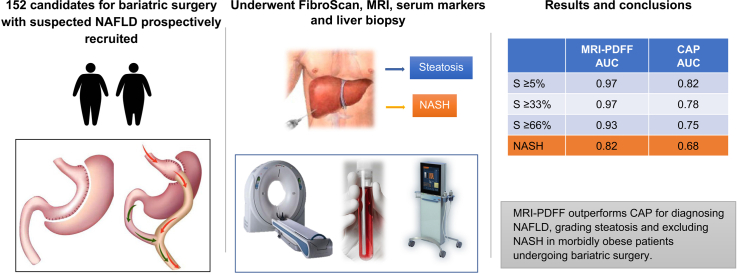
Abstract
Background & Aims
Tools for the non-invasive diagnosis of non-alcoholic steatohepatitis (NASH) in morbidly obese patients with suspected non-alcoholic fatty liver disease (NAFLD) are an unmet clinical need. We prospectively compared the performance of transient elastography, MRI, and 3 serum scores for the diagnosis of NAFLD, grading of steatosis and detection of NASH in bariatric surgery candidates.
Methods
Of 186 patients screened, 152 underwent liver biopsy, which was used as a reference for NAFLD (steatosis [S]>5%), steatosis grading and NASH diagnosis. Biopsies were read by a single expert pathologist. MRI-based proton density fat fraction (MRI-PDFF) was measured in an open-bore, vertical field 1.0T scanner and controlled attenuation parameter (CAP) was measured by transient elastography, using the XL probe. Serum scores (SteatoTest, hepatic steatosis index and fatty liver index) were also calculated.
Results
The applicability of MRI was better than that of FibroScan (98% vs. 79%; p <0.0001). CAP had AUROCs of 0.83, 0.79, 0.73 and 0.69 for S>5%, S>33%, S>66% and NASH, respectively. Transient elastography had an AUROC of 0.80 for significant fibrosis (F0-F1 vs. F2-F3). MRI-PDFF had AUROCs of 0.97, 0.95, 0.92 and 0.84 for S>5%, S>33%, S>66% and NASH, respectively. When compared head-to-head in the 97 patients with all valid tests available, MRI-PDFF outperformed CAP for grading steatosis (S>33%, AUROC 0.97 vs. 0.78; p <0.0003 and S>66%, AUROC 0.93 vs. 0.75; p = 0.0015) and diagnosing NASH (AUROC 0.82 vs. 0.68; p = 0.0056). When compared in “intention to diagnose” analysis, MRI-PDFF outperformed CAP, hepatic steatosis index and fatty liver index for grading steatosis (S>5%, S>33% and S>66%).
Conclusion
MRI-PDFF outperforms CAP for diagnosing NAFLD, grading steatosis and excluding NASH in morbidly obese patients undergoing bariatric surgery.
Lay summary
Non-invasive tests for detecting fatty liver and steatohepatitis, the active form of the disease, have not been well studied in obese patients who are candidates for bariatric surgery. The most popular tests for this purpose are Fibroscan, which can be used to measure the controlled attenuation parameter (CAP), and magnetic resonance imaging, which can be used to measure the proton density fat fraction (MRI-PDFF). We found that, when taking liver biopsy as a reference, MRI-PDFF performed better than CAP for detecting and grading fatty liver as well as excluding steatohepatitis in morbidly obese patients undergoing bariatric surgery.
Keywords: Non-invasive diagnosis, steatosis, NAFLD, NASH, transient elastography, CAP, MRI-PDFF, bariatric surgery
Abbreviations: AUROC, area under the receiver operating characteristic curve; CAP, controlled attenuation parameter; FLI, fatty liver index; FLIP, fatty liver inhibition of progression; HSI, hepatic steatosis index; LSM, liver stiffness measurement; MRI-PDFF, MRI-proton density fat fraction; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity; ST, SteatoTest; TE, transient elastography
Graphical abstract
Highlights
-
•
NAFLD/NASH is common in morbidly obese patients undergoing bariatric surgery.
-
•
Non-invasive diagnosis of NAFLD/NASH is an unmet need in this population.
-
•
We compared MRI-PDFF, CAP and serum scores for grading steatosis and diagnosing NASH, using liver biopsy as a reference.
-
•
Applicability of magnetic resonance imaging was better than that of Fibroscan.
-
•
MRI-PDFF outperformed CAP for diagnosing and grading steatosis, as well as excluding NASH.
Introduction
Non-alcoholic fatty liver disease (NAFLD), encompassing a wide spectrum of lesions ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis, affects around one-quarter of the general population worldwide.1 NAFLD is frequently associated with metabolic comorbidities such as obesity, type 2 diabetes, hyperlipidemia, hypertension and metabolic syndrome. Although the most common cause of death in patients with NAFLD is cardiovascular disease, independent of other metabolic comorbidities, NAFLD is becoming a major cause of liver disease-related morbidity (e.g., cirrhosis, end-stage liver disease, hepatocellular carcinoma, and liver transplantation), as well as mortality.2,3 Patients with NASH, the active form of NAFLD, characterized histologically by steatosis, lobular inflammation and hepatocyte ballooning are those at greatest risk of developing complications of chronic liver disease.4
In morbidly obese patients, the prevalence of NAFLD and NASH is high: 91% (95% CI 85-98%) and 37% (24-98%), respectively, in a large series (n = 1,620) of candidates for bariatric surgery.5 However, in the preoperative phase of bariatric surgery, NAFLD is usually assessed only with routine ultrasonography, a procedure that not only is operator-dependent, but also has low sensitivity in obese patients and does not provide enough information about the severity of liver injury (NASH and fibrosis).6 Bariatric surgery procedures have increased regularly in most countries over the last 2 decades. For instance, approximately 50,000 procedures are currently performed each year in France.7 Thus, NAFLD is becoming an increasing issue and the key challenge in the management of these patients is to differentiate NASH from isolated steatosis as patients with the former are at high risk of developing cirrhosis and its complications.
Until now, liver biopsy has been the reference test for identifying NASH and fibrosis, but has well-known limitations, including invasiveness, rare but potentially life-threatening complications, poor acceptability; sampling variability, and cost.8,9 Over the past decade, there has been a growing interest in alternative novel non-invasive strategies, relying on 2 different but complementary approaches: serum biomarkers or imaging methods measuring liver fat content and stiffness with either ultrasound- or magnetic resonance-based methods.10 For instance, serum scores such as hepatic steatosis index (HSI), fatty liver index (FLI) and SteatoTest (ST) have been proposed for diagnosing and quantifying steatosis.11 Although they are easy to use, they have not been well evaluated before bariatric surgery.12 Transient elastography (TE) using the FibroScan (Echosens, Paris, France) is an ultrasound-based point of care technique that allows for liver stiffness measurement (LSM), and has good performance for diagnosing severe fibrosis and cirrhosis in patients with NAFLD.13 Additionally, the controlled attenuation parameter (CAP),14 measuring ultrasonic attenuation of the echo wave, has been shown to provide a standardized and rapid non-invasive quantification of hepatic steatosis with good performance.15 However, an important limitation of TE is the high failure rates when using the regular probe (M) in patients with a BMI >30 kg/m2, which applies to many patients with NAFLD.16 This has led to the development of the XL probe, which has been shown to reduce the failure rate for staging fibrosis and grading steatosis in obese patients with NAFLD with good performance.17,18 Regarding morbidly obese patients who are candidates to bariatric surgery, data are scarce so far.[19], [20], [21], [22], [23] MRI-based techniques, such as magnetic resonance elastography (MRE) and proton density fat fraction (PDFF) have been shown to accurately stage fibrosis and grade steatosis, respectively, in patients with NAFLD13 and to outperform TE-CAP for fibrosis staging and steatosis quantification.24,25 However, data in morbidly obese patients undergoing bariatric surgery remains limited.26 In addition, these patients are often difficult to examine in conventional MRI systems with 60 cm bore size and 1.5 or 3.0 T field strength,27 but low field (1T), open-bore systems are available.28 Finally, there has been no head-to-head comparison between MRI-PDFF and CAP in bariatric surgery candidates.
