Abstract
Access to safe, effective, quality-assured antivenom products that are tailored to endemic venomous snake species is a crucial component of recent coordinated efforts to reduce the global burden of snakebite envenoming. Multiple access barriers may affect the journey of antivenoms from manufacturers to the bedsides of patients. Our review describes the antivenom ecosystem at different levels and identifies solutions to overcome these challenges.
At the global level, there is insufficient manufacturing output to meet clinical needs, notably for antivenoms intended for use in regions with a scarcity of producers. At national level, variable funding and deficient regulation of certain antivenom markets can lead to the procurement of substandard antivenom. This is particularly true when producers fail to seek registration of their products in the countries where they should be used, or where weak assessment frameworks allow registration without local clinical evaluation. Out-of-pocket expenses by snakebite victims are often the main source of financing antivenoms, which results in the underuse or under-dosing of antivenoms, and a preference for low-cost products regardless of efficacy. In resource-constrained rural areas, where the majority of victims are bitten, supply of antivenom in peripheral health facilities is often unreliable. Misconceptions about treatment of snakebite envenoming are common, further reducing demand for antivenom and exacerbating delays in reaching facilities equipped for antivenom use.
Multifaceted interventions are needed to improve antivenom access in resource-limited settings. Particular attention should be paid to the comprehensive list of actions proposed within the WHO Strategy for Prevention and Control of Snakebite Envenoming.
Keywords: Antivenoms, Snakebite envenoming, Health services accessibility, Neglected tropical diseases, Regulatory capacity, Affordability
Highlights
-
•
The global antivenom market is fragmented into multiple sub-markets of region-specific products, leaving many needs unmet.
-
•
Weaknesses in national regulatory capacity and some manufacturing processes lead to procurement of substandard antivenoms.
-
•
Prompt access to antivenom in rural settings is challenging due to lack of transportation.
-
•
Scarcity of adequately resourced facilities with experienced staff to administer antivenom is another access barrier.
-
•
Fully-financed regional antivenom stockpiles have the potential to significantly improve access.
1. Introduction
The goal of achieving “access to safe, effective, quality and affordable essential medicines and vaccines for all” is embedded into UN Sustainable Development Goal (SDG) 3.8, and a central component of Universal Health Coverage (UHC) (United Nations, 2017). For hundreds of thousands of snakebite victims around the world, this basic human right is unattainable. Lack of access to essential medicines in resource-limited settings is multifaceted. Medicines may be unavailable (e.g.: due to shortages, stock-outs or discontinued production), unsuitable (e.g.: lacking specificity, or suitability to programmatic requirements in a given setting), unaffordable (e.g.: higher in price than the capacity or willingness to pay), and/or of low quality (e.g.: lacking in potency or failing to meet appropriate standards) (Pécoul et al., 1999; Wirtz et al., 2017). Effective strategies to enhance access to medicines in resource-limited settings need to be tailored to the specific product characteristics, the clinical context and the target population.
Snakebite envenoming (SBE) can cause life-threatening medical emergencies. The effects can include severe bleeding, paralysis, kidney injury, and damage to muscle and other local tissues that can result in permanent disability, amputation of limbs, or death. It is estimated that worldwide, SBE may kill up to 137,880 persons annually (Gutiérrez et al., 2017). Antivenoms have been recognized by the World Health Organization (WHO) as essential medicines since WHO released its first list of essential medicines in 1977 (WHO Expert Committee, 1977). The administration of antivenom has been the foundation of treatment of SBE for nearly 125 years. Well-designed antivenoms intended to neutralize the venoms of specific species of snakes (from national or regional populations) can be highly effective in reducing mortality and morbidity, in tandem with appropriate ancillary medical care. Current antivenoms are biological preparations of animal plasma-derived antibodies that differ from one another with regards to many characteristics, primarily the specific snake species they are intended to be used for [see Box 1].
Box 1. What are antivenoms? How are they made?
Antivenoms (or antivenins) are produced from hyperimmune plasma obtained by immunizing donor animals (e.g.: horses, sheep, camels, etc.) with venoms. Toxins present in snake venom generate an immune response in the donor animals that is largely specific to those toxins and others with high homology.
One of the key characteristics of an effective antivenom is that it must be manufactured using immunoglobulins raised against venoms from snakes that occur in the countries and regions where the product will be deployed (WHO, 2017). Antivenoms are bespoke biological products specific to a limited range of snake species. Antivenoms may also exert varying degrees of paraspecific recognition and neutralization of the venoms of related species containing similar toxins. However the clinical paraspecific effectiveness of these products needs to be robustly established before marketing authorizations are issued. Just because one species is related to another does not guarantee that an antivenom can be redeployed against bites by that species. In addition, for some wide-ranging species (i.e. Daboia russelii), intraspecific venom variation can be considerable and antivenom raised using venom from one region may differ widely in its ability to neutralize the components in venoms coming from other regions (Pla, 2019).
Alt-text: Box 1
Although precise data is scant, there is consensus that only a small fraction of the estimated 2.7 million people envenomed after a snake bite each year have access to antivenom therapy. WHO includes SBE in its Category A list of highest priority neglected tropical diseases (NTDs), and has launched an ambitious global Strategy for the Prevention and Control of Snakebite Envenoming, that aims to reduce deaths and disabilities by 50%, and deliver 3 million effective treatments per year by 2030 (Williams et al., 2019; WHO, 2019).
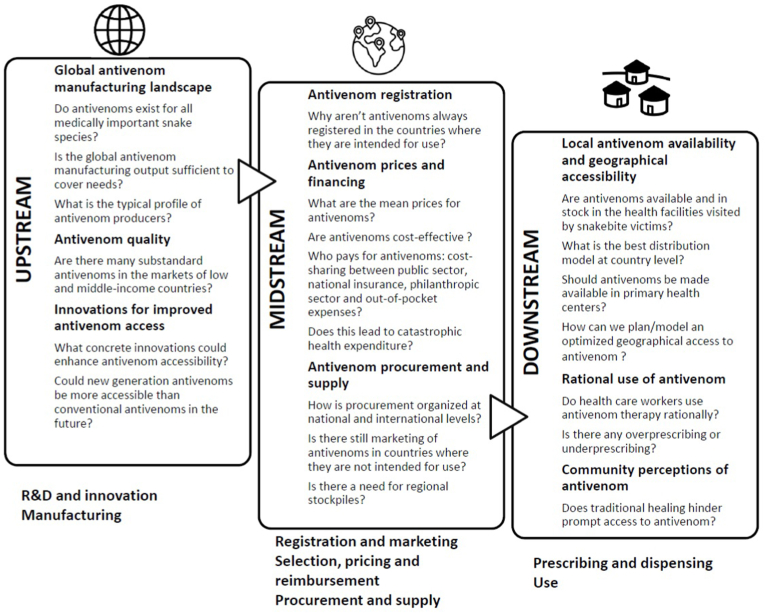
In light of WHO's plan to increase the accessibility, affordability, effectiveness and safety of antivenoms, this paper examines some of the most relevant barriers to these goals in resource-limited settings of Latin America, sub-Saharan Africa, South Asia and South-East Asia, and discusses actions to overcome them. We have drawn in part on an adapted framework on access to insulin (Beran et al., 2021), and how all three stages (e.g. upstream, midstream, downstream) of the antivenom journey from the manufacturing site to the patient bedside need to be taken into account. Specific access challenges are associated to each stage [See Fig. 1] and we will present these in a multi-disciplinary manner taking into account a range of perspectives. Our analysis is restricted to antivenom access but we also recognize the importance of access to good ancillary care alongside other components of SBE management.
Fig. 1.
Potential access barriers during the three stages of the journey of antivenom from the manufacturing site to the patient bedside.
2. Upstream: R&D and innovation/manufacturing
2.1. Global antivenom manufacturing landscape
Historically SBE has been considered a local issue by the majority of health authorities in affected countries. This has driven parallel manufacturing, often by public institutions, of antivenoms that were specific to local endemic species and requirements. In practice, antivenoms were developed to meet needs at national or sub-national levels, and occasionally at sub-regional level for a group of neighbouring countries. Some institutions (e.g. France's Institut Pasteur or Australia's Commonwealth Serum Laboratories, now CSL Limited) produced antivenoms with wider geographic coverage for political and strategic reasons, but commercial interest in antivenoms has generally been low.
Approximately 50 antivenom manufacturers are currently listed as active by WHO (WHO Website, a). The scale of antivenom production by each of these organizations is unclear. Manufacturer surveys about the number of vials produced annually capture only a proportion of the true number, due to commercial in-confidence limitations. Translation of this data into the number of effective treatments available is somewhat speculative, since few products have undergone well-designed dose-finding studies, and it is impossible to extrapolate the dose of one product to the dose that may be needed for another [See Box 2]. A global survey in 2020 obtained data from 22 manufacturers representing 65 distinct antivenom products currently in use (Global Snakebite Initiative, 2020). Just under 6 million antivenom vials or ampoules intended for markets in LMICs were produced by these companies. Based on self-reported, manufacturers’ recommended starting doses, this equates to approximately 1 million doses, probably an overestimate, since many producers claim that their antivenom requires low dosing in the absence of evidence. In any case, this is far below the global need to treat 2.7 million cases of SBE every year. The antivenom supply crisis is however not uniform. It is particularly serious in sub-Saharan Africa where previous surveys from 2007 to 2010 found that the availability of efficacious antivenom could be as low as 2.5% of the projected need (Brown, 2012).
Box 2. How to evaluate the antivenom effective dose?
Each antivenom, depending on its intrinsic potency and protein content, requires a specific effective dose, and this usually equates to more than a single vial or ampoule of antivenom to successfully treat a patient. Many manufacturers recommend “initial” or “starting” doses to be repeated over subsequent hours or days in case of poor clinical response. For most clinicians this is the primary source of antivenom dosing information, but it should be interpreted with caution. Independent analysis has shown that manufacturers’ dose recommendations are often unrealistic, and lack of published supporting data reinforces this view (Calvete et al., 2016; Harrison et al., 2017). Antivenoms should not be given registration or marketing authorization by national regulatory authorities in the absence of independent preclinical neutralization tests and well-designed, pragmatic clinical dose-finding and safety studies (Williams et al., 2018; Watson, 2020).
