patients and HCs are provided in Fig. S1. Fig. 1A shows the spatial distribution of all voxels in the brain that had cortisol-associated FCs (z-score>5) across subjects. The regions with a higher number of FCs (z-score>5) were primarily distributed in the bilateral visual cortex, inferior parietal cortex, medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, putamen and insula (Table S1). The regions most strongly associated with cortisol variation were located in the visual cortex (Table S1; peak coordinate (x, y, z) = -16, −76, 12, MA value = 194). Having aligned the cortisol association map with a previously well-validated large-scale functional brain network layout (Menon, 2011; Yeo et al., 2011) in which regions coupled with spontaneous neural signals at rest were clustered with each other to form an independent system, we observed that the regions with strong links to cortisol variations were primarily embedded in several neurocognitive networks. For example, the anterior cingulate cortex and insula constitute the salience network (SN), the medial prefrontal and posterior cingulate cortex are the core elements that make up the DMN, the inferior parietal cortex has been assigned to the frontoparietal network (FPN), and the visual cortex is localized to the VN (Fig. 1).
Fig. 1.
Cortisol-associated FC signatures and their relation with neuropsychological variables.
(A) Voxels with cortisol-associated FC (z-score>5). The value on a voxel represents the measure of association (MA), i.e., the number of z-score of functional connectivity related to this voxel greater than 5. No further statistical threshold was applied on the MA map. The regions associated with cortisol variations were prominently located in the bilateral insula, visual, medial prefrontal, inferior parietal and posterior cingulate cortices. (B) A schematic diagram showing 39 FC
clusters associated with cortisol levels across subjects. The yellow dots represent regions of interest, which are positioned at the peak coordinates of our identified voxel region of interest-based connectivity clusters (Table S2). The lines between yellow dots represent FC between brain regions. The red and blue lines represent positive and negative correlations between the FC and cortisol level across subjects, respectively. (C) Significant correlations between the cortisol-associated FC clusters and anxiety (left), depression (middle) and neuropsychiatric scores (right). X-axis represents the average of all the significant FC clusters identified in the BWAS analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
After applying the cluster-level inference approach, we identified 39 significant FC clusters. We found that the regions with significant cortisol-associated FC clusters were consistent with the MA map. Most of the significant FC clusters showed positive correlations with cortisol; these regions were primarily distributed in the DMN (e.g., medial prefrontal, posterior cingulate, superior temporal cortex), FPN (e.g., inferior parietal and dorsolateral prefrontal cortex), SN (e.g., insula and anterior cingulate cortex) and VN (e.g., visual cortex) (Fig. 1B and Table S2). We also observed six negative correlations with FC between regions of the DMN, including the medial prefrontal cortex, posterior cingulate cortex, and ventrolateral prefrontal cortex, as well as FC between the posterior cingulate cortex and the visual cortex (Fig. 1B and Table S2).
We next examined the effect of group on the identified FC clusters using the generalized linear regression model. The results showed that neither cortisol nor cortisol–group interaction effects of each FC cluster were significant after correction for multiple testing, suggesting that a mixture of the group difference and cortisol variance contributed to significant associations (Table S3).
3.3. Correlations of the cortisol-related functional brain signatures with clinical scores
To quantify the clinical relevance of cortisol-associated FC clusters, we performed partial correlation analysis with neuropsychological variables. We found that the mean correlation coefficient of identified cortisol-related FC clusters had significant positive correlations with anxiety (r = 0.30, p = 0.0006) and depression scores (r = 0.34, p = 0.0001) across all participants, as well as with Neuropsychiatric Inventory scores (r = 0.45, p = 0.0007) in CD patients (Fig. 1C and Table S4).
In addition, we did not find any significant correlations between the mean correlation coefficients of identified cortisol-related FC clusters and Self-Rating Depression Scale, Self-Rating Anxiety Scale, Mini-Mental State Examination or Montreal Cognitive Assessment scores within either the CD group or the HC group (Table S5).