Using a well-characterized, prospective cohort of morbidly obese French patients who underwent liver biopsy during bariatric surgery, we compared the performance of FibroScan-CAP, MRI-PDFF, and 3 serum scores (ST, HSI and FLI) for diagnosing NAFLD, grading steatosis and detecting NASH.
Patients and methods
Study population
This prospective study (NCT01695083) was part of an extensive perioperative data collection conducted in morbidly obese patients who were candidates for bariatric surgery in an expert center29 at Louis Mourier University Hospital, Colombes, France. The study was performed between October 2012 and November 2015 in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. The local ethics committee approved the study and all participants provided written informed consent. Inclusion criteria were morbid obesity (defined as BMI ≥40 kg/m2) or severe obesity (BMI ≥35 kg/m2) with at least 1 comorbid condition (e.g., sleep apnea syndrome, type 2 diabetes, cardiovascular disease or severe joint pain). Patients were considered to have significant sleep apnea syndrome if they were being treated with nocturnal continuous positive-airway pressure; type 2 diabetes if they were taking an antidiabetic treatment or if fasting glycemia was ≥7 mmol/L on at least 2 different occasions; hypertension if they were taking an antihypertensive treatment or if systolic blood pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mmHg on at least 2 different occasions; dyslipidemia if they were taking a lipid-lowering therapy or if total cholesterol was ≥5.7 mmol/L or triglyceride levels were ≥1.7 mmol/L.
All patients had failed to lose weight with non-surgical weight reduction programs (dietary and behavioral management) that had been properly conducted for at least 6 months, and during the study, they had stable weight. No specific diet was recommended before surgery. Exclusion criteria were: age <18 years old, medical or psychological contraindications to bariatric surgery, alcohol consumption above recommended limits (>14 units/week for women and >21 units/week for men; 1 unit = 8 g of ethanol), presence of liver disease other than NAFLD (hepatitis B or C, autoimmune liver disease, hemochromatosis), steatogenic medications (e.g., amiodarone, methotrexate, or corticosteroids).
Clinical and laboratory assessment
The following characteristics were recorded before bariatric surgery for each patient: age, sex, BMI, waist circumference, presence of type 2 diabetes, sleep apnea, hypertension, and dyslipidemia. The following laboratory parameters were also determined: platelet count, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, alkaline phosphatase, bilirubin, prothrombin time, ferritin, total cholesterol, triglycerides, glycated hemoglobin. The homeostasis model assessment of insulin resistance was calculated: [fasting insulin (mUI/ml) x fasting glycemia (mmol/L)]/22.5.
Steatosis scores
Two non-patented steatosis scores, FLI and HSI were calculated using the clinical, anthropometric and laboratory data available before bariatric surgery, according to the published formulas.30,31 In addition, a patented steatosis score, ST,32 was kindly provided by the inventor (Thierry Poynard).
FibroScan-CAP and LSM
CAP (dB/m) and LSM (kPa) were assessed using the FibroScan (Echosens, Paris, France), equipped with both M and XL probes. All examinations were performed within 12 weeks before surgery, after an overnight fast, by a trained operator (>100 exams) blinded to the results of other tests. When the study started, CAP was not available on the XL probe; therefore, the raw ultrasonic radiofrequency signals were stored in the FibroScan examination file to enable computation of CAP off-line. CAP computation was performed blinded to patients’ clinical and histological data using an identical configuration and algorithm to the one embedded in the commercial device. Examinations with fewer than 10 valid measurements or an IQR/median >30% or a success rate <60% were considered unreliable.16
MRI
MRI acquisition was performed in a Panorama Open 1.0T MRI system (Philips Healthcare, Best, The Netherlands) within 12 weeks before surgery, with bore width and height of patient opening of 160 cm and 45 cm, respectively. Multiecho gradient echo imaging was performed (field of view: 41 x 45 cm, reconstructed pixel size: 1.875 mm, three 10-mm thick contiguous slices, 14 echoes evenly spaced from 3.4 ms to 48.3 ms, flip angle: 10°, repetition time: 60 ms). Data processing was carried out with a numerical fitting procedure using Matlab (The Mathworks, Natick, USA). The multiecho gradient echo images were used to calculate the R2∗ corrected liver PDFF as described previously and explained in the supplementary material.33,34 Large regions of interest were drawn by 2 observers (P.G., 12 years’ experience with MRI, S.D., 16 years’ experience with MRI), both blinded to the other index and reference test values. Regions were positioned in the right liver lobe on the first echo image in all available slices by avoiding organ edges and large vessel structures. An average of 3,110 pixels were considered per patient.
Histological assessment
Liver biopsies were performed during bariatric surgery (laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass) in the left hepatic lobe. Biopsy specimens were fixed in formalin and paraffin embedded and stained with H&E, Picrosirius red and Masson’s trichrome for evaluation of fibrosis. Slides were analyzed by the same experienced pathologist (P.B.) blinded to the patient’s clinical data and the results of the different tests. Steatosis was defined according to the number of affected hepatocytes: S0 (<5%), S1 (5–33%), S2 (34–66%), S3 (>66%). Ballooning (0-2), lobular inflammation (0-3), the NAFLD activity score (NAS) (0-8) and fibrosis (0-4), were scored using the NASH CRN scoring system.35 NASH was diagnosed using the “fatty liver inhibition of progression” (FLIP) definition (presence of steatosis, hepatocyte ballooning, and lobular inflammation with at least 1 point for each category), itself based on the SAF (steatosis, activity and fibrosis) score.36,37
Statistical analyses
Continuous variables are reported as mean (SD); categorical variables as frequencies and percentages, n (%). Statistical tests are presented with their 95% CIs. Overall diagnostic accuracies of CAP, MRI-PDFF, and serum biomarkers were determined through the area under the receiver operating characteristic curve (AUROC) using histological data as reference standard. The optimal cut-off value was determined by the Youden’s index method. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were calculated along with their 95% CIs. In the subgroup of 97 patients with all available valid tests, pairwise and multiple comparisons of AUROCs were performed using the DeLong method.38 A pairwise comparative analysis of correctly classified patients (true negative plus true positive) was performed in the 152 patients. The comparison was performed in "intention to diagnose" by calculating summary diagnostic performance in a "worst case scenario" as described by Schuetz et al., i.e. non evaluable cases with a positive gold standard were counted towards false positives and non evaluable cases with a negative gold standard were counted towards false negatives.39
For the adjustment of type I error (alpha) for multiple comparisons, the Hochberg’s method was used. Statistical significance was set to a 2-sided alpha level of 0.05. All statistical analyses were performed using R software v3.6 (The R foundation for Statistical Computing).