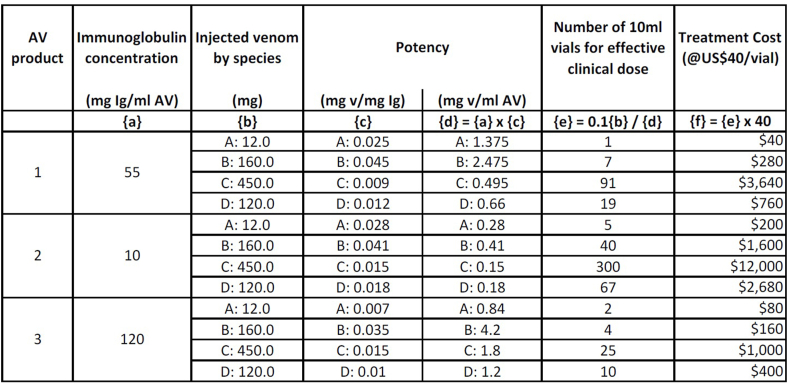
For healthcare providers and drug regulatory agencies to be able to determine the effective clinical dose of a given product, the amount of venom that is completely neutralized by a milliliter of antivenom should be indicated. In addition, it is recommended to indicate the immunoglobulin concentration of the reconstituted antivenom solution, not just the protein concentration. The amount of venom that individual species can inject when they bite humans needs to be taken into consideration. Depending on the specific immunoglobulin content and its potency (from one batch to another or one product to another), the number of vials could vary enormously as shown in the following example [See Fig. 3].
Alt-text: Box 2
Another survey in 2011 cautiously noted that annual production of antivenoms intended for use in India may have reached 2 million vials (Whitaker and Whitaker, 2012), which, based on current manufacturers’ dose recommendations (5–20 vials depending on severity), equates to 100–400 thousand initial treatments. Because the number of vials that constitutes an effective clinical dose is unclear [See Box 2] this likely translates into even fewer complete treatments. With between 1.11 and 1.77 million snakebite cases per year in India alone (Suraweera et al., 2020), and many more cases in the whole region of South Asia where these antivenoms are used, clearly the supply of antivenoms is woefully short of current needs.
Antivenom manufacturing represents an exceptionally heterogeneous industry. The majority of producers, particularly in Latin America and South East Asia, are not-for-profit, low volume public sector manufacturers that meet domestic or sub-regional needs. A small number of public and private sector manufacturers have larger capacity for distribution into both domestic and foreign markets (Gutiérrez, 2019). While small national public sector manufacturers may be a legacy of the past, they are also strategic investments, as the lack of national production capacity leaves endemic countries vulnerable through dependence on foreign supply [See Fig. 2]. One such example is Nepal, where an acute shortage of supply occurred in 2012 after an Indian court banned the export of antivenom due to shortages in India itself (Shrestha et al., 2017). Similarly, Papua New Guinea's (PNG) historical reliance on very expensive Australian antivenoms resulted in chronic shortages (McGain et al., 2004). Since 2018 the Australian manufacturer has donated 600 vials a year to the PNG government under a partnership with the Australian government. This approach still leaves PNG vulnerable to loss of product access, and in part contributed to the abandonment of efforts to introduce new low-cost products and pathways to local manufacturing in the future. Until recently, Myanmar had to import expensive Indian and Thai antivenoms of limited effectiveness against the medically most important species in Myanmar due to the limited production capacity of the country's public manufacturer (Williams et al., 2011; World Health Organization Regional Office for South-East Asia, 2002). However, following a collaboration with Australia to upgrade production, the national antivenom company in Myanmar has been able to meet the country's needs since 2017.
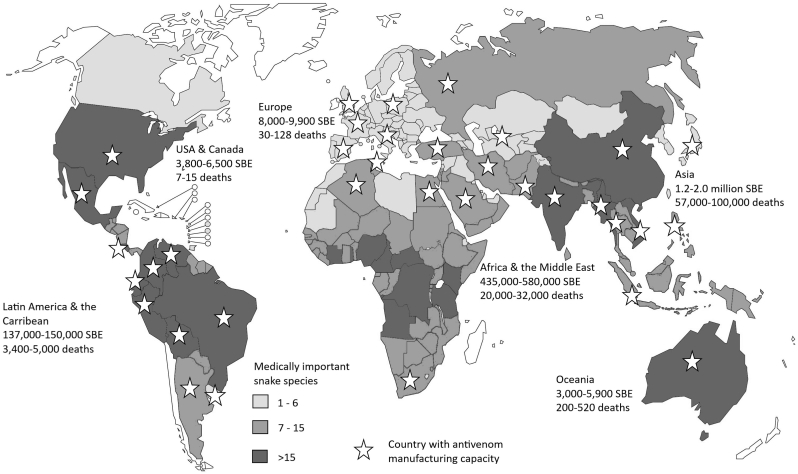
Fig. 2.
Regional burden of SBE, national abundance of medically important snake species and national antivenom manufacturing capacity.
Sources: Gutiérrez et al. (2017), Gutiérrez, 2012 and WHO (2019).
The small number of private antivenom manufacturers and the general lack of interest from large multinational pharmaceutical producers are indicative of the commercial realities of manufacturing nationally or regionally-specific products for unpredictable and unreliable markets. The decision made by Sanofi in 2010 to cease antivenom production (Chippaux and Habib, 2015) highlights how commercial imperatives trump corporate social responsibility when it comes to markets in low-to middle-income countries (LMICs).
One of the key weaknesses in the global market is the lack of diversified manufacturing of products that meet specific needs. This fragility can lead to severe consequences when a sole producer leaves the market, or where geopolitical and other constraints render access to products difficult. In Yemen, Médecins Sans Frontières (MSF), which admits more than 600 snakebite victims to its hospitals every year, has been unable on several occasions to access the only regionally relevant polyvalent antivenom, a product manufactured by the National Antivenom and Vaccine Production Center (NAVPC), a public sector laboratory in Saudi Arabia. Logistics and bureaucratic constraints are largely to blame. Similarly, the sole manufacturer of boomslang (Dispholidus typus) antivenom, South African Antivenom Producers (SAVP) based in Johannesburg, South Africa, produces only small quantities of this expensive antivenom, which has relatively small demand. Consequently, production gaps, stock shortages and logistics challenges mean that the product is unavailable to those who need it in other countries where the species is endemic (Gutiérrez, 2019). Efforts are needed to maintain appropriate supply and future-proof markets with multiple sources of efficacious and safe products for every region (Gutiérrez, 2019).
Within this ecosystem, it is not surprising that chronic shortages of antivenom are reported in regions with high burdens of SBE and a small number of producers [see Fig. 2]. After calls were made to address the antivenom supply crisis in Sub-Saharan Africa at the end of 20th century (Theakston and Warrell, 2000), greater commercial interest emerged, as entrepreneurial manufacturers in India, Latin America and elsewhere responded by broadening their market ambitions. Over the past 20 years, some manufacturers have moved into new markets, mostly with good intentions and a desire to improve treatment options for affected communities. Some of these new products have proven to be safe and effective treatments, while others have not (Visser et al., 2008; Abubakar et al., 2010).
New antivenoms are generally produced in response to specific priorities, resulting in products not being developed for species that cause, or are perceived to be responsible for, a low burden of snakebite injuries and deaths. South Asia's case is striking: all marketed antivenoms in the sub-region have been raised against the same “Big Four” snake species (Bungarus caeruleus, Daboia russelii, Echis carinatus and Naja) for more than 70 years, yet there are many more medically important species for which specific antivenoms have never been developed. For example, no specific antivenom for Naja kaouthia is available in the South Asian market, although the species accounts for most cobra bites in Bangladesh. Similarly, specific products to neutralize venoms of hump-nosed pit vipers (Hypnale hypnale), the commonest cause of snakebite envenoming in Sri Lanka, do not exist. Available products are usually ineffective in such instances despite the administration of large doses, and carry a high concomitant risk of allergic reactions (Ralph et al., 2019). The development of an investigational product able to neutralize the venom of Hypnale hypnale is therefore an encouraging prospect (Petras et al., 2011; Villalta et al., 2016). Finally, there exists no specific antivenom for Bungarus walli and B. niger, now recognized as medically important krait species in Bangladesh and Nepal. The antivenoms currently marketed in these countries are prepared in India and are raised against B. caeruleus, and no preclinical or clinical evidence of paraspecific neutralization against B. walli and B. niger exists for these products (Shrestha et al., 2017). It should however be noted that current antivenoms are generally considered poorly effective against krait venoms (Bungarus spp.) (Alirol et al., 2010), so it is unsure if an antivenom raised specifically against B. walli and B. niger would be any more effective than the antivenoms currently available in Nepal and Bangladesh.
In addition, only one major producer of venom has been licensed to supply antivenom manufacturers in India, and these venoms, sourced from snake specimens only from Tamil Nadu in Southern India, have not historically been produced to standards that apply to pharmaceutical starting materials. This may be one of the reasons why current antivenoms are believed to be significantly less effective in Northern India (Ralph et al., 2019).
Similar issues are also found outside South Asia. The tri-specific antivenom made and marketed in Indonesia by a state-owned enterprise is another example of mismatch. It neutralizes venoms from Indonesian populations of Calloselasma rhodostoma, Naja sputatrix and Bungarus fasciatus, but it excludes coverage against the venom of B. candidus which is actually responsible for far more bites than B. fasciatus (Tan et al., 2016; Williams et al., 2011).
In order to improve antivenom access at a global level, the reliability, stability and security of antivenom supply lines, the elimination of monopolies and the development of a competitive, dynamic market are all needed. A sustainable future market requires product standardization to specific market needs, and consortia or networks of Good Manufacturing Practice (GMP) compliant manufacturers who share research and development, quality control and other common costs while pursuing process optimization and rationalization goals aimed at sustainable, low-cost, high volume, commercially viable antivenom output (Williams et al., 2011).
Achieving an optimized antivenom producer ecosystem requires a mixed approach, depending on technical capacities and political will in the different endemic regions. Consolidation of production effort would likely generate economies of scale and facilitate a range of improvements, although there will continue to be single manufacturer business case models, especially for very low-volume products. Collaboration between producers can lead to rapid technological improvements, product diversification and increased production: the well-coordinated network of public manufacturers in Latin America offers multiple sources of pan-specific antivenoms adapted to all sub-regions of Latin America, enables high-volume antivenom production and economies of scale, and incentivizes sharing of reference venoms from different geographical origins (Gutiérrez, 2019). Likewise, the international collaborations undertaken by public institutions and private companies in Australia, Brazil, Costa Rica, India and the United Kingdom for production and development of antivenoms for use in other regions of the world have been critical to address unmet antivenom needs (Di Fabio et al., 2021).
