Abstract
Background
The length of sphincter which can be divided during fistulotomy for perianal fistula is unclear. The aim was to quantify sphincter damage during fistulotomy and determine the relationship between such damage with symptoms and severity of faecal incontinence and long-term quality of life (QOL).
Methods
A prospective cohort study was performed over a 2-year period. Patients with intersphincteric and mid to low transsphincteric perianal fistulas without risk factors for faecal incontinence were scheduled for fistulotomy. All patients underwent 3D endoanal ultrasound (3D-EAUS) pre-operatively and 8 weeks postoperatively. Measurements were taken of pre- and postoperative anal sphincter involvement and division. Anal continence was assessed using the Jorge-Wexner scale and QOL scores pre, 6 and 12 months postoperatively.
Results
Forty-nine patients were selected. A strong correlation between pre- and postoperative measurements was found p < 0.001. A median length of 41% of the external anal sphincter and 32% of the internal anal sphincter was divided during fistulotomy. Significant differences in mild symptoms of anal continence were found with increasing length of external anal sphincter division. But there was no significant deterioration in continence, soiling, or quality of life scores at the 1-year follow-up. Division of over two-thirds of the external anal sphincter was associated with the highest incontinence rates.
Conclusions
3D-EAUS is a valuable tool for quantifying the extent of sphincter involvement pre- and postoperatively. Post-fistulotomy faecal incontinence is mild and increases with increasing length of sphincter division but does not affect long-term quality of life.
Keywords : Fistulotomy, Anal ultrasound, Faecal incontinence, Perianal fistulae, Fistula, Incontinence, Fistulotomy
Introduction
Fistulotomy is frequently used for the treatment of simple perianal fistula; however, the fistula height and sphincter length which can be divided during fistulotomy remain controversial. This technique is generally reserved for low, uncomplicated fistulae, albeit the exact amount of sphincter which defines a high or low fistula has not been established. Murad-Regadas et al. reported tracts crossing the external anal sphincter (EAS) at the same height or above the internal opening (IO) involved over 50% of the EAS and defined them as high fistulous tracts [1]. Conversely, other authors such as van Koperen [2] defined low fistulas as those which crossed the inferior third of the EAS. The classification of fistulas with respect to where they cross the EAS is purely arbitrary and varies between surgeons. In the majority of cases, it is related to the treatment considered most appropriate to obtain the highest cure rate and lowest postoperative sequelae. There is an overall 45% rate of postoperative faecal incontinence (FI). Risk of FI is associated with females, the surgical technique, a previous history of fistula surgery and high fistulae [3]. There is currently no firm evidence correlating rates of postoperative FI with length of sphincter division, and this remains a grey area of colorectal surgery [4, 5].
Three-dimensional endoanal ultrasound (3D-EAUS) has been shown to provide reliable, quantitative measurements of the anal sphincters both pre- and post-fistulotomy [4, 6–8] and is increasingly used by surgeons to help choose the best treatment options for patients with perianal fistulae [1, 5].
The aim of this study was to quantify sphincter damage during fistulotomy and determine the relationship between such damage with symptoms and severity of FI and quality of life (QOL).
Materials and methods
A prospective cohort, single-centre, consecutive study was performed over a 2-year period including patients from a highly specialized colorectal unit in a tertiary referral centre. The study protocol was approved by the hospital ethics committee. All patients signed an informed consent form prior to inclusion in the study and were followed up for 1 year. Patients diagnosed with a simple cryptoglandular perianal fistula scheduled for fistulotomy were selected. Patients were excluded if operated in another centre, diagnosed with inflammatory bowel disease, had risk factors for FI, supra- or extrasphincteric fistulas or were currently undergoing treatment with other non-surgical techniques or medication.
A simple fistula was defined as an intersphincteric fistula or transsphincteric fistula which involved less than 66% of the total length of the external anal sphincter (EAS) in patients without risk factors for FI [5]. Risk factors for FI included female patients with anterior transsphincteric fistulas, obstetric injuries to the anal sphincters, inflammatory bowel disease and patients with prior anal surgery and major FI according to the Jorge-Wexner score [9]. Any extent of impairment in FI even in its mildest form was taken into account. Minor incontinence was defined as involuntary passage of gas or liquid and major incontinence as the involuntary loss of stools. Soiling was defined as the involuntary loss of small quantities of stool after defecation in patients who were otherwise continent [10]. Recurrence was defined as postoperative persistence or reappearance of symptoms. Pre- and postoperative 3D-EAUS measurements were taken together with assessment of FI and QOL.
Study protocol
Preoperative assessment
A full medical history was taken with particular attention to previous anal surgeries, obstetric history and risk factors for FI measured by the Jorge-Wexner score [9] and QOL as previously mentioned. During the same visit, the presence of an IO, external opening (EO) and location of the fistula were documented by digital rectal examination.