Results
Patient characteristics
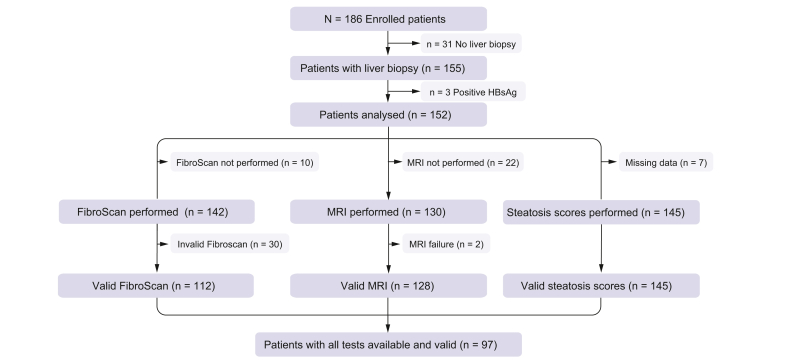
The study flow chart is represented in Fig. 1. Of 186 patients screened, 152 meeting the inclusion criteria and who underwent a liver biopsy were analyzed. Patients baseline characteristics are described in Table 1, Table 2. Most of the patients were female (84%), with a mean age of 42 ± 11 years and a mean BMI of 44.1 ± 5.3 kg/m2. Hypertension, type 2 diabetes, and dyslipidemia were present in 36%, 21%, and 23% of patients, respectively (Table 1). As shown in Table 2, mean liver biopsy length was 13.6 ± 6.3 mm, with biopsies smaller than 10 mm in 24% of patients or with less than 10 portal tracts in 34%. NAFLD (steatosis >5%) was present in 74% of patients and NASH in 30%. Moderate steatosis (>33% = S2-3) was present in 50% of patients and severe steatosis (>66% = S3) in 24%. Fibrosis was present in 51% of patients (F1: 42%; F2: 7%; F3: 2%) and cirrhosis in none.
Fig. 1.
Study flow chart.
Table 1.
Clinical and biological characteristics of the 152 patients with liver biopsy and of the 97 patients with all valid tests available and results on non-invasive tests.
| Variables | Liver biopsy (n = 152) | All valid tests available (n = 97) | NASH (n = 30) |
No NASH (n = 67) |
p value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 42 ± 11 | 41 ± 10 | 43 ± 9 | 40 ± 10 | 0.19 |
| Sex, women, n (%) | 128 (84) | 83 (86) | 19 (63) | 64 (96) | 1.0×10-4 |
| BMI, kg/m2, mean ± SD | 44.1 ± 5.3 | 44.4 ± 5.4 | 46.6 ± 5.8 | 43.4 ± 4.9 | 1.4×10-2 |
| WC, cm, mean ± SD | 124.6 ± 14.5 | 124.5 ± 14.2 | 131.3 ± 11.8 | 121.4 ± 14.2 | 6.9×10-4 |
| Hypertension, n (%) | 55 (36) | 33 (34) | 12 (40) | 21 (31) | 0.40 |
| Sleep apnea, n (%) | 85 (56) | 56 (58) | 22 (73) | 34 (51) | 3.7×10-2 |
| Diabetes, n (%) | 32 (21) | 16 (16) | 9 (30) | 7 (10) | 3.5×10-2 |
| Dyslipidemia, n (%) | 35 (23) | 21 (22) | 9 (30) | 12 (18) | 0.18 |
| Platelets (Giga/L) | 270 ± 63 | 273 ± 64 | 257 ± 67 | 280 ± 62 | 0.10 |
| ALT (IU/L) | 38.7 ± 21.6 | 38.5 ± 21.8 | 52.9 ± 27.5 | 32.1 ± 15.0 | 4.1×10-4 |
| AST (IU/L) | 23.9 ± 9.8 | 23.9 ± 9.5 | 28.7 ± 10.5 | 21.8 ± 8.3 | 2.6×10-3 |
| GGT (IU/L) | 45.4 ± 32.9 | 44.1 ± 31.9 | 57.3 ± 48.6 | 38.2 ± 18.2 | 4.5×10-2 |
| ALP (IU/L) | 83.2 ± 23.4 | 82.2 ± 25.8 | 85.4 ± 26.3 | 80.7 ± 25.6 | 0.41 |
| Bilirubin (μmol/L) | 11.0 ± 3.7 | 10.9 ± 3.9 | 11.7 ± 4.2 | 10.6 ± 3.8 | 0.37 |
| Prothrombin time (%) | 104 ± 8 | 103 ± 8 | 104 ± 7 | 102 ± 8 | 0.38 |
| Ferritin (μg/L) | 116 ± 110 | 118 ± 111 | 170 ± 134 | 95 ± 91 | 8.2×10-3 |
| Total cholesterol (mmol/L) | 5.1 ± 1.03 | 5.18 ± 1.04 | 5.21 ± 1.04 | 5.16 ± 1.04 | 0.85 |
| Triglycerides (mmol/L) | 1.45 ± 0.9 | 1.49 ± 0.8 | 1.86 ± 0.99 | 1.32 ± 0.6 | 9.0×10-3 |
| Glycated hemoglobin (%) | 5.89 ± 0.84 | 5.81 ± 0.68 | 6.10 ± 0.84 | 5.68 ± 0.56 | 1.7×10-2 |
| HOMA-IR | 6.27 ± 4.24 | 6.12 ± 3.97 | 7.98 ± 4.43 | 5.29 ± 3.47 | 5.1×10-3 |
| SteatoTest | 0.60 ± 0.17 | 0.60 ± 0.17 | 0.71 ± 0.12 | 0.56 ± 0.17 | 3.6×10-6 |
| Hepatic steatosis index | 59.6 ± 6.9 | 59.6 ± 7.3 | 64.0 ± 7.0 | 57.7 ± 6.5 | 1.0×10-4 |
| Fatty liver index | 45.4 ± 28.2 | 46.7 ± 28.6 | 64.4 ± 25.7 | 38.8 ± 26.3 | 3.5×10-5 |
| CAP (dB/m) | 325 ± 62 | 324 ± 63 | 351 ± 40 | 312 ± 68 | 7.1×10-4 |
| LSM (kPa) | 6.6 [4.6-9.5] | 6.2 [4.2-8.1] | 7.9 [5.9-8.9] | 5.4 [4.0-7.0] | 6.7×10-2 |
| MRI-PDFF (%) | 15.3 ± 10.5 | 15.9 ± 10.9 | 24.1 ± 8.2 | 12.2 ± 10.1 | 5.3×10-8 |
p values are those of the t tests between NASH and no NASH groups for each variable.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; GGT, gamma-glutamyltransferase; HOMA-IR, homeostasis model assessment of insulin resistance; LSM, liver stiffness measurement; MRI-PDFF, MRI-proton density fat fraction; NASH, non-alcoholic steatohepatitis; WC, waist circumference.
Table 2.