2.2. Antivenom quality
A recent review observed that regulatory affairs-related antivenom issues were rarely discussed in peer-reviewed literature (Di Fabio et al., 2021). To address the gap in regulatory aspects, WHO published Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins designed to assist both manufacturers and regulatory authorities in 2010, followed by a revision in 2017 (WHO, 2017). The WHO guidelines attempt to establish a minimum set of design, production, quality control and regulatory parameters to support standardization and harmonization efforts. As there are shortcomings, a third edition is planned in 2022. For example, data on venom potency and venom yield, which could substantially inform rational design of products with adequate neutralizing potency, is not included. There is also a strong case to be made for closer scrutiny of antivenom production by the WHO Expert Committee on Biological Standardization (ECBS), and by networks of regulatory authorities. Many regulators lack the technical capacity and human resources to adequately regulate and control the safety, effectiveness and quality of the antivenoms that are imported or exported. Future editions of the guidelines will focus more on improving these capabilities.
The underlying principle for the production of all medical products is the adherence to GMP and other international standards (e.g. Good Laboratory Practice, GLP), and antivenoms are no exception. Manufacturers with deficient GMP are vulnerable to serious deficiencies that can result in release of inferior, defective, ineffective or even potentially dangerous products. Pyrogenic reactions, hypersensitivity reactions and other adverse events due to possible presence of endotoxins, protein aggregates and other impurities are often a consequence of poor GMP, notably during the plasma fractionation and subsequent manufacturing processes (Morais and Massaldi, 2009). Lack of standardization methodology for quality control can lead to variable results, even when compliant with the same specifications, and lack of reference venoms established and validated by competent authorities continue to hamper reproducibility and quality assurance improvements (León et al., 2018). Many products are marketed without prior independent preclinical efficacy testing (Ainsworth et al., 2020), nor data of safety and clinical effectiveness from well designed, pragmatic clinical trials (Alirol et al., 2015).
Evidence of poor preclinical efficacy (Calvete et al., 2016; Harrison et al., 2017) of products with high market penetration (Potet et al., 2019; Brown, 2012) and the abandonment of other products with acceptable profiles, such as Sanofi's FAV-Afrique in 2010, have led to efforts to reshape the market and improve compliance. WHO launched a pilot programme to develop a risk-benefit assessment procedure for antivenoms in 2015, focused on products for Sub-Saharan Africa. The first product recommendation was issued in August 2018 and four other products are completing the process in 2021 (WHO Website, b). The comprehensive nature of the assessment process has uncovered one unscrupulous antivenom producer having presented fabricated clinical trial data in documents submitted to WHO (Imani, 2019). Clearly there remains considerable work to be done to improve quality and safety of antivenom products, especially in weakly regulated markets such as sub-Saharan Africa.
WHO's risk-benefit assessment programme will continue to evaluate products for sub-Saharan Africa, South-East Asia and the Western Pacific regions, and WHO has a number of activities aimed at strengthening the capacity of regulatory agencies to evaluate and improve regulation of antivenoms. Under the WHO strategy for snakebite envenoming, a formal prequalification procedure to support procurement of quality-assured products will be developed within the next 3–4 years (Williams et al., 2019). These measures are expected to increase the supply and sustainability of well-designed, safe and effective antivenoms. Achieving them will require external support and technical assistance for manufacturers who need to invest in upgrading infrastructure and manufacturing technologies in order to meet the requirements for participation in this new environment. Support from governments, funding agencies, philanthropic foundations and development banks should be considered within the context of SDGs related to health as well as national innovation and infrastructure policy (United Nations, 2017).
2.3. Innovations for improved antivenom access
WHO has prioritized the need for academia and antivenom manufacturers to cooperate and align research priorities on practical issues that need to be overcome to improve the production, quality control and regulation of antivenoms, as many manufacturers lack the resources and capacity to undertake R&D activities on their own. Incremental modification of manufacturing processes can greatly improve the safety, effectiveness and accessibility of final products (León et al., 2018). One example of this is the emergence of lyophilization as a means of producing antivenoms that do not depend on cold-chain, something that is often absent in remote resource-limited settings. Lyophilization avoids the need for refrigeration, increasing the range of deployable locations for antivenoms. Research to improve stability can further extend the effective life of some antivenoms, reducing wastage and boosting antivenom availability.
A wider range of innovations to traditional antivenoms are now being developed, including new adjuvants, improved immunizing mixtures, quality control tools and assays, and the use of toxicovenomic technology-aided antivenom design, alongside new generation SBE treatments based on small molecules or cocktails of neutralizing antibody cocktails (Gutiérrez et al., 2017). Likewise, the availability of new rapid tests to identify the offending snake species may have an impact on antivenom designs in the future (Knudsen et al., 2021). The novel antivenoms that arise from these innovations will likely have very different characteristics in terms of geographic coverage, administration routes and costs, compared to conventional antivenoms. This will bring new perspectives and challenges regarding access, but ultimately will lead to increased access to safer, more effective and affordable treatments for many more people around the globe.
3. Midstream: registration and marketing/selection, pricing and reimbursement/procurement and supply
3.1. Antivenom registration
Effective regulation of medicines is designed to ensure that products in the marketplace are safe, effective and represent high value care for specific illnesses or diseases. Registration at country level is essential for national procurement agencies to have confidence that products are safe, effective and truly adapted to the medically most important snake species in the country. Antivenoms are subject to registration by competent regulatory authorities in virtually all jurisdictions, but the degree to which the registration process independently and robustly establishes the validity and reliability of manufacturer's claims is extremely variable. The Collaborative Registration Procedure has been designed by the WHO to aid in precisely this process: to accelerate registration of prequalified essential medicines in multiple countries (Ahonkhai et al., 2016). The planned introduction of prequalification procedures for antivenoms will further facilitate their entry into regional registration programmes.
Countries that lack local manufacturing capacity are particularly vulnerable if the antivenoms used are unregistered and lack marketing authorization. Suppliers generally give priority to markets where products are registered, leaving other countries without reliable access. SAIMR-Polyvalent, a polyspecific antivenom produced by SAVP in South Africa and considered by some as the current “Gold Standard” for treating envenomings by many African snake species, is only registered in South Africa. Export of supplies to other countries such as Eswatini and Tanzania are limited (Harrison et al., 2017; Yates et al., 2010; Habib et al., 2020; Erickson et al., 2020). Many manufacturers eschew the financial and administrative implications of registering products in multiple jurisdictions.
Regional collaboration between regulatory authorities, and shared registration programmes that facilitate broader market penetration across regions are needed to address this issue, following experiences that have been applied to other essential medicines. Inclusion of antivenoms in joint assessment initiatives would change the landscape considerably, inviting greater participation by manufacturers (Arik et al., 2020).
3.2. Antivenom prices and financing
The cost of antivenom has a major impact on accessibility and affordability. Very few countries regulate price, and a lack of adequate price regulation can result in price-gouging and profiteering by manufacturers or intermediaries involved in the supply chain. For example, the cost of antivenom in the USA can exceed more than US$10,000 per vial (Theakston and Warrell, 2000; Boyer, 2015). While costs are generally lower in LMICs, antivenoms are often prohibitively expensive relative to the incomes of often impoverished snakebite victims. In such settings even treatment costing as little as US$4 per vial might be unaffordable to patients in need (Theakston and Warrell, 2000). In Kenya, when calculating the daily wage of a lowest-paid government worker (LPGW), a study found just one vial of antivenom was unaffordable, costing an LPGW 2.3 days in the public sector, 16.9 days in the private sector and 7.7 days in the mission sector (Ooms et al., 2021), keeping in mind that to be effective multiple vials are needed.
Indeed, unit price is a completely unreliable indicator of the total cost of providing an effective clinical dose. The actual price of an effective treatment needs to be based upon the number of vials/ampoules that are confirmed in independent studies to be necessary. It varies from product to product based on potency, immunoglobulin concentration, mass of injected venom (per species) and other factors [see Box 2]. Attempts have been made to estimate costs of an effective dose for different products. In sub-Saharan Africa this was highly variable, ranging from US$55 to US$640 (Brown, 2012). A 2020 global survey of 22 antivenom manufacturers found that the average price per starting dose equated to US$463 (Global Snakebite Initiative, 2020). These numbers should be taken with caution as the reality is that against some of the most medically important species, some antivenoms may be ineffective even at currently recommended doses [See Fig. 3].
Fig. 3.
Hypothetical immunoglobulin content and potency of three antivenoms (1, 2, 3) against four venoms (A, B, C, D), with estimated impact on dose and cost of effective treatment. While snakes generally inject less venom in defensive bites than they do when venom is extracted in the laboratory, using average venom yield as the basis for initial dose estimation ensures that every patient receives a clinically effective dose as soon as possible. Products 1 and 2 would be ineffective against venom C (product 2 also ineffective against venom D). Product 3 with the highest immunoglobulin content could be effective at volumes of 20–250 ml against all four species, but at a cost ranging from US$80–1000 per treatment, depending on the species. Acronyms: mg Ig/ml AV = milligrams of immunoglobulin per milliliter of antivenom; mg v/mg Ig = milligrams of venom neutralized by 1 mg of immunoglobulin; mg v/ml AV = milligrams of venom neutralized by 1 mL of antivenom.
Some LMICs provide antivenoms either free or highly subsidized to patients in public hospitals, but the resources allocated and volumes supplied are often inadequate. A scheme in Burkina Faso provided only enough antivenom to treat around 4% of patients (Gampini et al., 2016). In many resource-limited settings, particularly in sub-Saharan Africa and South Asia, national insurance schemes do not have high coverage, are often restricted to employees in the public sector and do not extend to farming communities. This leads to enormous out-of-pocket expenses for individuals requiring them to sell assets such as land or livestock (Vaiyapuri et al., 2013). Few supplies of antivenom are provided by not-for-profit non-governmental organizations. Recent examples are scarce and include Rotary International in north-eastern Nigeria, and Médecins Sans Frontières in parts of Central African Republic, Ethiopia, South Sudan and Yemen (den Boer, 2021). Humanitarian organizations are often best prepared to provide antivenom therapy during humanitarian emergencies, particularly natural disasters and other crises causing population displacement, which carry a high risk of both snakebite epidemics and disruption of health services and medical supply chains (Ochoa et al., 2020; Igawe et al., 2020).