3.4. Cognitive function of the cortisol functional network
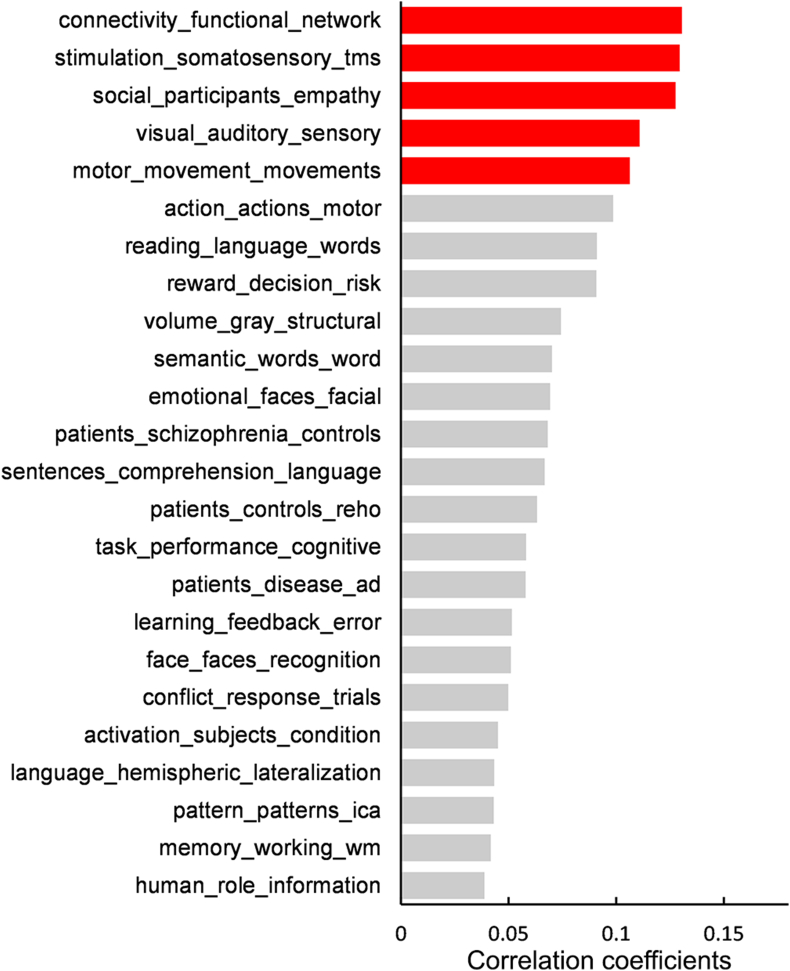
To explore behavioral characterization of the cortisol-associated brain circuit, we screened for specific cognitive topics with associated brain activation maps that overlapped with cortisol-biased MA map. After Bonferroni correction of p < 0.05, we found 24 significant cognitive domains that were associated with the MA map derived from the BWAS (Fig. 2). With reference to the spatial profile of cognitive activation enrichment, we further emphasized five cognitive topics with |r|>0.1, such as functional brain hubs, social communication and primary sensory perception (Fig. 2, Table S6 and Fig. S2). Specifically, the top topic was “connectivity_functional_network” (that is, the functional hub; r = 0.13, p = 2.05 × 10−33), which was likely contributed to by the regions medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex and temporoparietal junction. These hub regions also constitute the core element of the DMN, which is involved in self-reference processing and thinking about others (Jenkins, 2019). The supplementary motor and visual areas identified in our cortisol-sensitive brain map could be activated by tasks involving sensorimotor and visual perception, annotated by the terms “stimulation_somatosensory_tms” (r = −0.13, p = 6.08 × 10−19), “motor_movement_movements” (r = −0.11, p = 1.73 × 10−16) and “visual_auditory_sensory” (r = 0.11, p = 7.24 × 10−20). The “social_participants_empathy” item (r = 0.13, p = 2.98 × 10−28) might account for the cognitive functions implicated by the inferior parietal cortex, ventrolateral prefrontal cortex and middle temporal cortex.
Fig. 2.
Neurosynth cognitive topics correlated with the cortisol network.
The histogram illustrated details for Neurosynth cognitive topics showing significant spatial correlations with the MA map derived from the BWAS. Each topic is named after three text terms with top loadings. The red columns represent cognitive domains with a correlation coefficient >0.1 or < -0.1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Transcriptional signature underlying cortisol-sensitive functional architecture
To investigate gene expression underlying cortisol-related brain functional variations, we analyzed the relationship between the transcription levels of a glucocorticoid receptor (NR3C1) and the MA map and between a mineralocorticoid receptor (NR3C2) and the MA map. Fig. 3A and B illustrate that the spatial expression patterns of these two genes varied across the 306 brain parcellations defined by Whitaker et al. (2016), with higher expression in the visual, parietal, dorsolateral prefrontal and posterior cingulate cortices. We also observed significantly positive correlations between both the NR3C1 and NR3C2 expression patterns and regions significantly associated with cortisol across the brain (NR3C1: r = 0.41, p = 8.71 × 10−14; NR3C2: r = 0.45, p = 2.36 × 10−16; Fig. 3C and D), suggesting that the cortisol-associated functional network is restricted by the spatial distribution of cortisol receptor systems.