3D-EAUS
All 3D-EAUS were performed by the same surgeon during a second visit to the outpatient clinic. The B&K Medical Systems Pro Focus 2202® and B-K 2050 transducer (B-K Medical, Herlev, Denmark) were used. A preoperative diagnostic ultrasound and 8-week post-fistulotomy scan was performed for all patients. Patients were examined in the prone jack-knife position, and the scan was systematically performed from the upper to the lower thirds of the anal canal. An initial 2D-EAUS was carried out and immediately followed by a 3D-EAUS examination. All examinations were performed at a 10 MHz frequency with 0.2 mm spacing taking 300 sequential images which were subsequently reconstructed in a 3D cube. These examinations were repeated after instillation of 10% hydrogen peroxide in patients who had an open EO. Quantitative measurements of the following variables were taken in all patients: total length of the anal canal, total length of the EAS and internal anal sphincter (IAS), preoperative sphincter involvement (Fig. 1) and postoperative sphincter defect for both the EAS and IAS (Fig. 2). The percentage of sphincter length involved or divided pre- and postoperatively was calculated for both sphincters. Whilst the 3D-EAUS is performed, it is important to keep the patient’s buttocks separated in order to obtain accurate images of the inferior third of the anal canal which could otherwise be confused with subcutaneous tissue, thus overestimating the total length of the anal canal and EAS. The separation between the EAS and puborectalis muscle can be seen on midline sagittal section as a thin hypoechoic line, which combined with the transverse axial image, defines the exact length of the EAS. When the fistula did not cross perpendicular to the EAS, a midway measure to calculate the length of EAS involved was taken. The proximal limit of the IAS was the anorectal junction.
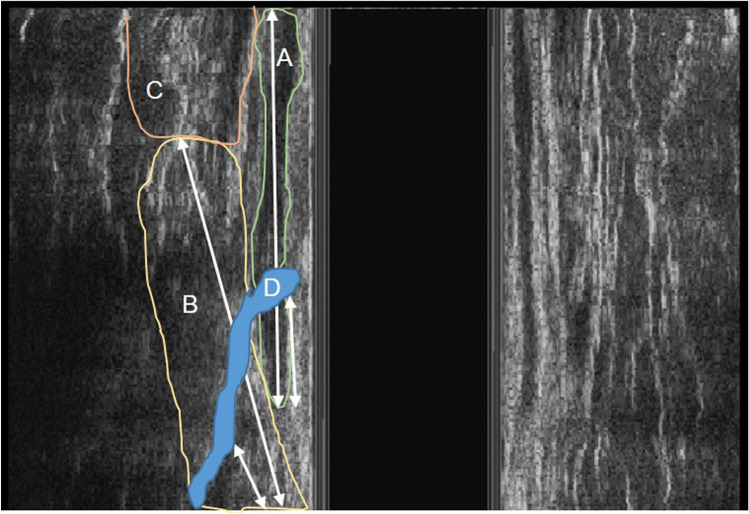
Fig. 1.
Endoanal ultrasound image showing a coronal section of a right lateral transphincteric fistula and arrows indicating total length and involved sphincter of both the internal and external anal sphincters. A internal anal sphincter, B external anal sphincter, C puborectalis muscle, D fistula tract
Fig. 2.
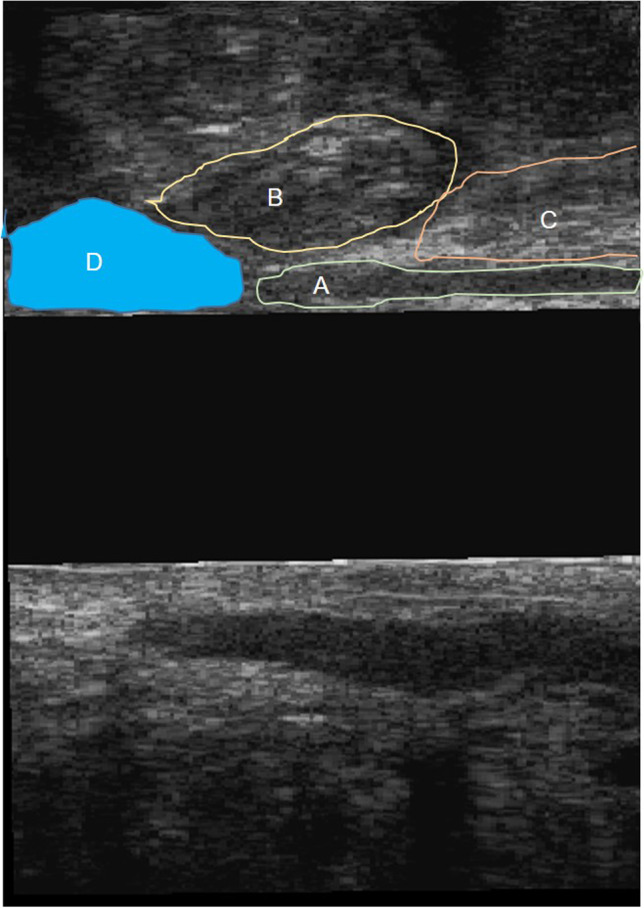
Endoanal ultrasound image showing a sagittal section of an anterior post-fistulotomy external and internal anal sphincter defects and remaining sphincters. A internal anal sphincter, B external anal sphincter, C puborectalis muscle, D fistulotomy defect
Surgery
All patients included in the study were operated on in the prone jack-knife position. Patients were prepped with an enema and locoregional anaesthesia was used. 3D-EAUS was used to guide surgery, but final decision to perform a fistulotomy was the surgeon’s criteria. An initial physical examination was performed followed by fistulotomy taking note of IO and fistula location and height.