Despite the challenge around high costs and affordability, antivenoms are among the most cost-effective interventions in developing countries (Brown and Landon, 2010). Analysis of data from 16 west and central African countries shows it to be highly cost-effective (Habib et al., 2015; Hamza et al., 2016). But these data need to be expanded. Further health economic modelling and sensitivity analyses are required to better articulate the cost-effectiveness of antivenoms and to ensure their appropriate usage across multiple geographic regions. These studies should investigate the potential for gains that could be achieved by improving antivenom potency, production costs, economies of scale and quality of life utilities for different treatment options (including ancillary treatments when available). Furthermore, these assessments could also be used to better highlight the downstream medical costs and socio-economic impacts of untreated snakebite on victims and their families, as well as the cost-benefits of improved distribution and storage programs, staff training and improved utilization of antivenom.
While domestic public resources to finance antivenom access are limited, the launch of the new WHO Roadmap for neglected tropical diseases in 2021 (WHO, 2020), which reiterates the ambitious objective to cut snakebite mortality in half by 2030, will hopefully attract additional financial support for preventing and treating SBE from both donor and affected country governments, as well as philanthropic foundations.
3.3. Antivenom procurement and supply
Procurement processes can vary substantially depending on whether supply is provided by a national public sector manufacturer operating as a monopoly, several national public and private sector manufacturers competing against each other, or if there is no national manufacturer and supply is imported from overseas. All countries in sub-Saharan Africa, except South Africa, belong to the latter category. To ensure that products are fit-for-purpose, procurement agencies need to carefully define product specifications based on expert consensus. Where products are registered by a national regulatory authority, or where WHO recommendations exist for particular products, this process may be somewhat easier, but when there are no products registered, or in the absence of evidence-based recommendations, procurement of appropriate products may be especially challenging. Purchasing and distribution of inappropriate antivenoms that were not specific for the venomous species endemic in the country has been reported in West Africa, PNG (Warrell, 2008) and more recently in Ethiopia (den Boer, 2021), suggesting that antivenoms may be particularly amenable to violations of Good Distribution Practices by wholesale distributors.
The misunderstanding of what represents effective treatment with antivenom [See Box 2], creates another challenge for national procurement agencies. Procurement models and supply contracts should be restricted to dealing with quantities in terms of “effective treatments” or “effective doses”, rather than “single vial/ampoule doses”. Well-designed clinical dose-finding and safety studies are essential to determine what volume of a product constitutes an “effective clinical dose”, and should be a prerequisite to product registration and marketing authorization.
Within this context, LMICs would benefit from multilateral antivenom procurement mechanisms. Examples exist for other products: the Strategic Fund for the acquisition of Essential Medicines and the Revolving Fund of Vaccines of the Pan American Health Organization consolidate procurement on behalf of participating countries in the Americas, and the International Coordination Group on Vaccine provision manages stockpiles of vaccines for prompt delivery for outbreak response (DeRoeck et al., 2006; Yen et al., 2015). Coordinated demand forecasts and pooled procurement of specific antivenoms at continental level could increase supply security and optimize pricing. Such a mechanism would however depend on a continuous and sustainable provision of antivenoms. Long term contracts could entice manufacturers to commit to producing their products, particularly those from the public sector who depend on a government budget to support a periodic production program. Along those lines, WHO has begun work to establish a stockpile of effective antivenoms for sub-Saharan Africa.
4. Downstream: prescribing and dispensing/use
4.1. Local antivenom availability and geographical accessibility
Snakebite envenoming is a time-critical medical emergency. A rapid response with access to effective treatment is essential in the first hours after the bite. Delayed treatment is a recognized risk factor for complications and death (Feitosa et al., 2015a, 2015b; da Silva Souza et al., 2018; Iliyasu et al., 2015).
Unfortunately, antivenom availability and accessibility remain distant possibilities for large proportions of at-risk populations around the world. Variable policies on use, distribution and clinical environments restrict access by limiting the number and types of health facilities where antivenom can be held and used. Rather than being available at primary health centres, antivenoms are often only available in secondary or tertiary referral centres under medical prescription (Habib et al., 2020). Surveys in Kenya (Okumu et al., 2019; Ooms et al., 2021), Uganda and Zambia (Ooms et al., 2020) paint a depressing picture. In Kenya antivenom was available at one-third of the healthcare facilities, and stock-outs were reported even in large urban referral hospitals such as Kisumu. Only 4.2% and 7.6% of healthcare workers in Uganda and Zambia respectively reported available antivenom stock when surveyed. The situation is equally bleak in parts of South-East Asia. A community-based survey on snakebite incidence from Lao PDR highlighted the lack of antivenom in district and provincial hospitals (Vongphoumy et al., 2015). Similarly, in Vietnam, antivenom products can only be accessed from certain prominent tertiary hospitals in the Mekong Delta and are largely unavailable in district and provincial hospitals in some provinces of central Vietnam (Blessmann et al., 2018).
In many countries access to antivenom is restricted to facilities staffed by doctors, where resources for effective management of complications such as adverse drug reactions, airway and breathing emergencies, kidney injury and local tissue injury are available. This high bar for initial treatment can be a barrier to access especially in rural settings. In India, there have been calls to decentralize access to antivenom in every primary health center in order to drastically improve geographical accessibility; however, strengthening of the health workforce in these facilities will be required, so that an officer can be available during night hours and that all workers be properly trained on SBE management (Bawaskar et al., 2020). In Ecuador and Tanzania, successful management of SBE was achieved in a severely resource-constrained area by improving access to treatment in nurse-led clinics (Gaus et al., 2013; Yates et al., 2010). In Nigeria decentralization of antivenom supply through a “hub-and-spoke” distribution and utilization network model, wherein rural facilities serve as satellites or spokes and are linked to major hospitals in urban hubs for referrals, linkages, support, training and antivenom supplies has been proposed (Habib, 2013). Lack of communication also can lead to tragedy. During a snakebite outbreak in 2016 in Donga, Nigeria, most victims and their relatives were unfortunately not aware of the free antivenom provided at the referral hospital in the city and did not seek care accordingly (Igawe et al., 2020).
Many victims of snakebite must travel long distances to access even primary health care, and the distance to facilities where antivenom is available can be even greater (Feitosa et al., 2015a, 2015b; da Silva Souza et al., 2018; Schioldann et al., 2018). Shortages of qualified health workers able to administer antivenoms and provide ancillary treatments compound the situation in many settings. In the Brazilian Amazon, transport to health facilities may involve several different means of travel, significant time delays and sometimes exposure to dangerous conditions on land, water and in the air (Cristino et al., 2021). Even when antivenom is available locally, some patients may perceive that quality of care will be better at more distant facilities, and travel hundreds of kilometres further away (to Manaus for example), often suffering poorer outcomes as a result (Guimarães et al., 2018; Cristino et al., 2021). Unfortunately, patient waiting times, ineffective triage and workforce shortages can lead to delays in access to treatment even when a patient arrives at a health facility (Bajpai, 2014; Sharma, 2015; Simpson, 2007; Islam and Biswas, 2014).
In India, distances to antivenom treatment centres are generally shorter, but snakebite victims in rural areas sometimes travel over 100 km to access basic healthcare (Singh and Badaya, 2014). Free ambulance services established through public-private partnerships sometimes provide antivenom for critically ill patients during transport but the impact of these initiatives is hindered by a shortage of services in most rural areas, suboptimal response times or non-attendance, inadequately trained paramedics in standardized resuscitation protocols (Bharti and Singh, 2015; Ralph et al., 2019). In Nepal community education and motorcycles have been used to shorten the delay between bite and access to antivenom treatment, successfully reducing case fatality rates from 10.5% to 0.5% (Sharma et al., 2013).
Solving the challenges posed by physical geography requires the use of tools that improve our understanding of factors influencing antivenom accessibility. Geographic Information Systems (GIS) in particular have emerged as powerful technological advances for the measurement of geographic access to healthcare over the past 3 decades (Neutens, 2015; Delamater et al., 2012). One particularly well-suited approach for modeling timely physical accessibility to health services uses a least-cost path approach informed by local travelling constraints (e.g., terrain, road network, barriers to movement, modes and speeds of transport) (Ray and Ebener, 2008). It is currently being used to evaluate access to snakebite treating facilities in Cameroon and Nepal (Alcoba et al., 2021). When SBE risk is not uniformly distributed in a region of interest, modeling the vulnerability of the population to SBE can be instrumental in helping to plan antivenom distribution and referral networks (Longbottom et al., 2018). In Costa Rica high resolution geospatial data, snakebite incidence data, locations of health facilities and ambulance stations, and data on the geographical extent of habitat suitable for Bothrops asper enabled identification of areas in need of improved access to antivenom (Hansson et al., 2013). Prioritizing collection of geospatial data on snake ecology and distribution (Pintor, 2021) and developing innovative methods to collect field data may help to enable improved prediction of snakebite hotspots (Geneviève et al., 2018; Goldstein et al., 2021). Central to all these issues is the need to improve health systems and infrastructure, particularly in rural areas, and ensure that UHC is accessible and affordable for all.
4.2. Rational use of antivenom
According to the WHO, rational use of medicines requires that “patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community” (WHO Website, c). In this context a snakebite patient's real need for antivenom must first be considered, especially since not all snakebites lead to envenoming, and not all cases of envenoming are serious enough to warrant antivenom. Snakebites that do not require antivenom treatment, such as dry bites, in which no venom is injected, and bites caused by snakes of no medical importance, may represent up to 60% of all snakebites (WHO, 2019). In these cases, the mistaken administration of antivenom has no clinical benefit to the patient, but may still potentially lead to early or late adverse reactions. Inappropriate clinical judgements for antivenom treatment have been documented in several countries, leading to unnecessary antivenom usage (Fung et al., 2009; da Silva et al., 2019b). At the same time, more rational use has also been reported after implementation of new treatment protocols, notably in Bangladesh and India (Harris et al., 2010; Ghosh et al., 2008). Furthermore, we can never forget that SBEs are time-critical medical emergencies. The risk of possible overuse of antivenoms needs to be balanced with the imperative that safe, effective and affordable antivenoms are available as close to the patient as possible, in adequate doses that can be administered early.
Conversely, inadequate treatment with antivenom results in incomplete neutralization of toxins and poor clinical outcomes. The cost of antivenoms was blamed for under-dosing of patients in Cameroon, while in Myanmar rationing due to shortages of products meant that less severe cases were administered lower doses (Einterz and Bates, 2003; Alfred et al., 2019). In the Amazon 52% of patients with severe envenoming caused by Bothrops spp., and 82% of those with severe Lachesis spp. envenoming were under-dosed (Feitosa et al., 2015a, 2015b). In this region, increased lethality was significantly associated to lack of antivenom administration (53.5% of the fatal cases) and antivenom underuse (63.3% of fatal cases using antivenom) (da Silva Souza et al., 2018). Antivenom under-dosing was more common in indigenous populations compared to urban and countryside populations, although antivenom is available free-of-charge across the country (Fan and Monteiro, 2018; Monteiro et al., 2020). Several factors can lead to under-dosing, especially inferior potency and low immunoglobulin content in poorly designed or low quality products. The variability of products, even from one batch to another, can result in considerable uncertainty when it comes to estimating dose at the bedside [See Box 2].