Fig. 3.
Transcriptional signatures capture cortisol-associated brain functional variations.
Spatial distribution of NR3C1 (A) and NR3C2 (B) expression; red and blue indicate the brain regions with high and low gene expression, respectively. The results showed positive correlations between expression of both NR3C1 (C) and NR3C2 (D) and the cortisol-associated MA map derived from the BWAS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Sensitivity analysis
First, given that cortisol levels vary across different bodily fluids, we next explored whether the cortisol-sensitive neural network identified by our BWAS was affected by different sources from which cortisol was acquired. We reanalyzed the BWAS data using urinary cortisol measured by 24-h UFC. The MA map illustrated that most of the cortisol-associated regions were consistent with our main findings, such as the anterior and posterior cingulate cortices, the insula and the inferior parietal cortex (Fig. S3). Second, given that global signal regression could have an impact on the brain FC pattern, we then reperformed the BWAS using the fMRI data without global signal removal. The results illustrated that the regions affected by cortisol were still evident in the MA map, indicating that the cortisol-sensitive brain regions were independent of those involved in preprocessing analytic strategies (Fig. S4). Third, because the distinct time gap between MRI scanning and cortisol sampling (8 a.m.) across participants might have implications for the BWAS results, we next carried out a time-dependent BWAS. We found significant associations, primarily in regions important for primary sensory perception, particularly the visual cortex and sensorimotor cortex (Fig. S5).
4. Discussion
Using a cross-sectional cohort of CD patients and HCs, the present study comprehensively investigated cortisol-related functional relevance in human brains and linked the neural signature with underlying patterns of glucocorticoid receptor gene expression. Specifically, (i) the BWAS identified cortisol-related functional brain regions distributed in the medial prefrontal, inferior parietal, posterior cingulate and visual cortices; (ii) the specific cortisol-related functional brain network plays a critical role in cognitive functions, such as self-reference, social communication and sensory perception; and (iii) the cortisol-related functional network was shown to be restricted by the global distribution of mineralocorticoid and glucocorticoid receptor gene expression profiles. These findings add to our understanding of the chronic effect of cortisol on human brain function.
Growing evidence suggests that functional interactions within and between a few major large-scale brain networks play crucial roles in cognitive functions, including the effects of the cortisol stress response on the human brain (Menon, 2011; Sha et al., 2019; W. Zhang et al., 2020). Among these brain systems, the DMN is anchored in the medial prefrontal cortex and posterior cingulate cortex and is thought to support various self-related cognitive activities and mental simulations; additionally, the SN consists of the anterior cingulate cortex and insula and is important for orienting toward salient internal events and external inputs (Anticevic et al., 2012; Uddin, 2015). Interestingly, the main brain regions of the cortisol-related functional network identified by BWAS in this study are core components of these neurocognitive networks, which might be integral to linking cortisol variations to cognitive deficits of psychiatric disorders.
Previous neuroimaging studies have identified cortisol-related brain circuits focused on three major domains. First, by employing acute psychological stressors, such as tasks involving uncontrollability and/or social-evaluative threats, several studies have found that stress-induced cortisol increases are associated with connectivity changes in regions from the SN and DMN, including the anterior cingulate cortex, amygdala, posterior cingulate cortex, and precuneus (W. Zhang et al., 2019; W. Zhang et al., 2020). Second, studies of exogenous cortisol using oral or intravenous administration found that cortisol elevation is related to altered activity in the prefrontal cortex, anterior cingulate cortex, amygdala and hippocampus (Harrewijn et al., 2020; Nakataki et al., 2017). Finally, normative variations in endogenous cortisol levels are associated with functional coupling between the amygdala and multiple brain regions involved in sensory processing and integration, emotion regulation, and DMN activity (Harrewijn et al., 2020; Veer et al., 2012). Our current study is in line with prior findings and compatible with theories of neural resource reallocations, that is, overloading more information between the SN and DMN to respond to excessive cortisol (Maron-Katz et al., 2016; W. Zhang et al., 2019). Moreover, cortisol is the end product of the HPA axis and plays an important role in the stress response (Maron-Katz et al., 2016). This opens up the possibility that these specific cortisol-related network responses could serve as a promising biomarker for future investigations on individual neural resilience and vulnerability to acute or chronic stress exposure.