Histological characteristics of the 152 patients with liver biopsy and of the 97 patients with all valid tests available.
| Variables | Liver biopsy (n = 152) | All valid tests available (n = 97) |
NASH (n = 30) |
No NASH (n = 67) |
p value |
|---|---|---|---|---|---|
| Fragment length (mm) | 13.6 ± 6.3 | 14.3 ± 6.4 | 16.4 ± 6.0 | 13.4 ± 6.4 | 3.0×10-2 |
| Number of portal tracts | 13.6 ± 8.0 | 14.1 ± 7.9 | 17.2 ± 7.7 | 12.7 ± 7.6 | 1.1×10-2 |
| Steatosis grade, n (%) | 7.3×10-8 | ||||
| 0 (<5%) | 40 (26) | 23 (24) | 0 (0) | 23 (34) | |
| 1 (5-33%) | 37 (24) | 21 (22) | 0 (0) | 21 (31) | |
| 2 (34-66%) | 40 (26) | 25 (26) | 14 (47) | 11 (16) | |
| 3 (>66%) | 35 (24) | 28 (28) | 16 (53) | 12 (19) | |
| Lobular inflammation, n (%) | 4.9×10-19 | ||||
| 0 | 98 (64) | 60 (62) | 0 (0) | 60 (90) | |
| 1 | 46 (30) | 31 (32) | 26 (87) | 5 (7) | |
| 2-3 | 8 (5) | 6 (6) | 4 (13) | 2 (3) | |
| Ballooning grade, n (%) | 1.1×10-15 | ||||
| 0 | 87 (57) | 54 (56) | 0 (0) | 54 (81) | |
| 1 | 59 (39) | 39 (40) | 26 (87) | 13 (20) | |
| 2 | 6 (4) | 4 (4) | 4 (13) | 0 (0) | |
| NAS score, n (%) | 7.8×10-17 | ||||
| <3 | 79 (52) | 45 (46) | 0 (0) | 45 (67) | |
| 3-4 | 46 (30) | 34 (35) | 12 (40) | 22 (33) | |
| ≥5 | 27 (18) | 18 (19) | 18 (60) | 0 (0) | |
| Fibrosis stage, n (%) | 9.1×10-11 | ||||
| F0 | 75 (49) | 47 (48) | 1 (3) | 46 (69) | |
| F1 | 63 (42) | 38 (39) | 19 (63) | 19 (28) | |
| F2 | 11 (7) | 10 (10) | 8 (27) | 2 (3) | |
| F3 | 3 (2) | 2 (2) | 2 (7) | 0 (0) | |
| F4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
p values are those of the t tests between NASH and no NASH groups for each variable.
NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.
As for the 97 patients with all valid tests available, their clinical characteristics did not differ from those of the 152 patients: female (86%), mean age 41 ± 19 years, mean BMI 44.4 ± 5.4 kg/m2, NAFLD 76% and NASH 31%. Mean liver biopsy length was 14.3 ± 6.4 mm, with biopsies smaller than 10 mm in 21% of patients or with less than 10 portal tracts in 30%.
As shown in Table 1, Table 2, patients with NASH were more often male, had higher BMI, higher prevalence of sleep apnea, dyslipidemia and diabetes, higher levels of aminotransferases and gamma-glutamyltransferase (Table 1), and had more severe fibrosis stage (Table 2) than those without NASH.
Applicability of FibroScan and MRI
Of 142 patients evaluated using the FibroScan (Fig. 1), readings were valid in 112 patients leading to an applicability value of 79%. Of 130 patients evaluated with MRI, readings were valid in 128 patients leading to an applicability value of 98%. MRI applicability was significantly better than that of FibroScan (p <0.0001).
Diagnostic performance of FibroScan for steatosis, NASH and fibrosis
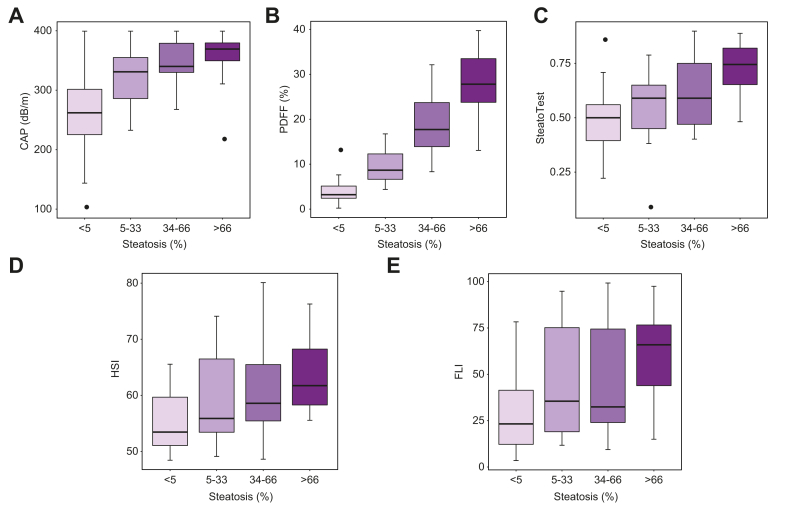
In the 112 patients with valid FibroScan, the boxplot of CAP vs. steatosis grade is shown in Fig. 2A. The diagnostic performance of CAP (including AUROCs) is detailed in Table 3. Accuracy was highest to distinguish S0 from S1-3 (>5%) with an AUROC of 0.83 (95% CI 0.72–0.93). At a cut-off of 316 dB/m, CAP had Se of 0.79 (0.69–0.87), Sp of 0.84 (0.64– 0.95), PPV of 0.95 (0.87-0.98) and NPV of 0.54 (0.37–0.70) for detecting NAFLD. Accuracy was lower to distinguish S0-1 from S2-3 (>33%) and S0-2 from S3 (>66%) with AUROCs of 0.79 (0.70–0.88) and of 0.73 (0.63–0.83), respectively. For diagnosing NASH, CAP had an AUROC of 0.69 (0.59–0.79), with Se of 0.86 (0.71-0.95) and Sp of 0.47 (0.35-0.59), at a cut-off of 318 dB/m.
Fig. 2.
Boxplots comparing non-invasive measures to steatosis grade on histology.
Boxplots of (A) CAP vs. steatosis grade, (B) MRI-PDFF vs. steatosis grade, (C) SteatoTest vs. steatosis grade, (D) HSI vs. steatosis grade, (E) FLI vs. steatosis grade. CAP, controlled attenuation parameter; FLI, fatty liver index; HSI, hepatic steatosis index; MRI-PDFF, MRI-proton density fat fraction.
Table 3.
Diagnostic performance of CAP for grading steatosis and diagnosing NASH (n = 112).
| AUROC (95% CI∗) | Cut-off (dB/m) | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| S0 vs. S1-3 (>5%) | 0.83 (0.72–0.93) | 316 | 0.79 (0.69–0.87) | 0.84 (0.64–0.95) | 0.95 (0.87–0.98) | 0.54 (0.37–0.70) |
| S0-1 vs. S2-3 (>33%) | 0.79 (0.70–0.88) | 316 | 0.87 (0.76–0.94) | 0.61 (0.46–0.74) | 0.73 (0.61–0.82) | 0.79 (0.64–0.91) |
| S0-2 vs. S3 (>66%) | 0.73 (0.63–0.83) | 343 | 0.77 (0.59–0.90) | 0.64 (0.53–0.75) | 0.45 (0.32–0.60) | 0.88 (0.77–0.95) |
| NASH | 0.69 (0.59–0.79) | 318 | 0.86 (0.71–0.95) | 0.47 (0.35–0.59) | 0.44 (0.33–0.57) | 0.88 (0.73–0.96) |
Values are provided with their 95% CI.
AUROC, area under the receiver operating characteristic curve; CAP, controlled attenuation parameter; NASH, non-alcoholic steatohepatitis; NPV, negative predictive value; PPV positive predictive value; Se, sensitivity; Sp, specificity.