In countries where substandard antivenoms have dominated the market for decades, the confidence of health care workers is eroded, which may lead to antivenom underuse. Health workers in remote settings may be apprehensive about treating snakebites, for fear of not being able to manage antivenom-associated adverse reactions should they occur (Ralph et al., 2019). Knowledge about snakebite management, antivenom use and management of antivenom-associated adverse reactions is often poor (Michael et al., 2018; Taieb et al., 2018; Bala et al., 2020; Sapkota et al., 2020; Ameade et al., 2021). Developing new or improved treatment guidelines, supporting training programs for public and private health workers and improving the quality, safety and effectiveness of antivenoms are key steps towards optimising use of antivenom and achieving consistent, improved outcomes.
4.3. Community perceptions of antivenom
The first pillar of the WHO snakebite envenoming strategy is engagement with, and the empowerment of, affected communities. In LMIC settings there are a multitude of cultural, social and economic barriers that contribute to delayed access and it is important to consider these contextual factors in relation to the patient. Large proportions of patients choose traditional or faith healers ahead of allopathic medicine with a range of associated outcomes (Sloan et al., 2007; Lam et al., 2016; Alcoba et al., 2020). Plants, animals and mineral-based therapies, blessings and prayers, as well as self-medication with orthodox medications, are commonly used by patients before making the decision to search for the health service (Pierini et al., 1996; da Silva et al., 2019a). Use of these self-care practices are recorded across the world as the cause of late medical assistance and poor outcomes in SBE (da Silva et al., 2019a; Schioldann et al., 2018; Mahmood et al., 2019; Longkumer et al., 2017; Alirol et al., 2010).
The fact that snakebite victims resort to traditional medicine does not necessarily mean that they mistrust modern medicine. In Kenya, 60% of community members that were interviewed believed that antivenom works for the treatment of snakebite and 91% believed in the effectiveness of medicines in general (Ooms et al., 2021). But inadequate knowledge about appropriate first aid methods is widespread (da Silva et al., 2019a; Silva et al., 2020; Michael et al., 2011) and high costs of treatment also influence decision-making.
In Bangladesh envenomed victims of snakebite requiring antivenom spent more time with traditional healers than victims of non-venomous snakebite (Harris et al., 2010). The perception of the seriousness of SBE often varies, some victims seeking help only after more severe symptoms (e.g. onset of unbearable pain, disfiguring oedema, bleeding or decreasing functional mobility) develop (Cristino et al., 2021). On some occasions, utilization of healthcare services by snakebite victims was a reflection of the resistance of the snakebite victim to seek medical assistance, which was only overcome by pressure from family members. For some traditional populations, the displacement of an indigenous patient to a hospital setting to receive antivenom after a snakebite is a radical event (Guimarães, 2015). Engagement with traditional healers at community level is needed to reduce the occurrence of harmful care practices and encourage prompter referrals to a healthcare facility equipped with antivenom.
5. Conclusion
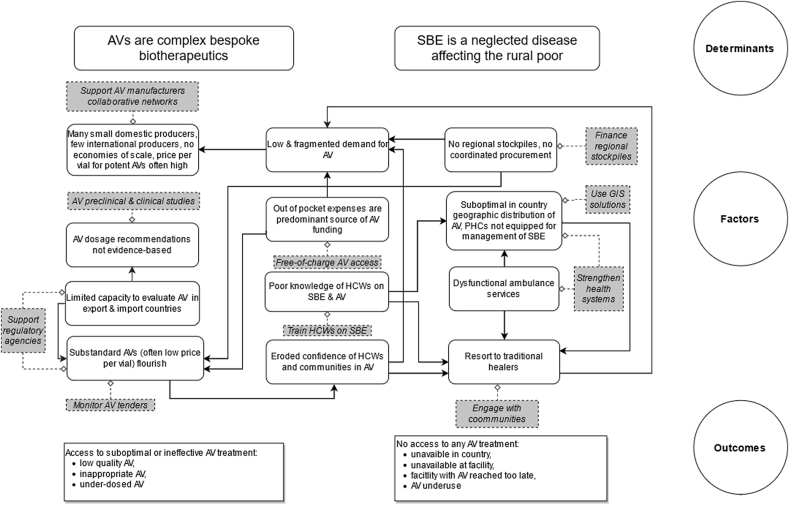
SBE is a neglected disease that is most endemic in rural areas of LMICs, where health infrastructure is often deficient, and antivenoms are complex, bespoke biotherapeutics supplied through inconsistent and fragmented markets challenged by variable regulatory compliance. There are multiple barriers to antivenom access, but they can be overcome with appropriate measures [See Fig. 4]. We hereafter propose a list of concrete recommendations requiring a fully-financed, coordinated response [See Box 3]. WHO has estimated that programme costs for SBE will be US$136.76 million between 2019 and 2030. This does not include the cost of commodities such as antivenoms, other treatments, medical consumables, or investments by countries themselves. Incorporation of SBE into the national health plans of affected countries, along with appropriate resource allocation and investment across a broad range of activities is essential. Modernized infrastructure, incorporating new technology and pragmatic collaboration between academia, manufacturers and government could reduce costs per capita of antivenom production and drastically improve quality, production capacity, sustainability and clinical effectiveness. North-South and South-South models of technology transfer should be pursued. With almost 140,000 deaths and hundreds of thousands of disabilities caused each year, SBE is a threat to the health and economic growth of LMIC communities in all parts of the world. Concerted action, led by WHO and strongly supported by governments, NGOs, donors and the pharmaceutical community is imperative.
Fig. 4.
Determinants, factors and outcomes of antivenom access. Not all these factors are at play for all antivenom products. Arrows represent cause and effect relations between the different factors. Grey boxes and dotted lines represent possible actions to mitigate detrimental factors. Acronyms: AV = antivenom; HCWs = healthcare workers; PHCs = primary health centres; SBE = snakebite envenoming.
Box 3. Recommendations.
-
•Build global and national capacities to better regulate antivenoms:
-
○Develop the technical capacity of national authorities to evaluate safety, effectiveness, and quality of antivenom products;
-
○Encourage regional regulatory collaborations, particularly for countries that are not producing antivenoms;
-
○Develop WHO prequalification of antivenoms and collaborative registration of antivenoms;
-
○Establish a WHO-led network of regulators, national control laboratories and reference laboratories to develop reference standards for antivenom production, quality control and regulation;
-
○Determine the effective clinical dose of antivenoms through robust independent pre-clinical testing and dose-finding clinical trials;
-
○Make antivenoms part of existing national pharmacovigilance programs for assured drug safety.
-
○
-
•Improve procurement of antivenoms:
-
○Encourage transparent and consistent antivenom procurement procedures;
-
○Support independent monitoring by civil society organizations;
-
○Share good practices of antivenom procurement, using number of effective treatments, not number of vials;
-
○Conduct evidence-based needs assessment of antivenom procurement requirements in regions with poor access to effective antivenoms, based on incorporation of improved snakebite epidemiological data (through mandatory reporting), and establish regional antivenom stockpiles, particularly in sub-Saharan Africa.
-
○
-
•Strengthen the antivenom supplier base:
-
○List GMP-compliant antivenom manufacturers, evaluate their manufacturing capacity;
-
○Mandate the use of geographically-representative venom pools for antivenom production to address problem of intra-species variations, particularly for medically important snake species of South Asia and sub-Saharan Africa;
-
○Incentivize collaborations between networks of manufacturers to mutualize costs, achieve economies of scale, and increase production of effective antivenom at affordable cost, particularly in and for sub-Saharan Africa, where effective products are in short supplies.
-
○
-
•Increase community demand for antivenom:
-
○Engage with traditional healers for prompt referral of SBE patients to a facility equipped with antivenom;
-
○Educate communities about local species that are dangerous, appropriate situationally relevant first aid measures, and the benefits of early medical care and use of effective antivenoms;
-
○Provide accessible information about the availability of treatment in affected communities using a range of tools, media and community engagement;
-
○Provide free access to emergency SBE treatment (including antivenoms) to affected communities, incorporate coverage for out-of-pockets into national health care subsidies or insurance schemes, and include SBE in national efforts to achieve UHC.
-
○
-
•Improve in country antivenom distribution:
-
○Use GIS mapping tools to model populations at risk of SBE and antivenom needs, and optimize antivenom procurement and distribution;
-
○Strengthen first responder health services such as village first aid providers, primary health care centres and decentralized rural ambulance services;
-
○Improve the training and education of rural health workers in the diagnosis, treatment and care of snakebite emergencies, including rational antivenom use and appropriate early management of adverse drug events.
-
○
Alt-text: Box 3
Credit author statement
Julien Potet: design of the study, collection of information, preparation of the first draft, editing of the first draft, revision of the final draft, validation of the final draft. David Beran: design of the study, collection of information, editing of the first draft, revision of the final draft, validation of the final draft. Nicolas Ray: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Gabriel Alcoba: collection of information, editing of the first draft, validation of the final draft. Abdulrazaq Garba Habib: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Garba Iliyasu: collection of information, editing of the first draft, validation of the final draft. Benjamin Waldmann: collection of information, editing of the first draft, validation of the final draft. Ravikar Ralph: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Mohammad Abul Faiz: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Wuelton Marcelo Monteiro: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Jacqueline de Almeida Gonçalves Sachett: collection of information, editing of the first draft, validation of the final draft. Jose Luis di Fabio: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. María de los Ángeles Cortés: collection of information, preparation of the first draft, editing of the first draft, validation of the final draft. Nicholas I. Brown: collection of information, preparation of the first draft, editing of the first draft, revision of the final draft, validation of the final draft. David J. Williams: design of the study, collection of information, preparation of the first draft, editing of the first draft, revision of the final draft, validation of the final draft.
Ethical statement
On behalf of the group of co-authors, Julien Potet confirms that the manuscript “Access to antivenoms in the developing world: A multidisciplinary analysis” was prepared following standard ethical guidelines for scientific publications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Nicolas Ray acknowledges the support of the Swiss National Science Foundation (SNSF) [project number 315130_176271, website: http://p3.snf.ch/project-176271]. Abdulrazaq Garba Habib and Garba Iliyasu are members of the African Snakebite Research Group (ASRG) project and the Scientific Research Partnership for Neglected Tropical Snakebite (SRPNTS) that are supported by NIHR (UK) and DfID (UK) respectively. Wuelton Monteiro has project funding from the Brazilian Ministry of Health (no. 733781/19–035).