Quality of life assessment
Patients completed QOL (SF-36 [11] and Faecal Incontinence Quality of Life Score FIQOLS [12]) pre, immediately postoperatively, and 6 and 12 months after surgery.
Statistical analysis
Spearman’s correlation coefficient was used to correlate the preoperative fistula height with the extent of fistulotomy. The Wilcoxon test was used to compare pre and postoperative continence. The chi-squared test or Likelihood ratio were used to assess the level of division of the sphincters and deterioration in anal continence. A p value greater than 0.05 was considered statistically significant. Statistical analysis was performed using the IBM® SPSS® version 26 for Windows (SPSS, Chicago, Illinois, USA).
Results
Patient distribution is shown in Fig. 3. A total of 49 patients (37 male and 12 female) with a mean age of 49 years (range 21–77) were selected. Patient characteristics are listed in Table 1. Thirteen patients had intersphincteric tracts, 28 low transsphincteric tracts and 8 high transsphincteric tracts in this group of patients. Preoperative 3D-EAUS measurements and the percentage of IAS and EAS involved pre- and postoperatively are shown in Table 2. There was a strong correlation between preoperative sphincter involvement and postoperative sphincter division, with no significant differences between pre- and postoperative values (Spearman’s correlation IAS Rho = 0.639; EAS Rho = 0.633, p < 0.001) (Fig. 4a and b).
Fig. 3.
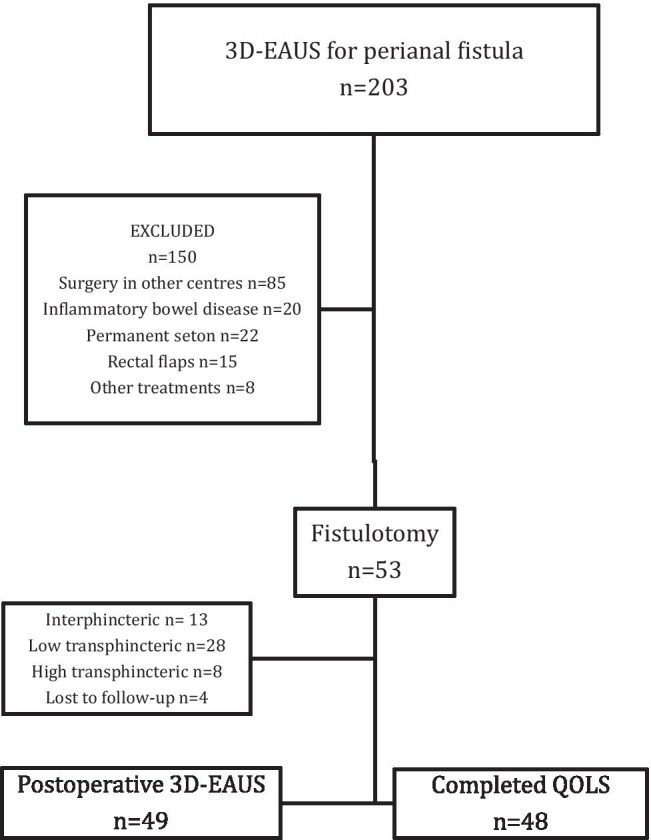
Patient flow diagram. 3D-EAUS = three dimensional endoanal ultrasonography; QOLS = quality of life scales
Table 1.
Patient characteristics
| Total | n = 49 | |
| Mean length of symptoms (months) (range) * | 11.9 (0–120) | |
| Drained abscesses | 25 (51) | |
| Loose seton | 13 (26.5) | |
| Fistulotomy | 3 (6.1) | |
| Fistulectomy | 1 (2) | |
| Internal lateral sphincterotomy | 5 (10.2) | |
| Hemorrhoidectomy | 3 (6.1) | |
| Female | n = 12 | |
| Vaginal deliveries | 6 (0) | |
| Episiotomy | 3 (25) | |
| Hysterectomies | 1 (8,3) | |
Values are expressed as n (%) unless otherwise staed *from diagnosis to surgery
Table 2.
Pre- and postoperative three-dimensional endoanal ultrasound measurements of sphincter involvement and division, respectively
| IAS | EAS | |||
|---|---|---|---|---|
| Median % (range) | Median mm (range) | Median % (range) | Median mm. (range) | |
| Preoperative | 31 (0–66) | 9 (0–24) | 41.2 (0–89) | 10 (0–28) |
| Postoperative | 32,4 (0–75) | 8 (0–25) | 41.7 (0–100) | 11 (0–29) |
IAS internal anal sphincter; EAS external anal sphincter p > 0.05
Fig. 4.