For detecting the presence of any fibrosis (F0 vs. F1-F2-F3), LSM had an AUROC of 0.78 (0.70-0.87), with Se of 0.76 (0.63-0.86), Sp of 0.68 (0.54-0.80), PPV of 0.73 (0.60-0.83) and NPV of 0.72 (0.58-0.84), at a cut-off of 5.6 kPa. For detecting the presence of significant fibrosis (F0-F1 vs. F2-F3), LSM had an AUROC of 0.80 (0.70-0.90), with Se of 0.75 (0.43-0.95), Sp of 0.67 (0.57-0.76), PPV of 0.21 (0.10-0.37) and NPV of 0.96 (0.88-0.99), at a cut-off of 6.8 kPa.
Diagnostic performance of MRI for steatosis and NASH
In the 128 patients with valid MRI, the boxplot of MRI-PDFF vs. steatosis grade is shown in Fig. 2B. The diagnostic performance of MRI-PDFF (including AUROCs) is detailed in Table 4. MRI-PDFF had excellent diagnostic performance for all steatosis grades, with AUROCs of 0.97 (0.94-1.00), 0.95 (0.91-0.98), and 0.92 (0.87-0.97) for distinguishing S0 from S1-3 (>5%), S0-1 from S2-3 (>33%) and S0-2 from S3 (>66%), respectively. At a cut-off of 6%, MRI-PDFF had Se of 0.95 (0.88–0.98), Sp of 0.84 (0.66–0.95), PPV of 0.95 (0.88–0.98) and NPV of 0.84 (0.66–0.95) for detecting NAFLD.
Table 4.
Diagnostic performance of MRI-PDFF for grading steatosis and diagnosing NASH (n = 128).
| AUROC (95% CI) | Cut-off | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| S0 vs. S1-3 (>5%) | 0.97 (0.94–1.00) | 6% | 0.95 (0.88–0.98) | 0.84 (0.66–0.95) | 0.95 (0.88–0.98) | 0.84 (0.66–0.95) |
| S0-1 vs. S2-3 (>33%) | 0.95 (0.91–0.98) | 11% | 0.95 (0.87–0.99) | 0.80 (0.68–0.89) | 0.82 (0.72–0.90) | 0.94 (0.85–0.99) |
| S0-2 vs. S3 (>66%) | 0.92 (0.87–0.97) | 20% | 0.83 (0.65–0.94) | 0.84 (0.75–0.90) | 0.61 (0.45–0.76) | 0.94 (0.87–0.98) |
| NASH | 0.84 (0.77–0.91) | 12% | 0.97 (0.86–1.00) | 0.60 (0.50–0.71) | 0.50 (0.38–0.62) | 0.98 (0.90–1.00) |
Values are provided with their 95% CI.
AUROC, area under the receiver operating characteristic curve; MRI-PDFF, MRI-proton density fat fraction; NASH, non-alcoholic steatohepatitis; NPV, negative predictive value; PPV positive predictive value; Se, sensitivity; Sp, specificity.
For diagnosing NASH, MRI-PDFF had an AUROC of 0.84 (0.77–0.91), with Se of 0.97 (0.86–1.00) and Sp of 0.60 (0.50–0.71), PPV of 0.50 (0.38–0.62) and NPV of 0.98 (0.90–1.00) at a cut-off of 12%.
Diagnostic performance of serum scores for steatosis
In the 145 patients with available results (missing data n = 7) of serum scores, the boxplots of ST, HSI and FLI vs. steatosis grade are shown in Fig. 2C-E. AUROCs and diagnostic performances of scores are detailed in Table 5. ST had the highest AUROCs for steatosis grading: AUROCs of 0.74 (0.66-0.83), 0.77 (0.69-0.85), and 0.79 (0.71-0.87) for distinguishing S0 from S1-3 (>5%), S0-1 from S2-3 (>33%) and S0-2 from S3 (>66%), respectively, with Se/Sp of 0.69/0.65, 0.58/0.83 and 0.71/0.73 at cut-offs of 0.54, 0.66 and 0.65, respectively.
Table 5.
Diagnostic performance of serum scores for grading steatosis (n = 145).
| AUROC (95% CI) | Cut-off | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| SteatoTest | ||||||
| S0 vs. S1-3 (>5%) | 0.74 (0.66–0.83) | 0.54 | 0.69 (0.60–0.78) | 0.65 (0.47–0.80) | 0.85 (0.76–0.92) | 0.42 (0.29–0.56) |
| S0-1 vs. S2-3 (>33%) | 0.77 (0.69–0.85) | 0.66 | 0.58 (0.45–0.69) | 0.83 (0.73–0.91) | 0.78 (0.64–0.88) | 0.66 (0.55–0.76) |
| S0-2 vs. S3 (>66%) | 0.79 (0.71–0.87) | 0.65 | 0.71 (0.54–0.85) | 0.73 (0.63–0.81) | 0.45 (0.32–0.59) | 0.89 (0.81–0.95) |
| HSI | ||||||
| S0 vs. S1-3 (>5%) | 0.73 (0.64–0.83) | 55 | 0.78 (0.70–0.86) | 0.59 (0.42–0.74) | 0.84 (0.76–0.91) | 0.49 (0.34–0.64) |
| S0-1 vs. S2-3 (>33%) | 0.68 (0.59–0.76) | 55 | 0.86 (0.77–0.93) | 0.49 (0.37–0.60) | 0.62 (0.52–0.72) | 0.79 (0.64–0.89) |
| S0-2 vs. S3 (>66%) | 0.72 (0.64–0.80) | 56 | 0.94 (0.81–0.99) | 0.45 (0.36–0.55) | 0.34 (0.25–0.45) | 0.96 (0.87–1.00) |
| FLI | ||||||
| S0 vs. S1-3 (>5%) | 0.72 (0.62–0.81) | 28.5 | 0.77 (0.68–0.84) | 0.62 (0.45–0.77) | 0.85 (0.76–0.91) | 0.49 (0.34–0.64) |
| S0-1 vs. S2-3 (>33%) | 0.65 (0.56–0.74) | 29.1 | 0.77 (0.65–0.86) | 0.50 (0.38–0.62) | 0.60 (0.50–0.70) | 0.69 (0.54–0.80) |
| S0-2 vs. S3 (>66%) | 0.68 (0.59–0.77) | 31.2 | 0.80 (0.63–0.92) | 0.54 (0.45–0.64) | 0.35 (0.25–0.47) | 0.90 (0.80–0.96) |
Values are provided with their 95% CIs.
AUROC, area under the receiver operating characteristic curve; FLI fatty liver index; HSI, hepatic steatosis index; NPV, negative predictive value; PPV positive predictive value; Se, sensitivity; Sp, specificity.
Head-to-head comparison of performance of CAP, MRI-PDFF and serum scores for steatosis and NASH
Pairwise comparisons using the DeLong methods with adjustment for multiple comparisons of AUROCs of CAP, MRI-PDFF and ST, HIS and FLI for grading steatosis are shown in Table 6. MRI-PDFF significantly outperformed CAP for distinguishing S0-1 from S2-3 (>33%) (AUROC 0.97 vs. 0.78 respectively; p <0.0003) and S0-2 from S3 (>66%) (AUROC 0.93 vs. 0.75, respectively; p = 0.0015). As for distinguishing S0 from S1-3 (≥5%), the difference did not reach statistical significance (AUROC 0.97 vs. 0.82, respectively; p = 0.053). MRI-PDFF significantly outperformed all serum scores for all steatosis grades, except ST for distinguishing S0-2 from S3 (>66%). The performance of the 3 serum scores did not differ for any of the steatosis grades. MRI-PDFF significantly outperformed CAP for detecting NASH (AUROC 0.82 vs. 0.68, respectively, p = 0.0056). MRI-PDFF also outperformed CAP for detecting severe NASH (defined as NAS score >5) (AUROC 0.87 vs. 0.72, respectively, p = 0.043).