Handling Editor: Dr. Glenn King
References
- Abubakar I.S., Abubakar S.B., Habib A.G., Nasidi A., Durfa N., Yusuf P.O., Larnyang S., Garnvwa J., Sokomba E., Salako L., Theakston R.D.G., Juszczak E., Alder N., Warrell D.A. Randomised controlled double-blind non-inferiority trial of two antivenoms for Saw-scaled or carpet viper (Echis ocellatus) envenoming in Nigeria. PLoS Neglected Trop. Dis. 2010;4:8–17. doi: 10.1371/journal.pntd.0000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonkhai V., Martins S.F., Portet A., Lumpkin M., Hartman D. Speeding access to vaccines and medicines in low- and middle-income countries: a case for change and a framework for optimized product market authorization. PLoS One. 2016;11:1–12. doi: 10.1371/journal.pone.0166515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth S., Menzies S.K., Casewell N.R., Harrison R.A. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Neglected Trop. Dis. 2020;14:1–25. doi: 10.1371/journal.pntd.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoba G., Chabloz M., Eyong J., Wanda F., Ochoa C., Comte E., Nkwescheu A., Chappuis F. Snakebite epidemiology and health-seeking behavior in akonolinga health district, Cameroon: cross-sectional study. PLoS Neglected Trop. Dis. 2020;14:1–15. doi: 10.1371/journal.pntd.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoba G., Ochoa C., Babo Martins S., Ruiz de Castañeda R., Bolon I., Wanda F., Comte E., Subedi M., Shah B., Ghimire A., Gignoux E., Luquero F., Nkwescheu A.S., Sharma S.K., Chappuis F., Ray N. Novel transdisciplinary methodology for cross-sectional analysis of snakebite epidemiology at national scale. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfred S., Bates D., White J., Mahmood M.A., Warrell D.A., Thwin K.T., Thein M.M., Sint San S.S., Myint Y.L., Swe H.K., Kyaw K.M., Zaw A., Peh C.A. Acute kidney injury following eastern russell's viper (Daboia siamensis) snakebite in Myanmar. Kidney Int. Rep. 2019;4:1337–1341. doi: 10.1016/j.ekir.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirol E., Lechevalier P., Zamatto F., Chappuis F., Alcoba G., Potet J. Antivenoms for snakebite envenoming: what is in the research pipeline? PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirol E., Sharma S.K., Bawaskar H.S., Kuch U., Chappuis F. Snake bite in South Asia: a review. PLoS Neglected Trop. Dis. 2010;4:e603. doi: 10.1371/journal.pntd.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameade E.P.K., Bonney I., Boateng E.T. Health professionals' overestimation of knowledge on snakebite management, a threat to the survival of snakebite victims—a cross-sectional study in Ghana. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arik M., Bamenyekanye E., Fimbo A., Kabatende J., Kijo A.S., Simai B., Siyoi F., Azatyan S., Ambali A., Cooke E., Mashingia J.H., Mwesigye J.P., Ndomondo-Sigonda M., Sillo H., Sonoiya S., Tanui P., Ward M., Delano T. Optimizing the East african community's medicines regulatory harmonization initiative in 2020–2022: a Roadmap for the future. PLoS Med. 2020;17:1–11. doi: 10.1371/JOURNAL.PMED.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai V. The challenges confronting public hospitals in India, their origins, and possible solutions. Adv. Publ. Health. 2014;2014:1–27. doi: 10.1155/2014/898502. [DOI] [Google Scholar]
- Bala A.A., Jatau A.I., Yunusa I., Mohammed M., Mohammed A.K.H., Isa A.M., Sadiq W.A., Gulma K.A., Bello I., Malami S., Michael G.C., Chedi B.A.Z. Development and validation of antisnake venom knowledge assessment tool (AKAT) for healthcare practitioners. Toxicon X. 2020;8 doi: 10.1016/j.toxcx.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawaskar H.S., Bawaskar Pramodini Himmatrao, Bawaskar Parag Himmatrao. Primary health care for snakebite in India is inadequate. Lancet. 2020;395:112. doi: 10.1016/S0140-6736(19)31909-9. [DOI] [PubMed] [Google Scholar]
- Beran D., Lazo-Porras M., Mba C.M., Mbanya J.C. A global perspective on the issue of access to insulin. Diabetologia. 2021 doi: 10.1007/s00125-020-05375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti O.K., Singh G. Snakebite management through free emergency ambulance service in Himachal saves lives. Indian J. Appl. Res. 2015;5 doi: 10.1371/journal.pntd.0001018. (INDIAN) [DOI] [Google Scholar]
- Blessmann J., Nguyen T.P.N., Bui T.P.A., Krumkamp R., Vo V.T., Nguyen H.L. Incidence of snakebites in 3 different geographic regions in Thua Thien Hue province, central Vietnam: green pit vipers and cobras cause the majority of bites. Toxicon. 2018;156:61–65. doi: 10.1016/j.toxicon.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Boyer L.V. On 1000-fold pharmaceutical price markups and why drugs cost more in the United States than in Mexico. Am. J. Med. 2015 doi: 10.1016/j.amjmed.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Brown N., Landon J. Antivenom: the most cost-effective treatment in the world? Toxicon. 2010 doi: 10.1016/j.toxicon.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Brown N.I. Consequences of neglect: analysis of the sub-Saharan African snake antivenom market and the global context. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J.J., Arias A.S., Rodríguez Y., Quesada-Bernat S., Sánchez L.V., Chippaux J.P., Pla D., Gutiérrez J.M. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: neutralization of toxic activities and antivenomics. Toxicon. 2016;119:280–288. doi: 10.1016/j.toxicon.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Chippaux J.P., Habib A.G. Antivenom shortage is not circumstantial but structural. Trans. R. Soc. Trop. Med. Hyg. 2015;109:747–748. doi: 10.1093/trstmh/trv088. [DOI] [PubMed] [Google Scholar]
- Cristino J.S., Salazar G.M., Machado V.A., Honorato E., Farias A.S., Vissoci J.R.N., Silva Neto A.V., Lacerda M., Wen F.H., Monteiro W.M., Sachett J.A.G. A painful journey to antivenom: the therapeutic itinerary of snakebite patients in the Brazilian Amazon (The QUALISnake Study) PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A.M., Colombini M., Moura-da-Silva A.M., de Souza R.M., Monteiro W.M., Bernarde P.S. Ethno-knowledge and attitudes regarding snakebites in the alto juruá region, western Brazilian amazonia. Toxicon. 2019;171:66–77. doi: 10.1016/j.toxicon.2019.10.238. [DOI] [PubMed] [Google Scholar]
- da Silva A.M., Mendes V.K. da G., Monteiro W.M., Bernarde P.S. Non-venomous snakebites in the western Brazilian amazon. Rev. Soc. Bras. Med. Trop. 2019;52:2–5. doi: 10.1590/0037-8682-0120-2019. [DOI] [PubMed] [Google Scholar]
- da Silva Souza A., de Almeida Gonçalves Sachett J., Alcântara J.A., Freire M., Alecrim M., das G.C., Lacerda M., de Lima Ferreira L.C., Fan H.W., de Souza Sampaio V., Monteiro W.M. Snakebites as cause of deaths in the western Brazilian amazon: why and who dies? Deaths from snakebites in the amazon. Toxicon. 2018;145:15–24. doi: 10.1016/j.toxicon.2018.02.041. [DOI] [PubMed] [Google Scholar]
- Delamater P.L., Messina J.P., Shortridge A.M., Grady S.C. Measuring geographic access to health care: raster and network-based methods. Int. J. Health Geogr. 2012;11:15. doi: 10.1186/1476-072X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer M. 2021. Overcoming Neglect. Finding Ways to Manage and Control Neglected Tropical Diseases.https://www.msf.org/overcoming-neglect-report-ntds [WWW document]. URL. [Google Scholar]
- DeRoeck D., Bawazir S.A., Carrasco P., Kaddar M., Brooks A., Fitzsimmons J., Andrus J. Regional group purchasing of vaccines: review of the Pan American Health Organization EPI revolving fund and the Gulf Cooperation Council group purchasing program. Int. J. Health Plann. Manag. 2006;21:23–43. doi: 10.1002/hpm.822. [DOI] [PubMed] [Google Scholar]
- Di Fabio J.L., de los Ángeles Cortés Castillo M., Griffiths E. Landscape of research, production, and regulation in venoms and antivenoms: a bibliometric analysis. Rev. Panam. Salud Publica/Pan Am. J. Publ. Health. 2021;45:1–13. doi: 10.26633/RPSP.2021.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einterz E.M., Bates M.E. Snakebite in northern Cameroon: 134 victims of bites by the saw-scaled or carpet viper, Echis ocellatus. Trans. R. Soc. Trop. Med. Hyg. 2003;97:693–696. doi: 10.1016/S0035-9203(03)80105-0. [DOI] [PubMed] [Google Scholar]
- Erickson L.T., Litschka-Koen T., Pons J., Bulfone T.C., Bhendile G., Fuller S., Harrington E., Harrison J., Samuel S.L.M. The “Snake song”: a pilot study of musical intervention in Eswatini. Rural Rem. Health. 2020 doi: 10.22605/RRH5494. [DOI] [PubMed] [Google Scholar]
- Fan H.W., Monteiro W.M. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon. 2018;151:15–23. doi: 10.1016/j.toxicon.2018.06.070. [DOI] [PubMed] [Google Scholar]
- Feitosa E.L., Sampaio V.S., Salinas J.L., Queiroz A.M., Da Silva I.M., Gomes A.A., Sachett J., Siqueira A.M., Ferreira L.C.L., Dos Santos M.C., Lacerda M., Monteiro W., Gutiérrez J.M. Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: a case-control study. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0132237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitosa E.S., Sampaio V., Sachett J., De Castro D.B., Noronha M., das D.N., Lozano J.L.L., Muniz E., De Ferreira L.C.L., De Lacerda M.V.G., Monteiro W.M. Snakebites as a largely neglected problem in the brazilian amazon: highlights of the epidemiological trends in the state of amazonas. Rev. Soc. Bras. Med. Trop. 2015;48:34–41. doi: 10.1590/0037-8682-0105-2013. [DOI] [PubMed] [Google Scholar]
- Fung H.T.J., Lam S.K.T., Lam K.K., Kam C.W., Simpson I.D. A survey of snakebite management knowledge amongst select physicians in Hong Kong and the implications for snakebite training. Wilderness Environ. Med. 2009;20:364–372. doi: 10.1580/1080-6032-020.004.0364. [DOI] [PubMed] [Google Scholar]