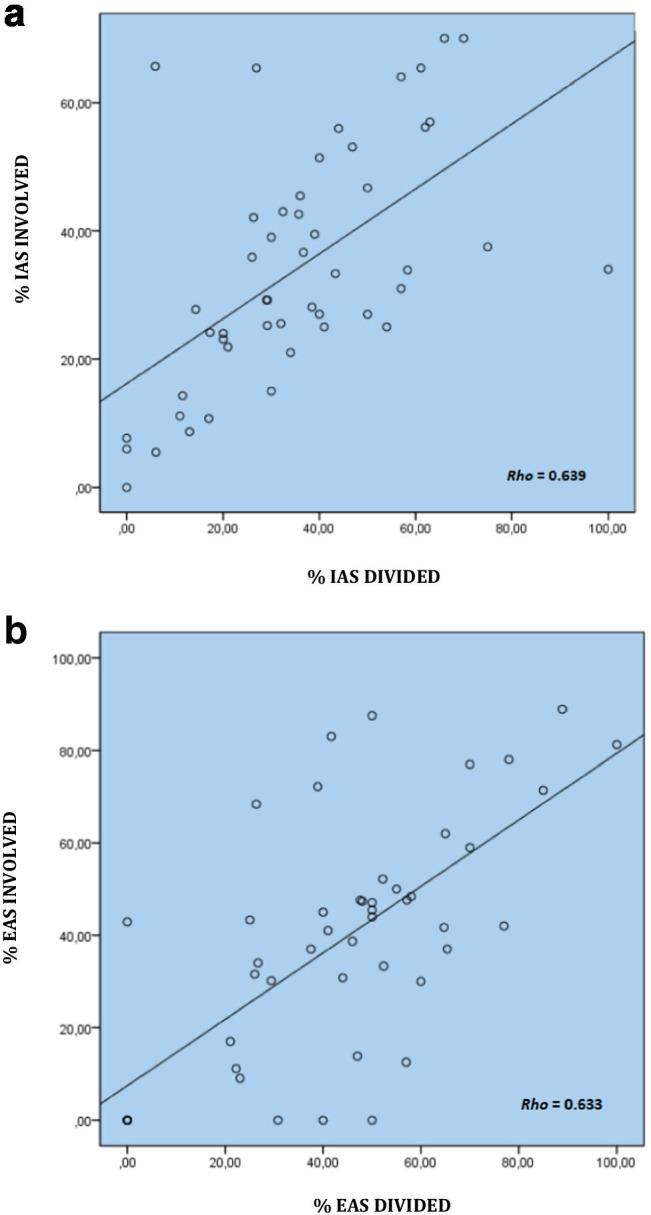
Three-dimensional endoanal ultrasound measurements and their correlation between the percentage of involved sphincter and percentage of divided sphincter pre- and postoperative respectively. a Internal anal sphincter. b External anal sphincter. (Spearman’s correlation p < 0.001)
Anal continence was analysed in relation to the percentage of sphincter divided during fistulotomy. There were significant differences with regards to the thirds of the EAS divided (Likehood ratio = 0.049). When division of the EAS was less than 66%, only 8/33 patients (24.2%) showed deterioration in FI (including mild incontinence) or soiling. However, when more than 66% of the anal sphincter was divided, 5/8 patients (62.8%) showed deterioration in FI (Tables 3 and 4). Division of over 50% of the IAS was associated with significant differences in FI pre- and postoperatively (Table 5). Five of the 8 cases with a deterioration in FI had a division of over 50% and 66% of the IAS and EAS, respectively. Figure 5 shows the relationship between the amount of sphincter involved and the Jorge-Wexner score prior to and 1 year after surgery.
Table 3.
Subdivision of the level of division of the external anal sphincter and immediate postoperative deterioration in anal continence defined as any extent of impairment of faecal incontinence. Divided into thirds
| Deterioration in anal continence | |||
|---|---|---|---|
| No | Yes | ||
| EAS | Low | 11(30.6) | 2 (15.4) |
| Medium | 22 (61.1) | 6 (46.2) | |
| High | 3 (8.3) | 5 (38.5) | |
| Total | 36 | 13 | |
Values are expressed as n (% of subgroup deterioration in anal continence)
low (≤ 33%); medium (33–66%); high (≥ 66%). EAS external anal sphincter. p = 0.049
Table 4.
Subdivision of the level of division of the external anal sphincter and immediate postoperative deterioration in anal continence defined as any extent of impairment of fecal incontinence. Divided into subgroups (below or above 66%)
| Deterioration in anal continence | |||
|---|---|---|---|
| No | Yes | ||
| EAS | < 66% | 33 (91.7) | 8 (61.5) |
| ≥ 66% | 3 (8.3) | 5 (38.5) | |
| Total | 36 | 13 | |
Values are expressed as n (% of subgroup deterioration in anal continence)
EAS external anal sphincter. p = 0.018
Table 5.