Table 6.
Comparison of performance of CAP, MRI-PDFF and serum scores for steatosis grading (n = 97 patients) with DeLong test.
| S0 vs. S1-3 (>5%) |
S0-1 vs. S2-3 (>33%) |
S0-2 vs. S3 (>66%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DeLong test p value |
0.0012 |
2.31×10-6 |
2.7×10-5 |
||||||
| Pairwise comparison | AUROC test 1 |
AUROC test 2 |
p value | AUROC test 1 |
AUROC test 2 |
p value | AUROC test 1 |
AUROC test 2 |
p value |
| CAP vs. MRI-PDFF | 0.82 | 0.97 | 0.053 | 0.78 | 0.97 | 3.0×10-4 | 0.75 | 0.93 | 0.0015 |
| CAP vs. ST | 0.82 | 0.77 | 0.89 | 0.78 | 0.77 | 0.83 | 0.75 | 0.81 | 0.66 |
| CAP vs. HSI | 0.82 | 0.74 | 0.61 | 0.78 | 0.72 | 0.68 | 0.75 | 0.72 | 0.66 |
| CAP vs. FLI | 0.82 | 0.74 | 0.89 | 0.78 | 0.68 | 0.3 | 0.75 | 0.70 | 0.66 |
| MRI-PDFF vs. ST | 0.97 | 0.77 | 5.5×10-4 | 0.97 | 0.77 | 4.0×10-5 | 0.93 | 0.81 | 0.071 |
| MRI-PDFF vs. HSI | 0.97 | 0.74 | 1.9×10-4 | 0.97 | 0.72 | 3.0×10-6 | 0.93 | 0.72 | 6.6×10-4 |
| MRI-PDFF vs. FLI | 0.97 | 0.74 | 5.5×10-4 | 0.97 | 0.68 | 8.7×10-8 | 0.93 | 0.70 | 3.7×10-4 |
| ST vs. HSI | 0.77 | 0.74 | 0.89 | 0.77 | 0.72 | 0.68 | 0.81 | 0.72 | 0.65 |
| ST vs. FLI | 0.77 | 0.74 | 0.89 | 0.77 | 0.68 | 0.19 | 0.81 | 0.70 | 0.12 |
| HSI vs. FLI | 0.74 | 0.74 | 0.89 | 0.72 | 0.68 | 0.68 | 0.72 | 0.70 | 0.66 |
For each pair of compared tests, the individual test comparisons p values (after adjustment for alpha risk) are indicated, along with the AUROC of each respective test.
AUROC, area under the receiver operating characteristic curve; CAP controlled attenuation parameter; FLI, fatty liver index; HSI, Hepatic steatosis index; MRI-PDFF magnetic resonance proton density fat fraction; ST, SteatoTest.
When compared in “intention to diagnose” analysis (Table S1), MRI-PDFF outperformed CAP for distinguishing S0 from S1-3 (≥5%) (correctly classified 91.1% vs. 63.4%, respectively; p = 3.0 x 10-6), S0-1 from S2-3 (≥33%) (correctly classified 81.1% vs. 59.2%, respectively; p = 6.7 x 10-4) and S0-2 from S3 (≥66%) (correctly classified 82.3% vs. 53.5%, respectively; p = 1.3 x 10-5). MRI-PDFF significantly outperformed all serum scores for all steatosis grades, except ST for distinguishing S0-1 (≥33%) and S0-2 from S3 (≥66%).
In patients with biopsies of more than 10 mm (Table S2), MRI-PDFF significantly outperformed all tests for all steatosis grades, except CAP for distinguishing S0 from S1-3 (≥5%). Similarly, in patients with biopsies with more than 10 portal tracts (Table S2), MRI-PDFF significantly outperformed all tests for all steatosis grades, except CAP for distinguishing S0 from S1-3 (≥5%).
Discussion
Using a prospective, rigorously characterized, cohort of morbidly obese candidates for bariatric surgery seen in an expert center, the present study shows that MRI-PDFF, using an open-bore 1T scanner, despite similar accuracy for detecting NAFLD, has higher applicability and is more accurate than CAP with the XL probe for grading steatosis and diagnosing NASH. The key novelty of this study is that it is the first study, to our knowledge, to perform head-to-head comparison between CAP and MRI-PDFF for diagnosing NAFLD, grading steatosis and detecting NASH. These results may have important implications for developing an optimal clinical approach for the non-invasive assessment of NAFLD and NASH in morbidly obese candidates for bariatric surgery.
The general characteristics of our population (mostly women in their forties with a mean BMI of 44 kg/m2) are similar to those reported in the literature[19], [20], [21],23 and can be considered representative of typical candidates for bariatric surgery. Similarly, the prevalence of NAFLD, NASH and severe steatosis (74%, 30% and 24%, respectively) in our population is consistent with previous reports.[19], [20], [21] Liver histology, with reading by a single expert pathologist (P.B.), was used as the reference standard for steatosis grading and NASH diagnosis, using the NASH-Clinical Research Network scoring system and FLIP algorithm, respectively. Finally, we used MRI with an open-bore scanner and CAP with the XL probe, to minimize the failure rates in a morbidly obese population.
Despite the use of the XL probe, applicability of FibroScan (79%) was significantly lower than that of MRI (98%) in our population. This rate is lower than the that reported (97-98%) in 2 recent multicenter non-bariatric cohorts with the XL probe.17,18 It is consistent however with rates reported with the XL probe in morbidly obese patients, ranging from 63% in patients with mean BMI above 50 kg/m240 to 88% in patients with lower BMI.20 MRI is known to have high applicability in patients with chronic liver disease.41,42 In patients with NAFLD without morbid obesity (mean BMI between 28 and 30 kg/m2), MRI applicability has been reported to be better than that of Fibroscan.24,25 However, in the only study comparing FibroScan and MR spectroscopy (using a 3T, 60 cm bore, MRI scanner) in bariatric surgery candidates,27 TE applicability (80%) was similar to our finding but higher than that of MRI (63%). The high MRI applicability in our study might be related to the fact that we used MRI-PDFF rather than MR spectroscopy for grading steatosis, but might also be explained by our use of an open-bore, vertical field 1.0T scanner, more suited for morbidly obese patients, because of its higher accessibility and lower sensitivity to artefacts relative to a conventional 3T scanner.28
The performance of CAP in our study is consistent with those recently reported with the XL probe in 2 large multicenter non-bariatric cohorts.17,18 Indeed, CAP had good performance for diagnosing NAFLD (AUROC of 0.83) but poor performance for grading steatosis (AUROCs of 0.73 for S>66%) and detecting NASH (AUROC of 0.69). At a cut-off of 316 dB/m, CAP had 79% Se, 84% Sp and 95% and 54% PPV and NPV for diagnosing NAFLD, a finding in keeping with those of Eddowes et al.17 These results suggest that CAP is better at ruling in than ruling out NAFLD in candidates for bariatric surgery. The poor CAP performances for detecting severe steatosis (with AUROCS lower than for detecting NAFLD) are consistent with the findings in non-bariatric cohorts17,18,24,25,43 but in contrast with those (AUROCs 0.82-0.84) in the 2 studies in bariatric cohorts.20,21 Although we have no clear explanation for this discrepancy, differences in severe steatosis prevalence between the studied populations might play a role. Regarding NASH diagnosis, there are no data available for CAP in morbidly obese patients so far, but our results are not in favor of using CAP for this purpose. Overall, our results suggest that CAP with the XL probe, is feasible and accurate to detect NAFLD in morbidly obese patients before bariatric surgery but is not sufficient to score its severity.