- Gampini S., Nassouri S., Chippaux J.P., Semde R. Retrospective study on the incidence of envenomation and accessibility to antivenom in Burkina Faso. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016;22:1–5. doi: 10.1186/s40409-016-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus D.P., Herrera D.F., Troya C.J., Guevara A.H. Management of snakebite and systemic envenomation in rural Ecuador using the 20-minute whole blood clotting test. Wilderness Environ. Med. 2013;24:345–350. doi: 10.1016/j.wem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Geneviève L.D., Ray N., Chappuis F., Alcoba G., Mondardini M.R., Bolon I., Ruiz de Castañeda R. Participatory approaches and open data on venomous snakes: a neglected opportunity in the global snakebite crisis? PLoS Neglected Trop. Dis. 2018;12:1–10. doi: 10.1371/journal.pntd.0006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Maisnam I., Murmu B.K., Mitra P.K., Roy A., Simpson I.D. A locally developed snakebite management protocol significantly reduces overall anti snake venom utilization in West Bengal, India. Wilderness Environ. Med. 2008;19:267–274. doi: 10.1580/08-WEME-OR-219.1. [DOI] [PubMed] [Google Scholar]
- Global Snakebite Initiative . A Report to the World Health Organization; 2020. A Global Survey of Snake Antivenom Manufacturers. (Unpublished) [Google Scholar]
- Goldstein E., Erinjery J.J., Martin G., Kasturiratne A., Ediriweera D.S., de Silva H.J., Diggle P., Lalloo D.G., Murray K.A., Iwamura T. Integrating human behavior and snake ecology with agent-based models to predict snakebite in high risk landscapes. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães S.M.F. The Sanumá-Yanomami medical system and indigenous peoples' health policy in Brazil. Cad. Saúde Pública. 2015;31:2148–2156. doi: 10.1590/0102-311X00194414. [DOI] [PubMed] [Google Scholar]
- Guimarães W.S.G., Parente R.C.P., Guimarães T.L.F., Garnelo L. Access to prenatal care and quality of care in the family health strategy: infrastructure, care, and management. Cad. Saúde Pública. 2018;34:1–13. doi: 10.1590/0102-311x00110417. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60 doi: 10.1016/j.toxicon.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Global availability of antivenoms: the relevance of public manufacturing laboratories. Toxins. 2019;11 doi: 10.3390/toxins11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017;3:17063. doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- Habib A.G. Public health aspects of snakebite care in West Africa: perspectives from Nigeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013;19:27. doi: 10.1186/1678-9199-19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G., Lamorde M., Dalhat M.M., Habib Z.G., Kuznik A. Cost-effectiveness of antivenoms for snakebite envenoming in Nigeria. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G., Musa B.M., Iliyasuid G., Hamza M., Kuznik A., Chippauxid J.P. Challenges and prospects of snake antivenom supply in Sub-Saharan Africa. PLoS Neglected Trop. Dis. 2020;14:1–10. doi: 10.1371/journal.pntd.0008374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza M., Idris M.A., Maiyaki M.B., Lamorde M., Chippaux J.P., Warrell D.A., Kuznik A., Habib A.G. Cost-effectiveness of antivenoms for snakebite envenoming in 16 countries in West africa. PLoS Neglected Trop. Dis. 2016;10:1–16. doi: 10.1371/journal.pntd.0004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E., Sasa M., Mattisson K., Robles A., Gutiérrez J.M. Using geographical information systems to identify populations in need of improved accessibility to antivenom treatment for snakebite envenoming in Costa Rica. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.B., Faiz M.A., Rahman M.R., Jalil M.M.A., Ahsan M.F., Theakston R.D.G., Warrell D.A., Kuch U. Snake bite in Chittagong Division, Bangladesh: a study of bitten patients who developed no signs of systemic envenoming. Trans. R. Soc. Trop. Med. Hyg. 2010;104:320–327. doi: 10.1016/j.trstmh.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Harrison R.A., Oluoch G.O., Ainsworth S., Alsolaiss J., Bolton F., Arias A.S., Gutiérrez J.M., Rowley P., Kalya S., Ozwara H., Casewell N.R. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Neglected Trop. Dis. 2017;11:1–24. doi: 10.1371/journal.pntd.0005969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawe P.B., Muhammad J.O., Nwokoro U.U., Abubakar J.D., Isah S.I., Aketemo U., Balogun M.S., Nguku P. Snakebite outbreak and associated risk factors in Donga, taraba state, Nigeria, june, 2016. Pan Afr. Med. J. 2020;37:1–8. doi: 10.11604/pamj.2020.37.82.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliyasu G., Tiamiyu A.B., Daiyab F.M., Tambuwal S.H., Habib Z.G., Habib A.G. Effect of distance and delay in access to care on outcome of snakebite in rural north-eastern Nigeria. Rural Rem. Health. 2015;15:1–6. [PubMed] [Google Scholar]
- Imani . 2019. Still on Ineffective Anti-snake Serum and Procurement Breaches - Evidence Finally.https://imaniafrica.org/2019/02/28/still-on-ineffective-anti-snake-serum-and-procurement-breaches-evidence-finally/ [WWW document]. URL. [Google Scholar]
- Islam A., Biswas T. Health system in Bangladesh: challenges and opportunities. Am. J. Health Res. 2014;2:366. doi: 10.11648/j.ajhr.20140206.18. [DOI] [Google Scholar]
- Knudsen C., Jürgensen J.A., Føns S., Haack A.M., Friis R.U.W., Dam S.H., Bush S.P., White J., Laustsen A.H. Snakebite envenoming diagnosis and diagnostics. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.661457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam A., Camara B., Kane O., Diouf A., Chippaux J.P. Epidemiology of snakebites in Kédougou region (eastern Senegal): comparison of various methods for assessment of incidence and mortality. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016;22:1–6. doi: 10.1186/s40409-016-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León G., VargasM, Segura A., Herrera M., Villalta M., Sánchez M., Solano M., Gómez A., Sánchez M., Estrada R., G J. Current technology for the industrial manufacture of snake antivenoms. Toxicon. 2018;151:63–73. doi: 10.1016/j.toxicon.2018.06.084. [DOI] [PubMed] [Google Scholar]
- Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., Ray S.E., Ray N., Warrell D.A., Ruiz de Castañeda R., Williams D.J., Hay S.I., Pigott D.M. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longkumer T., Armstrong L.J., Finny P. Outcome determinants of snakebites in North Bihar, India: a prospective hospital based study. J. Venom Res. 2017;8:14–18. [PMC free article] [PubMed] [Google Scholar]
- Mahmood M.A., Halliday D., Cumming R., Thwin K.T., Myitzu M., White J., Alfred S., Warrell D.A., Bacon D., Naing W., Aung H., Thein M.M., Chit N.N., Serhal S., Nwe M.T., Aung P.P., Peh C.A. Inadequate knowledge about snakebite envenoming symptoms and application of harmful first aid methods in the community in high snakebite incidence areas of Myanmar. PLoS Neglected Trop. Dis. 2019;13:1–10. doi: 10.1371/journal.pntd.0007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGain F., Limbo A., Williams D.J., Didei G., Winkel K.D. Snakebite mortality at port moresby general hospital, Papua New Guinea, 1992-2001. Med. J. Aust. 2004;181:687–691. doi: 10.5694/j.1326-5377.2004.tb06525.x. [DOI] [PubMed] [Google Scholar]
- Michael G.C., Grema B.A., Aliyu I., Alhaji M.A., Lawal T.O., Ibrahim H., Fikin A.G., Gyaran F.S., Kane K.N., Thacher T.D., Badamasi A.K., Ogwuche E. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria: a cross-sectional multicentre study. Trans. R. Soc. Trop. Med. Hyg. 2018;112:47–56. doi: 10.1093/trstmh/try028. [DOI] [PubMed] [Google Scholar]
- Michael G.C., Thacher T.D., Shehu M.I.L. The effect of pre-hospital care for venomous snake bite on outcome in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2011;105:95–101. doi: 10.1016/j.trstmh.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Monteiro W.M., Farias A.S., De Val F., Neto A.V.S., Sachett A., Lacerda M., Sampaio V., Cardoso D., Garnelo L., Vissoci J.R.N., Sachett J., Wen F.H. Providing antivenom treatment access to all Brazilian amazon indigenous areas: “every life has equal value”. Toxins (Basel) 2020 doi: 10.3390/toxins12120772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais V.M., Massaldi H. Snake antivenoms: adverse reactions and production technology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009 doi: 10.1590/S1678-91992009000100002. [DOI] [Google Scholar]
- Neutens T. Accessibility, equity and health care: review and research directions for transport geographers. J. Transport Geogr. 2015;43:14–27. doi: 10.1016/j.jtrangeo.2014.12.006. [DOI] [Google Scholar]
- Ochoa C., Bolon I., Durso A.M., Ruiz De Castañeda R., Alcoba G., Babo Martins S., Chappuis F., Ray N. Assessing the increase of snakebite incidence in relationship to flooding events. J. Environ. Public Health. 2020;2020 doi: 10.1155/2020/6135149. [DOI] [Google Scholar]
- Okumu M.O., Patel M.N., Bhogayata F.R., Ochola F.O., Olweny I.A., Onono J.O., Gikunju J.K. Management and cost of snakebite injuries at a teaching and referral hospital in Western Kenya. F1000Research. 2019;8:1588. doi: 10.12688/f1000research.20268.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G.I., van Oirschot J., Waldmann B. 2021. Snakebite in Kenya: Evidence from the Field. Research data from 2019–2020. [Google Scholar]
- Ooms G.I., van Oirschot J., Waldmann B., von Bernus S., van den Ham H.A., Mantel-Teeuwisse A.K., Reed T. The current state of snakebite care in Kenya, Uganda, and Zambia: healthcare workers' perspectives and knowledge, and health facilities' treatment capacity. Am. J. Trop. Med. Hyg. 2020 doi: 10.4269/ajtmh.20-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécoul B., Chirac P., Trouiller P., Pinel J. Access to essential drugs in poor countries: a lost battle? J. Am. Med. Assoc. 1999;281:361–367. doi: 10.1001/jama.281.4.361. [DOI] [PubMed] [Google Scholar]
- Petras D., Sanz L., Segura A., Herrera M., Villalta M., Solano D., Vargas M., León G., Warrell D. a, Theakston R.D.G., Harrison R. a, Durfa N., Nasidi A., Gutiérrez J.M., Calvete J.J. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011;10:1266–1280. doi: 10.1021/pr101040f. [DOI] [PubMed] [Google Scholar]