Subdivision of the level of division of the internal anal sphincter (below or above 50%) and deterioration in anal continence defined as any extent of impairment of faecal incontinence in the immediate postoperative period
| Deterioration in anal continence | |||
|---|---|---|---|
| No | Yes | ||
| IAS | < 50% | 32 (88.9) | 5 (38.5) |
| ≥ 50% | 4 (11.1) | 8 (61.5) | |
| Total | 36 | 13 | |
Values are expressed as n (% of subgroup deterioration in anal continence)
IAS internal anal sphincter, p = 0.001
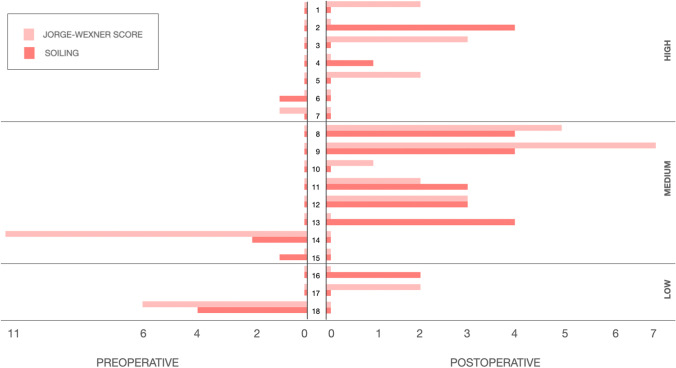
Fig. 5.
The relationship between the amount of sphincter involved and the Jorge-Wexner score prior to and 1 year after surgery
Five (10.2%) patients had some degree of preoperative FI, and 8 (16.3%) had a deterioration in Jorge-Wexner score 1 year after surgery, of which 6 had mild incontinence with a Jorge-Wexner score < 3. The 2 patients with a major degree of incontinence were the following: the first case had a transsphincteric fistula which crossed above 47.4% of the EAS and 26.9% of the IAS pre, with division of 70% of the EAS and 50% of the IAS postoperatively. The second case had a fistula that involved 47.6% of EAS and 29% of the IAS pre with division of 48% of the EAS and 29.2% of the IAS divided postoperatively. No patients had a Jorge-Wexner score > 4 at 1-year follow-up.
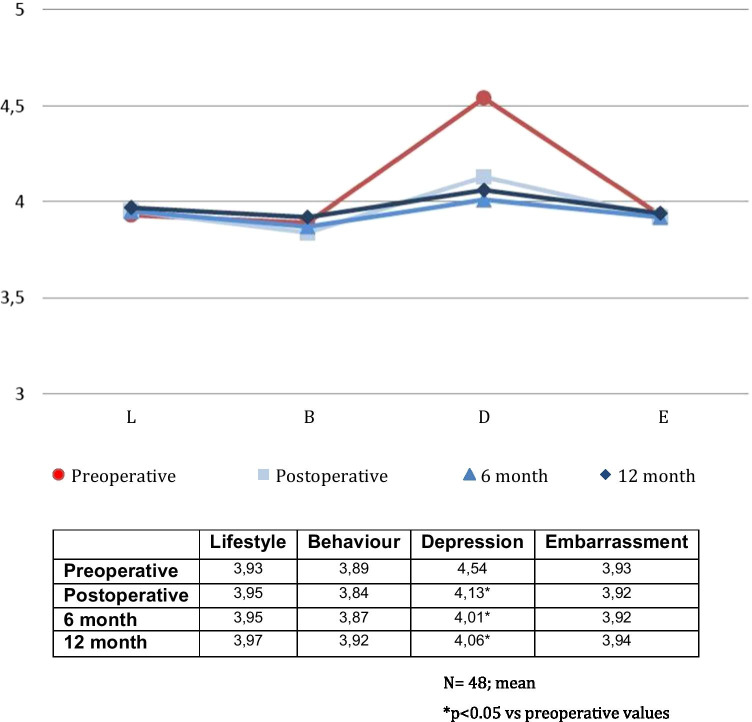
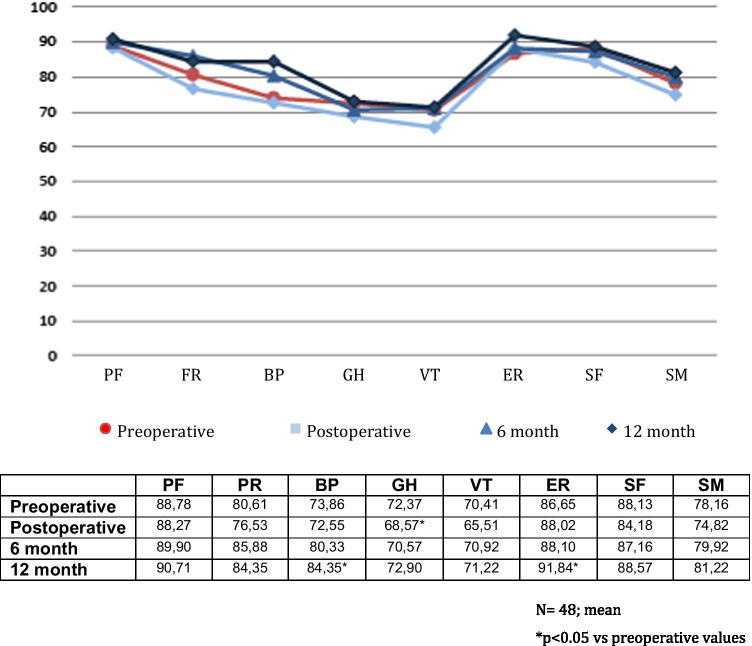
One patient did not complete the QOL questionnaires. QOL analysis of 48 patients showed no significant differences in FIQOL in the immediate, 6- and 12-month follow-ups (p > 0.05). Figure 6 shows the results of the FIQOLS. All domains showed an initial deterioration which slowly improved over time. Depression was the only domain which showed a statistically significant deterioration and improvement. The SF-36 score showed an improvement at annual follow-up, most evident in the body pain and emotional role areas, both of which were statistically significant as shown in Fig. 7. There was 1 fistula recurrence.