The performance of MRI-PDFF in the present study is consistent with that reported in non-bariatric cohorts.24,25,43 Indeed, MRI-PDFF had excellent performances for detecting NAFLD (AUROC of 0.97), grading steatosis (AUROCs of 0.95 and 0.92 for S>33% and S>66%) and detecting NASH (AUROC 0.84). At a cut-off of 6%, MRI-PDFF had 95% Se, 84% Sp, 95% PPV and 84% NPV for diagnosing NAFLD, a finding in accordance with the results of studies by Imajo et al.24 and Park et al.25 The excellent performance of MRI-PDFF for grading steatosis is consistent with that reported in patients with NAFLD in a recent meta-analysis.44
There are no published data regarding MRI-PDFF performance in morbidly obese patients. In our study, MRI-PDFF had 97% Se, 60% Sp, 50% PPV and 98% NPV for diagnosing NASH at a cut-off of 12%. These results suggest that MRI-PDFF is better at ruling out than ruling in NASH. These good performances for NASH exclusion are in keeping with those recently reported by Allen et al.,26 combining MRI-PDFF with multifrequency 3D-MR-elastography in a bariatric cohort.
Overall, the 3 serum scores assessed (ST, FLI and HSI) in our study performed poorly to detect NAFLD and grade steatosis with AUROCs consistently below 0.8. When compared to each other (Table 5), no difference was observed. Data regarding serum steatosis scores in patients with morbid obesity are very limited so far. Our findings regarding FLI and HSI are consistent with those reported in bariatric27 and non-bariatric45 cohorts. Similarly, the performance of ST in our population is consistent with those reported by 2 studies in candidates for bariatric surgery.11,37 A possible explanation for the poor performance of steatosis scores could be the heavy weighting of BMI and waist circumference measurements in their algorithms, which can distort the overall score when applied to a morbidly obese cohort.
As mentioned before, the present study is the first to provide head-to-head comparison between CAP and MRI-PDFF for diagnosing NAFLD, grading steatosis and detecting NASH. Interestingly, MRI-PDFF outperformed CAP for grading steatosis and diagnosing NASH. MRI-PDFF also outperformed serum scores for grading steatosis, except ST for distinguishing S0-1 (≥33%) and S0-2 from S3 (≥66%), in “intention to diagnose” analysis. The subgroup of 97 patients with all valid tests available did not differ from the total group of patients for most characteristics, including NAFLD, steatosis grades and NASH prevalence. The better performances for grading steatosis of MRI-PDFF compared to CAP are in keeping with those reported in the 3 published comparative studies in non-bariatric cohorts.24,25,43 In addition, MRI-PDFF outperformed CAP for detecting NASH but was better at ruling out than ruling in NASH. Thus MRI-PDFF, given its high applicability, appears to be the method of choice to grade steatosis and exclude NASH in patients with morbid obesity.
Regarding NAFLD diagnosis, although MRI-PDFF AUROCs were higher than those of CAP, the difference did not reach statistical significance. Similarly, when MRI-PDFF and CAP performances were compared according to biopsy length and number of portal tracts,46 they did not differ in patients with good quality liver biopsies (greater than 10 mm or with more than 10 portal tracts). However, when analyzed in “intention to diagnose” analysis, keeping the patients with CAP and MRI-PDFF failures, MRI-PDFF clearly outperformed CAP for diagnosing NAFLD. Despite its high accuracy, cost and limited availability are limitations to MRI-PDFF use in clinical practice. In contrast, CAP is widely available, can be performed by nurses after short training, with immediate results. Therefore, CAP might be a reasonable alternative option for detecting NAFLD in morbidly obese candidates for bariatric surgery.
FibroScan performances for staging fibrosis with AUROCs of 0.78 to 0.80 are consistent with those previously reported in bariatric surgery candidates.19,20,27,47 Nevertheless, caution is warranted regarding the clinical relevance of these findings, not only because mortality in patients with NAFLD is mainly related to the presence of advanced fibrosis and cirrhosis,48 but also because performances of FibroScan for staging fibrosis in our population are subject to the well-known spectrum bias.49 This bias is however inherent to all bariatric cohorts, in which most patients have mild fibrosis and no cirrhosis.
This study has several limitations that should be acknowledged. First, because this was a single-center study in a highly specialized setting, the generalizability of the findings in other clinical settings is unknown. Second, MRI-PDFF was performed using an open MRI that is more suited to morbidly obese patients but not widely available. Third, MR-elastography was not performed as the probe was not available at the time the study started. Fourth, liver biopsy specimen quality was suboptimal with biopsy length less than 10 mm in 1 in 5 patients and less than 10 portal tracts in 1 in 3. Finally, the cross-sectional design of the study did not allow for the assessment of MRI-PDFF and CAP for monitoring longitudinal changes in steatosis.
In conclusion, the present study provides novel findings that are relevant for developing an optimal clinical approach to the non-invasive assessment of NAFLD and NASH in morbidly obese candidates for bariatric surgery. MRI-PDFF, using an open-bore 1T scanner, has high applicability and outperforms CAP for detecting and grading steatosis as well as detecting NASH. These findings suggest that MRI-PDFF is the method of choice for detecting NASH and grading steatosis in morbidly obese candidates for bariatric surgery. CAP with the XL probe, given its wide availability and simplicity, might however be a reasonable alternative option for detecting NAFLD in settings where MRI-PDFF is not available.
Financial support
The project was funded by a Programme Hospitalier de Recherche Clinique – PHRC from the Ministère de la Santé N° AOR10 086.
Authors’ contributions
Study concept and design: PJ, BVB. Patient’s recruitment and acquisition of data: SL, MC, DC, SM, SD, PG. Analysis and interpretation of data: PB, SD, MDB, LC, PG, BVB, PJ, FD. Drafting of the manuscript: LC, PG, PJ, FD. Critical revision of the manuscript for important intellectual content: MC, SL, FD, BVB. Statistical analysis: MEF, FD. Obtained funding: PJ. Study supervision: PJ.
Data availability statement
Anonymised data available on request.