Regarding the behavioral annotations of cortisol-sensitive brain patterns, our identified cortisol-related functional network likely relates to self-processing, social communication and primary sensory perception. Based on the BWAS results, we observed that the regions affected by cortisol variations prominently overlapped with well-validated functional hubs (Buckner et al., 2009), including the medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex and temporoparietal junction, which account for the majority of the DMN (Yeo et al., 2011). Recent studies in humans have also revealed that individual differences in stress-induced cortisol levels are linked to the DMN and implicated in self-processing and homeostasis (W. Zhang et al., 2019). Moreover, alterations in the functional hub regions have consistently been implicated in various psychiatric conditions and particularly in stress-related disorders (Admon et al., 2013; Yan et al., 2019). We also reported that the “social_participants_empathy” item accounts for behavioral profiles implicated by the regions of the inferior parietal cortex, lateral prefrontal cortex and middle temporal cortex. An increasing number of studies have associated the influence of stress with human social and emotional behavior, hinting toward a prosocial tendency in terms of a “tend-and-befriend” response following cortisol administration (Margittai et al., 2018; Taylor, 2006; von Dawans et al., 2021). Under psychosocial stress, the salivary cortisol level was thought to be related to stronger frontoparietal activation after the administration of androstadienone in a mental arithmetic task containing social-evaluative threat and uncontrollability elements (Chung et al., 2016). Moreover, recent studies reported that acute cortisol was associated with attenuated activity of the inferior parietal cortex and middle temporal cortex during emotion processing in patients with negative mood disorders (Peters et al., 2016). Based on the above evidence, we speculate that frontoparietal regions is selectively vulnerable to the adaptive protection of circuits supporting psychosocial perception and emotion regulation in response to the aggregated cortisol burden.
It is worth noting that some brain regions in the VN are also involved in cortisol-related functional brain circuits. The observation of occipital activity or FC of the VN in stress-induced cortisol effects has recently been reported in neuroimaging studies (Henckens et al., 2009; Soares et al., 2013; van Marle et al., 2009). Moreover, functional alterations in this system have also been found in patients who have been exposed to prolonged hypercortisolism. For example, resting-state functional imaging studies have demonstrated increased connectivity between the visual cortex and the DMN in CD patients (van der Werff et al., 2015). This result is also in accordance with our previous studies, which showed decreased cerebral blood flow in the visual cortex in CD (Y. Zhang et al., 2021). It is possible that the effects of cortisol on the brain are mediated, at least partially, by underlying sensory perception, such as visual and other sensory modalities, including primary sensorimotor perception (Hoenen et al., 2017; Weckesser et al., 2016).
The molecular mechanisms through which cortisol exerts its effects on the human brain remain unclear. To bridge this gap, we initially provided evidence that cortisol-related functional networks were spatially associated with the transcription levels of two specific cortisol receptor genes, i.e., NR3C2 (encoding a mineralocorticoid receptor) and NR3C1 (encoding a glucocorticoid receptor). On a cellular level, cortisol enters the brain and binds mineralocorticoid receptors with high affinity and glucocorticoid receptors with lower affinity (Keller et al., 2017). Both receptors act as ligand-dependent transcription factors that translocate to the nucleus and regulate cortisol-related genes, including FK506-binding protein 5, proopiomelanocortin and corticotropin-releasing factor (Gatta et al., 2021; McGowan et al., 2009). As mineralocorticoid and glucocorticoid receptors mediate cortisol signaling, variations in the epigenetic regulation of NR3C1 and NR3C2 expression may introduce changes in cortisol signaling dynamics and subsequent changes in stress/reward-responsive brain networks, such as the DMN, SN and sensory system. This triangular cortisol-gene-brain framework might underlie the pathogenesis of stress-related disorders.