Fig. 6.
Preoperative, immediate postoperative and 6- and 12-month faecal incontinence quality of life core (L, lifestyle; B, behaviour; D, depression; E, embarrassment)
Fig. 7.
Preoperative, immediate postoperative and 6- and 12-month SF-36 score (PF, physical function; RF, physical role; BP, body pain; GH, general heath; VT, vitality; ER, emotional role; SF, social function; SM, mental health)
Discussion
The present study shows a strong correlation between pre- and postoperative, IAS and EAS measurements. A median length of 41% of EAS and 32% of IAS was divided during fistulotomy without a significant long-term deterioration in FI, soiling or QOLS. Division of the lower two-thirds of the EAS was associated with a deterioration in FI in 8/33 (24,2%) of patients, whilst division above this cut-off point was associated with a deterioration in FI in 5/8 (62,8%) of patients. There are currently no studies using 3D-EAUS to quantify the extent of sphincter division after fistulotomy and correlate this with postoperative FI. A study in 40 patients which evaluates fistulotomies/fistulectomies with 2D-EAUS concludes that fistulotomies produce less damage to the sphincters, but there is no mention of cure or incontinence rates [13]. Voyvodic et al. published a study in 330 patients and found no correlation between the extent of fistulotomy measured by 2D-EAUS, manometry and postoperative incontinence [14]. These studies were performed using 2D-EAUS, a subjective, observer-dependant technique which fails to objectively quantify sphincter division, as opposed to 3D-EAUS. Recently, Murad-Regadas published the results of a cohort of patients with less than 40–50% of EAS involvement preoperatively, who were scheduled for fistulotomy, and reported a 33% rate of FI but did not correlate the length of division with FI [4]. The present study reports the value of 3D-EAUS to quantify the length of IAS and EAS divided during fistulotomy and its correlation with postoperative FI. These measurements are helpful to indicate the appropriate surgical technique for each type of fistula, minimizing the risk of postoperative FI and recurrence. The same group published a study in 2012 conducted in 36 fistulotomy patients and found a strong correlation between preoperative 3D-EAUS measurement of fistula height with intra and postoperative 3D-EAUS measurement of IAS and EAS division [5]. Similar results were found in 16 patients undergoing rectal mucosal advancement flap for perianal fistulae [15]. 3D-EAUS therefore becomes a fundamental tool for pre- and postoperative evaluation of perianal fistulae and sphincter division.
Some authors have modified the Parks classification dividing fistulas in intersphincteric, low, mid and high transsphincteric, suprasphincteric and extrasphincteric [16]. The subdivision of transsphincteric fistulas according to the level at which they cross the EAS, dividing the latter in thirds tends to be arbitrary. Like other authors, we believe fistulotomy is the treatment of choice for intersphincteric and low transsphincteric fistulas, but those that affect the mid or high portions of the EAS remain a surgical challenge [17].
FI was mild for all patients with a Jorge-Wexner score < 4 in all cases, similar to the FI rates ranging from 7 to 44% reported by other authors [2, 4, 18–20]. The aetiology of FI is multifactorial, and the rate of reported incontinence also depends on the definition and arduousness with which it is sought. We took into account even the mildest forms of incontinence. In addition, there was no significant difference between pre- and postoperative FIQOLS in the long term except for depression which showed a significant deterioration and subsequent improvement. Likewise, all domains of the SF-36 scores deteriorated in the immediate postoperative period and improved to baseline or above values 1 year after follow-up. Significant differences in the emotional role, general health or body pain scores could be explained by the fear of surgery-related incontinence or that patients were more aware of their body in the immediate postoperative period just after going through surgery. Other authors have found that the severity of incontinence increases with the complexity of the fistula, negatively influencing quality of life in 141 patients who underwent fistula surgery [21].
2D-EAUS has also been used to evaluate the extent of sphincterotomy for treatment of chronic anal fissure and compare this to postoperative incontinence and other clinical parameters [22–25]. 3D-EAUS allows us to quantitatively measure the length of lateral internal sphincterotomy or fistulotomy, determining the percentage of sphincter which has been divided and correlate this with postoperative results. 3D-EAUS therefore becomes a fundamental tool for pre and postoperative evaluation of perianal fistulae and sphincter division.