Conflict of interest
LC has received lecture’s fees from Abbvie, Echosens, Intercept, Gilead, and Novo Nordisk and consultancy fees from Allergan, Intercept, Gilead, MSD, Novo Nordisk, Pfizer and Servier. No other authors have relevant conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors are thankful to Echosens for providing the FibroScan device with CAP and XL probe. The authors did not receive funding from Echosens for performing their research. The authors would also like to thank Doryssemma TCHATAT for her help.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100381.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., Venkatesh S.K., Wang Z., Miller F.H., Motosugi U., Low R.N., et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2015;13:440–451 e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado M., Marques-Vidal P., Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Cazzo E., de Felice Gallo F., Pareja J.C., Chaim E.A. Nonalcoholic fatty liver disease in morbidly obese subjects: correlation among histopathologic findings, biochemical features, and ultrasound evaluation. Obes Surg. 2014;24:666–668. doi: 10.1007/s11695-014-1183-4. [DOI] [PubMed] [Google Scholar]
- 7.Oberlin P., de Peretti C. Surg Obes Relat Dis; 2020. Bariatric Surgery in France from 1997 to 2018. [DOI] [PubMed] [Google Scholar]
- 8.Castera L., Negre I., Samii K., Buffet C. Patient-administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: a randomized placebo-controlled trial. Am J Gastroenterol. 2001;96:1553–1557. doi: 10.1111/j.1572-0241.2001.03776.x. [DOI] [PubMed] [Google Scholar]
- 9.Castera L., Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59:861–866. doi: 10.1136/gut.2010.214650. [DOI] [PubMed] [Google Scholar]
- 10.EASL-ALEH Clinical Practice Guidelines Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Lassailly G., Caiazzo R., Hollebecque A., Buob D., Leteurtre E., Arnalsteen L., et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol. 2011;23:499–506. doi: 10.1097/MEG.0b013e3283464111. [DOI] [PubMed] [Google Scholar]
- 12.Stern C., Castera L. Non-invasive diagnosis of hepatic steatosis. Hepatol Int. 2017;11:70–78. doi: 10.1007/s12072-016-9772-z. [DOI] [PubMed] [Google Scholar]
- 13.Castera L., Friedrich-Rust M., Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–12681 e4. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Karlas T., Petroff D., Sasso M., Fan J.-G., Mi Y.-Q., de Lédinghen V., et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Castera L., Foucher J., Bernard P.H., Carvalho F., Allaix D., Merrouche W., et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 17.Eddowes P.J., Sasso M., Allison M., Tsochatzis E., Anstee Q.M., Sheridan D., et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui M.S., Vuppalanchi R., Van Natta M.L., Hallinan E., Kowdley K.V., Abdelmalek M., et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2019;17:156–163 e2. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barsamian C., Carette C., Sasso M., Poghosyan T., Bedossa P., Emile J.F., et al. Diagnostic of hepatic fibrosis with the XL probe of the Fibroscan versus biopsies in patients candidates to bariatric surgery. Clin Nutr ESPEN. 2020;37:226–232. doi: 10.1016/j.clnesp.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Garg H., Aggarwal S., Shalimar Yadav R., Datta Gupta S., Agarwal L., et al. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg Obes Relat Dis. 2018;14:81–91. doi: 10.1016/j.soard.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Naveau S., Voican C.S., Lebrun A., Gaillard M., Lamouri K., Njike-Nakseu M., et al. Controlled attenuation parameter for diagnosing steatosis in bariatric surgery candidates with suspected nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2017;29:1022–1030. doi: 10.1097/MEG.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 22.Somda S., Lebrun A., Tranchart H., Lamouri K., Prevot S., Njike-Nakseu M., et al. Adaptation of controlled attenuation parameter (CAP) measurement depth in morbidly obese patients addressed for bariatric surgery. PloS one. 2019;14 doi: 10.1371/journal.pone.0217093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Barros F., Setubal S., Martinho J.M., Leite N.C., Guarana T., Monteiro A.B.S., et al. The correlation between obesity-related diseases and non-alcoholic fatty liver disease in women in the pre-operative evaluation for bariatric surgery assessed by transient hepatic elastography. Obes Surg. 2016;26:2089–2097. doi: 10.1007/s11695-016-2054-y. [DOI] [PubMed] [Google Scholar]
- 24.Imajo K., Kessoku T., Honda Y., Tomeno W., Ogawa Y., Mawatari H., et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637 e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 25.Park C.C., Nguyen P., Hernandez C., Bettencourt R., Ramirez K., Fortney L., et al. Magnetic resonance elastography vs. Transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607 e2. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen A.M., Shah V.H., Therneau T.M., Venkatesh S.K., Mounajjed T., Larson J.J., et al. The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology. 2020;71:510–521. doi: 10.1002/hep.30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooi G.J., Earnest A., Kemp W.W., Burton P.R., Laurie C., Majeed A., et al. Evaluating feasibility and accuracy of non-invasive tests for nonalcoholic fatty liver disease in severe and morbid obesity. Int J Obes (Lond) 2018;42:1900–1911. doi: 10.1038/s41366-018-0007-3. [DOI] [PubMed] [Google Scholar]
- 28.de Bucourt M., Streitparth F., Wonneberger U., Rump J., Teichgraber U. Obese patients in an open MRI at 1.0 Tesla: image quality, diagnostic impact and feasibility. Eur Radiol. 2011;21:1004–1015. doi: 10.1007/s00330-010-2005-2. [DOI] [PubMed] [Google Scholar]
- 29.Ledoux S., Sami O., Calabrese D., Le Gall M., Flamant M., Coupaye M. Gastric bypass specifically impairs liver parameters as compared with sleeve gastrectomy, independently of evolution of metabolic disorders. Surg Obes Relat Dis. 2019;15:220–226. doi: 10.1016/j.soard.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.H., Kim D., Kim H.J., Lee C.H., Yang J.I., Kim W., et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T., Ratziu V., Naveau S., Thabut D., Charlotte F., Messous D., et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. doi: 10.1186/1476-5926-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campo C.A., Hernando D., Schubert T., Bookwalter C.A., Pay A.J.V., Reeder S.B. Standardized approach for ROI-based measurements of proton density fat fraction and R2∗ in the liver. AJR Am J Roentgenol. 2017;209:592–603. doi: 10.2214/AJR.17.17812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parente D.B., Rodrigues R.S., Paiva F.F., Oliveira Neto J.A., Machado-Silva L., Lanzoni V., et al. Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PloS one. 2014;9 doi: 10.1371/journal.pone.0112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 36.Bedossa P., Consortium F.P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 37.Poynard T., Lassailly G., Diaz E., Clement K., Caiazzo R., Tordjman J., et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PloS One. 2012;7 doi: 10.1371/journal.pone.0030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 39.Schuetz G.M., Schlattmann P., Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ. 2012;345 doi: 10.1136/bmj.e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss J., Rau M., Meertens J., Hering I., Reichert L., Kudlich T., et al. Feasibility of liver stiffness measurement in morbidly obese patients undergoing bariatric surgery using XL probe. Scand J Gastroenterol. 2016;51:1263–1268. doi: 10.1080/00365521.2016.1191084. [DOI] [PubMed] [Google Scholar]
- 41.Wagner M., Corcuera-Solano I., Lo G., Esses S., Liao J., Besa C., et al. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology. 2017;284:401–412. doi: 10.1148/radiol.2016160863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J., Yin M., Talwalkar J.A., Oudry J., Glaser K.J., Smyrk T.C., et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology. 2017;283:418–428. doi: 10.1148/radiol.2016160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runge J.H., Smits L.P., Verheij J., Depla A., Kuiken S.D., Baak B.C., et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology. 2017:162931. doi: 10.1148/radiol.2017162931. [DOI] [PubMed] [Google Scholar]
- 44.Gu J., Liu S., Du S., Zhang Q., Xiao J., Dong Q., et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564–3573. doi: 10.1007/s00330-019-06072-4. [DOI] [PubMed] [Google Scholar]
- 45.Fedchuk L., Nascimbeni F., Pais R., Charlotte F., Housset C., Ratziu V., et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–1222. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 46.Poynard T., Halfon P., Castera L., Charlotte F., Le Bail B., Munteanu M., et al. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25:733–739. doi: 10.1111/j.1365-2036.2007.03252.x. [DOI] [PubMed] [Google Scholar]
- 47.Naveau S., Lamouri K., Pourcher G., Njike-Nakseu M., Ferretti S., Courie R., et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg. 2014;24:1693–1701. doi: 10.1007/s11695-014-1235-9. [DOI] [PubMed] [Google Scholar]
- 48.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z., et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ransohoff D.F., Feinstein A.R. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299:926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data available on request.