In the sensitivity analysis, even though the brain pattern in relation to urinary cortisol measured by 24-h UFC was generally consistent with that estimated by serum cortisol at 8 a.m., the effect appeared to be prominently diminished. This could be the result of varying sampling times and sampling sources. It is well known that cortisol in humans typically exhibits a diurnal rhythm, characterized by high levels upon waking, a rise immediately after awakening, a gradual decline through the day, and a lower level at night before sleep (Weitzman et al., 1971). However, 24-h UFC refers to the total cortisol collected from urine over a complete day (24 h), which takes the cortisol fluctuation at different times into account. We therefore speculated that the lower degree of cortisol variation in this measurement could weaken its effect in the sensitivity analysis. In other words, the intrinsic functional architecture of the human brain could be very sensitive to subtle changes in cortisol secretion. Therefore, future works of cortisol acquired with frequent samplings should be required to validate our findings and understand the neurobiological mechanisms underlying the effects of cortisol on brain functional organization. In addition, we also need to note that the association of the visual cortex was not replicated by the BWAS of 24-h UFC. Combining the results from the time-dependent BWAS, we observed significant associations between brain connectivity and the time lag of fMRI scanning and cortisol sampling (8 a.m.), primarily in regions of the visual cortex. These results imply that the association of cortisol with FC of the visual cortex could be driven by fluctuations in brain activity over time following cortisol sampling. Nevertheless, the results from 24-h UFC still showed a whole-brain connectivity pattern associated with time-independent cortisol levels.
Several limitations of the present study should be mentioned. First, despite the scarceness of CD patients, we collected a relatively large number of samples. However, the absolute sample size remained limited. Future studies with larger sample sizes, including samples from both CD patients and HCs, are warranted to replicate the findings of our study. Second, we explored the effects of a wide range of cortisol concentrations on human brain function by drawing on a cohort of CD within in vivo hyperexpression of cortisol, together with HCs, which had a typical range in cortisol variation. However, the associations of brain connectivity with cortisol could be attributable to a mixture of group differences and cortisol variance. Future work is needed to validate and generalize our findings in a general population (e.g., UK Biobank) or postoperative CD patients with a wide range of cortisol levels. Third, even though bandpass filtering (0.01–0.08 Hz) was used to reduce the effects of physiological noise (e.g., heart rate and respiration noise) in this study, this noise still could not be thoroughly removed due to the low sampling time of the fMRI scan (TR = 2 s). Future work will be needed to validate our findings by using more state-of-art technology that addresses physiological noise. Fourth, head motion issues have been debated since the term FC was proposed and onwards. Although this study controlled for head motion effects to some extent by commonly used strategies (e.g., scrubbing procedure), we also observed that head motion parameters of some of the fMRI data, particularly for individuals with CD, might not fully meet the more stringent frame-to-frame displacement (FD) threshold (e.g., 0.2 mm), which was reported by a recent methodological study (Power et al., 2015). Thus, more fMRI data with better-controlled head motion effects is required to confirm whether the cortisol-brain association is independent of artifact effects arising from the fMRI scan. Fifth, Neurosynth was used to explore the cognitive functions implicated by the brain regions sensitive to cortisol variation. However, we should note that these functional annotations were only based on the fMRI meta-analyzed data, rather than the independent neuroimaging scan of CD patients. Thus, in the future, a large group of CD patients with extensive task-based functional MRI scans and well-documented neurocognitive scales should be considered to quantify the functional relevance in relation to the cortisol-affected brain regions. Sixth, the difference in psychological symptoms in cases and controls could have biased the brain representation of cortisol. Given that the number of participants accomplishing the complete psychological assessment in our study was underpowered to sensitively detect the association signal with cortisol variation, future analyses with more psychological and physiological variables in a large sample size will be required to help clarify the effects of neuropsychological health on the association between cortisol and human brain function. Last, the Allen Human Brain Atlas is the only source of high-resolution gene expression data across the human brain to date. This atlas is, however, limited by its sampling of only six donors with six left hemispheres and two right hemispheres (Parker et al., 2020). Future studies using a larger dataset of whole-brain gene expression profiles could better address this issue.
In conclusion, the findings of our current study suggest that the cortisol-affected regions were mainly distributed in brain functional hubs (e.g., the medial prefrontal, inferior parietal, visual and posterior cingulate cortices). These cortisol-affected regions are involved in reward, social communication and primary sensory perception. Finally, the cortisol-related brain network is specifically and significantly coupled to regional expression of the cortisol receptor system. These findings suggest that cortisol can modulate the reconfiguration of large-scale brain-wide networks involving neurocognitive functions by restricting the spatial expression of cortisol receptors.