This study has several limitations such as the number of patients included even though this is equal to or superior to other studies [26, 27]. All scans and measurements in this study have been performed by the same surgeon, which can reduce interobserver variability on the one hand but also be a source of bias. Notwithstanding, the strengths of this study include the thorough pre- and postoperative evaluation of fistulotomies with 3D-EAUS and extensive search for and follow-up of postoperative FI using the Jorge-Wexner score and QOLS.
In conclusion, 3D-EAUS is a valuable tool for quantifying the extent of sphincter involvement pre- and postoperatively. Post-fistulotomy faecal incontinence is mild and increases with increasing length of sphincter division, with the highest probability when over two-thirds of the EAS is divided. This does not affect long-term quality of life.
Authors’ Contributions
All the authors have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and final approval of the version to be published.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.www.for2240.de); EU COST BM1302 (CC, DH); EU Horizon 2020 ARREST BLINDNESS (CC).
Data availability
Data is available on request.
Declarations
Ethics approval
The study protocol was approved by the hospital ethics committee.
Informed consent
Informed consent was obtained from all individual participants included in the study to participate and for publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murad-Regadas SM, Regadas FS, Rodrigues LV, Holanda Ede C, Barreto RG, Oliveira L. The role of 3-dimensional anorectal ultrasonography in the assessment of anterior transsphincteric fistula. Dis Colon Rectum. 2010;53(7):1035–1040. doi: 10.1007/DCR.0b013e3181dce163. [DOI] [PubMed] [Google Scholar]
- 2.van Koperen PJ, Wind J, Bemelman WA, Bakx R, Reitsma JB, Slors JF. Long-term functional outcome and risk factors for recurrence after surgical treatment for low and high perianal fistulas of cryptoglandular origin. Dis Colon Rectum. 2008;51(10):1475–1481. doi: 10.1007/s10350-008-9354-9. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39(7):723–9. doi: 10.1007/BF02054434. [DOI] [PubMed] [Google Scholar]
- 4.Murad-Regadas SM, Regadas Filho FSP, Holanda EC, Veras LB, Vilarinho ADS, Lopes MS. Can three-dimensional anorectal ultrasonography be included as a diagnostic tool for the assessment of anal fistula before and after surgical treatment? Arq Gastroenterol. 2018;55Suppl 1(Suppl 1):18–24. doi: 10.1590/S0004-2803.201800000-42. [DOI] [PubMed] [Google Scholar]
- 5.Garcés-Albir M, García-Botello SA, Esclapez-Valero P, Sanahuja-Santafé A, Raga-Vázquez J, Espi-Macías A, Ortega-Serrano J. Quantifying the extent of fistulotomy. How much sphincter can we safely divide? A three-dimensional endosonographic study. Int J Colorectal Dis. 2012;27(8):1109–16. doi: 10.1007/s00384-012-1437-3. [DOI] [PubMed] [Google Scholar]
- 6.Garcés-Albir M, García-Botello SA, Espi A, Pla-Martí V, Martin-Arevalo J, Moro-Valdezate D, Ortega J. Three-dimensional endoanal ultrasound for diagnosis of perianal fistulas: reliable and objective technique. World J Gastrointest Surg. 2016;8(7):513–520. doi: 10.4240/wjgs.v8.i7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brillantino A, Iacobellis F, Di Sarno G, D’Aniello F, Izzo D, Paladino F, De Palma M, Castriconi M, Grassi R, Di Martino N, Renzi A. Role of tridimensional endoanal ultrasound (3D-EAUS) in the preoperative assessment of perianal sepsis. Int J Colorectal Dis. 2015;30(4):535–542. doi: 10.1007/s00384-015-2167-0. [DOI] [PubMed] [Google Scholar]
- 8.Garcés Albir M, GarcíaBotello S, Esclápez Valero P, SanahujaSantafé A, EspíMacías A, Flor Lorente B, García-Granero E. Evaluación de las fístulas perianales mediante ecografía endoanal tridimensional y correlación con los hallazgos intraoperatorios [Evaluation of three-dimensional endoanal endosonography of perianal fistulas and correlation with surgical findings] Cir Esp. 2010;87(5):299–305. doi: 10.1016/j.ciresp.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 10.Keighley MRB, Williams NS (2008) Fecal incontinence. In: Surgery of the anus, rectum and colon, 3rd edn. Elsevier, Philadelphia, p 593
- 11.Ware JE, GandaK B, the IQOLA project group The SF-36® Health survey: development and use in mental health research and the IQOLA Project. Int J Mental Health. 1994;23:49–73. doi: 10.1080/00207411.1994.11449283. [DOI] [Google Scholar]