- Pierini S.V., Warrell D.A., De Paulo A., Theakston R.D.G. High incidence of bites and stings by snakes and other animals among rubber tappers and Amazonian Indians of the Jurua valley, acre state, Brazil. Toxicon. 1996;34:225–236. doi: 10.1016/0041-0101(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Pintor A. Solving the snake bite crisis with geospatial analyses – novel approaches and future directions in times of ‘big data’. Toxicon X. 2021 (in press) [Google Scholar]
- Pla D., et al. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. J. Proteomics. 2019;207 doi: 10.1016/j.jprot.2019.103443. [DOI] [PubMed] [Google Scholar]
- Potet J., Smith J., McIver L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-saharan africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph R., Sharma S.K., Faiz M.A., Ribeiro I., Rijal S., Chappuis F., Kuch U. The timing is right to end snakebite deaths in South Asia. BMJ. 2019;364:1–6. doi: 10.1136/bmj.k5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N., Ebener S. AccessMod 3.0: computing geographic coverage and accessibility to health care services using anisotropic movemen of patients. Int. J. Health Geogr. 2008;7:1–17. doi: 10.1186/1476-072X-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota S., Pandey D.P., Dhakal G.P., Gurung D.B. Knowledge of health workers on snakes and snakebite management and treatment seeking behavior of snakebite victims in Bhutan. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioldann E., Mahmood M.A., Kyaw M.M., Halliday D., Thwin K.T., Chit N.N., Cumming R., Bacon D., Alfred S., White J., Warrell D., Peh C.A. Why snakebite patients in Myanmar seek traditional healers despite availability of biomedical care at hospitals? Community perspectives on reasons. PLoS Neglected Trop. Dis. 2018;12:1–14. doi: 10.1371/journal.pntd.0006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.C. India still struggles with rural doctor shortages. Lancet. 2015;386:2381–2382. doi: 10.1016/S0140-6736(15)01231-3. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Bovier P., Jha N., Alirol E., Loutan L., Chappuis F. Effectiveness of rapid transport of victims and community health education on snake bite fatalities in rural Nepal. Am. J. Trop. Med. Hyg. 2013;89:145–150. doi: 10.4269/ajtmh.12-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B.R., Pandey D.P., Acharya K.P., Thapa-Magar C., Mohamed F., Isbister G.K. Effective, polyvalent, affordable antivenom needed to treat snakebite in Nepal. Bull. World Health Organ. 2017;95:718–719. doi: 10.2471/BLT.17.195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.L. da, Fonseca W.L. da, Mota da Silva A., Amaral G.L.G. do, Ortega G.P., Oliveira A. de S., Correa R.R., Oliveira I., Monteiro W.M., Bernarde P.S. Venomous snakes and people in a floodplain forest in the Western Brazilian Amazon: potential risks for snakebites. Toxicon. 2020;187:232–244. doi: 10.1016/j.toxicon.2020.09.007. [DOI] [PubMed] [Google Scholar]
- Simpson I.D. Snakebite management in India, the first few hours: a guide for primary care physicians. J. Indian Med. Assoc. 2007;105 [PubMed] [Google Scholar]
- Singh S., Badaya S. Health care in rural India: a lack between need and feed. South Asian J. cancer. 2014;3:143–144. doi: 10.4103/2278-330X.130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D.J., Dedicoat M.J., Lalloo D.G. Healthcare-seeking behaviour and use of traditional healers after snakebite in Hlabisa sub-district, KwaZulu Natal. Trop. Med. Int. Health. 2007;12:1386–1390. doi: 10.1111/j.1365-3156.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- Suraweera W., Warrell D., Whitaker R., Menon G., Rodrigues R., Fu S.H., Begum R., Sati P., Piyasena K., Bhatia M., Brown P., Jha P. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife. 2020;9:1–37. doi: 10.7554/eLife.54076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb F., Dub T., Madec Y., Tondeur L., Chippaux J.P., Lebreton M., Medang R., Foute F.N.N., Tchoffo D., Potet J., Alcoba G., Comte E., Einterz E.M., Nkwescheu A.S. Knowledge, attitude and practices of snakebite management amongst health workers in Cameroon: need for continuous training and capacity building. PLoS Neglected Trop. Dis. 2018;12:1–10. doi: 10.1371/journal.pntd.0006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.H., Liew J.L., Tan K.Y., Tan N.H. Assessing SABU (Serum Anti Bisa Ular), the sole Indonesian antivenom: a proteomic analysis and neutralization efficacy study. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep37299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D.G., Warrell D.A. Crisis in snake antivenom supply for Africa [14] Lancet. 2000;356:2104. doi: 10.1016/S0140-6736(05)74319-1. [DOI] [PubMed] [Google Scholar]
- United Nations . 2017. Work of the Statistical Commission Pertaining to the 2030 Agenda for Sustainable Development.https://undocs.org/A/RES/71/313 (A/RES/71/313) [WWW document]. Resolut. A/RES/71/313. URL. [Google Scholar]
- Vaiyapuri S., Vaiyapuri R., Ashokan R., Ramasamy K., Nattamaisundar K., Jeyaraj A., Chandran V., Gajjeraman P., Baksh M.F., Gibbins J.M., Hutchinson E.G. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PLoS One. 2013;8:10–13. doi: 10.1371/journal.pone.0080090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta M., Sánchez A., Herrera M., Vargas M., Segura A., Cerdas M., Estrada R., Gawarammana I., Keyler D.E., McWhorter K., Malleappah R., Alape-Girón A., León G., Gutiérrez J.M. Development of a new polyspecific antivenom for snakebite envenoming in Sri Lanka: analysis of its preclinical efficacy as compared to a currently available antivenom. Toxicon. 2016;122:152–159. doi: 10.1016/j.toxicon.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Visser L.E., Kyei-Faried S., Belcher D.W., Geelhoed D.W., van Leeuwen J.S., van Roosmalen J. Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans. R. Soc. Trop. Med. Hyg. 2008;102:445–450. doi: 10.1016/j.trstmh.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Vongphoumy I., Phongmany P., Sydala S., Prasith N., Reintjes R., Blessmann J. Snakebites in two rural districts in Lao PDR: community-based surveys disclose high incidence of an invisible public health problem. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D. a. Unscrupulous marketing of snake bite antivenoms in Africa and Papua New Guinea: choosing the right product--‘what's in a name?’. Trans. R. Soc. Trop. Med. Hyg. 2008;102:397–399. doi: 10.1016/j.trstmh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Watson J.A., et al. A Bayesian phase 2 model based adaptive design to optimise antivenom dosing: Application to a dose-finding trial for a novel Russell’s viper antivenom in Myanmar. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R., Whitaker S. Venom, antivenom production and the medically important snakes of India. Curr. Sci. 2012;103:635–643. [Google Scholar]
- WHO . 2020. Ending the Neglect to Attain the Sustainable Development Goals.https://www.who.int/publications/i/item/WHO-UCN-NTD-2020.01 A road map for neglected tropical diseases 2021–2030. Overview [WWW Document]. URL. [Google Scholar]
- WHO . 2019. Snakebite Envenoming: A Strategy for Prevention and Control.https://www.who.int/snakebites/resources/9789241515641/en/ [WWW Document]. URL. [DOI] [PubMed] [Google Scholar]
- Who . 2017. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins.https://www.who.int/bloodproducts/AntivenomGLrevWHO_TRS_1004_web_Annex_5.pdf [WWW Document]. WHO Tech. Rep. Ser. URL. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee . 1977. The Selection of Essential Drugs.https://apps.who.int/iris/handle/10665/41272 [WWW Document]. WHO Tech. Rep. Ser. URL. [Google Scholar]
- Who Website a Venomous snakes distribution and species risk categories. https://apps.who.int/bloodproducts/snakeantivenoms/database/ (n.d.) [WWW Document]. URL.
- Who Website b WHO issues new recommendation on antivenom for snakebites. https://www.who.int/news/item/19-08-2018-who-issues-new-recommendation-on-antivenom-for-snakebites (n.d.) [WWW Document]. URL.
- Who Website c Promoting rational use of medicines. https://www.who.int/activities/promoting-rational-use-of-medicines (n.d.) [WWW Document]. URL.
- Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., Harrison R.A., Warrell D.A. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Neglected Trop. Dis. 2019;13:12–14. doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.J., Gutiérrez J.-M., Calvete J.J., Wüster W., Ratanabanangkoon K., Paiva O., Brown N.I., Casewell N.R., Harrison R. a, Rowley P.D., O'Shea M., Jensen S.D., Winkel K.D., Warrell D. a. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics. 2011;74:1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Williams D.J., Habib A.G., Warrell D.A., et al. Clinical studies of the effectiveness and safety of antivenoms. Toxicon. 2018;150 doi: 10.1016/j.toxicon.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Wirtz V.J., Hogerzeil H.V., Gray A.L., Bigdeli M., de Joncheere C.P., Ewen M.A., Gyansa-Lutterodt M., Jing S., Luiza V.L., Mbindyo R.M., Möller H., Moucheraud C., Pécoul B., Rägo L., Rashidian A., Ross-Degnan D., Stephens P.N., Teerawattananon Y., Hoen E.F.M., Wagner A.K., Yadav P., Reich M.R. Essential medicines for universal health coverage. Lancet. 2017;389:403–476. doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Regional Office for South-East Asia . 2002. Management of Snakebite and Research. Report and Working Papers of a Seminar Yangon, Myanmar. [Google Scholar]
- Yates V.M., Lebas E., Orpiay R., Bale B.J. Management of snakebites by the staff of a rural clinic: the impact of providing free antivenom in a nurse-led clinic in Meserani, Tanzania. Ann. Trop. Med. Parasitol. 2010;104:439–448. doi: 10.1179/136485910X12743554760306. [DOI] [PubMed] [Google Scholar]
- Yen C., Hyde T.B., Costa A.J., Fernandez K., Tam J.S., Hugonnet S., Huvos A.M., Duclos P., Dietz V.J., Burkholder B.T. The development of global vaccine stockpiles. Lancet Infect. Dis. 2015;15:340–347. doi: 10.1016/S1473-3099(14)70999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]