CRediT authorship contribution statement
Yanyang Zhang: Study conception and design, acquisition of data, statistical analysis, Formal analysis and interpretation of data, Tcritical revision, drafting of manuscript, All authors approved the final version of the article. Tao Zhou: acquisition of data, critical revision, All authors approved the final version of the article. Shiyu Feng: acquisition of data, critical revision, All authors approved the final version of the article. Xinyun Liu: critical revision, All authors approved the final version of the article. Fuyu Wang: critical revision, All authors approved the final version of the article. Zhiqiang Sha: Study conception and design, Formal analysis and interpretation of data, drafting of manuscript, critical revision, All authors approved the final version of the article. Xinguang Yu: Study conception and design, Formal analysis and interpretation of data, critical revision, All authors approved the final version of the article.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 82001798 and No. 81871087), Military Young Scholar Medical Research Fund of Chinese PLA General Hospital (No. QNF19071) and Medical Big Data and Artificial Intelligence Development Fund of Chinese PLA General Hospital (No. 2019MBD-039).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100414.
Contributor Information
Yanyang Zhang, Email: sjwkzyy@163.com.
Zhiqiang Sha, Email: sha.zhiqiang@163.com.
Declarations of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Submission declaration and verification
This work has not been published previously. The submission is approved by all authors. If accepted, it will not be published elsewhere in the same form, in English or in any other language.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abercrombie H.C., Jahn A.L., Davidson R.J., Kern S., Kirschbaum C., Halverson J. Cortisol's effects on hippocampal activation in depressed patients are related to alterations in memory formation. J. Psychiatr. Res. 2011;45(1):15–23. doi: 10.1016/j.jpsychires.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Milad M.R., Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cognit. Sci. 2013;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cognit. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.N., Gohel S., Kelly C., Smith S.M., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cognit. Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., ffytche D.H. The rises and falls of disconnection syndromes. Brain. 2005;128(Pt 10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Gu H., Zhang J., Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain. 2015;138(Pt 5):1382–1393. doi: 10.1093/brain/awv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Qiu J., Liu W., Tang Y., Huang C.C., Feng J. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139(Pt 12):3296–3309. doi: 10.1093/brain/aww255. [DOI] [PubMed] [Google Scholar]
- Chung K.C., Springer I., Kogler L., Turetsky B., Freiherr J., Derntl B. The influence of androstadienone during psychosocial stress is modulated by gender, trait anxiety and subjective stress: an fMRI study. Psychoneuroendocrinology. 2016;68:126–139. doi: 10.1016/j.psyneuen.2016.02.026. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R. Functional profile of the binary brain corticosteroid receptor system: mediating, multitasking, coordinating, integrating. Eur. J. Pharmacol. 2013;719(1–3):53–62. doi: 10.1016/j.ejphar.2013.04.053. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Fan Y.S., Eddy R.L., Byers M.G., Haley L.L., Henry W.M., Nowak N.J., Shows T.B. The human mineralocorticoid receptor gene (MLR) is located on chromosome 4 at q31.2. Cytogenet. Cell Genet. 1989;52(1–2):83–84. doi: 10.1159/000132846. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16(3):159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Gatta E., Grayson D.R., Auta J., Saudagar V., Dong E., Chen Y., Guidotti A. Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1) Mol. Psychiatr. 2021;26(3):1029–1041. doi: 10.1038/s41380-019-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W., Wan L., Lu W., Ma L., Cheng F., Cheng W., Feng J. Statistical testing and power analysis for brain-wide association study. Med. Image Anal. 2018;47:15–30. doi: 10.1016/j.media.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E., Leblanc G.G. National Institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Harkness K.L., Stewart J.G., Wynne-Edwards K.E. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36(2):173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Harrewijn A., Vidal-Ribas P., Clore-Gronenborn K., Jackson S.M., Pisano S., Pine D.S., Stringaris A. Associations between brain activity and endogenous and exogenous cortisol - a systematic review. Psychoneuroendocrinology. 