- 12.Minguez M, Garrigues V, Soria MJ, Andreu M, Mearin F, Clave P. Adaptation to Spanish language and validation of the fecal incontinence quality of life scale. Dis Colon Rectum. 2006;49(4):490–499. doi: 10.1007/s10350-006-0514-5. [DOI] [PubMed] [Google Scholar]
- 13.Belmonte Montes C, Ruiz Galindo GH, Montes Villalobos JL, Decanini Terán C. C. Fistulotomía vs fistulectomía. Valoración ultrasonográfica de lesión al mecanismo de esfínter anal [Fistulotomy vs fistulectomy. Ultrasonographic evaluation of lesion of the anal sphincter function] Rev Gastroenterol Mex. 1999;64(4):167–70. [PubMed] [Google Scholar]
- 14.Voyvodic F, Rieger NA, Skinner S, Schloithe AC, Saccone GT, Sage MR, Wattchow DA. Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum. 2003;46(6):735–741. doi: 10.1007/s10350-004-6650-x. [DOI] [PubMed] [Google Scholar]
- 15.Garcés Albir M, García-Botello SA, Pla-Martí V, Martín-Arévalo J, Moro-Valdezate D, Espi A, Ortega J. Rectal advancement flaps for the treatment of transphincteric perianal fistulas: a three-dimensional endoanal ultrasound and quality of life assessment. Rev Esp Enferm Dig. 2020;112(11):860–863. doi: 10.17235/reed.2020.7187/2020. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Luna A, García-Domingo MI, Rius-Macías J, Marco-Molina C. Ultrasound study of anal fistulas with hydrogen peroxide enhancement. Dis Colon Rectum. 2004;47(1):108–114. doi: 10.1007/s10350-003-0015-8. [DOI] [PubMed] [Google Scholar]
- 17.Schouten WR (2008) Abscess, Fistula. In: Harold A, Lehur PA, Matzel KE, O´Connell PR (eds) European Manual of Medicine. Coloproctology. Verlag Berlin Heidelberg: Springer, pp 53–9.
- 18.Westerterp M, Volkers NA, Poolman RW, van Tets WF. Anal fistulotomy between Scylla and Charybdis. Colorectal Dis. 2003;5(6):549–551. doi: 10.1046/j.1463-1318.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Hagen SJ, Baeten CG, Soeters PB, van Gemert WG. Long-term outcome following mucosal advancement flap for high perianal fistulas and fistulotomy for low perianal fistulas: recurrent perianal fistulas: failure of treatment or recurrent patient disease? Int J Colorectal Dis. 2006;21(8):784–790. doi: 10.1007/s00384-005-0072-7. [DOI] [PubMed] [Google Scholar]
- 20.Pascual Migueláñez I, García-Olmo D, Martínez-Puente MC, Pascual Montero JA. Is routine endoanal ultrasound useful in anal fistulas? Rev Esp Enferm Dig. 2005;97(5):323–7. doi: 10.4321/s1130-01082005000500004. [DOI] [PubMed] [Google Scholar]
- 21.Visscher AP, Schuur D, Roos R, Van der Mijnsbrugge GJ, Meijerink WJ, Felt-Bersma RJ. Long-term follow-up after surgery for simple and complex cryptoglandular fistulas: fecal incontinence and impact on quality of life. Dis Colon Rectum. 2015;58(5):533–539. doi: 10.1097/DCR.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 22.García-Granero E, Sanahuja A, García-Armengol J, Jiménez E, Esclapez P, Mínguez M, Espí A, López F, Lledó S. Anal endosonographic evaluation after closed lateral subcutaneous sphincterotomy. Dis Colon Rectum. 1998;41(5):598–601. doi: 10.1007/BF02235266. [DOI] [PubMed] [Google Scholar]
- 23.García-Granero E, Sanahuja A, García-Botello SA, Faiz O, Esclápez P, Espí A, Flor B, Minguez M, Lledó S. The ideal lateral internal sphincterotomy: clinical and endosonographic evaluation following open and closed internal anal sphincterotomy. Colorectal Dis. 2009;11(5):502–507. doi: 10.1111/j.1463-1318.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- 24.Menteş BB, Ege B, Leventoglu S, Oguz M, Karadag A. Extent of lateral internal sphincterotomy: up to the dentate line or up to the fissure apex? Dis Colon Rectum. 2005;48(2):365–370. doi: 10.1007/s10350-004-0812-8. [DOI] [PubMed] [Google Scholar]
- 25.Menteş BB, Güner MK, Leventoglu S, Akyürek N. Fine-tuning of the extent of lateral internal sphincterotomy: spasm-controlled vs. up to the fissure apex. Dis Colon Rectum. 2008;51(1):128–33. doi: 10.1007/s10350-007-9121-3. [DOI] [PubMed] [Google Scholar]
- 26.Law PJ, Bartram CI (1989 Fall) Anal endosonography: technique and normal anatomy. Gastrointest Radiol 14(4):349–53. 10.1007/BF01889235 [DOI] [PubMed]
- 27.West RL, Zimmerman DD, Dwarkasing S, Hussain SM, Hop WC, Schouten WR, Kuipers EJ, Felt-Bersma RJ. Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis Colon Rectum. 2003;46(10):1407–1415. doi: 10.1007/s10350-004-6758-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request.