2020;120:104775. doi: 10.1016/j.psyneuen.2020.104775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Radua J., Mataix-Cols D., Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neurosci. Biobehav. Rev. 2012;36(10):2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A., Jones A.R. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J., Hermans E.J., Pu Z., Joëls M., Fernández G. Stressed memories: how acute stress affects memory formation in humans. J. Neurosci. 2009;29(32):10111–10119. doi: 10.1523/jneurosci.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn J.N., Daly M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 2005;6(2):95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- Hoenen M., Wolf O.T., Pause B.M. The impact of stress on odor perception. Perception. 2017;46(3–4):366–376. doi: 10.1177/0301006616688707. [DOI] [PubMed] [Google Scholar]
- Hollenberg S.M., Weinberger C., Ong E.S., Cerelli G., Oro A., Lebo R.…Evans R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.C. Rethinking cognitive load: a default-mode network perspective. Trends Cognit. Sci. 2019;23(7):531–533. doi: 10.1016/j.tics.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroids and the brain. J. Endocrinol. 2018;238(3):R121–R130. doi: 10.1530/JOE-18-0226. [DOI] [PubMed] [Google Scholar]
- Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G.M., Jr., Schatzberg A.F. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatr. 2017;22(4):527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner V.L., Wolf O.T., Merz C.J. Cortisol alters reward processing in the human brain. Horm. Behav. 2016;84:75–83. doi: 10.1016/j.yhbeh.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Klok M.D., Alt S.R., Irurzun Lafitte A.J., Turner J.D., Lakke E.A., Huitinga I., Derijk R.H. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J. Psychiatr. Res. 2011;45(7):871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Hanisch C., Fink G.R., Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol. Psychiatr. 2006;59(7):643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Leung V.P., Lam L.C., Chiu H.F., Cummings J.L., Chen Q.L. Validation study of the Chinese version of the neuropsychiatric inventory (CNPI) Int. J. Geriatr. Psychiatr. 2001;16(8):789–793. doi: 10.1002/gps.427. [DOI] [PubMed] [Google Scholar]
- Lyons D.M., Lopez J.M., Yang C., Schatzberg A.F. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J. Neurosci. 2000;20(20):7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margittai Z., van Wingerden M., Schnitzler A., Joels M., Kalenscher T. Dissociable roles of glucocorticoid and noradrenergic activation on social discounting. Psychoneuroendocrinology. 2018;90:22–28. doi: 10.1016/j.psyneuen.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Maron-Katz A., Vaisvaser S., Lin T., Hendler T., Shamir R. A large-scale perspective on stress-induced alterations in resting-state networks. Sci. Rep. 2016;6:21503. doi: 10.1038/srep21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P.O., Sasaki A., D'Alessio A.C., Dymov S., Labonte B., Szyf M., Meaney M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Tabbert K., Schweckendiek J., Klucken T., Vaitl D., Stark R., Wolf O.T. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35(1):33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Montoya E.R., Bos P.A., Terburg D., Rosenberger L.A., van Honk J. Cortisol administration induces global down-regulation of the brain's reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakataki M., Soravia L.M., Schwab S., Horn H., Dierks T., Strik W., Morishima Y. Glucocorticoid administration improves aberrant fear-processing networks in spider phobia. Neuropsychopharmacology. 2017;42(2):485–494. doi: 10.1038/npp.2016.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.M., Forsythe A., McLeod L., Pulgar S., Maldonado M., Coles T., Badia X. Psychometric evaluation of the Cushing's Quality-of-Life questionnaire. Patient. 2013;6(2):113–124. doi: 10.1007/s40271-013-0012-5. [DOI] [PubMed] [Google Scholar]
- Oei N.Y., Everaerd W.T., Elzinga B.M., van Well S., Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9(3):133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Parker N., Patel Y., Jackowski A.P., Pan P.M., Salum G.A., Pausova Z., the I.C. Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry. 2020;77(11):1127–1136. doi: 10.1001/jamapsychiatry.2020.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman W.R., Webster M.J., Herman M.M., Kleinman J.E., Weickert C.S. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol. Aging. 2007;28(3):447–458. doi: 10.1016/j.neurobiolaging.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Peters A.T., Van Meter A., Pruitt P.J., Briceno E.M., Ryan K.A., Hagan M., Langenecker S.A. Acute cortisol reactivity attenuates engagement of fronto-parietal and striatal regions during emotion processing in negative mood disorders. Psychoneuroendocrinology. 2016;73:67–78. doi: 10.1016/j.psyneuen.2016.07.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Hermans E.J., van Marle H.J., Luo J., Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatr. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Quidé Y., Girshkin L., Watkeys O.J., Carr V.J., Green M.J. The relationship between cortisol reactivity and emotional brain function is differently moderated by childhood trauma, in bipolar disorder, schizophrenia and healthy individuals. Eur. Arch. Psychiatr. Clin. Neurosci. 2020 doi: 10.1007/s00406-020-01190-3. [DOI] [PubMed] [Google Scholar]
- Romero-Garcia R., Warrier V., Bullmore E.T., Baron-Cohen S., Bethlehem R.A.I. Synaptic and transcriptionally downregulated genes are associated with cortical thickness differences in autism. Mol. Psychiatr. 2019;24(7):1053–1064. doi: 10.1038/s41380-018-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatr. 2019;85(5):379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Soares J.M., Sampaio A., Ferreira L.M., Santos N.C., Marques P., Marques F., Sousa N. Stress impact on resting state brain networks. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E. Tend and befriend: biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15(6):273–277. [Google Scholar]
- Tombaugh T.N. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch. Clin. Neuropsychol. 2005;20(4):485–503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. A cross-disorder connectome landscape of brain dysconnectivity. Nat. Rev. Neurosci. 2019;20(7):435–446. doi: 10.1038/s41583-019-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff S.J., Pannekoek J.N., Andela C.D., Meijer O.C., van Buchem M.A., Rombouts S.A., van der Wee N.J. Resting-state functional connectivity in patients with long-term remission of Cushing's disease. Neuropsychopharmacology. 2015;40(8):1888–1898. doi: 10.1038/npp.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle H.J., Hermans E.J., Qin S., Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol. Psychiatr. 2009;66(7):649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37(7):1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- von Dawans B., Strojny J., Domes G. The effects of acute stress and stress hormones on social cognition and behavior: current state of research and future directions. Neurosci. Biobehav. Rev. 2021;121:75–88. doi: 10.1016/j.neubiorev.2020.11.026. [DOI] [PubMed] [Google Scholar]
- Weckesser L.J., Alexander N.C., Kirschbaum C., Mennigen E., Miller R. Hydrocortisone counteracts adverse stress effects on dual-task performance by improving visual sensory processes. J. Cognit. Neurosci. 2016;28(11):1784–1803. doi: 10.1162/jocn_a_01006. [DOI] [PubMed] [Google Scholar]
- Weerda R., Muehlhan M., Wolf O.T., Thiel C.M. Effects of acute psychosocial stress on working memory related brain activity in men. Hum. Brain Mapp. 2010;31(9):1418–1429. doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman E.D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T.F., Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 1971;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Wellman C.L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Whitaker K.J., Vértes P.E., Romero-Garcia R., Váša F., Moutoussis M., Prabhu G., Bullmore E.T. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc. Natl. Acad. Sci. U. S. A. 2016;113(32):9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann B.C., Schott B.H., Guderian S., Frey J.U., Heinze H.J., Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Xia C.H., Ma Z., Ciric R., Gu S., Betzel R.F., Kaczkurkin A.N., Satterthwaite T.D. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 2018;9(1):3003. doi: 10.1038/s41467-018-05317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Chen X., Li L., Castellanos F.X., Bai T.J., Bo Q.J., Zang Y.F. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 2019;116(18):9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Hashemi M.M., Kaldewaij R., Koch S.B.J., Beckmann C., Klumpers F., Roelofs K. Acute stress alters the 'default' brain processing. Neuroimage. 2019;189:870–877. doi: 10.1016/j.neuroimage.2019.01.063. [DOI] [PubMed] [Google Scholar]
- Zhang W., Llera A., Hashemi M.M., Kaldewaij R., Koch S.B.J., Beckmann C.F., Roelofs K. Discriminating stress from rest based on resting-state connectivity of the human brain: a supervised machine learning study. Hum. Brain Mapp. 2020;41(11):3089–3099. doi: 10.1002/hbm.25000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhou T., Feng S., Wang W., Liu H., Wang P., Yu X. The chronic effect of cortisol on orchestrating cerebral blood flow and brain functional connectivity: evidence from Cushing's disease. Metabolism. 2021;115:154432. doi: 10.1016/j.metabol.2020.154432. [DOI] [PubMed] [Google Scholar]
- Zung W.W. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- Zung W.W. The depression status inventory: an adjunct to the self-rating depression scale. J. Clin. Psychol. 1972;28(4):539–543. doi: 10.1002/1097-4679(197210)28:4<539::aid-jclp2270280427>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.